The Immune-Stimulating and Anti-Diabetic Effects of Allium hookeri Leaves Grown in a Plant Factory with Artificial Lights in Immunosuppressed Obese C57BL/6 Mice
Abstract
1. Introduction
2. Results and Discussion
2.1. Concentration of Alliin and Cycloalliin
2.2. Effects of A. hookeri Leaf Extracts on α-Amylase and α-Glucosidase Inhibitory Activities
2.3. Effects of A. hookeri Leaf Extracts on Body and Organ Weights of the Immunosuppressed Obese Mice
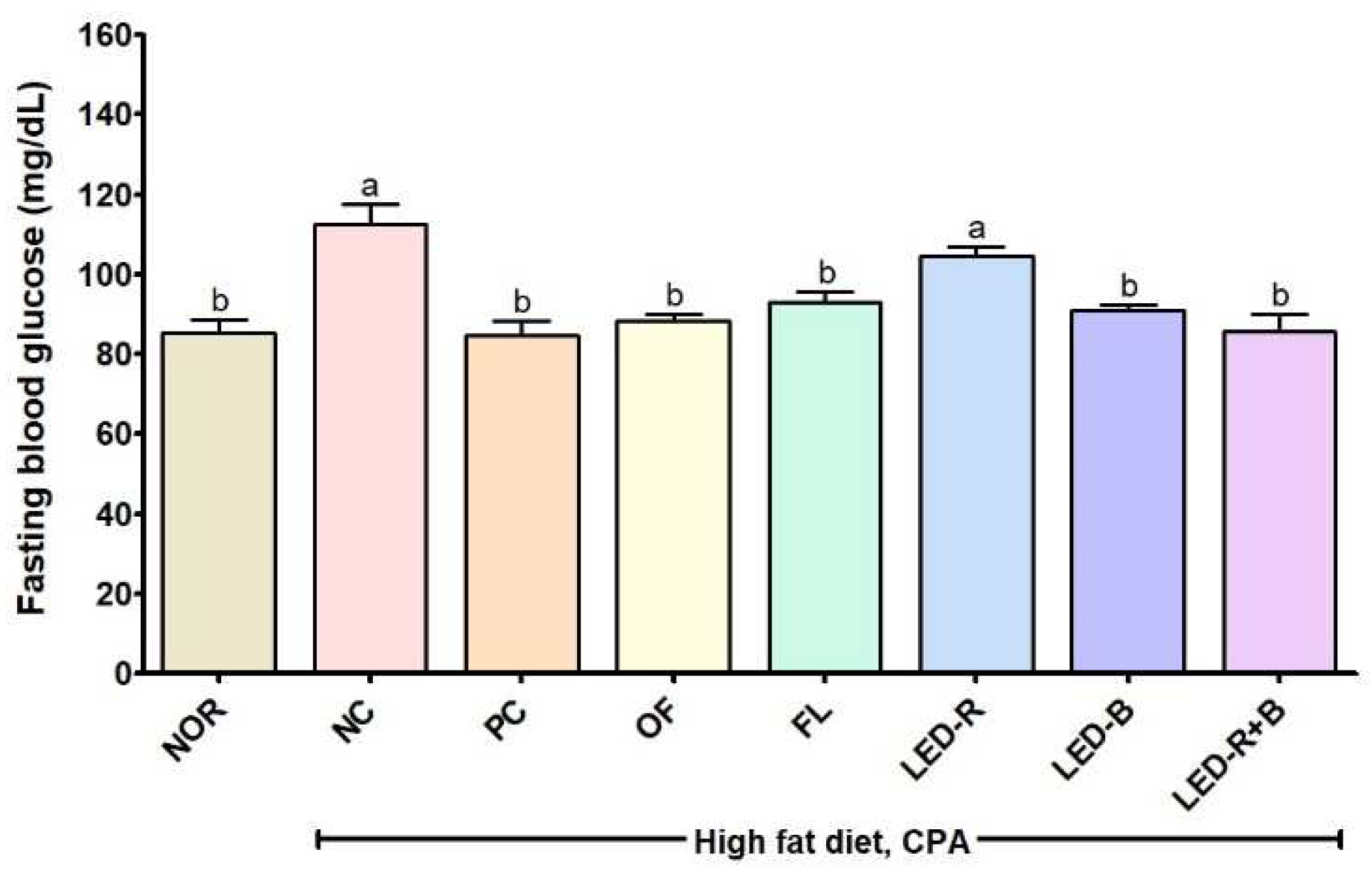
2.4. Effects of A. hookeri Leaf Extracts on Fasting Blood Glucose of the Immunosuppressed Obese Mice
2.5. Effects of A. hookeri Leaf Extracts on Hematological Factors of the Immunosuppressed Obese Mice
2.6. Effects of A. hookeri Leaf Extracts on Biochemical Factors of the Immunosuppressed Obese Mice
2.7. Effects of A. hookeri Leaf Extracts on the Splenocyte Proliferation in Immunosuppressed Obese Mice
2.8. Effects of A. hookeri Leaf Extracts on the NK Cell Activity in Immunosuppressed Obese Mice
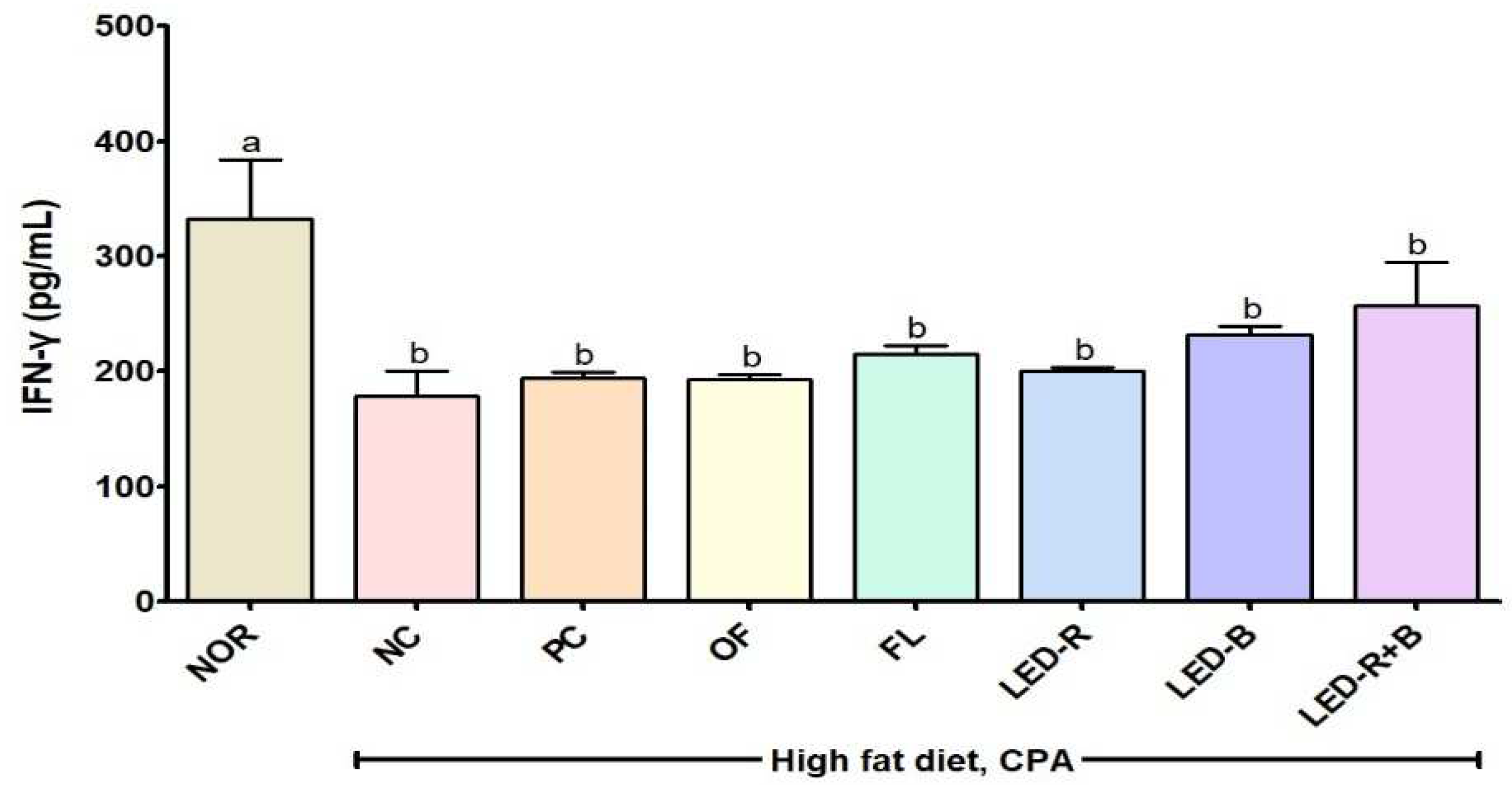
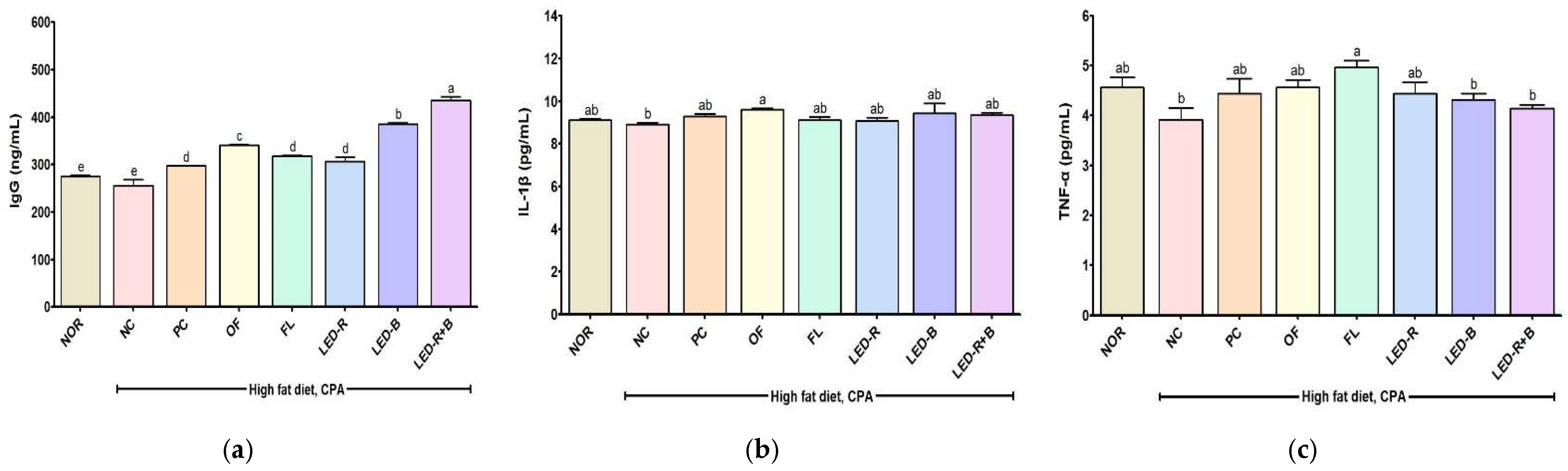
2.9. Effects of A. hookeri Leaf Extracts on Serum IgG and Cytokines Levels of Immunosuppressed Obese Mice
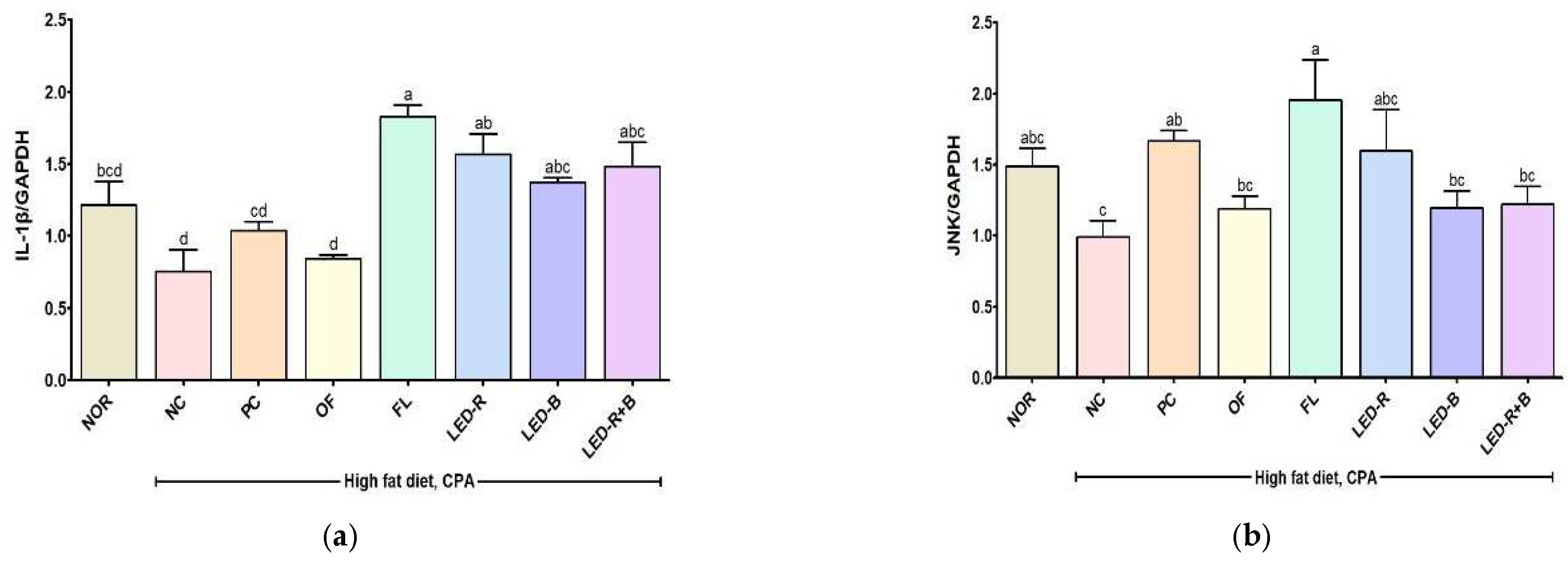
2.10. Effects of A. hookeri Leaf Extracts Grown in Plant Factories with Artificial Lights on MAPK and NF-κB Signaling Pathways in Immunosuppressed Obese Mice
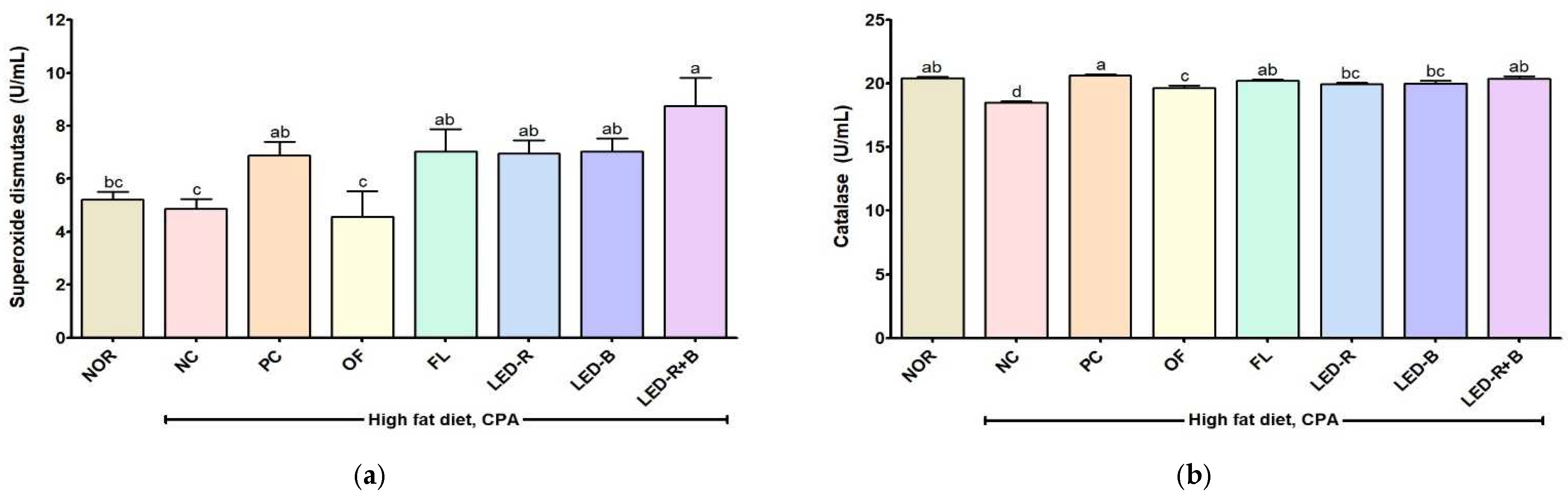
2.11. Effects of A. hookeri Leaf Extracts Grown in Plant Factories with Artificial Lights on Superoxide Dismutase and Catalase Activities in Immunosuppressed Obese Mice
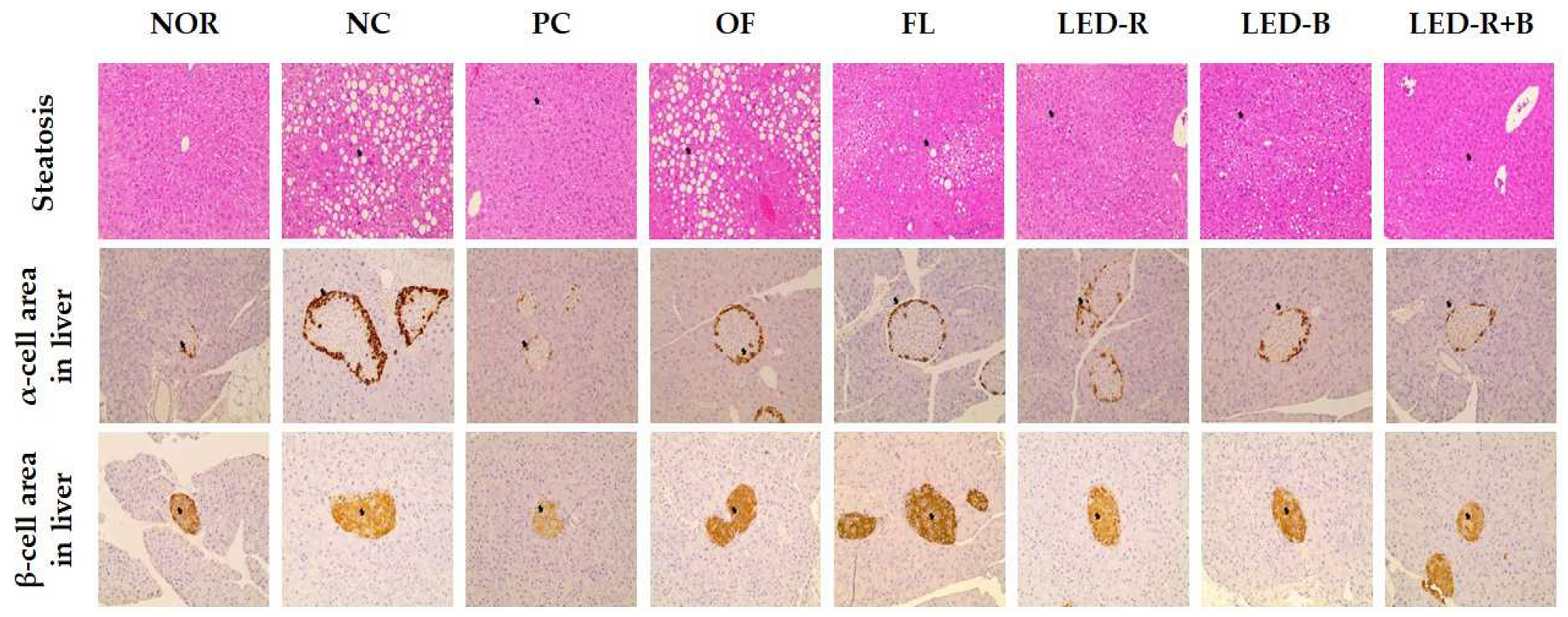
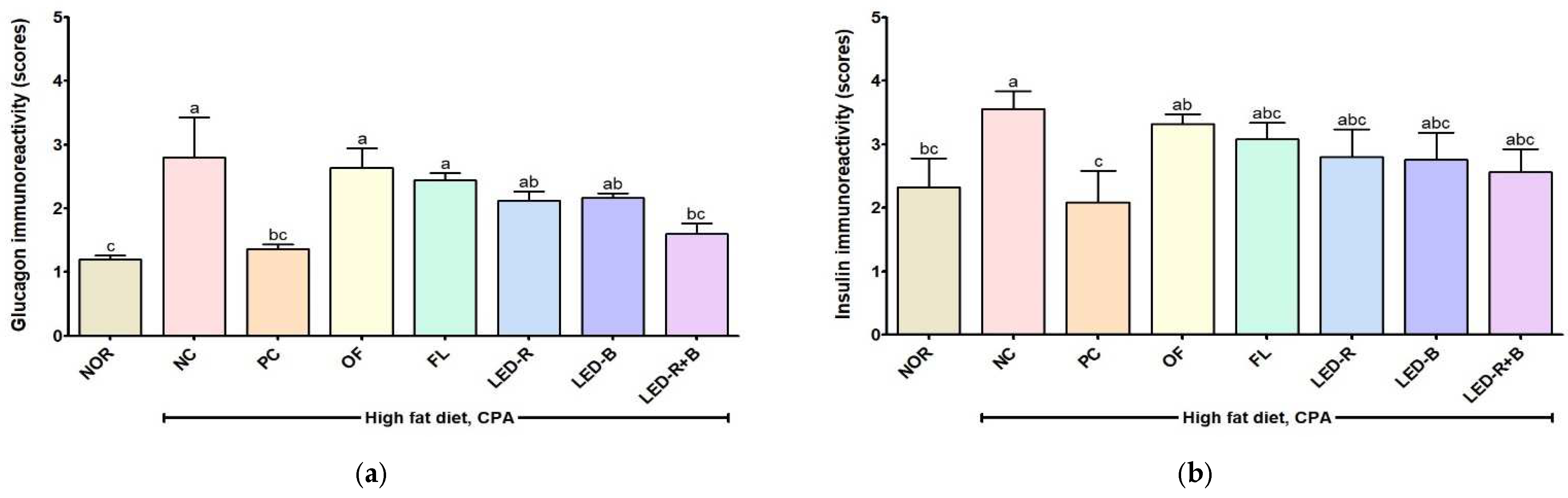
2.12. Effects of A. hookeri Leaf Extracts Grown in Plant Factories with Artificial Lights on Histopathological Properties in Liver of Immunosuppressed Obese Mice
3. Materials and Methods
3.1. Preparation of Plant Materials
3.1.1. Cultivated Condition
3.1.2. Preparation of Samples
3.2. Evaluation of Functional Compound and Diastatic Enzyme Inhibitory Activity
3.2.1. Alliin and Cycloalliin Concentrations
3.2.2. α-Amylase Inhibition Assay
3.2.3. α-Glucosidase Inhibition Assay
3.3. Animal Experiment
3.3.1. Experimental Design
- Group 1:
- NOR (normal control; distilled water, DW).
- Group 2:
- NC (negative control with high-fat diet; CPA; DW).
- Group 3:
- PC (positive control with high-fat diet; CPA; β-glucan 50 mg/kg BW).
- Group 4:
- OF (high-fat diet; CPA; A. hookeri leaves grown in outfield, extract 300 mg/kg BW).
- Group 5:
- FL (high-fat diet; CPA; A. hookeri leaves grown in plant factory with FL, extract 300 mg/kg BW).
- Group 6:
- LED-R (high-fat diet; CPA; A. hookeri leaves grown in plant factory with LED-R, extract 300 mg/kg BW).
- Group 7:
- LED-B (high-fat diet; CPA; A. hookeri leaves grown in plant factory with LED-B, extract 300 mg/kg BW).
- Group 8:
- LED-R+B (high-fat diet; CPA; A. hookeri leaves grown in plant factory with LED-R+B, extract 300 mg/kg BW).
3.3.2. Determination of Fasting Blood Glucose
3.3.3. Biochemical and Hematological Analysis
3.3.4. Splenocyte Proliferation
3.3.5. NK Cell Activity
3.3.6. Immunoglobulin G (IgG) and Cytokine Concentrations in Serum
3.3.7. Western Blot Analysis Using the Thymus of Immunosuppressed Obese Mice
3.3.8. Superoxide Dismutase and Catalase in Serum
3.3.9. Histopathological and Immunohistochemical Investigations
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koh, Y.M.; Jang, S.W.; Ahn, T.W. Anti-obesity effect of Yangkyuksanwhatang in high-fat diet-induced obese mice. BMC Complement. Altern. Med. 2019, 19, 246. [Google Scholar] [CrossRef] [PubMed]
- Jee, Y.M. Korea Health Statistics 2021: Korea National Health and Nutrition Examination Survey (KNHANES VIII-3); Korea Disease Control and Prevention Agency: Cheongju-si, Republic of Korea, 2020; pp. 159–161.
- Sung, Y.Y.; Kim, D.S.; Kim, S.H.; Kim, H.K. Aqueous and ethanolic extracts of welsh onion, Allium fistulosum, attenuate high-fat diet-induced obesity. BMC Complement. Altern. Med. 2018, 18, 105. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Li, H.; Cui, T.; Cui, M.; Piao, C.; Wang, S.; Ju, M.; Liu, X.; Zhou, G.; Xu, H.; et al. Onion (Allium cepa L.) peel extract effects on 3T3-L1 adipocytes and high-fat diet-induced obese mice. Food Biosci. 2021, 41, 101019. [Google Scholar] [CrossRef]
- Klein, S.; Gastaldelli, A.; Yki-Järvinen, H.; Scherer, P.E. Why does obesity cause diabetes? Cell Metab. 2022, 34, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Marti, A.; Marcos, A.; Martínez, J.A. Obesity and immune function relationships. Obes. Rev. 2001, 2, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.H.; Ku, J.M.; Kim, H.I.; Ahn, C.W.; Park, S.H.; Seo, H.S.; Shin, Y.C.; Ko, S.G. The immune-enhancing activity of Cervus nippon mantchuricus extract (NGE) in RAW264.7 macrophage cells and immunosuppressed mice. Food Res. Int. 2017, 99, 623–629. [Google Scholar] [PubMed]
- Zhang, H.; Gao, J.; Tang, Y.; Jin, T.; Tao, J. Inflammasomes cross-talk with lymphocytes to connect the innate and adaptive immune response. J. Adv. Res. 2023, 54, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.S.; Warrington, R.; Watson, W.; Kim, H.L. An introduction to immunology and immunopathology. Allergy Asthma Clin. Immunol. 2018, 14, 49. [Google Scholar] [CrossRef]
- Bonilla, F.A.; Oettgen, H.C. Adaptive immunity. J. Allergy Clin. Immunol. 2010, 125, S33–S40. [Google Scholar] [CrossRef]
- Tong, T.; Wang, Y.N.; Zhang, C.M.; Kang, S.G. In vitro and in vivo antihypertensive and antioxidant activities of fermented roots of Allium hookeri. Chin. Herb. Med. 2021, 13, 541–548. [Google Scholar] [CrossRef]
- Deka, B.; Manna, P.; Borah, J.C.; Talukdar, N.C. A review on phytochemical, pharmacological attributes and therapeutic uses of Allium hookeri. Phytomed. Plus 2022, 2, 100262. [Google Scholar] [CrossRef]
- Kim, J.S. Effects of solvents with different polarities on the antioxidant activities of the leaves and roots of Allium hookeri. J. East. Asian Soc. Diet. Life 2020, 30, 363–373. [Google Scholar] [CrossRef]
- Jang, J.Y.; Lee, M.J.; You, B.R.; Jin, J.S.; Lee, S.H.; Yun, Y.R.; Kim, H.J. Allium hookeri root extract exerts anti-inflammatory effects by nuclear factor-κB down-regulation in lipopolysaccharide-induced RAW264.7 cells. BMC Complement. Altern. Med. 2017, 17, 126. [Google Scholar]
- Kim, H.J.; Lee, M.J.; Jang, J.Y.; Lee, S.H. Allium hookeri root extract inhibits adipogenesis by promoting lipolysis in high fat diet-induced obese mice. Nutrients 2019, 11, 2262. [Google Scholar] [CrossRef] [PubMed]
- Chun, H.; Han, S. A study on the design and operation method of plant factory using artificial intelligence. Nanotechnol. Environ. Eng. 2021, 6, 41. [Google Scholar]
- Arcel, M.M.; Lin, X.; Huang, J.; Wu, J.; Zheng, S. The application of LED illumination and intelligent control in plant factory, a new direction for modern agriculture: A review. J. Phys. Conf. Ser. 2021, 1732, 012178. [Google Scholar] [CrossRef]
- Chen, X.L.; Guo, W.Z.; Xue, X.Z.; Wang, L.C.; Qiao, X.J. Growth and quality responses of ‘green oak leaf’ lettuce as affected by monochromic or mixed radiation provided by fluorescent lamp (FL) and light-emitting diode (LED). Sci. Hortic. 2014, 172, 168–175. [Google Scholar] [CrossRef]
- Ma, Y.; Xu, A.; Cheng, Z.M. Effects of light emitting diode lights on plant growth, development and traits a meta-analysis. Hortic. Plant J. 2021, 7, 552–564. [Google Scholar] [CrossRef]
- Razzak, M.A.; Asaduzzaman, M.; Tanaka, H.; Asao, T. Effects of supplementing green light to red and blue light on the growth and yield of lettuce in plant factories. Sci. Hortic. 2022, 305, 111429. [Google Scholar] [CrossRef]
- Kim, Y.M.; Sung, J.K.; Lee, Y.J.; Lee, D.B.; Yoo, C.H.; Lee, S.B. Varying effects of artificial light on plant functional metabolites. Korean J. Environ. Agric. 2019, 38, 61–67. [Google Scholar] [CrossRef]
- Cho, J.Y.; Yoo, K.S.; Kim, J.; Choi, B.J.; Oh, W. Growth and bioactive compounds of lettuce as affected by light intensity and photoperiod in a plant factory using external electrode fluorescent lamps. Hortic. Sci. Technol. 2020, 38, 645–659. [Google Scholar] [CrossRef]
- Jung, J.; Heo, J.W.; Kim, J.S.; Jeong, U.Y.; Bae, U.J.; Jang, H.N.; Shim, C.K.; Joung, Y.; Lee, S.H. Functionality of Allium hookeri leaves and roots grown in a hydroponic plant factory using artificial lights. J. Korean Soc. Food Sci. Nutr. 2022, 51, 1355–1363. [Google Scholar] [CrossRef]
- Jung, J.; Heo, J.W.; Kim, J.S.; Jeong, U.J.; Kim, H.B.; Shim, C.K.; Lee, S.H. Comparison of the antioxidant and anti-inflammatory effects of Allium hookeri leaves grown in an outfield and a plant factory using different artificial lights. Korean J. Community Living Sci. 2022, 33, 657–669. [Google Scholar] [CrossRef]
- Park, S.H.; Bae, U.J.; Choi, E.K.; Jung, S.J.; Lee, S.H.; Yang, J.H.; Kim, Y.S.; Jeong, D.Y.; Kim, H.J.; Park, B.H.; et al. A randomized, double-blind, placebo-controlled crossover clinical trial to evaluate the anti-diabetic effects of Allium hookeri extract in the subjects with prediabetes. BMC Complement. Med. Ther. 2020, 20, 211. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Suh, H.J.; Han, S.H.; Hong, J.; Choi, H.S. Optimization of extraction of cycloalliin from garlic (Allium sativum L.) by using principal components analysis. Prev. Nutr. Food Sci. 2016, 21, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Kalita, D.; Holm, D.G.; LaBarbera, D.V.; Petrash, J.M.; Jayanty, S.S. Inhibition of α-glucosidase, α-amylase, and aldose reductase by potato polyphenolic compounds. PLoS ONE 2018, 13, e0191025. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Thrimawithana, T.; Shukla, R.; Adhikari, B. Inhibition of enzymes associated with obesity by the polyphenol-rich extracts of Hibiscus sabdariffa. Food Biosci. 2022, 50, 101992. [Google Scholar] [CrossRef]
- Wu, Y.X.; Kim, Y.J.; Li, S.; Yun, M.C.; Yoon, J.M.; Kim, J.Y.; Cho, S.I.; Son, K.H.; Kim, T. Anti-obese effects of mulberry (Morus alba L.) root bark through the inhibition of digestive enzymes and 3T3-L1 adipocyte differentiation. Korean J. Food Preserv. 2015, 22, 27–35. [Google Scholar] [CrossRef]
- Unuofin, J.O.; Otunola, G.A.; Afolayan, A.J. In vitro α-amylase, α-glucosidase, lipase inhibitory and cytotoxic activities of tuber extracts of Kedrostis africana (L.) Cogn. Heliyon 2018, 4, e00810. [Google Scholar] [CrossRef]
- Ardeshirlarijani, E.; Namazi, N.; Jalili, R.B.; Saeedi, M.; Imanparast, S.; Adhami, H.R.; Faramarzi, M.A.; Ayati, M.H.; Mahdavi, M.; Larijani, B. Potential anti-obesity effects of some medicinal herb: In vitro α-amylase, α-glucosidase and lipase inhibitory activity. Int. Biol. Biomed. J. 2019, 5, 1–8. [Google Scholar]
- Kurniawan, H.; Dacamis, E.S.; Simamora, A.; Tobing, P.S.D.L.; Hanapiah, A.; Santoso, A.W. Antioxidant, antidiabetic, and anti-obesity potential of Ipomoea reptans poir leaves. Borneo J. Pharm. 2020, 3, 216–226. [Google Scholar] [CrossRef]
- Vo, T.N.; Luong, T.D.M.; Le, T.P.H.; Trinh, K.S. Control of obesity, blood glucose, and blood lipid with Olax imbricate Roxb. root extract in high-fat diet-induced obese mice. J. Toxicol. 2022, 2022, 7781723. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Kim, S.M.; Jung, J.I.; Lim, J.; Woo, M.; Kim, E.J. Immune-enhancing effect of hydrolyzed and fermented Platycodon grandiflorum extract in cyclophosphamide-induced immunosuppressed BALB/c mice. Nutr. Res. Pract. 2023, 17, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Jeong, U.Y.; Jung, J.; Lee, E.B.; Choi, J.H.; Kim, J.S.; Jang, H.H.; Park, S.Y.; Lee, S.H. Antioxidant and immune stimulating effects of Allium hookeri extracts in the RAW 264.7 cells and immune-depressed C57BL/6 mice. Antioxidants 2022, 11, 1927. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Kim, S.H.; Lee, E.B.; Kim, J.S.; Jung, J.E.; Jeong, U.Y.; Kim, J.H.; Jang, H.H.; Park, S.Y.; Kim, G.C.; et al. Anti-diabetic effects of Allium hookeri extracts prepared by different methods in type 2 C57BL/J-db/db mice. Pharmaceuticals 2022, 15, 486. [Google Scholar] [CrossRef] [PubMed]
- Qi, Q.; Dong, Z.; Sun, Y.; Li, S.; Zhao, Z. Protective effect of bergenin against cyclophosphamide-induced immunosuppression by immunomodulatory effect and antioxidation in Balb/c mice. Molecules 2018, 23, 2668. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, J.W.; Kim, Y.K.; Ku, S.K.; Lee, H.J. Immunoenhancement effects of the herbal formula hemomine on cyclophosphamide-induced immunosuppression in mice. Appl. Sci. 2022, 12, 4935. [Google Scholar] [CrossRef]
- Park, Y.H.; An, M.; Kim, J.K.; Lim, Y.H. Antiobesity effect of ethanolic extract of Ramulus mori in differentiated 3T3-L1 adipocytes and high-fat diet-induced obese mice. J. Ethnopharmacol. 2020, 251, 112542. [Google Scholar] [CrossRef]
- Ülger, T.G.; Çakiroglu, F.P. The effects of onion (Allium cepa L.) dried by different heat treatments on plasma lipid profile and fasting blood glucose level in diabetic rats. Avicenna J. Phytomed 2020, 10, 325–333. [Google Scholar]
- Silva-Santana, G.; Bax, J.C.; Fernandes, D.C.S.; Bacellar, D.T.L.; Hooper, C.; Dias, A.A.S.O.; Silva, C.B.; Souza, A.M.; Ramos, S.; Santos, R.A.; et al. Clinical hematological and biochemical parameters in Swiss, BALB/c, C57BL/6 and B6D2F1 Mus musculus. Anim. Models Exp. Med. 2020, 3, 304–315. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, E.B.; Choi, J.H.; Jung, J.; Jeong, U.Y.; Bae, U.J.; Jang, H.H.; Park, S.Y.; Cha, Y.S.; Lee, S.H. Antioxidant and immune stimulating effects of Allium cepa skin in the RAW 264.7 cells and in the C57BL/6 mouse immunosuppressed by cyclophosphamide. Antioxidants 2023, 12, 892. [Google Scholar] [CrossRef]
- Kim, H.I.; Kim, D.S.; Jung, Y.; Sung, N.Y.; Kim, M.; Han, I.J.; Nho, E.Y.; Hong, J.H.; Lee, J.K.; Boo, M.; et al. Immune-enhancing effect of Sargassum horneri on cyclophosphamide-induced immunosuppression in BALB/c mice and primary cultured splenocytes. Molecules 2022, 27, 8253. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.H.; Lee, Y.S.; Ku, S.K.; Lee, H.J. Phenllius baumii enhances the immune response in cyclophosphamide-induced immunosuppressed mice. Nutr. Res. 2020, 75, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; No, K.; Lee, J. Anti-obesity effect of Allium hookeri leaf extract in high-fat diet-fed mice. J. Med. Food 2018, 21, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Momoh, B.J.; Okere, S.O.; Anyanwu, G.O. The anti-obesity effect of Allium cepa L. leaves on high fat diet induced obesity in male Wistar rats. Clin. Complement. Med. Pharmacol. 2022, 2, 100035. [Google Scholar] [CrossRef]
- Chae, J.; Lee, E.; Oh, S.M.; Ryu, H.W.; Kim, S.; Nam, J.O. Aged black garlic (Allium sativum L.) and aged black elephant garlic (Allium ampeloprasum L.) alleviate obesity and attenuate obesity-induced muscle atrophy in diet-induced obese C57BL/6 mice. Biomed. Pharmacother. 2023, 163, 114810. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.M.; Lee, S.H.; Lee, D.S.; You, M.J.; Chung, I.K.; Cheon, W.H.; Kwon, Y.S.; Lee, Y.J.; Ku, S.K. Fermented garlic protects diabetic, obese mice when fed a high-fat diet by antioxidant effects. Nut. Res. 2011, 31, 387–396. [Google Scholar] [CrossRef]
- Sung, Y.Y.; Kim, S.H.; Yoo, B.W.; Kim, H.K. The nutritional composition and anti-obesity effects of an herbal mixed extract containing Allium fistulosum and Viola mandshurica in high-fat-diet-induced obese mice. BMC Complement. Altern. Med. 2015, 15, 370. [Google Scholar] [CrossRef]
- Ryu, D.S.; Kim, S.H.; Lee, D.S. Effect of Salicornia herbacea polysaccharides on the activation of immune cells in vitro and in vivo. Food Sci. Biotechnol. 2009, 18, 1481–1486. [Google Scholar]
- Lee, J.; Park, K.H.; Ryu, J.H.; Bae, H.J.; Choi, A.; Lee, H.; Lim, J.; Han, K.; Park, C.H.; Jung, E.S.; et al. Natural killer cell activity for IFN-gamma production as a supportive diagnostic marker for gastric cancer. Oncotarget 2017, 8, 70431–70440. [Google Scholar] [CrossRef]
- Kaneko, Y.; Nimmerjahn, F.; Ravetch, J.V. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science 2006, 313, 670–673. [Google Scholar] [CrossRef]
- Choi, J.H.; Lee, E.B.; Park, Y.G.; Lee, H.K.; Jang, H.H.; Choe, J.; Hwang, K.A.; Park, S.Y.; Hwang, I.G.; Hong, H.C.; et al. Aged doraji (Platycodon grandiflorum) ameliorates cyclophosphamide-induced immunosuppression in mice. Korean J. Pharmacogn. 2019, 50, 219–225. [Google Scholar]
- Pal, P.P.; Begum, A.S.; Basha, S.A.; Araya, H.; Fujimoto, Y. New natural pro-inflammatory cytokines (TNF-α, IL-6 and IL-1β) and iNOS inhibitors identified from Penicillium polonicum through in vitro and in vivo studies. Int. Immunopharmacol. 2023, 117, 109940. [Google Scholar]
- Seo, H.J.; Jeong, J.B. Immune-enhancing effects of green lettuce (Lactuca sativa L.) extracts through the TLR4-MAPK/NF-κB signaling pathways in RAW264.7 macrophage cells. Korean J. Plant Res. 2020, 33, 183–193. [Google Scholar]
- Kim, S.J.; Shin, M.S.; Kim, M.; Baek, S.H.; Kang, K.S. Characterization of an immune-enhancing polysaccharide fraction isolated from heat-processed ginseng derived from Panax ginseng C.A. Meyer. Appl. Sci. 2021, 11, 10835. [Google Scholar] [CrossRef]
- Zhao, R.; Jiang, X.X.; Zhao, Q.L.; Ye, H.W.; Lin, Y.; Huang, J.; Tang, Y.P. Immunoenhancing effects of Cyclina sinensis pentadecapeptide through modulation of signaling pathways in mice with cyclophosphamide-induced immunosuppression. Mar. Drugs 2022, 20, 560. [Google Scholar] [CrossRef] [PubMed]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPS): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar]
- Masood, S.; Rehman, A.; Bashir, S.; Shazly, M.E.; Imran, M.; Khalil, P.; Ifthikar, F.; Jaffar, H.M.; Khursheed, T. Investigation of the anti-hyperglycemic and antioxidant effects of wheat bread supplemented with onion peel extract and onion powder in diabetic rats. J. Diabetes Metab. Disord. 2021, 20, 485–495. [Google Scholar] [CrossRef]
- Kim, J.S.; Kim, H.J.; Lee, E.B.; Choi, J.H.; Jung, J.; Jang, H.H.; Park, S.Y.; Ha, K.C.; Park, Y.K.; Joo, J.C.; et al. Supplementary effects of Allium hookeri extract on glucose tolerance in prediabetic subjects and C57BL/KsJ-db/db mice. Pharmaceuticals 2023, 16, 1364. [Google Scholar] [CrossRef]
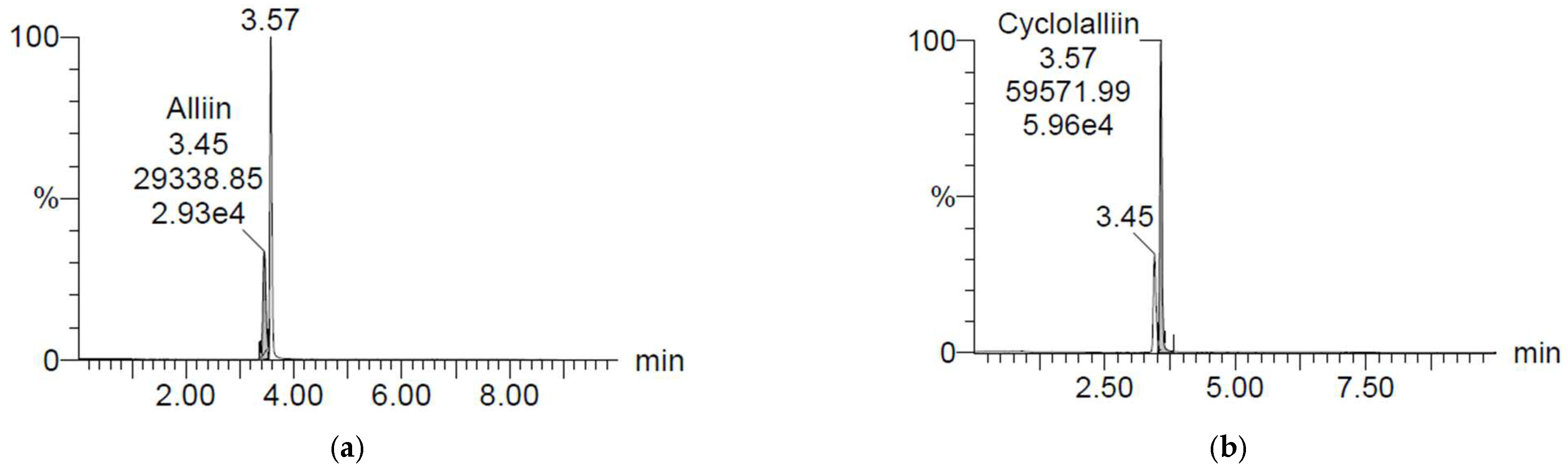




| Sample | Alliin | Cycloallin | ||
|---|---|---|---|---|
| Peak Area | Concentration (μg/g) | Peak Area | Concentration (μg/g) | |
| OF 1 | 3,817,751 | 1669.66 | 697,544 | 442.20 |
| FL | 458,234 | 200.19 | 227,163 | 143.77 |
| LED-R | 269,700 | 117.72 | 159,380 | 100.77 |
| LED-B | 162,735 | 70.93 | 103,263 | 65.17 |
| LED-R+B | 260,231 | 113.58 | 158,170 | 100.00 |
| NOR 1 | Immunosuppressed Obese Mice 2 | |||||||
|---|---|---|---|---|---|---|---|---|
| NC | PC | OF | FL | LED-R | LED-B | LED-R+B | ||
| Initial body weight (g) | 21.27 ± 0.28 ns | 21.37 ± 0.12 | 21.17 ± 0.12 | 21.40 ± 0.35 | 21.07 ± 0.15 | 21.00 ± 0.31 | 21.00 ± 0.10 | 21.47 ± 0.03 |
| Final body weight (g) | 25.43 ± 0.57 ab | 23.90 ± 0.22 c | 26.33 ± 0.45 a | 26.28 ± 0.21 a | 25.30 ±0.41 ab | 25.10 ± 0.35 b | 24.95 ± 0.33 bc | 25.08 ± 0.14 b |
| Tissue weight (% of BW) | ||||||||
| Spleen | 0.26 ± 0.01 ab | 0.23 ± 0.01 b | 0.30 ± 0.01 a | 0.30 ± 0.01 a | 0.30 ± 0.02 a | 0.27 ± 0.00 a | 0.28 ± 0.01 a | 0.28 ± 0.01 a |
| Thymus | 0.22 ± 0.02 a | 0.13 ± 0.01 c | 0.20 ± 0.01 ab | 0.20 ± 0.01 ab | 0.17 ± 0.02 bc | 0.19 ± 0.02 ab | 0.19 ± 0.00 ab | 0.20 ± 0.00 ab |
| Liver | 4.28 ± 0.29 b | 4.72 ± 0.10 a | 4.25 ± 0.14 b | 4.39 ± 0.07 ab | 4.36 ± 0.08 ab | 4.38 ± 0.08 ab | 4.10 ± 0.13 b | 4.19 ± 0.02 b |
| Heart | 0.46 ± 0.01 b | 0.52 ± 0.01 a | 0.46 ± 0.00 b | 0.46 ± 0.01 b | 0.46 ± 0.02 b | 0.47 ± 0.01 b | 0.46 ± 0.01 b | 0.47 ± 0.00 b |
| Epididymal fat | 1.27 ± 0.04 c | 2.35 ± 0.27 a | 1.37 ± 0.05 bc | 1.52 ± 0.07 bc | 1.29 ± 0.07 c | 1.51 ± 0.07 bc | 1.49 ± 0.09 bc | 1.71 ± 0.13 b |
| Pancreas | 1.06 ± 0.04 c | 1.87 ± 0.10 a | 1.71 ± 0.05 ab | 1.73 ± 0.04 ab | 1.77 ± 0.08 ab | 1.69 ± 0.02 b | 1.77 ± 0.04 ab | 1.69 ± 0.03 b |
| NOR 1 | Immunosuppressed Obese Mice 2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NC | PC | OF | FL | LED-R | LED-B | LED-R+B | ||||
| RBC (×106 cells/μL) 3 | 10.76 ± 0.23 a | 9.68 ± 0.05 e | 10.22 ± 0.09 cd | 10.18 ± 0.14 cd | 10.00 ± 0.04 de | 10.62 ± 0.15 ab | 10.46 ± 0.12 abc | 10.33 ± 0.05 bcd | ||
| HGB (g/dL) | 15.60 ± 0.30 a | 14.17 ± 0.13 d | 14.50 ± 0.15 cd | 14.80 ± 0.26 bc | 14.73 ± 0.12 cd | 15.40 ± 0.25 ab | 15.00 ± 0.12 abc | 14.97 ± 0.09 bc | ||
| HCT (%) | 56.93 ± 1.41 a | 46.10 ± 0.17 d | 50.47 ± 0.20 c | 53.40 ± 0.76 bc | 52.37 ± 0.91 bc | 53.93 ± 1.55 b | 52.57 ± 0.90 bc | 51.80 ± 0.66 bc | ||
| RBC Index | MCV (fL) | 54.10 ± 0.46 a | 46.37 ± 0.27 d | 51.73 ± 0.09 c | 53.47 ± 0.41 ab | 53.43 ± 0.23 ab | 52.67 ± 0.35 abc | 52.40 ± 0.96 bc | 51.50 ± 0.31 c | |
| MCH (pg) | 14.30 ± 0.10 b | 14.83 ± 0.13 a | 14.10 ± 0.25 b | 14.33 ± 0.03 b | 14.37 ± 0.03 b | 14.47 ± 0.03 b | 14.20 ± 0.10 b | 14.27 ± 0.09 b | ||
| MCHC (g/dL) | 27.17 ± 0.09 b | 31.53 ± 0.18 a | 27.47 ± 0.39 b | 27.20 ± 0.26 b | 27.53 ± 0.18 b | 27.43 ± 0.24 b | 27.57 ± 0.32 b | 27.60 ± 0.35 b | ||
| PLT (×103 cells/μL) | 1803.33 ± 110.09 a | 1100.00 ± 16.92 c | 1336.67 ± 16.02 b | 1254.33 ± 15.30 b | 1263.67 ± 12.03 b | 1278.33 ± 22.82 b | 1279.33 ± 14.15 b | 1209.00 ± 53.98 bc | ||
| WBC (×103 cells/μL) | 3.74 ± 0.06 a | 1.51 ± 0.17 c | 2.44 ± 0.36 b | 2.66 ± 0.05 b | 2.11 ± 0.03 bc | 2.65 ± 0.29 b | 2.68 ± 0.16 b | 2.58 ± 0.60 b | ||
| WBC Differential Counting (%) | NEU | 3.57 ± 0.73 d | 8.97 ± 0.90 a | 7.80 ± 0.53 ab | 4.67 ± 0.13 cd | 6.23 ± 0.60 bc | 6.00 ± 0.67 bc | 6.20 ± 0.45 bc | 5.00 ± 0.25 cd | |
| LYM | 91.77 ± 1.23 a | 72.13 ± 2.20 b | 86.90 ± 1.22 a | 89.70 ± 1.55 a | 89.40 ± 1.29 a | 90.37 ± 0.67 a | 86.80 ± 2.71 a | 89.73 ± 0.50 a | ||
| MONO | 3.10 ± 0.56 ab | 2.40 ± 0.15 b | 3.33 ± 0.19 ab | 3.40 ± 0.46 ab | 3.97 ± 0.37 a | 3.93 ± 0.09 a | 3.83 ± 0.30 a | 3.23 ± 0.49 ab | ||
| EOS | 0.70 ± 0.10 b | 1.87 ± 0.58 a | 0.90 ± 0.17 b | 0.60 ± 0.06 b | 0.70 ± 0.12 b | 0.67 ± 0.12 b | 0.97 ± 0.09 b | 1.23 ± 0.13 ab | ||
| BASO | 0.00 ± 0.00 b | 0.20 ± 0.10 a | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b | ||
| NOR 1 | Immunosuppressed Obese Mice 2 | |||||||
|---|---|---|---|---|---|---|---|---|
| NC | PC | OF | FL | LED-R | LED-B | LED-R+B | ||
| ALT (U/mL) 3 | 4.80 ± 1.29 b | 21.33 ± 6.86 a | 6.40 ± 2.81 b | 3.33 ± 0.48 b | 5.73 ± 1.96 b | 4.00 ± 0.23 b | 5.40 ± 2.39 b | 2.13 ± 0.13 b |
| AST (U/mL) | 36.00 ± 1.89 b | 95.73 ± 33.39 a | 39.33 ± 1.48 b | 39.87 ± 2.36 b | 41.47 ± 2.43 b | 41.87 ± 3.58 b | 48.73 ± 3.28 b | 40.93 ± 1.39 b |
| T-Chol (mg/dL) | 106.67 ± 3.53 b | 148.00 ± 6.93 a | 148.00 ± 0.00 a | 134.67 ± 8.11 ab | 120.00 ± 28.38 ab | 128.00 ± 2.31 ab | 136.67 ±11.33 ab | 144.00 ± 0.00 ab |
| TG (mg/dL) | 37.33 ± 3.53 a | 37.33 ± 6.67 a | 18.67 ± 3.53 b | 20.00 ± 2.31 b | 14.67 ± 3.53 b | 20.00 ± 6.93 b | 17.33 ± 3.53 b | 18.67 ± 4.81 b |
| HDL (mg/dL) | 73.07 ± 2.15 ab | 59.20 ± 19.69 b | 88.27 ± 5.47 a | 85.73 ± 3.10 a | 85.60 ± 5.06 a | 80.93 ± 3.58 ab | 88.40 ± 1.29 a | 83.60 ± 0.83 ab |
| LDL (mg/dL) | 5.47 ± 0.13 b | 14.53 ±1.87 a | 13.73 ± 0.67 a | 10.40 ± 1.80 a | 9.87 ± 2.92 a | 11.33 ± 0.48 a | 13.80 ± 0.76 a | 14.40 ± 0.40 a |
| GLU (mg/dL) | 317.33 ± 9.61 ab | 384.00 ± 50.12 a | 306.67 ± 11.62 ab | 309.33 ± 30.05 ab | 273.33 ± 55.44 b | 270.67 ± 24.69 b | 250.00 ± 11.02 b | 270.67 ± 13.92 b |
| HbA1c (%) | 4.23 ± 0.06 ns | 4.14 ± 0.02 | 4.14 ± 0.02 | 4.15 ± 0.05 | 4.10 ± 0.00 | 4.13 ± 0.03 | 4.18 ± 0.02 | 4.13 ± 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, J.; Kim, J.-S.; Jeong, U.-Y.; Bae, U.-J.; Kim, M.; Park, S.-Y.; Hwang, I.-G.; Heo, J.-W.; Shim, C.-K.; Ham, J.-S.; et al. The Immune-Stimulating and Anti-Diabetic Effects of Allium hookeri Leaves Grown in a Plant Factory with Artificial Lights in Immunosuppressed Obese C57BL/6 Mice. Pharmaceuticals 2024, 17, 91. https://doi.org/10.3390/ph17010091
Jung J, Kim J-S, Jeong U-Y, Bae U-J, Kim M, Park S-Y, Hwang I-G, Heo J-W, Shim C-K, Ham J-S, et al. The Immune-Stimulating and Anti-Diabetic Effects of Allium hookeri Leaves Grown in a Plant Factory with Artificial Lights in Immunosuppressed Obese C57BL/6 Mice. Pharmaceuticals. 2024; 17(1):91. https://doi.org/10.3390/ph17010091
Chicago/Turabian StyleJung, Jieun, Ji-Su Kim, Un-Yul Jeong, Ui-Jin Bae, Mina Kim, Shin-Young Park, In-Guk Hwang, Jeong-Wook Heo, Chang-Ki Shim, Jun-Sang Ham, and et al. 2024. "The Immune-Stimulating and Anti-Diabetic Effects of Allium hookeri Leaves Grown in a Plant Factory with Artificial Lights in Immunosuppressed Obese C57BL/6 Mice" Pharmaceuticals 17, no. 1: 91. https://doi.org/10.3390/ph17010091
APA StyleJung, J., Kim, J.-S., Jeong, U.-Y., Bae, U.-J., Kim, M., Park, S.-Y., Hwang, I.-G., Heo, J.-W., Shim, C.-K., Ham, J.-S., & Lee, S.-H. (2024). The Immune-Stimulating and Anti-Diabetic Effects of Allium hookeri Leaves Grown in a Plant Factory with Artificial Lights in Immunosuppressed Obese C57BL/6 Mice. Pharmaceuticals, 17(1), 91. https://doi.org/10.3390/ph17010091







