Anti-Inflammatory Activity of APPA (Apocynin and Paeonol) in Human Articular Chondrocytes
Abstract
1. Introduction
2. Results
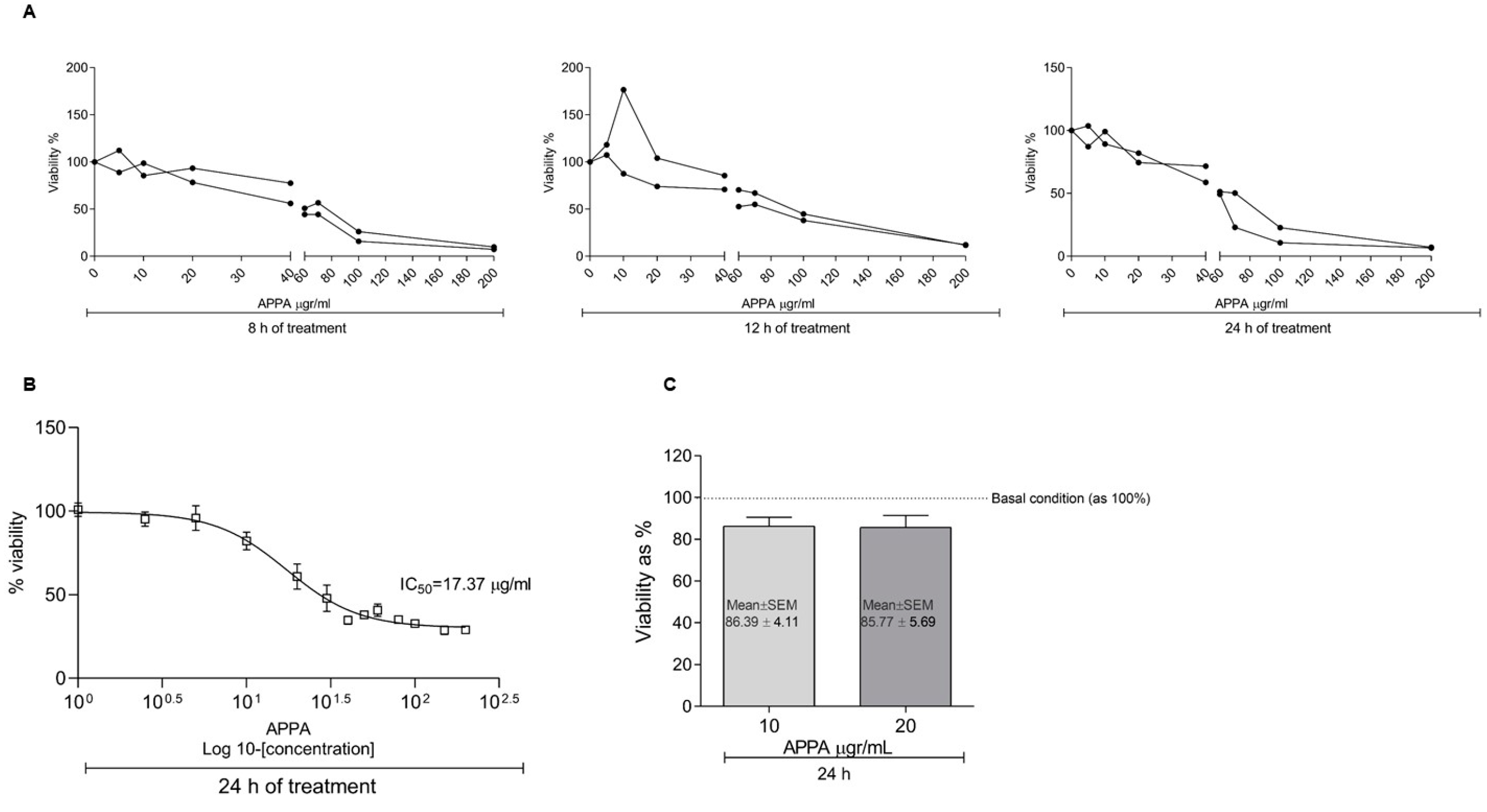
2.1. APPA Effect on Cell Proliferation
2.2. APPA Effect over ROS Production in Human Chondrocytes
2.3. APPA Anti-Inflammatory Capacity
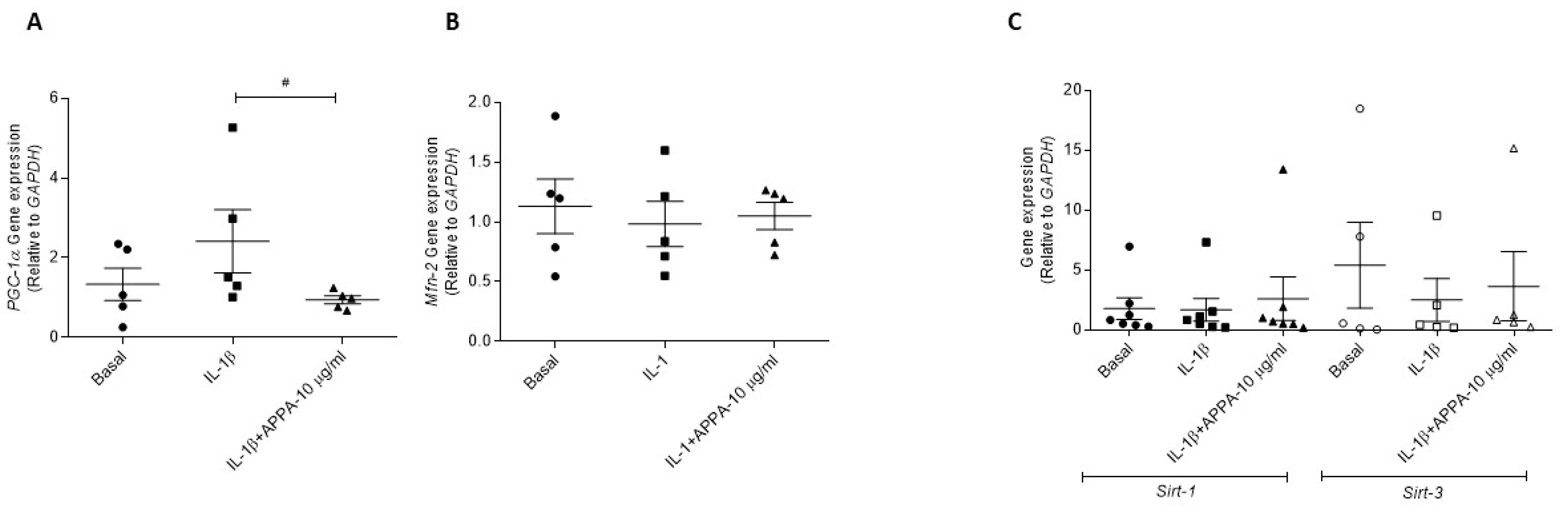
2.4. APPA Effect in the Mitogensis
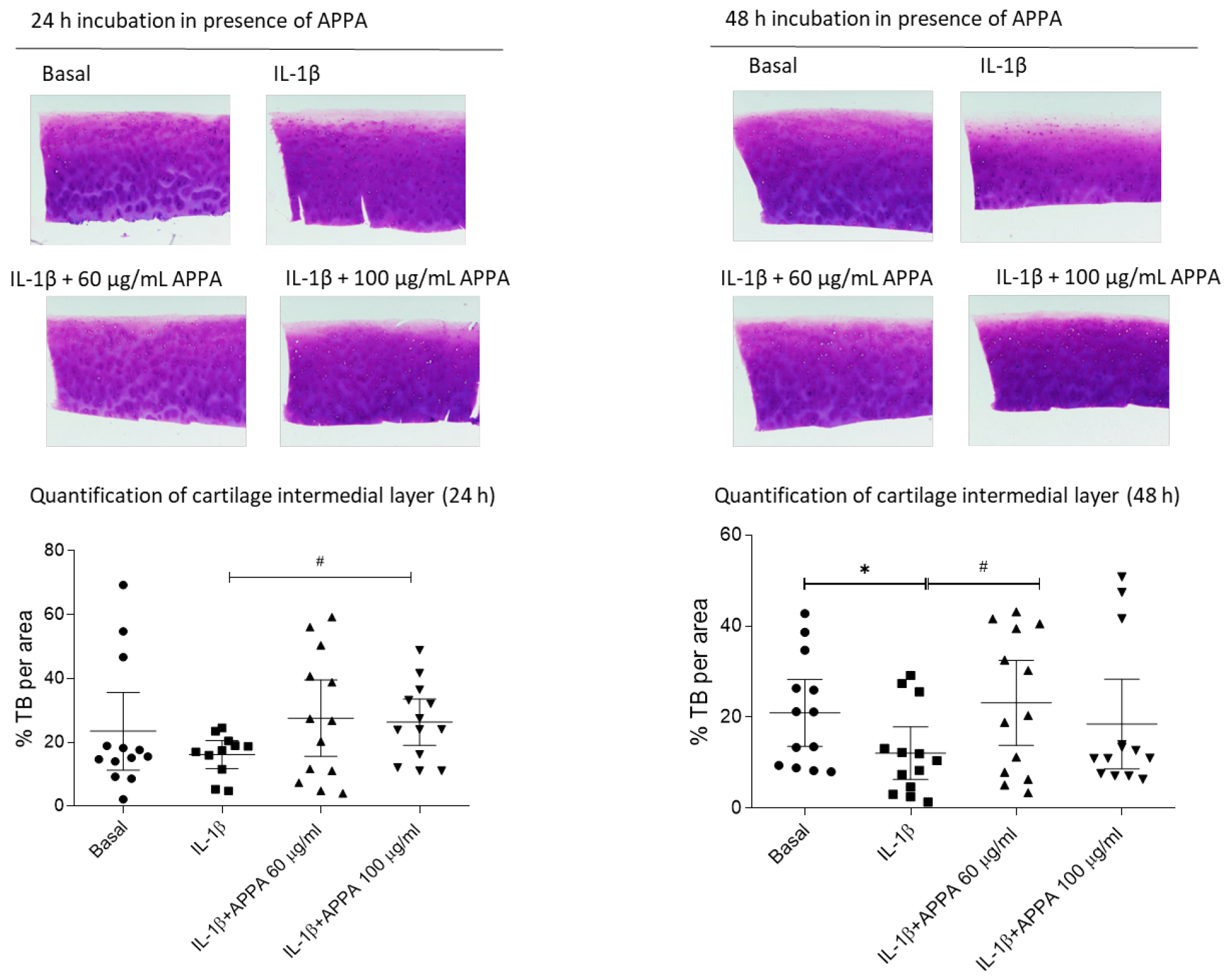
2.5. APPA Effect over the Human Articular Cartilage Degradation
3. Discussion
4. Materials and Methods
4.1. Drug Preparation
4.2. Cartilage and Chondrocytes Isolation
4.3. Chondrocyte Viability (MTT)
4.4. Cytoplasmic Reactive Oxygen Species (ROS) and Mitochondrial Anion Superoxide (O2−) Production
4.5. Quantitative Real-Time PCR (qRT-PCR)
4.6. Histological Analysis
4.7. Sulfated Glycosaminoglycan Assay (GAG)
4.8. Statistical Analyses
4.9. Study Limitations
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Conaghan, P.G.; Peloso, P.M.; Everett, S.V.; Rajagopalan, S.; Black, C.M.; Mavros, P.; Arden, N.K.; Phillips, C.J.; Rannou, F.; van de Laar, M.A.F.J.; et al. Inadequate pain relief and large functional loss among patients with knee osteoarthritis: Evidence from a prospective multinational longitudinal study of osteoarthritis real-world therapies. Rheumatology 2015, 54, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Abramoff, B.; Caldera, F.E. Osteoarthritis: Pathology, Diagnosis, and Treatment Options. Med. Clin. N. Am. 2020, 104, 293–311. [Google Scholar] [CrossRef] [PubMed]
- Long, H.; Liu, Q.; Yin, H.; Wang, K.; Diao, N.; Zhang, Y.; Lin, J.; Guo, A. Prevalence Trends of Site-Specific Osteoarthritis From 1990 to 2019: Findings From the Global Burden of Disease Study 2019. Arthritis Rheumatol. 2022, 74, 1172–1183. [Google Scholar] [CrossRef] [PubMed]
- Conley, B.; Bunzli, S.; Bullen, J.; O’Brien, P.; Persaud, J.; Gunatillake, T.; Dowsey, M.M.; Choong, P.F.M.; Lin, I. Core Recommendations for Osteoarthritis Care: A Systematic Review of Clinical Practice Guidelines. Arthritis Care Res. 2023, 75, 1897–1907. [Google Scholar] [CrossRef] [PubMed]
- Berenbaum, F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthr. Cartil. 2013, 21, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Robinson, W.H.; Lepus, C.M.; Wang, Q.; Raghu, H.; Mao, R.; Lindstrom, T.M.; Sokolove, J. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 580–592. [Google Scholar] [CrossRef]
- Mora, J.C.; Przkora, R.; Cruz-Almeida, Y. Knee osteoarthritis: Pathophysiology and current treatment modalities. J. Pain Res. 2018, 11, 2189–2196. [Google Scholar] [CrossRef] [PubMed]
- Loeser, R.F.; Collins, J.A.; Diekman, B.O. Ageing and the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 412–420. [Google Scholar] [CrossRef]
- Toh, W.S.; Brittberg, M.; Farr, J.; Foldager, C.B.; Gomoll, A.H.; Hui, J.H.; Richardson, J.B.; Roberts, S.; Spector, M. Cellular senescence in aging and osteoarthritis. Acta Orthop. 2016, 87, 6–14. [Google Scholar] [CrossRef]
- Goldring, M.B.; Berenbaum, F. Emerging targets in osteoarthritis therapy. Curr. Opin. Pharmacol. 2015, 22, 51–63. [Google Scholar] [CrossRef]
- Goldring, S.R.; Goldring, M.B. Changes in the osteochondral unit during osteoarthritis: Structure, function and cartilage-bone crosstalk. Nat. Rev. Rheumatol. 2016, 12, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Mathiessen, A.; Conaghan, P.G. Synovitis in osteoarthritis: Current understanding with therapeutic implications. Arthritis Res. Ther. 2017, 19, 18. [Google Scholar] [CrossRef]
- Kraus, V.B.; Blanco, F.J.; Englund, M.; Karsdal, M.A.; Lohmander, L.S. Call for standardized definitions of osteoarthritis and risk stratification for clinical trials and clinical use. Osteoarthr. Cartil. 2015, 23, 1233–1241. [Google Scholar] [CrossRef] [PubMed]
- Blanco, F.J. Osteoarthritis and atherosclerosis in joint disease. Reumatol. Clin. 2018, 14, 251–253. [Google Scholar] [CrossRef]
- Attur, M.; Krasnokutsky, S.; Statnikov, A.; Samuels, J.; Li, Z.; Friese, O.; Le Graverand-Gastineau, M.H.; Rybak, L.; Kraus, V.B.; Jordan, J.M.; et al. Low-grade inflammation in symptomatic knee osteoarthritis: Prognostic value of inflammatory plasma lipids and peripheral blood leukocyte biomarkers. Arthritis Rheumatol. 2015, 67, 2905–2915. [Google Scholar] [CrossRef] [PubMed]
- Blanco, F.J.; Fernandez-Moreno, M. Mitochondrial biogenesis: A potential therapeutic target for osteoarthritis. Osteoarthr. Cartil. 2020, 28, 1003–1006. [Google Scholar] [CrossRef]
- Blanco, F.J.; López-Armada, M.J.; Maneiro, E. Mitochondrial dysfunction in osteoarthritis. Mitochondrion 2004, 4, 715–728. [Google Scholar] [CrossRef]
- Blanco, F.J.; Valdes, A.M.; Rego-Pérez, I. Mitochondrial DNA variation and the pathogenesis of osteoarthritis phenotypes. Nat. Rev. Rheumatol. 2018, 14, 327–340. [Google Scholar] [CrossRef]
- Dalmao-Fernández, A.; Hermida-Gómez, T.; Lund, J.; Vazquez-Mosquera, M.E.; Rego-Pérez, I.; Garesse, R.; Blanco, F.J.; Fernández-Moreno, M. Mitochondrial DNA from osteoarthritic patients drives functional impairment of mitochondrial activity: A study on transmitochondrial cybrids. Cytotherapy 2021, 23, 399–410. [Google Scholar] [CrossRef]
- Johnson, K.; Jung, A.; Murphy, A.; Andreyev, A.; Dykens, J.; Terkeltaub, R. Mitochondrial oxidative phosphorylation is a downstream regulator of nitric oxide effects on chondrocyte matrix synthesis and mineralization. Arthritis Rheum. 2000, 43, 1560–1570. [Google Scholar] [CrossRef]
- Terkeltaub, R.; Johnson, K.; Murphy, A.; Ghosh, S. Invited review: The mitochondrion in osteoarthritis. Mitochondrion 2002, 1, 301–319. [Google Scholar] [CrossRef] [PubMed]
- Latourte, A.; Kloppenburg, M.; Richette, P. Emerging pharmaceutical therapies for osteoarthritis. Nat. Rev. Rheumatol. 2020, 16, 673–688. [Google Scholar] [CrossRef]
- Fang, C.; Guo, J.-W.; Wang, Y.-J.; Li, X.-Q.; Zhang, H.; Cui, J.; Hu, Y.; Jing, Y.-Y.; Chen, X.; Su, J.-C. Diterbutyl phthalate attenuates osteoarthritis in ACLT mice via suppressing ERK/c-fos/NFATc1 pathway, and subsequently inhibiting subchondral osteoclast fusion. Acta Pharmacol. Sin. 2022, 43, 1299–1310. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xu, H.; Li, X.; Chen, H.; Zhang, H.; Zhu, X.; Lin, Z.; Guo, S.; Bao, Z.; Rui, H.; et al. Cucurbitacin E reduces IL-1β-induced inflammation and cartilage degeneration by inhibiting the PI3K/Akt pathway in osteoarthritic chondrocytes. J. Transl. Med. 2023, 21, 880. [Google Scholar] [CrossRef]
- Wang, W.; Mai, H.; Xu, H.; Jing, B.; Yu, C.; Li, X.; Chen, D.; Huang, Y.; Shao, M.; Pan, T. 4,8-Dicarboxyl-8,9-iridoid-1-glycoside inhibits apoptosis in human osteoarthritis chondrocytes via enhanced c-MYC-mediated cholesterol metabolism in vitro. Arthritis Res. Ther. 2023, 25, 240. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, X.; Liu, L.; Guo, X.; Zhang, H.; Yin, J.; Lin, R.; Shao, Y.; Cai, D. Fructose-bisphosphatase1 (FBP1) alleviates experimental osteoarthritis by regulating Protein crumbs homolog 3 (CRB3). Arthritis Res. Ther. 2023, 25, 235. [Google Scholar] [CrossRef]
- Zhou, D.; Zhang, H.; Xue, X.; Tao, Y.; Wang, S.; Ren, X.; Su, J. Safety Evaluation of Natural Drugs in Chronic Skeletal Disorders: A Literature Review of Clinical Trials in the Past 20 years. Front. Pharmacol. 2021, 12, 801287. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Wang, K.; Wei, X.; Gong, X.; Tang, H.; Xue, H.; Wang, J.; Yin, P.; Zhang, L.; Ma, Z.; et al. Replenishing decoy extracellular vesicles inhibits phenotype remodeling of tissue-resident cells in inflammation-driven arthritis. Cell Rep. Med. 2023, 4, 101228. [Google Scholar] [CrossRef]
- Savla, S.R.; Laddha, A.P.; Kulkarni, Y.A. Pharmacology of apocynin: A natural acetophenone. Drug Metab. Rev. 2021, 53, 542–562. [Google Scholar] [CrossRef]
- Stefanska, J.; Pawliczak, R. Apocynin: Molecular aptitudes. Mediat. Inflamm. 2008, 2008, 106507. [Google Scholar] [CrossRef]
- Zhang, L.; Li, D.C.; Liu, L.F. Paeonol: Pharmacological effects and mechanisms of action. Int. Immunopharmacol. 2019, 72, 413–421. [Google Scholar] [CrossRef]
- Bravo-Sánchez, E.; Peña-Montes, D.; Sánchez-Duarte, S.; Saavedra-Molina, A.; Sánchez-Duarte, E.; Montoya-Pérez, R. Effects of Apocynin on Heart Muscle Oxidative Stress of Rats with Experimental Diabetes: Implications for Mitochondria. Antioxidants 2021, 10, 335. [Google Scholar] [CrossRef]
- Impellizzeri, D.; Esposito, E.; Mazzon, E.; Paterniti, I.; Di Paola, R.; Bramanti, P.; Cuzzocrea, S. Effect of apocynin, a NADPH oxidase inhibitor, on acute lung inflammation. Biochem. Pharmacol. 2011, 81, 636–648. [Google Scholar] [CrossRef] [PubMed]
- Impellizzeri, D.; Mazzon, E.; Esposito, E.; Paterniti, I.; Bramanti, P.; Cuzzocrea, S. Effect of Apocynin, an inhibitor of NADPH oxidase, in the inflammatory process induced by an experimental model of spinal cord injury. Free Radic. Res. 2011, 45, 221–236. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.; Wang, C.; Tang, Q.; Zheng, W.; Feng, Z.; Yu, X.; Guo, X.; Wang, J. Paeonol Inhibits IL-1beta-Induced Inflammation via PI3K/Akt/NF-kappaB Pathways: In Vivo and Vitro Studies. Inflammation 2017, 40, 1698–1706. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xu, X.; Kang, Z.; Zhang, Z.; Li, Y. Paeonol prevents IL-1β-induced inflammatory response and degradation of type II collagen in human primary chondrocytes. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2139–2145. [Google Scholar] [CrossRef]
- Ye, W.; Zhong, Z.; Zhu, S.; Zheng, S.; Xiao, J.; Song, S.; Yu, H.; Wu, Q.; Lin, Z.; Chen, J. Advanced oxidation protein products induce catabolic effect through oxidant-dependent activation of NF-κ B pathway in human chondrocyte. Int. Immunopharmacol. 2016, 39, 149–157. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Pan, S.; Zhang, L.; He, L.; Niu, Y. Paeonol attenuates ligation-induced periodontitis in rats by inhibiting osteoclastogenesis via regulating Nrf2/NF-κB/NFATc1 signaling pathway. Biochimie 2019, 156, 129–137. [Google Scholar] [CrossRef]
- Cross, A.L.; Hawkes, J.; Wright, H.L.; Moots, R.J.; Edwards, S.W. APPA (apocynin and paeonol) modulates pathological aspects of human neutrophil function, without supressing antimicrobial ability, and inhibits TNFα expression and signalling. Inflammopharmacology 2020, 28, 1223–1235. [Google Scholar] [CrossRef]
- Glasson, S.; Larkins, N. APPA provides symptom relief in clinical canine osteoarthritis. Osteoarthr. Cartil. 2012, 20 (Suppl. 1), S287. [Google Scholar] [CrossRef]
- Larkins, N.; King, C. Effectiveness of apocynin-paeonol (APPA) for the management of osteoarthritis in dogs: Comparisons with placebo and meloxicam in client-owned dogs. Matters 2017, 3, e201608000001. [Google Scholar] [CrossRef]
- Glasson, S.; Bendele, A.; Larkins, N. APPA provides disease modification in preclinical osteoarthritis. Osteoarthr. Cartil. 2012, 20, S72–S73. [Google Scholar] [CrossRef]
- Gerwin, N.; Bendele, A.; Glasson, S.; Carlson, C.S. The OARSI histopathology initiative—Recommendations for histological assessments of osteoarthritis in the rat. Osteoarthr. Cartil. 2010, 18 (Suppl. 3), S24–S34. [Google Scholar] [CrossRef]
- Bihlet, A.R.; Byrjalsen, I.; Andersen, J.R.; Metnik, A.; Reynolds, A.; Larkins, N.; Alexandersen, P.; Rovsing, H.; Schmidt, U.; Moots, R.; et al. The efficacy and safety of a fixed-dose combination of apocynin and paeonol in symptomatic knee oa: A double-blind, randomized, placebo-controlled clinical trial. Ann. Rheum. Dis. 2022, 81, 321. [Google Scholar] [CrossRef]
- Gratal, P.; Lamuedra, A.; Medina, J.P.; Bermejo-Álvarez, I.; Largo, R.; Herrero-Beaumont, G.; Mediero, A. Purinergic System Signaling in Metainflammation-Associated Osteoarthritis. Front. Med. 2020, 7, 506. [Google Scholar] [CrossRef]
- Lepetsos, P.; Papavassiliou, A.G. ROS/oxidative stress signaling in osteoarthritis. Biochim. Biophys. Acta 2016, 1862, 576–591. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Luo, P.; Yang, M.; Wang, J.; Hou, W.; Xu, P. The role of oxidative stress in the development of knee osteoarthritis: A comprehensive research review. Front. Mol. Biosci. 2022, 9, 1001212. [Google Scholar] [CrossRef]
- Motta, F.; Barone, E.; Sica, A.; Selmi, C. Inflammaging and Osteoarthritis. Clin. Rev. Allergy Immunol. 2023, 64, 222–238. [Google Scholar] [CrossRef]
- Coryell, P.R.; Diekman, B.O.; Loeser, R.F. Mechanisms and therapeutic implications of cellular senescence in osteoarthritis. Nat. Rev. Rheumatol. 2021, 17, 47–57. [Google Scholar] [CrossRef]
- Mehana, E.-S.E.; Khafaga, A.F.; El-Blehi, S.S. The role of matrix metalloproteinases in osteoarthritis pathogenesis: An updated review. Life Sci. 2019, 234, 116786. [Google Scholar] [CrossRef]
- Bolduc, J.A.; Collins, J.A.; Loeser, R.F. Reactive oxygen species, aging and articular cartilage homeostasis. Free Radic. Biol. Med. 2019, 132, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Tudorachi, N.B.; Totu, E.E.; Fifere, A.; Ardeleanu, V.; Mocanu, V.; Mircea, C.; Isildak, I.; Smilkov, K.; Cărăuşu, E.M. The Implication of Reactive Oxygen Species and Antioxidants in Knee Osteoarthritis. Antioxidants 2021, 10, 985. [Google Scholar] [CrossRef]
- O’Reilly, S.; Stratton, R.; Larkins, N. APPA: A novel pharmaceutical in systemic sclerosis treatment. In Proceedings of the 16th Intternational Workshop on Scleroderma Research, Cambridge, UK, 27–31 July 2019. [Google Scholar]
- Hougee, S.; Hartog, A.; Sanders, A.; Graus, Y.M.; Hoijer, M.A.; Garssen, J.; Berg, W.B.v.D.; van Beuningen, H.M.; Smit, H.F. Oral administration of the NADPH-oxidase inhibitor apocynin partially restores diminished cartilage proteoglycan synthesis and reduces inflammation in mice. Eur. J. Pharmacol. 2006, 531, 264–269. [Google Scholar] [CrossRef]
- Beswick, R.A.; Dorrance, A.M.; Leite, R.; Webb, R.C. NADH/NADPH oxidase and enhanced superoxide production in the mineralocorticoid hypertensive rat. Hypertension 2001, 38, 1107–1111. [Google Scholar] [CrossRef]
- Cotter, M.A.; Cameron, N.E. Effect of the NAD(P)H oxidase inhibitor, apocynin, on peripheral nerve perfusion and function in diabetic rats. Life Sci. 2003, 73, 1813–1824. [Google Scholar] [CrossRef]
- Liu, C.; Han, Y.; Gu, X.; Li, M.; Du, Y.; Feng, N.; Li, J.; Zhang, S.; Maslov, L.N.; Wang, G.; et al. Paeonol promotes Opa1-mediated mitochondrial fusion via activating the CK2α-Stat3 pathway in diabetic cardiomyopathy. Redox Biol. 2021, 46, 102098. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Abu-Amer, Y.; O’keefe, R.J.; McAlinden, A. Inflammation and epigenetic regulation in osteoarthritis. Connect. Tissue Res. 2017, 58, 49–63. [Google Scholar] [CrossRef]
- Goldring, M.B.; Otero, M. Inflammation in osteoarthritis. Curr. Opin. Rheumatol. 2011, 23, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Thudium, C.S.; Bay-Jensen, A.C.; Gantzel, T.; Dziegiel, M.; Larkins, N.; Reynolds, A. Characterizing the effect of APPA on tissue turnover in cartilage and bone tissue cultures. Osteoarthr. Cartil. 2021, 29 (Suppl. S1), S152. [Google Scholar] [CrossRef]
- Su, S.Y.; Cheng, C.Y.; Tsai, T.H.; Hsiang, C.Y.; Ho, T.Y.; Hsieh, C.L. Paeonol attenuates H₂O₂-induced NF-κB-associated amyloid precursor protein expression. Am. J. Chin. Med. 2010, 38, 1171–1792. [Google Scholar] [CrossRef]
- Popov, L.D. Mitochondrial biogenesis: An update. J. Cell. Mol. Med. 2020, 24, 4892–4899. [Google Scholar] [CrossRef]
- Rius-Pérez, S.; Torres-Cuevas, I.; Millán, I.; Ortega, Á.L.; Pérez, S. PGC-1α, Inflammation, and Oxidative Stress: An Integrative View in Metabolism. Oxid. Med. Cell. Longev. 2020, 2020, 1452696. [Google Scholar] [CrossRef]
- Eisele, P.S.; Handschin, C. Functional crosstalk of PGC-1 coactivators and inflammation in skeletal muscle pathophysiology. Semin. Immunopathol. 2014, 36, 27–53. [Google Scholar] [CrossRef] [PubMed]
- Malfait, A.M.; Little, C.B. On the predictive utility of animal models of osteoarthritis. Arthritis Res. Ther. 2015, 17, 225. [Google Scholar] [CrossRef]
- McCoy, A.M. Animal Models of Osteoarthritis: Comparisons and Key Considerations. Vet. Pathol. 2015, 52, 803–818. [Google Scholar] [CrossRef] [PubMed]
- Zaki, S.; Blaker, C.; Little, C.B. OA foundations—Experimental models of osteoarthritis. Osteoarthr. Cartil. 2022, 30, 357–380. [Google Scholar] [CrossRef]
- Cook, J.; Kuroki, K.; Visco, D.; Pelletier, J.-P.; Schulz, L.; Lafeber, F. The OARSI histopathology initiative—Recommendations for histological assessments of osteoarthritis in the dog. Osteoarthr. Cartil. 2010, 18 (Suppl. 3), S66–S79. [Google Scholar] [CrossRef]
- Maneiro, E.; Martín, M.A.; de Andres, M.C.; López-Armada, M.J.; Fernández-Sueiro, J.L.; del Hoyo, P.; Galdo, F.; Arenas, J.; Blanco, F.J. Mitochondrial respiratory activity is altered in osteoarthritic human articular chondrocytes. Arthritis Rheum. 2003, 48, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]




| Gene Expression | Gene Expression | ||||
|---|---|---|---|---|---|
| Basal | LPS | p Value | IL-1β+ APPA 10 µg/mL | p Value | |
| NFE2L2 | 0.721 ± 0.07 | 1.33 ± 0.26 | ns (p = 0.057) | 1.05 ± 0.13 | |
| SOD-2 | 0.566 ± 0.17 | 3.74 ± 1.03 | ** (0.007) | 1.72 ± 0.60 | ns (p = 0.055) |
| Gene Expression | Gene Expression | ||||
| Basal | IL-1β | p Value | IL-1β+ APPA 10 µg/mL | p Value | |
| NFE2L2 | 1.01 ± 0.33 | 1.38 ± 0.69 | 0.82 ± 0.19 | ||
| SOD-2 | 0.255 ± 0.11 | 2.43 ± 0.86 | ** (0.007) | 1.11 ± 0.07 | ## (0.007) |
| iNOS | 0.16 ± 0.09 | 3.25 ± 0.55 | ** (0.007) | 1.87 ± 0.31 | ns (p = 0.092) |
| Mean ± SEM, p value (Mann Whitney) | |||||
| **, ## p ≤ 0.01 | |||||
| Joint | Sex | Age | ||
|---|---|---|---|---|
| Mean | ± SD | |||
| Cartilage | Hip | F (N = 5) | 72.20 | 11.14 |
| Chondrocytes | Knee | M (N = 2) | 71.50 | 3.53 |
| Hip | M (N = 5) | 76.33 | 9.81 | |
| F (N = 22) | 79.835 | 15.455 | ||
| Gene Name | Symbol | Fw | Rv |
|---|---|---|---|
| Nuclear Factor, Erythroid 2 Like 2 | NFE2L2 | gcaacaggacattgagcaag | tggacttggaaccatggtagt |
| Superoxide Dismutase 2 | SOD-2 | ctggacaaacctcagcccta | tgatggcttccagcaactc |
| Inducible Nitric Oxide Synthase | iNOS | gctgccaagctgaaattga | gatagcgcttctggctcttg |
| Glyceraldehyde-3-Phosphate Dehydrogenase | GAPDH | gagtccactggcgtcttcac | gttcacacccatgacgaaca |
| Interleukin 6 | IL6 | gatgagtacaaaagtcctgatcca | ctgcagccactggttctgt |
| Interleukin 8 | IL8 | gagcactccataaggcacaaa | atggttccttccggtggt |
| Tumor Necrosis Factor alpha | TNF-α | gcaacaggacattgagcaag | tggacttggaaccatggtagt |
| Matrix Metallopeptidase 13 | MMP-13 | ccagtctccgaggagaaaca | aaaaacagctccgcatcaac |
| Matrix Metallopeptidase 3 | MMP-3 | caaaacatatttctttgtagaggacaa | ttcagctatttgcttgggaaa |
| Peroxisome Proliferator-Activated Receptor Gamma Coactivator 1-Alpha | PGC1-α | tgagagggccaagcaaag | ataaatcacacggcgctctt |
| Mitofusin-2 | Mfn2 | tcagctacactggctccaac | caaaggtcccagacagttcc |
| Sirtuin-1 | Sirt-1 | aaatgctggcctaatagagtgg | tggcaaaaacagatactgattacc |
| Sirtuin-3 | Sirt-3 | ccacctgctggattgtgac | ggagcctgtgcagaagtagc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Moreno, M.; Hermida-Gómez, T.; Larkins, N.; Reynolds, A.; Blanco, F.J. Anti-Inflammatory Activity of APPA (Apocynin and Paeonol) in Human Articular Chondrocytes. Pharmaceuticals 2024, 17, 118. https://doi.org/10.3390/ph17010118
Fernández-Moreno M, Hermida-Gómez T, Larkins N, Reynolds A, Blanco FJ. Anti-Inflammatory Activity of APPA (Apocynin and Paeonol) in Human Articular Chondrocytes. Pharmaceuticals. 2024; 17(1):118. https://doi.org/10.3390/ph17010118
Chicago/Turabian StyleFernández-Moreno, Mercedes, Tamara Hermida-Gómez, Nicholas Larkins, Alan Reynolds, and Francisco J. Blanco. 2024. "Anti-Inflammatory Activity of APPA (Apocynin and Paeonol) in Human Articular Chondrocytes" Pharmaceuticals 17, no. 1: 118. https://doi.org/10.3390/ph17010118
APA StyleFernández-Moreno, M., Hermida-Gómez, T., Larkins, N., Reynolds, A., & Blanco, F. J. (2024). Anti-Inflammatory Activity of APPA (Apocynin and Paeonol) in Human Articular Chondrocytes. Pharmaceuticals, 17(1), 118. https://doi.org/10.3390/ph17010118






