Isoliquiritigenin Inhibits the Growth of Colorectal Cancer Cells through the ESR2/PI3K/AKT Signalling Pathway
Abstract
:1. Introduction
2. Results
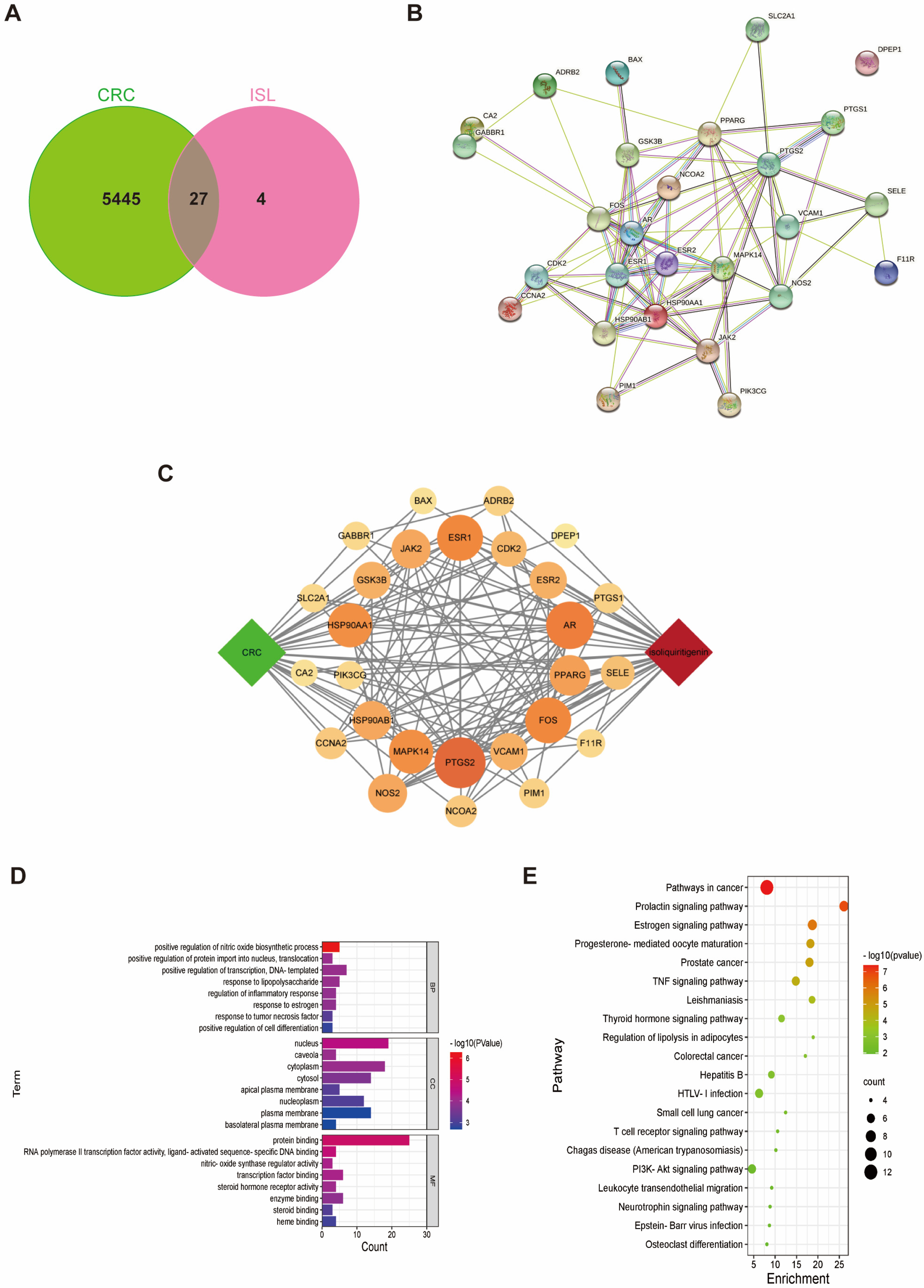
2.1. Target Prediction of ISL for Prevention and Treatment of CRC
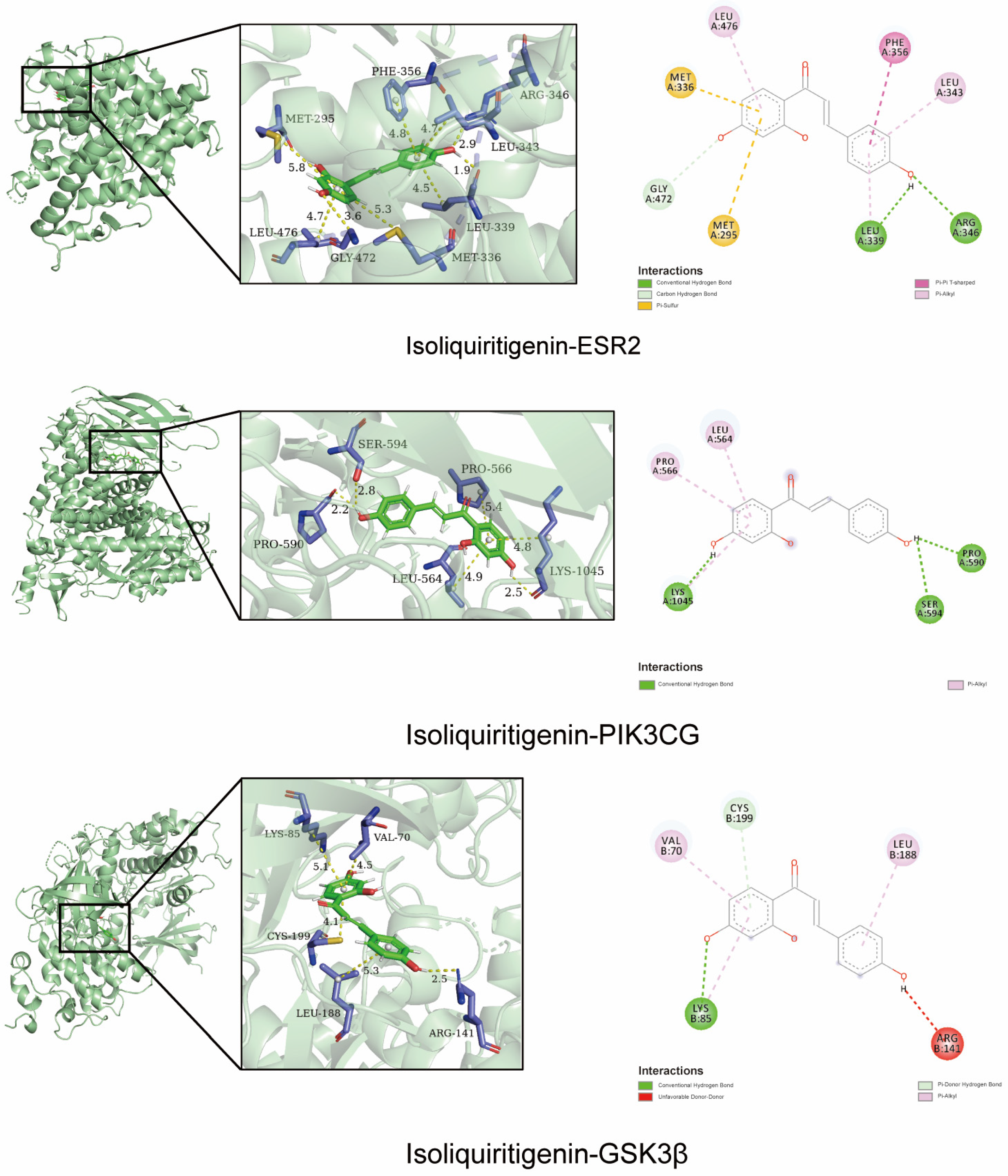
2.2. Molecular Docking Validation of the Predicted Key Targets
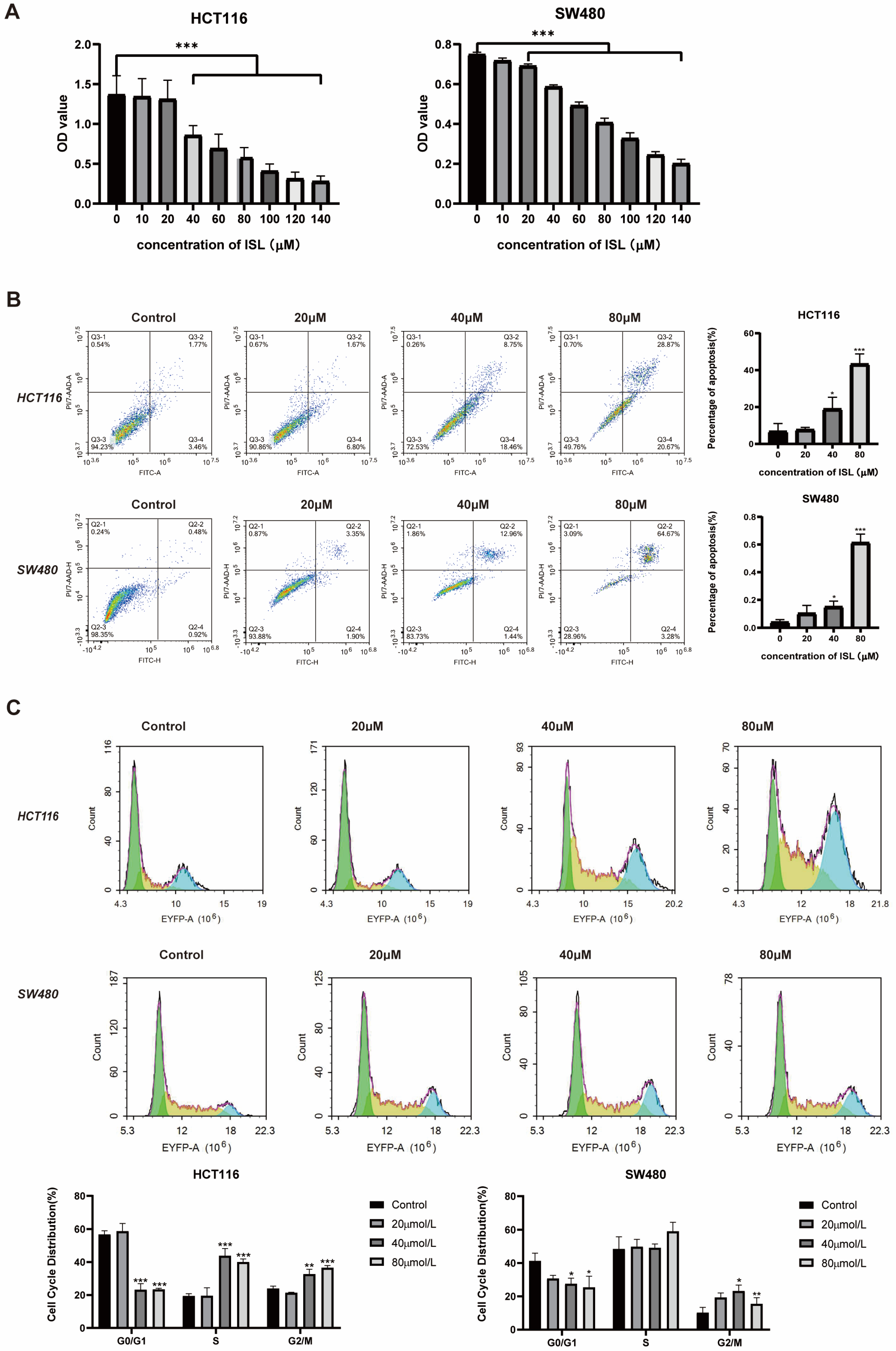
2.3. ISL Inhibits CRC Cell Viability, Induces Cell Apoptosis and Blocks Cell Cycle
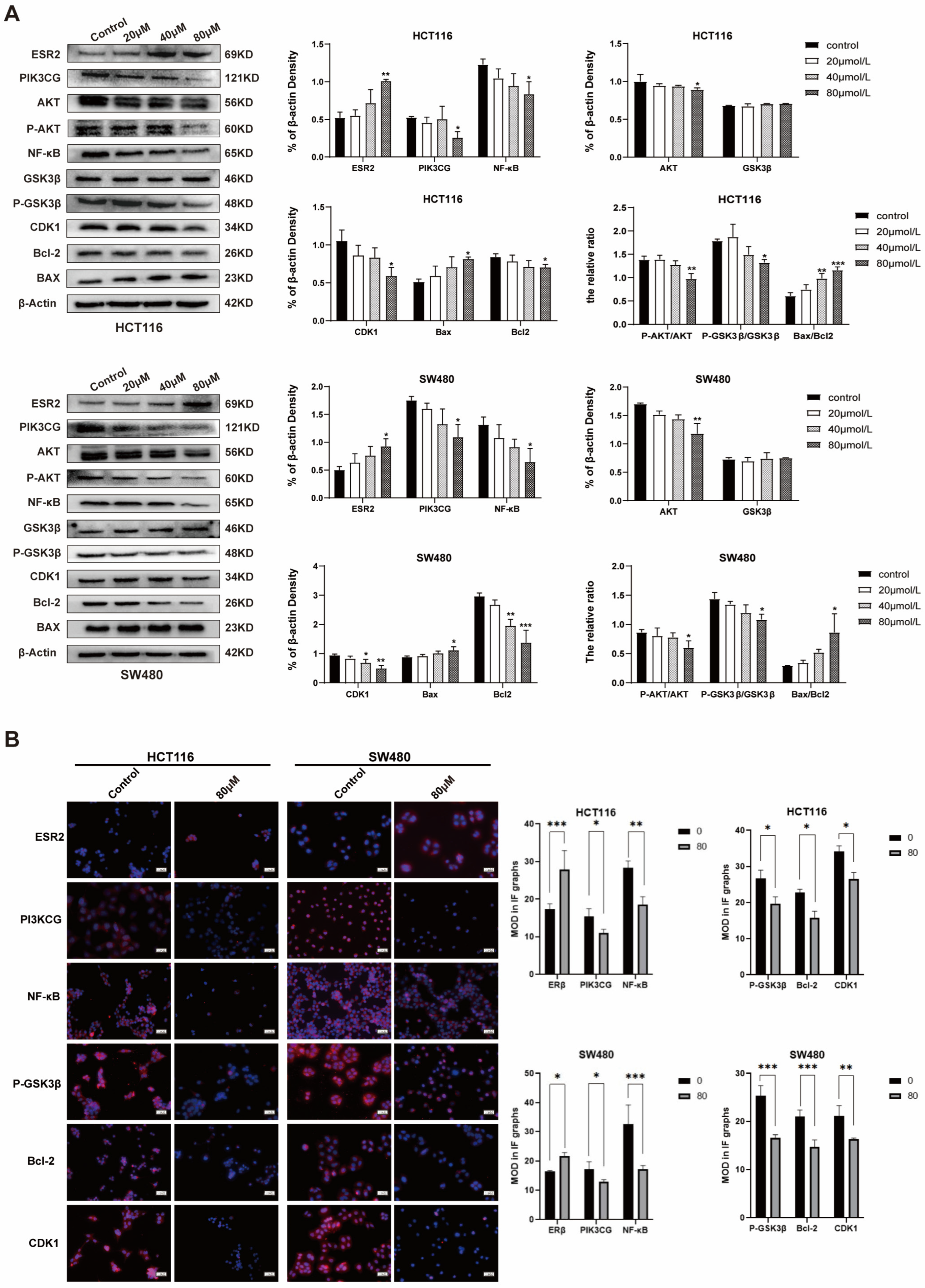
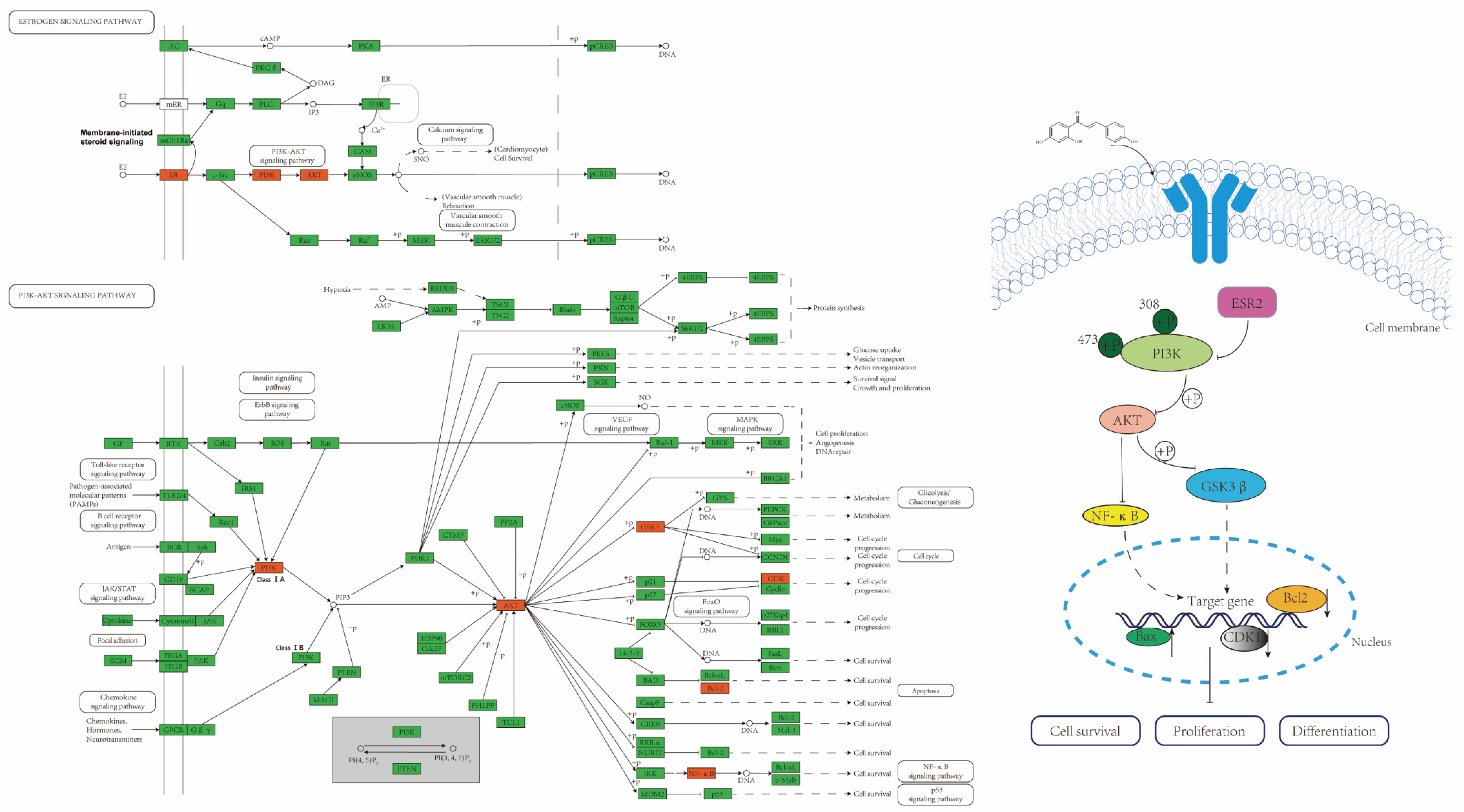
2.4. ISL Up-Regulates ESR2 and Inhibits the PI3K/AKT Signalling Pathway in CRC Cells
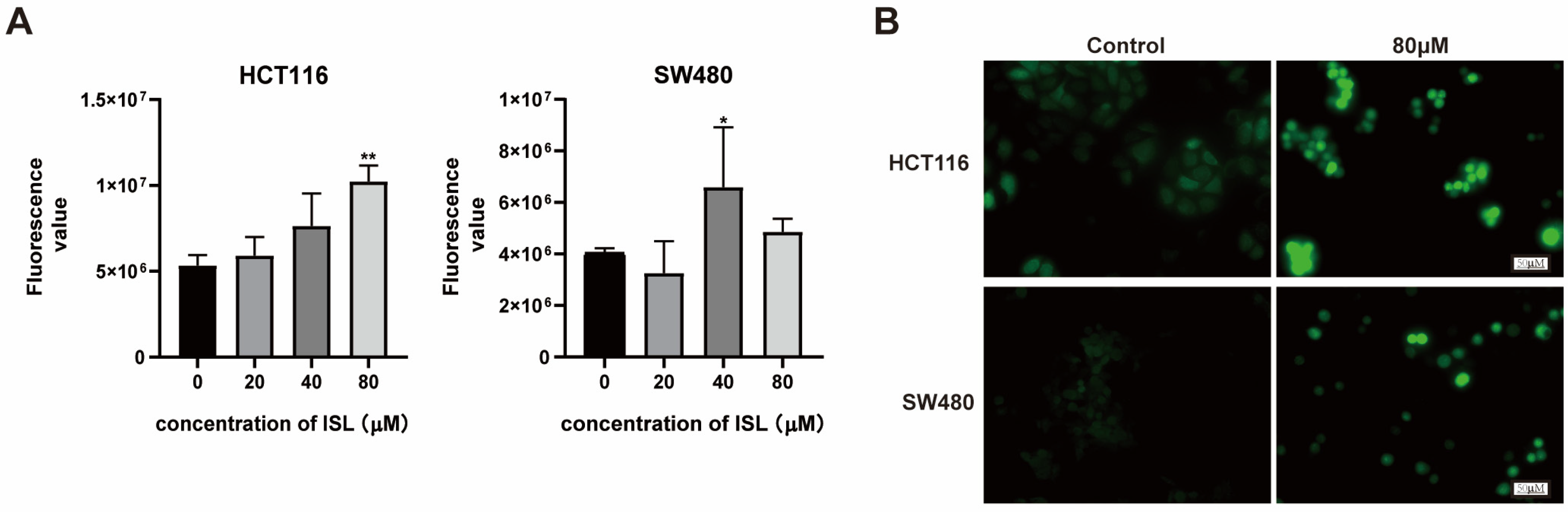
2.5. ISL Induces ROS Accumulation in CRC Cells
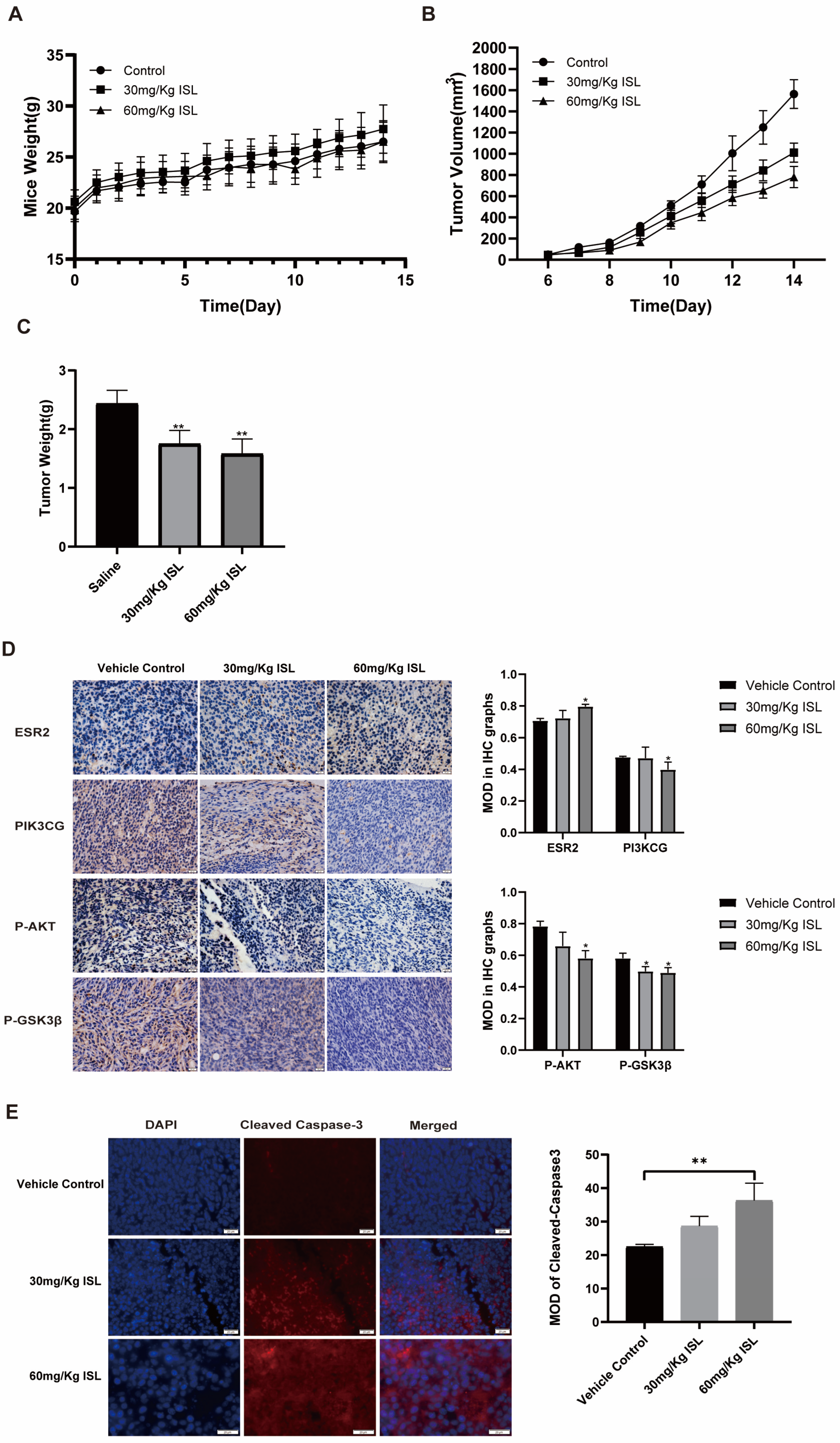
2.6. ISL Inhibits the Tumour Growth In Vivo
3. Discussion
4. Materials and Methods
4.1. Prediction of Targets of ISL against CRC
4.2. Molecular Docking
4.3. Treatment of ISL
4.4. Culture of CRC Cell SW480 and HCT116
4.5. Cell Viability Assay
4.6. Apoptosis and Cell Cycle Assays
4.7. Western Blot
4.8. Immunofluorescence
4.9. ROS Detection
4.10. Animals and Treatment
4.11. Immunohistochemistry and Immunofluorescence Experiments
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Byrne, S.; Boyle, T.; Ahmed, M.; Lee, S.H.; Benyamin, B.; Hyppönen, E. Lifestyle, genetic risk and incidence of cancer: A prospective cohort study of 13 cancer types. Int. J. Epidemiol. 2023, 52, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, Z.; Li, J. The current status of treatment for colorectal cancer in China: A systematic review. Medicine 2017, 96, e8242. [Google Scholar] [CrossRef] [PubMed]
- Slevin, F.; Hanna, C.R.; Appelt, A.; Cunningham, C.; Marijnen, C.A.; Sebag-Montefiore, D.; Muirhead, R. The Long and the Short of it: The Role of Short-course Radiotherapy in the Neoadjuvant Management of Rectal Cancer. Clin. Oncol. 2022, 34, e210–e217. [Google Scholar] [CrossRef] [PubMed]
- Ohishi, T.; Kaneko, M.K.; Yoshida, Y.; Takashima, A.; Kato, Y.; Kawada, M. Current Targeted Therapy for Metastatic Colorectal Cancer. Int. J. Mol. Sci. 2023, 24, 1702. [Google Scholar] [CrossRef]
- Mikhail, S.; Bekaii-Saab, T. Maintenance Therapy for Colorectal Cancer: Which Regimen and Which Patients? Drugs 2015, 75, 1833–1842. [Google Scholar] [CrossRef]
- Johdi, N.A.; Sukor, N.F. Colorectal Cancer Immunotherapy: Options and Strategies. Front. Immunol. 2020, 11, 1624. [Google Scholar] [CrossRef]
- Ma, S.; Sun, B.; Li, M.; Han, T.; Yu, C.; Wang, X.; Zheng, X.; Li, S.; Zhu, S.; Wang, Q. High-precision detection and navigation surgery of colorectal cancer micrometastases. J. Nanobiotechnol. 2023, 21, 403. [Google Scholar] [CrossRef]
- Biao, Z.; Xinying, D. Application of traditional Chinese medicine in integrated treatment of colorectal cancer. Negative 2021, 12, 18–23. [Google Scholar] [CrossRef]
- Lin, S.; An, X.; Guo, Y.; Gu, J.; Xie, T.; Wu, Q.; Sui, X. Meta-Analysis of Astragalus-Containing Traditional Chinese Medicine Combined with Chemotherapy for Colorectal Cancer: Efficacy and Safety to Tumor Response. Front. Oncol. 2019, 9, 749. [Google Scholar] [CrossRef] [PubMed]
- Naeem, A.; Hu, P.; Yang, M.; Zhang, J.; Liu, Y.; Zhu, W.; Zheng, Q. Natural Products as Anticancer Agents: Current Status and Future Perspectives. Molecules 2022, 27, 8367. [Google Scholar] [CrossRef] [PubMed]
- Langdon, S.P. Estrogen Receptor Signaling in Cancer. Cancers 2020, 12, 2744. [Google Scholar] [CrossRef] [PubMed]
- Caiazza, F.; Ryan, E.J.; Doherty, G.; Winter, D.C.; Sheahan, K. Estrogen Receptors and Their Implications in Colorectal Carcinogenesis. Front. Oncol. 2015, 5, 19. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.; DiLeo, A.; Niv, Y.; Gustafsson, J.-Å. Estrogen receptor beta as target for colorectal cancer prevention. Cancer Lett. 2016, 372, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Ceccarelli, I.; Bioletti, L.; Peparini, S.; Solomita, E.; Ricci, C.; Casini, I.; Miceli, E.; Aloisi, A.M. Estrogens and phytoestrogens in body functions. Neurosci. Biobehav. Rev. 2022, 132, 648–663. [Google Scholar] [CrossRef]
- Chen, Z.; Ding, W.; Yang, X.; Lu, T.; Liu, Y. Isoliquiritigenin, a potential therapeutic agent for treatment of inflammation-associated diseases. J. Ethnopharmacol. 2024, 318 Pt B, 117059. [Google Scholar] [CrossRef]
- Wang, K.-L.; Yu, Y.-C.; Hsia, S.-M. Perspectives on the Role of Isoliquiritigenin in Cancer. Cancers 2021, 13, 115. [Google Scholar] [CrossRef]
- Zhao, T.-T.; Xu, Y.-Q.; Hu, H.-M.; Gong, H.-B.; Zhu, H.-L. Isoliquiritigenin (ISL) and its Formulations: Potential Antitumor Agents. Curr. Med. Chem. 2019, 26, 6786–6796. [Google Scholar] [CrossRef]
- Nogales, C.; Mamdouh, Z.M.; List, M.; Kiel, C.; Casas, A.I.; Schmidt, H.H. Network pharmacology: Curing causal mechanisms instead of treating symptoms. Trends Pharmacol. Sci. 2022, 43, 136–150. [Google Scholar] [CrossRef]
- Xin, W.A.; Zi-Yi, W.A.; Zheng, J.H.; Shao, L.I. TCM network pharmacology: A new trend towards combining computational, experimental and clinical approaches. Chin. J. Nat. Med. 2021, 19, 1–11. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, H.; Li, N.; Chen, J.; Xu, H.; Wang, Y.; Liang, Q. Network pharmacology, a promising approach to reveal the pharmacology mechanism of Chinese medicine formula. J. Ethnopharmacol. 2023, 309, 116306. [Google Scholar] [CrossRef] [PubMed]
- Maharati, A.; Moghbeli, M. PI3K/AKT signaling pathway as a critical regulator of epithelial-mesenchymal transition in colorectal tumor cells. Cell Commun. Signal. CCS 2023, 21, 201. [Google Scholar] [CrossRef] [PubMed]
- Sanaei, M.J.; Baghery Saghchy Khorasani, A.; Pourbagheri-Sigaroodi, A.; Shahrokh, S.; Zali, M.R.; Bashash, D. The PI3K/Akt/mTOR axis in colorectal cancer: Oncogenic alterations, non-coding RNAs, therapeutic opportunities, and the emerging role of nanoparticles. J. Cell. Physiol. 2022, 237, 1720–1752. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, A.; Khazaei, M.; Hasanzadeh, M.; ShahidSales, S.; Joudi Mashhad, M.; Farazestanian, M.; Sadeghnia, H.R.; Rezayi, M.; Maftouh, M.; Hassanian, S.M.; et al. Therapeutic Potential of Targeting PI3K/AKT Pathway in Treatment of Colorectal Cancer: Rational and Progress. J. Cell. Biochem. 2018, 119, 2460–2469. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Tang, H.; Du, J.; Chen, J.; Peng, C. Isoliquiritigenin Suppresses EMT-Induced Metastasis in Triple-Negative Breast Cancer through miR-200c/C-JUN/β-Catenin. Am. J. Chin. Med. 2021, 49, 505–523. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, C.; Yang, Q.-Q.; Ma, R.-B.; Ke, Y.; Dong, F.; Wu, X.-E. Isoliquiritigenin Suppresses Osteosarcoma U2OS Cell Proliferation and Invasion by Regulating the PI3K/Akt Signalling Pathway. Chemotherapy 2018, 63, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Wu, Y.; Wang, C.; Wang, Z.; Li, Y.; Jiang, Z.; Zhao, W.; Pan, Z. Isoliquiritigenin inhibits non-small cell lung cancer progression via m6A/IGF2BP3-dependent TWIST1 mRNA stabilization. Phytomed. Int. J. Phytother. Phytopharm. 2022, 104, 154299. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, S.; Liao, Y.-F.; Sheng, Y.-M.; He, J.-C.; Cai, Z.-X.; Man, Q.; Wu, Y.-Y. Calycosin suppresses colorectal cancer progression by targeting ERβ, upregulating PTEN, and inhibiting PI3K/Akt signal pathway. Cell Biol. Int. 2022, 46, 1367–1377. [Google Scholar] [CrossRef]
- Zheng, K.; Ga Lu Ma, Y.-H.; Yang, H.; Zhao, Y.; Luo, H. Research progress of reactive oxygen species (ROS)-dependent anticancer drugs. J. Guangdong Pharm. Univ. 2022, 38, 130–136. [Google Scholar] [CrossRef]
- Lin, P.-H.; Chiang, Y.-F.; Shieh, T.-M.; Chen, H.-Y.; Shih, C.-K.; Wang, T.-H.; Wang, K.-L.; Huang, T.-C.; Hong, Y.-H.; Li, S.-C.; et al. Dietary Compound Isoliquiritigenin, an Antioxidant from Licorice, Suppresses Triple-Negative Breast Tumor Growth via Apoptotic Death Program Activation in Cell and Xenograft Animal Models. Antioxidants 2020, 9, 228. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Huang, S.; Chen, C.L.; Su, S.B.; Fang, D.D. Isoliquiritigenin Inhibits Ovarian Cancer Metastasis by Reversing Epithelial-to-Mesenchymal Transition. Molecules 2019, 24, 3725. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Lai, Y.; Li, Y.; Shu, N.; Wang, Z.; Wang, Y.; Li, Y.; Chen, Z. Antineoplastic activity of isoliquiritigenin, a chalcone compound, in androgen-independent human prostate cancer cells linked to G2/M cell cycle arrest and cell apoptosis. Eur. J. Pharmacol. 2018, 821, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.-H.; Li, R.-P.; Chen, X.-Y.; Yang, Y.; Guo, Y.-Z. Progress in the study of anti-tumor effects of isoglycyrrhizin and its mechanism. Pract. Drugs Clin. 2020, 23, 371–375. [Google Scholar] [CrossRef]
- Tian, T.; Sun, J.; Wang, J.; Liu, Y.; Liu, H. Isoliquiritigenin inhibits cell proliferation and migration through the PI3K/AKT signaling pathway in A549 lung cancer cells. Oncol. Lett. 2018, 16, 6133–6139. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Kim, H.S.; Yu, S.T.; Shin, S.R.; Lee, S.H.; Seo, G.S. Synergistic anticancer effect of docosahexaenoic acid and isoliquiritigenin on human colorectal cancer cells through ROS-mediated regulation of the JNK and cytochrome c release. Mol. Biol. Rep. 2021, 48, 1171–1180. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Zhu, J.; Zhang, P. Mechanisms of main components in Curcuma longa L. on hepatic fibrosis based on network pharmacology and molecular docking: A review. Medicine 2023, 102, e34353. [Google Scholar] [CrossRef]
- Song, X.; Zhang, Y.; Dai, E.; Wang, L.; Du, H. Prediction of triptolide targets in rheumatoid arthritis using network pharmacology and molecular docking. Int. Immunopharmacol. 2020, 80, 106179. [Google Scholar] [CrossRef]
- Luo, T.-T.; Lu, Y.; Yan, S.-K.; Xiao, X.; Rong, X.-L.; Guo, J. Network Pharmacology in Research of Chinese Medicine Formula: Methodology, Application and Prospective. Chin. J. Integr. Med. 2020, 26, 72–80. [Google Scholar] [CrossRef]
- Miziak, P.; Baran, M.; Błaszczak, E.; Przybyszewska-Podstawka, A.; Kałafut, J.; Smok-Kalwat, J.; Dmoszyńska-Graniczka, M.; Kiełbus, M.; Stepulak, A. Estrogen Receptor Signaling in Breast Cancer. Cancers 2023, 15, 4689. [Google Scholar] [CrossRef]
- Jia, M.; Dahlman-Wright, K.; Gustafsson, J.-Å. Estrogen receptor alpha and beta in health and disease. Best Pract. Res. Clin. Endocrinol. Metab. 2015, 29, 557–568. [Google Scholar] [CrossRef] [PubMed]
- Noorolyai, S.; Shajari, N.; Baghbani, E.; Sadreddini, S.; Baradaran, B. The relation between PI3K/AKT signalling pathway and cancer. Gene 2019, 698, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Dai, Q.; Su, X.; Fu, J.; Feng, X.; Peng, J. Role of PI3K/AKT pathway in cancer: The framework of malignant behavior. Mol. Biol. Rep. 2020, 47, 4587–4629. [Google Scholar] [CrossRef] [PubMed]
- Tewari, D.; Patni, P.; Bishayee, A.; Sah, A.N.; Bishayee, A. Natural products targeting the PI3K-Akt-mTOR signaling pathway in cancer: A novel therapeutic strategy. In Seminars in Cancer Biology; Academic Press: Cambridge, MA, USA, 2022; Volume 80, pp. 1–17. [Google Scholar] [CrossRef]
- Gabbouj, S.; Ryhänen, S.; Marttinen, M.; Wittrahm, R.; Takalo, M.; Kemppainen, S.; Martiskainen, H.; Tanila, H.; Haapasalo, A.; Hiltunen, M.; et al. Altered Insulin Signaling in Alzheimer’s Disease Brain—Special Emphasis on PI3K-Akt Pathway. Front. Neurosci. 2019, 13, 629. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.-L.; Yin, W.-C.; Zhong, Y.-C.; Luo, J.-Q.; Liu, L.-L.; Liu, W.-Y.; Zhao, K. The role of PI3K/Akt signalling pathway in spinal cord injury. Biomed. Pharmacother. Biomed. Pharmacother. 2022, 156, 113881. [Google Scholar] [CrossRef]
- Lindberg, K.; Helguero, L.A.; Omoto, Y.; Gustafsson, J.-Å.; Haldosén, L.-A. Estrogen receptor β represses Akt signaling in breast cancer cells via downregulation of HER2/HER3 and upregulation of PTEN: Implications for tamoxifen sensitivity. Breast Cancer Res. BCR 2011, 13, R43. [Google Scholar] [CrossRef]
- Tian, W.; Wang, Z.-W.; Yuan, B.-M.; Bao, Y.-G. Calycosin induces apoptosis in osteosarcoma cell line via ERβ-mediated PI3K/Akt signaling pathways. Mol. Med. Rep. 2020, 21, 2349–2356. [Google Scholar] [CrossRef]
- Kaloni, D.; Diepstraten, S.T.; Strasser, A.; Kelly, G.L. BCL-2 protein family: Attractive targets for cancer therapy. Apoptosis: Int. J. Program. Cell Death 2023, 28, 20–38. [Google Scholar] [CrossRef]
- Tang, R.; Xu, J.; Zhang, B.; Liu, J.; Liang, C.; Hua, J.; Meng, Q.; Yu, X.; Shi, S. Ferroptosis, necroptosis, and pyroptosis in anticancer immunity. J. Hematol. Oncol. 2020, 13, 110. [Google Scholar] [CrossRef]
- Yan, H.; Talty, R.; Johnson, C.H. Targeting ferroptosis to treat colorectal cancer. Trends Cell Biol. 2023, 33, 185–188. [Google Scholar] [CrossRef]
- Xiong, H.; Zhu, Y.-H.; Hu, Z.-Q.; Yuan, Z.-M.; Hu, H.-Q.; Tang, Q.-C. Research progress of ferroptosis in colorectal diseases. Chin. J. Color. Dis (Electron. Ed.) 2021, 10, 86–89. [Google Scholar] [CrossRef]
- Wang, C.X.; Chen, L.H.; Zhuang, H.B.; Shi, Z.S.; Chen, Z.C.; Pan, J.P.; Hong, Z.S. Auriculasin enhances ROS generation to regulate colorectal cancer cell apoptosis, ferroptosis, oxeiptosis, invasion and colony formation. Biochem. Biophys. Res. Commun. 2022, 587, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Yao, D.; Dong, M.; Dai, C.; Wu, S. Inflammation and Inflammatory Cytokine Contribute to the Initiation and Development of Ulcerative Colitis and Its Associated Cancer. Inflamm. Bowel Dis. 2019, 25, 1595–1602. [Google Scholar] [CrossRef] [PubMed]
- Stolfi, C.; Pallone, F.; Monteleone, G. Molecular targets of TRAIL-sensitizing agents in colorectal cancer. Int. J. Mol. Sci. 2012, 13, 7886–7901. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Horinaka, M.; Takara, M.; Tsuchihashi, M.; Mukai, N.; Wakada, M.; Sakai, T. Combination of isoliquiritigenin and tumor necrosis factor-related apoptosis-inducing ligand induces apoptosis in colon cancer HT29 cells. Environ. Health Prev. Med. 2008, 13, 281–287. [Google Scholar] [CrossRef]
- Liao, Y.F.; Luo, F.L.; Tang, S.S.; Huang, J.W.; Yang, Y.; Wang, S.; Jiang, T.Y.; Man, Q.; Liu, S.; Wu, Y.Y. Network analysis and experimental pharmacology study explore the protective effects of Isoliquiritigenin on 5-fluorouracil-Induced intestinal mucositis. Front. Pharmacol. 2022, 13, 1014160. [Google Scholar] [CrossRef]
- Man, Q.; Deng, Y.; Li, P.; Ma, J.; Yang, Z.; Yang, X.; Zhou, Y.; Yan, X. Licorice Ameliorates Cisplatin-Induced Hepatotoxicity Through Antiapoptosis, Antioxidative Stress, Anti-Inflammation, and Acceleration of Metabolism. Front. Pharmacol. 2020, 11, 563750. [Google Scholar] [CrossRef]
- Li, K.; You, G.; Jiang, K.; Wang, R.; Li, W.; Meng, Y.; Fang, Y.; Chen, W.; Zhu, G.; Song, J.; et al. Root extract of Hemsleya amabilis Diels suppresses renal cell carcinoma cell growth through inducing apoptosis and G2/M phase arrest via PI3K/AKT signaling pathway. J. Ethnopharmacol. 2024, 318 Pt B, 117014. [Google Scholar] [CrossRef]
- Zhang, L.; Liang, B.; Xu, H.; Gong, Y.; Hu, W.; Jin, Z.; Wu, X.; Chen, X.; Li, M.; Shi, L.; et al. Cinobufagin induces FOXO1-regulated apoptosis, proliferation, migration, and invasion by inhibiting G9a in non-small-cell lung cancer A549 cells. J. Ethnopharmacol. 2022, 291, 115095. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, F.; Tang, Y.; Zheng, L.; Yang, Y.; Gao, H.; Tian, S.; Chen, H.; Tang, C.; Tang, S.; Man, Q.; et al. Isoliquiritigenin Inhibits the Growth of Colorectal Cancer Cells through the ESR2/PI3K/AKT Signalling Pathway. Pharmaceuticals 2024, 17, 43. https://doi.org/10.3390/ph17010043
Luo F, Tang Y, Zheng L, Yang Y, Gao H, Tian S, Chen H, Tang C, Tang S, Man Q, et al. Isoliquiritigenin Inhibits the Growth of Colorectal Cancer Cells through the ESR2/PI3K/AKT Signalling Pathway. Pharmaceuticals. 2024; 17(1):43. https://doi.org/10.3390/ph17010043
Chicago/Turabian StyleLuo, Fenglin, Yimeng Tang, Lin Zheng, Ying Yang, Haoyue Gao, Shiya Tian, Hongyu Chen, Chenxi Tang, Shanshan Tang, Qiong Man, and et al. 2024. "Isoliquiritigenin Inhibits the Growth of Colorectal Cancer Cells through the ESR2/PI3K/AKT Signalling Pathway" Pharmaceuticals 17, no. 1: 43. https://doi.org/10.3390/ph17010043
APA StyleLuo, F., Tang, Y., Zheng, L., Yang, Y., Gao, H., Tian, S., Chen, H., Tang, C., Tang, S., Man, Q., & Wu, Y. (2024). Isoliquiritigenin Inhibits the Growth of Colorectal Cancer Cells through the ESR2/PI3K/AKT Signalling Pathway. Pharmaceuticals, 17(1), 43. https://doi.org/10.3390/ph17010043





