Researching New Drug Combinations with Senolytic Activity Using Senescent Human Lung Fibroblasts MRC-5 Cell Line
Abstract
1. Introduction
2. Results
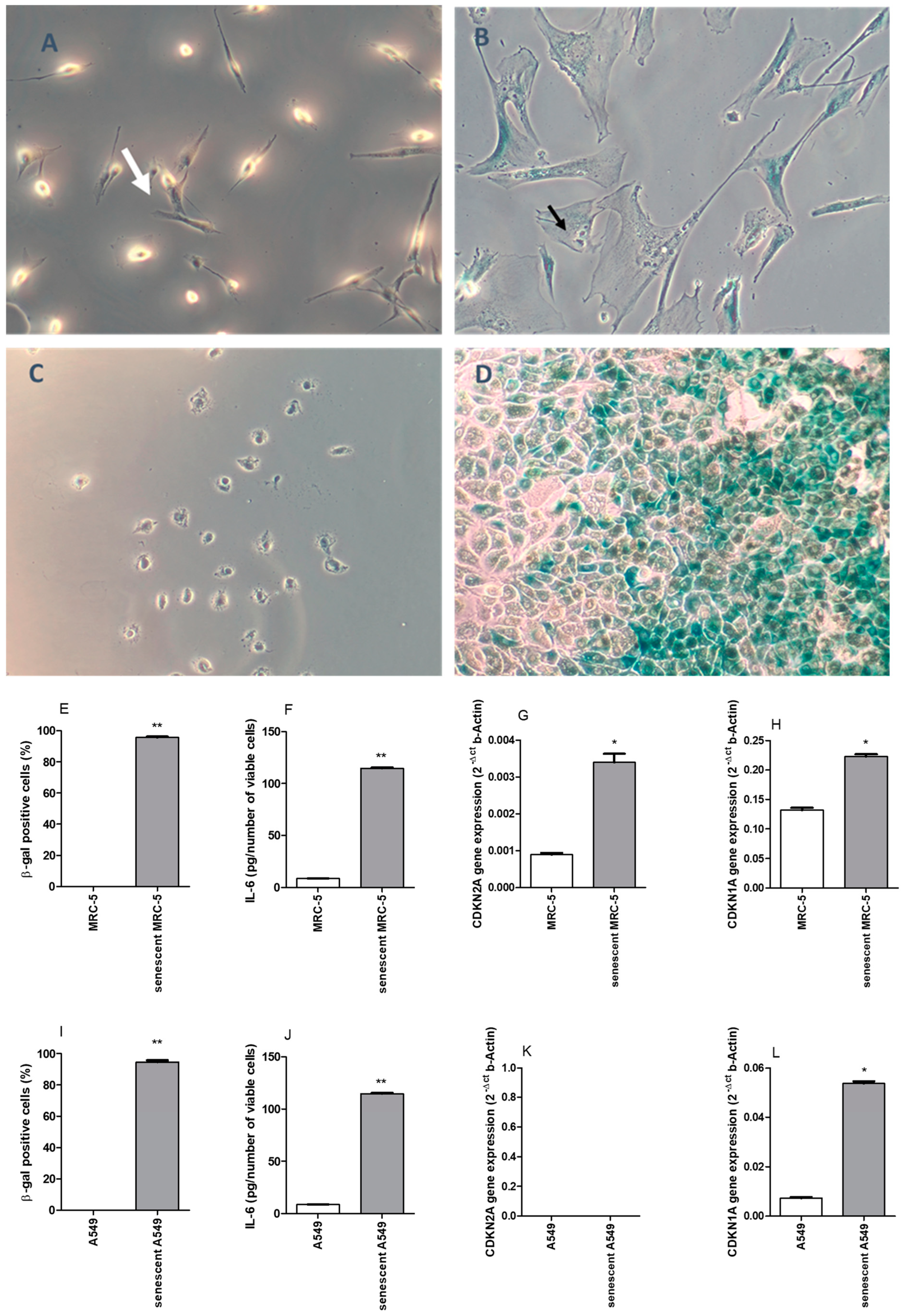
2.1. Doxorubicin (DOXO)-Induced Senescence in MRC-5 and A549 Cell Lines
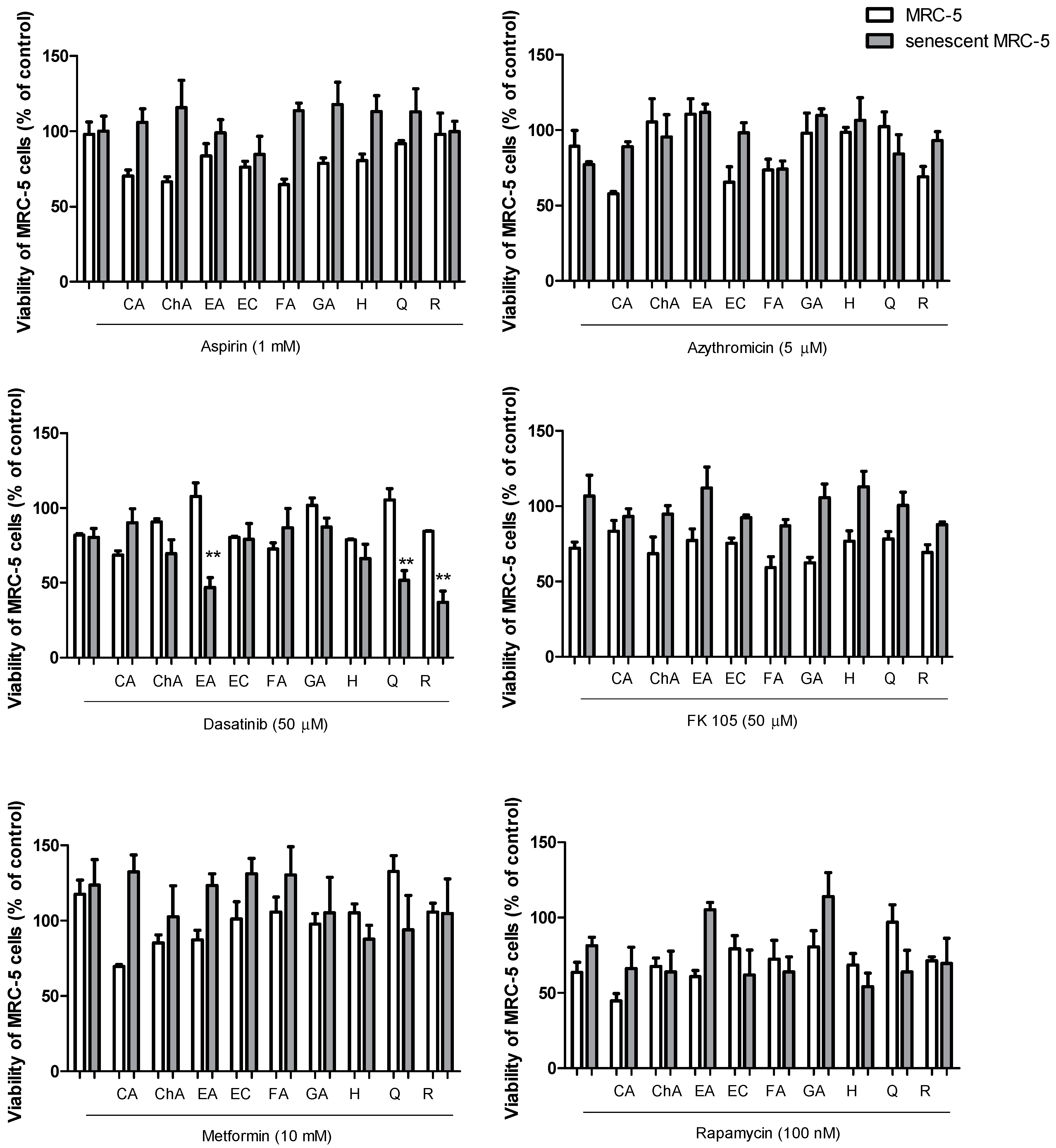
2.2. Senolytic Activity of Drug and Polyphenol Combinations
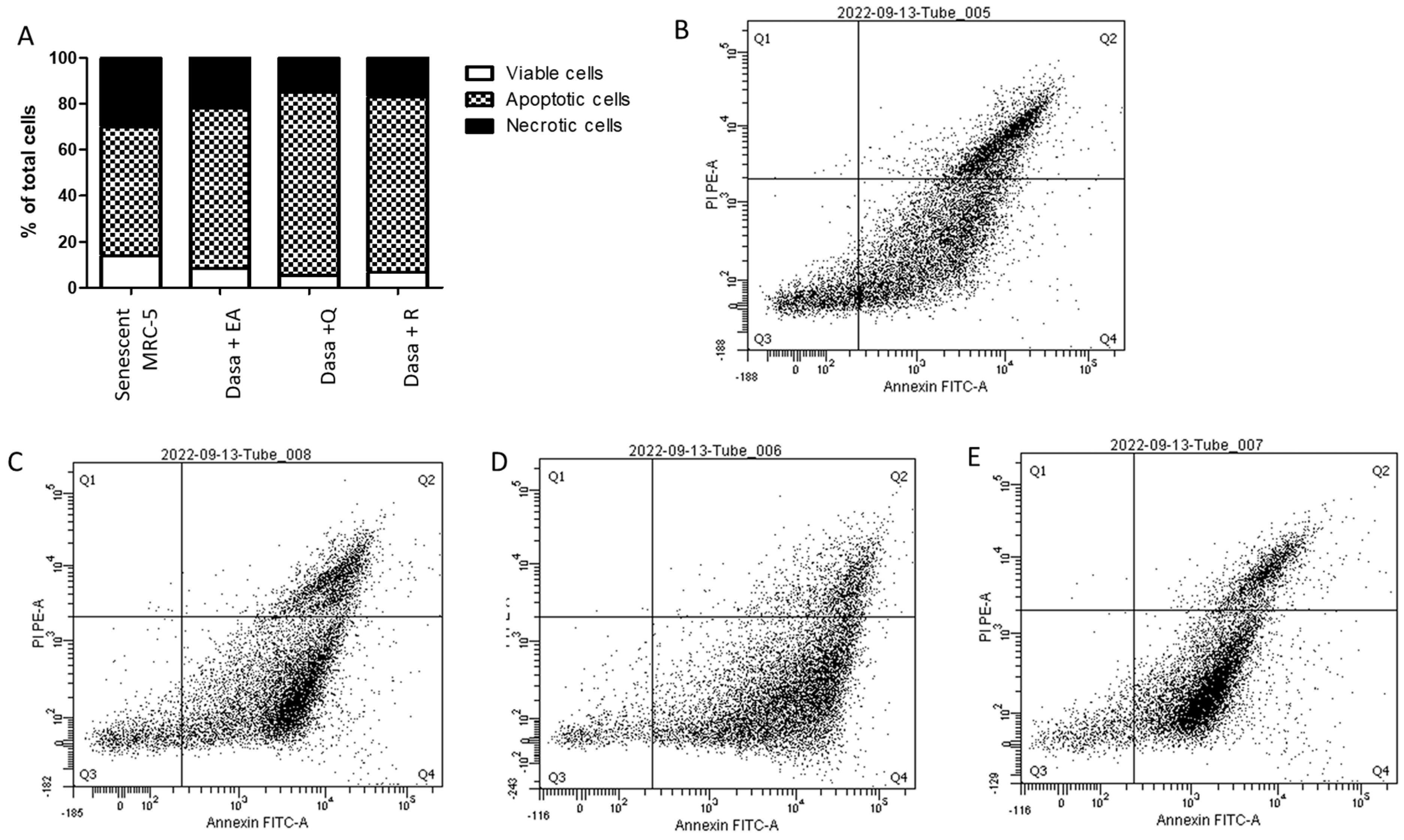
2.3. Evaluation of Senolytic Mechanism
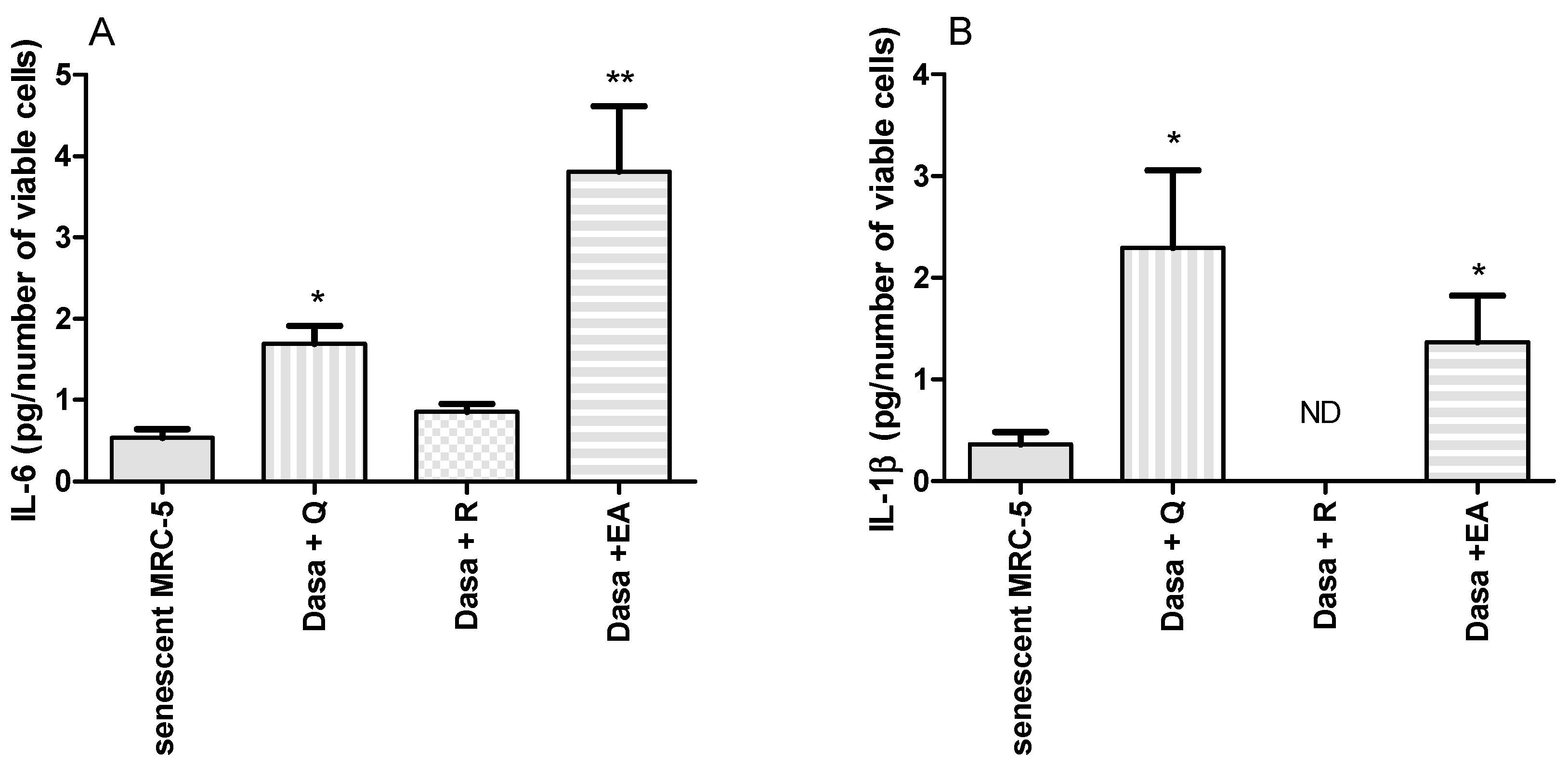
2.4. Inflammatory Markers Released during Senolysis
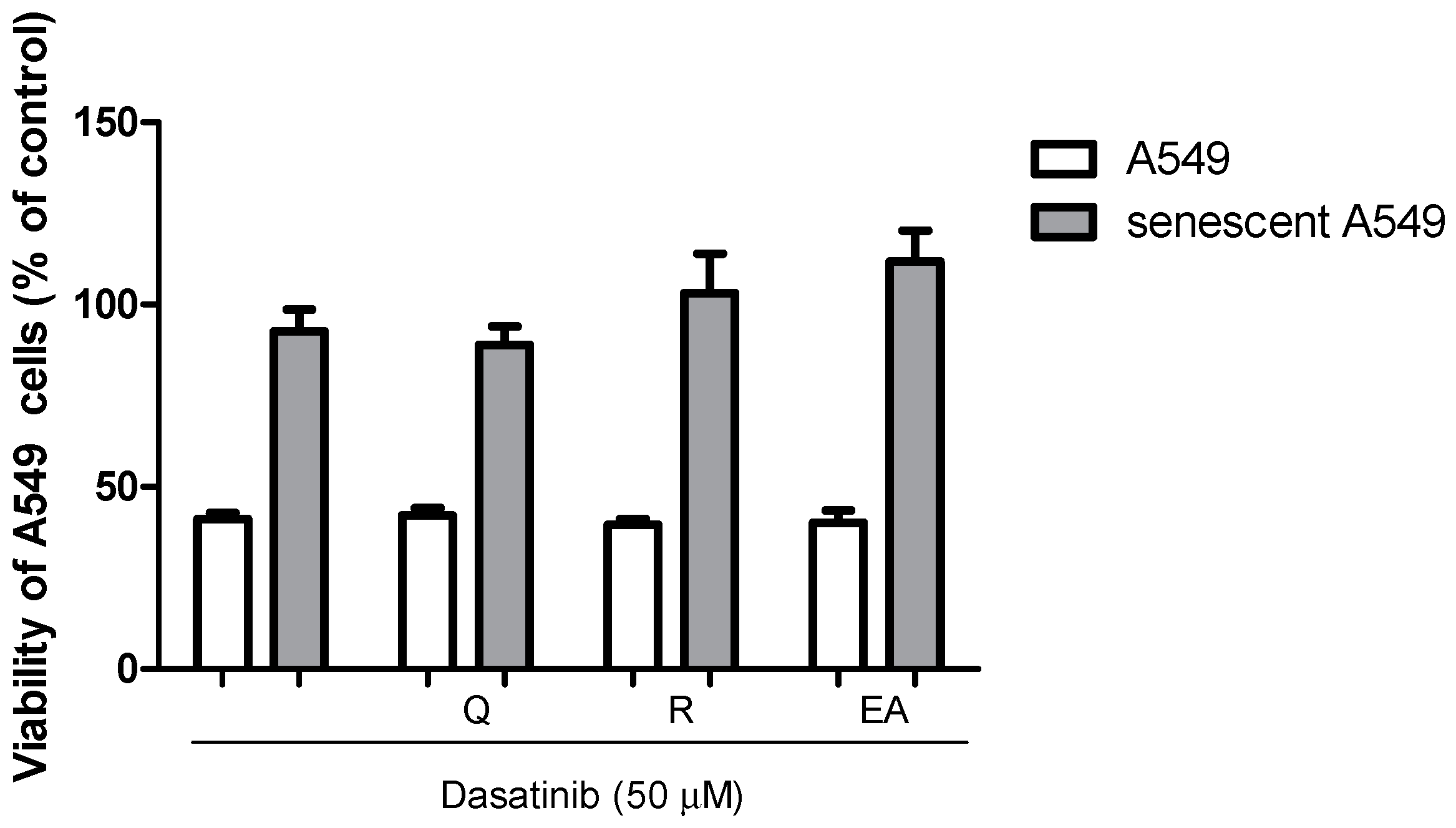
2.5. Senolytic Activity of Dasatinib and Polyphenols in A549 Cells
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Senescence Induced by Doxorubicin
4.3. Screening for Senolytic Activity
4.4. Apoptosis Detection Assays
4.5. BCL-2/BCL-xl, IL-6, and IL-1β Quantification
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ruiz, A.; Flores-Gonzalez, J.; Buendia-Roldan, I.; Chavez-Galan, L. Telomere Shortening and Its Association with Cell Dysfunction in Lung Diseases. Int. J. Mol. Sci. 2021, 23, 425. [Google Scholar] [CrossRef] [PubMed]
- Waters, D.W.; Schuliga, M.; Pathinayake, P.S.; Wei, L.; Tan, H.Y.; Blokland, K.E.C.; Jaffar, J.; Westall, G.P.; Burgess, J.K.; Prele, C.M.; et al. A Senescence Bystander Effect in Human Lung Fibroblasts. Biomedicines 2021, 9, 1162. [Google Scholar] [CrossRef] [PubMed]
- Campisi, J.; d’Adda di Fagagna, F. Cellular senescence: When bad things happen to good cells. Nat. Rev. Mol. Cell Biol. 2007, 8, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Kumari, R.; Jat, P. Mechanisms of Cellular Senescence: Cell Cycle Arrest and Senescence Associated Secretory Phenotype. Front. Cell Dev. Biol. 2021, 9, 645593. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Li, H.; Zi, M.; Li, W.; Liu, J.; Yang, Y.; Zhou, D.; Kong, Q.P.; Zhang, Y.; He, Y. Why Senescent Cells Are Resistant to Apoptosis: An Insight for Senolytic Development. Front. Cell Dev. Biol. 2022, 10, 822816. [Google Scholar] [CrossRef]
- de Mera-Rodriguez, J.A.; Alvarez-Hernan, G.; Ganan, Y.; Martin-Partido, G.; Rodriguez-Leon, J.; Francisco-Morcillo, J. Is Senescence-Associated beta-Galactosidase a Reliable in vivo Marker of Cellular Senescence during Embryonic Development? Front. Cell Dev. Biol. 2021, 9, 623175. [Google Scholar] [CrossRef] [PubMed]
- Parikh, P.; Wicher, S.; Khandalavala, K.; Pabelick, C.M.; Britt, R.D., Jr.; Prakash, Y.S. Cellular senescence in the lung across the age spectrum. Am. J. Physiol. Lung Cell Mol. Physiol. 2019, 316, L826–L842. [Google Scholar] [CrossRef]
- Bjorklund, G.; Shanaida, M.; Lysiuk, R.; Butnariu, M.; Peana, M.; Sarac, I.; Strus, O.; Smetanina, K.; Chirumbolo, S. Natural Compounds and Products from an Anti-Aging Perspective. Molecules 2022, 27, 7084. [Google Scholar] [CrossRef]
- Schafer, M.J.; White, T.A.; Iijima, K.; Haak, A.J.; Ligresti, G.; Atkinson, E.J.; Oberg, A.L.; Birch, J.; Salmonowicz, H.; Zhu, Y.; et al. Cellular senescence mediates fibrotic pulmonary disease. Nat. Commun. 2017, 8, 14532. [Google Scholar] [CrossRef]
- Spagnolo, P.; Semenzato, U. Revealing the pathogenic and ageing-related mechanisms of the enigmatic idiopathic pulmonary fibrosis (and chronic obstructive pulmonary disease). Curr. Opin. Pulm. Med. 2022, 28, 296–302. [Google Scholar] [CrossRef]
- Zhang, L.M.; Zhang, J.; Zhang, Y.; Fei, C.; Wang, L.; Yi, Z.W.; Zhang, Z.Q. Interleukin-18 promotes fibroblast senescence in pulmonary fibrosis through down-regulating Klotho expression. Biomed. Pharmacother. 2019, 113, 108756. [Google Scholar] [CrossRef] [PubMed]
- Wiley, C.D.; Brumwell, A.N.; Davis, S.S.; Jackson, J.R.; Valdovinos, A.; Calhoun, C.; Alimirah, F.; Castellanos, C.A.; Ruan, R.; Wei, Y.; et al. Secretion of leukotrienes by senescent lung fibroblasts promotes pulmonary fibrosis. JCI Insight 2019, 4, e130056. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, T.; Iwai, M.; Aoki, S.; Takimoto, K.; Maruyama, M.; Maruyama, W.; Motoyama, N. SIRT1 suppresses the senescence-associated secretory phenotype through epigenetic gene regulation. PLoS ONE 2015, 10, e0116480. [Google Scholar] [CrossRef] [PubMed]
- Aoshiba, K.; Tsuji, T.; Kameyama, S.; Itoh, M.; Semba, S.; Yamaguchi, K.; Nakamura, H. Senescence-associated secretory phenotype in a mouse model of bleomycin-induced lung injury. Exp. Toxicol. Pathol. 2013, 65, 1053–1062. [Google Scholar] [CrossRef] [PubMed]
- Woldhuis, R.R.; Heijink, I.H.; van den Berge, M.; Timens, W.; Oliver, B.G.G.; de Vries, M.; Brandsma, C.A. COPD-derived fibroblasts secrete higher levels of senescence-associated secretory phenotype proteins. Thorax 2021, 76, 508–511. [Google Scholar] [CrossRef]
- Woldhuis, R.R.; de Vries, M.; Timens, W.; van den Berge, M.; Demaria, M.; Oliver, B.G.G.; Heijink, I.H.; Brandsma, C.A. Link between increased cellular senescence and extracellular matrix changes in COPD. Am. J. Physiol. Lung Cell Mol. Physiol. 2020, 319, L48–L60. [Google Scholar] [CrossRef]
- Lehmann, M.; Korfei, M.; Mutze, K.; Klee, S.; Skronska-Wasek, W.; Alsafadi, H.N.; Ota, C.; Costa, R.; Schiller, H.B.; Lindner, M.; et al. Senolytic drugs target alveolar epithelial cell function and attenuate experimental lung fibrosis ex vivo. Eur. Respir. J. 2017, 50, 1602367. [Google Scholar] [CrossRef]
- Merkt, W.; Bueno, M.; Mora, A.L.; Lagares, D. Senotherapeutics: Targeting senescence in idiopathic pulmonary fibrosis. Semin. Cell Dev. Biol. 2020, 101, 104–110. [Google Scholar] [CrossRef]
- Tilstra, J.S.; Robinson, A.R.; Wang, J.; Gregg, S.Q.; Clauson, C.L.; Reay, D.P.; Nasto, L.A.; St Croix, C.M.; Usas, A.; Vo, N.; et al. NF-kappaB inhibition delays DNA damage-induced senescence and aging in mice. J. Clin. Investig. 2012, 122, 2601–2612. [Google Scholar] [CrossRef]
- Xu, M.; Tchkonia, T.; Ding, H.; Ogrodnik, M.; Lubbers, E.R.; Pirtskhalava, T.; White, T.A.; Johnson, K.O.; Stout, M.B.; Mezera, V.; et al. JAK inhibition alleviates the cellular senescence-associated secretory phenotype and frailty in old age. Proc. Natl. Acad. Sci. USA 2015, 112, E6301–E6310. [Google Scholar] [CrossRef]
- Ribaudo, G.; Gianoncelli, A. An Updated Overview on the Role of Small Molecules and Natural Compounds in the “Young Science” of Rejuvenation. Antioxidants 2023, 12, 288. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Doornebal, E.J.; Pirtskhalava, T.; Giorgadze, N.; Wentworth, M.; Fuhrmann-Stroissnigg, H.; Niedernhofer, L.J.; Robbins, P.D.; Tchkonia, T.; Kirkland, J.L. New agents that target senescent cells: The flavone, fisetin, and the BCL-X(L) inhibitors, A1331852 and A1155463. Aging 2017, 9, 955–963. [Google Scholar] [CrossRef] [PubMed]
- Justice, J.N.; Nambiar, A.M.; Tchkonia, T.; LeBrasseur, N.K.; Pascual, R.; Hashmi, S.K.; Prata, L.; Masternak, M.M.; Kritchevsky, S.B.; Musi, N.; et al. Senolytics in idiopathic pulmonary fibrosis: Results from a first-in-human, open-label, pilot study. EBioMedicine 2019, 40, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Kirkland, J.L.; Tchkonia, T. Senolytic drugs: From discovery to translation. J. Intern. Med. 2020, 288, 518–536. [Google Scholar] [CrossRef]
- Ozsvari, B.; Nuttall, J.R.; Sotgia, F.; Lisanti, M.P. Azithromycin and Roxithromycin define a new family of “senolytic” drugs that target senescent human fibroblasts. Aging 2018, 10, 3294–3307. [Google Scholar] [CrossRef]
- As Sobeai, H.M.; Alohaydib, M.; Alhoshani, A.R.; Alhazzani, K.; Almutairi, M.M.; Saleh, T.; Gewirtz, D.A.; Alotiabi, M.R. Sorafenib, rapamycin, and venetoclax attenuate doxorubicin-induced senescence and promote apoptosis in HCT116 cells. Saudi Pharm. J. 2022, 30, 91–101. [Google Scholar] [CrossRef]
- Xu, W.; Zhao, T.; Chen, H.; Huang, N.; Gong, H.; Zhang, J.; Yang, Y.; Li, T.; Zhang, G.; Gong, C.; et al. Pan-mTOR inhibitors sensitize the senolytic activity of navitoclax via mTORC2 inhibition-mediated apoptotic signaling. Biochem. Pharmacol. 2022, 200, 115045. [Google Scholar] [CrossRef]
- Feng, M.; Kim, J.; Field, K.; Reid, C.; Chatzistamou, I.; Shim, M. Aspirin ameliorates the long-term adverse effects of doxorubicin through suppression of cellular senescence. FASEB Bioadv. 2019, 1, 579–590. [Google Scholar] [CrossRef]
- Petrocelli, J.J.; de Hart, N.; Lang, M.J.; Yee, E.M.; Ferrara, P.J.; Fix, D.K.; Chaix, A.; Funai, K.; Drummond, M.J. Cellular senescence and disrupted proteostasis induced by myotube atrophy are prevented with low-dose metformin and leucine cocktail. Aging 2023, 15, 1808–1832. [Google Scholar] [CrossRef]
- Kang, S.W.; Kim, J.; Shin, D.Y. Inhibition of senescence and promotion of the proliferation of chondrocytes from articular cartilage by CsA and FK506 involves inhibition of p38MAPK. Mech. Ageing Dev. 2016, 153, 7–13. [Google Scholar] [CrossRef]
- Nambiar, A.; Kellogg, D., 3rd; Justice, J.; Goros, M.; Gelfond, J.; Pascual, R.; Hashmi, S.; Masternak, M.; Prata, L.; LeBrasseur, N.; et al. Senolytics dasatinib and quercetin in idiopathic pulmonary fibrosis: Results of a phase I, single-blind, single-center, randomized, placebo-controlled pilot trial on feasibility and tolerability. EBioMedicine 2023, 90, 104481. [Google Scholar] [CrossRef] [PubMed]
- Hickson, L.J.; Langhi Prata, L.G.P.; Bobart, S.A.; Evans, T.K.; Giorgadze, N.; Hashmi, S.K.; Herrmann, S.M.; Jensen, M.D.; Jia, Q.; Jordan, K.L.; et al. Senolytics decrease senescent cells in humans: Preliminary report from a clinical trial of Dasatinib plus Quercetin in individuals with diabetic kidney disease. EBioMedicine 2019, 47, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Orr, M.; Gonzales, M.; Garbarino, V.; Kautz, T.; Palavicini, J.; Lopez-Cruzan, M.; Dehkordi, S.K.; Mathews, J.; Zare, H.; Xu, P.; et al. Senolytic therapy to modulate the progression of Alzheimer’s Disease (SToMP-AD)-Outcomes from the first clinical trial of senolytic therapy for Alzheimer’s disease. Res. Sq. 2023. [Google Scholar] [CrossRef]
- Aaij, R.; Adeva, B.; Adinolfi, M.; Affolder, A.; Ajaltouni, Z.; Akar, S.; Albrecht, J.; Alessio, F.; Alexander, M.; Ali, S.; et al. Evidence for CP violation in B+ --> ppK+ decays. Phys. Rev. Lett. 2014, 113, 141801. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Li, H.; Wang, X.W.; Wu, D.C.; Chen, X.Y.; Liu, J. Resveratrol promotes differentiation and induces Fas-independent apoptosis of human medulloblastoma cells. Neurosci. Lett. 2003, 351, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Kohandel, Z.; Darrudi, M.; Naseri, K.; Samini, F.; Aschner, M.; Pourbagher-Shahri, A.M.; Samarghandian, S. The Role of Resveratrol in Aging and Senescence: A Focus on Molecular Mechanisms. Curr. Mol. Med. 2023; ahead of print. [Google Scholar] [CrossRef]
- Fuhrmann-Stroissnigg, H.; Ling, Y.Y.; Zhao, J.; McGowan, S.J.; Zhu, Y.; Brooks, R.W.; Grassi, D.; Gregg, S.Q.; Stripay, J.L.; Dorronsoro, A.; et al. Identification of HSP90 inhibitors as a novel class of senolytics. Nat. Commun. 2017, 8, 422. [Google Scholar] [CrossRef]
- Naiki-Ito, A.; Chewonarin, T.; Tang, M.; Pitchakarn, P.; Kuno, T.; Ogawa, K.; Asamoto, M.; Shirai, T.; Takahashi, S. Ellagic acid, a component of pomegranate fruit juice, suppresses androgen-dependent prostate carcinogenesis via induction of apoptosis. Prostate 2015, 75, 151–160. [Google Scholar] [CrossRef]
- Chung, Y.C.; Lu, L.C.; Tsai, M.H.; Chen, Y.J.; Chen, Y.Y.; Yao, S.P.; Hsu, C.P. The inhibitory effect of ellagic acid on cell growth of ovarian carcinoma cells. Evid. Based Complement. Alternat Med. 2013, 2013, 306705. [Google Scholar] [CrossRef]
- Zhu, H.; Yan, Y.; Jiang, Y.; Meng, X. Ellagic Acid and Its Anti-Aging Effects on Central Nervous System. Int. J. Mol. Sci. 2022, 23, 937. [Google Scholar] [CrossRef]
- Cho, M.; Kim, Y.; You, S.; Hwang, D.Y.; Jang, M. Chlorogenic Acid of Cirsium japonicum Resists Oxidative Stress Caused by Aging and Prolongs Healthspan via SKN-1/Nrf2 and DAF-16/FOXO in Caenorhabditis elegans. Metabolites 2023, 13, 224. [Google Scholar] [CrossRef]
- Wang, X.; Liu, J.; Xie, Z.; Rao, J.; Xu, G.; Huang, K.; Li, W.; Yin, Z. Chlorogenic acid inhibits proliferation and induces apoptosis in A498 human kidney cancer cells via inactivating PI3K/Akt/mTOR signalling pathway. J. Pharm. Pharmacol. 2019, 71, 1100–1109. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Choi, Y.J.; Suh, S.I.; Baek, W.K.; Suh, M.H.; Jin, I.N.; Min, D.S.; Woo, J.H.; Chang, J.S.; Passaniti, A.; et al. Bcl-2 overexpression attenuates resveratrol-induced apoptosis in U937 cells by inhibition of caspase-3 activity. Carcinogenesis 2001, 22, 1633–1639. [Google Scholar] [CrossRef] [PubMed]
- Brockmueller, A.; Buhrmann, C.; Shayan, P.; Shakibaei, M. Resveratrol induces apoptosis by modulating the reciprocal crosstalk between p53 and Sirt-1 in the CRC tumor microenvironment. Front. Immunol. 2023, 14, 1225530. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, L.; Tang, H.; Liu, Z.; Tian, Y.; Gao, X.; Peng, T.; Wang, Z.; Lan, X.; Shen, W.; Xiao, D.; et al. Resveratrol inhibits LPS-induced apoptosis in bovine mammary epithelial cells: The role of PGC1alpha-SIRT3 axis. Vitr. Cell Dev. Biol. Anim. 2023, 59, 264–276. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zheng, H.; Fu, Y.; Gu, Y.; Zou, H.; Yuan, Y.; Gu, J.; Liu, Z.; Bian, J. Role of PI3K/Akt-Mediated Nrf2/HO-1 Signaling Pathway in Resveratrol Alleviation of Zearalenone-Induced Oxidative Stress and Apoptosis in TM4 Cells. Toxins 2022, 14, 733. [Google Scholar] [CrossRef] [PubMed]
- Szondy, Z.; Sarang, Z.; Kiss, B.; Garabuczi, E.; Koroskenyi, K. Anti-inflammatory Mechanisms Triggered by Apoptotic Cells during Their Clearance. Front. Immunol. 2017, 8, 909. [Google Scholar] [CrossRef] [PubMed]
- Vanden Berghe, T.; Kalai, M.; Denecker, G.; Meeus, A.; Saelens, X.; Vandenabeele, P. Necrosis is associated with IL-6 production but apoptosis is not. Cell Signal 2006, 18, 328–335. [Google Scholar] [CrossRef]
- Vernot, J.P. Senescence-Associated Pro-inflammatory Cytokines and Tumor Cell Plasticity. Front. Mol. Biosci. 2020, 7, 63. [Google Scholar] [CrossRef]
- Cao, S.; Fu, X.; Yang, S.; Tang, S. The anti-inflammatory activity of resveratrol in acute kidney injury: A systematic review and meta-analysis of animal studies. Pharm. Biol. 2022, 60, 2088–2097. [Google Scholar] [CrossRef]
- Huo, R.; Huang, X.; Yang, Y.; Yang, Y.; Lin, J. Potential of resveratrol in the treatment of interstitial lung disease. Front. Pharmacol. 2023, 14, 1139460. [Google Scholar] [CrossRef]
- Abdelgawad, I.Y.; Agostinucci, K.; Ismail, S.G.; Grant, M.K.O.; Zordoky, B.N. EA.hy926 Cells and HUVECs Share Similar Senescence Phenotypes but Respond Differently to the Senolytic Drug ABT-263. Cells 2022, 11, 1992. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jin, Y.; Tao, X.; Bai, M. Effects of exogenous p16(ink4a) gene on biological behaviors of human lung cancer cells. J. Huazhong Univ. Sci. Technol. Med. Sci. 2007, 27, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Liao, W.; Xia, H.; Wang, S.; Sun, G. The Effect of Resveratrol on Blood Lipid Profile: A Dose-Response Meta-Analysis of Randomized Controlled Trials. Nutrients 2022, 14, 3755. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-Lozada, G.M.; Villarruel-Lopez, A.; Martinez-Abundis, E.; Vazquez-Paulino, O.; Gonzalez-Ortiz, M.; Perez-Rubio, K.G. Ellagic Acid Effect on the Components of Metabolic Syndrome, Insulin Sensitivity and Insulin Secretion: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. J. Clin. Med. 2022, 11, 5741. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Godoy, M.C.X.; Macedo, J.A.; Gambero, A. Researching New Drug Combinations with Senolytic Activity Using Senescent Human Lung Fibroblasts MRC-5 Cell Line. Pharmaceuticals 2024, 17, 70. https://doi.org/10.3390/ph17010070
de Godoy MCX, Macedo JA, Gambero A. Researching New Drug Combinations with Senolytic Activity Using Senescent Human Lung Fibroblasts MRC-5 Cell Line. Pharmaceuticals. 2024; 17(1):70. https://doi.org/10.3390/ph17010070
Chicago/Turabian Stylede Godoy, Maria Carolina Ximenes, Juliana Alves Macedo, and Alessandra Gambero. 2024. "Researching New Drug Combinations with Senolytic Activity Using Senescent Human Lung Fibroblasts MRC-5 Cell Line" Pharmaceuticals 17, no. 1: 70. https://doi.org/10.3390/ph17010070
APA Stylede Godoy, M. C. X., Macedo, J. A., & Gambero, A. (2024). Researching New Drug Combinations with Senolytic Activity Using Senescent Human Lung Fibroblasts MRC-5 Cell Line. Pharmaceuticals, 17(1), 70. https://doi.org/10.3390/ph17010070





