Roles of Prostaglandins and Hydrogen Sulfide in an Outflow Model of the Porcine Ocular Anterior Segment Ex Vivo
Abstract
1. Introduction
2. Results
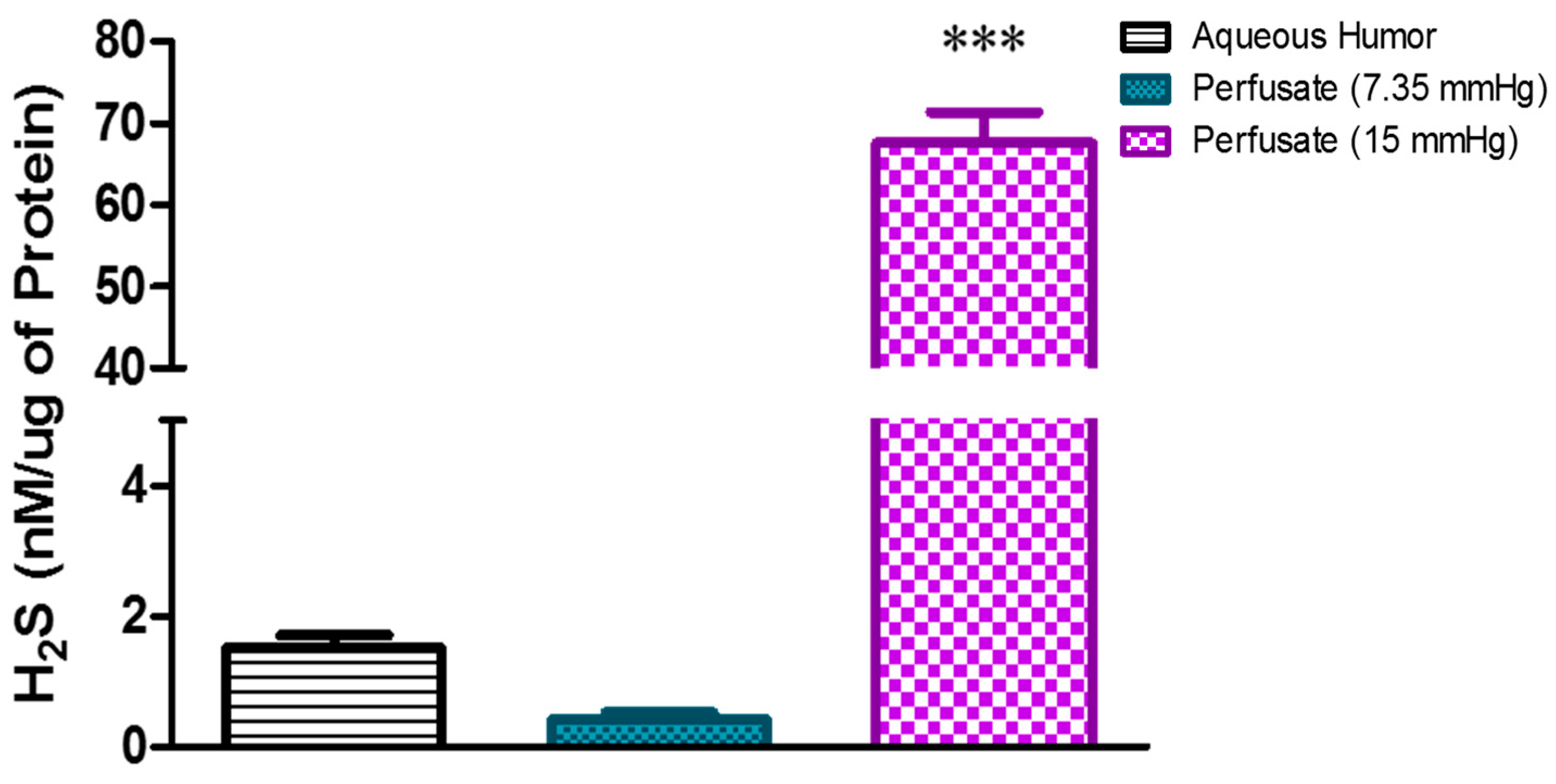
2.1. Effect of Elevated Perfusion Pressure on Hydrogen Sulfide Levels in TM Outflow Pathway
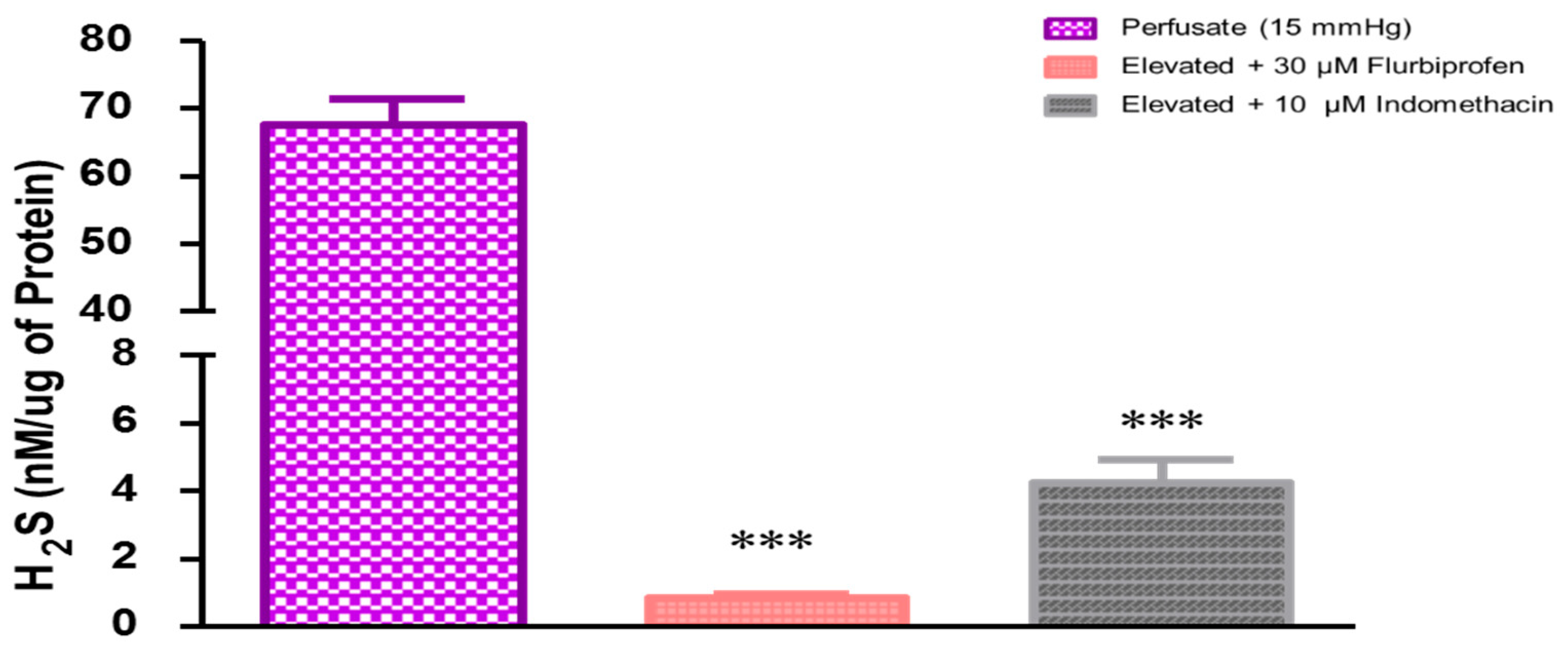
2.2. Role of Prostaglandins in Elevated Perfusion Pressure-Induced Increase in Hydrogen Sulfide Levels in TM Outflow Pathways
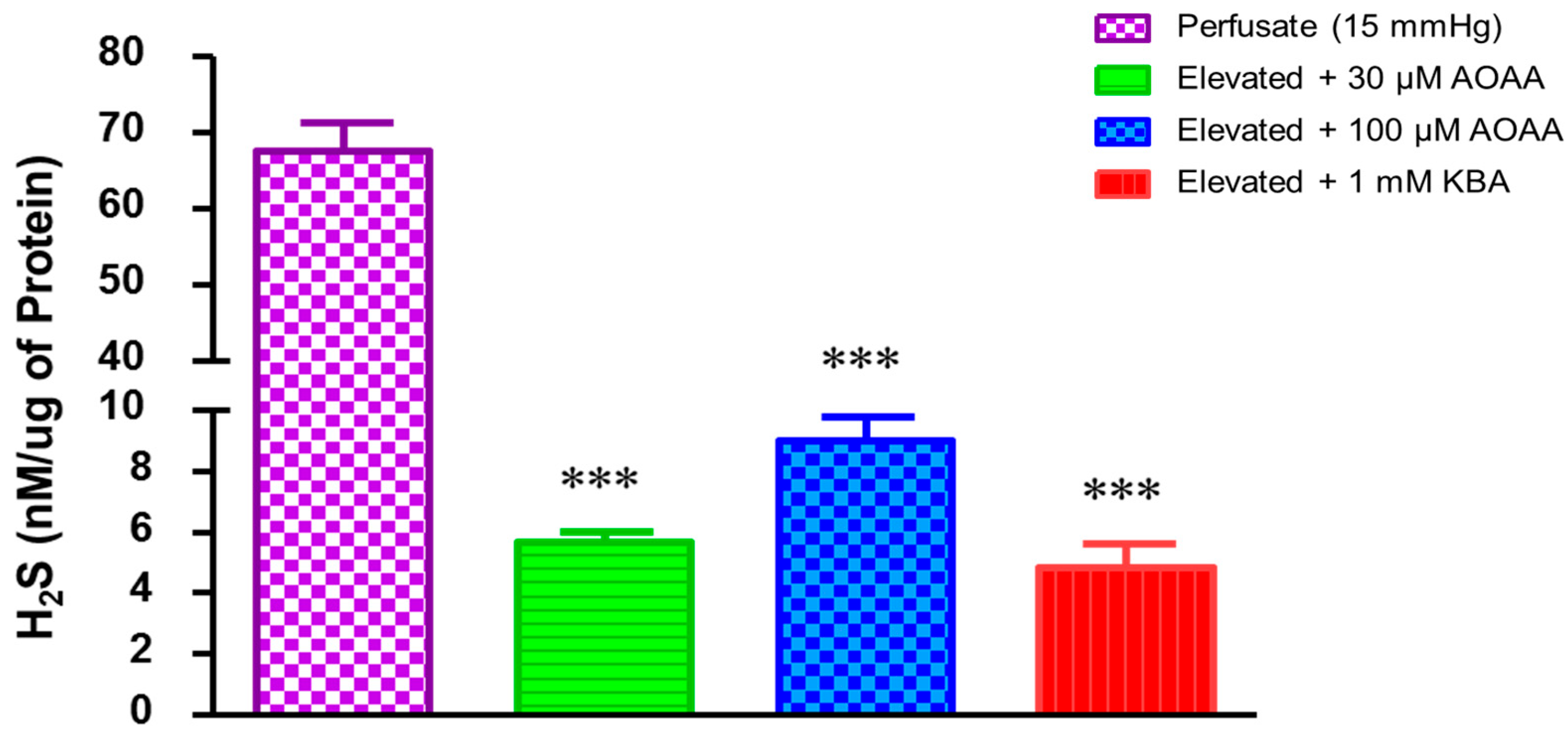
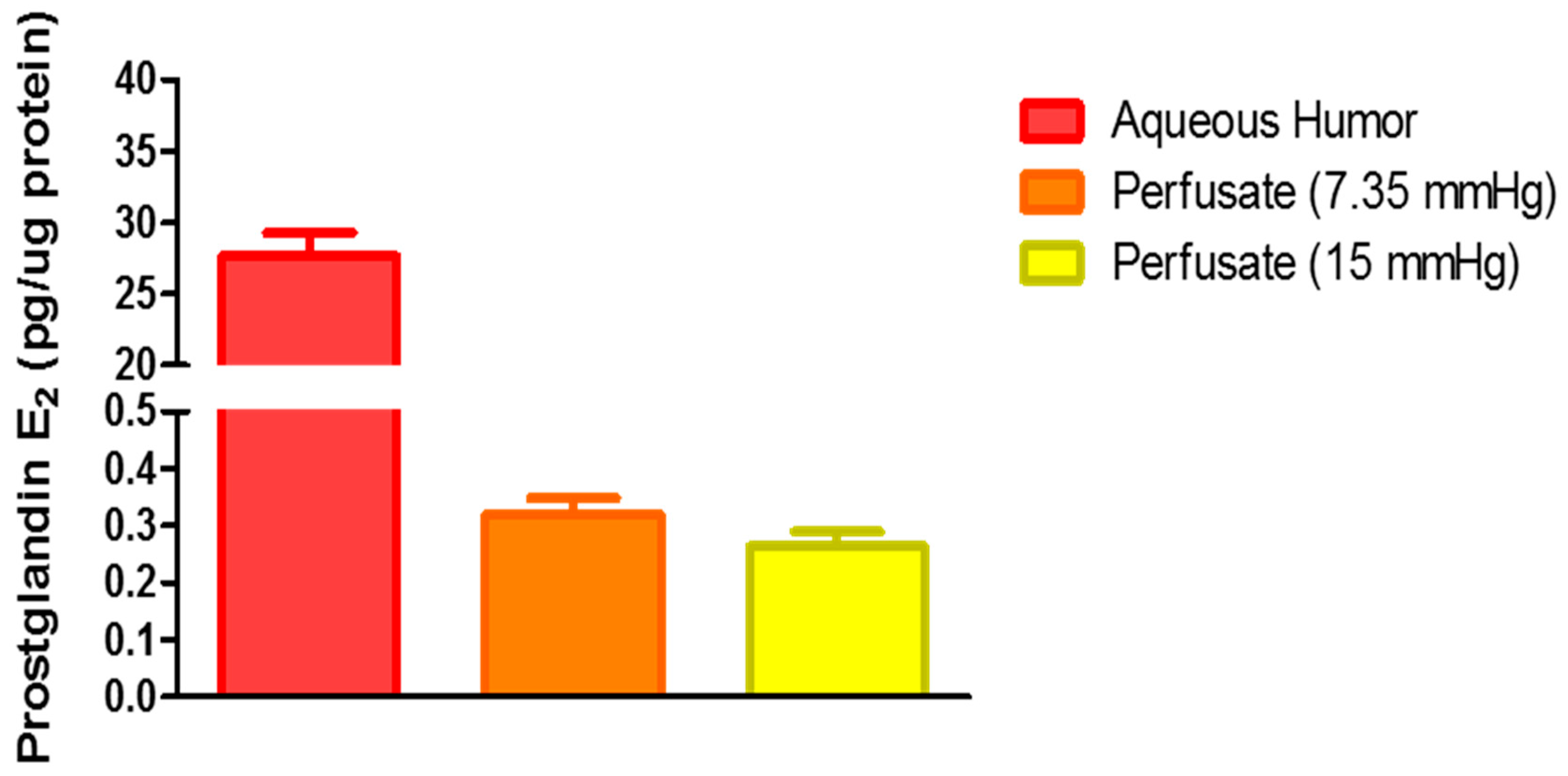
2.3. Role of Hydrogen Sulfide in Elevated Perfusion Pressure on Prostaglandin Levels in TM Outflow Pathways
3. Discussion
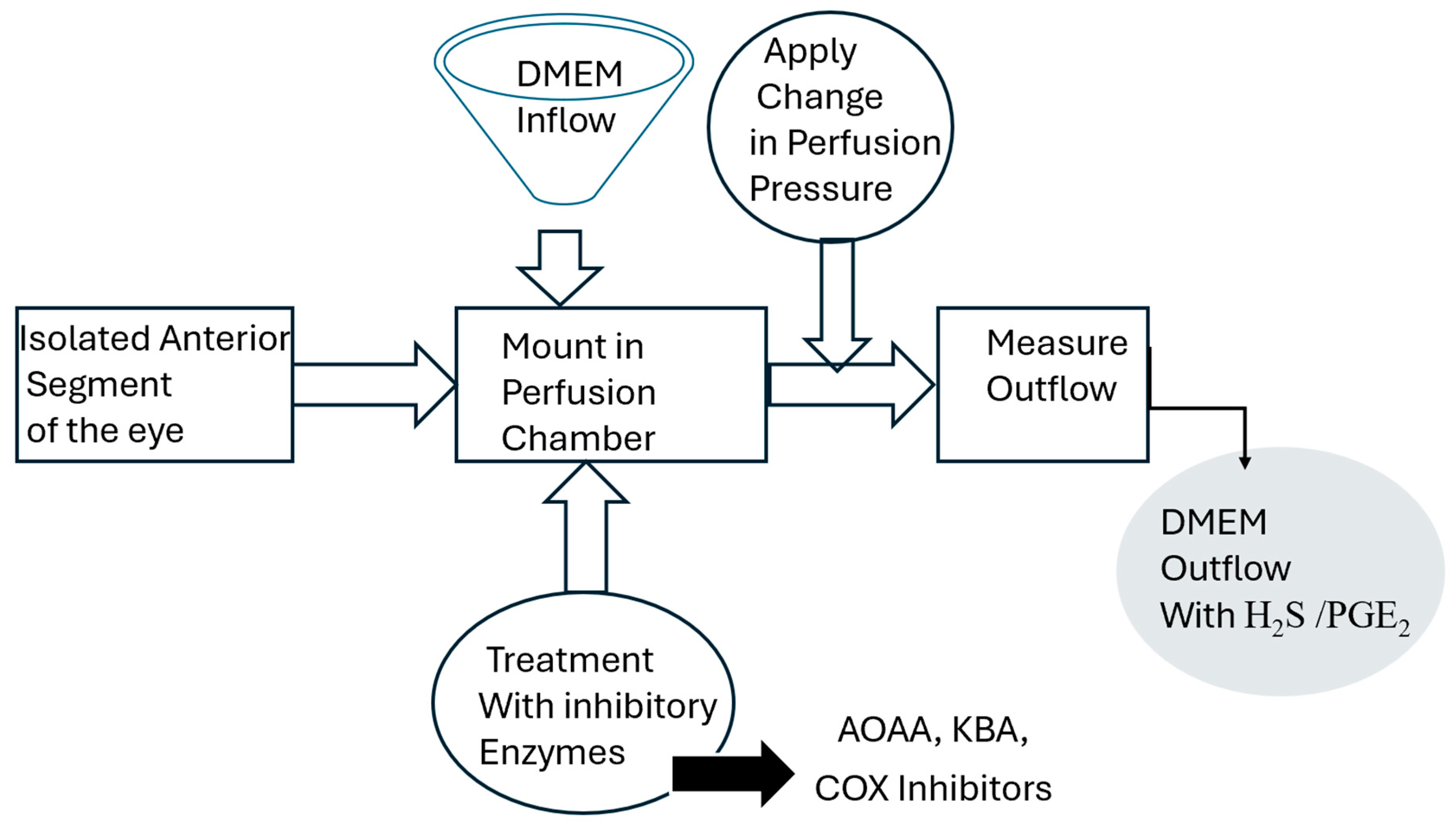
4. Materials and Methods
4.1. Chemicals
4.2. Porcine Anterior Segment Perfusion Model, Ex Vivo
4.3. Measurement of H2S and Prostaglandin E2 Concentrations
4.4. Protein Measurement
4.5. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kimura, H. Hydrogen sulfide: Its production, release and functions. Amino Acids 2011, 41, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H. Hydrogen Sulfide (H2S) and Polysulfide (H2Sn) Signaling: The First 25 Years. Biomolecules 2021, 11, 896. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.H.; Wong, P.T.; Bian, J.S. Hydrogen sulfide: A novel signaling molecule in the central nervous system. Neurochem. Int. 2010, 56, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Rossoni, G.; Sparatore, A.; Lee, L.C.; Del Soldato, P.; Moore, P.K. Anti-inflammatory and gastrointestinal effects of a novel diclofenac derivative. Free Radic. Biol. Med. 2007, 42, 706–719. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H. Hydrogen sulfide as a neuromodulator. Mol. Neurobiol. 2002, 26, 13–19. [Google Scholar] [CrossRef]
- Mikami, Y.; Shibuya, N.; Kimura, Y.; Nagahara, N.; Yamada, M.; Kimura, H. Hydrogen sulfide protects the retina from light-induced degeneration by the modulation of Ca2+ influx. J. Biol. Chem. 2011, 286, 39379–39386. [Google Scholar] [CrossRef]
- Shibuya, N.; Tanaka, M.; Yoshida, M.; Ogasawara, Y.; Togawa, T.; Ishii, K.; Kimura, H. 3-Mercaptopyruvate sulfurtransferase produces hydrogen sulfide and bound sulfane sulfur in the brain. Antioxid. Redox Signal. 2009, 11, 703–714. [Google Scholar] [CrossRef]
- Mikami, Y.; Shibuya, N.; Ogasawara, Y.; Kimura, H. Hydrogen sulfide is produced by cystathionine γ-lyase at the steady-state low intracellular Ca2+ concentrations. Biochem. Biophys. Res. Commun. 2013, 431, 131–135. [Google Scholar] [CrossRef]
- Beard, M.E.; Davies, T.; Holloway, M.; Holtzman, E. Peroxisomes in pigment epithelium and Müller cells of amphibian retina possess D-amino acid oxidase as well as catalase. Exp. Eye Res. 1988, 47, 795–806. [Google Scholar] [CrossRef]
- Koga, R.; Miyoshi, Y.; Sakaue, H.; Hamase, K.; Konno, R. Mouse d-Amino-Acid Oxidase: Distribution and Physiological Substrates. Front. Mol. Biosci. 2017, 4, 82. [Google Scholar] [CrossRef]
- Buffault, J.; Labbé, A.; Hamard, P.; Brignole-Baudouin, F.; Baudouin, C. The trabecular meshwork: Structure, function and clinical implications. A review of the literature. J. Fr. D’ophtalmol. 2020, 43, e217–e230. [Google Scholar] [CrossRef] [PubMed]
- Stamer, W.D.; Acott, T.S. Current understanding of conventional outflow dysfunction in glaucoma. Curr. Opin. Ophthalmol. 2012, 23, 135–143. [Google Scholar] [CrossRef]
- Salvi, A.; Bankhele, P.; Jamil, J.M.; Kulkarni-Chitnis, M.; Njie-Mbye, Y.F.; Ohia, S.E.; Opere, C.A. Pharmacological Actions of Hydrogen Sulfide Donors on Sympathetic Neurotransmission in the Bovine Anterior Uvea, In Vitro. Neurochem. Res. 2016, 41, 1020–1028. [Google Scholar] [CrossRef]
- Robinson, J.; Okoro, E.; Ezuedu, C.; Bush, L.; Opere, C.A.; Ohia, S.E.; Njie-Mbye, Y.F. Effects of Hydrogen Sulfide-Releasing Compounds on Aqueous Humor Outflow Facility in Porcine Ocular Anterior Segments, Ex Vivo. J. Ocul. Pharmacol. Ther. Off. J. Assoc. Ocul. Pharmacol. Ther. 2017, 33, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Huang, P.; Liu, X.; Lin, Z.; Wang, J.; Xu, S.; Guo, L.; Leung, C.K.; Zhong, Y. Relevant variations and neuroprotective effect of hydrogen sulfide in a rat glaucoma model. Neuroscience 2017, 341, 27–41. [Google Scholar] [CrossRef]
- Freedman, J.; Goddard, D. Elevated levels of transforming growth factor beta and prostaglandin E2 in aqueous humor from patients undergoing filtration surgery for glaucoma. Can. J. Ophthalmol. 2008, 43, 370. [Google Scholar]
- Mermoud, A.; Baerveldt, G.; Minckler, D.S.; Rao, N.A. Prostaglandines E2 et F2-alpha au cours du glaucome uvéitique chez le rat Lewis [Prostaglandins E2 and F2-alpha in uveitic glaucoma in the Lewis rat]. Klin. Monatsblatter Augenheilkd. 1995, 206, 409–412. [Google Scholar] [CrossRef] [PubMed]
- Monjok, E.M.; Kulkarni, K.H.; Kouamou, G.; McKoy, M.; Opere, C.A.; Bongmba, O.N.; Njie, Y.F.; Ohia, S.E. Inhibitory action of hydrogen sulfide on muscarinic receptor-induced contraction of isolated porcine irides. Exp. Eye Res. 2008, 87, 612–616. [Google Scholar] [CrossRef]
- Ohia, S.E.; Opere, C.A.; Monjok, E.M.; Kouamou, G.; Leday, A.M.; Njie-Mbye, Y.F. Role of hydrogen sulfide production in inhibitory action of L-cysteine on isolated porcine irides. Curr. Eye Res. 2010, 35, 402–407. [Google Scholar] [CrossRef]
- Asimakopoulou, A.; Panopoulos, P.; Chasapis, C.T.; Coletta, C.; Zhou, Z.; Cirino, G.; Giannis, A.; Szabo, C.; Spyroulias, G.A.; Papapetropoulos, A. Selectivity of commonly used pharmacological inhibitors for cystathionine β synthase (CBS) and cystathionine γ lyase (CSE). Br. J. Pharmacol. 2013, 169, 922–932. [Google Scholar] [CrossRef]
- Porter, D.W.; Baskin, S.I. The effect of three alpha-keto acids on 3-mercaptopyruvate sulfurtransferase activity. J. Biochem. Toxicol. 1996, 11, 45–50. [Google Scholar] [CrossRef]
- Chitnis, M.K.; Njie-Mbye, Y.F.; Opere, C.A.; Wood, M.E.; Whiteman, M.; Ohia, S.E. Pharmacological actions of the slow-release hydrogen sulfide donor GYY4137 on phenylephrine-induced tone in isolated bovine ciliary artery. Exp. Eye Res. 2013, 116, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni-Chitnis, M.; Njie-Mbye, Y.F.; Mitchell, L.; Robinson, J.; Whiteman, M.; Wood, M.E.; Opere, C.A.; Ohia, S.E. Inhibitory action of novel hydrogen sulfide donors on bovine isolated posterior ciliary arteries. Exp. Eye Res. 2015, 134, 73–79. [Google Scholar] [CrossRef]
- Pong, W.W.; Stouracova, R.; Frank, N.; Kraus, J.P.; Eldred, W.D. Comparative localization of cystathionine beta-synthase and cystathionine gamma-lyase in retina: Differences between amphibians and mammals. J. Comp. Neurol. 2007, 505, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Acott, T.S.; Kelley, M.J.; Keller, K.E.; Vranka, J.A.; Abu-Hassan, D.W.; Li, X.; Aga, M.; Bradley, J.M. Intraocular pressure homeostasis: Maintaining balance in a high-pressure environment. J. Ocul. Pharmacol. Ther. 2014, 30, 94–101. [Google Scholar] [CrossRef]
- Vranka, J.A.; Acott, T.S. Pressure-induced expression changes in segmental flow regions of the human trabecular meshwork. Exp. Eye Res. 2017, 158, 67–72. [Google Scholar] [CrossRef]
- Vranka, J.A.; Staverosky, J.A.; Raghunathan, V.; Acott, T.S. Elevated pressure influences relative distribution of segmental regions of the trabecular meshwork. Exp. Eye Res. 2020, 190, 107888. [Google Scholar] [CrossRef]
- Schneemann, A.; Leusink-Muis, A.; van den Berg, T.; Hoyng, P.F.; Kamphuis, W. Elevation of nitric oxide production in human trabecular meshwork by increased pressure. Graefes Arch. Clin. Exp. 2003, 241, 321–326. [Google Scholar] [CrossRef]
- Kulkarni, M.; Njie-Mbye, Y.F.; Okpobiri, I.; Zhao, M.; Opere, C.A.; Ohia, S.E. Endogenous production of hydrogen sulfide in isolated bovine eye. Neurochem. Res. 2011, 36, 1540–1545. [Google Scholar] [CrossRef]
- Abe, K.; Kimura, H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J. Neurosci. Off. J. Soc. Neurosci. 1996, 16, 1066–1071. [Google Scholar] [CrossRef]
- Kolluru, G.K.; Shen, X.; Bir, S.C.; Kevil, C.G. Hydrogen sulfide chemical biology: Pathophysiological roles and detection. Nitric Oxide Biol. Chem. 2013, 35, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Wang, Z.; Gu, Y.; Hu, Y.; Ji, Z.; Wang, S.; Lin, Z.; Li, X.; Xie, Z.; Pan, S. Glibenclamide Is Comparable to Target Temperature Management in Improving Survival and Neurological Outcome After Asphyxial Cardiac Arrest in Rats. J. Am. Heart Assoc. 2016, 5, e003465. [Google Scholar] [CrossRef] [PubMed]
- Winkler, N.S.; Fautsch, M.P. Effects of prostaglandin analogues on aqueous humor outflow pathways. J. Ocul. Pharmacol. Ther. 2014, 30, 102–109. [Google Scholar] [CrossRef]
- Toris, C.B.; Gabelt, B.T.; Kaufman, P.L. Update on the mechanism of action of topical prostaglandins for intraocular pressure reduction. Surv. Ophthalmol. 2008, 53 (Suppl. S1), S107–S120. [Google Scholar] [CrossRef] [PubMed]
- Bahler, C.K.; Howell, K.G.; Hann, C.R.; Fautsch, M.P.; Johnson, D.H. Prostaglandins increase trabecular meshwork outflow facility in cultured human anterior segments. Am. J. Ophthalmol. 2008, 145, 114–119. [Google Scholar] [CrossRef]
- Maihöfner, C.; Schlötzer-Schrehardt, U.; Gühring, H.; Zeilhofer, H.U.; Naumann, G.O.; Pahl, A.; Mardin, C.; Tamm, E.R.; Brune, K. Expression of cyclooxygenase-1 and -2 in normal and glaucomatous human eyes. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2616–2624. [Google Scholar]
- Kwiatkoski, M.; Soriano, R.N.; Araujo, R.M.; Azevedo, L.U.; Batalhao, M.E.; Francescato, H.D.; Coimbra, T.M.; Carnio, E.C.; Branco, L.G. Hydrogen sulfide inhibits preoptic prostaglandin E2 production during endotoxemia. Exp. Neurol. 2013, 240, 88–95. [Google Scholar] [CrossRef]
- Sun, Q.; Chen, Z.; He, P.; Li, Y.; Ding, X.; Huang, Y.; Gu, H.; Ni, X. Reduced Expression of Hydrogen Sulfide-Generating Enzymes Down-Regulates 15-Hydroxyprostaglandin Dehydrogenase in Chorion during Term and Preterm Labor. Am. J. Pathol. 2018, 188, 63–71. [Google Scholar] [CrossRef]
- Ang, S.F.; Sio, S.W.; Moochhala, S.M.; MacAry, P.A.; Bhatia, M. Hydrogen sulfide upregulates cyclooxygenase-2 and prostaglandin E metabolite in sepsis-evoked acute lung injury via transient receptor potential vanilloid type 1 channel activation. J. Immunol. 2011, 187, 4778–4787. [Google Scholar] [CrossRef]
- Njie, Y.F.; Qiao, Z.; Xiao, Z.; Wang, W.; Song, Z.H. N-arachidonylethanolamide-induced increase in aqueous humor outflow facility. Investig. Ophthalmol. Vis. Sci. 2008, 49, 4528–4534. [Google Scholar] [CrossRef]
- Yang, Y.F.; Sun, Y.Y.; Acott, T.S.; Keller, K.E. Effects of induction and inhibition of matrix cross-linking on remodeling of the aqueous outflow resistance by ocular trabecular meshwork cells. Sci. Rep. 2016, 6, 30505. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.M.; Pluth, M.D. Advances and Opportunities in H2S Measurement in Chemical Biology. JACS Au 2023, 3, 2677–2691. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Shimizu, T.; Shimizu, S.; Higashi, Y.; Nakamura, K.; Ono, H.; Aratake, T.; Saito, M. Possible role of hydrogen sulfide as an endogenous relaxation factor in the rat bladder and prostate. Neurourol. Urodyn. 2018, 37, 2519–2526. [Google Scholar] [CrossRef] [PubMed]
- Kruger, N.J. The Bradford method for protein quantitation. Methods Mol. Biol. 1994, 32, 9–15. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robinson, J.; Bush, L.; Okolie, A.; Muili, F.; Ohia, S.; Opere, C.; Mbye, Y.F.N. Roles of Prostaglandins and Hydrogen Sulfide in an Outflow Model of the Porcine Ocular Anterior Segment Ex Vivo. Pharmaceuticals 2024, 17, 1262. https://doi.org/10.3390/ph17101262
Robinson J, Bush L, Okolie A, Muili F, Ohia S, Opere C, Mbye YFN. Roles of Prostaglandins and Hydrogen Sulfide in an Outflow Model of the Porcine Ocular Anterior Segment Ex Vivo. Pharmaceuticals. 2024; 17(10):1262. https://doi.org/10.3390/ph17101262
Chicago/Turabian StyleRobinson, Jenaye, Leah Bush, Anthonia Okolie, Fatima Muili, Sunny Ohia, Catherine Opere, and Ya Fatou Njie Mbye. 2024. "Roles of Prostaglandins and Hydrogen Sulfide in an Outflow Model of the Porcine Ocular Anterior Segment Ex Vivo" Pharmaceuticals 17, no. 10: 1262. https://doi.org/10.3390/ph17101262
APA StyleRobinson, J., Bush, L., Okolie, A., Muili, F., Ohia, S., Opere, C., & Mbye, Y. F. N. (2024). Roles of Prostaglandins and Hydrogen Sulfide in an Outflow Model of the Porcine Ocular Anterior Segment Ex Vivo. Pharmaceuticals, 17(10), 1262. https://doi.org/10.3390/ph17101262





