Illuminating Dersimelagon: A Novel Agent in the Treatment of Erythropoietic Protoporphyria and X-Linked Protoporphyria
Abstract
1. Introduction
2. Previous Therapies
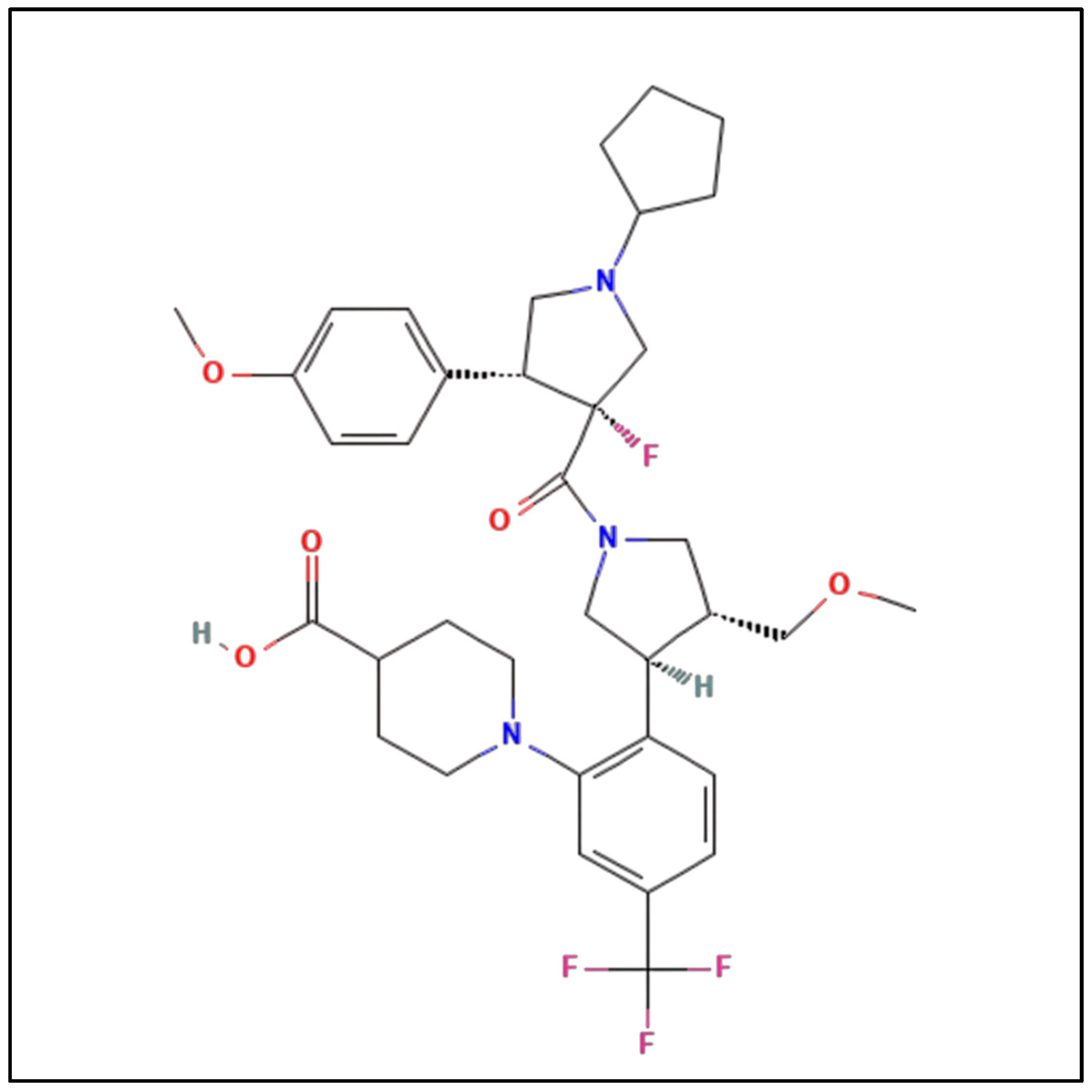
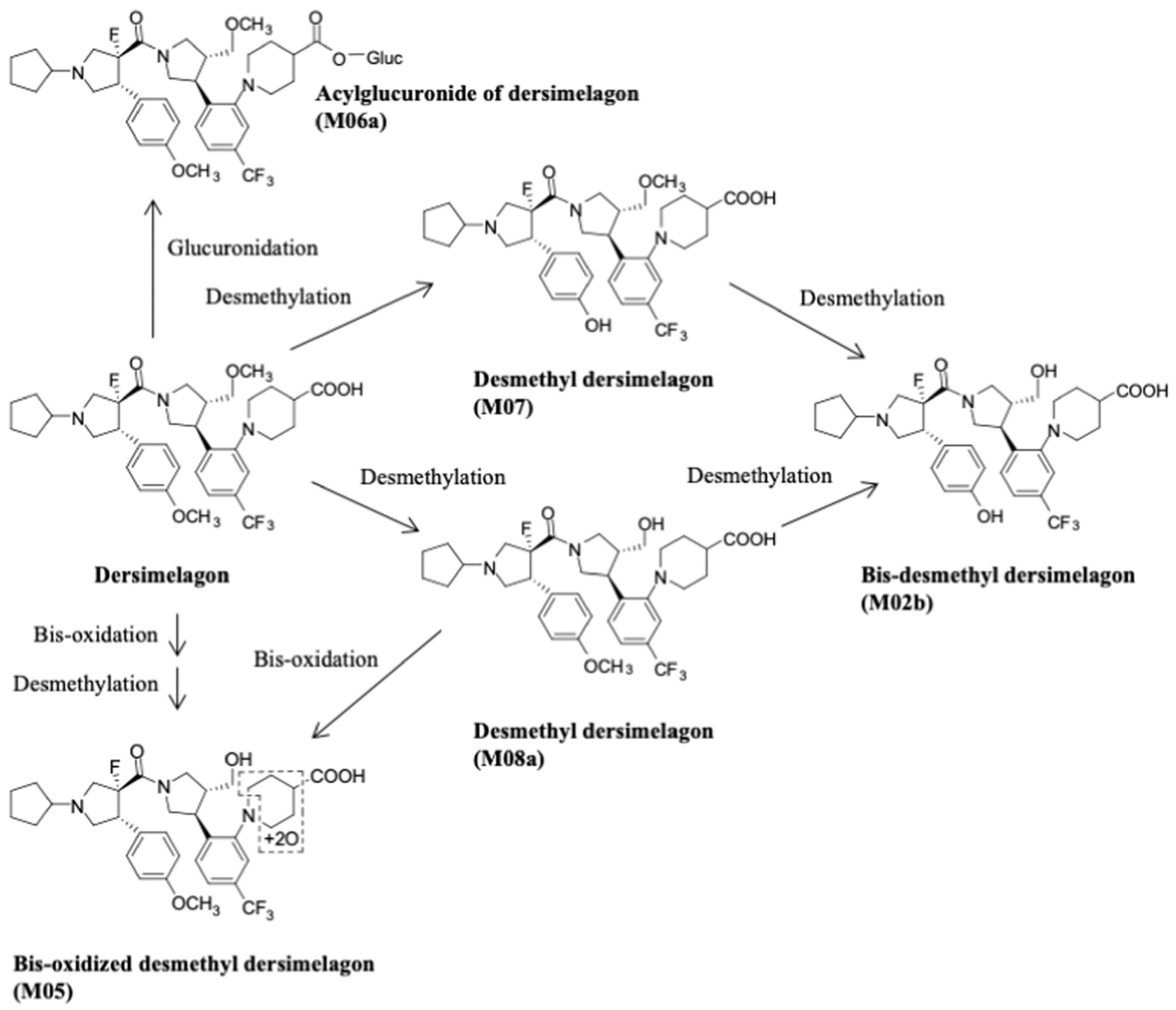
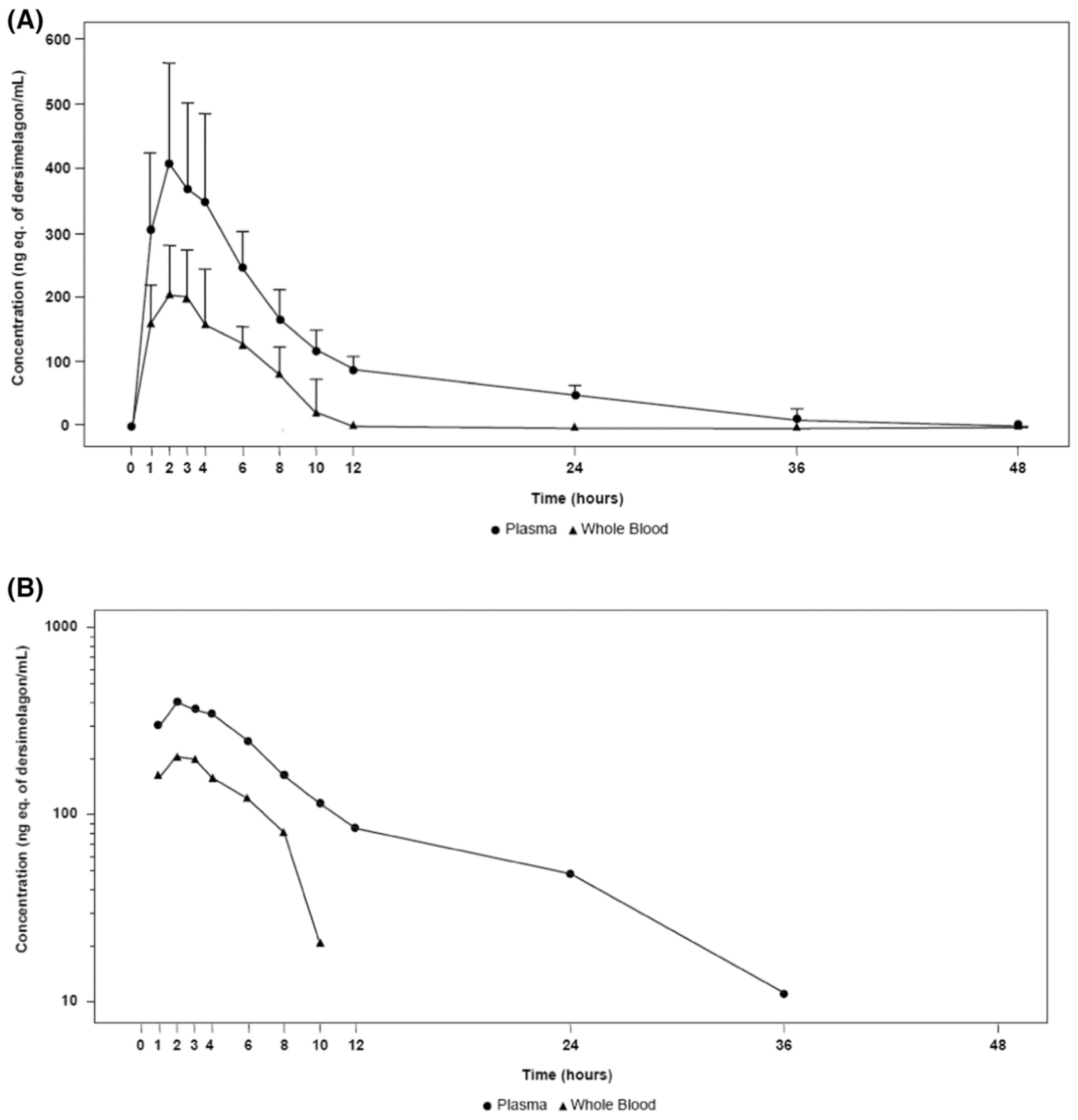
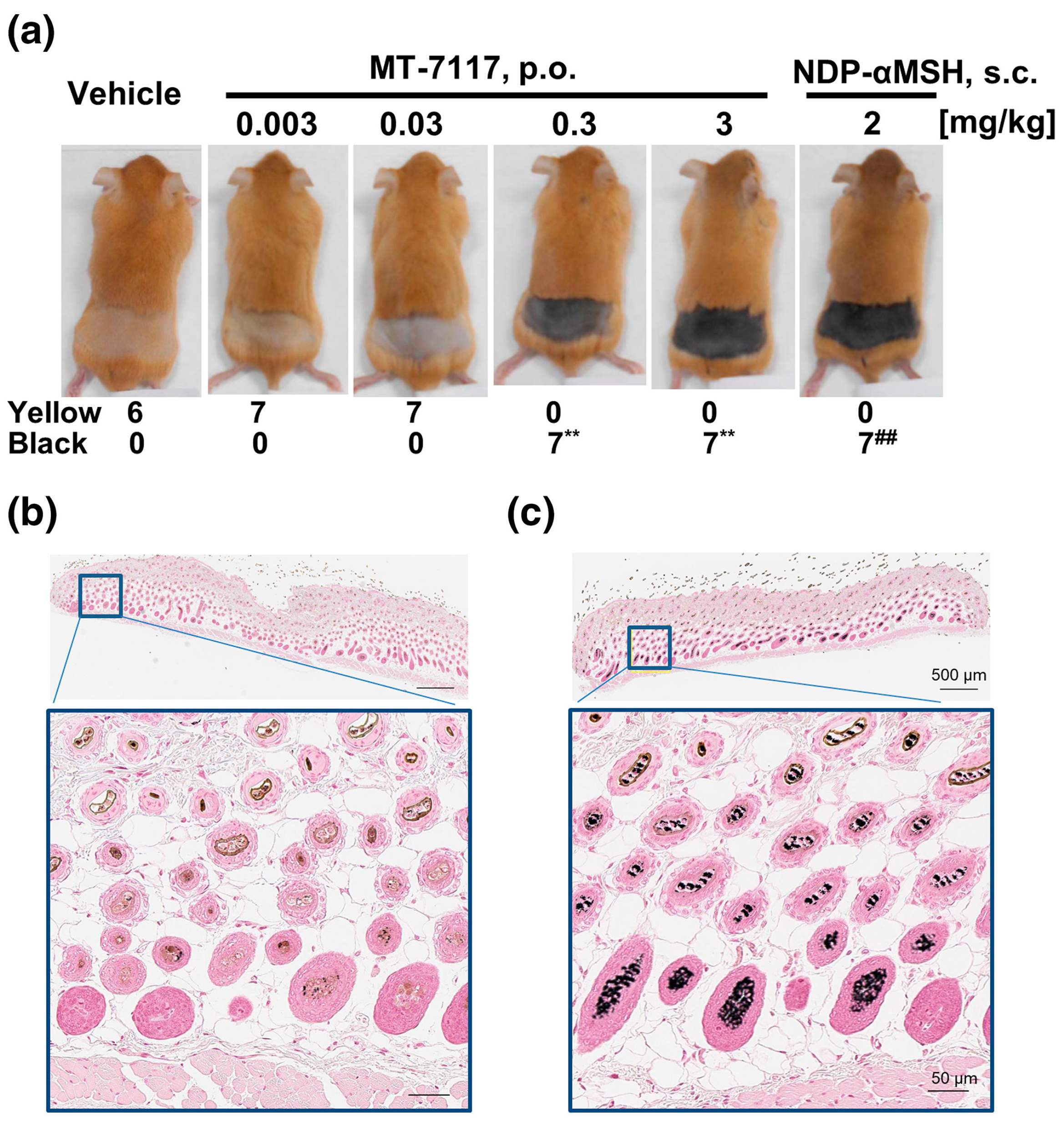
3. Metabolism and Pharmacokinetics of Dersimelagon
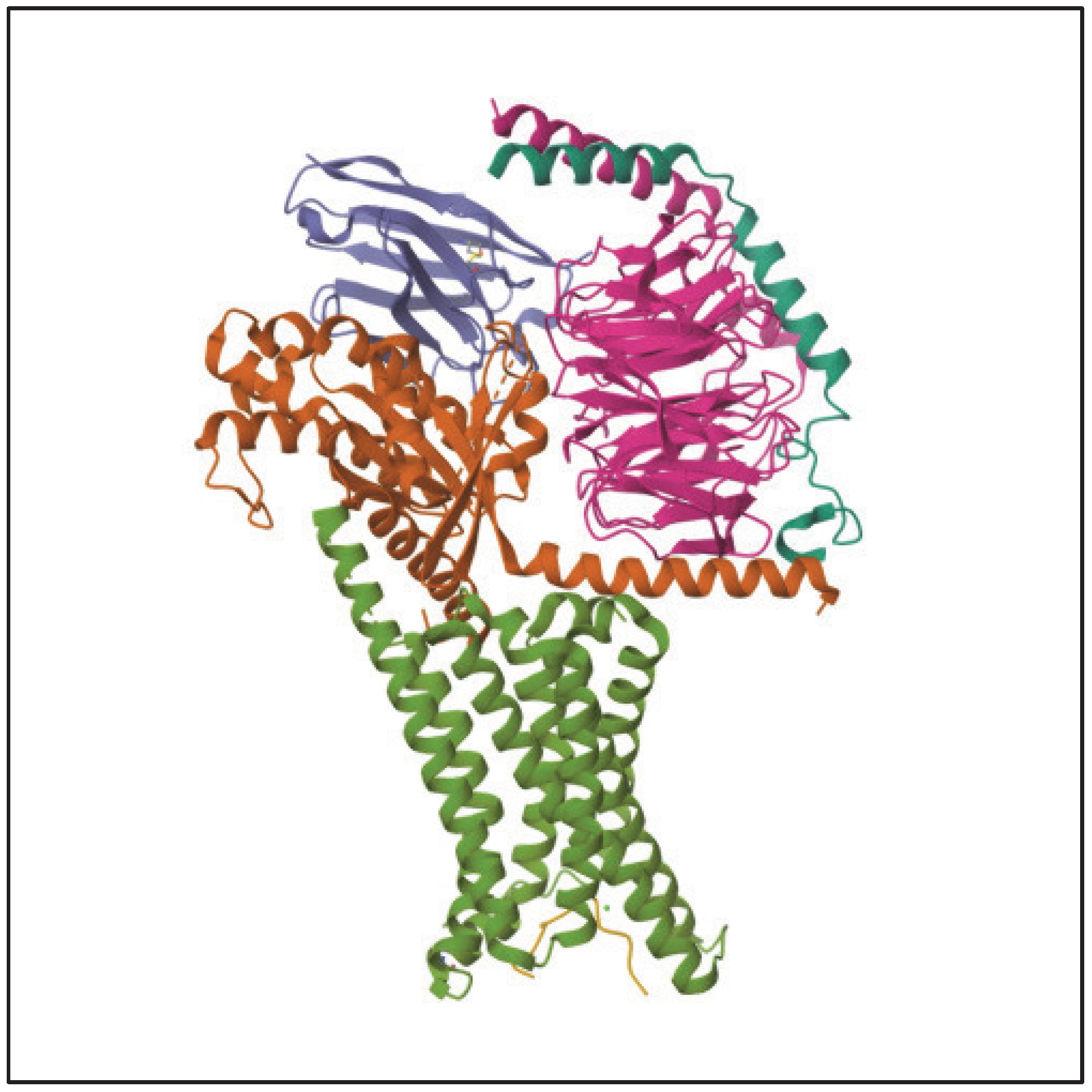
4. Mechanism of Action
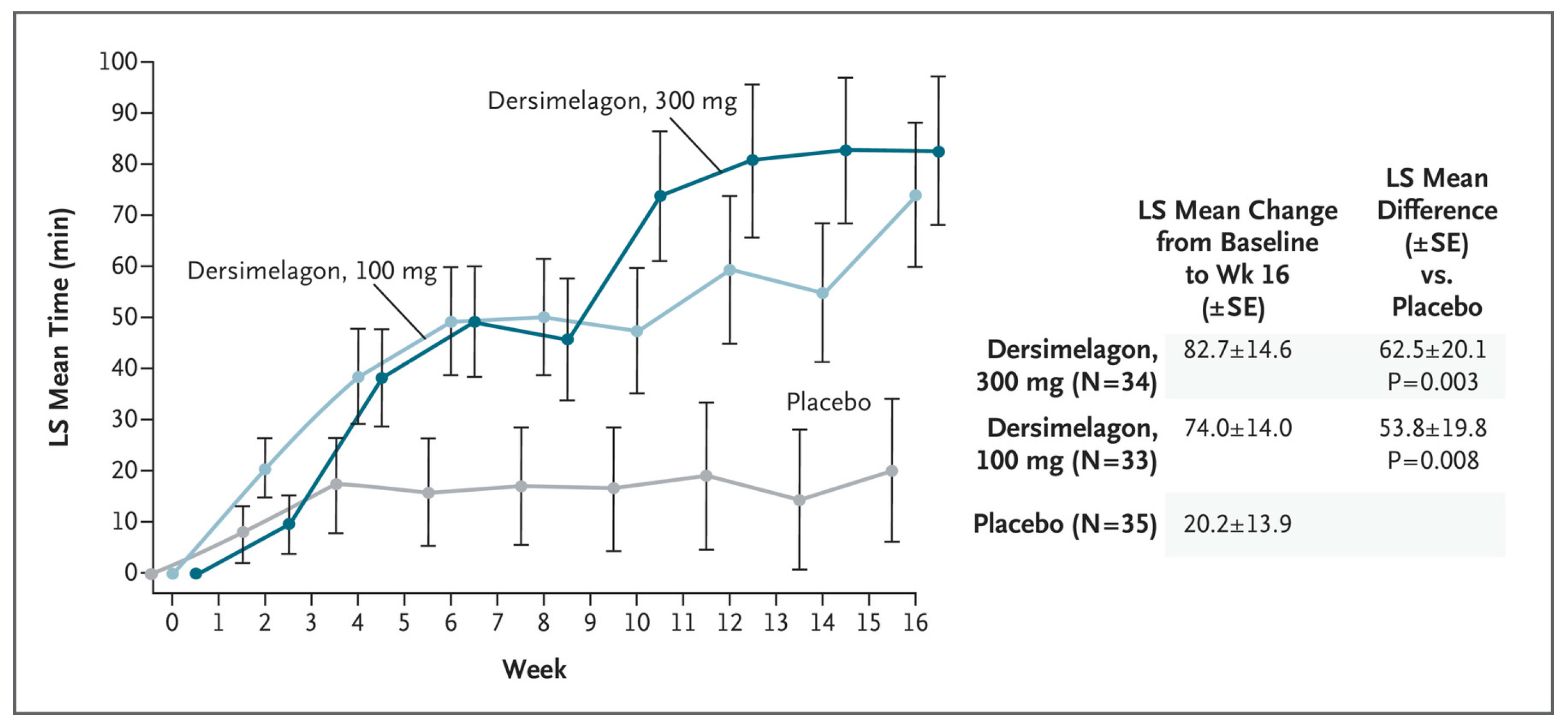
5. Key Clinical Trial Results for Dersimelagon
6. Limitations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heerfordt, I.M.; Philipsen, P.A.; Wulf, H.C. Bringing the gentle properties of daylight photodynamic therapy indoors: A systematic review of efficacy and safety. Photodiagn. Photodyn. Ther. 2022, 39, 102858. [Google Scholar] [CrossRef] [PubMed]
- Heerfordt, I.M.; Lerche, C.M.; Wulf, H.C. Cimetidine for erythropoietic protoporphyria. Photodiagn. Photodyn. Ther. 2022, 38, 102793. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.W. Mechanisms of phototoxicity in porphyria cutanea tarda and erythropoietic protoporphyria. Immunol. Ser. 1989, 46, 671–685. [Google Scholar] [PubMed]
- Bottomley, S.S.; Tanaka, M.; Everett, M. Diminished erythroid ferrochelatase activity in protoporphyria. J. Lab. Clin. Med. 1975, 86, 126–131. [Google Scholar] [PubMed]
- Bonkowsky, H.L.; Bloomer, J.R.; Ebert, P.S.; Mahoney, M.J. Heme synthetase deficiency in human protoporphyria. Demonstration of the defect in liver and cultured skin fibroblasts. J. Clin. Investig. 1975, 56, 1139–1148. [Google Scholar] [CrossRef] [PubMed]
- Levy, C. Overview of liver involvement in patients with erythropoietic protoporphyria. Gastroenterol. Hepatol. 2023, 19, 104–107. [Google Scholar]
- Todd, D. Erythropoietic protoporphyria. Br. J. Dermatol. 1994, 131, 751–766. [Google Scholar] [CrossRef] [PubMed]
- Brancaleoni, V.; Balwani, M.; Granata, F.; Graziadei, G.; Missineo, P.; Fiorentino, V.; Fustinoni, S.; Cappellini, M.D.; Naik, H.; Desnick, R.; et al. X-chromosomal inactivation directly influences the phenotypic manifestation of X-linked protoporphyria. Clin. Genet. 2016, 89, 20–26. [Google Scholar] [CrossRef]
- Balwani, M.; Doheny, D.; Bishop, D.F.; Nazarenko, I.; Yasuda, M.; Dailey, H.A.; Anderson, K.E.; Bissell, D.M.; Bloomer, J.; Bonkovsky, H.L. Porphyrias Consortium of the National Institutes of Health Rare Diseases Clinical Research Network: Loss-of-function ferrochelatase and gain-of-function erythroid-specific 5-aminolevulinate synthase mutations causing erythropoietic protoporphyria and x-linked protoporphyria in North American patients reveal novel mutations and a high prevalence of X-linked protoporphyria. Mol. Med. 2013, 19, 26–35. [Google Scholar]
- Balwani, M. Erythropoietic protoporphyria and X-linked protoporphyria: Pathophysiology, genetics, clinical manifestations, and management. Mol. Genet. Metab. 2019, 128, 298–303. [Google Scholar] [CrossRef]
- Di Pierro, E.; Granata, F.; De Canio, M.; Rossi, M.; Ricci, A.; Marcacci, M.; De Luca, G.; Sarno, L.; Barbieri, L.; Ventura, P.; et al. Recognized and emerging features of erythropoietic and X-linked protoporphyria. Diagnostics 2022, 12, 151. [Google Scholar] [CrossRef] [PubMed]
- Sandberg, S.; Talstad, I.; Hovding, G.; Bjelland, N. Light-induced release of protoporphyrin, but not of zinc protoporphyrin, from erythrocytes in a patient with greatly elevated erythrocyte protoporphyrin. Blood 1983, 62, 846–851. [Google Scholar] [CrossRef] [PubMed]
- Balwani, M.; Bonkovsky, H.L.; Levy, C.; Anderson, K.E.; Bissell, D.M.; Parker, C.; Takahashi, F.; Desnick, R.J.; Belongie, K. Dersimelagon in Erythropoietic Protoporphyrias. N. Engl. J. Med. 2023, 388, 1376–1385. [Google Scholar] [CrossRef] [PubMed]
- Dickey, A.K.; Quick, C.; Ducamp, S.; Zhu, Z.; Feng, Y.C.A.; Naik, H.; Balwani, M.; Anderson, K.E.; Lin, X.; Phillips, J.E.; et al. Evidence in the UK Biobank for the underdiagnosis of erythropoietic protoporphyria. Genet. Med. 2021, 23, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Elder, G.; Harper, P.; Badminton, M.; Sandberg, S.; Deybach, J. The incidence of inherited porphyrias in Europe. J. Inherit. Metab. Dis. 2013, 36, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Schulenburg-Brand, D.; Katugampola, R.; Anstey, A.V.; Badminton, M.N. The cutaneous porphyrias. Dermatol. Clin. 2014, 32, 369–384. [Google Scholar] [CrossRef] [PubMed]
- Dickey, A.K.; Naik, H.; Keel, S.B.; Levy, C.; Beaven, S.W.; Elmariah, S.B.; Erwin, A.L.; Goddu, R.J.; Hedstrom, K.; Leaf, R.K.; et al. Porphyrias Consortium of the Rare Diseases Clinical Research Network. Evidence-based consensus guidelines for the diagnosis and management of erythropoietic protoporphyria and X-linked protoporphyria. J. Am. Acad. Dermatol. 2023, 89, 1227–1237. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, M.; Ogawa, K.; Endou, T.; Goto, T.; Ogasawara, Y.; Ogasawara, A. Absorption, metabolism, and excretion of [14C] dersimelagon, an investigational oral selective melanocortin 1 receptor agonist, in preclinical species and healthy volunteers. Pharmacol. Res. Perspect. 2023, 11, e01084. [Google Scholar] [CrossRef]
- Kaye, E.T.; Levin, J.A.; Blank, I.H.; Arndt, K.A.; Anderson, R.R. Efficiency of opaque photoprotective agents in the visible light range. Arch. Dermatol. 1991, 127, 351–355. [Google Scholar] [CrossRef]
- Sivaramakrishnan, M.; Woods, J.; Dawe, R. Narrowband ultraviolet B phototherapy in erythropoietic protoporphyria: Case series. Br. J. Dermatol. 2014, 170, 987–988. [Google Scholar] [CrossRef]
- Mathews-Roth, M.M.; Pathak, M.A.; Fitzpatrick, T.B.; Harber, L.C.; Kass, E.H. β-Carotene as an oral photoprotective agent in erythropoietic protoporphyria. JAMA 1974, 228, 1004–1008. [Google Scholar] [CrossRef] [PubMed]
- Mathews-Roth, M.M. Treatment of erythropoietic protoporphyria with beta-carotene. Photo-Dermatology 1984, 1, 318–321. [Google Scholar] [CrossRef] [PubMed]
- Mathews-Roth, M.M.; Pathak, M.A.; Fitzpatrick, T.B.; Harber, L.H.; Kass, E.H. Beta carotene therapy for erythropoietic protoporphyria and other photosensitivity diseases. Arch. Dermatol. 1977, 113, 1229–1232. [Google Scholar] [CrossRef] [PubMed]
- Minder, E. Afamelanotide, an antagonistic analog of alpha-melanocyte-stimulating hormone, dermal phototoxicity of erythropoietic protoporphyria. Expert Opin. Investig. Drugs 2010, 19, 1591–1602. [Google Scholar] [CrossRef] [PubMed]
- Krook, G.; Haeger-Aronsen, B. Beta-carotene in the treatment of erythropoietic protoporphyria. A short review. Acta Derm. Venereol. Suppl. 1982, 100, 125–129. [Google Scholar]
- Collins, P.; Ferguson, J. Narrow-band UVB (TL-01) phototherapy: An effective preventative treatment for the photodermatoses. Br. J. Dermatol. 1995, 132, 956–963. [Google Scholar] [CrossRef] [PubMed]
- Bijlmer-Iest, J.C.; De La Faille, H.B.; Van Asbeck, B.S.; Van Hattum, J.; Van Weelden, H.; Marx, J.J.; Koningsberger, J.C. Protoporphyrin photosensitivity cannot be attenuated by oral N-acetylcysteine. Photodermatol. Photoimmunol. Photomed. 1993, 9, 245–249. [Google Scholar]
- McGuire, B.M.; Bonkovsky, H.L.; Carithers, R.L.; Chung, R.T.; Goldstein, L.I.; Lake, J.R.; Lok, A.S.; Potter, C.J.; Rand, E.; Voigt, M.D.; et al. Liver transplantation for erythropoietic protoporphyria liver disease. Liver Transpl. 2005, 11, 1590–1596. [Google Scholar] [CrossRef]
- Corbett, M.F.; Herxheimer, A.; Magnus, I.A.; Ramsay, C.A.; Kobza-Black, A. The long-term treatment with beta-carotene in erythropoietic protoporphyria: A controlled trial. Br. J. Dermatol. 1977, 97, 655–662. [Google Scholar] [CrossRef]
- Wensink, D.; Wagenmakers, M.A.; Langendonk, J.G. Afamelanotide for prevention of phototoxicity in erythropoietic protoporphyria. Expert Rev. Clin. Pharmacol. 2021, 14, 151–160. [Google Scholar] [CrossRef]
- European Medicines Agency. EU/3/22/2585—Orphan Designation for Treatment of Erythropoietic Protoporphyria. Available online: https://www.ema.europa.eu/en/medicines/human/orphan-designations/eu-3-22-2585 (accessed on 24 July 2023).
- Minder, A.-E.; Schneider-Yin, X.; Zulewski, H.; Minder, C.E.; Minder, E.I. Afamelanotide is associated with dose-dependent protective effect from liver damage related to erythropoietic protoporphyria. Life 2023, 13, 1066. [Google Scholar] [CrossRef] [PubMed]
- Levine, N.; Sheftel, S.N.; Eytan, T.; Dorr, R.T.; Hadley, M.E.; Weinrach, J.C.; Ertl, G.A.; Toth, K.; McGee, D.L.; Hruby, V. Induction of skin tanning by subcutaneous administration of a potent synthetic melanotropin. JAMA 1991, 266, 2730–2736. [Google Scholar] [CrossRef] [PubMed]
- García-Borrón, J.C.; Abdel-Malek, Z.; Jiménez-Cervantes, C. MC1R, the cAMP pathway, and the response to solar UV: Extending the horizon beyond pigmentation. Pigment Cell Melanoma Res 2014, 27, 699–720. [Google Scholar] [PubMed]
- Lane, A.M.; McKay, J.T.; Bonkovsky, H.L. Advances in the management of erythropoietic protoporphyria—The role of afamelanotide. Appl. Clin. Genet. 2016, 9, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Swetlitz, I. Clinuvel Limits the Availability of Scenesse for Rare Genetic Disease. Bloomberg. 2023. Available online: https://www.bloomberg.com/news/articles/2023-05-01/clinuvel-limits-availability-of-scenesse-for-rare-genetic-disease#xj4y7vzkg (accessed on 25 July 2023).
- Yao, J.-F.; Yang, H.; Zhao, Y.-Z.; Xue, M. Metabolism of peptide drugs and strategies to improve their metabolic stability. Curr. Drug Metab. 2018, 19, 892–901. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Kawano, Y.; Matsumoto, A.; Kondo, M.; Funayama, K.; Tanemura, S.; Miyashiro, M.; Nishi, A.; Yamada, K.; Tsuda, M.; et al. Melanogenic effect of dersimelagon (MT-7117), a novel oral melanocortin 1 receptor agonist. Skin Health Dis. 2022, 2, e78. [Google Scholar] [CrossRef]
- Ogawa, K.; Ide, R.; Belongie, K.; Tsuda, M.; Kawanishi, H.; Teng, R.; Ogasawara, A. The oral bioavailability and effect of various gastric conditions on the pharmacokinetics of dersimelagon in healthy adult volunteers. Clin. Pharmacol. Drug Dev. 2023, 12, 493–501. [Google Scholar] [CrossRef]
- Mun, Y.; Woo, K.; Dongyun, S. Melanocortin 1 Receptor (MC1R): Potentials as therapeutic targets. Int. J. Mol. Sci. 2023, 24, 12152. [Google Scholar] [CrossRef]
- Horrell, E.M.W.; Boulanger, M.C.; D’orazio, J.A. Melanocortin 1 Receptor: Structure, function, and regulation. Front. Genet. 2016, 7, 95. [Google Scholar] [CrossRef]
- Dorr, R.T.; Ertl, G.; Levine, N.; Brooks, C.; Bangert, J.L.; Powell, M.B.; Humphrey, S.; Alberts, D.S. Effects of a superpotent melanotropic peptide in combination with solar UV radiation on tanning of the skin in human volunteers. Arch. Dermatol. 2004, 140, 827–835. [Google Scholar][Green Version]
- Langendonk, J.G.; Balwani, M.; Anderson, K.E.; Bonkovsky, H.L.; Anstey, A.V.; Bissell, D.M.; Bloomer, J.; Edwards, C.; Neumann, N.J.; Parker, C.; et al. Afamelanotide for erythropoietic protoporphyria. N. Engl. J. Med. 2015, 373, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Ogasawara, A.; Ogawa, K.; Ide, R.; Ikenaga, Y.; Fukunaga, C.; Nakayama, S.; Tsuda, M. Results from a first-in-human study of dersimelagon, an investigational oral selective MC1R agonist. Eur. J. Clin. Pharmacol. 2023, 79, 801–813. [Google Scholar] [CrossRef] [PubMed]
- Belongie, K.; Takahashi, F.; Martin, S. 34420 Perspective of patients with erythropoietic protoporphyria treated with dersimelagon, a selective melanocortin-1 receptor agonist: Results of the ENDEAVOR study exit questionnaire. J. Am. Acad. Dermatol. 2002, 87, AB92. [Google Scholar] [CrossRef]
- Belongie, K.; Takahashi, F.; Mukai, S.; Endou, M.; Desnick, R. 33323 Dersimelagon, a melanocortin-1 receptor agonist, maintains efficacy regardless of seasons in subjects with erythropoietic protoporphyria: Results from a post hoc analysis of phase 2 clinical trial data. J. Am. Acad. Dermatol. 2022, 87, AB156. [Google Scholar] [CrossRef]
- Murata, J. Alpha-melanocyte-stimulating hormone blocks invasion of reconstituted basement membrane (Matrigel) by murine B16 melanoma cells. Invasion Metastasis 1997, 17, 82–93. [Google Scholar] [PubMed]
- Kondo, M.; Suzuki, T.; Kawano, Y.; Kojima, S.; Miyashiro, M.; Matsumoto, A.; Kania, G.; Błyszczuk, P.; Ross, R.L.; Mulipa, P.; et al. Dersimelagon, a novel oral melanocortin 1 receptor agonist, demonstrates disease-modifying effects in preclinical models of systemic sclerosis. Arthritis Res. Ther. 2022, 24, 210. [Google Scholar] [CrossRef] [PubMed]
- Fabrikant, J.; Touloei, K.; Brown, S. A review and update on melanocyte stimulating hormone therapy: Afamelanotide. J. Drugs Dermatol. 2013, 12, 775–779. [Google Scholar]
- Singal, A.K.; Parker, C.; Bowden, C.; Thapar, M.; Liu, L.; McGuire, B.M. Liver transplantation in the management of porphyria. Hepatology 2014, 60, 1082–1089. [Google Scholar] [CrossRef]
- Bonkovsky, H.L.; Schned, A.R. Fatal liver failure in protoporphyria: Synergism between ethanol excess and the genetic defect. Gastroenterology 1986, 90, 191–201. [Google Scholar] [CrossRef]

| The Protoporphyrias | ||||
| Disease | Inheritance | Enzyme/Genetic Abnormality | Clinical | Comment |
| Erythropoietic protoporphyria (EPP) | Autosomal Recessive (AR) | FECH (↓ activity) | Acute Photosensitivity—pain, redness, swelling Rarely, general paresis in setting of liver failure or after liver transplant | Most common is missense or nonsense mutation on 1 allele and IVS3-48T>C leading to ↓ expression on the other allele |
| X-linked protoporphyria (XLP) | X-linked | ALA-synthase-2 (Gain-of-function) | Acute Photosensitivity—pain, redness, swelling Rarely, general paresis in setting of liver failure of after liver transplant | Most common are deletions in Exon 11 |
| The Uroporphyrias | ||||
| Porphyria cutanea tarda (PCT) type 1 (acquired) | None —acquired | Hepatic uroporphyrinogen III decarboxylase (UROD) | Chronic blistering and bullae formation of sun-exposed skin; chronic actinic damage | Major risk factors: alcohol, estrogen, iron, HCV |
| PCT—type 2 (familial) | Autosomal recessive (AR) | UROD | Chronic blistering and bullae formation of sun-exposed skin; chronic actinic damage | 50% ↓ in enzyme activity insufficient to cause clinical disease; also need other risk factors (as above) |
| Hepato-erythropoietic porphyria (HEP) | AR homozygous or compound heterozygous | UROD | Severe blistering and bullae formation; hypertrichosis—occurring early in life- Infancy/childhood | Severe deficiency, leading to severe disease early in life |
| Congenital erythropoietic porphyria (CEP) | AR homozygous or compound heterozygous | UROS [aka URO3 Co-synthase] | Severe blistering and bullae formation; hypertrichosis—occurring early in life | Severe deficiency, with severe disease early in life; may also occur due to mutations in abnormal clones of developing red blood cells |
| Acute Porphyrias +/− Cutaneous Features | ||||
| Hereditary coproporphyria (HCP) | Autosomal dominant (AD) | Coproporphyrinogen oxidase (CPOX) | Blisters and bullae as in PCT + Acute attacks of generalized, poorly Localized abdominal pain and variable other neurological features | Cutaneous features rare |
| Variegate porphyria (VP) | AD | Protoporphyrinogen oxidase (PPOX) | Blisters and bullae as in PCT + Acute attacks of generalized, poorly Localized abdominal pain and variable other neurological features | Cutaneous features common, +/− symptoms of acute porphyria |
| Acute intermittent Porphyria (AIP) | AD | Hydroxymethylbilane Synthase [HMBS, aka PBG deaminase] | Acute attacks of generalized, poorly Localized abdominal pain and variable other neurological features + rarely Blisters and bullae as in PCT, HEP | Cutaneous features may occur in the setting of highly active AIP [homozygous or compound heterozygous severe HMBS deficiency] with very high ALA, PBG, and porphyrin overproduction and/or with ESRD leading to inability to excrete uroporphyrin |
| Pharmacokinetic Parameter | Plasma Total Radioactivity | Whole Blood Total Radioactivity |
|---|---|---|
| Cmax (ng/mL) a | 432.20 (151.20) | 219.00 (72.20) |
| Tmax (h) | 2.00 (2.01) | 2.00 (2.01) |
| AUC0−t (ng·h/mL) a | 3754.0 (1163.00) | 1158.00 (440.00) |
| AUC0−∞ (ng·h/mL) a | 4462.00 (1063.00) | 3311.00 (2268.00) |
| t1/2 (h) | 12.70 (5.32) | 15.73 (21.43) |
| Kel (/h) | 0.06 (0.02) | 0.11 (0.07) |
| Ki Value for Receptor Binding (nmol/L) | ||
|---|---|---|
| Human Recombinant Receptor | MT-7117 | NDP-α MSH |
| MC1R | 2.26 | 0.028 |
| MC3R | 1420 | 0.17 |
| MC4R | 32.9 | 0.20 |
| MC5R | 486 | 0.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madigan, K.E.; Rudnick, S.R.; Agnew, M.A.; Urooj, N.; Bonkovsky, H.L. Illuminating Dersimelagon: A Novel Agent in the Treatment of Erythropoietic Protoporphyria and X-Linked Protoporphyria. Pharmaceuticals 2024, 17, 31. https://doi.org/10.3390/ph17010031
Madigan KE, Rudnick SR, Agnew MA, Urooj N, Bonkovsky HL. Illuminating Dersimelagon: A Novel Agent in the Treatment of Erythropoietic Protoporphyria and X-Linked Protoporphyria. Pharmaceuticals. 2024; 17(1):31. https://doi.org/10.3390/ph17010031
Chicago/Turabian StyleMadigan, Katelyn E., Sean R. Rudnick, Matthew A. Agnew, Numra Urooj, and Herbert L. Bonkovsky. 2024. "Illuminating Dersimelagon: A Novel Agent in the Treatment of Erythropoietic Protoporphyria and X-Linked Protoporphyria" Pharmaceuticals 17, no. 1: 31. https://doi.org/10.3390/ph17010031
APA StyleMadigan, K. E., Rudnick, S. R., Agnew, M. A., Urooj, N., & Bonkovsky, H. L. (2024). Illuminating Dersimelagon: A Novel Agent in the Treatment of Erythropoietic Protoporphyria and X-Linked Protoporphyria. Pharmaceuticals, 17(1), 31. https://doi.org/10.3390/ph17010031







