A Mechanistic Review on How Berberine Use Combats Diabetes and Related Complications: Molecular, Cellular, and Metabolic Effects
Abstract
1. Introduction
2. Search Strategy and Study Selection
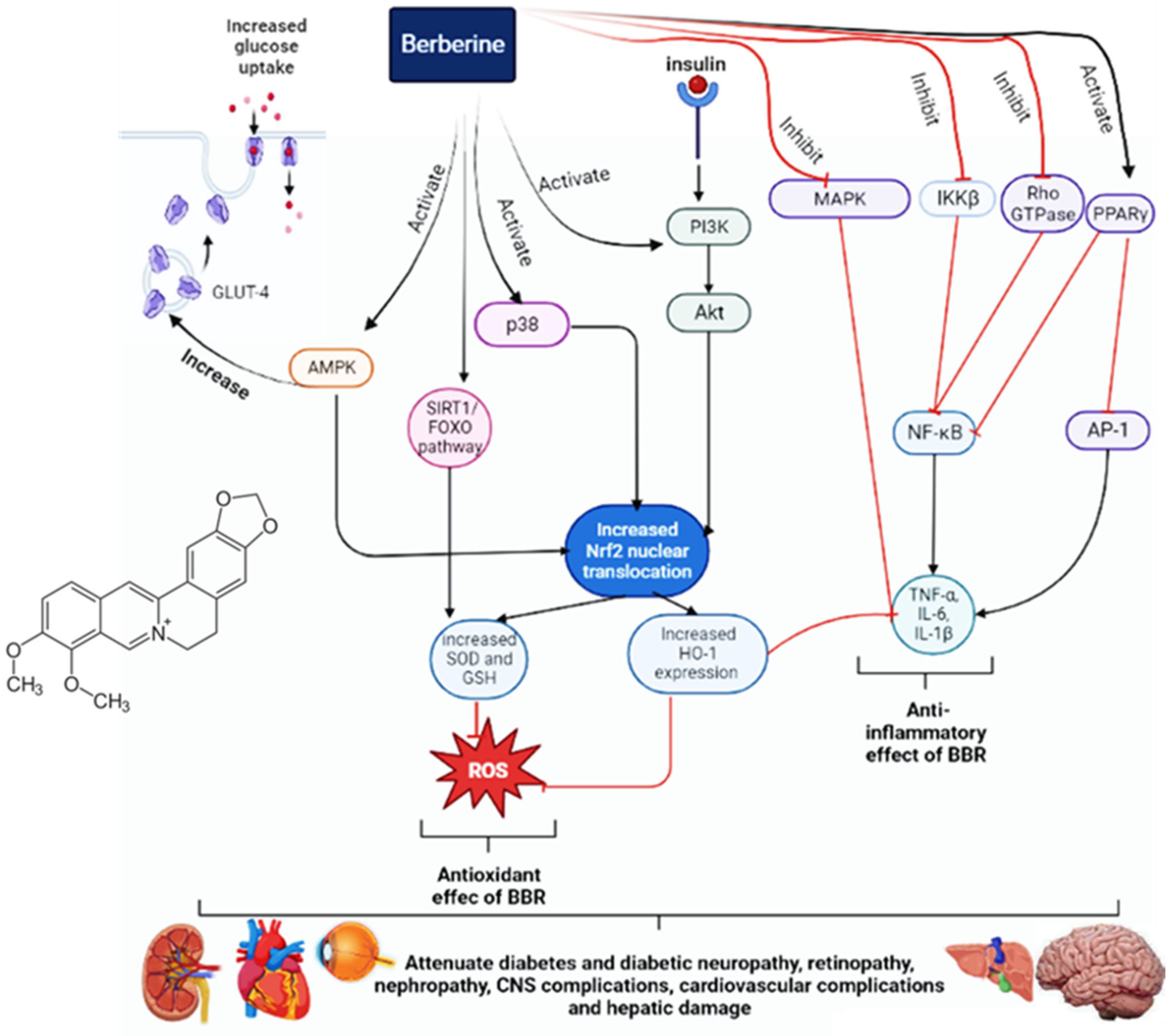
3. Results and Discussion
3.1. In Vitro Models of Diabetes Mellitus (DM)
3.2. Animal Models of Diabetes Mellitus (DM)
3.2.1. Protective Effects of Berberine against db/db and STZ-Induced DM
3.2.2. Protective Effects of Berberine against Alloxan-Induced DM
3.2.3. Protective Effects of Berberine against HFD-Induced DM
3.3. Effects of Berberine on Insulin Resistance and Secretion
3.4. Protective Effects of Berberine against Diabetes Complications
3.4.1. Diabetes-Induced Osteoporosis
3.4.2. Diabetes-Induced Gut Microbiota Alteration
3.4.3. Diabetic-Induced Hepatic Damage
3.4.4. Diabetic Retinopathy
3.4.5. Diabetic Vascular Complications
3.4.6. Diabetic-Induced Neuropathy
3.4.7. Diabetic-Induced Nephropathy
3.4.8. Diabetic-Induced Cardiovascular Disease
3.4.9. Diabetes-Induced Central Nervous System (CNS) Disorders
3.5. Clinical Investigations
3.6. Toxicity of and Cautionary Notes on Berberine
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Friedman, J.M. Modern science versus the stigma of obesity. Nat. Med. 2004, 10, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Wang, F.; Zhou, X.; Yang, H.; Wang, Y. Hypoglycemic and hypolipidemic properties of polysaccharides from Enterobacter cloacae Z0206 in KKAy mice. Carbohydr. Polym. 2015, 117, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Pang, B.; Zhao, L.-H.; Zhou, Q.; Zhao, T.-Y.; Wang, H.; Gu, C.-J.; Tong, X.-L. Application of berberine on treating type 2 diabetes mellitus. Int. J. Endocrinol. 2015, 2015, 905749. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Feng, X.; Chai, L.; Cao, S.; Qiu, F. The metabolism of berberine and its contribution to the pharmacological effects. Drug Metab. Rev. 2017, 49, 139–157. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wei, J.; Xue, R.; Wu, J.-D.; Zhao, W.; Wang, Z.-Z.; Wang, S.-K.; Zhou, Z.-X.; Song, D.-Q.; Wang, Y.-M.; et al. Berberine lowers blood glucose in type 2 diabetes mellitus patients through increasing insulin receptor expression. Metabolism 2010, 59, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Wen, W.; Qi, C.-L.; Zhao, R.-X.; Lü, J.-H.; Zhong, C.-Y.; Chen, Y.-Y. Ameliorative effect of berberine on renal damage in rats with diabetes induced by high-fat diet and streptozotocin. Phytomedicine 2012, 19, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Du, X.; Long, M.; Zhang, Z.; Zhou, S.; Zhou, J.; Qian, G. Neuroprotective effect of berberine is mediated by MAPK signaling pathway in experimental diabetic neuropathy in rats. Eur. J. Pharmacol. 2016, 774, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zhang, C.; Xu, Y.; Tan, H.-Y.; Chen, H.; Feng, Y. Berberine improves insulin-induced diabetic retinopathy through exclusively suppressing Akt/mTOR-mediated HIF-1α/VEGF activation in retina endothelial cells. Int. J. Biol. Sci. 2021, 17, 4316–4326. [Google Scholar] [CrossRef]
- Chen, Q.; Mo, R.; Wu, N.; Zou, X.; Shi, C.; Gong, J.; Li, J.; Fang, K.; Wang, D.; Yang, D.; et al. Berberine ameliorates diabetes-associated cognitive decline through modulation of aberrant inflammation response and insulin signaling pathway in DM rats. Front. Pharmacol. 2017, 8, 334. [Google Scholar] [CrossRef]
- Wang, L.; Yu, C.; Fu, Y.; Li, Q.; Sun, Y. Berberine elicits anti-arrhythmic effects via IK1/Kir2. 1 in the rat type 2 diabetic myocardial infarction model. Phytother. Res. 2011, 25, 33–37. [Google Scholar] [CrossRef]
- Wang, C.; Li, J.; Lv, X.; Zhang, M.; Song, Y.; Chen, L.; Liu, Y. Ameliorative effect of berberine on endothelial dysfunction in diabetic rats induced by high-fat diet and streptozotocin. Eur. J. Pharmacol. 2009, 620, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Kim, W.S.; Kim, K.H.; Yoon, M.J.; Cho, H.J.; Shen, Y.; Ye, J.-M.; Lee, C.H.; Oh, W.K.; Kim, C.T.; et al. Berberine, a natural plant product, activates AMP-activated protein kinase with beneficial metabolic effects in diabetic and insulin-resistant states. Diabetes 2006, 55, 2256–2264. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Li, G.; Geng, F.; Zhang, Z.; Li, J.; Yang, M.; Dong, L.; Gao, F. Berberine reduces ischemia/reperfusion-induced myocardial apoptosis via activating AMPK and PI3K–Akt signaling in diabetic rats. Apoptosis 2014, 19, 946–957. [Google Scholar] [CrossRef] [PubMed]
- Babaei Khorzoughi, R.; Namvarjah, F.; Teimouri, M.; Hosseini, H.; Meshkani, R. In-vitro synergistic effect of metformin and berberine on high glucose-induced lipogenesis. Iran. J. Pharm. Res. 2019, 18, 1921–1930. [Google Scholar] [CrossRef] [PubMed]
- Hang, W.; He, B.; Chen, J.; Xia, L.; Wen, B.; Liang, T.; Wang, X.; Zhang, Q.; Wu, Y.; Chen, Q.; et al. Berberine Ameliorates High Glucose-Induced Cardiomyocyte Injury via AMPK Signaling Activation to Stimulate Mitochondrial Biogenesis and Restore Autophagic Flux. Front. Pharmacol. 2018, 9, 1121. [Google Scholar] [CrossRef] [PubMed]
- Chandirasegaran, G.; Elanchezhiyan, C.; Ghosh, K.; Sethupathy, S. Berberine chloride ameliorates oxidative stress, inflammation and apoptosis in the pancreas of Streptozotocin induced diabetic rats. Biomed. Pharmacother. 2017, 95, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Leng, S.-H.; Lu, F.-E.; Xu, L.-J. Therapeutic effects of berberine in impaired glucose tolerance rats and its influence on insulin secretion. Acta Pharmacol. Sin. 2004, 25, 496–502. [Google Scholar]
- Cao, W.; Hu, L.; Chen, H.; Gao, Y.; Liang, Y.; Wu, Y.; Yang, Q.; Tang, N.; Cao, J.; Xiao, J. Berberine alleviates chronic inflammation of mouse model of type 2 diabetes by adjusting intestinal microbes and inhibiting TLR4 signaling pathway. Int. J. Clin. Exp. Med. 2017, 10, 10267–10276. [Google Scholar]
- Jiang, S.-J.; Dong, H.; Li, J.-B.; Xu, L.-J.; Zou, X.; Wang, K.-F.; Lu, F.-E.; Yi, P. Berberine inhibits hepatic gluconeogenesis via the LKB1-AMPK-TORC2 signaling pathway in streptozotocin-induced diabetic rats. World J. Gastroenterol. 2015, 21, 7777. [Google Scholar] [CrossRef]
- Kong, W.-J.; Zhang, H.; Song, D.-Q.; Xue, R.; Zhao, W.; Wei, J.; Wang, Y.-M.; Shan, N.; Zhou, Z.-X.; Yang, P.; et al. Berberine reduces insulin resistance through protein kinase C–dependent up-regulation of insulin receptor expression. Metabolism 2009, 58, 109–119. [Google Scholar] [CrossRef]
- Li, G.-S.; Liu, X.-H.; Zhu, H.; Huang, L.; Liu, Y.-L.; Ma, C.-M.; Qin, C. Berberine-improved visceral white adipose tissue insulin resistance associated with altered sterol regulatory element-binding proteins, liver X receptors, and peroxisome proliferator-activated receptors transcriptional programs in diabetic hamsters. Biol. Pharm. Bull. 2011, 34, 644–654. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, Y.; Zhang, J.; Sun, C.; Lopez, A. Berberine improves glucose homeostasis in streptozotocin-induced diabetic rats in association with multiple factors of insulin resistance. ISRN Endocrinol. 2011, 2011, 519371. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Jin, J.; Liu, P.; Song, Y.; Zhang, H.; Sheng, L.; Zhou, H.; Jiang, B. Berberine attenuates hyperglycemia by inhibiting the hepatic glucagon pathway in diabetic mice. Oxidative Med. Cell. Longev. 2020, 2020, 6210526. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhang, Y.; Huang, C. Berberine inhibits PTP1B activity and mimics insulin action. Biochem. Biophys. Res. Commun. 2010, 397, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-N.; Wang, X.; Lei, L.; Liu, M.-Z.; Li, R.-C.; Sun, S.-J.; Liu, S.-N.; Huan, Y.; Zhou, T.; Liu, Q.; et al. Berberine combined with stachyose induces better glycometabolism than berberine alone through modulating gut microbiota and fecal metabolomics in diabetic mice. Phytother. Res. 2020, 34, 1166–1174. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhang, X.; Liu, P.; Shen, X.; Lan, T.; Li, W.; Jiang, Q.; Xie, X.; Huang, H. Effects of berberine on matrix accumulation and NF-kappa B signal pathway in alloxan-induced diabetic mice with renal injury. Eur. J. Pharmacol. 2010, 638, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.; Li, J.-Y.; Gosby, A.; To, S.W.C.; Cheng, Z.; Miyoshi, H.; Taketo, M.M.; Cooney, G.J.; Kraegen, E.W.; James, D.E.; et al. Berberine and its more biologically available derivative, dihydroberberine, inhibit mitochondrial respiratory complex I: A mechanism for the action of berberine to activate AMP-activated protein kinase and improve insulin action. Diabetes 2008, 57, 1414–1418. [Google Scholar] [CrossRef]
- Sui, M.; Jiang, X.; Sun, H.; Liu, C.; Fan, Y. Berberine ameliorates hepatic insulin resistance by regulating microRNA-146b/SIRT1 pathway. Diabetes Metab. Syndr. Obes. 2021, 14, 2525–2537. [Google Scholar] [CrossRef]
- Teodoro, J.S.; Duarte, F.V.; Gomes, A.P.; Varela, A.T.; Peixoto, F.M.; Rolo, A.P.; Palmeira, C.M. Berberine reverts hepatic mitochondrial dysfunction in high-fat fed rats: A possible role for SirT3 activation. Mitochondrion 2013, 13, 637–646. [Google Scholar] [CrossRef]
- He, Q.; Chen, B.; Wang, G.; Zhou, D.; Zeng, H.; Li, X.; Song, Y.; Yu, X.; Liang, W.; Chen, H.; et al. Co-Crystal of Rosiglitazone With Berberine Ameliorates Hyperglycemia and Insulin Resistance Through the PI3K/AKT/TXNIP Pathway In Vivo and In Vitro. Front. Pharmacol. 2022, 13, 842879. [Google Scholar] [CrossRef]
- Wu, Y.S.; Li, Z.M.; Chen, Y.T.; Dai, S.J.; Zhou, X.J.; Yang, Y.X.; Lou, J.S.; Ji, L.T.; Bao, Y.T.; Xuan, L. Berberine improves inflammatory responses of diabetes mellitus in zucker diabetic fatty rats and insulin-resistant HepG2 cells through the PPM1B pathway. J. Immunol. Res. 2020, 2020, 2141508. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Cao, H.; Huan, Y.; Ji, W.; Liu, S.; Sun, S.; Liu, Q.; Lei, L.; Liu, M.; Gao, X.; et al. Berberine combined with stachyose improves glycometabolism and gut microbiota through regulating colonic microRNA and gene expression in diabetic rats. Life Sci. 2021, 284, 119928. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Li, J.; Dong, H.; Chen, G.; Qin, X.; Hu, M.; Yuan, F.; Fang, K.; Wang, D.; Jiang, S.; et al. Inhibitory effects of berberine on proinflammatory M1 macrophage polarization through interfering with the interaction between TLR4 and MyD88. BMC Complement. Altern. Med. 2019, 19, 314. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhao, Y.-B.; Wang, D.-K.; Zou, X.; Fang, K.; Wang, K.-F. Berberine relieves insulin resistance via the cholinergic anti-inflammatory pathway in HepG2 cells. J. Huazhong Univ. Sci. Technol. Med. Sci. 2016, 36, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Shen, N.; Huan, Y.; Shen, Z.-F. Berberine inhibits mouse insulin gene promoter through activation of AMP activated protein kinase and may exert beneficial effect on pancreatic β-cell. Eur. J. Pharmacol. 2012, 694, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Xin, T.; Li, D.; Wang, C.; Zhu, H.; Zhou, H. Therapeutic effect of Sirtuin 3 on ameliorating nonalcoholic fatty liver disease: The role of the ERK-CREB pathway and Bnip3-mediated mitophagy. Redox Biol. 2018, 18, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Lee, H.; Jung, C.H.; Choi, W.H.; Ha, T.Y. Zerumbone ameliorates high-fat diet-induced adiposity by restoring AMPK-regulated lipogenesis and microRNA-146b/SIRT1-mediated adipogenesis. Oncotarget 2017, 8, 36984–36995. [Google Scholar] [CrossRef]
- Chen, D.-L.; Yang, K.-Y. Berberine alleviates oxidative stress in islets of diabetic mice by inhibiting miR-106b expression and up-regulating SIRT1. J. Cell. Biochem. 2017, 118, 4349–4357. [Google Scholar] [CrossRef]
- Gong, M.; Duan, H.; Wu, F.; Ren, Y.; Gong, J.; Xu, L.; Lu, F.; Wang, D. Berberine Alleviates Insulin Resistance and Inflammation via Inhibiting the LTB4–BLT1 Axis. Front. Pharmacol. 2021, 12, 722360. [Google Scholar] [CrossRef]
- Johnson, A.M.F.; Hou, S.; Li, P. Inflammation and insulin resistance: New targets encourage new thinking. BioEssays 2017, 39, 1700036. [Google Scholar] [CrossRef]
- Sánchez-Galán, E.; Gómez-Hernández, A.; Vidal, C.; Martín-Ventura, J.L.; Blanco-Colio, L.M.; Muñoz-García, B.; Ortega, L.; Egido, J.; Tuñón, J. Leukotriene B4 enhances the activity of nuclear factor-κB pathway through BLT1 and BLT2 receptors in atherosclerosis. Cardiovasc. Res. 2009, 81, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, Q.; Xue, J.; Du, Y.; Liu, J.; Hong, Z. Vibrational Spectroscopic Investigation into Novel Ternary Eutectic Formed between Pyrazinamide, Fumaric Acid, and Isoniazid. ACS Omega 2020, 5, 17266–17274. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Zhao, Y.; Yang, X.; Han, H.; Ge, Y.; Zhang, M.; Zhang, H.; Zhang, M.; Chen, L. Berberine Potentiates Insulin Secretion and Prevents β-cell Dysfunction Through the miR-204/SIRT1 Signaling Pathway. Front. Pharmacol. 2021, 12, 720866. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.-J.; Lin, M.; Zhang, X.; Qin, L.-P. Combined Use of Astragalus Polysaccharide and Berberine Attenuates Insulin Resistance in IR-HepG2 Cells via Regulation of the Gluconeogenesis Signaling Pathway. Front. Pharmacol. 2019, 10, 1508. [Google Scholar] [CrossRef]
- Wang, Y.; Gong, W.; Lv, S.; Qu, H.; He, Y. Berberine improves insulin resistance in adipocyte models by regulating the methylation of hypoxia-inducible factor-3α. Biosci. Rep. 2019, 39, BSR20192059. [Google Scholar] [CrossRef] [PubMed]
- Yue, S.-J.; Liu, J.; Wang, A.-T.; Meng, X.-T.; Yang, Z.-R.; Peng, C.; Guan, H.-S.; Wang, C.-Y.; Yan, D. Berberine alleviates insulin resistance by reducing peripheral branched-chain amino acids. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E73–E85. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-H.; Li, X.-L.; Li, Q.; Fu, Y.; Yu, H.-J.; Sun, Y.-Q.; Zhang, L.; Shan, H.-L. Berberine alleviates ischemic arrhythmias via recovering depressed Ito and ICa currents in diabetic rats. Phytomedicine 2012, 19, 206–210. [Google Scholar] [CrossRef]
- Cole, L.K.; Sparagna, G.C.; Vandel, M.; Xiang, B.; Dolinsky, V.W.; Hatch, G.M. Berberine elevates cardiolipin in heart of offspring from mouse dams with high fat diet-induced gestational diabetes mellitus. Sci. Rep. 2021, 11, 15770. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, D.; Zhu, H.; Zhu, J.; Weng, S.; Dong, L.; Liu, T.; Hu, Y.; Shen, X. Berberine treatment increases Akkermansia in the gut and improves high-fat diet-induced atherosclerosis in Apoe−/− mice. Atherosclerosis 2018, 268, 117–126. [Google Scholar] [CrossRef]
- Shi, Y.; Hu, J.; Geng, J.; Hu, T.; Wang, B.; Yan, W.; Jiang, Y.; Li, J.; Liu, S. Berberine treatment reduces atherosclerosis by mediating gut microbiota in apoE−/− mice. Biomed. Pharmacother. 2018, 107, 1556–1563. [Google Scholar] [CrossRef]
- Li, G.; Xing, W.; Zhang, M.; Geng, F.-H.; Yang, H.; Zhang, H.; Zhang, X.; Li, J.; Dong, L.; Gao, F. Antifibrotic cardioprotection of berberine via downregulating myocardial IGF-1 receptor-regulated MMP-2/MMP-9 expression in diabetic rats. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H802–H813. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.-G.; Liang, L.; Zhang, Y.-B.; Wang, B.-F.; Bai, Y.-G.; Dai, Z.-J.; Xie, M.-J.; Wang, Z.-W. Berberine reduced blood pressure and improved vasodilation in diabetic rats. J. Mol. Endocrinol. 2017, 59, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zuo, J.; Chen, L.; Wei, Y. Combination of metformin and berberine represses the apoptosis of sebocytes in high-fat diet-induced diabetic hamsters and an insulin-treated human cell line. Cell Biochem. Funct. 2020, 38, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Bhutada, P.; Mundhada, Y.; Bansod, K.; Tawari, S.; Patil, S.; Dixit, P.; Umathe, S.; Mundhada, D. Protection of cholinergic and antioxidant system contributes to the effect of berberine ameliorating memory dysfunction in rat model of streptozotocin-induced diabetes. Behav. Brain Res. 2011, 220, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Adefegha, S.A.; Dada, F.A.; Oyeleye, S.I.; Oboh, G. Effects of berberine on cholinesterases and monoamine oxidase activities, and antioxidant status in the brain of streptozotocin (STZ)-induced diabetic rats. J. Basic. Clin. Physiol. Pharmacol. 2021, 33, 389–397. [Google Scholar] [CrossRef]
- Kim, S.O.; Kim, H.J. Berberine ameliorates cold and mechanical allodynia in a rat model of diabetic neuropathy. J. Med. Food. 2013, 16, 511–517. [Google Scholar] [CrossRef]
- Li, H.-Y.; Wang, X.-C.; Xu, Y.-M.; Luo, N.-C.; Luo, S.; Hao, X.-Y.; Cheng, S.-Y.; Fang, J.-S.; Wang, Q.; Zhang, S.-J.; et al. Berberine improves diabetic encephalopathy through the SIRT1/ER stress pathway in db/db mice. Rejuvenation Res. 2018, 21, 200–209. [Google Scholar] [CrossRef]
- Zhang, M.; Li, J.; Guo, X.; Wang, X.; Shi, D.; Cui, L.; Zhou, Y. Co-administration of berberine/gypenosides/bifendate ameliorates metabolic disturbance but not memory impairment in type 2 diabetic mice. Heliyon 2021, 7, e06004. [Google Scholar] [CrossRef]
- Liu, M.; Gao, L.; Zhang, N. Berberine reduces neuroglia activation and inflammation in streptozotocin-induced diabetic mice. Int. J. Immunopathol. Pharmacol. 2019, 33, 2058738419866379. [Google Scholar] [CrossRef]
- Ma, Y.-G.; Zhang, Y.-B.; Bai, Y.-G.; Dai, Z.-J.; Liang, L.; Liu, M.; Xie, M.-J.; Guan, H.-T. Berberine alleviates the cerebrovascular contractility in streptozotocin-induced diabetic rats through modulation of intracellular Ca2+ handling in smooth muscle cells. Cardiovasc. Diabetol. 2016, 15, 63. [Google Scholar] [CrossRef]
- Zhou, G.; Yan, M.; Guo, G.; Tong, N. Ameliorative effect of berberine on neonatally induced type 2 diabetic neuropathy via modulation of BDNF, IGF-1, PPAR-γ, and AMPK expressions. Dose Response 2019, 17, 1559325819862449. [Google Scholar] [CrossRef] [PubMed]
- Xuan, W.-T.; Wang, H.; Zhou, P.; Ye, T.; Gao, H.-W.; Ye, S.; Wang, J.-H.; Chen, M.-L.; Song, H.; Wang, Y.; et al. Berberine ameliorates rats model of combined Alzheimer’s disease and type 2 diabetes mellitus via the suppression of endoplasmic reticulum stress. 3 Biotech 2020, 10, 359. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Zuo, Z.; Yan, W.; Liu, W.; Zheng, Q.; Liu, X. Berberine ameliorates diabetic neuropathic pain in a rat model: Involvement of oxidative stress, inflammation, and μ-opioid receptors. Naunyn-Schmiedebergs Arch. Pharmacol. 2019, 392, 1141–1149. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, Y.; Qu, H.; Wang, Y. Methylation of HIF3A promoter CpG islands contributes to insulin resistance in gestational diabetes mellitus. Mol. Genet. Genom. Med. 2019, 7, e00583. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.; Zhou, Y.; Zhang, B.; Xue, B.; Zhao, Y. Berberine attenuates cerebral ischemia-reperfusion injury via activating PI3K-Akt signaling in a rat model of type 2 diabetes. Int. J. Clin. Exp. Med. 2017, 10, 16196–16202. [Google Scholar]
- Wei, S.; Zhang, M.; Yu, Y.; Lan, X.; Yao, F.; Yan, X.; Chen, L.; Hatch, G.M. Berberine Attenuates Development of the Hepatic Gluconeogenesis and Lipid Metabolism Disorder in Type 2 Diabetic Mice and in Palmitate-Incubated HepG2 Cells through Suppression of the HNF-4α miR122 Pathway. PLoS ONE 2016, 11, e0152097. [Google Scholar] [CrossRef] [PubMed]
- Geng, F.-H.; Li, G.-H.; Zhang, X.; Zhang, P.; Dong, M.-Q.; Zhao, Z.-J.; Zhang, Y.; Dong, L.; Gao, F. Berberine improves mesenteric artery insulin sensitivity through up-regulating insulin receptor-mediated signalling in diabetic rats. Br. J. Pharmacol. 2016, 173, 1569–1579. [Google Scholar] [CrossRef]
- Ding, B.; Geng, S.; Hou, X.; Ma, X.; Xu, H.; Yang, F.; Liu, K.; Liang, W.; Ma, G. Berberine reduces renal cell pyroptosis in golden hamsters with diabetic nephropathy through the Nrf2-NLRP3-Caspase-1-GSDMD pathway. Evid.-Based Complement. Altern. Med. 2021, 2021, 5545193. [Google Scholar] [CrossRef]
- Ma, Z.; Zhu, L.; Wang, S.; Guo, X.; Sun, B.; Wang, Q.; Chen, L. Berberine protects diabetic nephropathy by suppressing epithelial-to-mesenchymal transition involving the inactivation of the NLRP3 inflammasome. Ren. Fail. 2022, 44, 923–932. [Google Scholar] [CrossRef]
- Zhao, L.; Sun, L.-N.; Nie, H.-B.; Wang, X.-L.; Guan, G.-J. Berberine improves kidney function in diabetic mice via AMPK activation. PLoS ONE 2014, 9, e113398. [Google Scholar] [CrossRef]
- Xie, X.; Chang, X.; Chen, L.; Huang, K.; Huang, J.; Wang, S.; Shen, X.; Liu, P.; Huang, H. Berberine ameliorates experimental diabetes-induced renal inflammation and fibronectin by inhibiting the activation of RhoA/ROCK signaling. Mol. Cell. Endocrinol. 2013, 381, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Kumaş, M.; Eşrefoğlu, M.; Karataş, E.; Duymaç, N.; Kanbay, S.; Ergün, I.S.; Üyüklü, M.; Koçyiğit, A. Investigation of dose-dependent effects of berberine against renal ischemia/reperfusion injury in experimental diabetic rats. Nefrologia 2019, 39, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, H.; Zheng, M.; Yang, Y.; Ren, H.; Kong, Y.; Wang, S.; Wang, J.; Jiang, Y.; Yang, J. Berberine slows the progression of prediabetes to diabetes in Zucker diabetic fatty rats by enhancing intestinal secretion of glucagon-like peptide-2 and improving the gut microbiota. Front. Endocrinol. 2021, 12, 609134. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Chen, H.; Yan, L.; Wang, W.; Wang, D. Berberine alleviates type 2 diabetic symptoms by altering gut microbiota and reducing aromatic amino acids. Biomed. Pharmacother. 2020, 131, 110669. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Liu, S.; Zheng, X.; Chen, J.; Li, L.; Zhu, Z. Berberine promotes peri-implant osteogenesis in diabetic rats by ROS-mediated IRS-1 pathway. Biofactors 2021, 47, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhou, W.; Dang, Y.; Li, C.; Ji, G.; Zhang, L. Berberine compounds improves hyperglycemia via microbiome mediated colonic TGR5-GLP pathway in db/db mice. Biomed. Pharmacother. 2020, 132, 110953. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Ji, Y.; Yan, X.; Su, G.; Chen, L.; Xiao, J. Berberine attenuates apoptosis in rat retinal Müller cells stimulated with high glucose via enhancing autophagy and the AMPK/mTOR signaling. Biomed. Pharmacother. 2018, 108, 1201–1207. [Google Scholar] [CrossRef]
- Hao, M.H.; Li, S.-Y.; Sun, C.-K.; Xu, J.; Lin, Y.; Liu, K.-X.; Wang, L.; Li, C.-X.; Zhou, Q.; Du, J.-L.; et al. Amelioration effects of berberine on diabetic microendothelial injury model by the combination of high glucose and advanced glycation end products in vitro. Eur. J. Pharmacol. 2011, 654, 320–325. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Y.; Lam, K.S.; Li, Y.; Wong, W.T.; Ye, H.; Lau, C.-W.; Vanhoutte, P.M.; Xu, A. Berberine prevents hyperglycemia-induced endothelial injury and enhances vasodilatation via adenosine monophosphate-activated protein kinase and endothelial nitric oxide synthase. Cardiovasc. Res. 2009, 82, 484–492. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, C.-M.; Li, J.; Meng, Z.-J.; Wei, S.-N.; Li, J.; Bucala, R.; Li, Y.-L.; Chen, L. Berberine protects against palmitate-induced endothelial dysfunction: Involvements of upregulation of AMPK and eNOS and downregulation of NOX4. Mediat. Inflamm. 2013, 2013, 260464. [Google Scholar] [CrossRef]
- Hsu, Y.-Y.; Tseng, Y.-T.; Lo, Y.-C. Berberine, a natural antidiabetes drug, attenuates glucose neurotoxicity and promotes Nrf2-related neurite outgrowth. Toxicol. Appl. Pharmacol. 2013, 272, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Chen, X.; Liu, T.; Jiang, X.; Wu, Y.; Yang, S.; Hua, W.; Li, Z.; Huang, H.; Ruan, X.; et al. Berberine Protects Against Palmitate-Induced Apoptosis in Tubular Epithelial Cells by Promoting Fatty Acid Oxidation. Med. Sci. Moni. 2018, 24, 1484–1492. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Jiang, M.; Zhao, Y.; Gong, J.; Su, H.; Yuan, F.; Fang, K.; Yuan, X.; Yu, X.; Dong, H.; et al. Berberine protects against diabetic kidney disease via promoting PGC-1α-regulated mitochondrial energy homeostasis. Br. J. Pharmacol. 2020, 177, 3646–3661. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Li, J.; Xiong, F.; Huang, J.; Chen, C.; Liu, P.; Huang, H. Berberine attenuates high glucose-induced fibrosis by activating the G protein-coupled bile acid receptor TGR5 and repressing the S1P2/MAPK signaling pathway in glomerular mesangial cells. Exp. Cell Res. 2016, 346, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.; Zhang, M.; Li, J.; Meng, Z.; Wei, S.; Du, H.; Chen, L.; Hatch, G.M. Berberine improves insulin resistance in cardiomyocytes via activation of 5’-adenosine monophosphate-activated protein kinase. Metab. Clin. Exp. 2013, 62, 1159–1167. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.; Chen, L.; Hatch, G.M. Berberine treatment attenuates the palmitate-mediated inhibition of glucose uptake and consumption through increased 1,2,3-triacyl-sn-glycerol synthesis and accumulation in H9c2 cardiomyocytes. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2016, 1861, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Costantini, S.; Conte, C. Bone health in diabetes and prediabetes. World J. Diabetes 2019, 10, 421–445. [Google Scholar] [CrossRef]
- Fu, D.; Yu, J.Y.; Connell, A.R.; Yang, S.; Hookham, M.B.; McLeese, R.; Lyons, T.J. Beneficial Effects of Berberine on Oxidized LDL-Induced Cytotoxicity to Human Retinal Müller Cells. Investig. Ophthalmol. Vis. Sci. 2016, 57, 3369–3379. [Google Scholar] [CrossRef]
- Yu, J.Y.; Lyons, T.J. Modified Lipoproteins in Diabetic Retinopathy: A Local Action in the Retina. J. Clin. Exp. Ophthalmol. 2013, 4, 314. [Google Scholar] [CrossRef]
- Goh, S.-Y.; Cooper, M.E. Clinical review: The role of advanced glycation end products in progression and complications of diabetes. J. Clin. Endocrinol. Metab. 2008, 93, 1143–1152. [Google Scholar] [CrossRef]
- Libby, P.; Nathan, D.M.; Abraham, K.; Brunzell, J.D.; Fradkin, J.E.; Haffner, S.M.; Hsueh, W.; Rewers, M.; Roberts, B.T.; Savage, P.J.; et al. National Heart, Lung, and Blood Institute, National Institute of Diabetes and Digestive and Kidney Diseases Working Group on Cardiovascular Complications of Type 1 Diabetes Mellitus, Report of the National Heart, Lung, and Blood Institute-National Institute of Diabetes and Digestive and Kidney Diseases Working Group on Cardiovascular Complications of Type 1 Diabetes Mellitus. Circulation 2005, 111, 3489–3493. [Google Scholar] [CrossRef] [PubMed]
- Anselmi, L.; Huynh, J.; Duraffourd, C.; Jaramillo, I.; Vegezzi, G.; Saccani, F.; Boschetti, E.; Brecha, N.C.; De Giorgio, R.; Sternini, C. Activation of μ opioid receptors modulates inflammation in acute experimental colitis. Neurogastroenterol. Motil. 2015, 27, 509–523. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.X.; Edelstein, M.H.; Gafter, U.; Qiu, L.; Luo, Y.; Dobrinskikh, E.; Lucia, S.; Adorini, L.; D’Agati, V.D.; Levi, J.; et al. G Protein-Coupled Bile Acid Receptor TGR5 Activation Inhibits Kidney Disease in Obesity and Diabetes. J. Am. Soc. Nephrol. 2016, 27, 1362–1378. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.; Li, K.; Guan, F.; Yao, F.; Yu, Y.; Zhang, M.; Hatch, G.M.; Chen, L. Berberine pretreatment confers cardioprotection against ischemia–reperfusion injury in a rat model of type 2 diabetes. J. Cardiovasc. Pharmacol. Ther. 2016, 21, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.; Zhang, M.; Meng, Z.; Yu, Y.; Yao, F.; Hatch, G.M.; Chen, L. Berberine treatment prevents cardiac dysfunction and remodeling through activation of 5′-adenosine monophosphate-activated protein kinase in type 2 diabetic rats and in palmitate-induced hypertrophic H9c2 cells. Eur. J. Pharmacol. 2015, 769, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; DuGar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.-M.; et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Koren, O.; Spor, A.; Felin, J.; Fåk, F.; Stombaugh, J.; Tremaroli, V.; Behre, C.J.; Knight, R.; Fagerberg, B.; Ley, R.E.; et al. Human oral, gut, and plaque microbiota in patients with atherosclerosis. Proc. Natl. Acad. Sci. USA 2011, 108, 4592–4598. [Google Scholar] [CrossRef]
- Aydin, Ö.; Nieuwdorp, M.; Gerdes, V. The Gut Microbiome as a Target for the Treatment of Type 2 Diabetes. Curr. Diabetes Rep. 2018, 18, 55. [Google Scholar] [CrossRef]
- Pasterkamp, G.; Schoneveld, A.H.; Hijnen, D.J.; de Kleijn, D.P.; Teepen, H.; van der Wal, A.C.; Borst, C. Atherosclerotic arterial remodeling and the localization of macrophages and matrix metalloproteases 1, 2 and 9 in the human coronary artery. Atherosclerosis 2000, 150, 245–253. [Google Scholar] [CrossRef]
- Park, J.-G.; Ryu, S.Y.; Jung, I.-H.; Lee, Y.-H.; Kang, K.J.; Lee, M.-R.; Lee, M.-N.; Sonn, S.K.; Lee, J.H.; Lee, H.; et al. Evaluation of VCAM-1 antibodies as therapeutic agent for atherosclerosis in apolipoprotein E-deficient mice. Atherosclerosis 2013, 226, 356–363. [Google Scholar] [CrossRef]
- Wang, K.; Chen, Q.; Wu, N.; Li, Y.; Zhang, R.; Wang, J.; Gong, D.; Zou, X.; Liu, C.; Chen, J. Berberine ameliorates spatial learning memory impairment and modulates cholinergic anti-inflammatory pathway in diabetic rats. Front. Pharmacol. 2019, 10, 1003. [Google Scholar] [CrossRef]
- Wang, S.; Ren, H.; Zhong, H.; Zhao, X.; Li, C.; Ma, J.; Gu, X.; Xue, Y.; Huang, S.; Yang, J.; et al. Combined berberine and probiotic treatment as an effective regimen for improving postprandial hyperlipidemia in type 2 diabetes patients: A double blinded placebo controlled randomized study. Gut Microbes 2022, 14, 2003176. [Google Scholar] [CrossRef]
- Dai, P.; Wang, J.; Lin, L.; Zhang, Y.; Wang, Z. Renoprotective effects of berberine as adjuvant therapy for hypertensive patients with type 2 diabetes mellitus: Evaluation via biochemical markers and color Doppler ultrasonography. Exp. Ther. Med. 2015, 10, 869–876. [Google Scholar] [CrossRef]
- Harrison, S.A.; Gunn, N.; Neff, G.W.; Kohli, A.; Liu, L.; Flyer, A.; Goldkind, L.; Di Bisceglie, A.M. A phase 2, proof of concept, randomised controlled trial of berberine ursodeoxycholate in patients with presumed non-alcoholic steatohepatitis and type 2 diabetes. Nat. Commun. 2021, 12, 5503. [Google Scholar] [CrossRef]
- Chen, L.; Lu, W.; Li, Y. Berberine ameliorates type 2 diabetes via modulation of Bifidobacterium species, tumor necrosis factor-α, and lipopolysaccharide. Int. J. Clin. Exp. Med. 2016, 9, 9365–9372. [Google Scholar]
- Kulkarni, S.K.; Dandiya, P.C.; Varandani, N.L. Pharmacological investigations of berberine sulphate. Jpn. J. Pharmacol. 1972, 22, 11–16. [Google Scholar] [CrossRef]
- Lampe, D. Waste watch. Nat. Civ. Rev. 1992, 81, 192–194. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Zamani Taghizadeh Rabe, S.; Balali-Mood, M.; Karimi, G.; Memar, B.; Rahnama, M.; Tabasi, N.; Khazaee, M.; Riahi-Zanjani, B. Immunotoxicity induced in mice by subacute exposure to berberine. J. Immunotoxicol. 2016, 13, 255–262. [Google Scholar] [CrossRef]
- Li, K.; Yao, W.; Zheng, X.; Liao, K. Berberine promotes the development of atherosclerosis and foam cell formation by inducing scavenger receptor A expression in macrophage. Cell Res. 2009, 19, 1006–1017. [Google Scholar] [CrossRef]
- Ning, N.; Wang, Y.Z.; Zou, Z.Y.; Zhang, D.Z.; Wang, D.Z.; Li, X.G. Pharmacological and safety evaluation of fibrous root of Rhizoma Coptidis. Environ. Toxicol. Pharmacol. 2015, 39, 53–69. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.Y.; Zhou, S.W.; Zhang, K.B.; Tang, J.L.; Guang, L.X.; Ying, Y.; Xu, Y.; Zhang, L.; Li, D.D. Chronic effects of berberine on blood, liver glucolipid metabolism and liver PPARs expression in diabetic hyperlipidemic rats. Biol. Pharm. Bull. 2008, 31, 1169–1176. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Sharma, B. Toxicological Effects of Berberine and Sanguinarine. Front. Mol. Biosci. 2018, 5, 21. [Google Scholar] [CrossRef] [PubMed]
- Kwon, I.H.; Choi, H.S.; Shin, K.S.; Lee, B.K.; Lee, C.K.; Hwang, B.Y.; Lim, S.C.; Lee, M.K. Effects of berberine on 6-hydroxydopamine-induced neurotoxicity in PC12 cells and a rat model of Parkinson’s disease. Neurosci. Lett. 2010, 486, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.-F.; Shen, L. Molecular basis of inhibitory activities of berberine against pathogenic enzymes in Alzheimer’s disease. Sci. World J. 2012, 2012, 823201. [Google Scholar] [CrossRef]
- Philogène, B.J.; Arnason, J.T.; Towers, G.H.; Abramowski, Z.; Campos, F.; Champagne, D.; McLachlan, D. Berberine: A naturally occurring phototoxic alkaloid. J. Chem. Ecol. 1984, 10, 115–123. [Google Scholar] [CrossRef]
- Huang, K.; Hu, G.; Wang, R.; Zeng, Q.; Li, W.; Zou, H.; Wu, S.; Wang, G.; Li, M. In Vitro Assessment of Berberine against Ichthyophthirius multifiliis in Goldfish. Pathogens 2022, 11, 1207. [Google Scholar] [CrossRef]
| Type of Extract or Constituent | Cell Line | Results | Ref. |
|---|---|---|---|
| BBR | 3T3-L1 adipocytes and L6 myoblasts | Inhibited triglyceride accumulation in fully differentiated and undifferentiated adipocytes ↓ Adipogenic gene expression and levels of most lipogenic transcripts ↓ PPARs, CCAAT/enhancer binding proteins (C/EBPs), 11beta-hydroxysteroid dehydrogenase 1 (11β-HSD1), and aP2 ↑ AMPK and ACC in both adipocytes and myoblasts ↑ GLUT4 translocation in myoblasts | [12] |
| BBR | Neonatal rat cardiomyocytes exposed to hypoxia/reoxygenation with elevated glucose levels | ↓ Myocardial cell death ↑ Bcl-2/Bax ratio ↓caspase-3 Activated phosphoinositide 3-kinase (PI3K)–Akt and AMPK and eNOS phosphorylation | [13] |
| BBR with Metformin | High-glucose-induced HepG2 cell line | ↓ Total lipid content and triglyceride synergistic effects ↓ FAS and SREBP-1c | [14] |
| BBR | High-glucose-induced H9C2 cell | Reduced H9C2 cell line hypertrophy Promoted mitogenesis and destroyed damaged mitochondria Restored autophagic flux in damaged cardiomyocytes ↑AMPK | [15] |
| Type of Extract or Constituent | Dose/Concentration | Study Model | Results | Ref. |
|---|---|---|---|---|
| Berberine chloride | 50 mg/kg/day; orally for 45 days | STZ-induced diabetic rats | ↓ Blood glucose and HbAlc ↑ Plasma insulin, hemoglobin, and body weight ↑ Pancreatic levels of SOD, CAT, GPx, GSH, vitamin E, and vitamin C ↓ LOOH and TBARS ↓ TNF-a, NF-kB, phospho-NF-kB-p65, COX-2, and iNOS ↓ Caspase-8, t-Bid, Bax, cytochrome-c, and cleaved caspase-3 ↑ Bcl-2 ↑ Anti-inflammatory mediator IL-10 and GLUT-2 | [16] |
| 187.5 and 562.5 mg/kg; orally for 4 weeks | STZ-induced DM in rats | ↓ FBG, TGs, TC, FFAs, and apolipoprotein B ↑ HDL and apolipoprotein AI | [17] | |
| 100 mg/Kg per day; intragastrically for 6 weeks 10 mg/Kg/d; intraperitoneally for 4 weeks | STZ-induced DM in mice | ↓ FINS, HOMA-IR, and FPG, and expression of TLR4, TNF-α, IL-1β and IL-6 ↓ Pathological damage and macrophage (MΦ) infiltration in pancreatic islets of diabetic mice Regulated the probiotics in the intestinal tract Blocked the nuclear translocation of NF-κB in THP-1-derived MΦs | [18] | |
| 156 mg/kg per day; intragastrically for 12 weeks | STZ-induced DM in rats | ↓ FINS, HOMA-IR, hyperlipidemia ↑ p-TORC2 levels Up-regulated expression of liver kinase B1, AMPK, and phosphorylated AMPK Down-regulated expression of the key gluconeogenic enzymes Inhibited TORC2 nuclear translocation in the liver tissues | [19] | |
| Diabetic rats: 75 and 150 mg/kg/day; orally twice a day for 15 days Diabetic mice: 200 mg/kg/day; orally for 3 weeks | STZ-induced DM in rats and KK-Ay diabetic mice | ↓ FBG and FINS ↑ Expression of insulin receptor mRNA and PKC | [20] | |
| 150 mg/kg/d; orally for 9 weeks | STZ-induced T2D hamsters | ↑ Expression of LXRs and PPARs ↓ Expression of SREBPs in visceral white adipose tissue ↓ Body weight, total visceral white adipose tissue weight, blood glucose, FFAs, TC, LDL-c, and TGs ↑ Serum adiponectin ↓ Serum leptin, TNF-a, IL-6, and HOMA-IR ↓ Adipocyte size | [21] | |
| 100 mg/kg/d; orally for 7 weeks | STZ-induced diabetic rats | ↓ FBG, plasma-free fatty acids, CRP, TGs, and TC Improved glucose tolerance Inhibited DPP-4 and PTP-1B activities Moderately improved glucose homeostasis | [22] | |
| 5 mg/kg/day; intraperitoneally for 3 weeks | ob/ob and STZ-induced diabetic mice | Improved insulin, glucose tolerance, and glucose metabolism ↓ Blood glucose levels, cAMP, hepatic gluconeogenesis, and gluconeogenic gene expression Suppressed glucagon-induced CREB phosphorylation | [23] | |
| 5 mg/kg/day; intraperitoneally for 3 weeks | db/db mice | ↓ Body weight ↓ Fat mass and the size of fat cells Food intake did not change Improved glucose tolerance | [12] | |
| 100 mg/kg/d; orally for 2 weeks | db/db mice | Improved insulin resistance ↓ FBG Suppressed protein tyrosine phosphatase 1B ↑ Phosphorylation of insulin receptor, insulin receptor substrate1, and Akt | [24] | |
| Berberine 100mg/kg/d and Berberine 100 mg/kg/d+ stachyose 200 mg/kg/d; orally for 55 days | db/db mice | Improved glucose metabolism, the balance of α- and β-cells, and mucin-2 expression Increased abundance of Akkermansia muciniphila ↑ Fumaric acid level ↓ Metabolite all-transheptaprenyl diphosphate | [25] | |
| 300 mg/kg/day; orally for 12 weeks | Alloxan-induced diabetic mice with renal injury | ↓ NF-κB, and the ↑ IκB-α ↓ Levels of fibronectin, transforming growth factor-beta 1, and intercellular adhesion molecule-1 | [26] | |
| 380 mg/day; orally for 2 weeks | HFD-fed rats | ↓ Body weight, plasma triglycerides, and insulin resistance | [12] | |
| Dihydroberberine | 100 mg/kg/day; orally for 2 weeks | HFD-induced insulin resistance in mice and rat | Improved effectiveness of BBR Better oral bioavailability than BBR ↓ Augmented adiposity, TGs, and insulin resistance | [27] |
| 5, 10 mg/kg/day; intraperitoneal injections for 4 weeks | HFD-fed mice | ↓ Insulin resistance, body weight, and HOMA-IR ↑ Synthesis of liver glycogen and SIRT1 expression Regulated SIRT1/FOXO1 pathway | [28] | |
| 100 mg/kg/day; orally for 4 weeks | Mitochondria isolated from the liver of HFD-fed rats | ↑ Mitochondrial SirT3 activity Improved mitochondrial function Prevented a state of energetic deficit | [29] | |
| Berberine | RB 0.7 (RB-L), 2.11 (RB-M), or 6.33 mg/kg/day (RB-H); orally for 8 weeks | High-sugar, high-fat diet (HSHFD)-induced diabetic KKAy mice | Improved glucolipid metabolism, insulin resistance, OGTT, insulin tolerance test (ITT), and pathological changes in the pancreases and livers of mice ↓ FBG, white fat index, TGs, LDL, GIP, and insulin level ↑ GLP-1, HDL, and glycogen content in the liver and muscle ↑ p-PI3K and p-AKT levels ↓ TXNIP expression | [30] |
| 150 and 300 mg/kg/day; gavage for 12 weeks | HFD-fed rats | ↓ Body weight, urine volume, FBG, BUN, cholesterol, hepatic index levels, pathologic changes, and IR Improved albumin levels, glucose consumption, uptake, and inflammation ↑ Expression of PPM1B, PPARγ, LRP1, GLUT4, IRS-1, IRS-2, PI3K, AKT, and IKKβ Inhibited the phosphorylation of pIKKβ Ser181, total IKKβ, NF-κB p65, and JNK | [31] | |
| Berberine 100 mg/kg/d for 30 days then 150 mg/kg/d; berberine combined with stachyose; BBR 100 mg/kg/d + stachyose 200 mg/kg/d for 30 days then BBR 150 mg/kg/d + 300 mg/kg/d; 69 days in total | Zucker diabetic fatty rats | ↓ Blood glucose Improved impaired glucose tolerance ↑ Abundance of beneficial Akkermansiaceae, ↓ Abundance of pathogenic Enterobacteriaceae, Desulfovibrionaceae, and Proteobacteria ↓ Expression of intestinal Egr1 and Hbegf ↑ Expression of miR-10a-5p (just combination therapy) | [32] |
| Constituent | Dose | Study Model | Results | Ref. |
|---|---|---|---|---|
| BBR | 100 mg/kg; orally for 7 days | STZ-induced ischemic arrhythmias | Shortened prolonged QTc interval Returned the diminished K+ current and L-type Ca2+ current to their normal states | [47] |
| BBR | 60 mg/kg/day; intragastrically for 14 days | STZ-induced ischemic arrhythmias | Increased K+ current and current density Increased Kir2 | [10] |
| BBR | 160 mg/kg/day; orally for 12 weeks | Lean and GDM-exposed mice offspring | ↑ Cardiolipin remodeling enzyme tafazzin, tetra linoleoyl-cardiolipin, total cardiac cardiolipin ↓ NEFA, TGs, and ketones | [48] |
| Berberine chloride hydrate | 0.5 g/L; added to drinking water for 14 weeks | Apoe−/− HFD-fed mice | ↓ Akkermansia spp. and Bacteroides ↓ TNF-α and IL-1β Increased colonic mucus layer thickness ↓ TC, LPS, VCAM-1, and MMP-2 ↑ ZO-1 and Occludin in the ileum and colon, respectively | [49] |
| BBR | 50 mg/kg twice weekly; intragastrically for 12 weeks | BBR-treated Apoe−/− HFD-fed mice cohoused with non-BBR-treated Apoe−/− HFD-fed mice | ↓ FMO3 and TMAO Changed the abundance of Firmicutes and Verrucomicrobia | [50] |
| BBR | 200 mg/kg/day; orally for 4 weeks | STZ-induced cardiac dysfunction | Ameliorated cardiac fibrosis and dysfunction ↓ IGF-1R, MMP-2/MMP-9, alpha-smooth muscle actin, and collagen type I | [51] |
| BBR | 50, 100, and 200 mg/kg/day; intragastrically for 8 weeks | STZ-induced hypertension | ↓ Serum glucose and blood pressure Improved vascular relaxation Up-regulated expression of BKca | [52] |
| BBR | 50, 100 mg/kg; orally for 6 weeks | High-fat diet-induced diabetic hamsters | Reduced susceptibility to cardiovascular complications of diabetes ↓ Body weight, insulin, and glucose level Inhibited hepatic fat accumulation Increased glucose tolerance | [53] |
| BBR | 187.5 mg/Kg/d; intragastrically | STZ-induced cognitive decline | Down-regulated PI3K/Akt/mTOR and MAPK signaling pathway ↓ PKCη, PKCε, translocation of NF-κB, amyloid precursor protein, BACE-1, and Aβ42 ↑ GLUT3 and glucose uptake in the brain | [9] |
| BBR | 25–100 mg/kg; orally twice daily for 30 days | STZ-induced memory dysfunction | ↓ Hyperglycemia, oxidative stress, and AChE activity Improved cognitive performance, learning, and memory | [54] |
| BBR | 50 and 100 mg/kg; orally for 14 days | STZ-induced impaired neurochemicals | Restore impaired neurochemicals ↓ AChE, BChE, MAO activities, and MDA ↑ SOD, GPx activities, and GSH | [55] |
| BBR | 5, 10, and 20 mg/kg; intraperitoneally, single and repeated treatment (twice daily for 14 days) | STZ-induced neuropathy | ↓ MDA, SOD, catalase, and GPx activities | [56] |
| BBR | 50 mg/kg; orally for 10 weeks | db/db mice with encephalopathy | Improved learning and memory ability ↑ HDL, PSD95, SYN, NGF, and SIRT1 ↓ Body weight, FBG, TNF-α, NF-κB, TGs, TC, and LDL Down-regulated PERK, IRE-1α, eIF-2α, PDI, and CHOP | [57] |
| BBR | 200 mg/kg/day; orally for 4 weeks | STZ-induced diabetic rats with cerebral ischemia/reperfusion injury | ↑ Expression of PI3K, p-Akt, and Bcl-2 ↓ Cerebral infarct volume and cell apoptosis of cerebral infarct area ↓ NO and MDA ↓ Expression of Caspase-3 and Bax ↑ SOD | [58] |
| BBR | Dosage not mentioned; s.c. injection | STZ-induced neuropathy | Reduced neuropathy pain Suppressed the activation of microglia and astrocytes in the spinal cord ↓ Expression of TNF-α, IL-6, IL-1β, iNOS, and COX-2 | [59] |
| BBR | 50, 100, and 200 mg/kg/day; intragastrically for 8 weeks | STZ-induced vascular dysfunction | ↓ FBG, the augmented contractile function of the cerebral artery to KCl and 5-HT, Ca2+ channel current densities, α1C-subunit expressions of Ca2+ channels, and resting intracellular Ca2+ level ↓ Ca2+ release from RyRs in cerebral VSMCs | [60] |
| BBR | 187.75 mg/kg/day | STZ-induced cognitive impairment | Improved spatial learning memory Up-regulated α7nAchR expression ↓ AChE activity, inflammation, CSF/blood glucose, and Aβ | [61] |
| BBR | 150 mg/kg; for 4 weeks | STZ-induced diabetic Alzheimer’s | Restored the disordered arrangement of nerve cells and damage to neurons ↓ GRP78, CHOP, procaspase-12, procaspase-9, and procaspase-3 in the hippocampus ↓ FBG, TGs, TC, glycosylated serum protein levels, Aβ, and apoptosis rate | [62] |
| BBR | 5, 20, and 40 mg/kg/day; i.p. for 10 weeks | STZ-induced neuropathic pain | Increased mechanical and thermal nociception threshold ↓ ROS and MDA ↑ Catalase activity ↓ TNF-α and IL-6 Up-regulated expression of MOR | [63] |
| BBR | BBR 10 mg/kg+ gypenosides, 1 mg/kg+ bifendate 0.3 mg/kg; intragastrically for 14 weeks | db/db and STZ-induced diabetic mice | ↓ FBG, body weight, TGs, LDL No positive effects on memory impairment Synergistic effect | [64] |
| BBR | 10, 20, and 40 mg/kg; PO for 8 weeks | STZ-induced painful diabetic peripheral polyneuropathy | ↓ FBG, food intake, water intake, urine output, hepatic cholesterol, TGs, MDA, NO, glycosuria, aldose reductase, glycated Hb, oxide-nitrosative stress and pulse Ox levels, TNF-α, IL-1β, and IL-6 ↑ Body weight, serum insulin, pulse Ox, SOD, GSH, thermal hyperalgesia, motor nerve conduction velocity (MNCV), sensory nerve conduction velocity (SNCV), BDNF, IGF-1, and PPAR-γ ↑ Thr-172 expression ↓ PP2C-α expression ↓ Necrosis, edema, infiltration of inflammatory cells, congestion in the sciatic nerve, and atrophy in myelinated axons | [65] |
| BBR | 50 and 100 mg/kg; orally 0.2 and 0.4 μg/kg; ocular delivery for 12 weeks | Type 1 (STZ-induced) and type 2 (db/db) diabetic retinopathy mice treated with insulin | ↓ VEGF and HIF-1α Inhibited the Akt/mTOR pathway in insulin-treated retina endothelial cells Inhibited progression of retinopathy in types I and II diabetes | [8] |
| BBR | In vivo: 40, 160 mg/kg; orally for 4 weeks 2 | STZ-induced hepatic damage | ↓ TC, TGs, LDL, AST, ALT, FBG, ISI, HNF-4α, miR122, PEPCK, G6Pase, FAS-1, and ACCα ↑ HDL, FINS, and CPT1 Attenuated hepatic gluconeogenesis and lipid metabolism disorder | [66] |
| BBR | In vivo: 200 mg/kg/day; gavage for 4 weeks Ex vivo: 2.5–10 μM | STZ-induced endothelial dysfunction | Improved mesenteric arteries’ insulin sensitivity Improved endothelium-mediated vasodilatation Up-regulated insulin receptor-mediated signaling Synergistic effects between insulin and berberine ↑ Phosphorylation of InsR, AMPK, Akt, and eNOS | [67] |
| BBR | 200 mg/kg/day; for 10 weeks | HFD-fed mice | Improved insulin resistance Reduced the abundance of the bacteria that produce BCAAs Activated BCKDC ↓ Phosphorylation state of BCKDHA and BCKDK in the liver and epididymal white adipose tissues | [46] |
| BBR | 100 and 200 mg/kg | STZ-induced diabetic nephropathy hamster | ↓ Blood glucose, blood lipids, IL-1β, IL-6, NLRP3, Caspase-1, GSDMD, MDA, and the number of TUNEL-positive cells ↑ Nrf2 expression Improved NLRP3-Caspase-1-GSDMD signaling Inhibited diabetic nephropathic damage | [68] |
| BBR | 150 mg/kg/d orally; for 12 weeks | STZ-induced diabetic kidney disease | ↓ Microalbumin and renal pathologic changes and EMT Down-regulated NLRP3 | [69] |
| BBR | 200 mg/kg/day; for 8 weeks | STZ-induced diabetic nephropathy mice | Attenuated diabetic nephropathy Activated AMPK signaling pathway | [70] |
| BBR | 200 mg/kg/day; intragastrically for 12 weeks | Diabetic rat kidneys | Suppressed RhoA/ROCK signaling ↓ NF-κB ↓ Intercellular adhesion molecule-1, transforming growth factor-beta 1, and fibronectin | [71] |
| BBR | 50, 100, and 150 mg/kg; orally for 14 days | STZ-induced renal ischemic injury | ↓ BUN, creatinine, and LDH ↑ Ca2+-ATPase and Na+/K+-ATPase enzyme activities Antioxidant, anti-inflammatory, and antiapoptotic effects | [72] |
| Berberine hydrochloride | 100 mg/kg/d; gavage for 3 weeks | Zucker diabetic fatty rats | ↓ Food intake, FBG, insulin resistance, and LPS ↑ Fasting GLP-2, glutamine-induced intestinal GLP-2 secretion, goblet cell number, and villi length ↑ Mucin, occludin, and ZO-1 ↓ TLR-4, NF-κB, and TNF-α | [73] |
| BBR | 200 mg/kg/day; intragastrically for 6 weeks | STZ-induced diabetic rats | ↑ Bacteroidetes and Lactobacillaceae ↓ Proteobacteria and Verrucomicrobia ↓ Aromatic amino acids, such as tyrosine, tryptophan, and phenylalanine | [74] |
| BBR | 120 mg/kg/day; orally for 4 weeks | STZ-induced osteoporosis | Improved glucose and bone metabolism | [75] |
| BBR | BBR (210 mg/kg) BBR (210 mg/kg) + oryzanol (33.6 mg/kg) + vitamin B6 (7 mg/kg); orally (1 mL/100 g body weight) for 4 weeks | Diabetes-induced gut microbiota alteration db/db mice | ↓ FBG, HbA1c ↑ Bacteroidaceae and Clostridiaceae ↑ DCA, TGR5, GLP, and glucose, lipid, and energy metabolism | [76] |
| Study Model | Results | Ref. |
|---|---|---|
| H9C2 cell line | ↓ High-glucose-induced hypertrophy Improved mitochondrial function Promoted mitogenesis Activated AMPK signaling Restored autophagic flux | [15] |
| AML12 hepatocytes and 3T3-L1 adipocytes | ↓ BCAAs | [46] |
| High-glucose-induced BMSCs cell line | Increased osteogenesis Up-regulated ROS-mediated IRS-1 signaling pathway | [75] |
| Palmitate-incubated HepG2 cells | ↓ HNF-4α, miR122, PEPCK, G6Pase, FAS-1, and ACCα ↑ CPT1 | [66] |
| High-glucose-induced rat retinal Müller cells | Reduced apoptosis Increased autophagy ↓ Expression of Bax and caspase-3 ↑ Expression of Bcl-2 ↑ Beclin-1 and LC3II ↑ AMPK/mTOR signaling pathway | [77] |
| In vitro model of high-glucose-AGE-induced micro-endothelial injuries | ↑ Thrombomodulin, NOS, and NO Inhibited AGEs formation | [78] |
| High-glucose-induced endothelial dysfunction in endothelial cells and blood vessels isolated from rat aorta | ↑ eNOS and NO ↓ ROS, cellular apoptosis, NF-κB, and expression of adhesion molecules Inhibited monocyte attachment to endothelial cells Increased endothelium-dependent vasodilatation Activated AMPK | [79] |
| Palmitate-induced endothelial dysfunction in human umbilical vein endothelial cells (HUVECs) | ↑ Expression of eNOS ↓ Expression of NOX4 ↑ Expression of AMPK and p-AMPK ↑ NO ↓ ROS | [80] |
| High-glucose-induced SH-SY5Y human neuroblastoma cells | ↑ Nrf2, HO-1 and NGF Inhibited neuronal apoptosis ↓ Cytochrome c and ROS ↑ Bcl-2 expression and IGF-1/Akt/GSK-3β signaling pathway | [81] |
| High-glucose-induced HK-2 cells | ↓ EMT Down-regulated NLRP3 | [69] |
| Palmitate-induced lipid accumulation and apoptosis in HK-2 cells | ↑ CPT1A, PPARα, and PGC1α Reversed intracellular lipid accumulation and apoptosis Promoted fatty acid oxidation | [82] |
| Palmitic acid-induced cultured podocyte | Improved podocyte damage Inhibited lipid accumulation, excessive production of mitochondrial ROS, mitochondrial dysfunction, and deficient fatty acid oxidation Restored PGC-1α | [83] |
| High-glucose-induced renal fibrosis | Suppressed RhoA/ROCK signaling ↓ NF-κB ↓ Fibronectin overexpression ↓ Excessive reactive oxygens | [71] |
| High-glucose-induced renal fibrosis | Activated TGR5 Inhibited S1P2/MAPK signaling ↓ Fibronectin, ICAM-1, and TGF-β1 Down-regulated phosphorylation level of c-Jun/c-Fos | [84] |
| Insulin-resistant rat H9c2 cardiomyocyte | Reduced insulin resistance Increased glucose consumption and glucose uptake Activated AMPK | [85] |
| Palmitate-induced insulin-resistant H9c2 cardiomyocytes | ↑ Glucose uptake and consumption Activated AKT ↑ GLUT-4 ↓ DAG and TAG hydrolysis ↑ TAG and expression of DAG acyltransferase-2 Increased palmitic acid, [1,3-3H] glycerol, and [1-14C] glucose incorporation into TAG and decreased their incorporation into DAG | [86] |
| Insulin-treated human sebocytes (SEB-1) | Inhibited sebocyte apoptosis Reduced susceptibility to cardiovascular complications of diabetes ↓ Expression of BIK protein | [53] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Askari, V.R.; Khosravi, K.; Baradaran Rahimi, V.; Garzoli, S. A Mechanistic Review on How Berberine Use Combats Diabetes and Related Complications: Molecular, Cellular, and Metabolic Effects. Pharmaceuticals 2024, 17, 7. https://doi.org/10.3390/ph17010007
Askari VR, Khosravi K, Baradaran Rahimi V, Garzoli S. A Mechanistic Review on How Berberine Use Combats Diabetes and Related Complications: Molecular, Cellular, and Metabolic Effects. Pharmaceuticals. 2024; 17(1):7. https://doi.org/10.3390/ph17010007
Chicago/Turabian StyleAskari, Vahid Reza, Kimia Khosravi, Vafa Baradaran Rahimi, and Stefania Garzoli. 2024. "A Mechanistic Review on How Berberine Use Combats Diabetes and Related Complications: Molecular, Cellular, and Metabolic Effects" Pharmaceuticals 17, no. 1: 7. https://doi.org/10.3390/ph17010007
APA StyleAskari, V. R., Khosravi, K., Baradaran Rahimi, V., & Garzoli, S. (2024). A Mechanistic Review on How Berberine Use Combats Diabetes and Related Complications: Molecular, Cellular, and Metabolic Effects. Pharmaceuticals, 17(1), 7. https://doi.org/10.3390/ph17010007








