Mechanistic Exploration of Smilax glabra Roxb. in Osteoarthritis: Insights from Network Pharmacology, Molecular Docking, and In Vitro Validation
Abstract
1. Introduction
2. Results
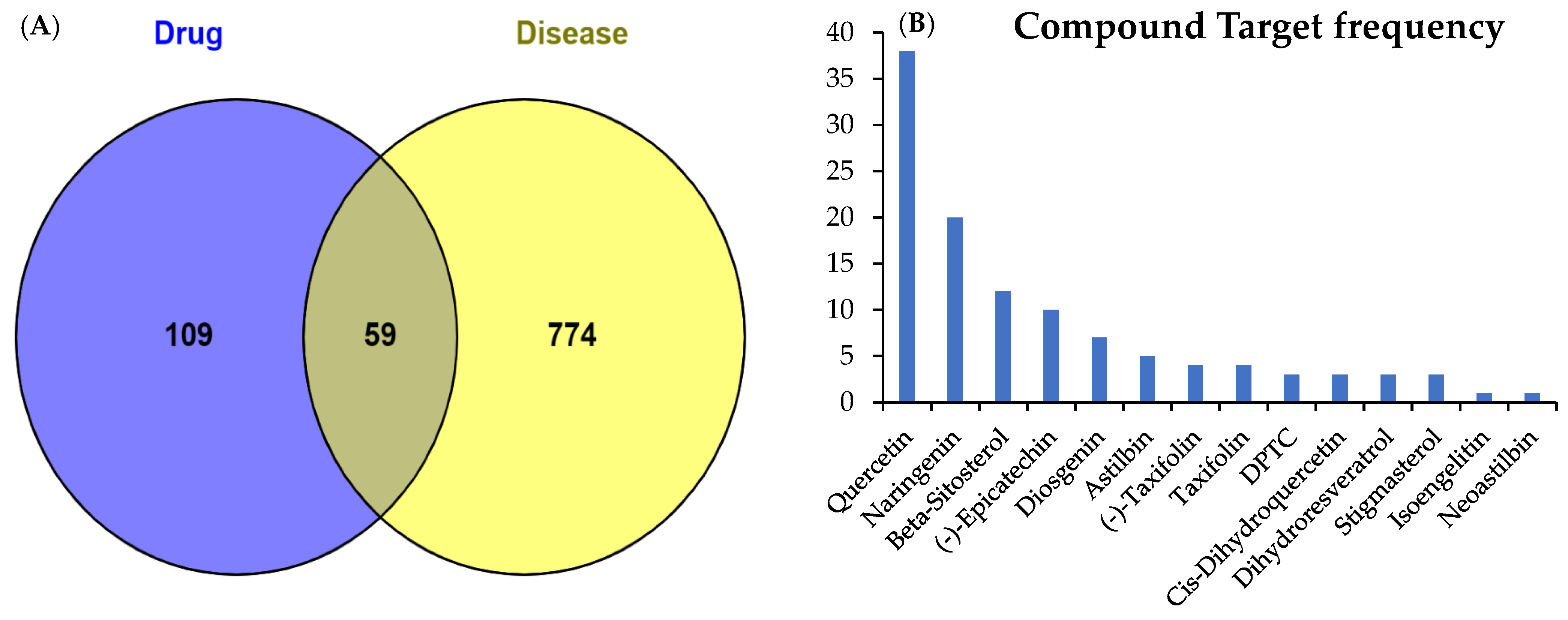
2.1. Active Compounds and Targets of SGR
2.2. Potential Targets of Arthritis
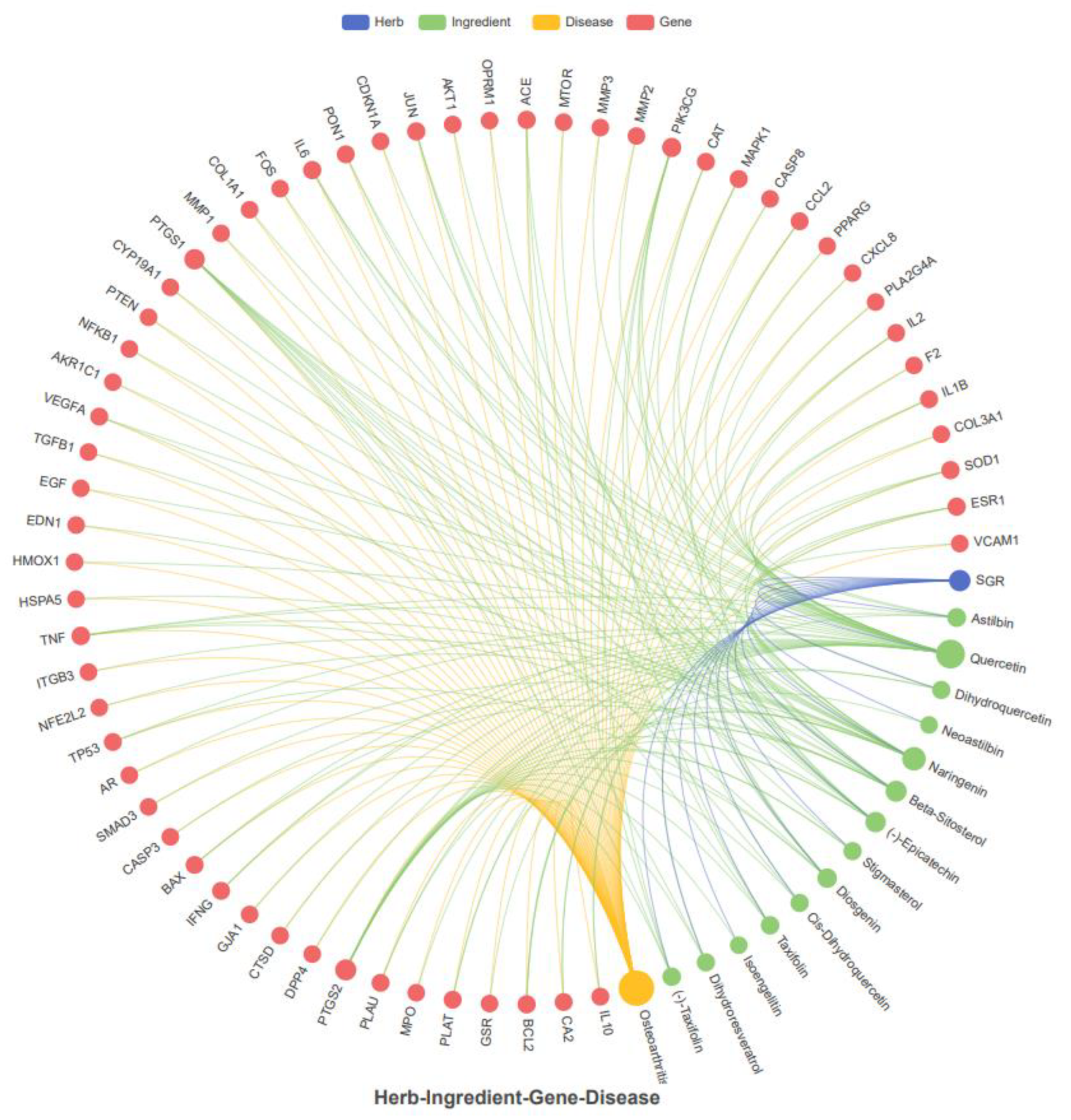
2.3. Common Target Determination and Network Construction
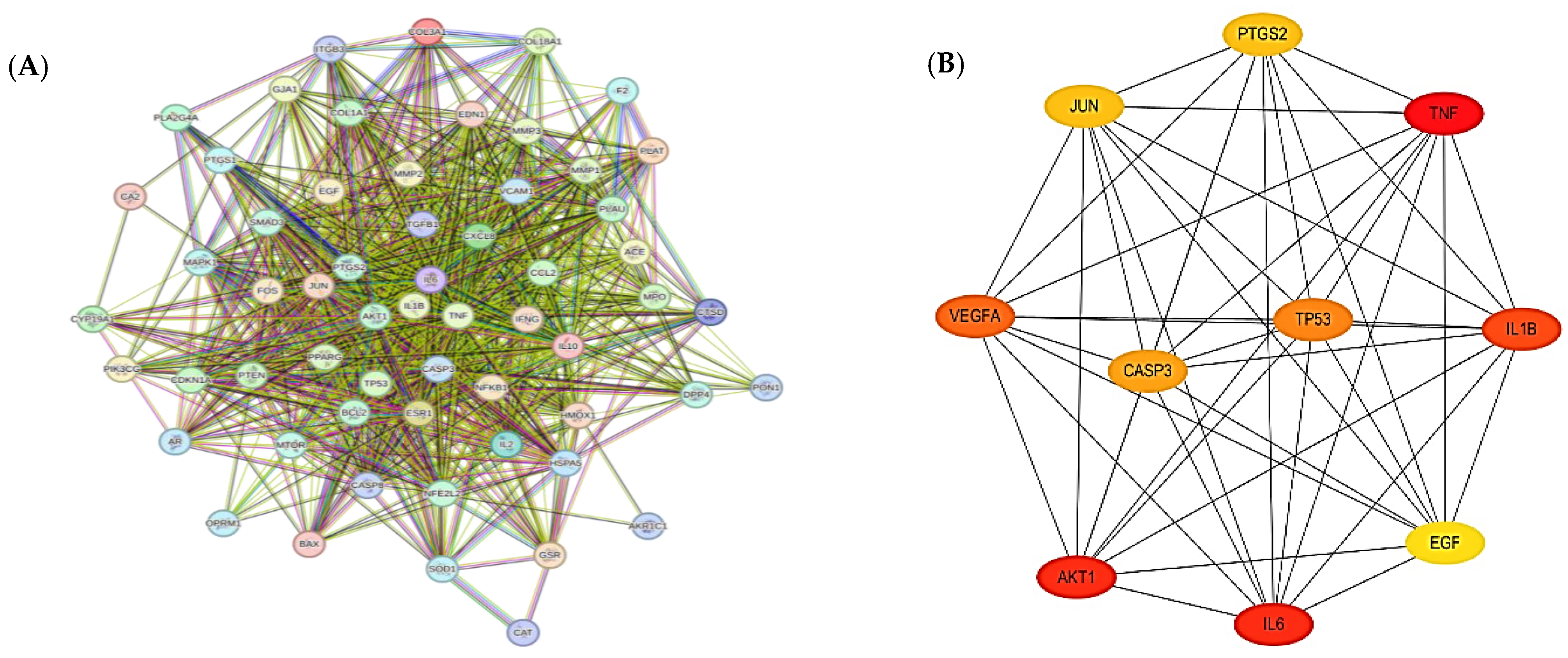
2.4. Network Visualization and Identification of Hub Targets
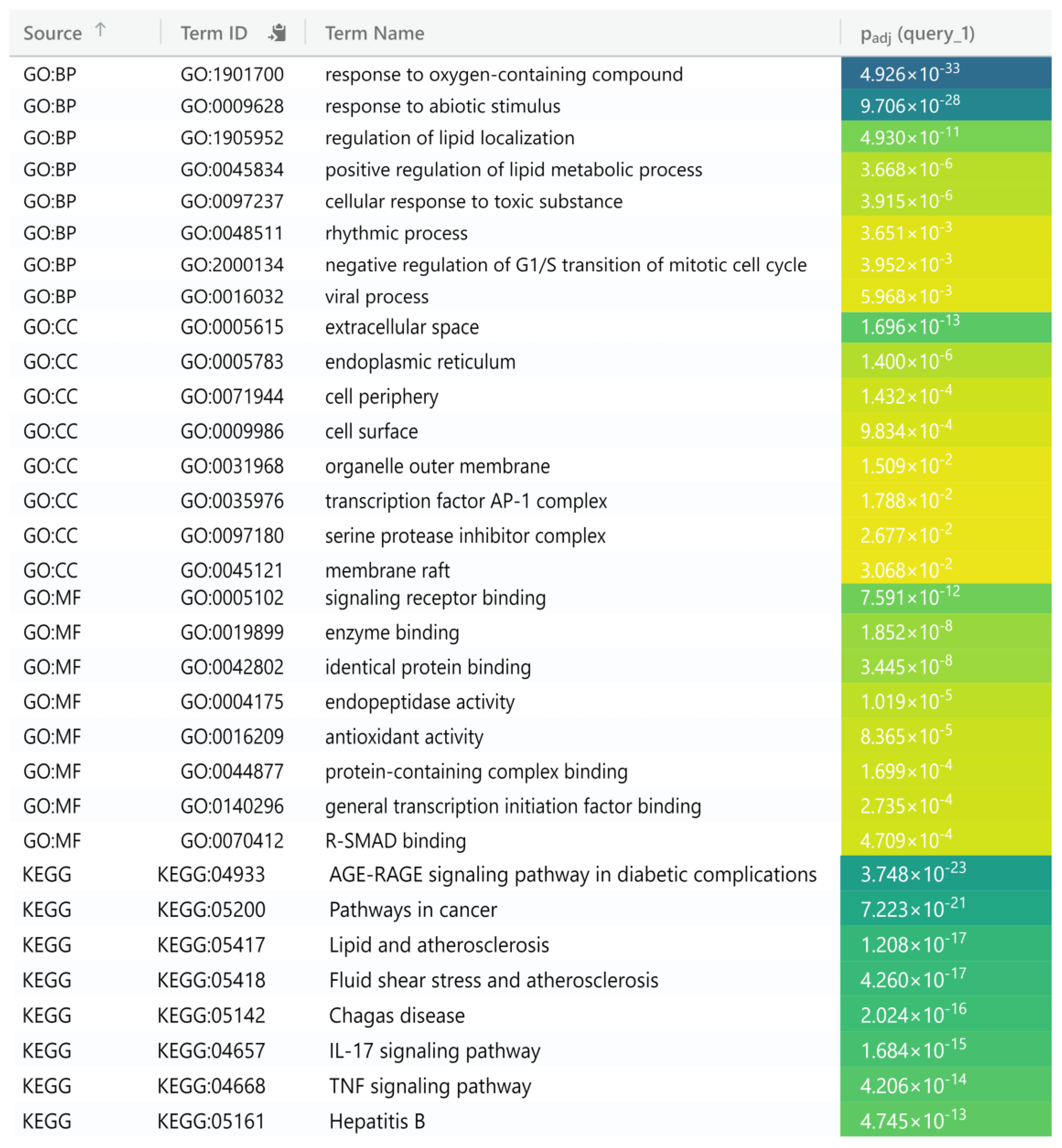
2.5. Functional Annotation of Common Target Genes
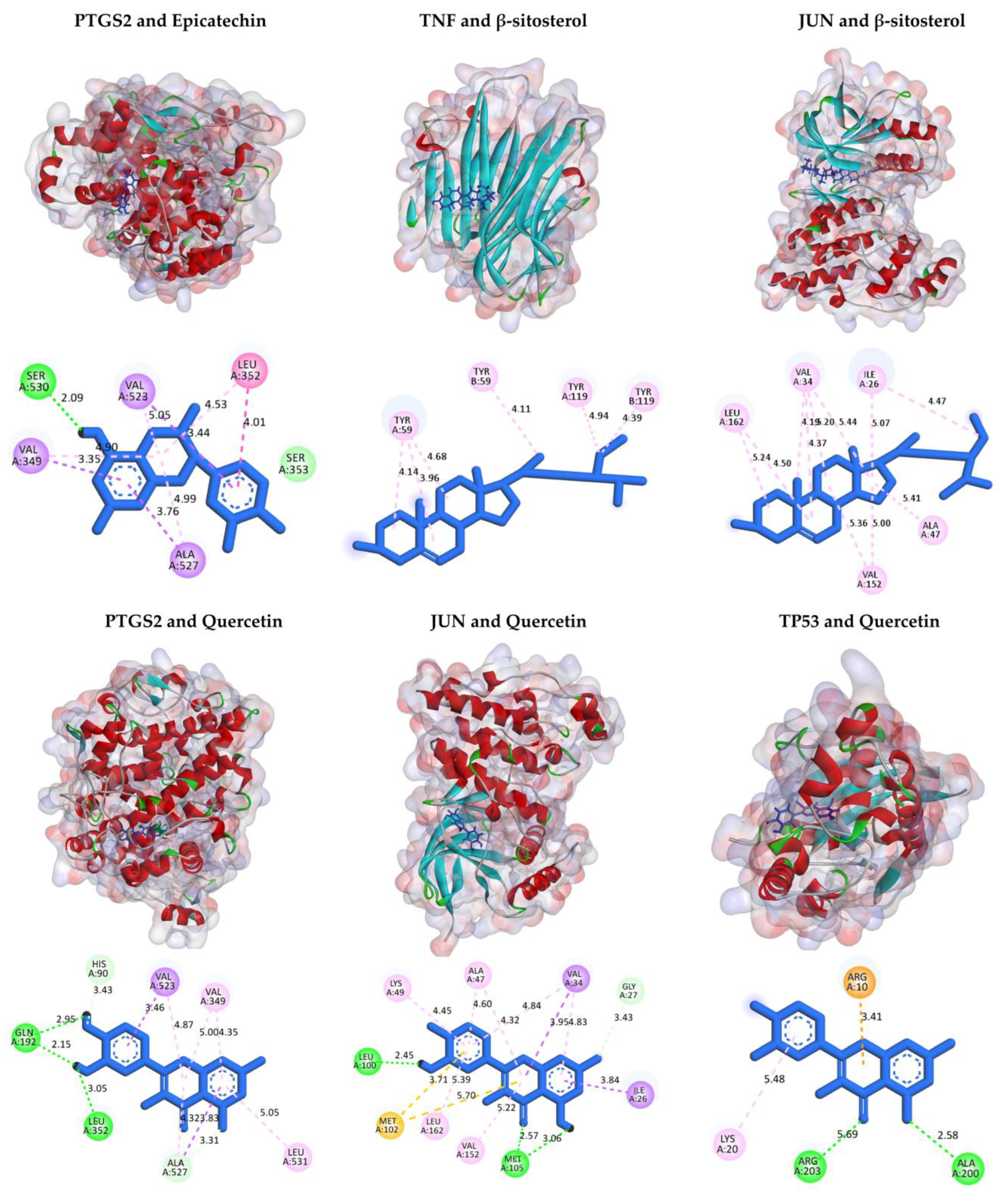
2.6. Molecular Docking
2.7. Compounds and Target Binding Affinity Prediction
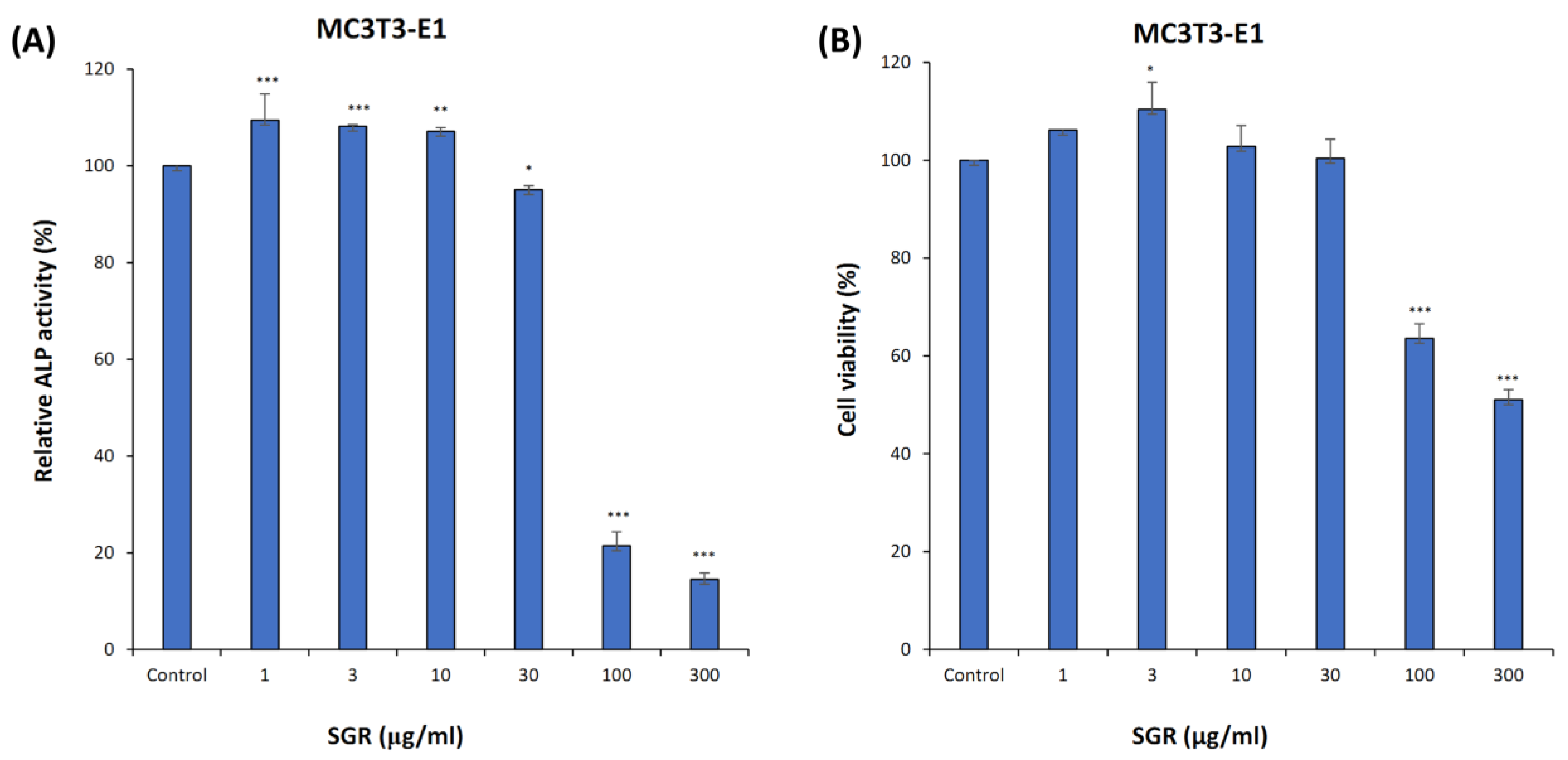
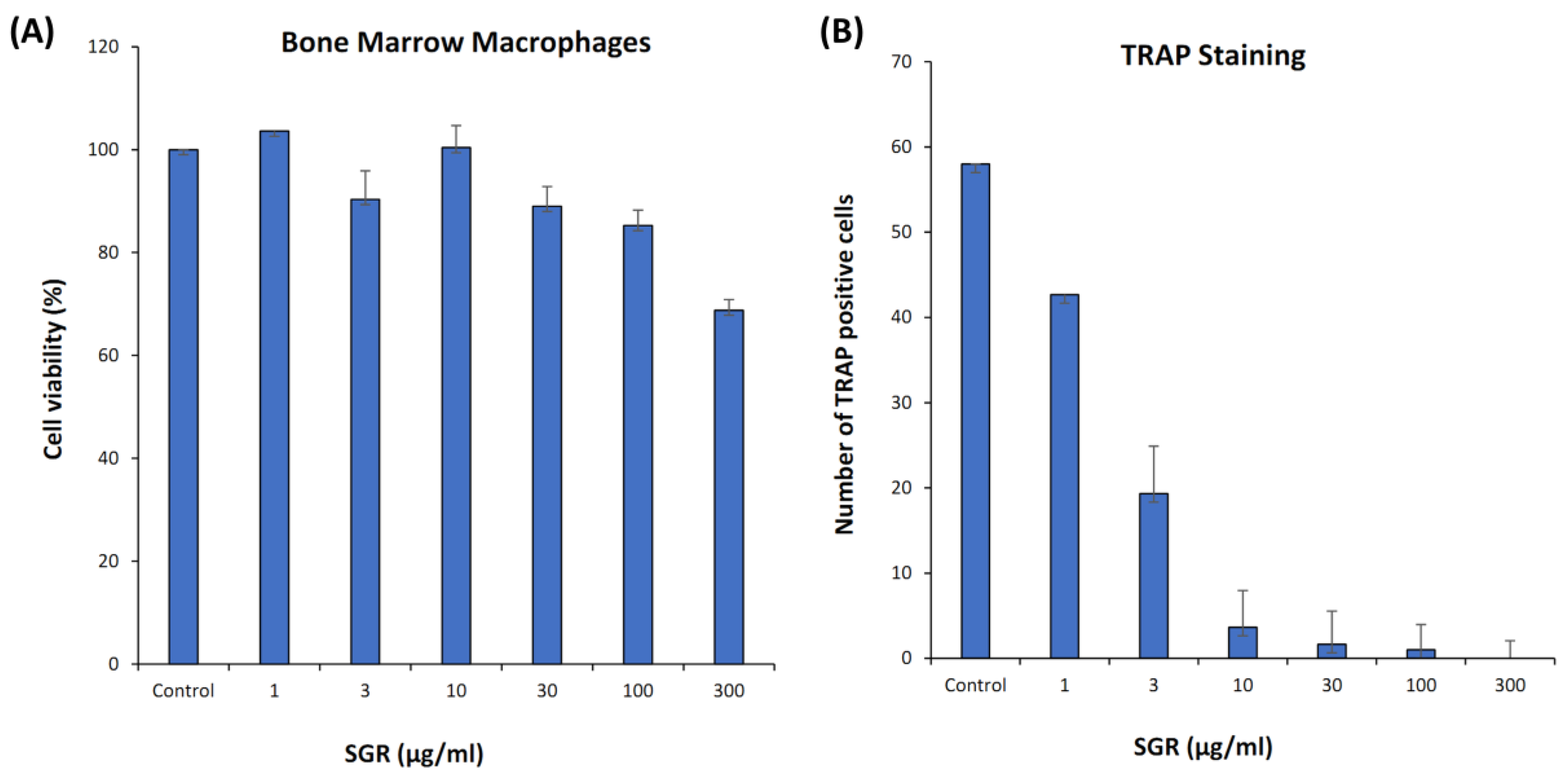
2.8. ALP Activity, Cell Viability, and TRAP Staining
3. Discussion
4. Material and Methods
4.1. Experimental Procedures
4.1.1. Data Acquisition of SGR
4.1.2. Potential Targets of Arthritis
4.1.3. Common Target Determination and Network Construction
4.1.4. Network Visualization and PPI Analysis
4.1.5. Functional Annotation of Common Target Genes
4.1.6. Molecular Docking
4.1.7. Compound Targets Predictions Based on MPNN and CNN
4.1.8. Plant Extract Preparation
4.1.9. Cell Culturing and ALP Activity
4.1.10. Cell Viability Assay
4.1.11. TRAP Staining
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hunter, D.J.; Nicolson, P.J.A.; Little, C.B.; Robbins, S.R.; Wang, X.; Bennell, K.L. Developing Strategic Priorities in Osteoarthritis Research: Proceedings and Recommendations Arising from the 2017 Australian Osteoarthritis Summit. BMC Musculoskelet. Disord. 2019, 20, 74. [Google Scholar] [CrossRef]
- McAlindon, T.E.; Bannuru, R.R. OARSI Recommendations for the Management of Hip and Knee Osteoarthritis: The Semantics of Differences and Changes. Osteoarthr. Cartil. 2010, 18, 473–475. [Google Scholar] [CrossRef] [PubMed]
- Bruyère, O.; Cooper, C.; Pelletier, J.-P.; Branco, J.; Luisa Brandi, M.; Guillemin, F.; Hochberg, M.C.; Kanis, J.A.; Kvien, T.K.; Martel-Pelletier, J.; et al. An Algorithm Recommendation for the Management of Knee Osteoarthritis in Europe and Internationally: A Report from a Task Force of the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO). Semin. Arthritis Rheum. 2014, 44, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Hochberg, M.C.; Altman, R.D.; April, K.T.; Benkhalti, M.; Guyatt, G.; McGowan, J.; Towheed, T.; Welch, V.; Wells, G.; Tugwell, P. American College of Rheumatology 2012 Recommendations for the Use of Nonpharmacologic and Pharmacologic Therapies in Osteoarthritis of the Hand, Hip, and Knee. Arthritis Care Res. 2012, 64, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Hermann, W.; Lambova, S.; Muller-Ladner, U. Current Treatment Options for Osteoarthritis. Curr. Rheumatol. Rev. 2018, 14, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Jo, H.G.; Seo, J.; Lee, D. Clinical Evidence Construction of East Asian Herbal Medicine for Inflammatory Pain in Rheumatoid Arthritis Based on Integrative Data Mining Approach. Pharmacol. Res. 2022, 185, 106460. [Google Scholar] [CrossRef]
- Guo, M.; Zeng, J.; Wang, Z.; Shen, Y. Advances in the Chemical Constituents, Pharmacological Activity, and Clinical Application of Smilacis Glabrae Rhizoma: A Review and Predictive Analysis of Quality Markers (Q-Markers). Heliyon 2024, 10, e29557. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Wu, S.; Wei, Y.; Liu, J.; Zhou, C.; Chen, T.; Zhu, J.; Tan, W.; Huang, C.; Feng, S.; et al. Exploring the Causal Relationship between Inflammatory Cytokines and Inflammatory Arthritis: A Mendelian Randomization Study. Cytokine 2024, 173, 156446. [Google Scholar] [CrossRef]
- Jo, H.-G.; Baek, C.Y.; Hwang, Y.; Baek, E.; Song, H.S.; Lee, D. Pain Relief, Functional Recovery, and Chondroprotective Effects of Angelica Gigas Nakai in Osteoarthritis Due to Its Anti-Inflammatory Property: An In Vitro and In Vivo Study. Nutrients 2024, 16, 2435. [Google Scholar] [CrossRef]
- Dong, L.; Zhu, J.; Du, H.; Nong, H.; He, X.; Chen, X. Astilbin from Smilax glabra Roxb. Attenuates Inflammatory Responses in Complete Freund’s Adjuvant-Induced Arthritis Rats. Evid. Based Complement. Altern. Med. 2017, 2017, 8246420, Erratum in Evid. Based Complement. Altern. Med. 2018, 2018, 6279328. [Google Scholar] [CrossRef]
- Jiang, J.; Xu, Q. Immunomodulatory Activity of the Aqueous Extract from Rhizome of Smilax Glabra in the Later Phase of Adjuvant-Induced Arthritis in Rats. J. Ethnopharmacol. 2003, 85, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wang, Y.; Zhang, B.; Li, Y.; Ren, Z.; Huang, J.; Zhang, Z.; Lin, Z.; Zhang, X. Smilax Glabra Roxb.: A Review of Its Traditional Usages, Phytochemical Constituents, Pharmacological Properties, and Clinical Applications. Drug Des. Dev. Ther. 2022, 16, 3621–3643. [Google Scholar] [CrossRef]
- Bao, Y.; Li, H.; Li, Q.Y.; Li, Y.; Li, F.; Zhang, C.F.; Wang, C.Z.; Yuan, C.S. Therapeutic Effects of Smilax Glabra and Bolbostemma Paniculatum on Rheumatoid Arthritis Using a Rat Paw Edema Model. Biomed. Pharmacother. 2018, 108, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Su, Y.; Qu, L.; Xu, S.; Meng, L.; Cai, S.-Q.; Shou, C. Mitochondrial Apoptosis Contributes to the Anti-Cancer Effect of Smilax Glabra Roxb. Toxicol. Lett. 2011, 207, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Diao, H.; Kang, Z.C.; Han, F.; Jiang, W.L. Astilbin Protects Diabetic Rat Heart against Ischemia-Reperfusion Injury via Blockade of HMGB1-Dependent NF-ΚB Signaling Pathway. Food Chem. Toxicol. 2014, 63, 104–110. [Google Scholar] [CrossRef]
- Zhang, Q.F.; Fu, Y.J.; Huang, Z.W.; Shangguang, X.C.; Guo, Y.X. Aqueous Stability of Astilbin: Effects of PH, Temperature, and Solvent. J. Agric. Food Chem. 2013, 61, 12085–12091. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-W.; Xu, Y.; Weng, Y.-Y.; Fan, X.-Y.; Bai, Y.-F.; Zheng, X.-Y.; Lou, L.-J.; Zhang, F. Astilbin Ameliorates Cisplatin-Induced Nephrotoxicity through Reducing Oxidative Stress and Inflammation. Food Chem. Toxicol. 2018, 114, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Gao, S.; Jie, W.; Ao, Q.; Huang, Y. Astilbin Inhibits Proliferation of Rat Aortic Smooth Muscle Cells Induced by Angiotensin II and Down-Regulates Expression of Protooncogene. J. Huazhong Univ. Sci. Technol. Med. Sci. 2012, 32, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Hayer, S.; Niederreiter, B.; Kalkgruber, M.; Wanic, K.; Maibner, J.; Smolen, J.S.; Aletaha, D.; Bluml, S.; Redlich, K. Analysis of Combined Deficiency of Interleukin-1 and -6 versus Single Deficiencies in TNF-Mediated Arthritis and Systemic Bone Loss. Bone Jt. Res. 2022, 11, 484–493. [Google Scholar] [CrossRef]
- Avci, A.B.; Feist, E.; Burmester, G.R. Targeting IL-6 or IL-6 Receptor in Rheumatoid Arthritis: What Have We Learned? BioDrugs 2024, 38, 61–71. [Google Scholar]
- Wang, M.; Zhou, Y.; Huang, W.; Zeng, Y.; Li, X. Association between Matrix Metalloproteinase-1 (Mmp-1) Protein Level and the Risk of Rheumatoid Arthritis and Osteoarthritis: A Meta-Analysis. Braz. J. Med. Biol. Res. 2021, 54, e10366. [Google Scholar] [CrossRef] [PubMed]
- Pulik, Ł.; Łęgosz, P.; Motyl, G. Matrix Metalloproteinases in Rheumatoid Arthritis and Osteoarthritis: A State of the Art Review. Reumatologia 2023, 61, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, S.; Singh, N.; Donnelly, D.; Migliore, M.; Johnson, P.; Fishwick, C.; Luke, B.T.; Martin, B.; Maudsley, S.; Fugmann, S.D.; et al. Pharmacophore Model of the Quercetin Binding Site of the SIRT6 Protein. J. Mol. Graph. Model. 2014, 49, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kim, Y.B. Uncovering Quercetin’s Effects against Influenza a Virus Using Network Pharmacology and Molecular Docking. Processes 2021, 9, 1627. [Google Scholar] [CrossRef]
- Wu, X.Y.; Chao, Z.M.; Wang, C.; Sun, W.; Zhang, G.F. Extraction and Crystal Structure of ß-Sitosterol. Jiegou Huaxue 2014, 33, 801–806. [Google Scholar]
- Kofink, M.; Papagiannopoulos, M.; Galensa, R. (-)-Catechin in Cocoa and Chocolate: Occurence and Analysis of an Atypical Flavan-3-Ol Enantiomer. Molecules 2007, 12, 1274–1288. [Google Scholar] [CrossRef]
- Duan, Z.W.; Zhang, J.; Chen, X.J.; Pang, X.; Ma, B.P. Non-Alkaloid Constituents of Hymenocallis Littoralis. Zhongguo Zhongyao Zazhi 2021, 46, 5304–5309. [Google Scholar] [CrossRef] [PubMed]
- Goyal, A.; Agrawal, N. Quercetin: A Potential Candidate for the Treatment of Arthritis. Curr. Mol. Med. 2022, 22, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-R.; Kim, B.-M.; Won, J.-Y.; Lee, K.-A.; Ko, H.M.; Kang, Y.S.; Lee, S.-H.; Kim, K.-W. Quercetin, a Plant Polyphenol, Has Potential for the Prevention of Bone Destruction in Rheumatoid Arthritis. J. Med. Food 2019, 22, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Weihan, Y.; Yaohua, H. Advances in the Mechanism of Quercetin in the Treatment of Osteoarthritis. Chin. J. Bone Jt. Surg. 2019, 12, 477–480. [Google Scholar]
- Wang, X.-P.; Xie, W.-P.; Bi, Y.-F.; Wang, B.-A.; Song, H.-B.; Wang, S.-L.; Bi, R.-X. Quercetin Suppresses Apoptosis of Chondrocytes Induced by IL-1β via Inactivation of P38 MAPK Signaling Pathway. Exp. Ther. Med. 2021, 21, 468. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yin, J.; Zhao, D.; Wang, C.; Zhang, Y.; Wang, Y.; Li, T. Therapeutic Effect and Mechanism of Action of Quercetin in a Rat Model of Osteoarthritis. J. Int. Med. Res. 2020, 48, 030006051987346. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, Y.; Tang, Y.; Lu, H.; Qi, Y.; Li, G.; He, H.; Lu, F.; Yang, Y.; Sun, H. Quercetin Alleviates Osteoarthritis Progression in Rats by Suppressing Inflammation and Apoptosis via Inhibition of IRAK1/NLRP3 Signaling. J. Inflamm. Res. 2021, 14, 3393–3403. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Gui, Z.; Zhou, Y.; Xia, L.; Lin, K.; Xu, Y. Quercetin Alleviates Rat Osteoarthritis by Inhibiting Inflammation and Apoptosis of Chondrocytes, Modulating Synovial Macrophages Polarization to M2 Macrophages. Free Radic. Biol. Med. 2019, 145, 146–160. [Google Scholar] [CrossRef] [PubMed]
- Samadi, F.; Kahrizi, M.S.; Heydari, F.; Arefnezhad, R.; Roghani-Shahraki, H.; Mokhtari Ardekani, A.; Rezaei-Tazangi, F. Quercetin and Osteoarthritis: A Mechanistic Review on the Present Documents. Pharmacology 2022, 107, 464–471. [Google Scholar] [CrossRef]
- Nguyen, H.T.T.; Ngo, Q.V.; Le, D.T.T.; Nguyen, M.T.H.; Nguyen, P.T.M. β-Sitosterol from Clinacanthus nutans Lindau Enhances Osteoblastogenic Activity via Upregulation of Differentiation Related Genes and Proteins. Biosci. Biotechnol. Biochem. 2022, 86, 1615–1622. [Google Scholar] [CrossRef]
- Wan Osman, W.N.F.; Che Ahmad Tantowi, N.A.; Lau, S.F.; Mohamed, S. Epicatechin and Scopoletin Rich Morinda Citrifolia (Noni) Leaf Extract Supplementation, Mitigated Osteoarthritis via Anti-Inflammatory, Anti-Oxidative, and Anti-Protease Pathways. J. Food Biochem. 2019, 43, e12755. [Google Scholar] [CrossRef]
- Imtiyaz, Z.; Lin, Y.T.; Cheong, U.H.; Jassey, A.; Liu, H.K.; Lee, M.H. Compounds Isolated from Euonymus Spraguei Hayata Induce Ossification through Multiple Pathways. Saudi J. Biol. Sci. 2020, 27, 2227–2237. [Google Scholar] [CrossRef]
- Byun, M.R.; Sung, M.K.; Kim, A.R.; Lee, C.H.; Jang, E.J.; Jeong, M.G.; Noh, M.; Hwang, E.S.; Hong, J.-H. (−)-Epicatechin Gallate (ECG) Stimulates Osteoblast Differentiation via Runt-Related Transcription Factor 2 (RUNX2) and Transcriptional Coactivator with PDZ-Binding Motif (TAZ)-Mediated Transcriptional Activation. J. Biol. Chem. 2014, 289, 9926–9935. [Google Scholar] [CrossRef]
- Jin, L.; Zeng, W.; Zhang, F.; Zhang, C.; Liang, W. Naringenin Ameliorates Acute Inflammation by Regulating Intracellular Cytokine Degradation. J. Immunol. 2017, 199, 3466–3477. [Google Scholar] [CrossRef]
- Nor Muhamad, M.L.; Ekeuku, S.O.; Wong, S.K.; Chin, K.Y. A Scoping Review of the Skeletal Effects of Naringenin. Nutrients 2022, 14, 4851. [Google Scholar] [CrossRef] [PubMed]
- Attur, M.; Al-Mussawir, H.E.; Patel, J.; Kitay, A.; Dave, M.; Palmer, G.; Pillinger, M.H.; Abramson, S.B. Prostaglandin E2 Exerts Catabolic Effects in Osteoarthritis Cartilage: Evidence for Signaling via the EP4 Receptor. J. Immunol. 2008, 181, 5082–5088. [Google Scholar] [CrossRef]
- Stannus, O.; Jones, G.; Cicuttini, F.; Parameswaran, V.; Quinn, S.; Burgess, J.; Ding, C. Circulating Levels of IL-6 and TNF-α Are Associated with Knee Radiographic Osteoarthritis and Knee Cartilage Loss in Older Adults. Osteoarthr. Cartil. 2010, 18, 1441–1447. [Google Scholar] [CrossRef]
- Li, Z.; Zhong, L.; Du, Z.; Chen, G.; Shang, J.; Yang, Q.; Wang, Q.; Song, Y.; Zhang, G. Network Analyses of Differentially Expressed Genes in Osteoarthritis to Identify Hub Genes. Biomed. Res. Int. 2019, 2019, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kitaura, H.; Marahleh, A.; Ohori, F.; Noguchi, T.; Nara, Y.; Pramusita, A.; Kinjo, R.; Ma, J.; Kanou, K.; Mizoguchi, I. Role of the Interaction of Tumor Necrosis Factor-α and Tumor Necrosis Factor Receptors 1 and 2 in Bone-Related Cells. Int. J. Mol. Sci. 2022, 23, 1481. [Google Scholar] [CrossRef] [PubMed]
- Roman-Blas, J.A.; Jimenez, S.A. NF-ΚB as a Potential Therapeutic Target in Osteoarthritis and Rheumatoid Arthritis. Osteoarthr. Cartil. 2006, 14, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Murahashi, Y.; Yano, F.; Chijimatsu, R.; Nakamoto, H.; Maenohara, Y.; Amakawa, M.; Miyake, Y.; Yamanaka, H.; Iba, K.; Yamashita, T.; et al. Oral Administration of EP4-Selective Agonist KAG-308 Suppresses Mouse Knee Osteoarthritis Development through Reduction of Chondrocyte Hypertrophy and TNF Secretion. Sci. Rep. 2019, 9, 20329. [Google Scholar] [CrossRef]
- Tchetina, E.V.; Di Battista, J.A.; Zukor, D.J.; Antoniou, J.; Poole, A.R. Prostaglandin PGE2 at Very Low Concentrations Suppresses Collagen Cleavage in Cultured Human Osteoarthritic Articular Cartilage: This Involves a Decrease in Expression of Proinflammatory Genes, Collagenases and COL10A1, a Gene Linked to Chondrocyte Hypertrophy. Arthritis Res. Ther. 2007, 9, R75. [Google Scholar] [CrossRef] [PubMed]
- Street, J.; Bao, M.; deGuzman, L.; Bunting, S.; Peale, F.V.; Ferrara, N.; Steinmetz, H.; Hoeffel, J.; Cleland, J.L.; Daugherty, A.; et al. Vascular Endothelial Growth Factor Stimulates Bone Repair by Promoting Angiogenesis and Bone Turnover. Proc. Natl. Acad. Sci. USA 2002, 99, 9656–9661. [Google Scholar] [CrossRef]
- Shatz, M.; Shats, I.; Menendez, D.; Resnick, M.A. P53 Amplifies Toll-like Receptor 5 Response in Human Primary and Cancer Cells through Interaction with Multiple Signal Transduction Pathways. Oncotarget 2015, 6, 16963–16980. [Google Scholar] [CrossRef]
- Komarova, E.A.; Krivokrysenko, V.; Wang, K.; Neznanov, N.; Chernov, M.V.; Komarov, P.G.; Brennan, M.; Golovkina, T.V.; Rokhlin, O.; Kuprash, D.V.; et al. P53 Is a Suppressor of Inflammatory Response in Mice. FASEB J. 2005, 19, 1030–1032. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Yang, S.; Lin, W.; Wang, L.; Ying, J.; Ding, Y.; Chen, X. Roles of Cell Cyle Regulators Cyclin D1, CDK4, and P53 in Knee Osteoarthritis. Genet. Test. Mol. Biomarkers 2016, 20, 529–534. [Google Scholar] [CrossRef]
- Basal, O.; Atay, T.; Ciris, İ.M.; Baykal, Y.B. Epidermal Growth Factor (EGF) Promotes Bone Healing in Surgically Induced Osteonecrosis of the Femoral Head (ONFH). Bosn. J. Basic. Med. Sci. 2018, 18, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Garces de los Fayos Alonso, I.; Liang, H.-C.; Turner, S.; Lagger, S.; Merkel, O.; Kenner, L. The Role of Activator Protein-1 (AP-1) Family Members in CD30-Positive Lymphomas. Cancers 2018, 10, 93. [Google Scholar] [CrossRef] [PubMed]
- Wagner, E.F.; Eferl, R. Fos/AP-1 Proteins in Bone and the Immune System. Immunol. Rev. 2005, 208, 126–140. [Google Scholar] [CrossRef]
- Kenner, L.; Hoebertz, A.; Beil, F.T.; Keon, N.; Karreth, F.; Eferl, R.; Scheuch, H.; Szremska, A.; Amling, M.; Schorpp-Kistner, M.; et al. Mice Lacking JunB Are Osteopenic Due to Cell-Autonomous Osteoblast and Osteoclast Defects. J. Cell Biol. 2004, 164, 613–623. [Google Scholar] [CrossRef]
- Miura, M.; Chen, X.-D.; Allen, M.R.; Bi, Y.; Gronthos, S.; Seo, B.-M.; Lakhani, S.; Flavell, R.A.; Feng, X.-H.; Robey, P.G.; et al. A Crucial Role of Caspase-3 in Osteogenic Differentiation of Bone Marrow Stromal Stem Cells. J. Clin. Investig. 2004, 114, 1704–1713. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, S.; Lee, J.; Lee, D. Emerging Roles of Natural Compounds in Osteoporosis: Regulation, Molecular Mechanisms and Bone Regeneration. Pharmaceuticals 2024, 17, 984. [Google Scholar] [CrossRef]
- Mukherjee, A.; Rotwein, P. Selective Signaling by Akt1 Controls Osteoblast Differentiation and Osteoblast-Mediated Osteoclast Development. Mol. Cell Biol. 2012, 32, 490–500. [Google Scholar] [CrossRef]
- Liao, P.; Chen, L.; Zhou, H.; Mei, J.; Chen, Z.; Wang, B.; Feng, J.Q.; Li, G.; Tong, S.; Zhou, J.; et al. Osteocyte Mitochondria Regulate Angiogenesis of Transcortical Vessels. Nat. Commun. 2024, 15, 2529. [Google Scholar] [CrossRef]

| Pubchem ID | Names | Structure | Category | Canonical Smiles | Oral Bioavailability OB (%) | Drug Likeness (DL) |
|---|---|---|---|---|---|---|
| 442437 | Neoastilbin | 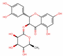 | Flavonoid | CC1C(C(C(C(O1)OC2C(OC3=CC(=CC(=C3C2=O)O)O)C4=CC(=C(C=C4)O)O)O)O)O | 40.54 | 0.74 |
| 129394 | 4,7-dihydroxy-5-methoxyl-6-methyl-8-formyl-flavan | 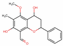 | Flavonoid | CC1=C(C(=C2C(=C1OC)C(CC(O2)C3=CC=CC=C3)O)C=O)O | 37.03 | 0.28 |
| 12303645 | Sitosterol | 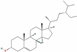 | Sterol | CCC(CCC(C)C1CCC2C1(CCC3C2CC=C4C3(CCC(C4)O)C)C)C(C)C | 36.91 | 0.75 |
| 222284 | Beta-sitosterol | 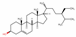 | Sterol | CCC(CCC(C)C1CCC2C1(CCC3C2CC=C4C3(CCC(C4)O)C)C)C(C)C | 36.91 | 0.75 |
| 119258 | Astilbin |  | Flavonoid | CC1C(C(C(C(O1)OC2C(OC3=CC(=CC(=C3C2=O)O)O)C4=CC(=C(C=C4)O)O)O)O)O | 36.46 | 0.74 |
| 101937309 | Isoengelitin | 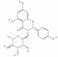 | Flavonoid | CC1C(C(C(C(O1)OC2C(OC3=CC(=CC(=C3C2=O)O)O)C4=CC=C(C=C4)O)O)O)O | 34.65 | 0.7 |
| 182232 | (+)-Epicatechin |  | Flavonoid | C1C(C(OC2=CC(=CC(=C21)O)O)C3=CC(=C(C=C3)O)O)O | 48.96 | 0.24 |
| 185914 | Dihydroresveratrol | 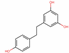 | Stilbene | C1=CC(=CC=C1CCC2=CC(=CC(=C2)O)O)O | 87.27 | 0.29 |
| 99474 | Diosgenin | 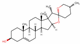 | Flavonoid | CC1CCC2(C(C3C(O2)CC4C3(CCC5C4CC=C6C5(CCC(C6)O)C)C)C)OC1 | 80.88 | 0.81 |
| 443758 | Cis-dihydroquercetin | 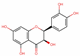 | Flavonoid | C1=CC(=C(C=C1C2C(C(=O)C3=C(C=C(C=C3O2)O)O)O)O)O | 66.44 | 0.27 |
| 5320468 | (2R,3R)-2-(3,5-dihydroxyphenyl)-3,5,7-trihydroxychroman-4-one | 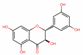 | Flavonoid | C1=C(C=C(C=C1O)O)C2C(C(=O)C3=C(C=C(C=C3O2)O)O)O | 63.17 | 0.27 |
| 712316 | (-)-Taxifolin | 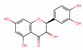 | Flavonoid | C1=CC(=C(C=C1C2C(C(=O)C3=C(C=C(C=C3O2)O)O)O)O)O | 60.51 | 0.27 |
| 439246 | Naringenin | 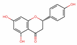 | Flavonoid | C1C(OC2=CC(=CC(=C2C1=O)O)O)C3=CC=C(C=C3)O | 59.29 | 0.21 |
| 439533 | Taxifolin | 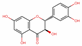 | Flavonoid | C1=CC(=C(C=C1C2C(C(=O)C3=C(C=C(C=C3O2)O)O)O)O)O | 57.84 | 0.27 |
| 5280343 | Quercetin | 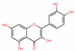 | Flavonoid | C1=CC(=C(C=C1C2=C(C(=O)C3=C(C=C(C=C3O2)O)O)O)O)O | 46.43 | 0.28 |
| 5280794 | Stigmasterol | 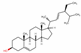 | Sterol | CCC(C=CC(C)C1CCC2C1(CCC3C2CC=C4C3(CCC(C4)O)C)C)C(C)C | 43.83 | 0.76 |
| 5377450 | Enhydrin | 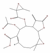 | Lactone | CC1C(O1)(C)C(=O)OC2C3C(C4C(O4)(CCC=C(C2OC(=O)C)C(=O)OC)C)OC(=O)C3=C | 40.56 | 0.74 |
| PDB ID | Target | Quercetin | Naringenin | β-Sitosterol | Epicatechin |
|---|---|---|---|---|---|
| 2AZ5 | TNF | −7.8 | −7.1 | −8.6 | −7.8 |
| 1UNQ | AKT1 | −5.7 | −5.4 | −5.4 | −5.7 |
| 1IL6 | IL6 | −7.2 | −6.9 | −4.8 | −6.8 |
| 6Y8M | IL-1β | −6.5 | −5.7 | −5.5 | −6.6 |
| 3QTK | VEGFA | −4.9 | −4.8 | −5.5 | −4.6 |
| 3DCY | TP53 | −8.0 | −7.6 | −7.4 | −7.6 |
| 1NMS | CASP3 | −6.7 | −6.1 | −6.9 | −6.5 |
| 5KIR | PTGS2 | −8.2 | −5.5 | −5.5 | −8.7 |
| 1PMN | JUN | −8.5 | −7.2 | −8.9 | −7.8 |
| Targets | Binding Affinity by Davis Model | Binding Affinity by KIBA Model | ||||||
|---|---|---|---|---|---|---|---|---|
| Quercetin | Naringenin | β-Sitosterol | Epicatechin | Quercetin | Naringenin | β-Sitosterol | Epicatechin | |
| TNF | 6.055 | 5.989 | 6.631 | 6.063 | 11.186 | 11.228 | 11.349 | 11.182 |
| AKT1 | 5.997 | 5.467 | 6.573 | 6.005 | 11.064 | 11.120 | 11.227 | 11.060 |
| IL6 | 5.569 | 5.958 | 6.601 | 5.514 | 11.205 | 11.247 | 11.368 | 11.201 |
| IL-1β | 5.650 | 5.965 | 6.608 | 6.040 | 11.218 | 11.260 | 11.381 | 11.214 |
| VEGFA | 6.131 | 6.064 | 6.706 | 6.138 | 11.378 | 11.419 | 11.540 | 11.374 |
| TP53 | 6.134 | 6.067 | 6.709 | 6.141 | 11.307 | 11.349 | 11.470 | 11.303 |
| CASP3 | 6.057 | 5.990 | 5.929 | 6.065 | 11.170 | 11.212 | 11.327 | 11.166 |
| PTGS2 | 6.068 | 6.001 | 6.644 | 6.076 | 11.342 | 11.383 | 11.504 | 11.338 |
| JUN | 5.697 | 6.017 | 5.712 | 5.642 | 11.265 | 11.306 | 11.428 | 11.261 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilyas, S.; Baek, C.Y.; Manan, A.; Choi, Y.; Jo, H.-G.; Lee, D. Mechanistic Exploration of Smilax glabra Roxb. in Osteoarthritis: Insights from Network Pharmacology, Molecular Docking, and In Vitro Validation. Pharmaceuticals 2024, 17, 1285. https://doi.org/10.3390/ph17101285
Ilyas S, Baek CY, Manan A, Choi Y, Jo H-G, Lee D. Mechanistic Exploration of Smilax glabra Roxb. in Osteoarthritis: Insights from Network Pharmacology, Molecular Docking, and In Vitro Validation. Pharmaceuticals. 2024; 17(10):1285. https://doi.org/10.3390/ph17101285
Chicago/Turabian StyleIlyas, Sidra, Chae Yun Baek, Abdul Manan, Yeojin Choi, Hee-Geun Jo, and Donghun Lee. 2024. "Mechanistic Exploration of Smilax glabra Roxb. in Osteoarthritis: Insights from Network Pharmacology, Molecular Docking, and In Vitro Validation" Pharmaceuticals 17, no. 10: 1285. https://doi.org/10.3390/ph17101285
APA StyleIlyas, S., Baek, C. Y., Manan, A., Choi, Y., Jo, H.-G., & Lee, D. (2024). Mechanistic Exploration of Smilax glabra Roxb. in Osteoarthritis: Insights from Network Pharmacology, Molecular Docking, and In Vitro Validation. Pharmaceuticals, 17(10), 1285. https://doi.org/10.3390/ph17101285









