Drug-Induced Anaphylaxis: National Database Analysis
Abstract
1. Introduction
2. Results
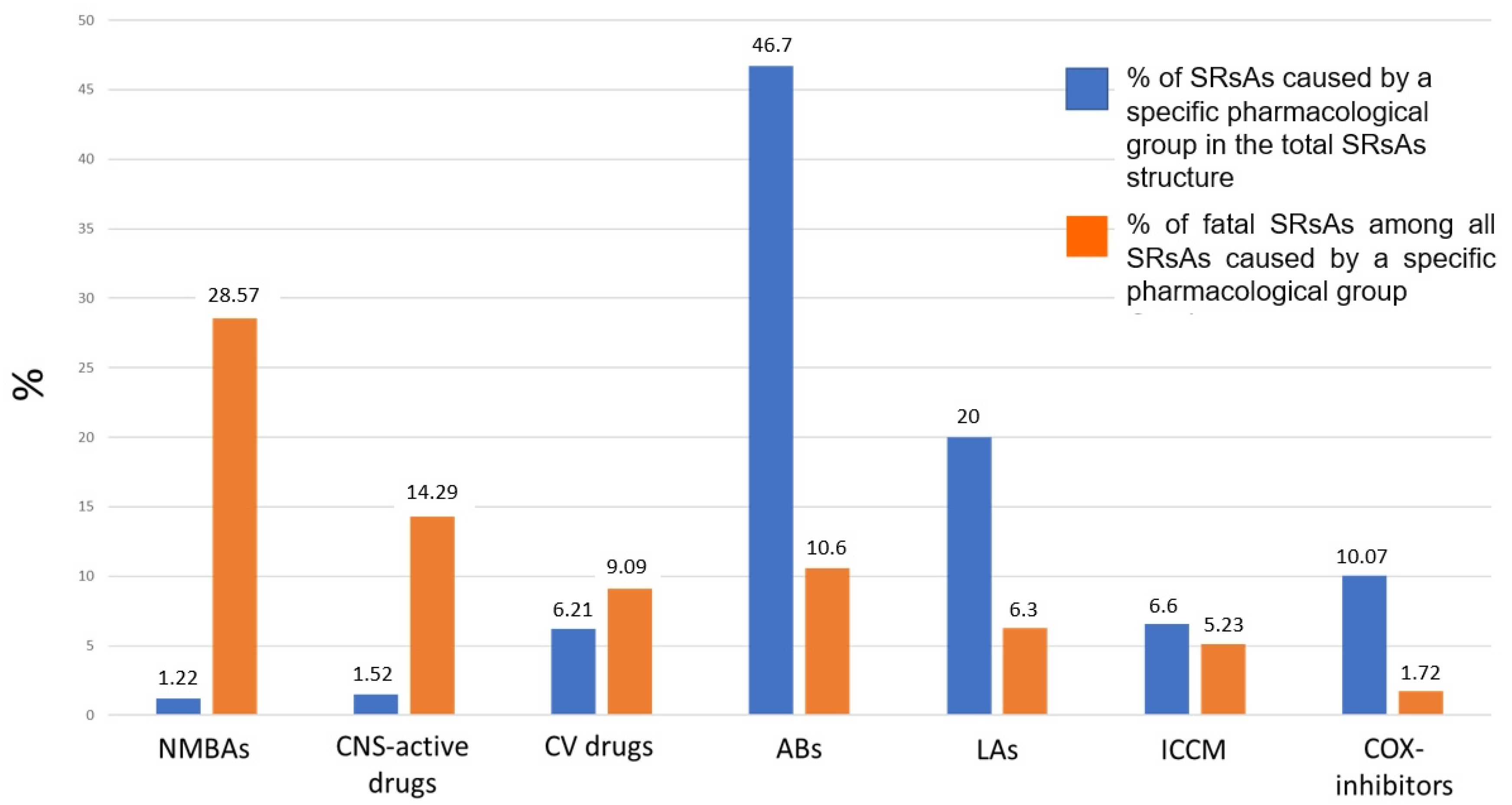
2.1. Analysis of SRsAs
2.1.1. Analysis of ABs Involved in Anaphylaxis
2.1.2. Analysis of LAs Involved in Anaphylaxis
2.1.3. Analysis of COX Inhibitors Involved in Anaphylaxis
2.1.4. Analysis of ICCM Involved in Anaphylaxis
2.1.5. Analysis of CV Drugs Involved in Anaphylaxis
2.1.6. Analysis of CNS-Active Drugs Involved in Anaphylaxis
2.1.7. Analysis of NMBAs Involved in Anaphylaxis
2.2. Analysis of Fatal SRsAs
2.3. Analysis of Pediatric SRsAs
2.4. Analysis of SRsAs in the Elderly
3. Discussion
4. Materials and Methods
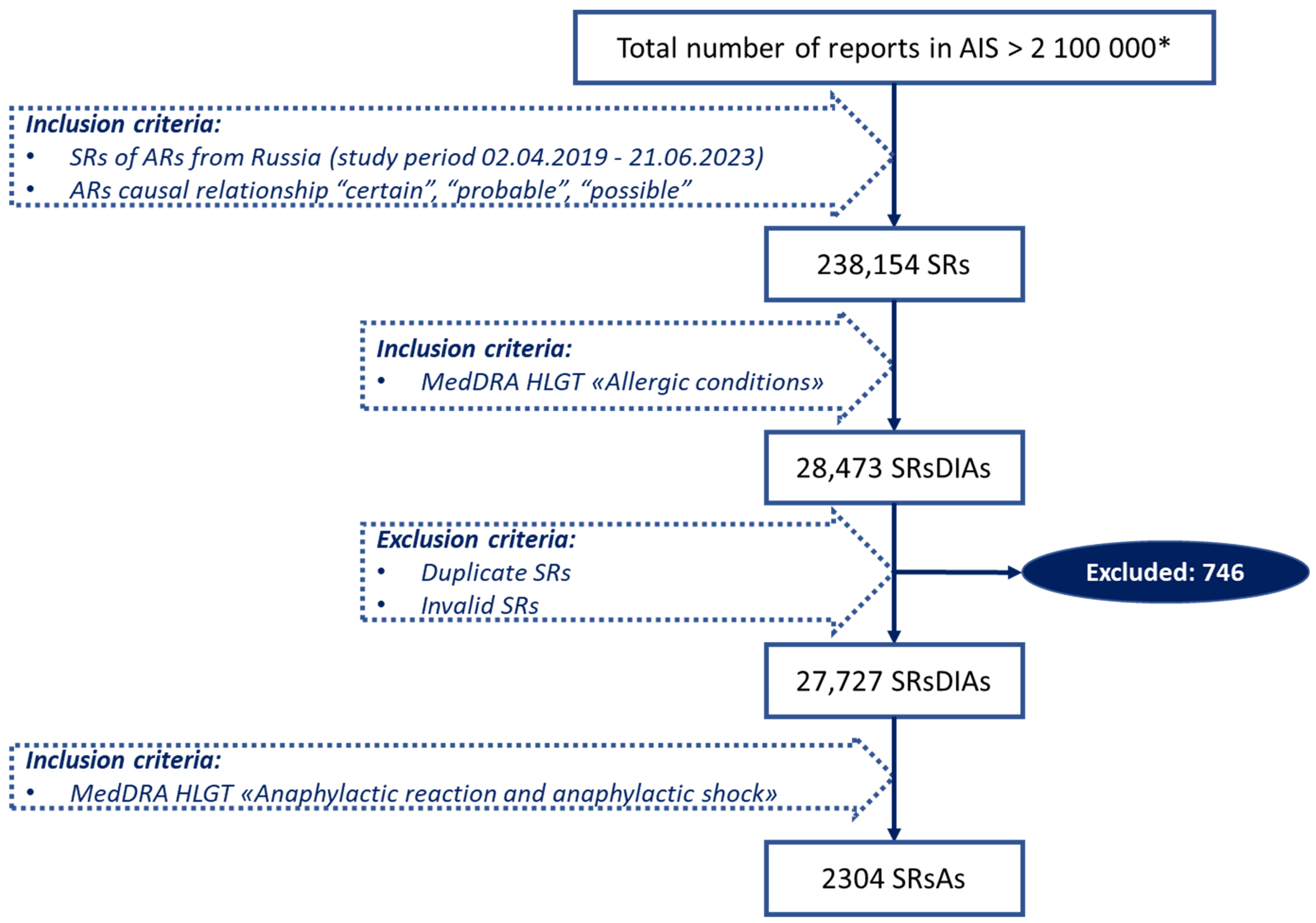
4.1. Data Source
4.2. Definitions
- “Adverse reaction—A response to a medicinal product, which is noxious and unintended. Adverse reaction may arise from use of the product within or outside the terms of the marketing authorization or from occupational exposure. Use outside the marketing authorization includes off-label use, overdose, misuse, abuse and medication errors.”
- “Causality: In accordance with ICH-E2A, the definition of an adverse reaction implies at least a reasonable possibility of a causal relationship between a suspected medicinal product and an adverse event. An adverse reaction, in contrast to an adverse event, is characterized by the fact that a causal relationship between a medicinal product and an occurrence is suspected. For regulatory reporting purposes, as detailed in ICH-E2D, if an event is spontaneously reported, even if the relationship is unknown or unstated, it meets the definition of an adverse reaction. Therefore, all spontaneous reports notified by healthcare professionals or consumers are considered suspected adverse reactions, since they convey the suspicions of the primary sources, unless the reporters specifically state that they believe the events to be unrelated or that a causal relationship can be excluded.”
- “A spontaneous report is an unsolicited communication by a healthcare professional, or consumer to a competent authority, marketing authorisation holder or other organization (e.g., regional pharmacovigilance center, poison control center) that describes one or more suspected adverse reactions in a patient who was given one or more medicinal products. It does not derive from a study or any organized data collection systems.”
4.3. Study Design and Data Selection
4.4. Drug Identification and Analyzed Categories
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cardona, V.; Ansotegui, I.J.; Ebisawa, M.; El-Gamal, Y.; Rivas, M.F.; Fineman, S.; Geller, M.; Gonzalez-Estrada, A.; Greenberger, P.A.; Borges, M.S.; et al. World Allergy Organization Anaphylaxis Guidance 2020. World Allergy Organ. J. 2020, 13, 100472. [Google Scholar] [CrossRef] [PubMed]
- Regateiro, F.S.; Marques, M.L.; Gomes, E.R. Drug-Induced Anaphylaxis: An Update on Epidemiology and Risk Factors. Int. Arch. Allergy Immunol. 2020, 181, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Villarreal-González, R.V.; Canel-Paredes, A.; Arias-Cruz, A.; Fraga-Olvera, A.; Delgado-Bañuelos, A.; Rico-Solís, G.A.; Ochoa-García, I.V.; Jiménez-Sandoval, J.O.; Heredia, J.R.; Flores-González, J.V.; et al. Alergia a medicamentos: Aspectos fundamentales en el diagnóstico y tratamiento. Drug allergy: Fundamental aspects in diagnosis and treatment. Rev. Alerg. Mex. 2023, 69, 195–213. [Google Scholar] [CrossRef]
- Bruhns, P.; Chollet-Martin, S. Mechanisms of human drug-induced anaphylaxis. J. Allergy Clin. Immunol. 2021, 147, 1133–1142. [Google Scholar] [CrossRef] [PubMed]
- Kiechle, E.S.; McKenna, C.M.; Carter, H.; Zeymo, A.; Gelfand, B.W.; DeGeorge, L.M.; Sauter, D.A.; Mazer-Amirshahi, M. Medication Allergy and Adverse Drug Reaction Documentation Discrepancies in an Urban, Academic Emergency Department. J. Med. Toxicol. 2018, 14, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.C. Prevalence of Antibiotic Allergy at a Spinal Cord Injury Center. Fed. Pract. 2023, 40, 142–145. [Google Scholar] [CrossRef]
- Alkanhal, R.; Alhoshan, I.; Aldakhil, S.; Alromaih, N.; Alharthy, N.; Salam, M.; Almutairi, A.F. Prevalence triggers and clinical severity associated with anaphylaxis at a tertiary care facility in Saudi Arabia: A cross-sectional study. Medicine 2018, 97, e11582. [Google Scholar] [CrossRef]
- Coutinho, I.A.; Ferreira, D.; Regateiro, F.; Pita, J.; Ferreira, M.; Martins, J.; Fonseca, I.; Loureiro, C.; Todo-Bom, A. Anaphylaxis in an emergency department: A retrospective 10-year study in a tertiary hospital. Minerva Anestesiol. 2020, 52, 23–34. [Google Scholar] [CrossRef]
- Wong, A.; Seger, D.L.; Lai, K.H.; Goss, F.R.; Blumenthal, K.G.; Zhou, L. Drug Hypersensitivity Reactions Documented in Electronic Health Records within a Large Health System. J. Allergy Clin. Immunol. Pract. 2019, 7, 1253–1260.e3. [Google Scholar] [CrossRef]
- Lerch, M.; Mainetti, C.; Beretta-Piccoli, B.T.; Harr, T. Current Perspectives on Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis. Clin. Rev. Allergy Immunol. 2018, 54, 147–176. [Google Scholar] [CrossRef]
- Dhopeshwarkar, N.; Sheikh, A.; Doan, R.; Topaz, M.; Bates, D.W.; Blumenthal, K.G.; Zhou, L. Drug-Induced Anaphylaxis Documented in Electronic Health Records. J. Allergy Clin. Immunol. Pract. 2019, 7, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, Z.; Yu, Y.; Feng, M.; Liu, A. Active surveillance and clinical analysis of anaphylaxis based on the China Hospital Pharmacovigilance System. Front. Pharmacol. 2023, 14, 1180685. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-C.; Hu, S.; Zhang, S.-Z.; Huang, J.-W.; Zhang, J.; Ji, C.; Cheng, B. The Epidemiology of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in China. J. Immunol. Res. 2018, 2018, 4320195. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.Y.; Knox, C.; Phillips, E.J. Worldwide Prevalence of Antibiotic-Associated Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis: A Systematic Review and Meta-analysis. JAMA Dermatol. 2023, 159, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Esh, C.J.; Chrismas, B.C.R.; Mauger, A.R.; Taylor, L. Pharmacological hypotheses: Is acetaminophen selective in its cyclooxygenase inhibition? Pharmacol. Res. Perspect. 2021, 9, e00835. [Google Scholar] [CrossRef] [PubMed]
- Van Diepen, A.T.N.; Simons, P.; Bos, J.M.; Kramers, C. Pijnbestrijding met metamizol in de Nederlandse praktijk [Metamizol: Current status in Dutch practice]. Ned. Tijdschr. Geneeskd. 2022, 166, D6182. [Google Scholar]
- Pagani, S.; Lombardi, N.; Crescioli, G.; Vighi, V.G.; Spada, G.; Andreetta, P.; Capuano, A.; Vannacci, A.; Venegoni, M.; Vighi, G.D.; et al. Drug-Related Hypersensitivity Reactions Leading to Emergency Department: Original Data and Systematic Review. J. Clin. Med. 2022, 11, 2811. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, S.; Li, X.; Ma, X.; Tang, H.; Sun, L.; Zhai, S.; Wang, T. Drug-induced anaphylaxis in China: A 10 year retrospective analysis of the Beijing Pharmacovigilance Database. Int. J. Clin. Pharm. 2018, 40, 1349–1358. [Google Scholar] [CrossRef]
- Poziomkowska-Gęsicka, I.; Kurek, M. Clinical Manifestations and Causes of Anaphylaxis. Analysis of 382 Cases from the Anaphylaxis Registry in West Pomerania Province in Poland. Int. J. Environ. Res. Public Health 2020, 17, 2787. [Google Scholar] [CrossRef]
- Ben Mansour, A.; Daghfous, H.; Ben Saad, S.; Slim, A.; Bellali, H.; Tritar, F. Drug related severe anaphylaxis investigation: A Tunisian retrospective study. Rev. Française d’Allergologie 2023, 63, 103309. [Google Scholar] [CrossRef]
- Nguyen, K.-D.; Nguyen, H.-A.; Vu, D.-H.; Le, T.T.-L.; Dang, B.-V.; Nguyen, T.-N.; Nguyen, D.-H.; Nguyen, T.-B.; Montastruc, J.-L.; Bagheri, H. Drug-Induced Anaphylaxis in a Vietnamese Pharmacovigilance Database: Trends and Specific Signals from a Disproportionality Analysis. Drug Saf. 2019, 42, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Ahn, K.-M.; Kim, B.-K.; Yang, M.-S. Risk factors of anaphylaxis in Korea: Identifying drug-induced anaphylaxis culprits using big data. Medicine 2022, 101, e30224. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.J.; Krantz, M.S.; Phillips, E.J.; Stone, C.A. Emerging Causes of Drug-Induced Anaphylaxis: A Review of Anaphylaxis-Associated Reports in the FDA Adverse Event Reporting System (FAERS). J. Allergy Clin. Immunol. Pract. 2021, 9, 819–829.e2. [Google Scholar] [CrossRef] [PubMed]
- Dogan, F.S.; Ozayd, V. Drug-Induced Anaphylaxis in the Emergency Department-A Prospective Observational Study. North. Clin. Istanb. 2021, 8, 595–600. [Google Scholar] [CrossRef]
- Demir, S.; Erdenen, F.; Gelincik, A.; Unal, D.; Olgac, M.; Coskun, R.; Colakoglu, B.; Buyukozturk, S. Evaluation of the Potential Risk Factors for Drug-Induced Anaphylaxis in Adult Patients. Int. Arch. Allergy Immunol. 2019, 178, 167–176. [Google Scholar] [CrossRef]
- Khan, D.A.; Banerji, A.; Bernstein, J.A.; Bilgicer, B.; Blumenthal, K.; Castells, M.; Ein, D.; Lang, D.M.; Phillips, E. Cephalosporin Allergy: Current Understanding and Future Challenges. J. Allergy Clin. Immunol. Pract. 2019, 7, 2105–2114. [Google Scholar] [CrossRef]
- D’errico, S.; Frati, P.; Zanon, M.; Valentinuz, E.; Manetti, F.; Scopetti, M.; Santurro, A.; Fineschi, V. Cephalosporins’ Cross-Reactivity and the High Degree of Required Knowledge. Case Report and Review of the Literature. Antibiotics 2020, 9, 209. [Google Scholar] [CrossRef] [PubMed]
- Romano, A.; Valluzzi, R.L.; Caruso, C.; Zaffiro, A.; Quaratino, D.; Gaeta, F. Evaluating Immediate Reactions to Cephalosporins: Time Is of the Essence. J. Allergy Clin. Immunol. Pract. 2021, 9, 1648–1657.e1. [Google Scholar] [CrossRef]
- Mori, F.; Liccioli, G.; Piccorossi, A.; Sarti, L.; Barni, S.; Giovannini, M.; Azzari, C.; Manfredi, M.; Novembre, E. The Diagnosis of Ceftriaxone Hypersensitivity in a Paediatric Population. Int. Arch. Allergy Immunol. 2019, 178, 272–276. [Google Scholar] [CrossRef]
- Jung, I.Y.; Kim, J.J.; Lee, S.J.; Kim, J.; Seong, H.; Jeong, W.; Choi, H.; Jeong, S.J.; Ku, N.S.; Han, S.H.; et al. Antibiotic-Related Adverse Drug Reactions at a Tertiary Care Hospital in South Korea. BioMed Res. Int. 2017, 2017, 4304973. [Google Scholar] [CrossRef]
- Rhyou, H.-I.; Nam, Y.-H.; Kim, S.-C.; Doo, G.-E.; Ha, C.-Y.; Nam, H.-J.; Woo, S.-D.; Lee, Y.; Jang, J.-H.; Lee, H.-Y.; et al. Cefaclor-induced hypersensitivity: Differences in the incidence of anaphylaxis relative to other 2nd and 3rd generation cephalosporins. PLoS ONE 2021, 16, e0254898. [Google Scholar] [CrossRef]
- Jevon, P.; Shamsi, S. Management of anaphylaxis in the dental practice: An update. Br. Dent. J. 2020, 229, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Zuo, J.; Gong, R.; Liu, X.; Zhao, J. Risk of True Allergy to Local Anesthetics: 10-Year Experience from an Anesthesia Allergy Clinic in China. Ther. Clin. Risk Manag. 2020, 16, 1297–1303. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Gong, R.; Xin, X.; Zhao, J. Clinical characteristics and allergen detection of perioperative anaphylaxis: A 12-year retrospective analysis from an anesthesia clinic in China. Perioper. Med. 2022, 11, 5. [Google Scholar] [CrossRef] [PubMed]
- Park, K.K.; Sharon, V.R. A Review of Local Anesthetics: Minimizing Risk and Side Effects in Cutaneous Surgery. Dermatol. Surg. 2017, 43, 173–187. [Google Scholar] [CrossRef]
- Bhole, M.V.; Manson, A.L.; Seneviratne, S.L.; Misbah, S.A. IgE-mediated allergy to local anaesthetics: Separating fact from perception: A UK perspective. Br. J. Anaesth. 2012, 108, 903–911. [Google Scholar] [CrossRef] [PubMed]
- Cherobin, A.C.F.P.; Tavares, G.T. Safety of local anesthetics. An. Bras. Dermatol. 2020, 95, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Hotta, K.; Inoue, S.; Takazawa, T.; Horiuchi, T.; Igarashi, T.; Takeuchi, M. Mepivacaine-induced anaphylactic shock in a pregnant woman undergoing combined spinal and epidural anesthesia for cesarean delivery: A case report. JA Clin. Rep. 2019, 5, 84. [Google Scholar] [CrossRef]
- Simionescu, A.A.; Danciu, B.M.; Stanescu, A.M.A. Severe Anaphylaxis in Pregnancy: A Systematic Review of Clinical Presentation to Determine Outcomes. J. Pers. Med. 2021, 11, 1060. [Google Scholar] [CrossRef]
- Hascoët, E.; Mahé, J.; Meillard, H.; Théophile, H.; Cloitre, A.; Lesclous, P. Anaphylactic reactions to local anesthetics in dental practice: A nationwide French retrospective study. Clin. Oral Investig. 2022, 26, 1667–1676. [Google Scholar] [CrossRef]
- Matveev, A.V.; Krasheninnikov, A.E.; Yagudina, R.I.; Egorova, E.A.; Konyaeva, E.I. Nezhelatel’nye reaktsii na mestnye anestetiki pri ikh primenenii v stomatologii. [Adverse drug reactions of local anesthetics used in dentistry]. Stomatologiya 2020, 99, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Blanca-López, N.; Soriano, V.; Martin, E.G.; Canto, G.; Blanca, M. NSAID-induced reactions: Classification, prevalence, impact, and management strategies. J. Asthma Allergy 2019, 12, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Cano, R.; Pascal, M.; Araujo, G.; Goikoetxea, M.J.; Valero, A.L.; Picado, C.; Bartra, J. Mechanisms, Cofactors, and Augmenting Factors Involved in Anaphylaxis. Front. Immunol. 2017, 8, 1193. [Google Scholar] [CrossRef] [PubMed]
- Cimen, S.S.; Suleyman, A.; Yucel, E.; Guler, N.; Tamay, Z.U. Evaluation of the triggers and the treatment models of anaphylaxis in pediatric patients. North. Clin. Istanb. 2023, 10, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Popiołek, I.; Piotrowicz-Wójcik, K.; Porebski, G. Hypersensitivity Reactions in Serious Adverse Events Reported for Paracetamol in the EudraVigilance Database, 2007–2018. Pharmacy 2019, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- Gabrielli, S.; Langlois, A.; Ben-Shoshan, M. Prevalence of Hypersensitivity Reactions in Children Associated with Acetaminophen: A Systematic Review and Meta-Analysis. Int. Arch. Allergy Immunol. 2018, 176, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Sachs, B.; Dubrall, D.; Fischer-Barth, W.; Schmid, M.; Stingl, J. Drug-induced anaphylactic reactions in children: A retrospective analysis of 159 validated spontaneous reports. Pharmacoepidemiol. Drug Saf. 2019, 28, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Cui, Q.; Gong, X.; Zhou, H. Anaphylaxis Following Contrast-Enhanced CT with Iodixanol: A Case Report and Literature Review. J. Asthma Allergy 2023, 16, 195–200. [Google Scholar] [CrossRef]
- Fukushima, Y.; Taketomi-Takahashi, A.; Suto, T.; Hirasawa, H.; Tsushima, Y. Clinical features and risk factors of iodinated contrast media (ICM)-induced anaphylaxis. Eur. J. Radiol. 2023, 164, 110880. [Google Scholar] [CrossRef]
- Sugizaki, C.; Sato, S.; Yanagida, N.; Ebisawa, M. Analysis of drug-induced anaphylaxis cases using the Japanese Adverse Drug Event Report (JADER) database—Secondary publication. Allergol. Int. 2023, 72, 580–587. [Google Scholar] [CrossRef]
- Kim, M.-H.; Lee, S.-Y.; Lee, S.-E.; Yang, M.-S.; Jung, J.-W.; Park, C.M.; Lee, W.; Cho, S.-H.; Kang, H.-R. Anaphylaxis to Iodinated Contrast Media: Clinical Characteristics Related with Development of Anaphylactic Shock. PLoS ONE 2014, 9, e100154. [Google Scholar] [CrossRef] [PubMed]
- Chiu, T.-M.; Chu, S.-Y. Hypersensitivity Reactions to Iodinated Contrast Media. Biomedicines 2022, 10, 1036. [Google Scholar] [CrossRef] [PubMed]
- Coop, C.A.; Schapira, R.S.; Freeman, T.M. Are ACE Inhibitors and Beta-blockers Dangerous in Patients at Risk for Anaphylaxis? J. Allergy Clin. Immunol. Pract. 2017, 5, 1207–1211. [Google Scholar] [CrossRef] [PubMed]
- Tejedor-Alonso, M.A.; Farias-Aquino, E.; Pérez-Fernández, E.; Grifol-Clar, E.; Moro-Moro, M.; Rosado-Ingelmo, A. Relationship Between Anaphylaxis and Use of Beta-Blockers and Angiotensin-Converting Enzyme Inhibitors: A Systematic Review and Meta-Analysis of Observational Studies. J. Allergy Clin. Immunol. Pr. 2018, 7, 879–897.e5. [Google Scholar] [CrossRef] [PubMed]
- Yeğit, O.O.; Aslan, A.F.; Coşkun, R.; Karadağ, P.; Toprak, I.D.; Can, A.; Öztop, N.; Demir, S.; Ünal, D.; Olgaç, M.; et al. Comparison of recent anaphylaxis diagnostic criteria in real life: Can more patients be diagnosed as having anaphylaxis? World Allergy Organ. J. 2023, 16, 100810. [Google Scholar] [CrossRef]
- Teshigawara, A.; Nishibe, S.; Horie, S.; Hakone, M.; Yamaji, Y.; Akasawa, A.; Yoshida, K.; Morikawa, E. Fentanyl-associated anaphylaxis in an infant with tetralogy of Fallot: A case report. JA Clin. Rep. 2019, 5, 34. [Google Scholar] [CrossRef] [PubMed]
- Laguna, J.; Archilla, J.; Doña, I.; Corominas, M.; Gastaminza, G.; Mayorga, C.; Berjes-Gimeno, P.; Tornero, P.; Martin, S.; Planas, A.; et al. Practical Guidelines for Perioperative Hypersensitivity Reactions. J. Investig. Allergol. Clin. Immunol. 2018, 28, 216–232. [Google Scholar] [CrossRef]
- Tomar, G.S.; Tiwari, A.K.; Chawla, S.; Mukherjee, A.; Ganguly, S. Anaphylaxis related to fentanyl citrate. J. Emergencies Trauma Shock. 2012, 5, 257–261. [Google Scholar] [CrossRef]
- Joo, J.; Bae, H.; Lee, J. Intraoperative allergic reaction to fentanyl—A case report. Korean J. Anesthesiol. 2009, 57, 776–779. [Google Scholar] [CrossRef]
- Belső, N.; Kui, R.; Szegesdi, I.; Kakuja, M.; Kapitány, K.; Kemény, L.; Bata-Csörgő, Z. Propofol and fentanyl induced perioperative anaphylaxis. Br. J. Anaesth. 2011, 106, 283–284. [Google Scholar] [CrossRef][Green Version]
- Haybarger, E.; Young, A.S.; Giovannitti, J.A., Jr. Benzodiazepine Allergy with Anesthesia Administration: A Review of Current Literature. Anesthesia Prog. 2016, 63, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Mali, S. Anaphylaxis during the perioperative period. Anesth. Essays Res. 2012, 6, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Mori, F.; Barni, S.; Manfredi, M.; Sarti, L.; Pecorari, L.; Pucci, N.; Novembre, E. Anaphylaxis to Intravenous Tramadol in a Child. Pharmacology 2015, 96, 256–258. [Google Scholar] [CrossRef] [PubMed]
- Brenning, G.; Hallberg, P. Angioedema induced by tramadol? a potentially life-threatening condition. Eur. J. Clin. Pharmacol. 2005, 60, 901–903. [Google Scholar] [CrossRef]
- Arslan, K. Tramadol-Induced Anaphylaxis: A Rare Case. Turk. Klin. J. Case Rep. 2022, 30, 238–241. [Google Scholar] [CrossRef]
- Baldo, B.A. Allergic and other adverse reactions to drugs used in anesthesia and surgery. Anesthesiol. Perioper. Sci. 2023, 1, 1–28. [Google Scholar] [CrossRef]
- Dejoux, A.; de Chaisemartin, L.; Bruhns, P.; Longrois, D.; Gouel-Chéron, A. Neuromuscular blocking agent induced hypersensitivity reaction exploration: An update. Eur. J. Anaesthesiol. 2023, 40, 95–104. [Google Scholar] [CrossRef]
- Di Leo, E.; Donne, P.D.; Calogiuri, G.F.; Macchia, L.; Nettis, E. Focus on the agents most frequently responsible for perioperative anaphylaxis. Clin. Mol. Allergy 2018, 16, 16. [Google Scholar] [CrossRef]
- Misbah, S.A.; Krishna, M.T. Peri-Operative Anaphylaxis—An Investigational Challenge. Front. Immunol. 2019, 10, 1117. [Google Scholar] [CrossRef]
- Zou, Y.; Shao, L.-J.; Xue, F.-S. Perioperative anaphylaxis: A potential hazard to the safety of surgical patients. Chin. Med. J. 2020, 133, 609–612. [Google Scholar] [CrossRef]
- Petitpain, N.; Argoullon, L.; Masmoudi, K.; Fedrizzi, S.; Cottin, J.; Latarche, C.; Mertes, P.M.; Gillet, P. Neuromuscular blocking agents induced anaphylaxis: Results and trends of a French pharmacovigilance survey from 2000 to 2012. Allergy 2018, 73, 2224–2233. [Google Scholar] [CrossRef] [PubMed]
- Turner, P.J.; Jerschow, E.; Umasunthar, T.; Lin, R.; Campbell, D.E.; Boyle, R.J. Fatal Anaphylaxis: Mortality Rate and Risk Factors. J. Allergy Clin. Immunol. Pract. 2017, 5, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Zhang, H.; Sun, S.; Ma, X.; Pleasants, R.A.; Tang, H.; Zheng, H.; Zhai, S.; Wang, T. Clinical features and treatment of pediatric patients with drug-induced anaphylaxis: A study based on pharmacovigilance data. Eur. J. Pediatr. 2017, 177, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Cavkaytar, O.; Karaatmaca, B.; Cetinkaya, P.G.; Esenboga, S.; Yilmaz, E.A.; Sahiner, U.M.; Sekerel, B.E.; Soyer, O. Characteristics of drug-induced anaphylaxis in children and adolescents. Allergy Asthma Proc. 2017, 38, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Bilò, M.B.; Corsi, A.; Martini, M.; Penza, E.; Grippo, F.; Bignardi, D. Fatal anaphylaxis in Italy: Analysis of cause-of-death national data, 2004-2016. Allergy 2020, 75, 2644–2652. [Google Scholar] [CrossRef]
- Perez-Codesido, S.; Rosado-Ingelmo, A.; Privitera-Torres, M.; Fernández, E.P.; Nieto-Nieto, A.; Gonzalez-Moreno, A.; Grifol-Clar, E.; Alberti-Masgrau, N.; Tejedor-Alonso, M. Incidence of Fatal Anaphylaxis: A Systematic Review of Observational Studies. J. Investig. Allergol. Clin. Immunol. 2022, 32, 245–260. [Google Scholar] [CrossRef]
- Jerschow, E.; Lin, R.Y.; Scaperotti, M.M.; McGinn, A.P. Fatal anaphylaxis in the United States, 1999-2010: Temporal patterns and demographic associations. J. Allergy Clin. Immunol. 2014, 134, 1318–1328.e7. [Google Scholar] [CrossRef]
- Tanno, L.K.; Molinari, N.; Annesi-Maesano, I.; Demoly, P.; Bierrenbach, A.L. Anaphylaxis in Brazil between 2011 and 2019. Clin. Exp. Allergy 2022, 52, 1071–1078. [Google Scholar] [CrossRef]
- Tejedor-Alonso, M.A.; Martínez-Fernandez, P.; Vallejo-De-Torres, G.; Navarro-Escayola, E.; Moro-Moro, M.; Alberti-Masgrau, N. Clinical and demographic characteristics of fatal anaphylaxis in Spain (1998–2011): A comparison between a series from the hospital system and a national forensic series. Clin. Exp. Allergy 2018, 49, 82–91. [Google Scholar] [CrossRef]
- Jares, E.J.; Cardona, V.; Gómez, R.M.; Bernstein, J.A.; Filho, N.A.R.; Cherrez-Ojeda, I.; Ensina, L.F.; De Falco, A.; Díaz, M.C.; Vereau, P.A.C.; et al. Latin American anaphylaxis registry. World Allergy Organ. J. 2023, 16, 100748. [Google Scholar] [CrossRef]
- Pauwels, I.; Versporten, A.; Drapier, N.; Vlieghe, E.; Goossens, H.; Koraqi, A.; Hoxha, I.; Tafaj, S.; Cornistein, W.; Quiros, R.; et al. Hospital antibiotic prescribing patterns in adult patients according to the WHO Access, Watch and Reserve classification (AWaRe): Results from a worldwide point prevalence survey in 69 countries. J. Antimicrob. Chemother. 2021, 76, 1614–1624. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. ICH E2B (R3) Electronic Transmission of Individual Case Safety Reports (ICSRs)—Data Elements and Message Specification—Implementation Guide—Scientific Guideline. Available online: https://www.ema.europa.eu/en/ich-e2b-r3-electronic-transmission-individual-case-safety-reports-icsrs-data-elements-message (accessed on 18 November 2023).
- MedDRA. Available online: https://www.meddra.org/how-to-use/support-documentation/english (accessed on 18 November 2023).
- European Medicines Agency. Guideline on Good Pharmacovigilance Practices (GVP) Module VI—Collection, Management and Submission of Reports of Suspected Adverse Reactions to Medicinal Products (Rev 2). Available online: https://www.ema.europa.eu/en/documents/regulatory-procedural-guideline/guideline-good-pharmacovigilance-practices-gvp-module-vi-collection-management-submission-reports_en.pdf (accessed on 18 November 2023).
- Eurasian Economic Union. “Good Pharmacovigilance Practice” Guideline (Edition No. 2 Dated 05/19/2022). Available online: https://docs.eaeunion.org/docs/ru-ru/01433831/err_09062022_81 (accessed on 18 November 2023).
- Bassir, F.; Varghese, S.; Wang, L.; Chin, Y.P.; Zhou, L. The Use of Electronic Health Records to Study Drug-Induced Hypersensitivity Reactions from 2000 to 2021: A Systematic Review. Immunol. Allergy Clin. N. Am. 2022, 42, 453–497. [Google Scholar] [CrossRef] [PubMed]
| ABs | n (Total—1028) | % |
|---|---|---|
| Beta-lactams | 902 | 87.7 |
| Ceftriaxone | 687 | 66.8 |
| Cefotaxime | 87 | 8.5 |
| Cefazolin | 39 | 3.8 |
| Ampicillin sulbactam | 17 | 1.7 |
| Cefepime | 15 | 1.5 |
| Cefuroxime | 10 | 1.0 |
| Meropenem | 10 | 1.0 |
| Amoxicillin clavulanate | 8 | 0.8 |
| Cefoperazone sulbactam | 7 | 0.7 |
| Ertapenem | 5 | 0.5 |
| Cefoperazone | 4 | 0.4 |
| Ampicillin | 4 | 0.4 |
| Cefepime sulbactam | 3 | 0.3 |
| Amoxicillin sulbactam | 2 | 0.2 |
| Piperacillin tazobactam | 2 | 0.2 |
| Cephalexin | 1 | 0.1 |
| Cefixime | 1 | 0.1 |
| Other | 126 | 12.3 |
| Vancomycin | 28 | 2.7 |
| Ciprofloxacin | 22 | 2.1 |
| Levofloxacin | 18 | 1.8 |
| Metronidazole | 14 | 1.4 |
| Linezolid | 7 | 0.7 |
| Amikacin | 7 | 0.7 |
| Nitrofurantoin | 7 | 0.7 |
| Fosfomycin | 5 | 0.5 |
| Sulfamethoxazole trimethoprim | 3 | 0.3 |
| Tigecycline | 3 | 0.3 |
| Polymyxin B | 2 | 0.2 |
| Kanamycin | 2 | 0.2 |
| Amphotericin B | 2 | 0.2 |
| Gentamicin | 1 | 0.1 |
| Ofloxacin | 1 | 0.1 |
| Erythromycin | 1 | 0.1 |
| Clindamycin | 1 | 0.1 |
| Rifampicin | 1 | 0.1 |
| Isoniazid | 1 | 0.1 |
| LAs | n (Total—460) | % |
|---|---|---|
| Lidocaine | 293 | 63.7 |
| Procaine | 68 | 14.8 |
| Articaine | 61 | 13.3 |
| Ropivacaine | 16 | 3.5 |
| Bupivacaine | 14 | 3.0 |
| Mepivacaine | 8 | 1.7 |
| COX-Inhibitors | n (Total—232) | % |
|---|---|---|
| Acetaminophen | 49 | 21.1 |
| Metamizole | 48 | 20.7 |
| Ibuprofen | 35 | 15.1 |
| Diclofenac | 29 | 12.5 |
| Ketorolac | 26 | 11.2 |
| Acetylsalicylic acid * | 25 | 10.8 |
| Ketoprofen | 6 | 3.0 |
| Celecoxib | 5 | 2.2 |
| Aceclofenac | 3 | 1.3 |
| Meloxicam | 3 | 1.3 |
| Lornoxicam | 2 | 0.9 |
| Nimesulide | 1 | 0.4 |
| ICCM | n (Total—153) | % |
|---|---|---|
| Iopromide | 97 | 63.4 |
| Iohexol | 39 | 25.5 |
| Iomeprol | 13 | 8.5 |
| Iodixanol | 2 | 1.3 |
| Ioversol | 1 | 0.7 |
| Iopamidol | 1 | 0.7 |
| CV Drug | n (Total—143) | % |
|---|---|---|
| ACEIs | 29 | 20.3 |
| Enalapril | 17 | 11.9 |
| Captopril | 11 | 7.8 |
| Perindopril | 1 | 0.7 |
| Beta-blockers | 22 | 15.4 |
| Bisoprolol | 13 | 9.1 |
| Metoprolol | 5 | 3.5 |
| Atenolol | 3 | 2.1 |
| Propranolol | 1 | 0.7 |
| Calcium Channel Blockers | 21 | 14.7 |
| Nifedipine | 9 | 6.3 |
| Amlodipine | 8 | 5.6 |
| Verapamil | 4 | 2.8 |
| Potassium-magnesium-asparaginate | 19 | 13.3 |
| Antiarrhythmics | 12 | 8.4 |
| Amiodarone | 10 | 7.0 |
| Digoxin | 1 | 0.7 |
| Propafenone | 1 | 0.7 |
| Diuretics | 11 | 7.7 |
| Furosemide | 7 | 4.9 |
| Spironolactone | 3 | 2.1 |
| Hydrochlorothiazide | 1 | 0.7 |
| Sartans (Losartan) | 8 | 5.6 |
| Statins | 6 | 4.2 |
| Rosuvastatin | 4 | 2.8 |
| Atorvastatin | 2 | 1.4 |
| Alpha-2 adrenergic receptor agonist (Clonidine) | 4 | 2.8 |
| Indirect oral anticoagulant (Warfarin) | 4 | 2.8 |
| Direct oral anticoagulants | 2 | 1.4 |
| Rivaroxaban | 1 | 0.7 |
| Apixaban | 1 | 0.7 |
| Unfractionated heparin | 2 | 1.4 |
| Antiplatelet drugs | 2 | 1.4 |
| Clopidogrel | 1 | 0.7 |
| Ticagrelor | 1 | 0.7 |
| Thrombolytic agent (Alteplase) | 1 | 0.7 |
| CNS-Active Drugs | n (Total—35) | % |
|---|---|---|
| Fentanyl | 20 | 57.1 |
| Diazepam | 4 | 11.4 |
| Tramadol | 4 | 11.4 |
| Midazolam | 3 | 8.6 |
| Venlafaxine | 1 | 2.9 |
| Droperidol | 1 | 2.9 |
| Carbamazepine | 1 | 2.9 |
| Levetiracetam | 1 | 2.9 |
| NMBAs | n (Total—28) | % |
|---|---|---|
| Rocuronium | 10 | 35.7 |
| Atracurium | 8 | 28.6 |
| Suxamethonium | 5 | 17.9 |
| Cisatracurium | 5 | 17.9 |
| Drug | n (Total—218) | % |
|---|---|---|
| ABs | 109 | 50.00 |
| Ceftriaxone | 68 | 31.2 |
| Cefotaxime | 13 | 6.0 |
| Fosfomycin | 5 | 2.3 |
| Amoxicillin clavulanate | 3 | 1.4 |
| Levofloxacin | 3 | 1.4 |
| Ciprofloxacin | 3 | 1.4 |
| Cefazolin | 3 | 1.4 |
| Ampicillin sulbactam | 2 | 0.9 |
| Ertapenem | 2 | 0.9 |
| Amphotericin B | 1 | 0.5 |
| Meropenem | 1 | 0.5 |
| Tigecycline | 1 | 0.5 |
| Sulfamethoxazole trimethoprim | 1 | 0.5 |
| Vancomycin | 1 | 0.5 |
| Metronidazole | 1 | 0.5 |
| LAs | 29 | 13.3 |
| Lidocaine | 19 | 8.7 |
| Bupivacaine | 7 | 3.2 |
| Articaine | 1 | 0.5 |
| Procaine | 1 | 0.5 |
| Ropivacaine | 1 | 0.5 |
| CV drugs | 13 | 6.0 |
| Beta-blockers | 3 | 1.4 |
| Bisoprolol | 2 | 0.9 |
| Metoprolol | 1 | 0.5 |
| ACEi (Enalapril) | 3 | 1.4 |
| Sartans (Losartan) | 1 | 0.5 |
| Calcium Channel Blockers (Amlodipine) | 2 | 0.9 |
| Antiarrhythmics (Amiodarone) | 1 | 0.5 |
| Statins (Rosuvastatin) | 1 | 0.5 |
| Antiplatelet drugs (Ticagrelor) | 1 | 0.5 |
| Alpha-2 adrenergic receptor agonist (Clonidine) | 1 | 0.5 |
| NMBAs | 8 | 3.7 |
| Rocuronium | 4 | 1.8 |
| Suxamethonium | 2 | 0.9 |
| Atracurium | 1 | 0.5 |
| Cisatracurium | 1 | 0.5 |
| ICCM | 8 | 3.7 |
| Iopromide | 4 | 1.8 |
| Iohexol | 3 | 1.4 |
| Iomeprol | 1 | 0.5 |
| CNS-active drugs | 5 | 2.3 |
| Fentanyl | 2 | 0.9 |
| Diazepam | 1 | 0.5 |
| Levetiracetam | 1 | 0.5 |
| Midazolam | 1 | 0.5 |
| COX inhibitors | 4 | 1.8 |
| Diclofenac | 2 | 0.9 |
| Ibuprofen | 1 | 0.5 |
| Acetylsalicylic acid | 1 | 0.5 |
| Other drugs | 42 | 19.3 |
| Causative Group of Drugs | Age | Females % (n) | |
|---|---|---|---|
| Mean (SD) | Min; Max | ||
| ABs | 48.2 (16.1) | 15 months; 85 years | 53.8 (50) |
| LAs | 39.4 (14.4) | 5 months; 68 years | 55.1 (16) |
| CV drugs | 62.6 (10.6) | 48 years; 86 years | 30.8 (4) |
| ICCM | 74.0 (8.2) | 65 years; 86 years | 62.5 (5) |
| NMBAs | 37.0 (12.7) | 22 years; 61 years | 75.0 (6) |
| COX inhibitors | 50.7 (0.4) | 50 years; 51 years | 75.0 (3) |
| CNS-active drugs | 45.6 (7.7) | 32 years; 55 years | 60.0 (3) |
| Other drugs | 51.4 (15.2) | 1 month; 80 years | 57.8 (41) |
| ABs | n (Total—57) | % |
|---|---|---|
| Beta-lactams | 50 | 87.7 |
| Ceftriaxone | 29 | 50.9 |
| Cefotaxime | 7 | 12.3 |
| Cefazolin | 5 | 8.8 |
| Ampicillin sulbactam | 4 | 7.0 |
| Cefepime | 2 | 3.5 |
| Cefoperazone sulbactam | 1 | 1.8 |
| Meropenem | 1 | 1.8 |
| Amoxicillin clavulanate | 1 | 1.8 |
| Other | 7 | 12.3 |
| Vancomycin | 4 | 7.0 |
| Metronidazole | 2 | 3.5 |
| Linezolid | 1 | 1.8 |
| LAs | n (Total—17) | % |
|---|---|---|
| Lidocaine | 9 | 52.9 |
| Articaine | 6 | 35.3 |
| Mepivacaine | 2 | 11.8 |
| Drug | n (Total—65) | % |
|---|---|---|
| ABs | 26 | 40.0 |
| Ceftriaxone | 23 | 35.4 |
| Amoxicillin clavulanate | 2 | 3.1 |
| Cefixime | 1 | 1.5 |
| CV drugs | 13 | 20.0 |
| Antiarrhythmic (Amiodarone) | 3 | 4.6 |
| Unfractionated heparin | 2 | 3.1 |
| Beta-blockers (Bisoprolol) | 2 | 3.1 |
| Calcium Channels Blockers | 3 | 4.6 |
| Amlodipine | 2 | 3.1 |
| Nifedipine | 1 | 1.5 |
| Diuretics | 2 | 3.1 |
| Furosemide | 1 | 1.5 |
| Hydrochlorothiazide | 1 | 1.5 |
| Thrombolytic agent (Alteplase) | 1 | 1.5 |
| ICCM | 8 | 12.3 |
| Iopromide | 4 | 6.2 |
| Iohexol | 3 | 4.6 |
| Iomeprol | 1 | 1.5 |
| COX inhibitors | 5 | 7.7 |
| Diclofenac | 3 | 4.6 |
| Lornoxicam | 1 | 1.5 |
| Metamizole | 1 | 1.5 |
| NMBAs | 4 | 6.2 |
| Rocuronium | 2 | 3.1 |
| Atracurium | 1 | 1.5 |
| Cisatracurium | 1 | 1.5 |
| LAs | 2 | 3.1 |
| Bupivacaine | 1 | 1.5 |
| Procaine | 1 | 1.5 |
| Other drugs | 7 | 10.8 |
| Prednisolone | 1 | 1.5 |
| Iron formulations | 1 | 1.5 |
| Ethyl-methyl-hydroxypyridine succinate | 1 | 1.5 |
| Venlafaxine | 1 | 1.5 |
| Oxaliplatin | 1 | 1.5 |
| Cisplatin | 1 | 1.5 |
| Pentoxifylline | 1 | 1.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Butranova, O.; Zyryanov, S.; Gorbacheva, A.; Asetskaya, I.; Polivanov, V. Drug-Induced Anaphylaxis: National Database Analysis. Pharmaceuticals 2024, 17, 90. https://doi.org/10.3390/ph17010090
Butranova O, Zyryanov S, Gorbacheva A, Asetskaya I, Polivanov V. Drug-Induced Anaphylaxis: National Database Analysis. Pharmaceuticals. 2024; 17(1):90. https://doi.org/10.3390/ph17010090
Chicago/Turabian StyleButranova, Olga, Sergey Zyryanov, Anastasia Gorbacheva, Irina Asetskaya, and Vitaly Polivanov. 2024. "Drug-Induced Anaphylaxis: National Database Analysis" Pharmaceuticals 17, no. 1: 90. https://doi.org/10.3390/ph17010090
APA StyleButranova, O., Zyryanov, S., Gorbacheva, A., Asetskaya, I., & Polivanov, V. (2024). Drug-Induced Anaphylaxis: National Database Analysis. Pharmaceuticals, 17(1), 90. https://doi.org/10.3390/ph17010090






