Optimization and Appraisal of Nintedanib-Loaded Mixed Polymeric Micelles as a Potential Nanovector for Non-Invasive Pulmonary Fibrosis Mitigation
Abstract
1. Introduction
2. Results and Discussion
2.1. Experimental Design
| Independent Factors | Levels | ||||||
| −1 | 0 | 1 | |||||
| X1: Soluplus concentration (mg) | 200 | 300 | 400 | ||||
| X2: Tween 80 concentration (mg) | 150 | 200 | 250 | ||||
| X3: SDC concentration (mg) | 0 | 20 | 40 | ||||
| Run | X1 | X2 | X3 | PS (Y1, nm) | EE (Y2, %) | ZP (Y3, mV) | CR (Y4, %) |
| R1 | 0 | 1 | 1 | 77.93 ± 3.49 | 76.59 ± 0.49 | –12.87 ± 0.25 | 40.25 ± 0.70 |
| R2 | 1 | 0 | 1 | 133.03 ± 4.66 | 75.44 ± 0.74 | –17.63 ± 1.05 | 21.24 ± 1.76 |
| R3 * | 0 | 0 | 0 | 59.53 ± 3.69 | 85.19 ± 1.86 | –10.53 ± 2.22 | 41.70 ± 3.05 |
| R4 | 1 | 0 | −1 | 122.03 ± 2.95 | 82.73 ± 0.43 | –14.40 ± 0.87 | 32.46 ± 2.08 |
| R5 | 0 | 1 | −1 | 71.47 ± 2.29 | 82.09 ± 1.17 | –8.40 ± 0.26 | 45.06 ± 0.98 |
| R6 | −1 | 1 | 0 | 102.87 ± 3.58 | 84.54 ± 0.77 | –4.03 ± 0.57 | 35.87 ± 1.94 |
| R7 * | 0 | 0 | 0 | 62.60 ± 2.16 | 85.17 ± 1.36 | –11.40 ± 0.75 | 49.62 ± 8.21 |
| R8 | 1 | 1 | 0 | 144.00 ± 1.73 | 74.21 ± 2.56 | –15.63 ± 0.85 | 24.72 ± 1.69 |
| R9 | −1 | 0 | −1 | 87.47 ± 2.15 | 93.51 ± 0.21 | –3.97 ± 0.64 | 49.28 ± 2.80 |
| R10 | 1 | −1 | 0 | 113.27 ± 2.25 | 83.31 ± 0.71 | –17.77 ± 1.10 | 38.78 ± 1.14 |
| R11 | −1 | 0 | 1 | 106.33 ± 3.31 | 86.47 ± 0.47 | –6.80 ± 0.69 | 41.69 ± 2.65 |
| R12 | 0 | −1 | −1 | 45.83 ± 3.91 | 94.30 ± 1.28 | –9.33 ± 0.21 | 77.14 ± 2.09 |
| R13 * | 0 | 0 | 0 | 61.67 ± 2.08 | 85.18 ± 1.58 | –11.40 ± 2.13 | 49.84 ± 8.45 |
| R14 | 0 | −1 | 1 | 59.40 ± 3.15 | 87.41 ± 1.11 | –13.60 ± 0.36 | 68.39 ± 1.88 |
| R15 | −1 | −1 | 0 | 94.80 ± 2.55 | 96.14 ± 1.21 | –5.70 ± 0.61 | 59.93 ± 2.71 |
| Source | PS (Y1) | EE (Y2) | ZP (Y3) | CR (Y4) | ||||
|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | F | p | |
| Model | 432.95 | <0.0001 | 404.06 | <0.0001 | 190.18 | <0.0001 | 55.73 | <0.0001 |
| X1: Soluplus (mg) | 563.37 | <0.0001 | 527.21 | <0.0001 | 513.21 | <0.0001 | 119.84 | <0.0001 |
| X2: Tween 80 (mg) | 265.45 | <0.0001 | 498.53 | <0.0001 | 4.91 | 0.0322 | 180.87 | <0.0001 |
| X3: SDC (mg) | 96.02 | <0.0001 | 186.43 | <0.0001 | 52.41 | <0.0001 | 23.67 | <0.0001 |
| Lack of Fit | 1.76 | 0.1755 | 1.13 | 0.3701 | 0.6757 | 0.7247 | 2.80 | 0.0556 |
| Model | Reduced Quadratic | Linear | Linear | Reduced Quadratic | ||||
| R2 | 0.9911 | 0.9673 | 0.9539 | 0.9348 | ||||
| Adjusted R2 | 0.9888 | 0.9649 | 0.9505 | 0.9180 | ||||
| Predicted R2 | 0.9850 | 0.9610 | 0.9474 | 0.9052 | ||||
| Adequate precision | 67.7579 | 62.0143 | 50.0291 | 26.5304 | ||||
| Standard deviation | 3.12 | 1.20 | 1.00 | 0.3190 | ||||
| %CV | 3.48 | 1.41 | 9.19 | 4.82 | ||||
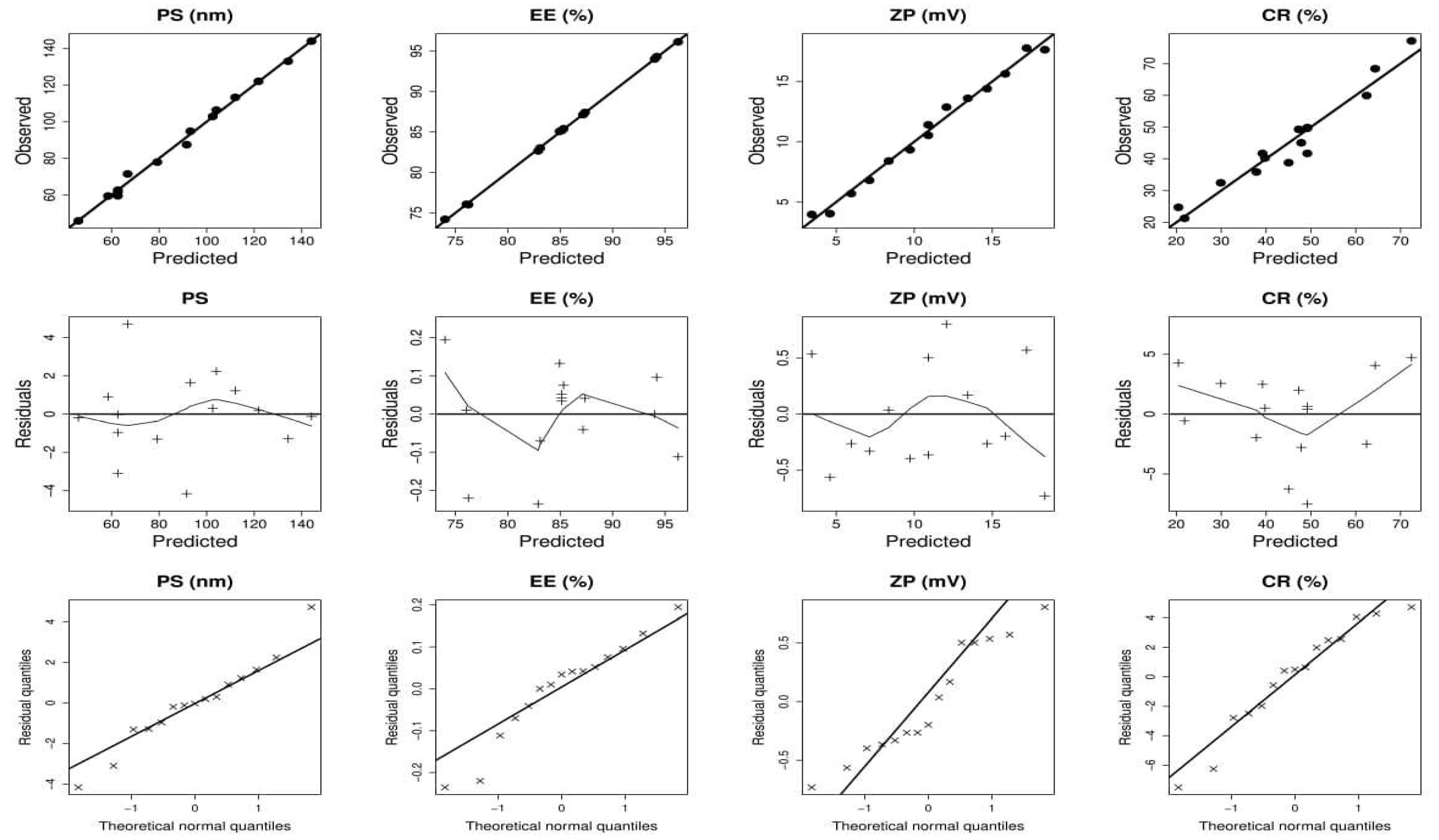
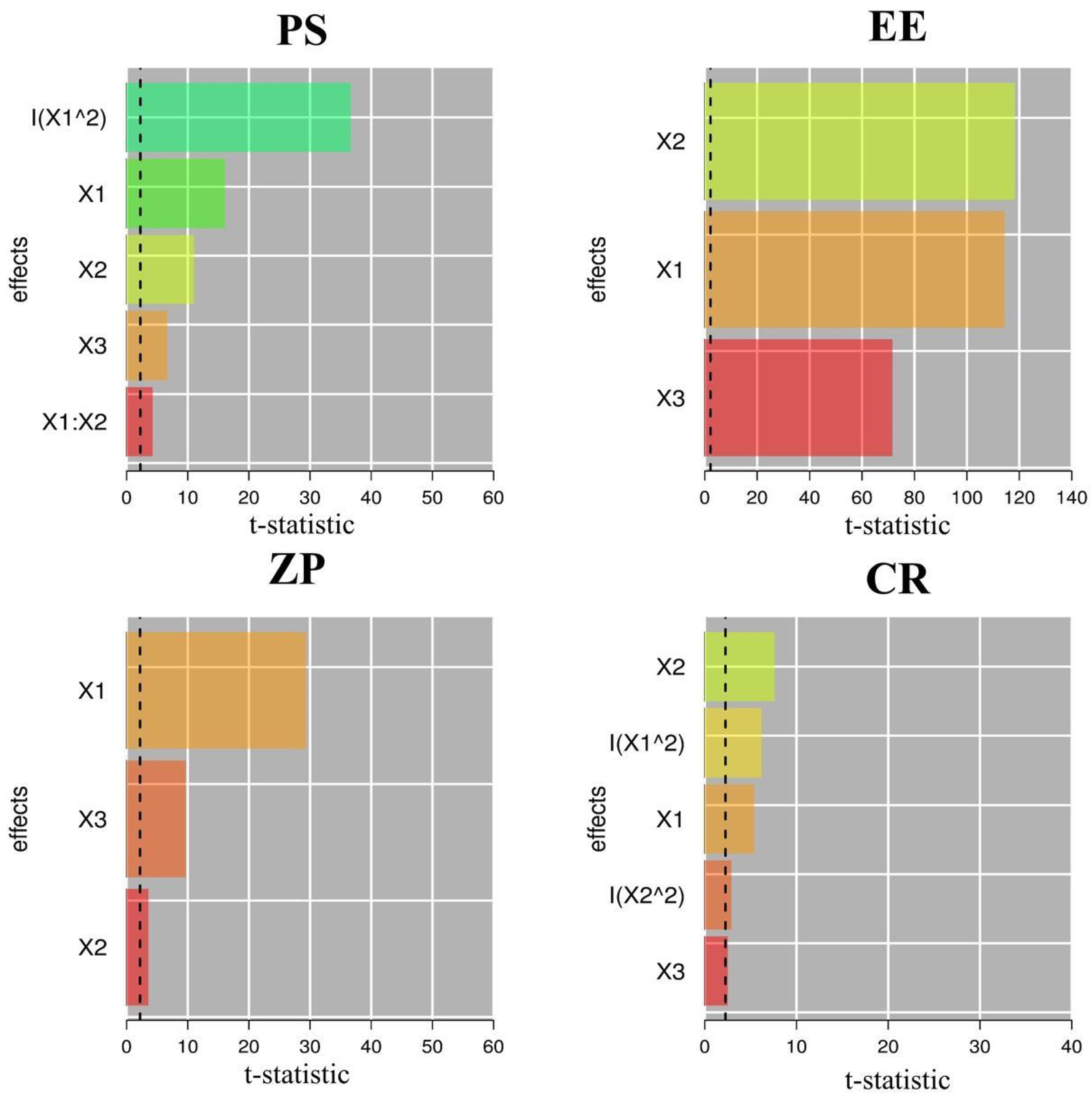
2.1.1. Effect of Independent Variables on Particle Size (PS)
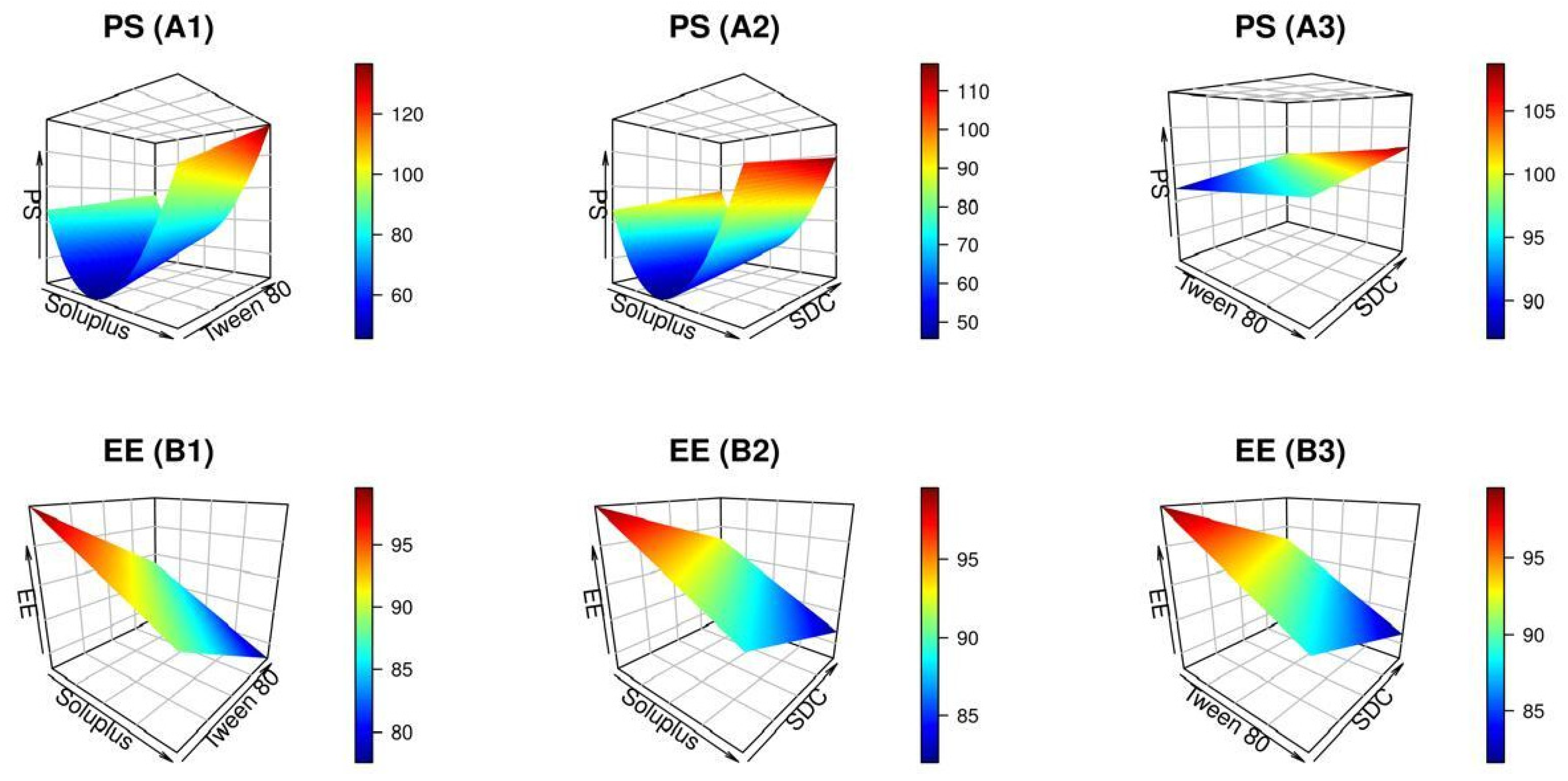
2.1.2. Effect of Independent Variables on EE
2.1.3. Effect of Independent Variables on ZP

2.1.4. Effect of Independent Variables on CR
2.2. Formulation Optimization
| X1: Soluplus Concentration (mg) | X2: Tween 80 Concentration (mg) | X3: SDC Concentration (mg) | |||
| Optimal values | 309.217 | 150 | 40 | ||
| Desirability | 0.756 | ||||
| PS (nm) | EE% | ZP (mV) | CR % | ||
| Predicted | 62.418 | 86.424 | −13.99 | 64.222 | |
| Experimental | 61.36 | 90.26 | −14.72 | 66.84 | |
| Prediction error (%) £ | 1.72 | 4.24 | 4.95 | 3.92 | |
2.3. Characterization of the Optimized Formulation
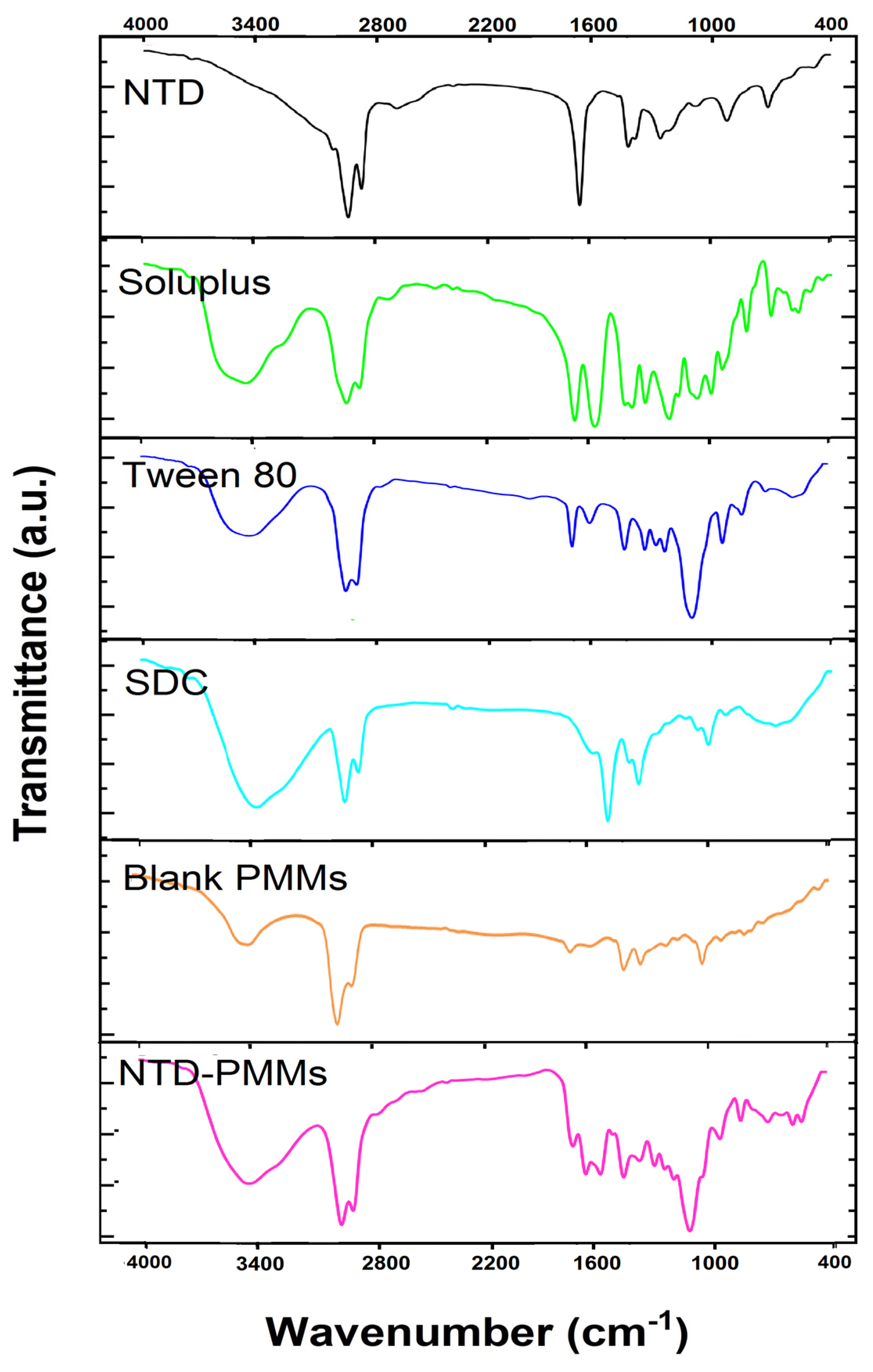
2.3.1. FTIR Analysis
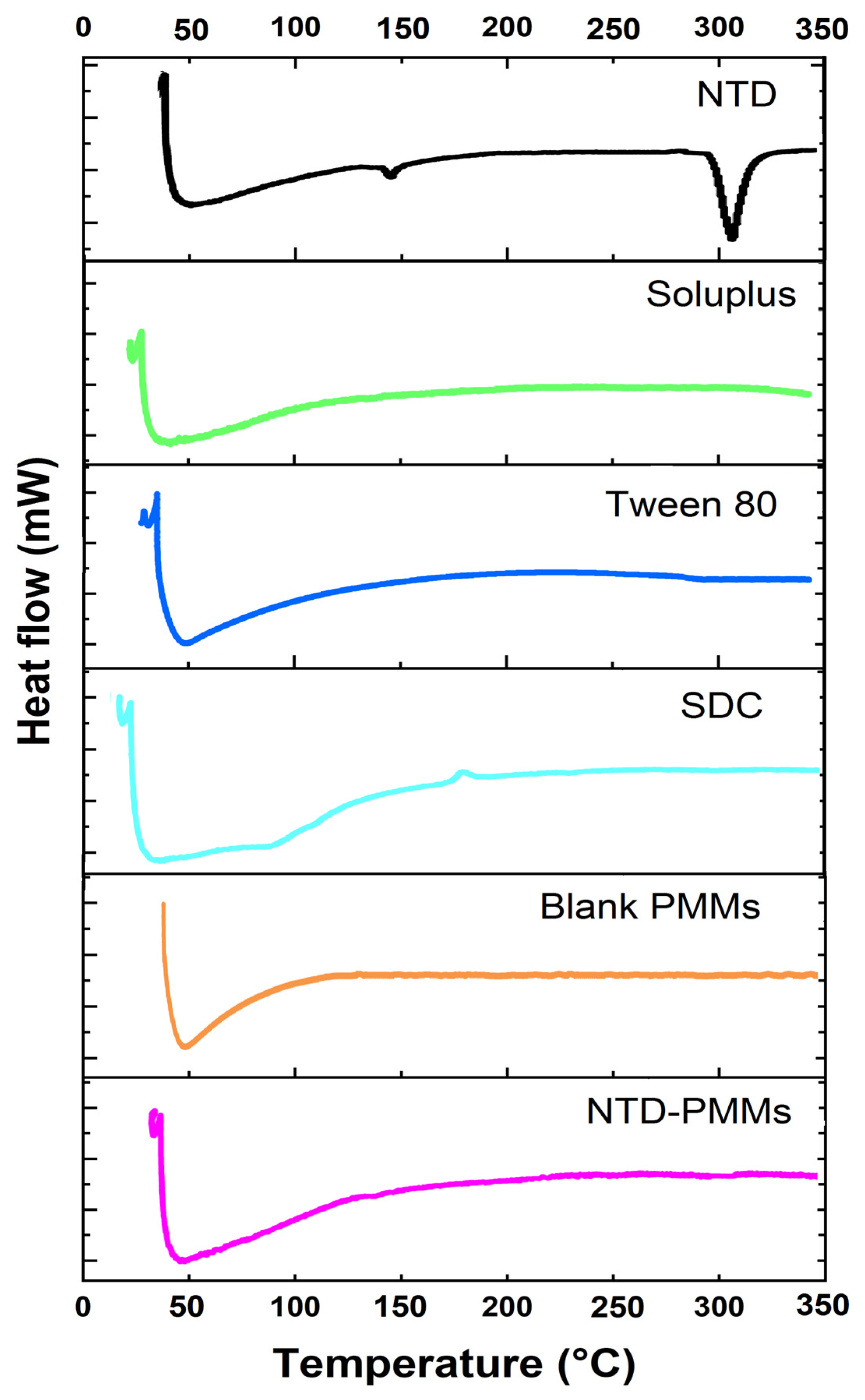
2.3.2. Differential Scanning Calorimetry
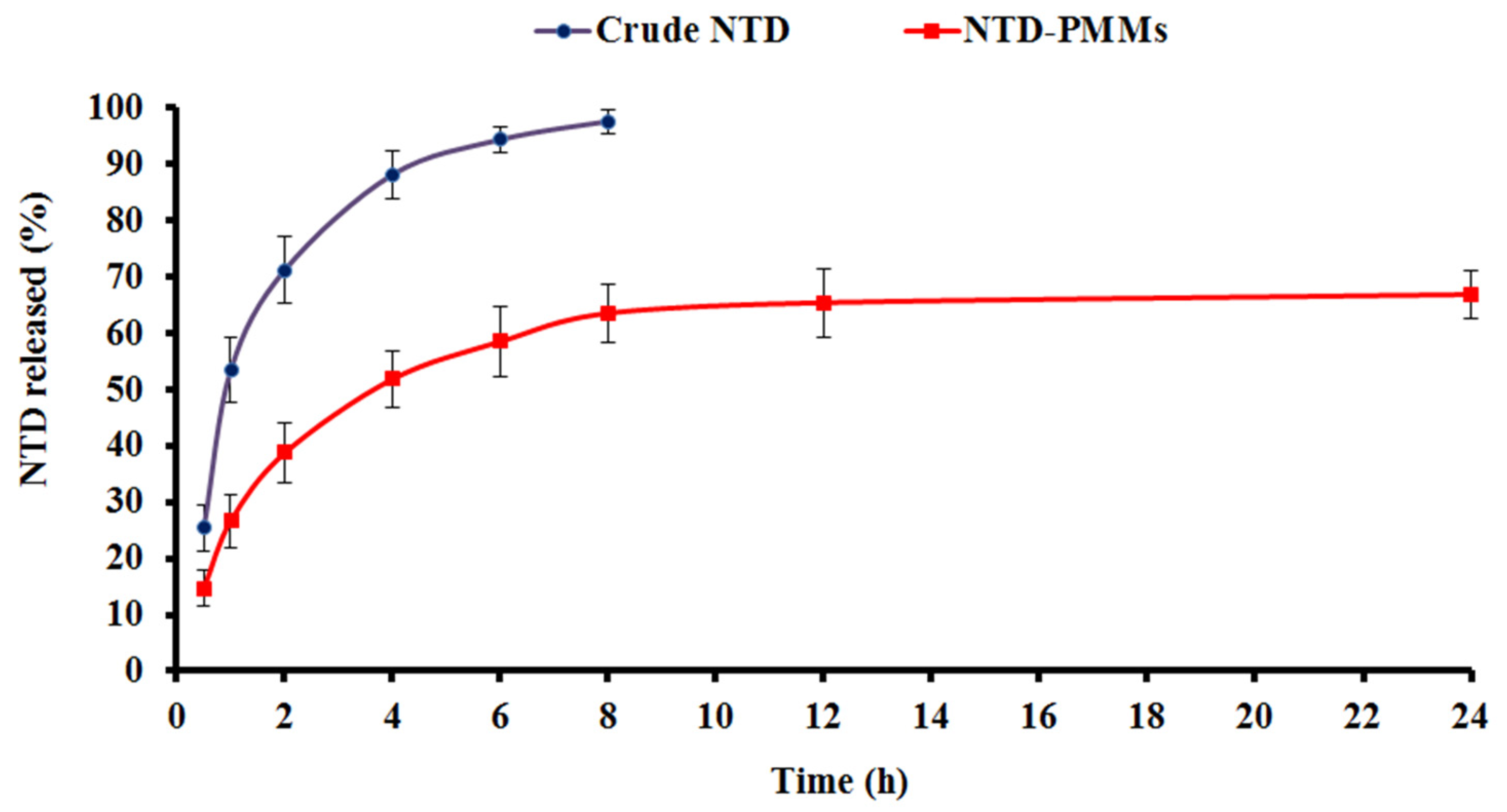
2.3.3. In Vitro Release
2.3.4. Morphological Analysis
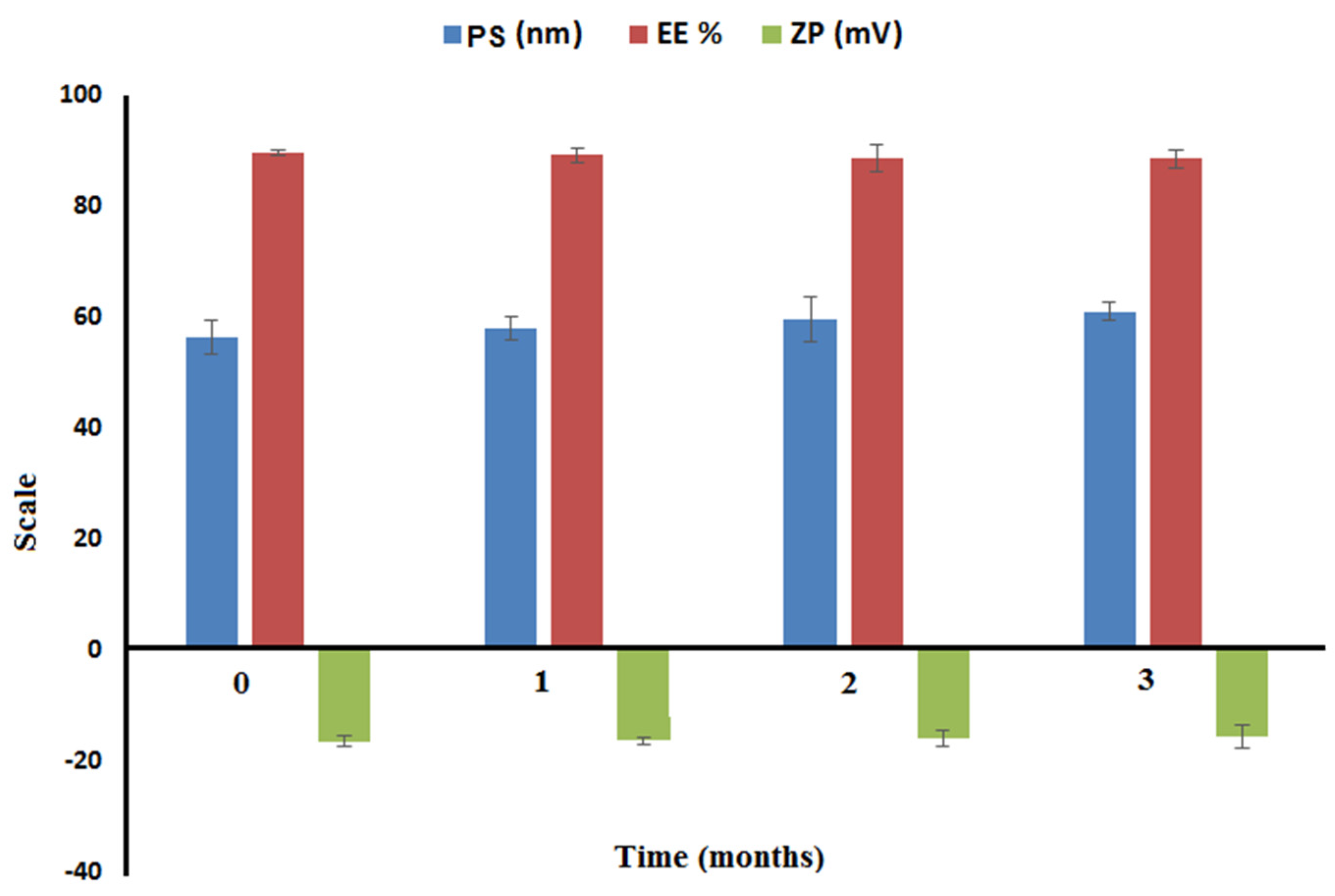
2.3.5. Short-Term Stability
2.4. In Vivo Studies
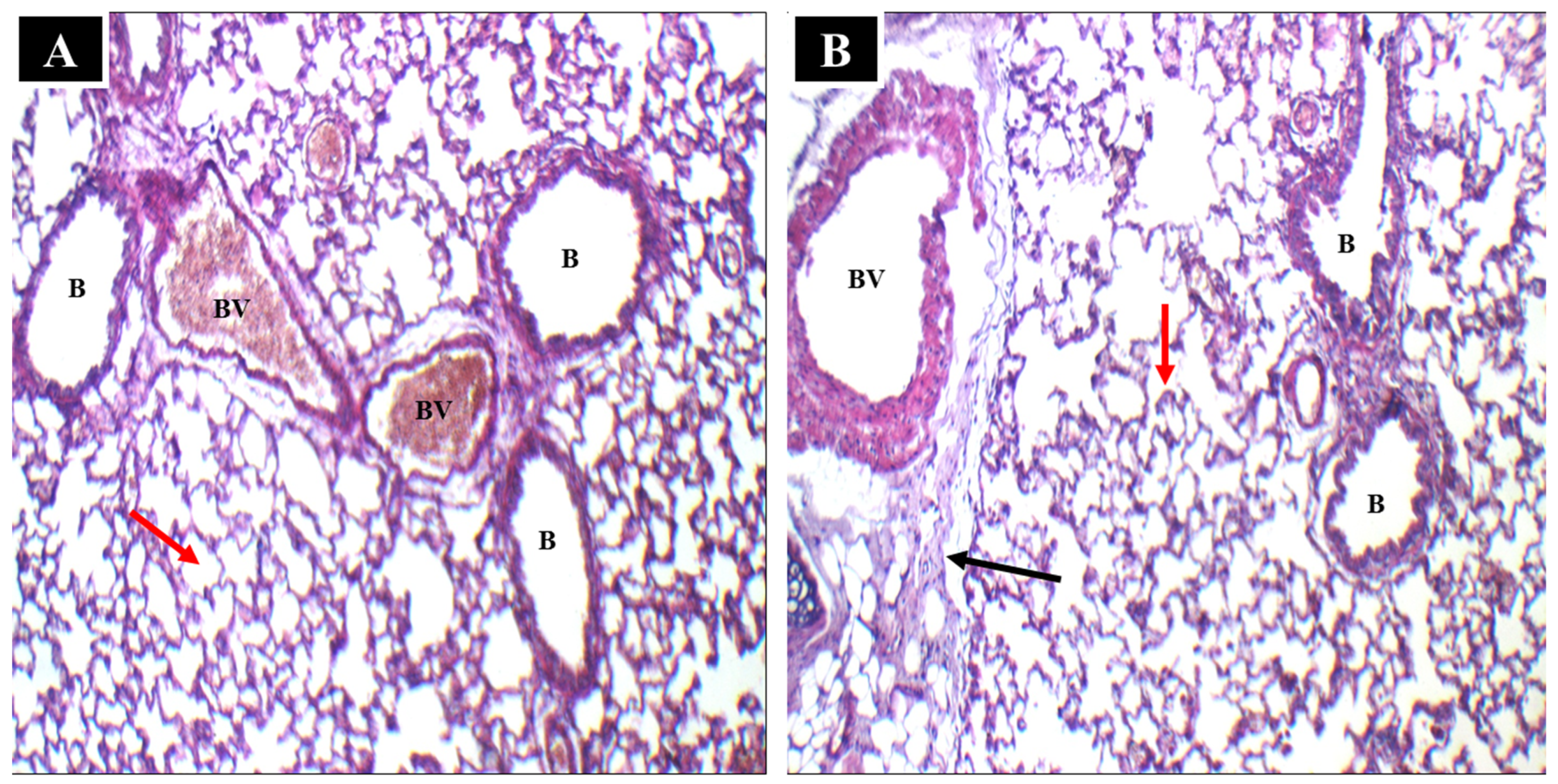
2.4.1. Histopathological Analysis
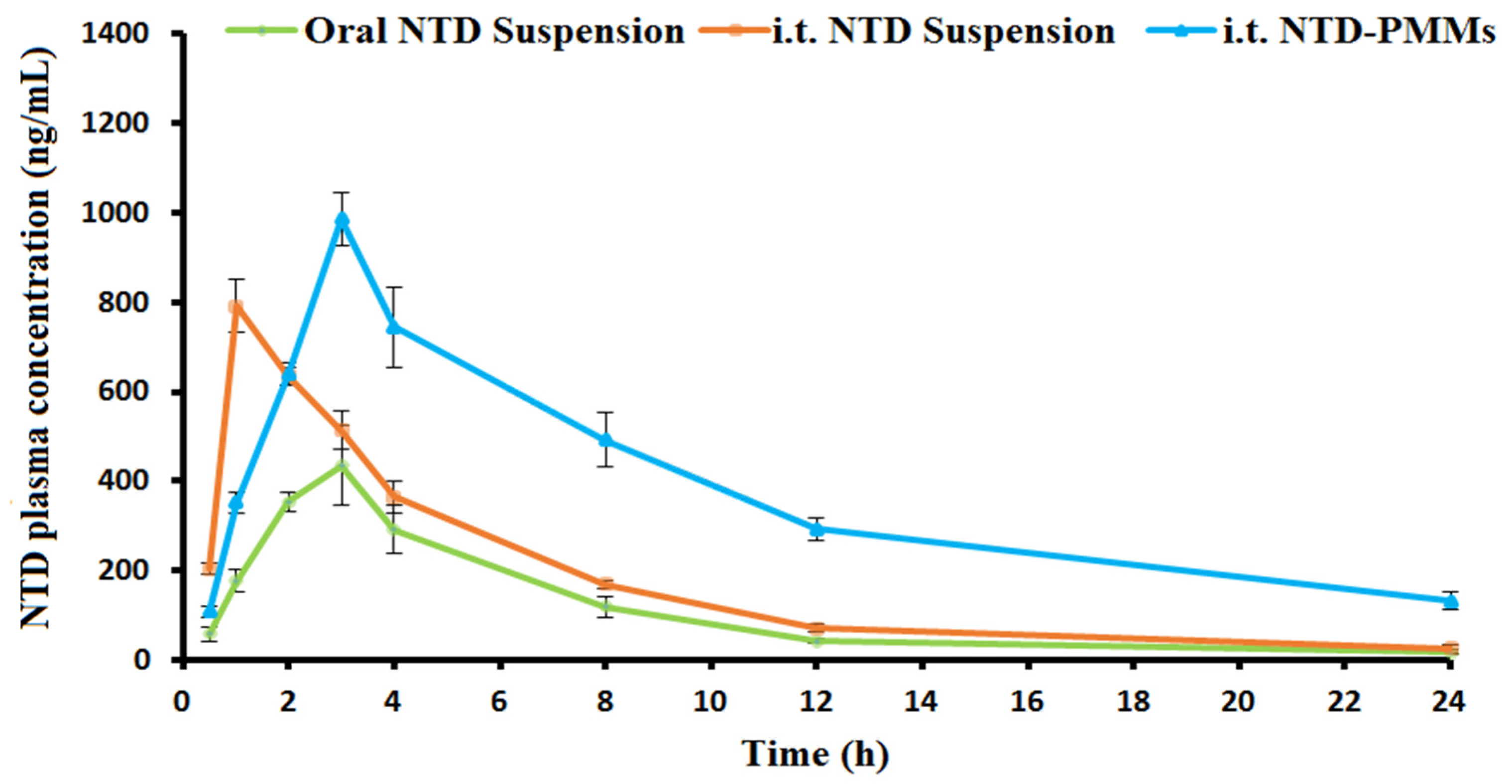
2.4.2. Pharmacokinetic Study
3. Materials and Methods
3.1. Materials
3.2. Design of Experiments
3.3. Preparation of NTD-PMMs
3.4. Optimization and Characterization of NTD-PMMs
3.4.1. Micelle Size and ZP Analysis
3.4.2. Entrapment Efficiency of NTD
3.4.3. Cumulative Release (CR) after 24 h
3.5. Characterization of the Optimized Formulation
3.5.1. Fourier Transform Infrared Spectroscopy (FTIR)
3.5.2. Differential Scanning Calorimetry (DSC)
3.5.3. In Vitro Release
3.5.4. Morphological Analysis
3.5.5. Short-Term Stability
3.6. In Vivo Studies
3.6.1. In Vivo Histopathological Analysis
3.6.2. Pharmacokinetic Study
Chromatographic Conditions
Data Analysis
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaur, R.; Shaikh, T.B.; Sripadi, H.P.; Kuncha, M.; Sarathi, U.V.; Kulhari, H.; Andugulapati, S.B.; Sistla, R. Nintedanib solid lipid nanoparticles improve oral bioavailability and ameliorate pulmonary fibrosis in vitro and in vivo models. Int. J. Pharm. 2024, 649, 123644. [Google Scholar] [CrossRef] [PubMed]
- Tzouvelekis, A.; Bouros, D.; Aidinis, V. Biomarkers in Idiopathic Pulmonary Fibrosis: A RAGE-ing Bull in the Arena; American Thoracic Society: New York, NY, USA, 2017; Volume 14, pp. 613–614. [Google Scholar]
- Maher, T.M.; Bendstrup, E.; Dron, L.; Langley, J.; Smith, G.; Khalid, J.M.; Patel, H.; Kreuter, M. Global incidence and prevalence of idiopathic pulmonary fibrosis. Respir. Res. 2021, 22, 197. [Google Scholar] [CrossRef] [PubMed]
- Alrajhi, N.N. Post-COVID-19 pulmonary fibrosis: An ongoing concern. Ann. Thorac. Med. 2023, 18, 173–181. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. Transforming growth factor–β in tissue fibrosis. J. Exp. Med. 2020, 217, e20190103. [Google Scholar] [CrossRef] [PubMed]
- Wuyts, W.A.; Agostini, C.; Antoniou, K.M.; Bouros, D.; Chambers, R.C.; Cottin, V.; Egan, J.J.; Lambrecht, B.N.; Lories, R.; Parfrey, H. The pathogenesis of pulmonary fibrosis: A moving target. Eur. Respir. J. 2013, 41, 1207–1218. [Google Scholar] [CrossRef]
- Ley, B.; Collard, H.R.; King, T.E., Jr. Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2011, 183, 431–440. [Google Scholar] [CrossRef]
- Leong, S.W.; Bos, S.; Lordan, J.L.; Nair, A.; Fisher, A.J.; Meachery, G. Lung transplantation for interstitial lung disease: Evolution over three decades. BMJ Open Respir. Res. 2023, 10, e001387. [Google Scholar] [CrossRef]
- Wollin, L.; Wex, E.; Pautsch, A.; Schnapp, G.; Hostettler, K.E.; Stowasser, S.; Kolb, M. Mode of action of nintedanib in the treatment of idiopathic pulmonary fibrosis. Eur. Respir. J. 2015, 45, 1434–1445. [Google Scholar] [CrossRef]
- Rao, L.; Zhu, P.; Guo, M.; Hu, M.; Guo, X.; Du, Y.; Xu, G. Nebulized inhalation of nintedanib-loaded biomimetic nano-liposomes attenuated bleomycin-induced interstitial lung fibrosis in mice. Nano Today 2024, 56, 102298. [Google Scholar] [CrossRef]
- Tepede, A.; Yogaratnam, D. Nintedanib for idiopathic pulmonary fibrosis. J. Pharm. Pract. 2019, 32, 199–206. [Google Scholar] [CrossRef]
- Kala, S.G.; Chinni, S. Bioavailability enhancement of vitamin E TPGS liposomes of nintedanib esylate: Formulation optimization, cytotoxicity and pharmacokinetic studies. Drug Deliv. Transl. Res. 2022, 12, 2856–2864. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Sasaki, S.; Nakamura, T.; Kurokawa, K.; Yamada, T.; Ochi, Y.; Ihara, H.; Takahashi, F.; Takahashi, K. Gastrointestinal adverse effects of nintedanib and the associated risk factors in patients with idiopathic pulmonary fibrosis. Sci. Rep. 2019, 9, 12062. [Google Scholar] [CrossRef]
- Wang, X.; Wan, W.; Lu, J.; Quan, G.; Pan, X.; Liu, P. Effects of L-leucine on the properties of spray-dried swellable microparticles with wrinkled surfaces for inhalation therapy of pulmonary fibrosis. Int. J. Pharm. 2021, 610, 121223. [Google Scholar] [CrossRef]
- Surber, M.W.; Beck, S.; Pham, S.; Marsden, A.T.; Gandi, S.K.; Baily, J.; McElroy, M.C. Inhaled nintedanib is well-tolerated and delivers key pharmacokinetic parameters required to treat bleomycin-induced pulmonary fibrosis. Pulm. Pharmacol. Ther. 2020, 63, 101938. [Google Scholar] [CrossRef]
- Ruggiero, V.; Aquino, G.; Del Gaudio, P.; Mencherini, T.; Tedesco, C.; Russo, P. Development and characterization of nintedanib inhalable powders as a potential pulmonary fibrosis treatment. J. Drug Deliv. Sci. Technol. 2024, 92, 105340. [Google Scholar] [CrossRef]
- Forest, V.; Pourchez, J. Nano-delivery to the lung-by inhalation or other routes and why nano when micro is largely sufficient? Adv. Drug Deliv. Rev. 2022, 183, 114173. [Google Scholar] [CrossRef]
- Grotz, E.; Tateosian, N.; Amiano, N.; Cagel, M.; Bernabeu, E.; Chiappetta, D.A.; Moretton, M.A. Nanotechnology in tuberculosis: State of the art and the challenges ahead. Pharm. Res. 2018, 35, 213. [Google Scholar] [CrossRef]
- Andrade da Silva, L.H.; Vieira, J.B.; Cabral, M.R.; Antunes, M.A.; Lee, D.; Cruz, F.F.; Hanes, J.; Rocco, P.R.M.; Morales, M.M.; Suk, J.S. Development of nintedanib nanosuspension for inhaled treatment of experimental silicosis. Bioeng. Transl. Med. 2023, 8, e10401. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gadhave, D.; Chauhan, G.; Gupta, V. Development and characterization of inhaled nintedanib-loaded PLGA nanoparticles using scalable high-pressure homogenization technique. J. Drug Deliv. Sci. Technol. 2024, 91, 105233. [Google Scholar] [CrossRef]
- Wang, X.; Goyal, M.; Gadhave, D.; Gupta, V. Inhaled nintedanib nanoparticles for enhanced efficacy in idiopathic pulmonary fibrosis (IPF) treatment–Evidence in disease-relevant in-vitro models. J. Drug Deliv. Sci. Technol. 2024, 95, 105615. [Google Scholar] [CrossRef]
- K Shukla, S.; Nguyen, V.; Goyal, M.; Gupta, V. Cationically modified inhalable nintedanib niosomes: Enhancing therapeutic activity against non-small-cell lung cancer. Nanomedicine 2022, 17, 935–958. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Karampuri, S.; Kansara, V.; Vyas, B. Inhalable dry powder containing lipid polymer hybrid nanoparticles of Nintedanib esylate: In vitro and in vivo evaluations. J. Drug Deliv. Sci. Technol. 2023, 86, 104716. [Google Scholar] [CrossRef]
- Owen, S.C.; Chan, D.P.; Shoichet, M.S. Polymeric micelle stability. Nano Today 2012, 7, 53–65. [Google Scholar] [CrossRef]
- Moretton, M.A.; Taira, C.; Flor, S.; Bernabeu, E.; Lucangioli, S.; Höcht, C.; Chiappetta, D.A. Novel nelfinavir mesylate loaded d-α-tocopheryl polyethylene glycol 1000 succinate micelles for enhanced pediatric anti HIV therapy: In vitro characterization and in vivo evaluation. Colloids Surf. B Biointerfaces 2014, 123, 302–310. [Google Scholar] [CrossRef]
- Cagel, M.; Tesan, F.C.; Bernabeu, E.; Salgueiro, M.J.; Zubillaga, M.B.; Moretton, M.A.; Chiappetta, D.A. Polymeric mixed micelles as nanomedicines: Achievements and perspectives. Eur. J. Pharm. Biopharm. 2017, 113, 211–228. [Google Scholar] [CrossRef]
- Li, S.-D.; Huang, L. Pharmacokinetics and biodistribution of nanoparticles. Mol. Pharm. 2008, 5, 496–504. [Google Scholar] [CrossRef]
- Feng, S.; Zhang, Z.; Almotairy, A.; Repka, M.A. Development and evaluation of polymeric mixed micelles prepared using hot-melt extrusion for extended delivery of poorly water-soluble drugs. J. Pharm. Sci. 2023, 112, 2869–2878. [Google Scholar] [CrossRef]
- Linn, M.; Collnot, E.-M.; Djuric, D.; Hempel, K.; Fabian, E.; Kolter, K.; Lehr, C.-M. Soluplus® as an effective absorption enhancer of poorly soluble drugs in vitro and in vivo. Eur. J. Pharm. Sci. 2012, 45, 336–343. [Google Scholar] [CrossRef]
- Bernabeu, E.; Gonzalez, L.; Cagel, M.; Gergic, E.P.; Moretton, M.A.; Chiappetta, D.A. Novel Soluplus®—TPGS mixed micelles for encapsulation of paclitaxel with enhanced in vitro cytotoxicity on breast and ovarian cancer cell lines. Colloids Surf. B Biointerfaces 2016, 140, 403–411. [Google Scholar] [CrossRef]
- Arafa, W.M.; Elkomy, M.H.; Aboud, H.M.; Ali, M.I.; Abdel Gawad, S.S.; Aboelhadid, S.M.; Mahdi, E.A.; Alsalahat, I.; Abdel-Tawab, H. Tunable polymeric mixed micellar nanoassemblies of lutrol F127/Gelucire 44/14 for oral delivery of praziquantel: A promising nanovector against hymenolepis nana in experimentally-infected rats. Pharmaceutics 2022, 14, 2023. [Google Scholar] [CrossRef]
- Salem, H.F.; Ali, A.A.; Hegazy, A.M.; Sadek, A.-R.A.; Aboud, H.M. Harnessing of doxylamine succinate/pyridoxine hydrochloride-dual laden bilosomes as a novel combinatorial nanoparadigm for intranasal delivery: In vitro optimization and in vivo pharmacokinetic appraisal. J. Pharm. Sci. 2022, 111, 794–809. [Google Scholar] [CrossRef] [PubMed]
- Elkomy, M.H.; El Menshawe, S.F.; Kharshoum, R.M.; Abdeltwab, A.M.; Hussein, R.R.; Hamad, D.S.; Alsalahat, I.; Aboud, H.M. Innovative pulmonary targeting of terbutaline sulfate-laded novasomes for non-invasive tackling of asthma: Statistical optimization and comparative in vitro/in vivo evaluation. Drug Deliv. 2022, 29, 2058–2071. [Google Scholar] [CrossRef] [PubMed]
- Pignatello, R.; Corsaro, R.; Bonaccorso, A.; Zingale, E.; Carbone, C.; Musumeci, T. Soluplus® polymeric nanomicelles improve solubility of BCS-class II drugs. Drug Deliv. Transl. Res. 2022, 12, 1991–2006. [Google Scholar] [CrossRef] [PubMed]
- Aboud, H.M.; Mahmoud, M.O.; Abdeltawab Mohammed, M.; Shafiq Awad, M.; Sabry, D. Preparation and appraisal of self-assembled valsartan-loaded amalgamated Pluronic F127/Tween 80 polymeric micelles: Boosted cardioprotection via regulation of Mhrt/Nrf2 and Trx1 pathways in cisplatin-induced cardiotoxicity. J. Drug Target. 2020, 28, 282–299. [Google Scholar] [CrossRef] [PubMed]
- El Menshawe, S.F.; Aboud, H.M.; Elkomy, M.H.; Kharshoum, R.M.; Abdeltwab, A.M. A novel nanogel loaded with chitosan decorated bilosomes for transdermal delivery of terbutaline sulfate: Artificial neural network optimization, in vitro characterization and in vivo evaluation. Drug Deliv. Transl. Res. 2020, 10, 471–485. [Google Scholar] [CrossRef]
- M Elsharkawy, F.; M Amin, M.; A Shamsel-Din, H.; Ibrahim, W.; Ibrahim, A.B.; Sayed, S. Self-assembling lecithin-based mixed polymeric micelles for nose to brain delivery of clozapine: In-vivo assessment of drug efficacy via radiobiological evaluation. Int. J. Nanomed. 2023, 18, 1577–1595. [Google Scholar] [CrossRef]
- Khallaf, R.A.; Aboud, H.M.; Sayed, O.M. Surface modified niosomes of olanzapine for brain targeting via nasal route; preparation, optimization, and in vivo evaluation. J. Liposome Res. 2020, 30, 163–173. [Google Scholar] [CrossRef]
- Aboud, H.M.; Hassan, A.H.; Ali, A.A.; Abdel-Razik, A.-R.H. Novel in situ gelling vaginal sponges of sildenafil citrate-based cubosomes for uterine targeting. Drug Deliv. 2018, 25, 1328–1339. [Google Scholar] [CrossRef]
- Elkomy, M.H.; Khallaf, R.A.; Mahmoud, M.O.; Hussein, R.R.; El-Kalaawy, A.M.; Abdel-Razik, A.-R.H.; Aboud, H.M. Intratracheally inhalable nifedipine-loaded chitosan-PLGA nanocomposites as a promising nanoplatform for lung targeting: Snowballed protection via regulation of TGF-β/β-catenin pathway in bleomycin-induced pulmonary fibrosis. Pharmaceuticals 2021, 14, 1225. [Google Scholar] [CrossRef]
- Duan, R.-l.; Sun, X.; Liu, J.; Gong, T.; Zhang, Z.-R. Mixed micelles loaded with silybin-polyene phosphatidylcholine complex improve drug solubility. Acta Pharmacol. Sin. 2011, 32, 108–115. [Google Scholar] [CrossRef]
- Pignatello, R.; Corsaro, R. Polymeric nanomicelles of Soluplus® as a strategy for enhancing the solubility, bioavailability and efficacy of poorly soluble active compounds. Curr. Nanomed. (Former. Recent Pat. Nanomed.) 2019, 9, 184–197. [Google Scholar] [CrossRef]
- El Menshawe, S.F.; Shalaby, K.; Elkomy, M.H.; Aboud, H.M.; Ahmed, Y.M.; Abdelmeged, A.A.; Elkarmalawy, M.; Abou Alazayem, M.A.; El Sisi, A.M. Repurposing celecoxib for colorectal cancer targeting via pH-triggered ultra-elastic nanovesicles: Pronounced efficacy through up-regulation of Wnt/β-catenin pathway in DMH-induced tumorigenesis. Int. J. Pharm. X 2024, 7, 100225. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Kassem, M.A.; Sayed, S. Bilosomes as promising nanovesicular carriers for improved transdermal delivery: Construction, in vitro optimization, ex vivo permeation and in vivo evaluation. Int. J. Nanomed. 2020, 15, 9783–9798. [Google Scholar] [CrossRef] [PubMed]
- Abdelbary, A.A.; Abd-Elsalam, W.H.; Al-Mahallawi, A.M. Fabrication of novel ultradeformable bilosomes for enhanced ocular delivery of terconazole: In vitro characterization, ex vivo permeation and in vivo safety assessment. Int. J. Pharm. 2016, 513, 688–696. [Google Scholar] [CrossRef] [PubMed]
- Parvataneni, D.M.; Devraj, R.; Mangamoori, L.N. Microparticles-entrapped micelles: A novel delivery system to improve solubility and dissolution rate of poorly water-soluble valsartan. J. Microencapsul. 2013, 30, 805–816. [Google Scholar] [CrossRef]
- Inoue, K.; Ogawa, K.; Okada, J.I.; Sugibayashi, K. Enhancement of skin permeation of ketotifen by supersaturation generated by amorphous form of the drug. J. Control Release 2005, 108, 306–318. [Google Scholar] [CrossRef]
- Salem, H.F.; Aboud, H.M.; Abdellatif, M.M.; Abou-Taleb, H.A. Nose-to-Brain Targeted Delivery of Donepezil Hydrochloride via Novel Hyaluronic Acid-Doped Nanotransfersomes for Alzheimer’s Disease Mitigation. J. Pharm. Sci. 2024, 113, 1934–1945. [Google Scholar] [CrossRef]
- El Menshawe, S.F.; Nafady, M.M.; Aboud, H.M.; Kharshoum, R.M.; Elkelawy, A.M.M.H.; Hamad, D.S. Transdermal delivery of fluvastatin sodium via tailored spanlastic nanovesicles: Mitigated Freund’s adjuvant-induced rheumatoid arthritis in rats through suppressing p38 MAPK signaling pathway. Drug Deliv. 2019, 26, 1140–1154. [Google Scholar] [CrossRef]
- Aboud, H.M.; Hussein, A.K.; Zayan, A.Z.; Makram, T.S.; Sarhan, M.O.; El-Sharawy, D.M. Tailoring of selenium-plated novasomes for fine-tuning pharmacokinetic and tumor uptake of quercetin: In vitro optimization and in vivo radiobiodistribution assessment in ehrlich tumor-bearing mice. Pharmaceutics 2022, 14, 875. [Google Scholar] [CrossRef]
- Liu, H.; Du, K.; Li, D.; Du, Y.; Xi, J.; Xu, Y.; Shen, Y.; Jiang, T.; Webster, T.J. A high bioavailability and sustained-release nano-delivery system for nintedanib based on electrospray technology. Int. J. Nanomed. 2018, 13, 8379–8393. [Google Scholar] [CrossRef]
- Eid, H.M.; Turkia, T.H.; Ali, A.A.; Aboud, H.M. A Novel Chitosan-coated Leciplex Loaded with Ambrisentan as a Possible Pulmonary Nanosystem: Optimization, Characterization, and Pharmacokinetics Assessments. J. Pharm. Sci. 2024, 113, 2320–2330. [Google Scholar] [CrossRef]
- Ali, A.A.; Hassan, A.H.; Eissa, E.M.; Aboud, H.M. Response surface optimization of ultra-elastic nanovesicles loaded with deflazacort tailored for transdermal delivery: Accentuated bioavailability and anti-inflammatory efficacy. Int. J. Nanomed. 2021, 16, 591–607. [Google Scholar] [CrossRef]
- Bivas-Benita, M.; Zwier, R.; Junginger, H.E.; Borchard, G. Non-invasive pulmonary aerosol delivery in mice by the endotracheal route. Eur. J. Pharm. Biopharm. 2005, 61, 214–218. [Google Scholar] [CrossRef]
- Bancroft, J.D.; Gamble, M. Theory and Practice of Histological Techniques; Elsevier Health Sciences: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Xu, D.; Zhang, Y.; Dai, J.; Bai, Y.; Xiao, Y.; Zhou, M.-t. A fast, sensitive, and high throughput method for the determination of nintedanib in mouse plasma by UPLC-MS/MS. Anal. Methods 2015, 7, 6561–6565. [Google Scholar] [CrossRef]

| Pharmacokinetic Parameter | Formulation | ||
|---|---|---|---|
| Oral NTD Suspension | i.t. NTD Suspension | i.t. NTD-PMMs | |
| Cmax (ng/mL) | 434.73 ± 76.95 | 790.71 ± 43.16 a | 986.13 ± 76.46 a,b |
| Tmax (h) | 3.00 ± 0.00 | 1.00 ± 0.00 a | 3.00 ± 0.00 b |
| MRT (h) | 7.16 ± 0.39 | 6.60 ± 0.66 | 12.33 ± 0.35 a,b |
| Ke (1/h) | 0.1411 ± 0.0078 | 0.1490 ± 0.01035 | 0.0873 ± 0.0016 a,b |
| t1/2 (h) | 4.91 ± 0.27 | 4.65 ± 0.32 | 7.94 ± 0.14 a,b |
| AUC0–t (ng h/mL) | 2605.12 ± 436.84 | 4160.18 ± 112.60 a | 8912.11 ± 332.49 a,b |
| AUC 0–∞ (ng h/mL) | 2729.95 ± 410.11 | 4339.16 ± 148.71 a | 10,427.60 ± 427.01 a,b |
| Frel | --------- | 158.95 | 381.97 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aboud, H.M.; El Menshawe, S.F.; Mohammed, N.H.; Tulbah, A.S.; Ali, A.A. Optimization and Appraisal of Nintedanib-Loaded Mixed Polymeric Micelles as a Potential Nanovector for Non-Invasive Pulmonary Fibrosis Mitigation. Pharmaceuticals 2024, 17, 1275. https://doi.org/10.3390/ph17101275
Aboud HM, El Menshawe SF, Mohammed NH, Tulbah AS, Ali AA. Optimization and Appraisal of Nintedanib-Loaded Mixed Polymeric Micelles as a Potential Nanovector for Non-Invasive Pulmonary Fibrosis Mitigation. Pharmaceuticals. 2024; 17(10):1275. https://doi.org/10.3390/ph17101275
Chicago/Turabian StyleAboud, Heba M., Shahira F. El Menshawe, Nada H. Mohammed, Alaa S. Tulbah, and Adel A. Ali. 2024. "Optimization and Appraisal of Nintedanib-Loaded Mixed Polymeric Micelles as a Potential Nanovector for Non-Invasive Pulmonary Fibrosis Mitigation" Pharmaceuticals 17, no. 10: 1275. https://doi.org/10.3390/ph17101275
APA StyleAboud, H. M., El Menshawe, S. F., Mohammed, N. H., Tulbah, A. S., & Ali, A. A. (2024). Optimization and Appraisal of Nintedanib-Loaded Mixed Polymeric Micelles as a Potential Nanovector for Non-Invasive Pulmonary Fibrosis Mitigation. Pharmaceuticals, 17(10), 1275. https://doi.org/10.3390/ph17101275






