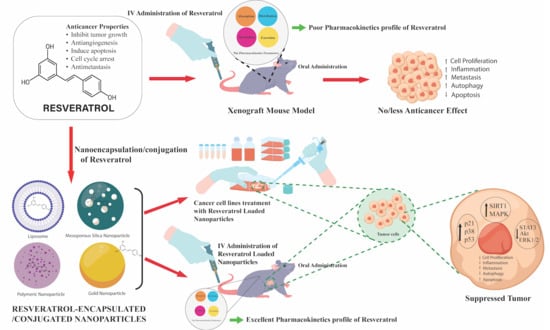
Recent Developments in Nanoparticle Formulations for Resveratrol Encapsulation as an Anticancer Agent
Abstract
1. Introduction
2. Resveratrol
2.1. The Structure and Physical Properties of Resveratrol
2.2. Metabolism of Resveratrol
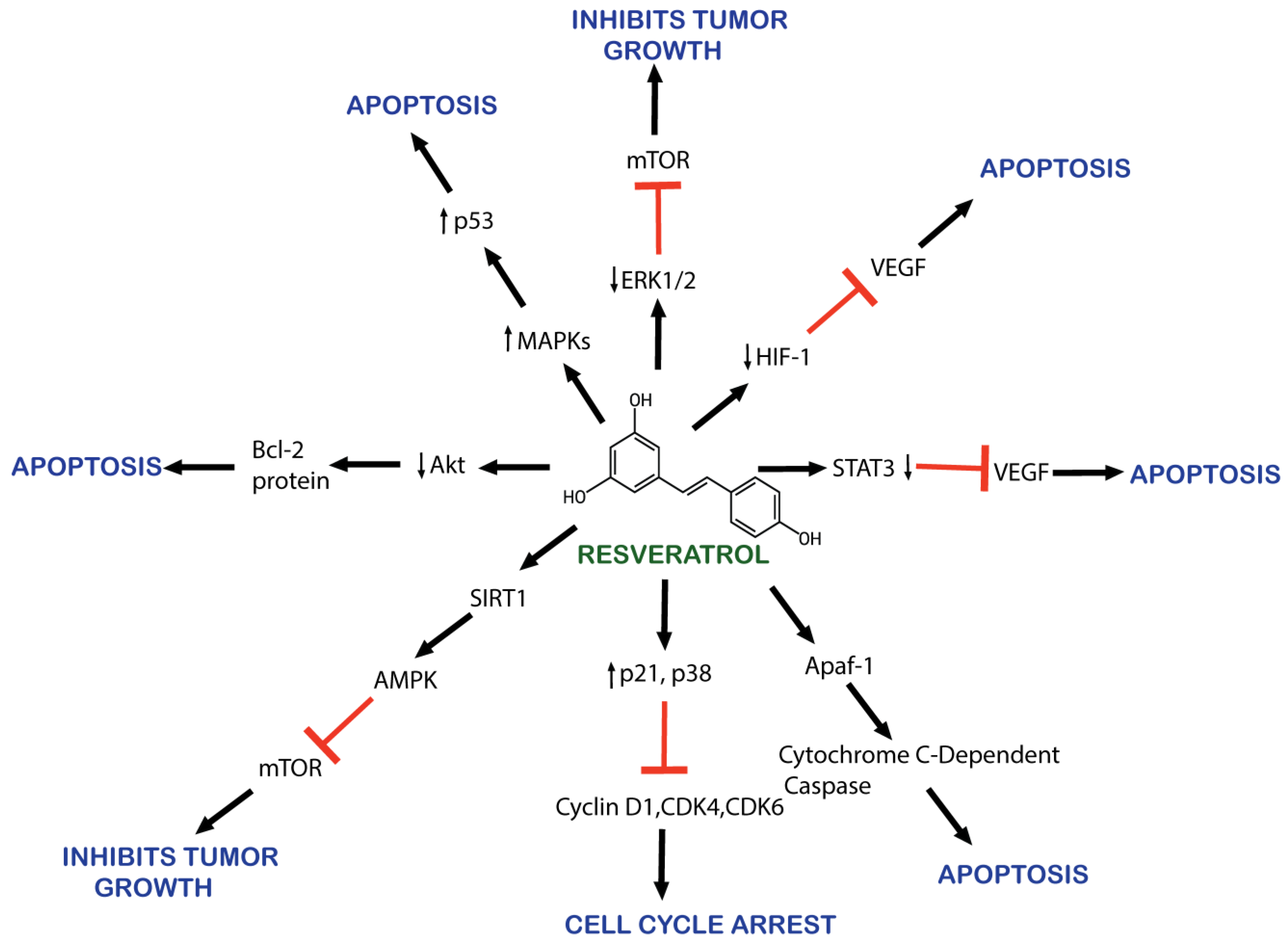
2.3. Mechanism of Action of Resveratrol against Cancer
3. Application of Nanoparticles to Improve the Therapeutic Potential of Resveratrol for Cancer
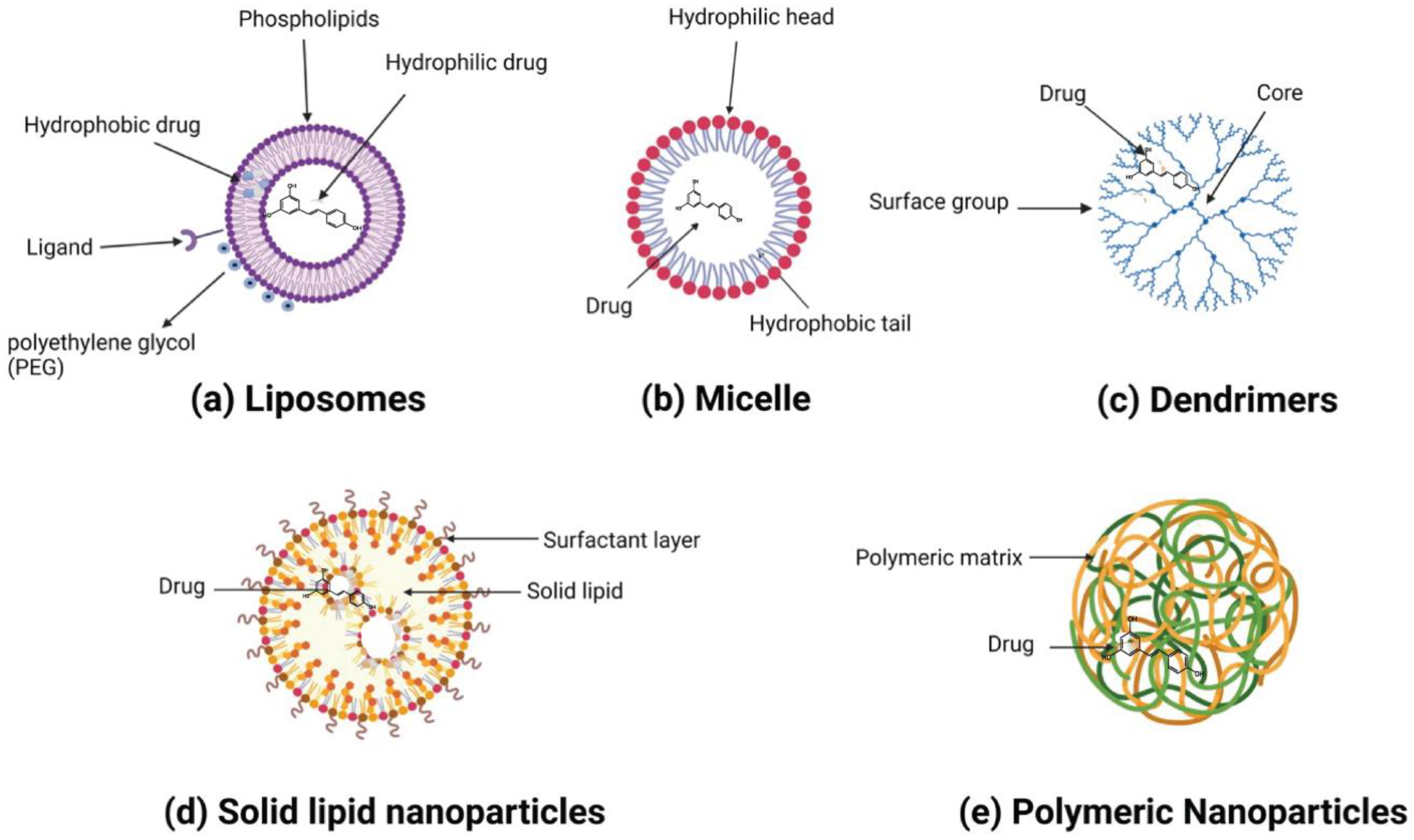
3.1. Organic Nanoparticles
3.1.1. Liposomes
3.1.2. Polymeric Nanoparticles
3.1.3. Solid Lipid Nanoparticles
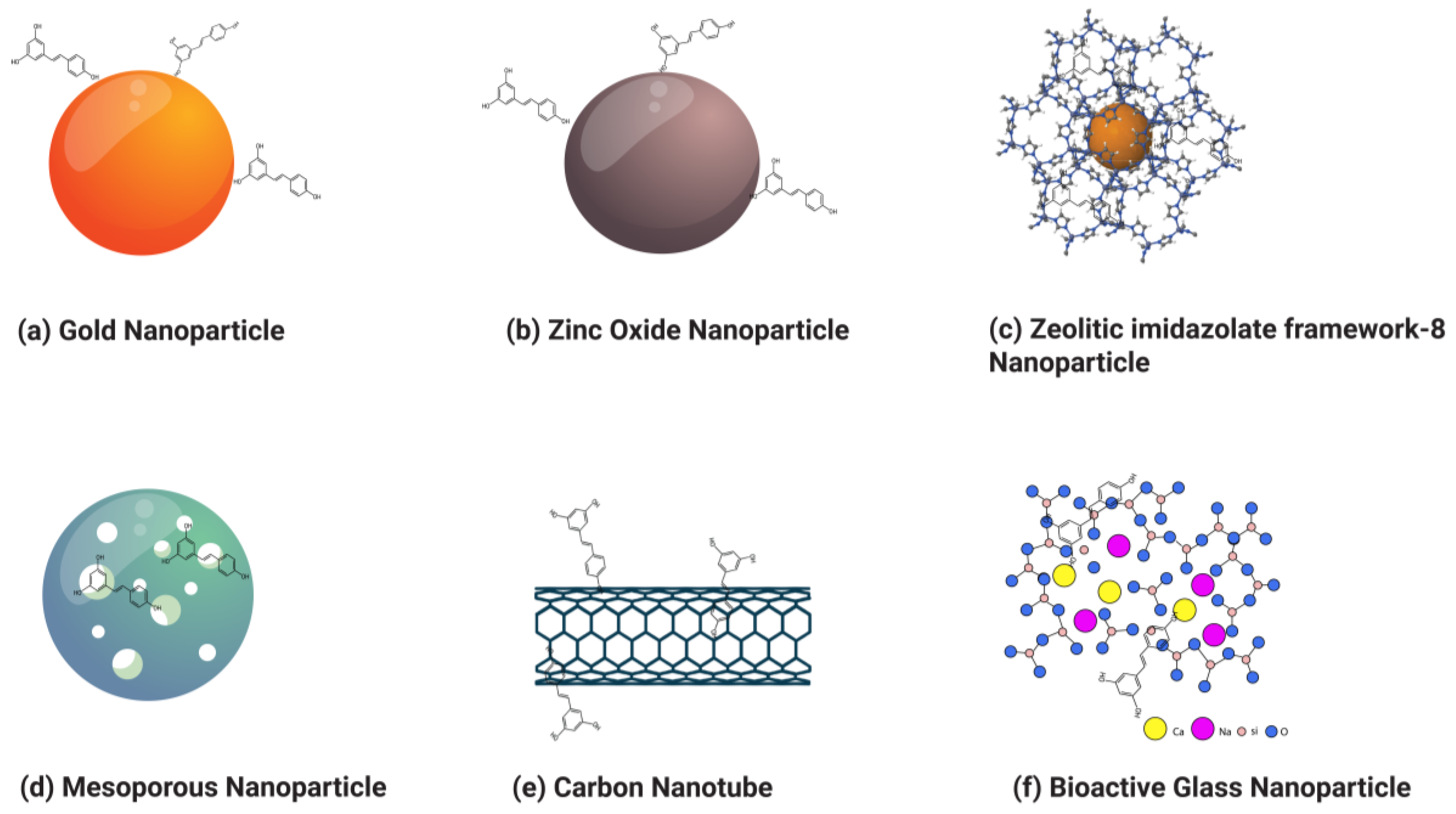
3.2. Inorganic Nanoparticles
3.2.1. Gold Nanoparticles
3.2.2. Zinc Oxide Nanoparticles
3.2.3. Zeolitic Imidazolate Framework-8 Nanoparticles (ZIF-8 NPs)
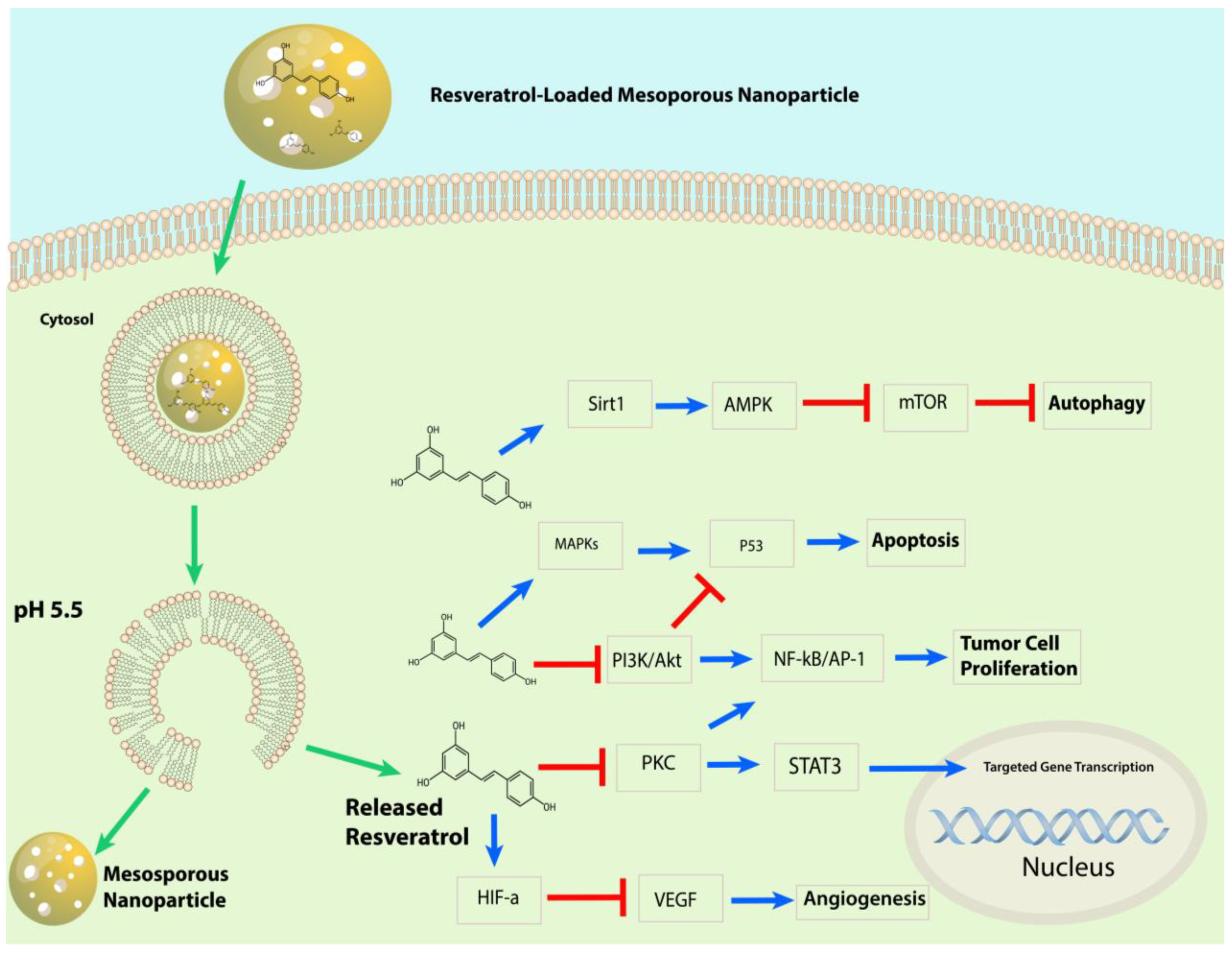
3.2.4. Mesoporous Silica Nanoparticles
3.2.5. Carbon Nanotubes
3.2.6. Bioactive Glass Nanoparticles
| Types of Nanoparticles | Preparation Techniques | Drug-Release Mechanism | Pros | Cons | Ref. |
|---|---|---|---|---|---|
| Liposomes | Reverse-phase evaporation–dehydration–rehydration, solvent injection, and microfluidic-based techniques. |
|
|
| [127] |
| Solid lipid nanoparticles | High-pressure homogenization, emulsification, high-speed stirring, and ultrasonication method. |
|
|
| [175] |
| Dendrimers | Divergent, convergent synthesis. |
|
|
| [168] |
| Polymeric nanoparticles | Solvent diffusion, solvent evaporation, ionic gelation, self-assembly, polymer electrostatic interaction, desolvation, and emulsion techniques. |
|
|
| [145] |
| Gold nanoparticles | Chemical reduction, citrate reduction, seed-mediated. |
|
|
| [240] |
| Zinc oxide nanoparticles | Sol–gel method, precipitation, hydrothermal synthesis |
|
|
| [241] |
| ZIF-8 nanoparticles | Solvothermal, microwave-assisted, co-precipitation. |
|
|
| [242] |
| Mesoporous silica nanoparticles | Sol–gel, co-condensation method. |
|
|
| [243] |
| Carbon nanotubes | Chemical vapor deposition, arc discharge, laser ablation method. |
|
|
| [244,245] |
| Bioactive glass nanoparticles | Sol–gel synthesis, flame synthesis, precipitation methods. |
|
|
| [233] |
| Drug | Organic Nanoparticle Formulation | Target System | Major Findings | Ref. |
|---|---|---|---|---|
| RSV |
Polymeric micelles | PC12 cell lines |
| [163] |
| RSV | Solid lipid nanoparticles (SLN) | NCTC2544 cell lines |
| [175] |
| RSV + QUE | Liposomes | HDFa cell lines and CD-1 mice |
| [130] |
| RSV | Lipid-core nanocapsules | C6 glioma cell lines and rats implanted with C6 glioma cells |
| [179] |
| Transferrin-modified PEGylated liposomes |
Xenograft mouse model of GBM and U-87 MG cell lines |
| [131] | |
| Transferrin (Tf) modified poly ethylene glycol-poly lactic acid (PEG-PLA) nanoparticles | C6, U87 cell lines and brain-glioma-bearing rat model |
| [159] | |
| Folate-modified nanostructured lipid carriers | MCF-7 cell lines and female Wistar rats |
| [138] | |
| PTX and RSV | PEGylated liposome | MCF-7 cell lines and BALB/c nude mice |
| [133] |
| EXM/RSV | Zein nano-capsules | MCF-7, 4T1 cell lines, and female Sprague Dawley rats |
| [162] |
| RSV | Solid lipid nanoparticles | SKBR3/PR, SKBR3/PR xenograft tumor models |
| [136] |
| RSV |
Dequalinium polyethylene glycol-distearoyl Phosphatidyl ethanolamine | Xenografted resistant A549/cDDP nude mice |
| [132] |
| RSV + CUR | Liposomes | PTEN-CaP8 cancer cell lines and B6C3F1/J mice |
| [96] |
| RSV | Poly(epsilon-caprolactone) (PCL) and poly (d,l-lactic-co-glycolic acid)-poly(ethylene glycol) conjugate (PLGA-PEG-COOH) | DU-145, PC-3, and LNCaP cell lines |
| [124] |
| DOX and RSV | PLGA nanoparticle | BALB/c nude mice and MCF-7/ADR and MDA-MB-231/ADR cell lines |
| [151] |
| RSV | PLGA-polyethylene glycol (PEG) NPs coated with chitosan | Athymic mice |
| [152] |
| RSV | Biomimetic nanocarrier | HT29 and HCT116 cell lines and C57/BL6j female nude mice |
| [154] |
| RSV | Epidermal growth factor (EGF) conjugated lipid–polymer hybrid nanoparticles | HCC827, NCIH2135, and HUVEC cell lines and BALB/c nude mice |
| [176] |
| Drug | Inorganic Nanoparticle Formulation | Target System | Major Findings | Reference |
|---|---|---|---|---|
| RSV | Gold nanoparticles | HepG2 cells |
| [180] |
| RSV | Gold nanoparticles | RAW264.7 |
| [182] |
| RSV | ZnO nanoparticles | PA1 cell lines and animal models |
| [43] |
| RSV | Zeolitic imidazolate framework-8 nanoparticles | MC38 cell line |
| [200] |
| RSV | Mesoporous silica nanoparticles | PC3 prostate cancer cell line |
| [209] |
| MGF-7 breast cancer cell line and in BALB/c nude mice |
| [210] | ||
| Gastric cancer cell line HGC-27 and HGC-27-tumor-bearing mice |
| [211] | ||
| Colon cancer cell lines HT-29 and LS147T |
| [212] | ||
| A375 and MNT-1 cell lines |
| [213] | ||
| RSV | Carbon nanotubes | Wistar rats |
| [220] |
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sleeman, K.E.; de Brito, M.; Etkind, S.; Nkhoma, K.; Guo, P.; Higginson, I.J.; Gomes, B.; Harding, R. The Escalating Global Burden of Serious Health-Related Suffering: Projections to 2060 by World Regions, Age Groups, and Health Conditions. Lancet Glob. Health 2019, 7, e883–e892. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Cao, Z.; Prettner, K.; Kuhn, M.; Yang, J.; Jiao, L.; Wang, Z.; Li, W.; Geldsetzer, P.; Bärnighausen, T.; et al. Estimates and Projections of the Global Economic Cost of 29 Cancers in 204 Countries and Territories from 2020 to 2050. JAMA Oncol. 2023, 9, 465. [Google Scholar] [CrossRef] [PubMed]
- Mathan, S.V.; Rajput, M.; Singh, R.P. CHAPTER 14—Chemotherapy and Radiation Therapy for Cancer. In Understanding Cancer; Jain, B., Pandey, S., Eds.; Academic Press: New York, NY, USA, 2022; pp. 217–236. ISBN 978-0-323-99883-3. [Google Scholar]
- Baskar, R.; Lee, K.A.; Yeo, R.; Yeoh, K.-W. Cancer and Radiation Therapy: Current Advances and Future Directions. Int. J. Med. Sci. 2012, 9, 193–199. [Google Scholar] [CrossRef]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled Drug Delivery Vehicles for Cancer Treatment and Their Performance. Signal Transduct. Target. Ther. 2018, 3, 7. [Google Scholar] [CrossRef]
- Miller, K.D.; Siegel, R.L.; Lin, C.C.; Mariotto, A.B.; Kramer, J.L.; Rowland, J.H.; Stein, K.D.; Alteri, R.; Jemal, A. Cancer Treatment and Survivorship Statistics, 2016. CA Cancer J. Clin. 2016, 66, 271–289. [Google Scholar] [CrossRef]
- Nagori, K.; Nakhate, K.T.; Yadav, K.; Ajazuddin; Pradhan, M. Unlocking the Therapeutic Potential of Medicinal Plants for Alzheimer’s Disease: Preclinical to Clinical Trial Insights. Future Pharmacol. 2023, 3, 877–907. [Google Scholar] [CrossRef]
- Singh Purewal, S.; Punia Bangar, S.; Kaur, P. (Eds.) Recent Advances in Citrus Fruits; Springer International Publishing: Cham, Switzerland, 2023; ISBN 978-3-031-37533-0. [Google Scholar]
- El-Saadony, M.T.; Zabermawi, N.M.; Zabermawi, N.M.; Burollus, M.A.; Shafi, M.E.; Alagawany, M.; Yehia, N.; Askar, A.M.; Alsafy, S.A.; Noreldin, A.E. Nutritional Aspects and Health Benefits of Bioactive Plant Compounds against Infectious Diseases: A Review. Food Rev. Int. 2023, 39, 2138–2160. [Google Scholar] [CrossRef]
- Adetuyi, B.O.; Odelade, K.A.; Odine, G.O.; Adetuyi, O.A.; Omowumi, S.O.; Ogunlana, O.O.; Egbuna, C. Neurorestorative Potential of Medicinal Plants and Their Phytochemicals. In Phytochemical Drug Discovery for Central Nervous System Disorders; Wiley: Hoboken, NJ, USA, 2023; pp. 291–310. ISBN 978-1-119-79412-7. [Google Scholar]
- Čižmárová, B.; Hubková, B.; Tomečková, V.; Birková, A. Flavonoids as Promising Natural Compounds in the Prevention and Treatment of Selected Skin Diseases. Int. J. Mol. Sci. 2023, 24, 6324. [Google Scholar] [CrossRef] [PubMed]
- Gorain, B.; Karmakar, V.; Sarkar, B.; Dwivedi, M.; Leong, J.T.L.; Toh, J.H.; Seah, E.; Ling, K.Y.; Chen, K.Y.; Choudhury, H.; et al. Biomacromolecule-Based Nanocarrier Strategies to Deliver Plant-Derived Bioactive Components for Cancer Treatment: A Recent Review. Int. J. Biol. Macromol. 2023, 253, 126623. [Google Scholar] [CrossRef]
- Abdallah, E.M.; Alhatlani, B.Y.; de Paula Menezes, R.; Martins, C.H. Back to Nature: Medicinal Plants as Promising Sources for Antibacterial Drugs in the Post-Antibiotic Era. Plants 2023, 12, 3077. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.R.; Jony, M.H.; Thufa, G.K.; Akash, S.; Dhar, P.S.; Rahman, M.M.; Afroz, T.; Ahmed, M.; Hemeg, H.A.; Rauf, A.; et al. A Clinical Study and Future Prospects for Bioactive Compounds and Semi-Synthetic Molecules in the Therapies for Huntington’s Disease. Mol. Neurobiol. 2023. [Google Scholar] [CrossRef]
- Gosline, G.; Bidault, E.; van der Burgt, X.; Cahen, D.; Challen, G.; Condé, N.; Couch, C.; Couvreur, T.L.; Dagallier, L.-P.M.; Darbyshire, I. A Taxonomically-Verified and Vouchered Checklist of the Vascular Plants of the Republic of Guinea. Sci. Data 2023, 10, 327. [Google Scholar] [PubMed]
- Schultz, F.; Garbe, L. How to Approach a Study in Ethnopharmacology? Providing an Example of the Different Research Stages for Newcomers to the Field Today. Pharmacol. Res. Perspect. 2023, 11, e01109. [Google Scholar] [CrossRef] [PubMed]
- Cadoná, F.C.; Dantas, R.F.; de Mello, G.H.; Silva, F.P., Jr. Natural Products Targeting into Cancer Hallmarks: An Update on Caffeine, Theobromine, and (+)-Catechin. Crit. Rev. Food Sci. Nutr. 2022, 62, 7222–7241. [Google Scholar]
- Priya, S.; Satheeshkumar, P. Natural Products from Plants: Recent Developments in Phytochemicals, Phytopharmaceuticals, and Plant-Based Neutraceuticals as Anticancer Agents. Funct. Preserv. Prop. Phytochem. 2020, 145–163. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar]
- Rudzińska, A.; Juchaniuk, P.; Oberda, J.; Wiśniewska, J.; Wojdan, W.; Szklener, K.; Mańdziuk, S. Phytochemicals in Cancer Treatment and Cancer Prevention—Review on Epidemiological Data and Clinical Trials. Nutrients 2023, 15, 1896. [Google Scholar]
- Bernitsa, S.; Dayan, R.; Stephanou, A.; Tzvetanova, I.D.; Patrikios, I.S. Natural Biomolecules and Derivatives as Anticancer Immunomodulatory Agents. Front. Immunol. 2023, 13, 1070367. [Google Scholar]
- Jang, J.-H.; Lee, T.-J. Mechanisms of Phytochemicals in Anti-Inflammatory and Anti-Cancer. Int. J. Mol. Sci. 2023, 24, 7863. [Google Scholar] [CrossRef]
- Majrashi, T.A.; Alshehri, S.A.; Alsayari, A.; Muhsinah, A.B.; Alrouji, M.; Alshahrani, A.M.; Shamsi, A.; Atiya, A. Insight into the Biological Roles and Mechanisms of Phytochemicals in Different Types of Cancer: Targeting Cancer Therapeutics. Nutrients 2023, 15, 1704. [Google Scholar] [CrossRef]
- Sohel, M.; Aktar, S.; Biswas, P.; Amin, M.A.; Hossain, M.A.; Ahmed, N.; Mim, M.I.H.; Islam, F.; Mamun, A.A. Exploring the Anti-cancer Potential of Dietary Phytochemicals for the Patients with Breast Cancer: A Comprehensive Review. Cancer Med. 2023, 12, 14556–14583. [Google Scholar] [CrossRef]
- Dogra, A.; Kumar, J. Biosynthesis of Anticancer Phytochemical Compounds and Their Chemistry. Front. Pharmacol. 2023, 14, 1136779. [Google Scholar] [CrossRef]
- Gahtori, R.; Tripathi, A.H.; Kumari, A.; Negi, N.; Paliwal, A.; Tripathi, P.; Joshi, P.; Rai, R.C.; Upadhyay, S.K. Anticancer Plant-Derivatives: Deciphering Their Oncopreventive and Therapeutic Potential in Molecular Terms. Future J. Pharm. Sci. 2023, 9, 14. [Google Scholar]
- Liang, Z.; Xu, Y.; Zhang, Y.; Zhang, X.; Song, J.; Jin, J.; Qian, H. Anticancer Applications of Phytochemicals in Gastric Cancer: Effects and Molecular Mechanism. Front. Pharmacol. 2023, 13, 1078090. [Google Scholar] [PubMed]
- Mandal, M.K.; Mohammad, M.; Parvin, S.I.; Islam, M.M.; Gazi, H.A.R. A Short Review on Anticancer Phytochemicals. Pharmacogn. Rev. 2023, 17, 11–23. [Google Scholar] [CrossRef]
- Curti, V.; Di Lorenzo, A.; Dacrema, M.; Xiao, J.; Nabavi, S.M.; Daglia, M. In Vitro Polyphenol Effects on Apoptosis: An Update of Literature Data. Semin. Cancer Biol. 2017, 46, 119–131. [Google Scholar] [CrossRef]
- Cook, M.T.; Mafuvadze, B.; Besch-Williford, C.; Ellersieck, M.R.; Goyette, S.; Hyder, S.M. Luteolin Suppresses Development of Medroxyprogesterone Acetate-Accelerated 7,12-Dimethylbenz(a)Anthracene-Induced Mammary Tumors in Sprague-Dawley Rats. Oncol. Rep. 2016, 35, 825–832. [Google Scholar] [CrossRef]
- Briguglio, G.; Costa, C.; Pollicino, M.; Giambò, F.; Catania, S.; Fenga, C. Polyphenols in Cancer Prevention: New Insights (Review). Int. J. Funct. Nutr. 2020, 1, 9. [Google Scholar] [CrossRef]
- Ali, M.; Benfante, V.; Stefano, A.; Yezzi, A.; Di Raimondo, D.; Tuttolomondo, A.; Comelli, A. Anti-Arthritic and Anti-Cancer Activities of Polyphenols: A Review of the Most Recent In Vitro Assays. Life 2023, 13, 361. [Google Scholar] [CrossRef]
- Jurczyk, M.; Kasperczyk, J.; Wrześniok, D.; Beberok, A.; Jelonek, K. Nanoparticles Loaded with Docetaxel and Resveratrol as an Advanced Tool for Cancer Therapy. Biomedicines 2022, 10, 1187. [Google Scholar] [CrossRef]
- Ren, B.; Kwah, M.X.-Y.; Liu, C.; Ma, Z.; Shanmugam, M.K.; Ding, L.; Xiang, X.; Ho, P.C.-L.; Wang, L.; Ong, P.S.; et al. Resveratrol for Cancer Therapy: Challenges and Future Perspectives. Cancer Lett. 2021, 515, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Walle, T. Bioavailability of Resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 9–15. [Google Scholar]
- Rauf, A.; Imran, M.; Butt, M.S.; Nadeem, M.; Peters, D.G.; Mubarak, M.S. Resveratrol as an Anti-Cancer Agent: A Review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1428–1447. [Google Scholar] [CrossRef]
- Bozorgi, A.; Haghighi, Z.; Khazaei, M.R.; Bozorgi, M.; Khazaei, M. The Anti-Cancer Effect of Chitosan/Resveratrol Polymeric Nanocomplex against Triple-Negative Breast Cancer; an in Vitro Assessment. IET Nanobiotechnol. 2023, 17, 91–102. [Google Scholar] [CrossRef]
- Muller, A.G.; Sarker, S.D.; Fatokun, A.A.; Hutcheon, G.A. Formulation of Resveratrol into PGA-Co-PDL Nanoparticles Increases Its Cytotoxic Potency against Lung Cancer Cells. RPS Pharm. Pharmacol. Rep. 2023, 2, rqac007. [Google Scholar] [CrossRef]
- Gagliardi, A.; Giuliano, E.; Venkateswararao, E.; Fresta, M.; Bulotta, S.; Awasthi, V.; Cosco, D. Biodegradable Polymeric Nanoparticles for Drug Delivery to Solid Tumors. Front. Pharmacol. 2021, 12, 601626. [Google Scholar] [CrossRef] [PubMed]
- Neves, A.R.; Queiroz, J.F.; Reis, S. Brain-Targeted Delivery of Resveratrol Using Solid Lipid Nanoparticles Functionalized with Apolipoprotein E. J. Nanobiotechnol. 2016, 14, 27. [Google Scholar] [CrossRef]
- Mohan, A.; Narayanan, S.; Sethuraman, S.; Krishnan, U.M. Novel Resveratrol and 5-Fluorouracil Coencapsulated in PEGylated Nanoliposomes Improve Chemotherapeutic Efficacy of Combination against Head and Neck Squamous Cell Carcinoma. BioMed Res. Int. 2014, 2014, 424239. [Google Scholar] [CrossRef]
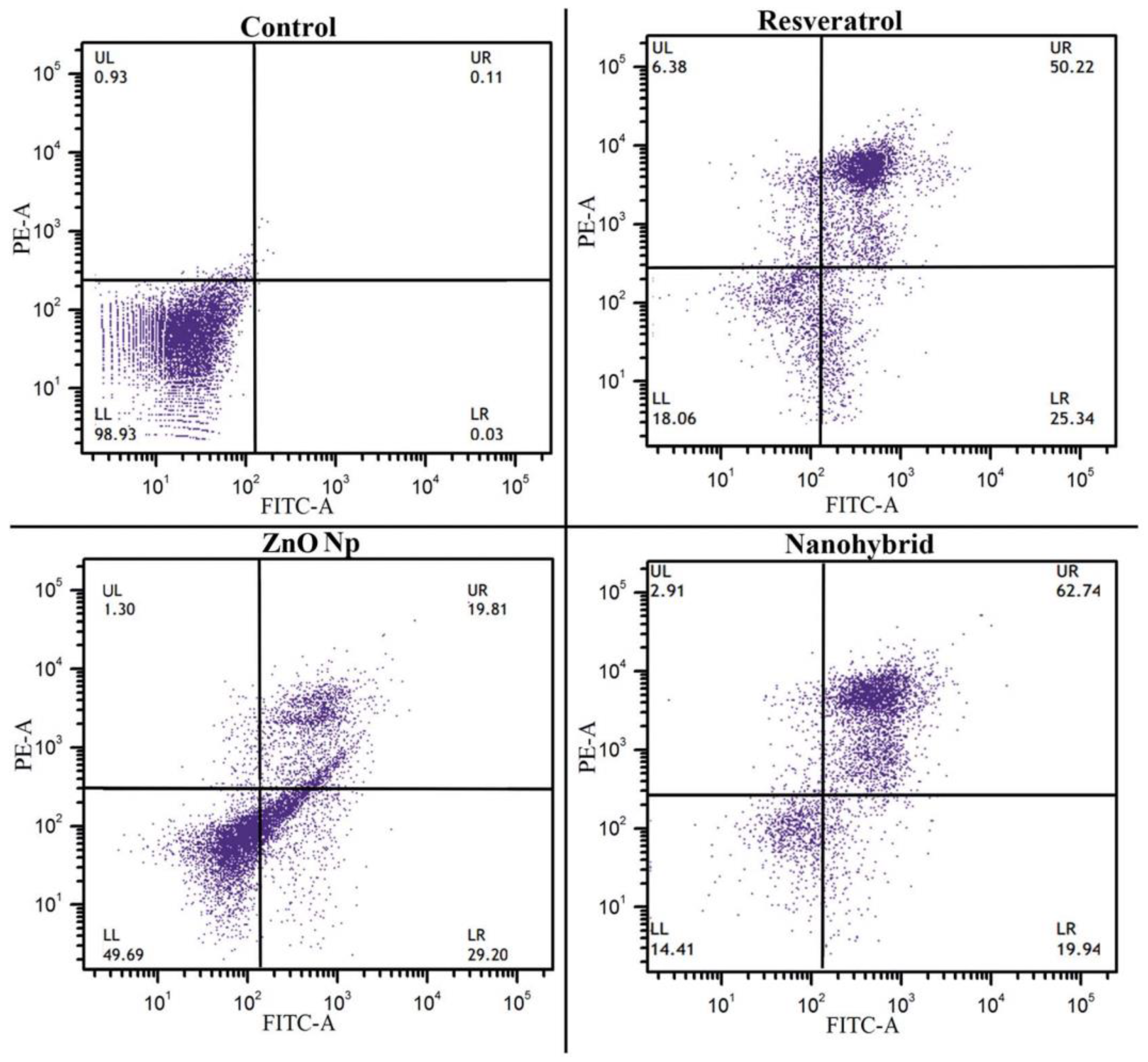
- Khatun, M.; Choudhury, S.; Liu, B.; Lemmens, P.; Pal, S.K.; Mazumder, S. Resveratrol–ZnO Nanohybrid Enhanced Anti-Cancerous Effect in Ovarian Cancer Cells through ROS. RSC Adv. 2016, 6, 105607–105617. [Google Scholar] [CrossRef]
- Aras, A.; Khokhar, A.R.; Qureshi, M.Z.; Silva, M.F.; Sobczak-Kupiec, A.; Pineda, E.A.G.; Hechenleitner, A.A.W.; Farooqi, A.A. Targeting Cancer with Nano-Bullets: Curcumin, EGCG, Resveratrol and Quercetin on Flying Carpets. Asian Pac. J. Cancer Prev. 2014, 15, 3865–3871. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, Applications and Toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Mekuye, B.; Abera, B. Nanomaterials: An Overview of Synthesis, Classification, Characterization, and Applications. Nano Sel. 2023, 4, 486–501. [Google Scholar] [CrossRef]
- Singh, V.; Yadav, S.S.; Chauhan, V.; Shukla, S.; Vishnolia, K.K. Applications of Nanoparticles in Various Fields: In Advances in Medical Technologies and Clinical Practice; Yadav, D., Bansal, A., Bhatia, M., Hooda, M., Morato, J., Eds.; IGI Global: Hershey, PA, USA, 2021; pp. 221–236. ISBN 978-1-79986-527-8. [Google Scholar]
- Kumari, S.; Raturi, S.; Kulshrestha, S.; Chauhan, K.; Dhingra, S.; András, K.; Thu, K.; Khargotra, R.; Singh, T. A Comprehensive Review on Various Techniques Used for Synthesizing Nanoparticles. J. Mater. Res. Technol. 2023, 27, 1739–1763. [Google Scholar] [CrossRef]
- Park, E.-J.; Pezzuto, J.M. The Pharmacology of Resveratrol in Animals and Humans. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2015, 1852, 1071–1113. [Google Scholar] [CrossRef]
- Tellone, E.; Galtieri, A.; Russo, A.; Giardina, B.; Ficarra, S. Resveratrol: A Focus on Several Neurodegenerative Diseases. Oxidative Med. Cell. Longev. 2015, 2015, 392169. [Google Scholar] [CrossRef]
- Morales, M.; Barcelo, A.; Pedreño, M. Plant Stilbenes: Recent Advances in Their Chemistry and Biology. Adv. Plant Physiol. 2000, 3, 39–70. [Google Scholar]
- Bostanghadiri, N.; Pormohammad, A.; Chirani, A.S.; Pouriran, R.; Erfanimanesh, S.; Hashemi, A. Comprehensive Review on the Antimicrobial Potency of the Plant Polyphenol Resveratrol. Biomed. Pharmacother. 2017, 95, 1588–1595. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.V.; Martins, N.; Sharifi-Rad, J. Resveratrol: A Double-Edged Sword in Health Benefits. Biomedicines 2018, 6, 91. [Google Scholar] [CrossRef]
- Jojima, K.; Tanaka, A.; Node, K. Resveratrol Supplementation: A Therapeutic Potential for Cardiac Remodeling in Hypertensive Heart Disease. Hypertens. Res. 2023, 46, 1596–1598. [Google Scholar] [CrossRef]
- Chen, X.; Song, X.; Zhao, X.; Zhang, Y.; Wang, Y.; Jia, R.; Zou, Y.; Li, L.; Yin, Z. Insights into the Anti-Inflammatory and Antiviral Mechanisms of Resveratrol. Mediat. Inflamm. 2022, 2022, 7138756. [Google Scholar] [CrossRef]
- Andrade, S.; Ramalho, M.J.; Pereira, M.D.C.; Loureiro, J.A. Resveratrol Brain Delivery for Neurological Disorders Prevention and Treatment. Front. Pharmacol. 2018, 9, 1261. [Google Scholar] [CrossRef]
- Clouser, C.L.; Chauhan, J.; Bess, M.A.; Van Oploo, J.L.; Zhou, D.; Dimick-Gray, S.; Mansky, L.M.; Patterson, S.E. Anti-HIV-1 Activity of Resveratrol Derivatives and Synergistic Inhibition of HIV-1 by the Combination of Resveratrol and Decitabine. Bioorganic Med. Chem. Lett. 2012, 22, 6642–6646. [Google Scholar] [CrossRef] [PubMed]
- Abba, Y.; Hassim, H.; Hamzah, H.; Noordin, M.M. Antiviral Activity of Resveratrol against Human and Animal Viruses. Adv. Virol. 2015, 2015, 184241. [Google Scholar] [CrossRef]
- Heredia, A.; Davis, C.; Redfield, R. Synergistic Inhibition of HIV-1 in Activated and Resting Peripheral Blood Mononuclear Cells, Monocyte-Derived Macrophages, and Selected Drug-Resistant Isolates with Nucleoside Analogues Combined with a Natural Product, Resveratrol. JAIDS J. Acquir. Immune Defic. Syndr. 2000, 25, 246–255. [Google Scholar] [CrossRef]
- Jang, M.; Cai, L.; Udeani, G.O.; Slowing, K.V.; Thomas, C.F.; Beecher, C.W.; Fong, H.H.; Farnsworth, N.R.; Kinghorn, A.D.; Mehta, R.G. Cancer Chemopreventive Activity of Resveratrol, a Natural Product Derived from Grapes. Science 1997, 275, 218–220. [Google Scholar]
- Robinson, K.; Mock, C.; Liang, D. Pre-Formulation Studies of Resveratrol. Drug Dev. Ind. Pharm. 2015, 41, 1464–1469. [Google Scholar]
- Amri, A.; Chaumeil, J.; Sfar, S.; Charrueau, C. Administration of Resveratrol: What Formulation Solutions to Bioavailability Limitations? J. Control. Release 2012, 158, 182–193. [Google Scholar] [PubMed]
- Perrone, D.; Fuggetta, M.P.; Ardito, F.; Cottarelli, A.; De Filippis, A.; Ravagnan, G.; De Maria, S.; Lo Muzio, L. Resveratrol (3,5,4′-Trihydroxystilbene) and Its Properties in Oral Diseases. Exp. Ther. Med. 2017, 14, 3–9. [Google Scholar]
- Pettit, G.R.; Grealish, M.P.; Jung, M.K.; Hamel, E.; Pettit, R.K.; Chapuis, J.-C.; Schmidt, J.M. Antineoplastic Agents. 465. Structural Modification of Resveratrol: Sodium Resverastatin Phosphate. J. Med. Chem. 2002, 45, 2534–2542. [Google Scholar] [CrossRef]
- Navarro-Orcajada, S.; Conesa, I.; Vidal-Sánchez, F.J.; Matencio, A.; Albaladejo-Maricó, L.; García-Carmona, F.; López-Nicolás, J.M. Stilbenes: Characterization, Bioactivity, Encapsulation and Structural Modifications. A Review of Their Current Limitations and Promising Approaches. Crit. Rev. Food Sci. Nutr. 2023, 63, 7269–7287. [Google Scholar] [CrossRef]
- Jeon, D.; Jo, M.; Lee, Y.; Park, S.-H.; Phan, H.T.L.; Nam, J.H.; Namkung, W. Inhibition of ANO1 by Cis-and Trans-Resveratrol and Their Anticancer Activity in Human Prostate Cancer PC-3 Cells. Int. J. Mol. Sci. 2023, 24, 1186. [Google Scholar] [CrossRef]
- Santos, A.C.; Pereira, I.; Magalhães, M.; Pereira-Silva, M.; Caldas, M.; Ferreira, L.; Figueiras, A.; Ribeiro, A.J.; Veiga, F. Targeting Cancer Via Resveratrol-Loaded Nanoparticles Administration: Focusing on In Vivo Evidence. AAPS J. 2019, 21, 57. [Google Scholar] [CrossRef]
- Dariya, B.; Girish, B.P.; Merchant, N.; Srilatha, M.; Nagaraju, G.P. Resveratrol: Biology, Metabolism, and Detrimental Role on the Tumor Microenvironment of Colorectal Cancer. Nutr. Rev. 2023, nuad133. [Google Scholar] [CrossRef]
- Santos, A.C.; Veiga, F.; Ribeiro, A.J. New Delivery Systems to Improve the Bioavailability of Resveratrol. Expert Opin. Drug Deliv. 2011, 8, 973–990. [Google Scholar] [CrossRef]
- Plauth, A.; Geikowski, A.; Cichon, S.; Wowro, S.J.; Liedgens, L.; Rousseau, M.; Weidner, C.; Fuhr, L.; Kliem, M.; Jenkins, G.; et al. Hormetic Shifting of Redox Environment by Pro-Oxidative Resveratrol Protects Cells against Stress. Free Radic. Biol. Med. 2016, 99, 608–622. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Pai, R.S. Optimized PLGA Nanoparticle Platform for Orally Dosed Trans-Resveratrol with Enhanced Bioavailability Potential. Expert Opin. Drug Deliv. 2014, 11, 647–659. [Google Scholar] [PubMed]
- Albuquerque, B.; Costa, M.S.; Peça, I.N.; Cardoso, M.M. Production of Double-walled Nanoparticles Containing Meloxicam. Polym. Eng. Sci. 2013, 53, 146–152. [Google Scholar] [CrossRef]
- Wenzel, E.; Somoza, V. Metabolism and Bioavailability of Trans-resveratrol. Mol. Nutr. Food Res. 2005, 49, 472–481. [Google Scholar]
- Walle, T.; Hsieh, F.; DeLegge, M.H.; Oatis, J.E.; Walle, U.K. High Absorption but Very Low Bioavailability of Oral Resveratrol in Humans. Drug Metab. Dispos. 2004, 32, 1377–1382. [Google Scholar] [CrossRef] [PubMed]
- Almeida, T.C.; Melo, A.S.; Lima, A.P.B.; Branquinho, R.T.; da Silva, G.N. Resveratrol Induces the Production of Reactive Oxygen Species, Interferes with the Cell Cycle, and Inhibits the Cell Migration of Bladder Tumour Cells with Different TP53 Status. Nat. Prod. Res. 2023, 37, 3838–3843. [Google Scholar] [CrossRef] [PubMed]
- Yousef, M.; Vlachogiannis, I.; Tsiani, E. Effects of Resveratrol against Lung Cancer: In Vitro and In Vivo Studies. Nutrients 2017, 9, 1231. [Google Scholar] [CrossRef]
- Bishayee, A.; Petit, D.M.; Samtani, K. Angioprevention Is Implicated in Resveratrol Chemoprevention of Experimental Hepatocarcinogenesis. J Carcinog. Mutagen. 2010, 1, 102. [Google Scholar] [CrossRef]
- Bhattacharjee, J.; Kirby, M.; Softic, S.; Miles, L.; Salazar-Gonzalez, R.-M.; Shivakumar, P.; Kohli, R. Hepatic Natural Killer T-Cell and CD8+ T-Cell Signatures in Mice with Nonalcoholic Steatohepatitis: Bhattacharjee, Kirby; et al. Hepatol. Commun. 2017, 1, 299–310. [Google Scholar] [CrossRef]
- Arablou, T.; Aryaeian, N.; Khodaverdi, S.; Kolahdouz-Mohammadi, R.; Moradi, Z.; Rashidi, N.; Delbandi, A.-A. The Effects of Resveratrol on the Expression of VEGF, TGF-β, and MMP-9 in Endometrial Stromal Cells of Women with Endometriosis. Sci. Rep. 2021, 11, 6054. [Google Scholar] [CrossRef]
- Pradhan, R.; Paul, S.; Das, B.; Sinha, S.; Dash, S.R.; Mandal, M.; Kundu, C.N. Resveratrol Nanoparticle Attenuates Metastasis and Angiogenesis by Deregulating Inflammatory Cytokines through Inhibition of CAFs in Oral Cancer by CXCL-12/IL-6-Dependent Pathway. J. Nutr. Biochem. 2023, 113, 109257. [Google Scholar] [CrossRef]
- Gołąbek-Grenda, A.; Kaczmarek, M.; Juzwa, W.; Olejnik, A. Natural Resveratrol Analogs Differentially Target Endometriotic Cells into Apoptosis Pathways. Sci. Rep. 2023, 13, 11468. [Google Scholar] [CrossRef]
- Khayat, M.T.; Zarka, M.A.; El-Telbany, D.; Farag, A.; El-Halawany, A.M.; Kutbi, H.I.; Elkhatib, W.F.; Noreddin, A.M.; Khayyat, A.N.; El-Telbany, R.F.A.; et al. Intensification of Resveratrol Cytotoxicity, pro-Apoptosis, Oxidant Potentials in Human Colorectal Carcinoma HCT-116 Cells Using Zein Nanoparticles. Sci. Rep. 2022, 12, 15235. [Google Scholar] [CrossRef]
- Singh, S.K.; Banerjee, S.; Acosta, E.P.; Lillard, J.W.; Singh, R. Resveratrol Induces Cell Cycle Arrest and Apoptosis with Docetaxel in Prostate Cancer Cells via a P53/p21WAF1/CIP1 and p27KIP1 Pathway. Oncotarget 2017, 8, 17216–17228. [Google Scholar] [CrossRef]
- Catania, A.; Barrajón-Catalán, E.; Nicolosi, S.; Cicirata, F.; Micol, V. Immunoliposome Encapsulation Increases Cytotoxic Activity and Selectivity of Curcumin and Resveratrol against HER2 Overexpressing Human Breast Cancer Cells. Breast Cancer Res. Treat. 2013, 141, 55–65. [Google Scholar] [CrossRef]
- Varoni, E.M.; Lo Faro, A.F.; Sharifi-Rad, J.; Iriti, M. Anticancer Molecular Mechanisms of Resveratrol. Front. Nutr. 2016, 3, 8. [Google Scholar] [CrossRef]
- Li, D.; Wang, G.; Jin, G.; Yao, K.; Zhao, Z.; Bie, L.; Guo, Y.; Li, N.; Deng, W.; Chen, X.; et al. Resveratrol Suppresses Colon Cancer Growth by Targeting the AKT/STAT3 Signaling Pathway. Int. J. Mol. Med. 2019, 43, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Ghafouri-Fard, S.; Bahroudi, Z.; Shoorei, H.; Hussen, B.M.; Talebi, S.F.; Baig, S.G.; Taheri, M.; Ayatollahi, S.A. Disease-Associated Regulation of Gene Expression by Resveratrol: Special Focus on the PI3K/AKT Signaling Pathway. Cancer Cell Int. 2022, 22, 298. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Bode, A.M.; Dong, Z.; Lee, M.-H. AKT as a Therapeutic Target for Cancer. Cancer Res. 2019, 79, 1019–1031. [Google Scholar] [CrossRef] [PubMed]
- Verzella, D.; Pescatore, A.; Capece, D.; Vecchiotti, D.; Ursini, M.V.; Franzoso, G.; Alesse, E.; Zazzeroni, F. Life, Death, and Autophagy in Cancer: NF-κB Turns up Everywhere. Cell Death Dis. 2020, 11, 210. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-Y.; Tang, H.-Y.; Davis, F.B.; Davis, P.J. Resveratrol and Apoptosis: Resveratrol-Induced Apoptosis. Ann. N. Y. Acad. Sci. 2011, 1215, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Chin, Y.-T.; Hsieh, M.-T.; Yang, S.-H.; Tsai, P.-W.; Wang, S.-H.; Wang, C.-C.; Lee, Y.-S.; Cheng, G.-Y.; HuangFu, W.-C.; London, D.; et al. Anti-Proliferative and Gene Expression Actions of Resveratrol in Breast Cancer Cells in Vitro. Oncotarget 2014, 5, 12891–12907. [Google Scholar] [CrossRef]
- Tang, H.-Y.; Shih, A.; Cao, H.J.; Davis, F.B.; Davis, P.J.; Lin, H.-Y. Resveratrol-Induced Cyclooxygenase-2 Facilitates P53-Dependent Apoptosis in Human Breast Cancer Cells. Mol. Cancer Ther. 2006, 5, 2034–2042. [Google Scholar] [CrossRef]
- Ko, J.-H.; Sethi, G.; Um, J.-Y.; Shanmugam, M.K.; Arfuso, F.; Kumar, A.P.; Bishayee, A.; Ahn, K.S. The Role of Resveratrol in Cancer Therapy. Int. J. Mol. Sci. 2017, 18, 2589. [Google Scholar] [CrossRef]
- Yang, X.; Jiang, T.; Wang, Y.; Guo, L. The Role and Mechanism of SIRT1 in Resveratrol-Regulated Osteoblast Autophagy in Osteoporosis Rats. Sci. Rep. 2019, 9, 18424. [Google Scholar] [CrossRef]
- Berman, A.Y.; Motechin, R.A.; Wiesenfeld, M.Y.; Holz, M.K. The Therapeutic Potential of Resveratrol: A Review of Clinical Trials. npj Precis. Oncol. 2017, 1, 35. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, N.K.; Nargi, D.; Randolph, C.; Narayanan, B.A. Liposome Encapsulation of Curcumin and Resveratrol in Combination Reduces Prostate Cancer Incidence in PTEN Knockout Mice. Int. J. Cancer 2009, 125, 1–8. [Google Scholar] [PubMed]
- Annaji, M.; Poudel, I.; Boddu, S.H.S.; Arnold, R.D.; Tiwari, A.K.; Babu, R.J. Resveratrol-loaded Nanomedicines for Cancer Applications. Cancer Rep. 2021, 4, e1353. [Google Scholar] [CrossRef]
- Sarvari, P.; Sarvari, P. Advances in Nanoparticle-Based Drug Delivery in Cancer Treatment. Glob. Transl. Med. 2023, 2, 0394. [Google Scholar] [CrossRef]
- Chenthamara, D.; Subramaniam, S.; Ramakrishnan, S.G.; Krishnaswamy, S.; Essa, M.M.; Lin, F.-H.; Qoronfleh, M.W. Therapeutic Efficacy of Nanoparticles and Routes of Administration. Biomater. Res. 2019, 23, 20. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Madhunapantula, S.V.; Robertson, G.P. Toxicological Considerations When Creating Nanoparticle-Based Drugs and Drug Delivery Systems. Expert Opin. Drug Metab. Toxicol. 2012, 8, 47–69. [Google Scholar] [CrossRef]
- Jin, C.; Wang, K.; Oppong-Gyebi, A.; Hu, J. Application of Nanotechnology in Cancer Diagnosis and Therapy—A Mini-Review. Int. J. Med. Sci. 2020, 17, 2964–2973. [Google Scholar] [CrossRef]
- Martínez-Ballesta, M.; Gil-Izquierdo, Á.; García-Viguera, C.; Domínguez-Perles, R. Nanoparticles and Controlled Delivery for Bioactive Compounds: Outlining Challenges for New “Smart-Foods” for Health. Foods 2018, 7, 72. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano Based Drug Delivery Systems: Recent Developments and Future Prospects 10 Technology 1007 Nanotechnology 03 Chemical Sciences 0306 Physical Chemistry (Incl. Structural) 03 Chemical Sciences 0303 Macromolecular and Materials Chemistry 11 Medical and Health Sciences 1115 Pharmacology and Pharmaceutical Sciences 09 Engineering 0903 Biomedical Engineering Prof Ueli Aebi, Prof Peter Gehr. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- Khushnud, T.; Mousa, S.A. Potential Role of Naturally Derived Polyphenols and Their Nanotechnology Delivery in Cancer. Mol. Biotechnol. 2013, 55, 78–86. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering Precision Nanoparticles for Drug Delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Jose, S.; Anju, S.S.; Cinu, T.A.; Aleykutty, N.A.; Thomas, S.; Souto, E.B. In Vivo Pharmacokinetics and Biodistribution of Resveratrol-Loaded Solid Lipid Nanoparticles for Brain Delivery. Int. J. Pharm. 2014, 474, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Liu, T.; Yang, C. Development of PLGA-Lipid Nanoparticles with Covalently Conjugated Indocyanine Green as a Versatile Nanoplatform for Tumor-Targeted Imaging and Drug Delivery. Int. J. Nanomed. 2016, 11, 5807–5821. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.S.; Schorzman, A.N.; Finniss, M.C.; Bowerman, C.J.; Peng, L.; Luft, J.C.; Madden, A.J.; Wang, A.Z.; Zamboni, W.C.; DeSimone, J.M. Nanoparticle Drug Loading as a Design Parameter to Improve Docetaxel Pharmacokinetics and Efficacy. Biomaterials 2013, 34, 8424–8429. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante de Freitas, P.G.; Rodrigues Arruda, B.; Araújo Mendes, M.G.; Barroso de Freitas, J.V.; da Silva, M.E.; Sampaio, T.L.; Petrilli, R.; Eloy, J.O. Resveratrol-Loaded Polymeric Nanoparticles: The Effects of D-α-Tocopheryl Polyethylene Glycol 1000 Succinate (TPGS) on Physicochemical and Biological Properties against Breast Cancer In Vitro and In Vivo. Cancers 2023, 15, 2802. [Google Scholar] [CrossRef] [PubMed]
- Chimento, A.; De Amicis, F.; Sirianni, R.; Sinicropi, M.; Puoci, F.; Casaburi, I.; Saturnino, C.; Pezzi, V. Progress to Improve Oral Bioavailability and Beneficial Effects of Resveratrol. IJMS 2019, 20, 1381. [Google Scholar] [CrossRef]
- Paiva-Santos, A.C.; Veiga, F.; Sequeira, J.; Fortuna, A.; Falcão, A.; Pereira, I.; Pattekari, P.; Ribeiro, C.; Ribeiro, A. First-Time Orally Administered Resveratrol-Loaded Layer-by-Layer Nanoparticles to Rats—A Pharmacokinetics Study. Analyst 2019, 144, 2062–2079. [Google Scholar] [CrossRef]
- Peñalva, R.; Morales, J.; González-Navarro, C.J.; Larrañeta, E.; Quincoces, G.; Peñuelas, I.; Irache, J.M. Increased Oral Bioavailability of Resveratrol by Its Encapsulation in Casein Nanoparticles. Int. J. Mol. Sci. 2018, 19, 2816. [Google Scholar] [CrossRef]
- Augustin, M.A.; Sanguansri, L.; Lockett, T. Nano- and Micro-Encapsulated Systems for Enhancing the Delivery of Resveratrol. Ann. N. Y. Acad. Sci. 2013, 1290, 107–112. [Google Scholar] [CrossRef]
- Intagliata, S.; Modica, M.N.; Santagati, L.M.; Montenegro, L. Strategies to Improve Resveratrol Systemic and Topical Bioavailability: An Update. Antioxidants 2019, 8, 244. [Google Scholar] [CrossRef]
- Vijayakumar, M.R.; Vajanthri, K.Y.; Balavigneswaran, C.K.; Mahto, S.K.; Mishra, N.; Muthu, M.S.; Singh, S. Pharmacokinetics, Biodistribution, in Vitro Cytotoxicity and Biocompatibility of Vitamin E TPGS Coated Trans Resveratrol Liposomes. Colloids Surf. B Biointerfaces 2016, 145, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Zhang, T.; Qin, S.; Huang, Z.; Zhou, L.; Shi, J.; Nice, E.C.; Xie, N.; Huang, C.; Shen, Z. Enhancing the Therapeutic Efficacy of Nanoparticles for Cancer Treatment Using Versatile Targeted Strategies. J. Hematol. Oncol. 2022, 15, 132. [Google Scholar] [CrossRef]
- AlSawaftah, N.M.; Awad, N.S.; Pitt, W.G.; Husseini, G.A. pH-Responsive Nanocarriers in Cancer Therapy. Polymers 2022, 14, 936. [Google Scholar] [CrossRef]
- Amin, M.; Huang, W.; Seynhaeve, A.L.B.; Ten Hagen, T.L.M. Hyperthermia and Temperature-Sensitive Nanomaterials for Spatiotemporal Drug Delivery to Solid Tumors. Pharmaceutics 2020, 12, 1007. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Katti, P.S.; Gu, Z. Enzyme-Responsive Nanomaterials for Controlled Drug Delivery. Nanoscale 2014, 6, 12273–12286. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Sun, W. Recent Advances in Redox-Responsive Nanoparticles for Combined Cancer Therapy. Nanoscale Adv. 2022, 4, 3504–3516. [Google Scholar] [CrossRef]
- Liu, J.F.; Jang, B.; Issadore, D.; Tsourkas, A. Use of Magnetic Fields and Nanoparticles to Trigger Drug Release and Improve Tumor Targeting. WIREs Nanomed. Nanobiotechnol. 2019, 11, e1571. [Google Scholar] [CrossRef]
- Awad, N.S.; Paul, V.; AlSawaftah, N.M.; Ter Haar, G.; Allen, T.M.; Pitt, W.G.; Husseini, G.A. Ultrasound-Responsive Nanocarriers in Cancer Treatment: A Review. ACS Pharmacol. Transl. Sci. 2021, 4, 589–612. [Google Scholar] [CrossRef]
- Khalid, K.; Tan, X.; Mohd Zaid, H.F.; Tao, Y.; Lye Chew, C.; Chu, D.-T.; Lam, M.K.; Ho, Y.-C.; Lim, J.W.; Chin Wei, L. Advanced in Developmental Organic and Inorganic Nanomaterial: A Review. Bioengineered 2020, 11, 328–355. [Google Scholar] [CrossRef]
- Sanna, V.; Siddiqui, I.A.; Sechi, M.; Mukhtar, H. Resveratrol-Loaded Nanoparticles Based on Poly(Epsilon-Caprolactone) and Poly(d,l-Lactic-Co-Glycolic Acid)–Poly(Ethylene Glycol) Blend for Prostate Cancer Treatment. Mol. Pharm. 2013, 10, 3871–3881. [Google Scholar] [CrossRef]
- Yao, Q.; Hou, S.-X.; He, W.-L.; Feng, J.-L.; Wang, X.-C.; Fei, H.-X.; Chen, Z.-H. Study on the preparation of resveratrol chitosan nanoparticles with free amino groups on the surface. Zhongguo Zhong Yao Za Zhi 2006, 31, 205–208. [Google Scholar] [PubMed]
- Huang, M.; Liang, C.; Tan, C.; Huang, S.; Ying, R.; Wang, Y.; Wang, Z.; Zhang, Y. Liposome Co-Encapsulation as a Strategy for the Delivery of Curcumin and Resveratrol. Food Funct. 2019, 10, 6447–6458. [Google Scholar] [CrossRef]
- Lombardo, D.; Kiselev, M.A. Methods of Liposomes Preparation: Formation and Control Factors of Versatile Nanocarriers for Biomedical and Nanomedicine Application. Pharmaceutics 2022, 14, 543. [Google Scholar] [CrossRef] [PubMed]
- Andra, V.V.S.N.L.; Pammi, S.V.N.; Bhatraju, L.V.K.P.; Ruddaraju, L.K. A Comprehensive Review on Novel Liposomal Methodologies, Commercial Formulations, Clinical Trials and Patents. BioNanoSci 2022, 12, 274–291. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, B.D.; Rodrigues, A.R.O.; Bañobre-López, M.; Almeida, B.G.; Amorim, C.O.; Amaral, V.S.; Coutinho, P.J.G.; Castanheira, E.M.S. Magnetoliposomes Based on Shape Anisotropic Calcium/Magnesium Ferrite Nanoparticles as Nanocarriers for Doxorubicin. Pharmaceutics 2021, 13, 1248. [Google Scholar] [CrossRef]
- Caddeo, C.; Nacher, A.; Vassallo, A.; Armentano, M.F.; Pons, R.; Fernàndez-Busquets, X.; Carbone, C.; Valenti, D.; Fadda, A.M.; Manconi, M. Effect of Quercetin and Resveratrol Co-Incorporated in Liposomes against Inflammatory/Oxidative Response Associated with Skin Cancer. Int. J. Pharm. 2016, 513, 153–163. [Google Scholar] [CrossRef]
- Jhaveri, A.; Deshpande, P.; Pattni, B.; Torchilin, V. Transferrin-Targeted, Resveratrol-Loaded Liposomes for the Treatment of Glioblastoma. J. Control. Release 2018, 277, 89–101. [Google Scholar] [CrossRef]
- Wang, X.-X.; Li, Y.-B.; Yao, H.-J.; Ju, R.-J.; Zhang, Y.; Li, R.-J.; Yu, Y.; Zhang, L.; Lu, W.-L. The Use of Mitochondrial Targeting Resveratrol Liposomes Modified with a Dequalinium Polyethylene Glycol-Distearoylphosphatidyl Ethanolamine Conjugate to Induce Apoptosis in Resistant Lung Cancer Cells. Biomaterials 2011, 32, 5673–5687. [Google Scholar] [CrossRef]
- Meng, J.; Guo, F.; Xu, H.; Liang, W.; Wang, C.; Yang, X.-D. Combination Therapy Using Co-Encapsulated Resveratrol and Paclitaxel in Liposomes for Drug Resistance Reversal in Breast Cancer Cells in Vivo. Sci. Rep. 2016, 6, 22390. [Google Scholar] [CrossRef]
- Yang, T.; Zhai, J.; Hu, D.; Yang, R.; Wang, G.; Li, Y.; Liang, G. “Targeting Design” of Nanoparticles in Tumor Therapy. Pharmaceutics 2022, 14, 1919. [Google Scholar] [CrossRef]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a Strategy for Improving Nanoparticle-Based Drug and Gene Delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef] [PubMed]
- Sanna, V.; Pala, N.; Sechi, M. Targeted Therapy Using Nanotechnology: Focus on Cancer. Int. J. Nanomed. 2014, 9, 467. [Google Scholar]
- Daniels, T.R.; Delgado, T.; Rodriguez, J.A.; Helguera, G.; Penichet, M.L. The Transferrin Receptor Part I: Biology and Targeting with Cytotoxic Antibodies for the Treatment of Cancer. Clin. Immunol. 2006, 121, 144–158. [Google Scholar] [CrossRef]
- Poonia, N.; Kaur Narang, J.; Lather, V.; Beg, S.; Sharma, T.; Singh, B.; Pandita, D. Resveratrol Loaded Functionalized Nanostructured Lipid Carriers for Breast Cancer Targeting: Systematic Development, Characterization and Pharmacokinetic Evaluation. Colloids Surf. B Biointerfaces 2019, 181, 756–766. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.T.; Zeng, X.F.; Yang, H.; Jia, M.L.; Zhang, W.; Liu, W.; Liu, S.Y. Resveratrol Loaded by Folate-Modified Liposomes Inhibits Osteosarcoma Growth and Lung Metastasis via Regulating JAK2/STAT3 Pathway. Int. J. Nanomed. 2023, 18, 2677–2691. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, L.; Le, Y.; Chen, J.-F.; Wang, J.; Yun, J. Synergistic Effect of PEGylated Resveratrol on Delivery of Anticancer Drugs. Int. J. Pharm. 2016, 498, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Feng, H.; Liu, L.; Peng, J.; Xiao, H.; Yu, T.; Zhou, Z.; Li, Y.; Zhang, Y.; Bai, X.; et al. Enhanced Antiproliferative Effect of Resveratrol in Head and Neck Squamous Cell Carcinoma Using GE11 Peptide Conjugated Liposome. Int. J. Mol. Med. 2019, 43, 1635–1642. [Google Scholar] [CrossRef]
- Zielińska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef]
- Pulingam, T.; Foroozandeh, P.; Chuah, J.-A.; Sudesh, K. Exploring Various Techniques for the Chemical and Biological Synthesis of Polymeric Nanoparticles. Nanomaterials 2022, 12, 576. [Google Scholar]
- Yee, Y.J.; Benson, H.A.E.; Dass, C.R.; Chen, Y. Evaluation of Novel Conjugated Resveratrol Polymeric Nanoparticles in Reduction of Plasma Degradation, Hepatic Metabolism and Its Augmentation of Anticancer Activity in Vitro and in Vivo. Int. J. Pharm. 2022, 615, 121499. [Google Scholar] [CrossRef]
- Madej, M.; Kurowska, N.; Strzalka-Mrozik, B. Polymeric Nanoparticles—Tools in a Drug Delivery System in Selected Cancer Therapies. Appl. Sci. 2022, 12, 9479. [Google Scholar] [CrossRef]
- Hao, J.; Tong, T.; Jin, K.; Zhuang, Q.; Han, T.; Bi, Y.; Wang, J.; Wang, X. Folic Acid-Functionalized Drug Delivery Platform of Resveratrol Based on Pluronic 127/D-α-Tocopheryl Polyethylene Glycol 1000 Succinate Mixed Micelles. IJN 2017, 12, 2279–2292. [Google Scholar] [CrossRef]
- Jadhav, P.; Bothiraja, C.; Pawar, A. Resveratrol-Piperine Loaded Mixed Micelles: Formulation, Characterization, Bioavailability, Safety and in Vitro Anticancer Activity. RSC Adv. 2016, 6, 112795–112805. [Google Scholar] [CrossRef]
- Lian, B.; Wu, M.; Feng, Z.; Deng, Y.; Zhong, C.; Zhao, X. Folate-Conjugated Human Serum Albumin-Encapsulated Resveratrol Nanoparticles: Preparation, Characterization, Bioavailability and Targeting of Liver Tumors. Artif. Cells Nanomed. Biotechnol. 2019, 47, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Benfante, V.; Stefano, A.; Ali, M.; Laudicella, R.; Arancio, W.; Cucchiara, A.; Caruso, F.; Cammarata, F.P.; Coronnello, C.; Russo, G.; et al. An Overview of In Vitro Assays of 64Cu-, 68Ga-, 125I-, and 99mTc-Labelled Radiopharmaceuticals Using Radiometric Counters in the Era of Radiotheranostics. Diagnostics 2023, 13, 1210. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.-H.; Lee, J.H.; Thien Quach, C.H.; Paik, J.-Y.; Oh, H.; Park, J.W.; Lee, E.J.; Moon, S.-H.; Lee, K.-H. Resveratrol Suppresses Cancer Cell Glucose Uptake by Targeting Reactive Oxygen Species–Mediated Hypoxia-Inducible Factor-1α Activation. J. Nucl. Med. 2013, 54, 2161. [Google Scholar] [CrossRef]
- Zhao, Y.; Huan, M.; Liu, M.; Cheng, Y.; Sun, Y.; Cui, H.; Liu, D.; Mei, Q.; Zhou, S. Doxorubicin and Resveratrol Co-Delivery Nanoparticle to Overcome Doxorubicin Resistance. Sci. Rep. 2016, 6, 35267. [Google Scholar] [CrossRef]
- Sudha, T.; El-Far, A.H.; Mousa, D.S.; Mousa, S.A. Resveratrol and Its Nanoformulation Attenuate Growth and the Angiogenesis of Xenograft and Orthotopic Colon Cancer Models. Molecules 2020, 25, 1412. [Google Scholar] [CrossRef]
- Aldawsari, H.M.; Alhakamy, N.A.; Padder, R.; Husain, M.; Md, S. Preparation and Characterization of Chitosan Coated PLGA Nanoparticles of Resveratrol: Improved Stability, Antioxidant and Apoptotic Activities in H1299 Lung Cancer Cells. Coatings 2020, 10, 439. [Google Scholar] [CrossRef]
- Zhang, Z.; Ji, Y.; Hu, N.; Yu, Q.; Zhang, X.; Li, J.; Wu, F.; Xu, H.; Tang, Q.; Li, X. Ferroptosis-Induced Anticancer Effect of Resveratrol with a Biomimetic Nano-Delivery System in Colorectal Cancer Treatment. Asian J. Pharm. Sci. 2022, 17, 751–766. [Google Scholar] [CrossRef]
- Zuo, H. iRGD: A Promising Peptide for Cancer Imaging and a Potential Therapeutic Agent for Various Cancers. J. Oncol. 2019, 2019, 9367845. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Su, T.; Shi, Q.; Feng, Y.; Tao, Z.; Huang, Q.; Li, L.; Hu, L.; Li, S.; Tan, H. Co-Administration of iRGD Enhances Tumor-Targeted Delivery and Anti-Tumor Effects of Paclitaxel-Loaded PLGA Nanoparticles for Colorectal Cancer Treatment. Int. J. Nanomed. 2019, 14, 8543–8560. [Google Scholar] [CrossRef] [PubMed]
- Geng, T.; Zhao, X.; Ma, M.; Zhu, G.; Yin, L. Resveratrol-Loaded Albumin Nanoparticles with Prolonged Blood Circulation and Improved Biocompatibility for Highly Effective Targeted Pancreatic Tumor Therapy. Nanoscale Res. Lett. 2017, 12, 437. [Google Scholar] [CrossRef] [PubMed]
- Long, Q.; Zhu, W.; Guo, L.; Pu, L. RGD-Conjugated Resveratrol HSA Nanoparticles as a Novel Delivery System in Ovarian Cancer Therapy. Drug Des. Dev. Ther. 2020, 14, 5747–5756. [Google Scholar] [CrossRef]
- Guo, W.; Li, A.; Jia, Z.; Yuan, Y.; Dai, H.; Li, H. Transferrin Modified PEG-PLA-Resveratrol Conjugates: In Vitro and in Vivo Studies for Glioma. Eur. J. Pharmacol. 2013, 718, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Paranthaman, S.; Rizvi, S.M.D.; Moin, A.; Gowda, D.V.; Subaiea, G.M.; Ansari, M.; Alanazi, A.S. Fabrication and Characterization of Paclitaxel and Resveratrol Loaded Soluplus Polymeric Nanoparticles for Improved BBB Penetration for Glioma Management. Polymers 2021, 13, 3210. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, S.; Prasad, N.R.; Ganamani, A.; Balamurugan, E. Anticancer Activity of Resveratrol-Loaded Gelatin Nanoparticles on NCI-H460 Non-Small Cell Lung Cancer Cells. Biomed. Prev. Nutr. 2013, 3, 64–73. [Google Scholar] [CrossRef]
- Elzoghby, A.O.; El-Lakany, S.A.; Helmy, M.W.; Abu-Serie, M.M.; Elgindy, N.A. Shell-Crosslinked Zein Nanocapsules for Oral Codelivery of Exemestane and Resveratrol in Breast Cancer Therapy. Nanomedicine 2017, 12, 2785–2805. [Google Scholar]
- Lu, X.; Ji, C.; Xu, H.; Li, X.; Ding, H.; Ye, M.; Zhu, Z.; Ding, D.; Jiang, X.; Ding, X.; et al. Resveratrol-Loaded Polymeric Micelles Protect Cells from Aβ-Induced Oxidative Stress. Int. J. Pharm. 2009, 375, 89–96. [Google Scholar] [CrossRef]
- Srinivasa-Gopalan, S.; Yarema, K. Nanotechnologies for the Life Sciences: Dendrimers in Cancer Treatment and Diagnosis. In Treatment Diagnosis; Wiley: Hoboken, NJ, USA, 2007. [Google Scholar]
- Santos, A.; Veiga, F.; Figueiras, A. Dendrimers as Pharmaceutical Excipients: Synthesis, Properties, Toxicity and Biomedical Applications. Materials 2020, 13, 65. [Google Scholar] [CrossRef]
- Sathe, R.Y.; Bharatam, P.V. Drug-Dendrimer Complexes and Conjugates: Detailed Furtherance through Theory and Experiments. Adv. Colloid Interface Sci. 2022, 303, 102639. [Google Scholar] [CrossRef] [PubMed]
- Tomalia, D.; Uppuluri, S.; Swanson, D.; Li, J. Dendrimers as Reactive Modules for the Synthesis of New Structure-Controlled, Higher-Complexity Megamers. Pure Appl. Chem. 2000, 72, 2343–2358. [Google Scholar] [CrossRef]
- An, H.; Deng, X.; Wang, F.; Xu, P.; Wang, N. Dendrimers as Nanocarriers for the Delivery of Drugs Obtained from Natural Products. Polymers 2023, 15, 2292. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T. Preparation and in Vitro Evaluation of Lactobionic Acid Modified Polyamide-Amine Dendrimer Grafted Resveratrol Nanoparticles. Chin. Tradit. Herb. Drugs 2020, 20, 4457–4463. [Google Scholar]
- Gu, Y.; Cai, Y.; Kou, Y.; Cheng, E.; Bi, H.; Hu, M.; Wu, S.; Jiang, Y.; Zhang, J.; Wu, Q.; et al. Construction of Multifunctional Targeted Nano-Prodrugs Based on PAMAM Dendrimers for Tumor Therapy. Eur. Polym. J. 2023, 200, 112486. [Google Scholar] [CrossRef]
- Kececiler-Emir, C.; Ilhan-Ayisigi, E.; Celen-Erden, C.; Nalbantsoy, A.; Yesil-Celiktas, O. Synthesis of Resveratrol Loaded Hybrid Silica-PAMAM Dendrimer Nanoparticles With Emphases on Inducible Nitric Oxide Synthase and Cytotoxicity. Plant Foods Hum. Nutr. 2021, 76, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Tenchov, R.; Bird, R.; Curtze, A.E.; Zhou, Q. Lipid Nanoparticles─From Liposomes to mRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement. ACS Nano 2021, 15, 16982–17015. [Google Scholar] [CrossRef] [PubMed]
- Üner, M.; Yener, G. Importance of Solid Lipid Nanoparticles (SLN) in Various Administration Routes and Future Perspectives. Int. J. Nanomed. 2007, 2, 289–300. [Google Scholar]
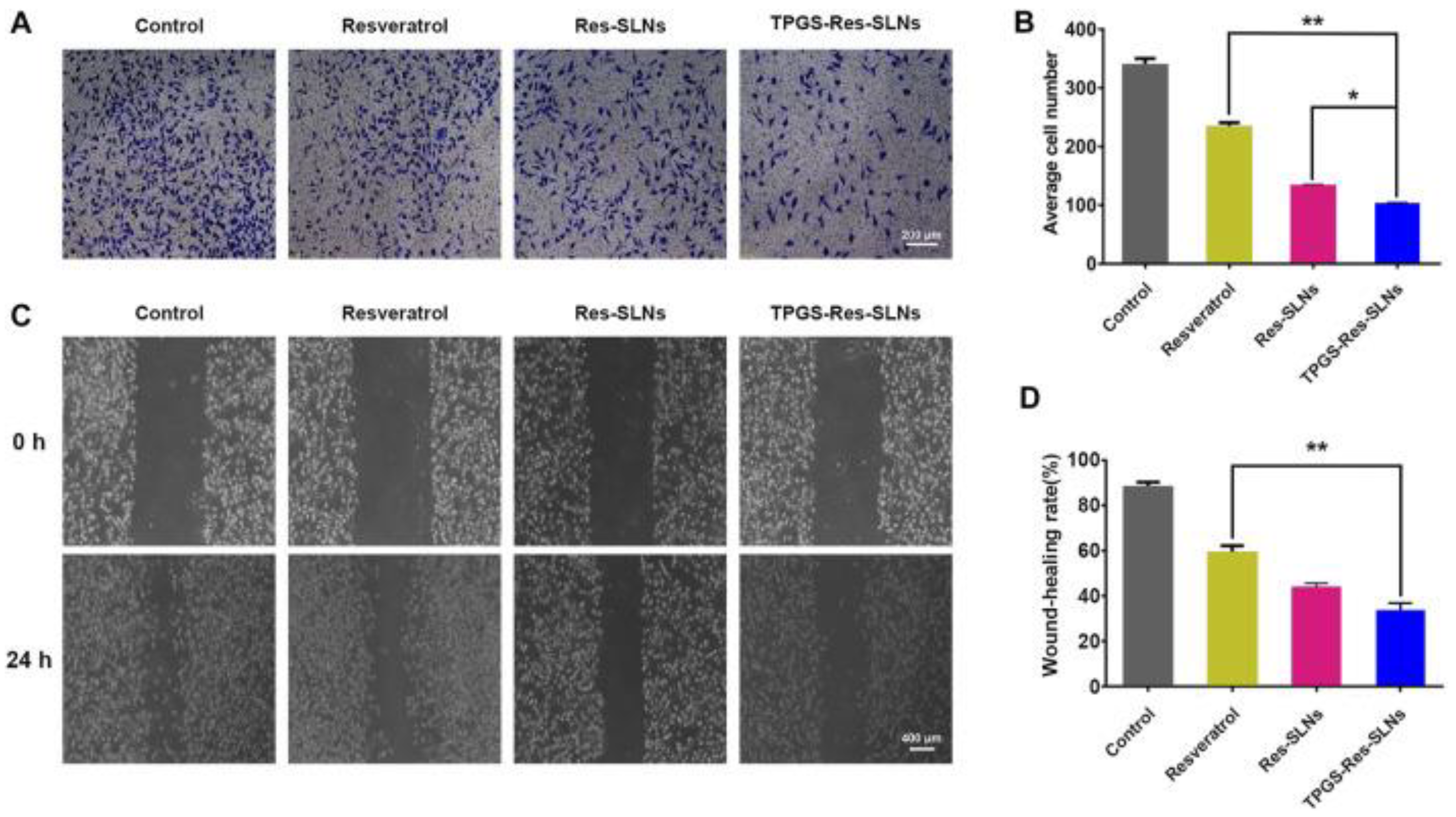
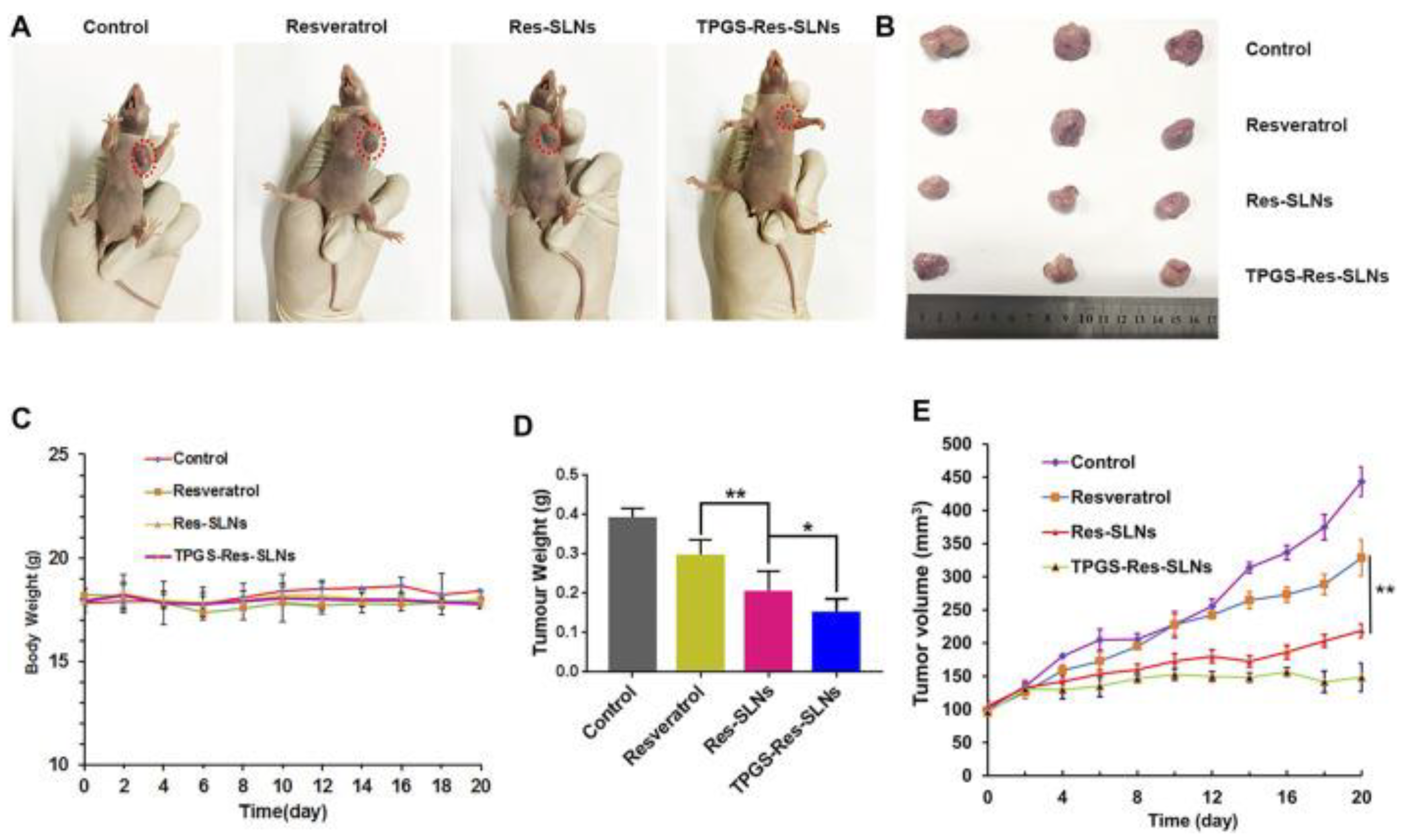
- Wang, W.; Zhou, M.; Xu, Y.; Peng, W.; Zhang, S.; Li, R.; Zhang, H.; Zhang, H.; Cheng, S.; Wang, Y.; et al. Resveratrol-Loaded TPGS-Resveratrol-Solid Lipid Nanoparticles for Multidrug-Resistant Therapy of Breast Cancer: In Vivo and In Vitro Study. Front. Bioeng. Biotechnol. 2021, 9, 762489. [Google Scholar] [CrossRef]
- Teskač, K.; Kristl, J. The Evidence for Solid Lipid Nanoparticles Mediated Cell Uptake of Resveratrol. Int. J. Pharm. 2010, 390, 61–69. [Google Scholar] [CrossRef]
- Song, Z.; Shi, Y.; Han, Q.; Dai, G. Endothelial Growth Factor Receptor-Targeted and Reactive Oxygen Species-Responsive Lung Cancer Therapy by Docetaxel and Resveratrol Encapsulated Lipid-Polymer Hybrid Nanoparticles. Biomed. Pharmacother. 2018, 105, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Giaccone, P.; Benfante, V.; Stefano, A.; Cammarata, F.P.; Russo, G.; Comelli, A. PET Images Atlas-Based Segmentation Performed in Native and in Template Space: A Radiomics Repeatability Study in Mouse Models. In Proceedings of the Image Analysis and Processing. ICIAP 2022 Workshops; Mazzeo, P.L., Frontoni, E., Sclaroff, S., Distante, C., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 351–361. [Google Scholar]
- Benfante, V.; Stefano, A.; Comelli, A.; Giaccone, P.; Cammarata, F.P.; Richiusa, S.; Scopelliti, F.; Pometti, M.; Ficarra, M.; Cosentino, S.; et al. A New Preclinical Decision Support System Based on PET Radiomics: A Preliminary Study on the Evaluation of an Innovative 64Cu-Labeled Chelator in Mouse Models. J. Imaging 2022, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Figueiró, F.; Bernardi, A.; Frozza, R.L.; Terroso, T.; Zanotto-Filho, A.; Jandrey, E.H.F.; Moreira, J.C.F.; Salbego, C.G.; Edelweiss, M.I.; Pohlmann, A.R.; et al. Resveratrol-Loaded Lipid-Core Nanocapsules Treatment Reduces In Vitro and In Vivo Glioma Growth. J. Biomed. Nanotechnol. 2013, 9, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhang, J.; Zeng, J.; Li, Z.; Zuo, H.; Huang, C.; Zhao, X. Nano-Gold Loaded with Resveratrol Enhance the Anti-Hepatoma Effect of Resveratrol In Vitro and In Vivo. J. Biomed. Nanotechnol. 2019, 15, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Chae, S.Y.; Park, J.O.; Lee, K.J.; Park, G. Gold-Conjugated Resveratrol Nanoparticles Attenuate the Invasion and MMP-9 and COX-2 Expression in Breast Cancer Cells. Oncol. Rep. 2016, 35, 3248–3256. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.G.; Lee, M.; Go, E.B.; Chung, N. Resveratrol-Loaded Gold Nanoparticles Enhance Caspase-Mediated Apoptosis in PANC-1 Pancreatic Cells via Mitochondrial Intrinsic Apoptotic Pathway. Cancer Nano 2022, 13, 34. [Google Scholar] [CrossRef]
- Anjum, S.; Hashim, M.; Malik, S.A.; Khan, M.; Lorenzo, J.M.; Abbasi, B.H.; Hano, C. Recent Advances in Zinc Oxide Nanoparticles (ZnO NPs) for Cancer Diagnosis, Target Drug Delivery, and Treatment. Cancers 2021, 13, 4570. [Google Scholar] [CrossRef] [PubMed]
- Raha, S.; Ahmaruzzaman, M. ZnO Nanostructured Materials and Their Potential Applications: Progress, Challenges and Perspectives. Nanoscale Adv. 2022, 4, 1868–1925. [Google Scholar] [CrossRef]
- Zhou, X.-Q.; Hayat, Z.; Zhang, D.-D.; Li, M.-Y.; Hu, S.; Wu, Q.; Cao, Y.-F.; Yuan, Y. Zinc Oxide Nanoparticles: Synthesis, Characterization, Modification, and Applications in Food and Agriculture. Processes 2023, 11, 1193. [Google Scholar] [CrossRef]
- Das, H.T.; Korkunda, T.B.; Babu, S.P.; Pal, A.K.; Joshi, D.N. 8—Chemical Methods for the Growth of Oxides. In Defect-Induced Magnetism in Oxide Semiconductors; Kumar, P., Pal Singh, J., Kumar, V., Eds.; Woodhead Publishing: Cambridge, UK, 2023; pp. 165–199. ISBN 978-0-323-90907-5. [Google Scholar]
- Jin, S.-E.; Jin, H.-E. Synthesis, Characterization, and Three-Dimensional Structure Generation of Zinc Oxide-Based Nanomedicine for Biomedical Applications. Pharmaceutics 2019, 11, 575. [Google Scholar] [CrossRef]
- Chung, I.-M.; Abdul Rahuman, A.; Marimuthu, S.; Vishnu Kirthi, A.; Anbarasan, K.; Rajakumar, G. An Investigation of the Cytotoxicity and Caspase-Mediated Apoptotic Effect of Green Synthesized Zinc Oxide Nanoparticles Using Eclipta Prostrata on Human Liver Carcinoma Cells. Nanomaterials 2015, 5, 1317–1330. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Lv, Y.; Li, N.; Huang, X.; Liu, X.; Li, H.; Wang, C.; Jia, Y.-F. Biological Investigations on Therapeutic Effect of Chitosan Encapsulated Nano Resveratrol against Gestational Diabetes Mellitus Rats Induced by Streptozotocin. Drug Deliv. 2020, 27, 953–963. [Google Scholar] [CrossRef] [PubMed]
- Bennett, T.D.; Yue, Y.; Li, P.; Qiao, A.; Tao, H.; Greaves, N.G.; Richards, T.; Lampronti, G.I.; Redfern, S.A.T.; Blanc, F.; et al. Melt-Quenched Glasses of Metal–Organic Frameworks. J. Am. Chem. Soc. 2016, 138, 3484–3492. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.C.; Bennett, T.D.; Cheetham, A.K. Chemical Structure, Network Topology, and Porosity Effects on the Mechanical Properties of Zeolitic Imidazolate Frameworks. Proc. Natl. Acad. Sci. USA 2010, 107, 9938–9943. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, M.J.; Hashemian, S.; Moktarian, N. Structural and Magnetic Properties of Zeolitic Imidazolate Framework Supported on Nickel Titanate. J. Mol. Struct. 2021, 1240, 130555. [Google Scholar] [CrossRef]
- Banerjee, R.; Phan, A.; Wang, B.; Knobler, C.; Furukawa, H.; O’Keeffe, M.; Yaghi, O.M. High-Throughput Synthesis of Zeolitic Imidazolate Frameworks and Application to CO2 Capture. Science 2008, 319, 939–943. [Google Scholar] [CrossRef] [PubMed]
- Phan, A.; Doonan, C.J.; Uribe-Romo, F.J.; Knobler, C.B.; O’Keeffe, M.; Yaghi, O.M. Synthesis, Structure, and Carbon Dioxide Capture Properties of Zeolitic Imidazolate Frameworks. Acc. Chem. Res. 2010, 43, 58–67. [Google Scholar] [CrossRef]
- Madsen, R.S.K.; Qiao, A.; Sen, J.; Hung, I.; Chen, K.; Gan, Z.; Sen, S.; Yue, Y. Ultrahigh-Field 67Zn NMR Reveals Short-Range Disorder in Zeolitic Imidazolate Framework Glasses. Science 2020, 367, 1473–1476. [Google Scholar] [CrossRef]
- Yang, W.; Kong, Y.; Yin, H.; Cao, M. Study on the Adsorption Performance of ZIF-8 on Heavy Metal Ions in Water and the Recycling of Waste ZIF-8 in Cement. J. Solid State Chem. 2023, 326, 124217. [Google Scholar] [CrossRef]
- Zheng, H.; Zhang, Y.; Liu, L.; Wan, W.; Guo, P.; Nyström, A.M.; Zou, X. One-Pot Synthesis of Metal–Organic Frameworks with Encapsulated Target Molecules and Their Applications for Controlled Drug Delivery. J. Am. Chem. Soc. 2016, 138, 962–968. [Google Scholar] [CrossRef]
- Sun, C.-Y.; Qin, C.; Wang, X.-L.; Yang, G.-S.; Shao, K.-Z.; Lan, Y.-Q.; Su, Z.-M.; Huang, P.; Wang, C.-G.; Wang, E.-B. Zeolitic Imidazolate Framework-8 as Efficient pH-Sensitive Drug Delivery Vehicle. Dalton Trans. 2012, 41, 6906–6909. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Semcheddine, F.; Jiang, H.; Wang, X. Acid-Responsive Multifunctional Zeolitic Imidazolate Framework-8 (ZIF-8) Nanocomposites for Tumor Chemo-Photothermal Synergistic Therapy. Bioconj. Chem. 2022, 33, 1405–1414. [Google Scholar] [CrossRef] [PubMed]
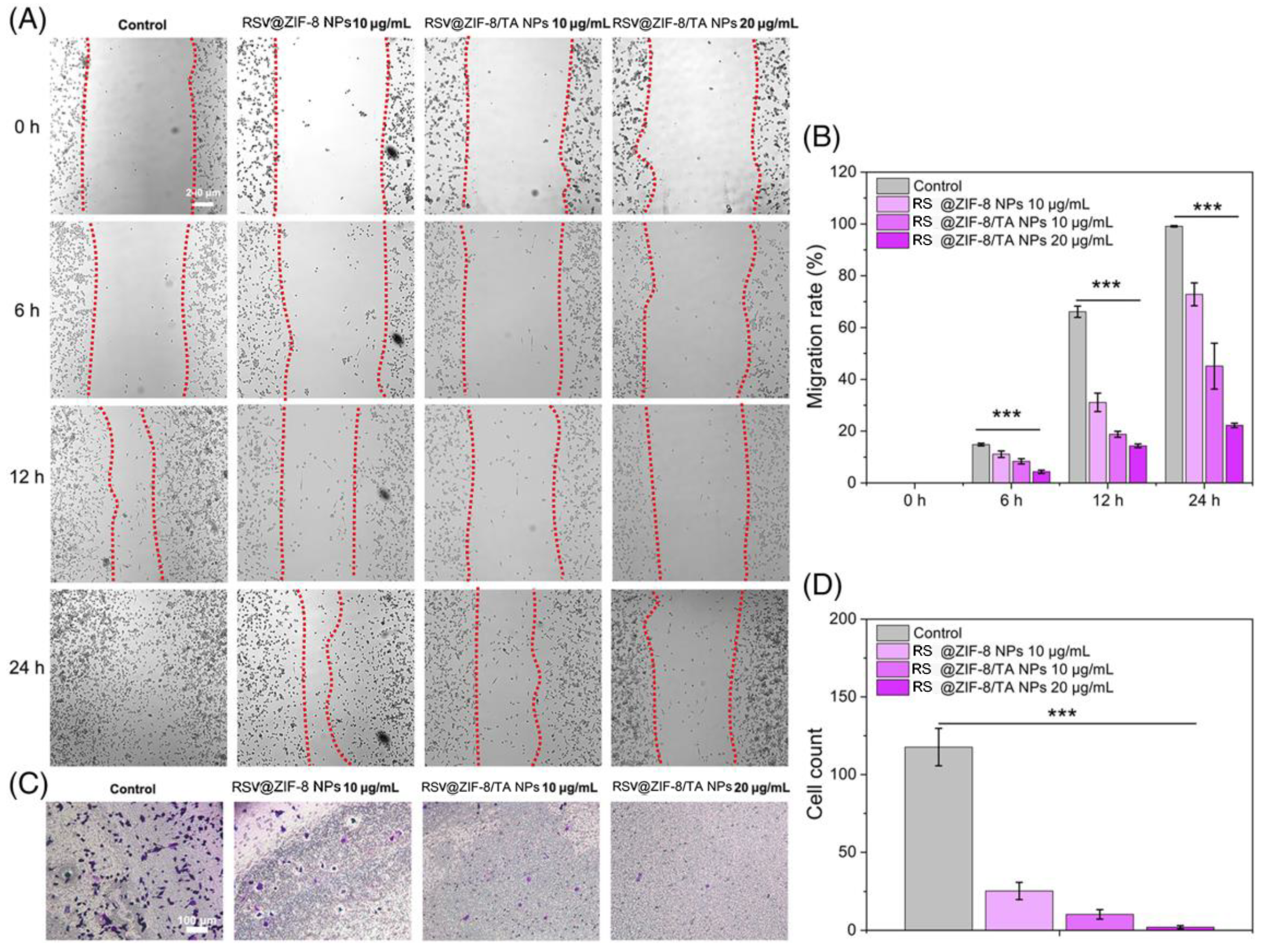
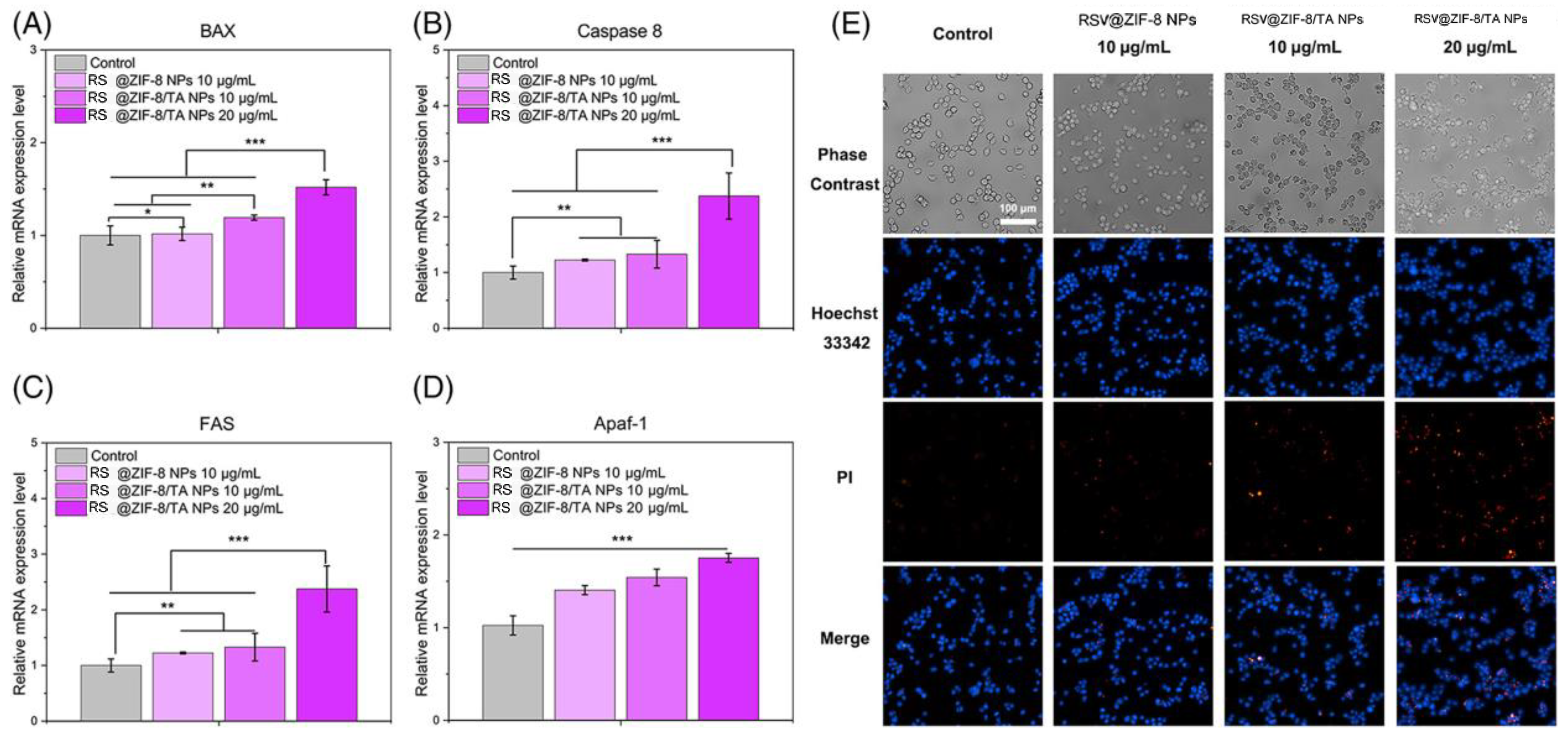
- Sun, X.; Li, F.; Yuan, L.; Bing, Z.; Li, X.; Yang, K. pH-Responsive Resveratrol-Loaded ZIF-8 Nanoparticles Modified with Tannic Acid for Promoting Colon Cancer Cell Apoptosis. J. Biomed. Mater. Res. Part B Appl. Biomater. 2024, 112, e35320. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Liao, Z.; Li, M.; Zhang, H.; Li, T.; Qin, X.; Li, S.; Wu, C.; You, F.; Liao, X.; et al. Mesoporous Silica Nanoparticles-Based Nanoplatforms: Basic Construction, Current State, and Emerging Applications in Anticancer Therapeutics. Adv. Healthc. Mater. 2023, 12, 2201884. [Google Scholar] [CrossRef]
- Moradi, Z.; Ghorbani-Choghamarani, A. Green Preparation and Characterization of AGC-ZM-2022 as a Novel Mesoporous Silica Material Using Palmitic Acid as a Natural Template. RSC Adv. 2023, 13, 2265–2268. [Google Scholar] [CrossRef] [PubMed]
- Lérida-Viso, A.; Estepa-Fernández, A.; García-Fernández, A.; Martí-Centelles, V.; Martínez-Máñez, R. Biosafety of Mesoporous Silica Nanoparticles; towards Clinical Translation. Adv. Drug Deliv. Rev. 2023, 201, 115049. [Google Scholar] [CrossRef]
- Nandiyanto, A.B.D.; Iskandar, F.; Okuyama, K. Nanosized Polymer Particle-Facilitated Preparation of Mesoporous Silica Particles Using a Spray Method. Chem. Lett. 2008, 37, 1040–1041. [Google Scholar] [CrossRef]
- Nandiyanto, A.B.D.; Kim, S.-G.; Iskandar, F.; Okuyama, K. Synthesis of Spherical Mesoporous Silica Nanoparticles with Nanometer-Size Controllable Pores and Outer Diameters. Microporous Mesoporous Mater. 2009, 120, 447–453. [Google Scholar] [CrossRef]
- Niculescu, V.-C. Mesoporous Silica Nanoparticles for Bio-Applications. Front. Mater. 2020, 7, 36. [Google Scholar] [CrossRef]
- Di Renzo, F.; Cambon, H.; Dutartre, R. A 28-Year-Old Synthesis of Micelle-Templated Mesoporous Silica. Microporous Mater. 1997, 10, 283–286. [Google Scholar] [CrossRef]
- Li, Q.; Zhou, Y. Brief History, Preparation Method, and Biological Application of Mesoporous Silica Molecular Sieves: A Narrative Review. Molecules 2023, 28, 2013. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, Z.; Subramaniam, S.; Khan, G.M.; Abeer, M.M.; Qu, Z.; Janjua, T.; Kumeria, T.; Batra, J.; Popat, A. Encapsulation and Controlled Release of Resveratrol Within Functionalized Mesoporous Silica Nanoparticles for Prostate Cancer Therapy. Front. Bioeng. Biotechnol. 2019, 7, 225. [Google Scholar] [CrossRef] [PubMed]
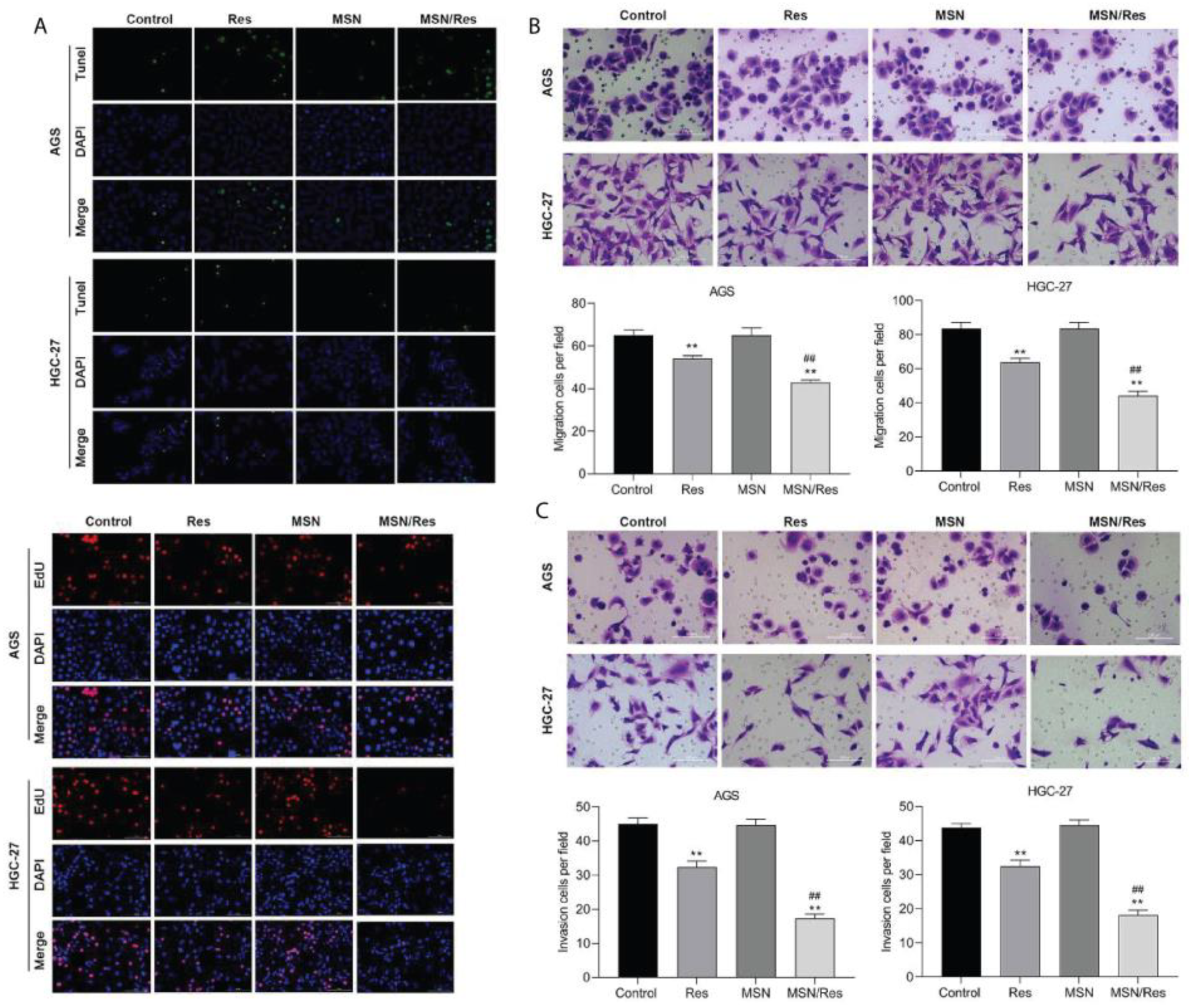
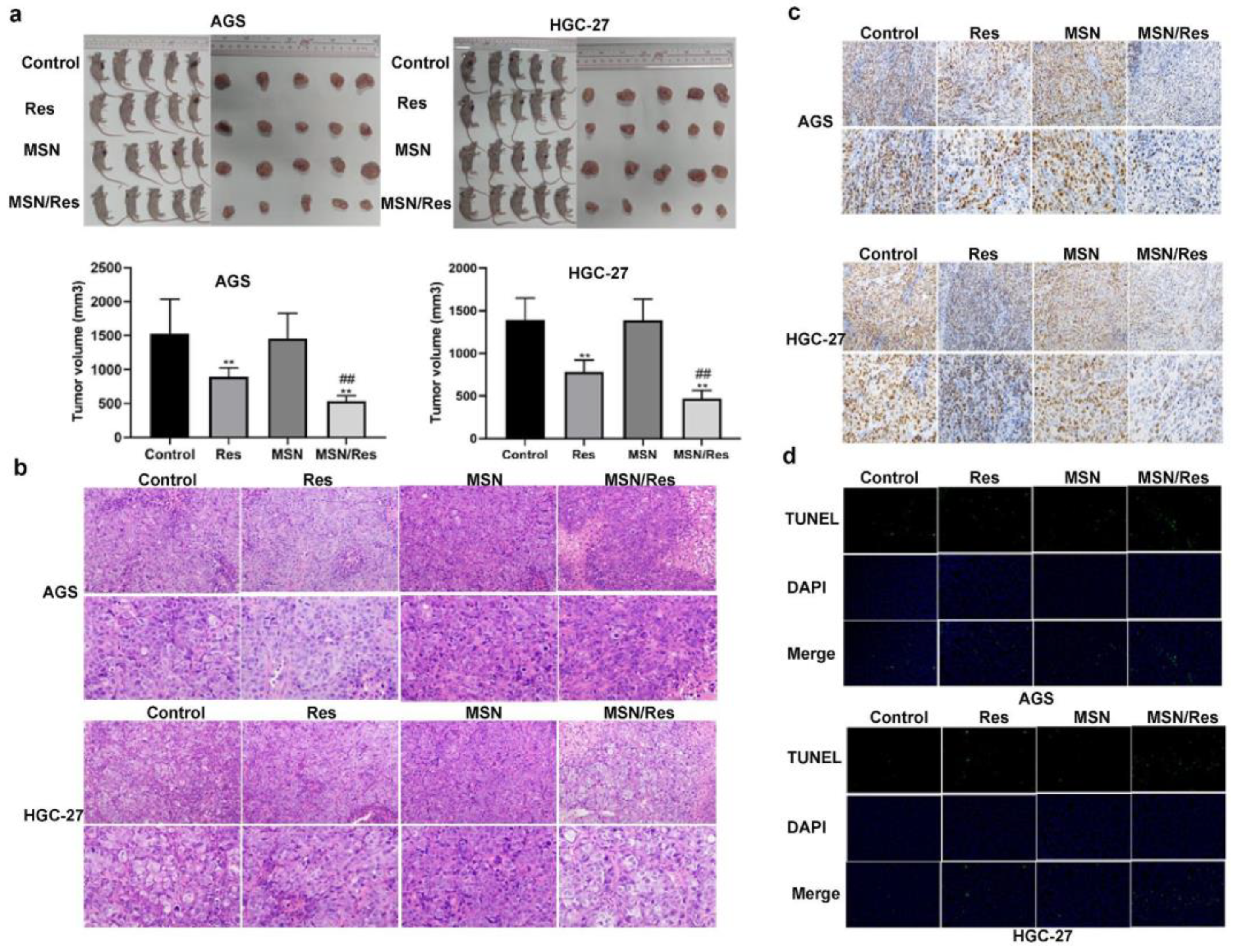
- Gu, Y.; Fei, Z. Mesoporous Silica Nanoparticles Loaded with Resveratrol Are Used for Targeted Breast Cancer Therapy. J. Oncol. 2022, 2022, 8471331. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Yao, W.; Xiao, Y.; Dong, Z.; Huang, W.; Zhang, F.; Zhou, X.; Liang, M. Resveratrol-Modified Mesoporous Silica Nanoparticle for Tumor-Targeted Therapy of Gastric Cancer. Bioengineered 2021, 12, 6343–6353. [Google Scholar] [CrossRef] [PubMed]
- Summerlin, N.; Qu, Z.; Pujara, N.; Sheng, Y.; Jambhrunkar, S.; McGuckin, M.; Popat, A. Colloidal Mesoporous Silica Nanoparticles Enhance the Biological Activity of Resveratrol. Colloids Surf. B Biointerfaces 2016, 144, 1–7. [Google Scholar] [CrossRef]
- Marinheiro, D.; Ferreira, B.; Oskoei, P.; Oliveira, H.; Daniel-da-Silva, A. Encapsulation and Enhanced Release of Resveratrol from Mesoporous Silica Nanoparticles for Melanoma Therapy. Materials 2021, 14, 1382. [Google Scholar] [CrossRef]
- Patila, M.; Chalmpes, N.; Dounousi, E.; Stamatis, H.; Gournis, D. Chapter Twelve—Use of Functionalized Carbon Nanotubes for the Development of Robust Nanobiocatalysts. In Methods in Enzymology; Kumar, C.V., Ed.; Academic Press: New York, NY, USA, 2020; Volume 630, pp. 263–301. ISBN 0076-6879. [Google Scholar]
- Ali, A.; Rahimian Koloor, S.S.; Alshehri, A.H.; Arockiarajan, A. Carbon Nanotube Characteristics and Enhancement Effects on the Mechanical Features of Polymer-Based Materials and Structures—A Review. J. Mater. Res. Technol. 2023, 24, 6495–6521. [Google Scholar] [CrossRef]
- Zare, H.; Ahmadi, S.; Ghasemi, A.; Ghanbari, M.; Rabiee, N.; Bagherzadeh, M.; Karimi, M.; Webster, T.J.; Hamblin, M.R.; Mostafavi, E. Carbon Nanotubes: Smart Drug/Gene Delivery Carriers. IJN 2021, 16, 1681–1706. [Google Scholar] [CrossRef]
- Pu, Z.; Wei, Y.; Sun, Y.; Wang, Y.; Zhu, S. Carbon Nanotubes as Carriers in Drug Delivery for Non-Small Cell Lung Cancer, Mechanistic Analysis of Their Carcinogenic Potential, Safety Profiling and Identification of Biomarkers. IJN 2022, 17, 6157–6180. [Google Scholar] [CrossRef]
- Loh, X.J.; Ong, S.J.; Tung, Y.T.; Choo, H.T. Co-Delivery of Drug and DNA from Cationic Dual-Responsive Micelles Derived from Poly (DMAEMA-Co-PPGMA). Mater. Sci. Eng. C 2013, 33, 4545–4550. [Google Scholar] [CrossRef]
- Kong, H.; Luo, P.; Gao, C.; Yan, D. Polyelectrolyte-Functionalized Multiwalled Carbon Nanotubes: Preparation, Characterization and Layer-by-Layer Self-Assembly. Polymer 2005, 46, 2472–2485. [Google Scholar]
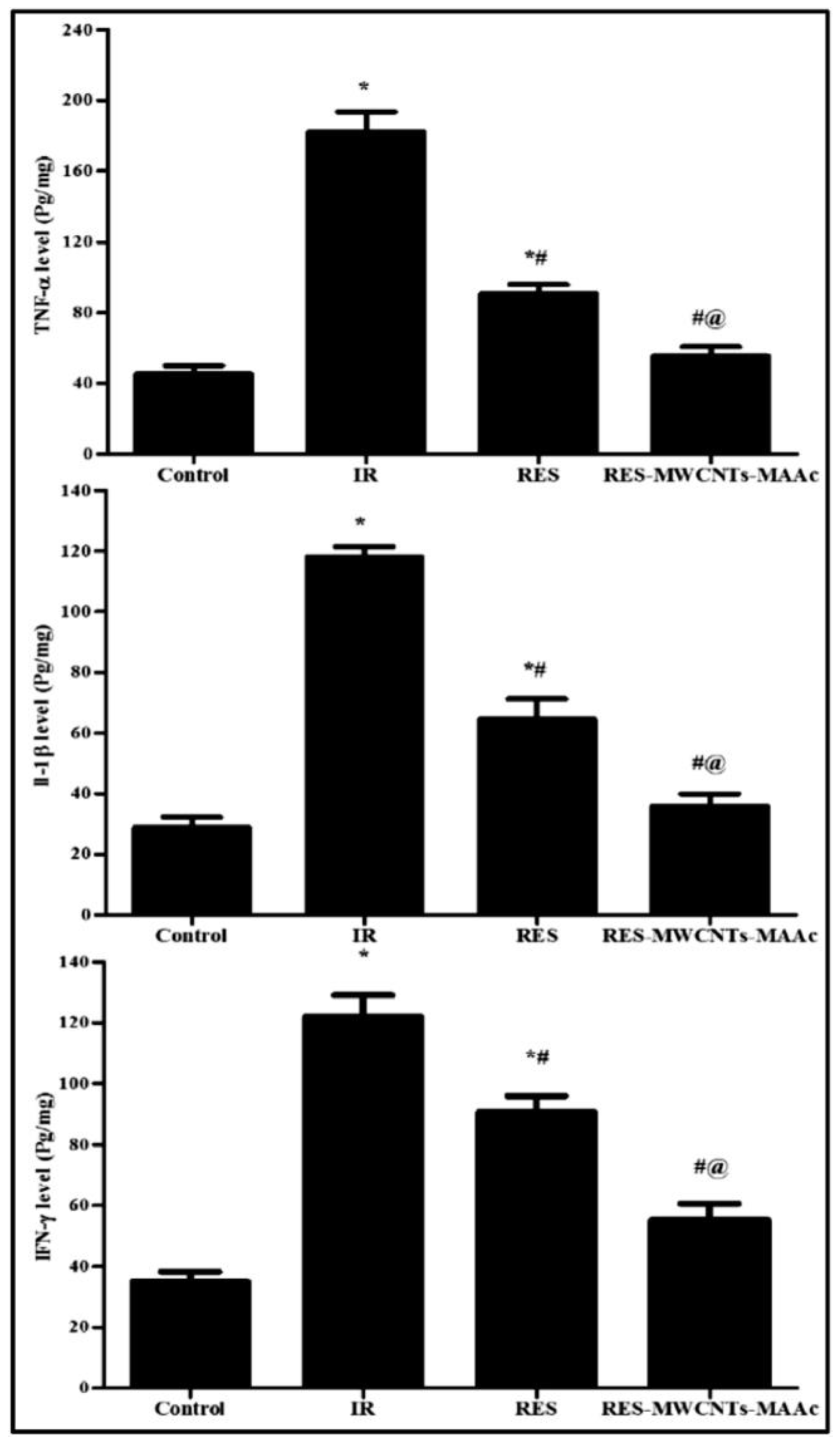
- Ali, H.E.; Radwan, R.R. Synthesis, Characterization and Evaluation of Resveratrol-Loaded Functionalized Carbon Nanotubes as a Novel Delivery System in Radiation Enteropathy. Eur. J. Pharm. Sci. 2021, 167, 106002. [Google Scholar] [CrossRef] [PubMed]
- Ding, N.; Dou, C.; Wang, Y.; Liu, F.; Guan, G.; Huo, D.; Li, Y.; Yang, J.; Wei, K.; Yang, M.; et al. Antishear Stress Bionic Carbon Nanotube Mesh Coating with Intracellular Controlled Drug Delivery Constructing Small-Diameter Tissue–Engineered Vascular Grafts. Adv. Healthc. Mater. 2018, 7, 1800026. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.R. 12—Bioactive Glass. In Bioceramics and Their Clinical Applications; Kokubo, T., Ed.; Woodhead Publishing: Cambridge, UK, 2008; pp. 266–283. ISBN 978-1-84569-204-9. [Google Scholar]
- Moghanian, A.; Koohfar, A.; Hosseini, S.; Hosseini, S.H.; Ghorbanoghli, A.; Sajjadnejad, M.; Raz, M.; Elsa, M.; Sharifianjazi, F. Synthesis, Characterization and in Vitro Biological Properties of Simultaneous Co-Substituted Ti+4/Li+1 58s Bioactive Glass. J. Non-Cryst. Solids 2021, 561, 120740. [Google Scholar] [CrossRef]
- Bellucci, D.; Anesi, A.; Salvatori, R.; Chiarini, L.; Cannillo, V. A Comparative in Vivo Evaluation of Bioactive Glasses and Bioactive Glass-Based Composites for Bone Tissue Repair. Mater. Sci. Eng. C 2017, 79, 286–295. [Google Scholar] [CrossRef]
- Lowe, B.; Ottensmeyer, M.P.; Xu, C.; He, Y.; Ye, Q.; Troulis, M.J. The Regenerative Applicability of Bioactive Glass and Beta-Tricalcium Phosphate in Bone Tissue Engineering: A Transformation Perspective. J. Funct. Biomater. 2019, 10, 16. [Google Scholar] [CrossRef]
- El-Rashidy, A.A.; Roether, J.A.; Harhaus, L.; Kneser, U.; Boccaccini, A.R. Regenerating Bone with Bioactive Glass Scaffolds: A Review of in Vivo Studies in Bone Defect Models. Acta Biomater. 2017, 62, 1–28. [Google Scholar] [CrossRef]
- Martelli, A.; Bellucci, D.; Cannillo, V. Additive Manufacturing of Polymer/Bioactive Glass Scaffolds for Regenerative Medicine: A Review. Polymers 2023, 15, 2473. [Google Scholar] [CrossRef]
- Gao, Y.; Seles, M.A.; Rajan, M. Role of Bioglass Derivatives in Tissue Regeneration and Repair: A Review. Rev. Adv. Mater. Sci. 2023, 62, 20220318. [Google Scholar] [CrossRef]
- Hoppe, A.; Mouriño, V.; Boccaccini, A. Therapeutic Inorganic Ions in Bioactive Glasses to Enhance Bone Formation and Beyond. Biomater. Sci. 2013, 1, 254–256. [Google Scholar] [CrossRef]
- Miola, M.; Pakzad, Y.; Banijamali, S.; Kargozar, S.; Vitale-Brovarone, C.; Yazdanpanah, A.; Bretcanu, O.; Ramedani, A.; Vernè, E.; Mozafari, M. Glass-Ceramics for Cancer Treatment: So Close, or yet so Far? Acta Biomater. 2019, 83, 55–70. [Google Scholar] [CrossRef] [PubMed]
- Kargozar, S.; Moghanian, A.; Rashvand, A.; Miri, A.K.; Hamzehlou, S.; Baino, F.; Mozafari, M.; Wang, A.Z. Nanostructured Bioactive Glasses: A Bird’s Eye View on Cancer Therapy. WIREs Nanomed. Nanobiotechnol. 2023, 15, e1905. [Google Scholar] [CrossRef]
- Shearer, A.; Montazerian, M.; Sly, J.J.; Hill, R.G.; Mauro, J.C. Trends and Perspectives on the Commercialization of Bioactive Glasses. Acta Biomater. 2023, 160, 14–31. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Colilla, M.; Izquierdo-Barba, I.; Vitale-Brovarone, C.; Fiorilli, S. Achievements in Mesoporous Bioactive Glasses for Biomedical Applications. Pharmaceutics 2022, 14, 2636. [Google Scholar] [CrossRef] [PubMed]
- Jayalekshmi, A.C.; Sharma, C.P. Gold Nanoparticle Incorporated Polymer/Bioactive Glass Composite for Controlled Drug Delivery Application. Colloids Surf. B Biointerfaces 2015, 126, 280–287. [Google Scholar] [CrossRef]
- Thomas, C.R.; Ferris, D.P.; Lee, J.-H.; Choi, E.; Cho, M.H.; Kim, E.S.; Stoddart, J.F.; Shin, J.-S.; Cheon, J.; Zink, J.I. Noninvasive Remote-Controlled Release of Drug Molecules in Vitro Using Magnetic Actuation of Mechanized Nanoparticles. J. Am. Chem. Soc. 2010, 132, 10623–10625. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, X.; Yang, Z.; Tan, X.; Wang, J.; Chen, Y. Preparation and Characterization of Folic Acid Functionalized Bioactive Glass for Targeted Delivery and Sustained Release of Methotrexate. J. Biomed. Mater. Res. Part A 2019, 107, 319–329. [Google Scholar] [CrossRef]
- Cazzola, M.; Vernè, E.; Cochis, A.; Sorrentino, R.; Azzimonti, B.; Prenesti, E.; Rimondini, L.; Ferraris, S. Bioactive Glasses Functionalized with Polyphenols: In Vitro Interactions with Healthy and Cancerous Osteoblast Cells. J. Mater. Sci. 2017, 52, 9211–9223. [Google Scholar] [CrossRef]
- Dziadek, M.; Dziadek, K.; Zagrajczuk, B.; Menaszek, E.; Cholewa-Kowalska, K. Poly(ε-Caprolactone)/Bioactive Glass Composites Enriched with Polyphenols Extracted from Sage (Salvia officinalis L.). Mater. Lett. 2016, 183, 386–390. [Google Scholar] [CrossRef]
- Li, L.; Yu, M.; Li, Y.; Li, Q.; Yang, H.; Zheng, M.; Han, Y.; Lu, D.; Lu, S.; Gui, L. Synergistic Anti-Inflammatory and Osteogenic n-HA/Resveratrol/Chitosan Composite Microspheres for Osteoporotic Bone Regeneration. Bioact. Mater. 2021, 6, 1255–1266. [Google Scholar] [CrossRef]
- Arvizo, R.; Bhattacharya, R.; Mukherjee, P. Gold Nanoparticles: Opportunities and Challenges in Nanomedicine. Expert Opin. Drug Deliv. 2010, 7, 753–763. [Google Scholar] [CrossRef] [PubMed]
- Hamada, A.M.; Radi, A.A.; Al-Kahtany, F.A.; Farghaly, F.A. A Review: Zinc Oxide Nanoparticles: Advantages and Disadvantages. J. Plant Nutr. 2023, 1–24. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, Y.; Li, S.; Zhang, P.; Yao, Q. Synthesis and Modification of ZIF-8 and Its Application in Drug Delivery and Tumor Therapy. RSC Adv. 2020, 10, 37600–37620. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, M.; Zarch, M.B.; Darroudi, M.; Sayyadi, K.; Keshavarz, S.T.; Sayyadi, J.; Fallah, A.; Maleki, H. Silica Mesoporous Structures: Effective Nanocarriers in Drug Delivery and Nanocatalysts. Appl. Sci. 2020, 10, 7533. [Google Scholar] [CrossRef]
- Dong, J. Signaling Pathways Implicated in Carbon Nanotube-Induced Lung Inflammation. Front. Immunol. 2020, 11, 552613. [Google Scholar] [CrossRef]
- Kushwaha, S.K.S.; Ghoshal, S.; Rai, A.K.; Singh, S. Carbon Nanotubes as a Novel Drug Delivery System for Anticancer Therapy: A Review. Braz. J. Pharm. Sci. 2013, 49, 629–643. [Google Scholar] [CrossRef]

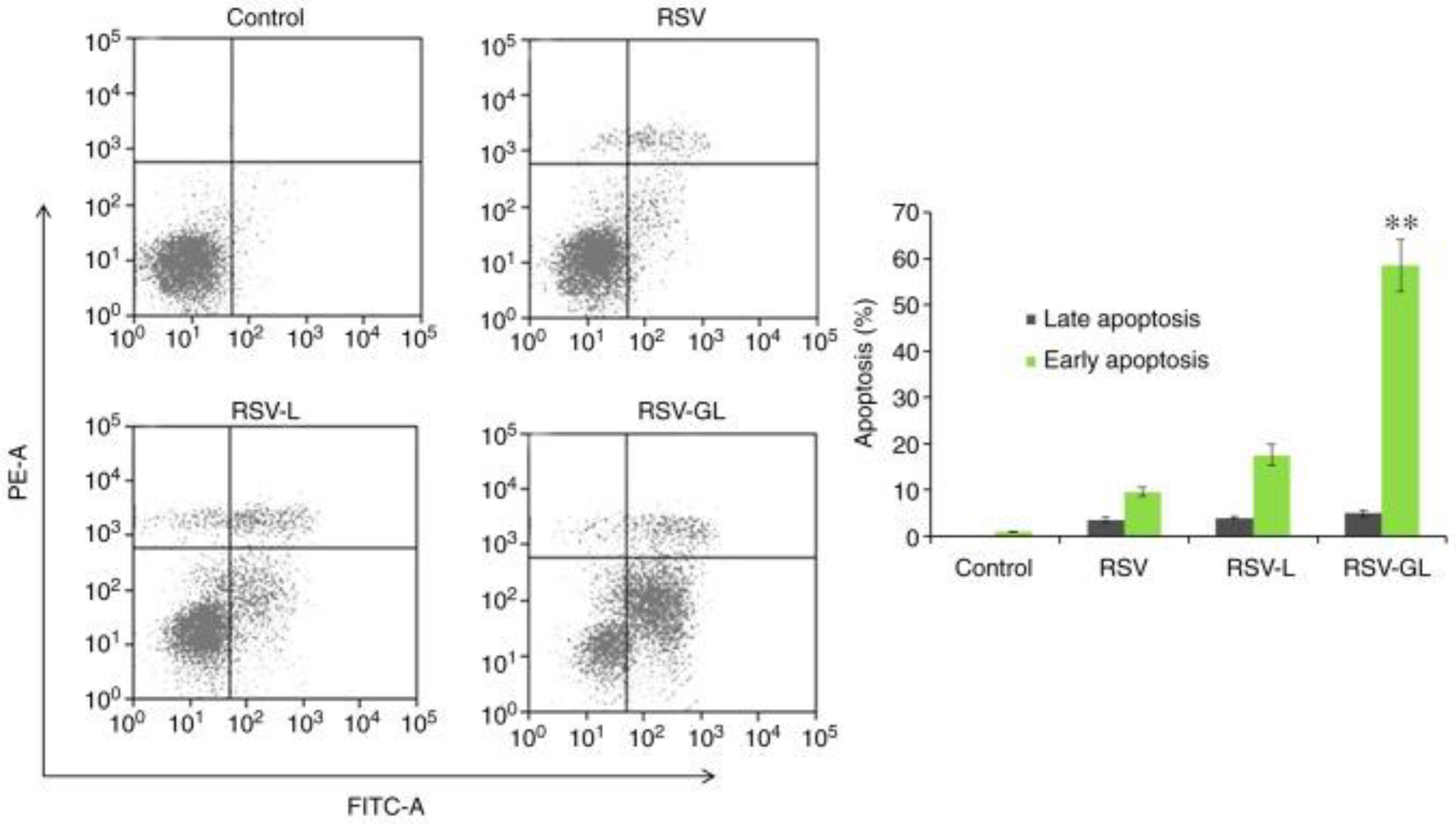
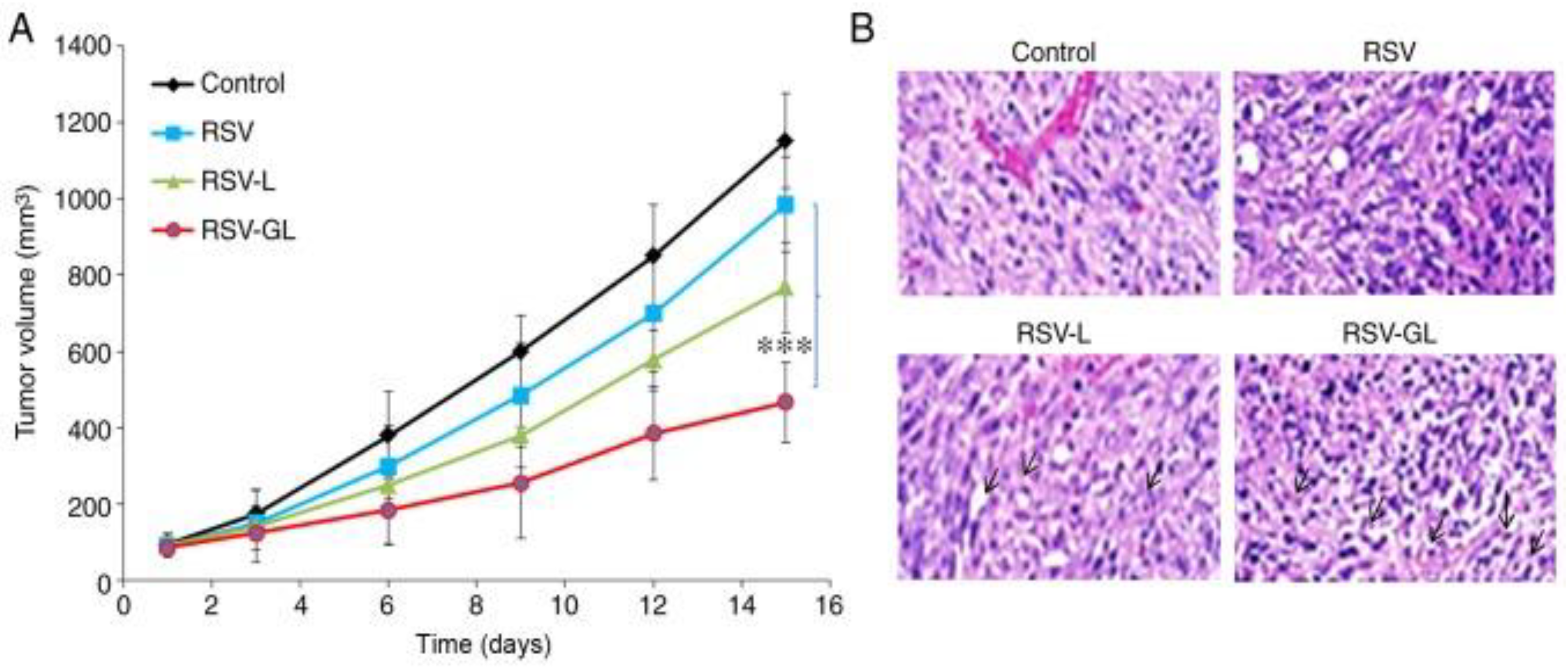
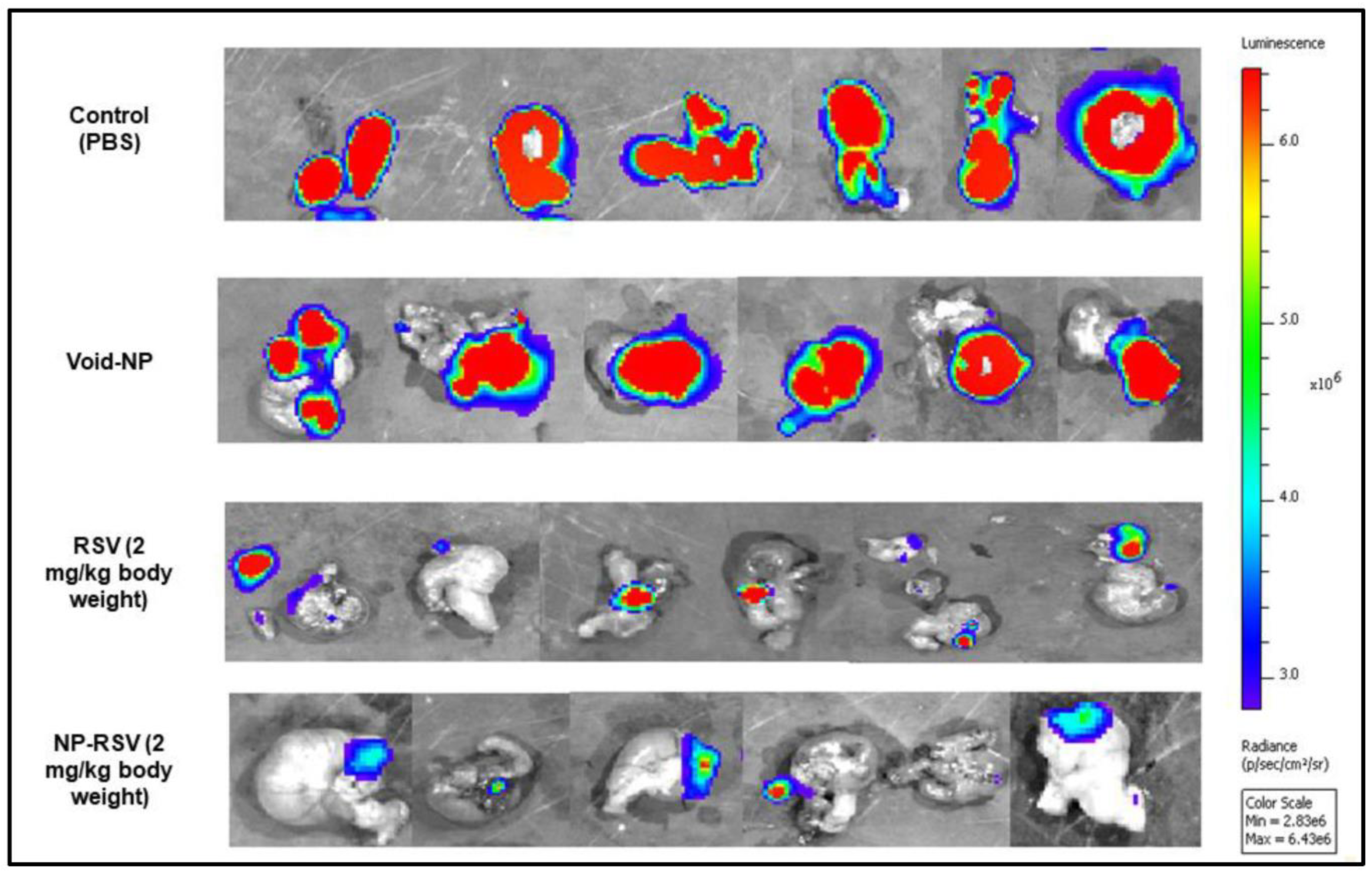
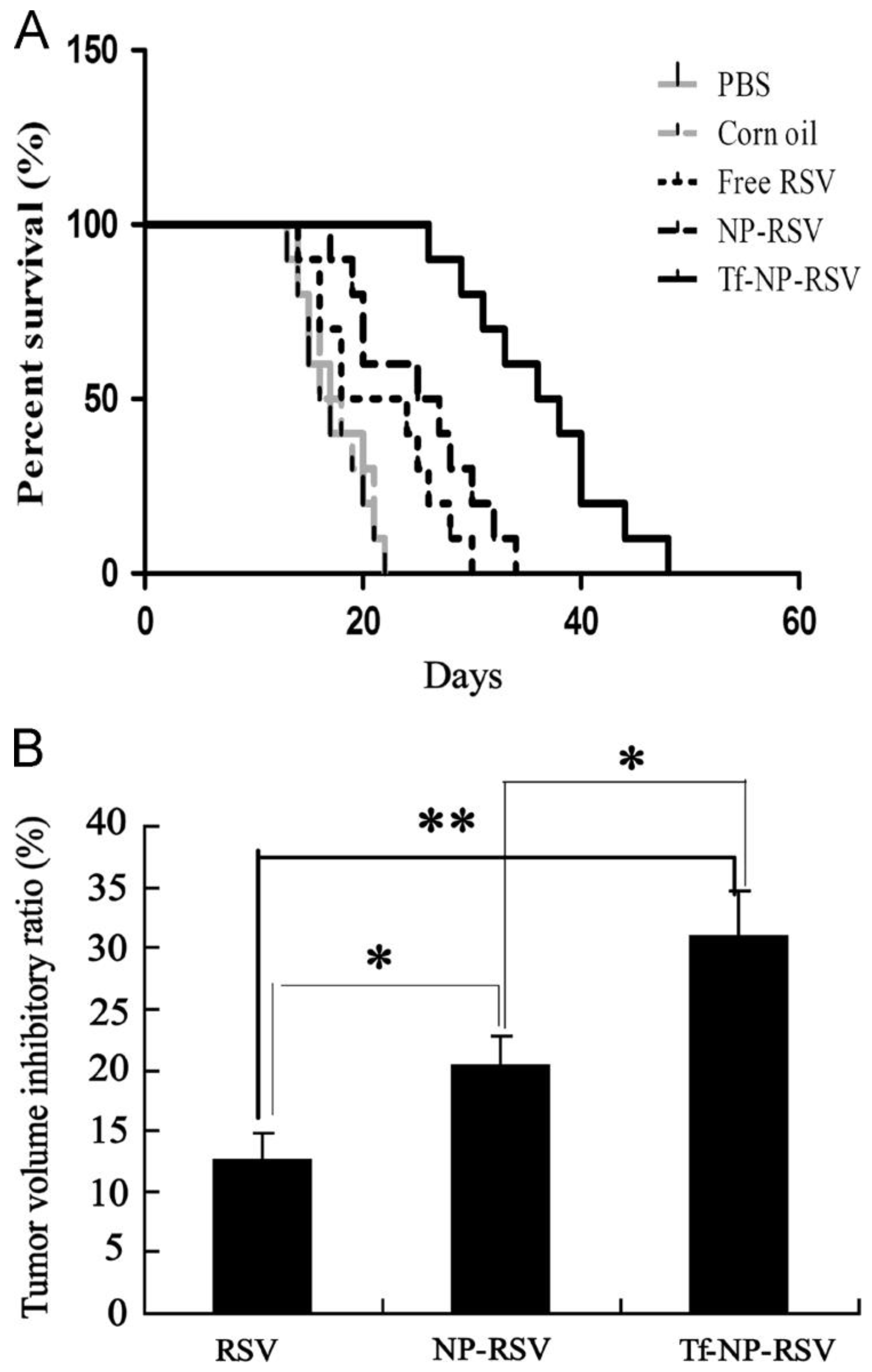


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, M.; Benfante, V.; Di Raimondo, D.; Salvaggio, G.; Tuttolomondo, A.; Comelli, A. Recent Developments in Nanoparticle Formulations for Resveratrol Encapsulation as an Anticancer Agent. Pharmaceuticals 2024, 17, 126. https://doi.org/10.3390/ph17010126
Ali M, Benfante V, Di Raimondo D, Salvaggio G, Tuttolomondo A, Comelli A. Recent Developments in Nanoparticle Formulations for Resveratrol Encapsulation as an Anticancer Agent. Pharmaceuticals. 2024; 17(1):126. https://doi.org/10.3390/ph17010126
Chicago/Turabian StyleAli, Muhammad, Viviana Benfante, Domenico Di Raimondo, Giuseppe Salvaggio, Antonino Tuttolomondo, and Albert Comelli. 2024. "Recent Developments in Nanoparticle Formulations for Resveratrol Encapsulation as an Anticancer Agent" Pharmaceuticals 17, no. 1: 126. https://doi.org/10.3390/ph17010126
APA StyleAli, M., Benfante, V., Di Raimondo, D., Salvaggio, G., Tuttolomondo, A., & Comelli, A. (2024). Recent Developments in Nanoparticle Formulations for Resveratrol Encapsulation as an Anticancer Agent. Pharmaceuticals, 17(1), 126. https://doi.org/10.3390/ph17010126