Promising Effects of Montelukast for Critically Ill Asthma Patients via a Reduction in Delirium
Abstract
1. Introduction
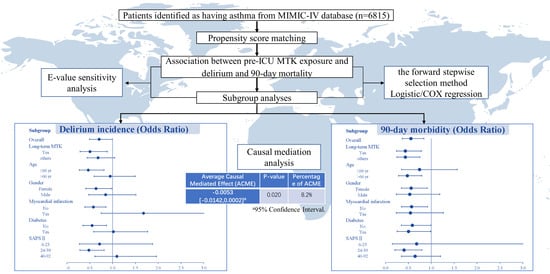
2. Results
2.1. Baseline Information and Clinical Outcomes
2.2. Subgroup Analyses
2.3. Interaction Effects and Regression Models
2.4. Further Analyses
3. Discussion
4. Materials and Methods
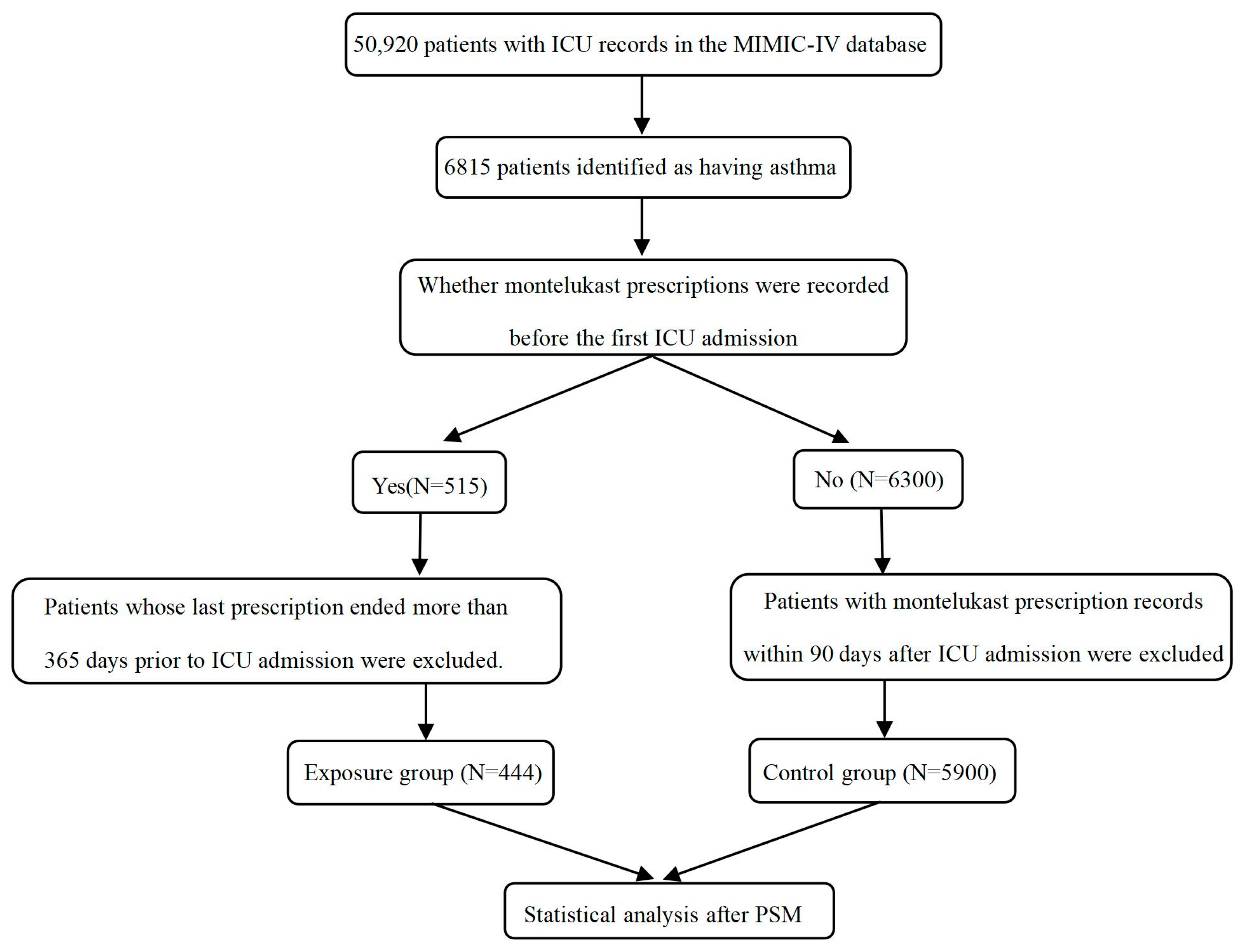
4.1. Data Source and Study Design
4.2. Data Extraction and Missing Value Processing
4.3. Statistical Analysis
4.4. Further Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wilson, J.E.; Mart, M.F.; Cunningham, C.; Shehabi, Y.; Girard, T.D.; MacLullich, A.M.J.; Slooter, A.J.C.; Ely, E.W. Delirium. Nat. Rev. Dis. Primers 2020, 6, 90. [Google Scholar] [CrossRef]
- Krewulak, K.D.; Stelfox, H.T.; Leigh, J.P.; Ely, E.W.; Fiest, K.M. Incidence and Prevalence of Delirium Subtypes in an Adult ICU: A Systematic Review and Meta-Analysis. Crit. Care Med. 2018, 46, 2029–2035. [Google Scholar] [CrossRef] [PubMed]
- Pisani, M.A.; Murphy, T.E.; Van Ness, P.H.; Araujo, K.L.B.; Inouye, S.K. Characteristics Associated With Delirium in Older Patients in a Medical Intensive Care Unit. Arch. Intern. Med. 2007, 167, 1629–1634. [Google Scholar] [CrossRef] [PubMed]
- Inouye, S.K.; Leo-Summers, L.; Zhang, Y.; Bogardus, S.T.; Agostini, J.V. A chart-based method for identification of delirium: Validation compared with interviewer ratings using the confusion assessment method. J. Am. Geriatr. Soc. 2010, 53, 312–318. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, Q.; Liu, X.; Hu, X.; Wang, L.; Zhou, F. Incidence and interaction factors of delirium as an independent risk of mortality in elderly patients in the intensive units: A retrospective analysis from MIMIC-IV database. Aging Clin. Exp. Res. 2022, 34, 2865–2872. [Google Scholar] [CrossRef]
- Sanchez, D.; Brennan, K.; Al Sayfe, M.; Shunker, S.A.; Bogdanoski, T.; Hedges, S.; Hou, Y.C.; Lynch, J.; Hunt, L.; Alexandrou, E.; et al. Frailty, delirium and hospital mortality of older adults admitted to intensive care: The Delirium (Deli) in ICU study. Crit. Care 2020, 24, 609. [Google Scholar] [CrossRef]
- Duprey, M.S.; Boogaard, M.V.D.; Hoeven, J.G.V.D.; Pickkers, P.; Devlin, J.W. Association between incident delirium and 28- and 90-day mortality in critically ill adults: A secondary analysis. Crit. Care 2020, 24, 161. [Google Scholar] [PubMed]
- Bramley, P.; McArthur, K.; Blayney, A.; McCullagh, I. Risk factors for postoperative delirium: An umbrella review of systematic reviews. Int. J. Surg. 2021, 93, 106063. [Google Scholar] [CrossRef]
- Li, Z.; Wang, J.; Ma, Y. Montelukast attenuates interleukin IL-1β-induced oxidative stress and apoptosis in chondrocytes by inhibiting CYSLTR1 (Cysteinyl Leukotriene Receptor 1) and activating KLF2 (Kruppel Like Factor 2). Bioengineered 2021, 12, 8476–8484. [Google Scholar] [CrossRef]
- Yates, A.G.; Kislitsyna, E.; Alfonso Martin, C.; Zhang, J.; Sewell, A.L.; Goikolea-Vives, A.; Cai, V.; Alkhader, L.F.; Skaland, A.; Hammond, B.; et al. Montelukast reduces grey matter abnormalities and functional deficits in a mouse model of inflammation-induced encephalopathy of prematurity. J. Neuroinflamm. 2022, 19, 265. [Google Scholar] [CrossRef]
- Rostevanov, I.S.; Betesh-Abay, B.; Nassar, A.; Rubin, E.; Uzzan, S.; Kaplanski, J.; Biton, L.; Azab, A.N. Montelukast induces beneficial behavioral outcomes and reduces inflammation in male and female rats. Front. Immunol. 2022, 13, 981440. [Google Scholar] [CrossRef] [PubMed]
- Abdelzaher, W.Y.; Mostafa-Hedeab, G.; Bahaa, H.A.; Mahran, A.; Atef Fawzy, M.; Abdel Hafez, S.M.N.; Welson, N.N.; Rofaeil, R.R. Leukotriene Receptor Antagonist, Montelukast Ameliorates L-NAME-Induced Pre-eclampsia in Rats through Suppressing the IL-6/Jak2/STAT3 Signaling Pathway. Pharmaceuticals 2022, 15, 914. [Google Scholar] [CrossRef]
- Paljarvi, T.; Forton, J.; Luciano, S.; Herttua, K.; Fazel, S. Analysis of Neuropsychiatric Diagnoses After Montelukast Initiation. JAMA Netw. Open 2022, 5, e2213643. [Google Scholar] [CrossRef]
- Glockler-Lauf, S.D.; Finkelstein, Y.; Zhu, J.; Feldman, L.Y.; To, T. Montelukast and Neuropsychiatric Events in Children with Asthma: A Nested Case-Control Study. J. Pediatr. 2019, 209, 176–182.e4. [Google Scholar] [CrossRef] [PubMed]
- Veronica, V. Sansing-Foster. In Neuropsychiatric Adverse Events and Montelukast: Observational Safety Analyse; FDA: Silver Spring, MD, USA, 2019; pp. 1–37. [Google Scholar]
- Ali, M.M.; O’Brien, C.E.; Cleves, M.A.; Martin, B.C. Exploring the possible association between montelukast and neuropsychiatric events among children with asthma: A matched nested case-control study. Pharmacoepidemiol. Drug Saf. 2015, 24, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Schumock, G.T.; Stayner, L.T.; Valuck, R.J.; Joo, M.J.; Gibbons, R.D.; Lee, T.A. Risk of suicide attempt in asthmatic children and young adults prescribed leukotriene-modifying agents: A nested case-control study. J. Allergy Clin. Immunol. 2012, 130, 368–375. [Google Scholar] [CrossRef]
- Maclullich, A.M.; Ferguson, K.J.; Miller, T.; de Rooij, S.E.; Cunningham, C. Unravelling the pathophysiology of delirium: A focus on the role of aberrant stress responses. J. Psychosom. Res. 2008, 65, 229–238. [Google Scholar] [CrossRef]
- Subramaniyan, S.; Terrando, N. Neuroinflammation and Perioperative Neurocognitive Disorders. Anesth. Analg. 2019, 128, 781–788. [Google Scholar] [CrossRef]
- Henjum, K.; Quist-Paulsen, E.; Zetterberg, H.; Blennow, K.; Nilsson, L.N.G.; Watne, L.O. CSF sTREM2 in delirium-relation to Alzheimer’s disease CSF biomarkers Abeta42, t-tau and p-tau. J. Neuroinflamm. 2018, 15, 304. [Google Scholar] [CrossRef]
- Skelly, D.T.; Griffin, E.W.; Murray, C.L.; Harney, S.; O’Boyle, C.; Hennessy, E.; Dansereau, M.A.; Nazmi, A.; Tortorelli, L.; Rawlins, J.N.; et al. Acute transient cognitive dysfunction and acute brain injury induced by systemic inflammation occur by dissociable IL-1-dependent mechanisms. Mol. Psychiatry 2019, 24, 1533–1548. [Google Scholar] [CrossRef]
- Varatharaj, A.; Galea, I. The blood-brain barrier in systemic inflammation. Brain Behav. Immun. 2017, 60, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Michael, J.; Zirknitzer, J.; Unger, M.S.; Poupardin, R.; Rieß, T.; Paiement, N.; Zerbe, H.; Hutter-Paier, B.; Reitsamer, H.; Aigner, L. The Leukotriene Receptor Antagonist Montelukast Attenuates Neuroinflammation and Affects Cognition in Transgenic 5xFAD Mice. Int. J. Mol. Sci. 2021, 22, 2782. [Google Scholar] [CrossRef]
- Zhou, L.; Sun, X.; Shi, Y.; Liu, J.; Luan, G.; Yang, Y. Cysteinyl leukotriene receptor type 1 antagonist montelukast protects against injury of blood-brain barrier. Inflammopharmacology 2019, 27, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Shi, W.Z.; Zhang, Y.M.; Fang, S.H.; Wei, E.Q. Montelukast, a cysteinyl leukotriene receptor-1 antagonist, attenuates chronic brain injury after focal cerebral ischaemia in mice and rats. J. Pharm. Pharmacol. 2011, 63, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.W.H.; Pathadka, S.; Qin, S.X.; Fung, L.W.Y.; Yan, V.K.C.; Yiu, H.H.E.; Bloom, C.I.; Wong, I.C.K.; Chan, E.W.Y. Neuropsychiatric events associated with montelukast in patients with asthma: A systematic review. Eur. Respir. Rev. 2023, 32, 169. [Google Scholar] [CrossRef]
- Wu, Z.; Li, H.; Liao, K.; Wang, Y. Association Between Dexamethasone and Delirium in Critically Ill Patients: A Retrospective Cohort Study of a Large Clinical Database. J. Surg. Res. 2021, 263, 89–101. [Google Scholar] [CrossRef]
- Kerget, B.; Kerget, F.; Aydın, M.; Karaşahin, Ö. Effect of montelukast therapy on clinical course, pulmonary function, and mortality in patients with COVID-19. J. Med. Virol. 2022, 94, 1950–1958. [Google Scholar] [CrossRef] [PubMed]
- Helmy, M.M.; El-Gowelli, H.M. Montelukast abrogates rhabdomyolysis-induced acute renal failure via rectifying detrimental changes in antioxidant profile and systemic cytokines and apoptotic factors production. Eur. J. Pharmacol. 2012, 683, 294–300. [Google Scholar] [CrossRef]
- Suddek, G.M. Montelukast ameliorates kidney function and urinary bladder sensitivity in experimentally induced renal dysfunction in rats. Fundam. Clin. Pharmacol. 2013, 27, 186–191. [Google Scholar] [CrossRef]
- Urcun, Y.S.; Altun, Y.; Pala, A.A. Early and late predictors of postoperative neurocognitive dysfunction in cardiac surgery. Ideggyógyászati Szemle 2022, 75, 231–240. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, J.; Chen, Y.; Lian, Q.; Shi, Z.; Zhang, Y. Incidence and risk factors of postoperative delirium following total knee arthroplasty: A retrospective Nationwide Inpatient Sample database study. Knee 2022, 35, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Mossello, E.; Baroncini, C.; Pecorella, L.; Giulietti, C.; Chiti, M.; Caldi, F.; Cavallini, M.C.; Simoni, D.; Baldasseroni, S.; Fumagalli, S.; et al. Predictors and prognosis of delirium among older subjects in cardiac intensive care unit: Focus on potentially preventable forms. Eur. Heart J. Acute Cardiovasc. Care 2020, 9, 771–778. [Google Scholar] [CrossRef]
- Johnson, A.B.L.; Pollard, T.; Horng, S.; Celi, L.A.; Mark, R. MIMIC-IV (version 2.0). PhysioNet. 2022. Available online: https://physionet.org/content/mimiciv/2.0/ (accessed on 6 April 2023).
- Stavem, K.; Hoel, H.; Skjaker, S.A.; Haagensen, R. Charlson comorbidity index derived from chart review or administrative data: Agreement and prediction of mortality in intensive care patients. Clin. Epidemiol. 2017, 9, 311–320. [Google Scholar] [CrossRef]
- Le Gall, J.R.; Lemeshow, S.; Saulnier, F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA 1993, 270, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.L.; Marshall, J.C.; Namendys-Silva, S.A.; François, B.; Martin-Loeches, I.; Lipman, J.; Reinhart, K.; Antonelli, M.; Pickkers, P.; Njimi, H.; et al. Assessment of the worldwide burden of critical illness: The intensive care over nations (ICON) audit. Lancet Respir. Med. 2014, 2, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.L.; Moreno, R.; Takala, J.; Willatts, S.; De Mendonça, A.; Bruining, H.; Reinhart, C.K.; Suter, P.M.; Thijs, L.G. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996, 22, 707–710. [Google Scholar] [CrossRef]
- Malone, D.L.; Kuhls, D.; Napolitano, L.M.; McCarter, R.; Scalea, T. Back to basics: Validation of the admission systemic inflammatory response syndrome score in predicting outcome in trauma. J. Trauma 2001, 51, 458–463. [Google Scholar] [CrossRef]
- Le Gall, J.R.; Klar, J.; Lemeshow, S.; Saulnier, F.; Alberti, C.; Artigas, A.; Teres, D. The Logistic Organ Dysfunction system. A new way to assess organ dysfunction in the intensive care unit. ICU Scoring Group. JAMA 1996, 276, 802–810. [Google Scholar] [CrossRef]
- Lieberman, O.J.; Lee, S.; Zabinski, J. Donepezil treatment is associated with improved outcomes in critically ill dementia patients via a reduction in delirium. Alzheimers Dement. 2023, 19, 1742–1751. [Google Scholar] [CrossRef]
- Su, X.; Wang, J.; Lu, X. The association between Monocyte-to-Lymphocyte ratio and postoperative delirium in ICU patients in cardiac surgery. J. Clin. Lab. Anal. 2022, 36, e24553. [Google Scholar] [CrossRef]
- Ely, E.W.; Margolin, R.; Francis, J.; May, L.; Truman, B.; Dittus, R.; Speroff, T.; Gautam, S.; Bernard, G.R.; Inouye, S.K. Evaluation of delirium in critically ill patients: Validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU). Crit. Care Med. 2001, 29, 1370–1379. [Google Scholar] [CrossRef] [PubMed]
- Puelle, M.R.; Kosar, C.M.; Xu, G.; Schmitt, E.; Jones, R.N.; Marcantonio, E.R.; Cooper, Z.; Inouye, S.K.; Saczynski, J.S. The Language of Delirium: Keywords for Identifying Delirium from Medical Records. J. Gerontol. Nurs. 2015, 41, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Figueiras, A.; Domenech-Massons, J.M.; Cadarso, C. Regression models: Calculating the confidence interval of effects in the presence of interactions. Stat. Med. 1998, 17, 2099–2105. [Google Scholar] [CrossRef] [PubMed]
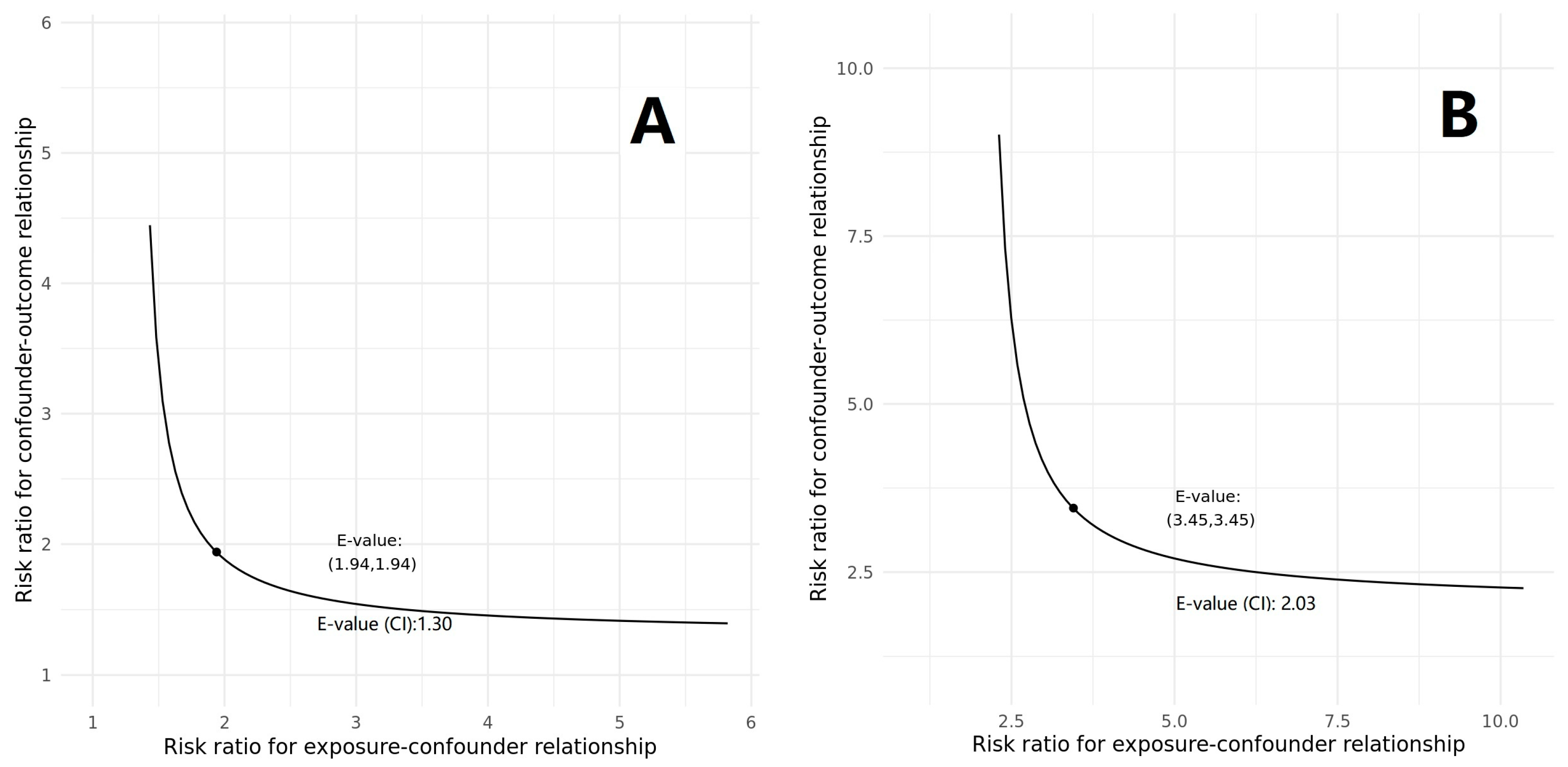
- VanderWeele, T.J.; Ding, P. Sensitivity Analysis in Observational Research: Introducing the E-Value. Ann. Intern. Med. 2017, 167, 268–274. [Google Scholar] [CrossRef]
- Mathur, M.B.; Ding, P.; Riddell, C.A.; VanderWeele, T.J. Web Site and R Package for Computing E-values. Epidemiology 2018, 29, e45–e47. [Google Scholar] [CrossRef]

| Characteristics | Before Matching | After Matching | ||||
|---|---|---|---|---|---|---|
| All Patients (n = 6344) | Exposure Group (n = 444) | Control Group (n = 5900) | p Value | Control Group (n = 443) | p Value | |
| Age (y) | 63.8 (51.8–75.0) | 66.1 (54.7–74.6) | 63.5 (51.4–75.0) | 0.012 | 66.2 (54.5–77.4) | 0.560 |
| Weight (kg) | 81.8 (68.4–98.0) | 85.3 (70.0–102.5) | 81.6 (68.2–98.0) | 0.015 | 83.4 (68.9–103) | 0.709 |
| Gender, n (%) | <0.001 | 0.905 | ||||
| Female | 3637 (57.3) | 290 (65.3) | 3347 (56.7) | 287 (64.9) | ||
| Male | 2707 (42.7) | 154 (34.7) | 2553 (43.3) | 155 (35.1) | ||
| Race, n (%) | 0.014 | 0.817 | ||||
| White | 4089 (64.5) | 310 (69.8) | 3379 (64.1) | 300 (67.9) | ||
| Black | 967 (15.2) | 67 (15.1) | 900 (15.3) | 72 (16.3) | ||
| Other a | 1288 (20.3) | 67 (15.1) | 1221 (20.7) | 70 (15.8) | ||
| Type of insurance, n (%) | 0.003 | 0.188 | ||||
| Medicaid | 617 (9.7) | 36 (8.1) | 581 (9.8) | 39 (8.8) | ||
| Medicare | 2681 (42.3) | 222 (50.0) | 2459 (41.7) | 194 (43.9) | ||
| Other b | 3046 (48.0) | 186 (41.9) | 2860 (48.5) | 209 (47.3) | ||
| Type of ICU for first admission, n (%) | 0.012 | 0.600 | ||||
| CCU/CVICU | 2225 (35.1) | 184 (41.4) | 2041 (34.6) | 178 (40.3) | ||
| MICU/SICU | 2337 (36.8) | 143 (32.2) | 2194 (37.2) | 156 (35.3) | ||
| Others c | 1782 (28.1) | 117 (26.4) | 1665 (28.2) | 108 (24.4) | ||
| Comorbidity, n (%) | ||||||
| Myocardial infarction | 887 (14.0) | 70 (15.8) | 817 (13.8) | 0.261 | 81 (18.3) | 0.311 |
| Congestive heart failure | 1733 (27.3) | 132 (29.7) | 1601 (27.1) | 0.237 | 123 (27.8) | 0.532 |
| Peripheral vascular disease | 629 (9.9) | 44 (9.9) | 585 (9.9) | 0.997 | 48 (10.9) | 0.643 |
| Cerebrovascular disease | 772 (12.2) | 38 (8.6) | 734 (12.4) | 0.016 | 40 (9.0) | 0.796 |
| Dementia | 154 (2.4) | 5 (1.1) | 149 (2.5) | 0.065 | 5 (1.1) | 0.994 |
| Chronic pulmonary disease | 4756 (75.0) | 407 (91.7) | 4349 (73.7) | <0.001 | 401 (90.7) | 0.620 |
| Rheumatic disease | 260 (4.1) | 32 (7.2) | 228 (3.9) | <0.001 | 26 (5.9) | 0.425 |
| Peptic ulcer disease | 139 (2.2) | 8 (1.8) | 131 (2.2) | 0.561 | 10 (2.3) | 0.627 |
| Mild liver disease | 711 (11.2) | 27 (6.1) | 684 (11.6) | <0.001 | 31 (7.0) | 0.575 |
| Diabetes | 1994 (31.4) | 161 (36.3) | 1833 (31.1) | 0.023 | 153 (34.6) | 0.609 |
| Paraplegia | 220 (3.5) | 12 (2.7) | 208 (3.5) | 0.361 | 13 (2.9) | 0.830 |
| Renal disease | 1243 (19.6) | 61 (13.7) | 1182 (20.0) | 0.001 | 56 (12.7) | 0.638 |
| Malignant cancer | 741 (11.7) | 62 (14.0) | 679 (11.5) | 0.120 | 46 (10.4) | 0.106 |
| Severe liver disease | 260 (4.1) | 9 (2.0) | 251 (4.3) | 0.022 | 9 (2.0) | 0.992 |
| Metastatic solid tumor | 360 (5.7) | 26 (5.9) | 334 (5.7) | 0.864 | 21 (4.8) | 0.463 |
| Aids | 51 (0.8) | 3 (0.7) | 48 (0.8) | 0.970 | 3 (0.7) | 1.000 |
| Scores | ||||||
| CCI | 5 (3–7) | 5 (3–7) | 5 (3–7) | 0.127 | 5 (3–7) | 0.722 |
| SAPS II | 31 (23–40) | 31 (23–39) | 32 (23–40) | 0.059 | 31 (24–38) | 0.443 |
| APS III | 38 (28–51) | 35 (27–47) | 38 (28–51) | 0.003 | 36 (28–48) | 0.407 |
| SOFA | 3 (2–6) | 3 (1–5) | 3 (2–6) | 0.001 | 3 (2–5) | 0.314 |
| SIRS | 3 (2–3) | 3 (2–3) | 3 (2–3) | 0.005 | 3 (2–3) | 0.494 |
| LODS | 3 (2–5) | 3 (2–5) | 3 (2–5) | 0.009 | 3 (2–5) | 0.824 |
| Laboratory tests | ||||||
| Hemoglobin concentration, g/dL | 11.1 ± 2.2 | 10.9 ± 2.1 | 11.1 ± 2.2 | 0.131 | 10.9 ± 2.2 | 0.926 |
| Hematocrit (%) | 33.3 ± 6.0 | 33.0 ± 5.7 | 33.3 ± 6.0 | 0.209 | 32.9 ± 5.8 | 0.887 |
| Platelet, K/μL | 206.5 (152.5–271.0) | 218.3 (157.6–285.1) | 206.5 (152.0–271.0) | 0.009 | 224 (160–287.8) | 0.619 |
| WBCs, K/μL | 10.8 (7.9–14.5) | 11.7 (8.5–14.9) | 10.8 (7.9–14.5) | 0.017 | 11.2 (8.2–15.3) | 0.815 |
| Bicarbonate, mEq/L | 23.7 ± 4.3 | 24.6 ± 4.6 | 23.6 ± 4.3 | <0.001 | 24.4 ± 4.3 | 0.510 |
| BUN, mg/dL | 17.5 (12.0–27.5) | 17.0 (12.0–23.5) | 17.5 (12.0–27.5) | 0.038 | 17.0 (12.0–24.6) | 0.835 |
| Calcium, mg/dL | 8.4 ± 0.8 | 8.5 ± 0.6 | 8.4 ± 0.8 | 0.445 | 8.5 ± 0.7 | 0.835 |
| Creatinine, mg/dL | 0.9 (0.7–1.3) | 0.9 (0.7–1.2) | 0.9 (0.7–1.4) | 0.002 | 0.9 (0.7–1.2) | 0.629 |
| Glucose, mg/dL | 128.0 (108.0–160.0) | 128.5 (109.1–159.4) | 128.0 (108.0–160.0) | 0.914 | 128.0 (108.8–163.5) | 0.878 |
| Sodium, mEq/L | 138.2 ± 4.4 | 137.9 ± 4.1 | 138.2 ± 4.4 | 0.131 | 138.0 ± 4.4 | 0.584 |
| Potassium, mEq/L | 4.2 ± 0.6 | 4.2 ± 0.6 | 4.2 ± 0.6 | 0.945 | 4.3 ± 0.6 | 0.601 |
| Outcomes, n (%) | ||||||
| Delirium | 1235 (19.5) | 67 (15.1) | 1168 (19.8) | 0.016 | 89 (20.1) | 0.049 |
| Hospital mortality | 439 (6.9) | 25 (5.6) | 414 (7.0) | 0.267 | 23 (5.2) | 0.779 |
| 90-day mortality rate | 820 (12.9) | 40 (9.0) | 780 (13.2) | 0.011 | 67 (15.2) | 0.005 |
| Variable | Coefficient | Standard Error | OR (95% CI) | * p-Value |
|---|---|---|---|---|
| Pre-ICU MTK exposure | β1: −0.534 | 0.217 | 0.586(0.383–0.896) b | 0.014 |
| Interaction item | β2: 1.119 a | 0.489 | - | 0.022 a |
| Total Effect | p-Value | Average Direct Effect (ADE) | p-Value | Average Causal Mediation Effect (ACME) | p-Value | Percentage of ACME |
|---|---|---|---|---|---|---|
| −0.0645 [−0.1120, −0.0200] a | <0.001 | −0.0592 [−0.1069, −0.0100] a | 0.020 | −0.0053 [−0.0142, 0.0002] a | 0.020 | 8.2% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Zhang, M.; Zhang, S.; Yang, G. Promising Effects of Montelukast for Critically Ill Asthma Patients via a Reduction in Delirium. Pharmaceuticals 2024, 17, 125. https://doi.org/10.3390/ph17010125
Li Y, Zhang M, Zhang S, Yang G. Promising Effects of Montelukast for Critically Ill Asthma Patients via a Reduction in Delirium. Pharmaceuticals. 2024; 17(1):125. https://doi.org/10.3390/ph17010125
Chicago/Turabian StyleLi, Yuan, Meilin Zhang, Shengnan Zhang, and Guoping Yang. 2024. "Promising Effects of Montelukast for Critically Ill Asthma Patients via a Reduction in Delirium" Pharmaceuticals 17, no. 1: 125. https://doi.org/10.3390/ph17010125
APA StyleLi, Y., Zhang, M., Zhang, S., & Yang, G. (2024). Promising Effects of Montelukast for Critically Ill Asthma Patients via a Reduction in Delirium. Pharmaceuticals, 17(1), 125. https://doi.org/10.3390/ph17010125