Assessment of Patient-Reported Outcomes at 48 Months of Treatment with Dupilumab for Severe Atopic Dermatitis: A Single-Center Real-Life Experience with 126 Patients
Abstract
1. Introduction
2. Results
2.1. Eczema Assessment and Severity Score (EASI)
2.2. Pruritus Numerical Rating Scale (Pruritus NRS) and Sleep Numerical Rating Scale (Sleep NRS)
2.3. Patient-Oriented Eczema Measure (POEM)
2.4. Atopic Dermatitis Control Tool (ADCT)
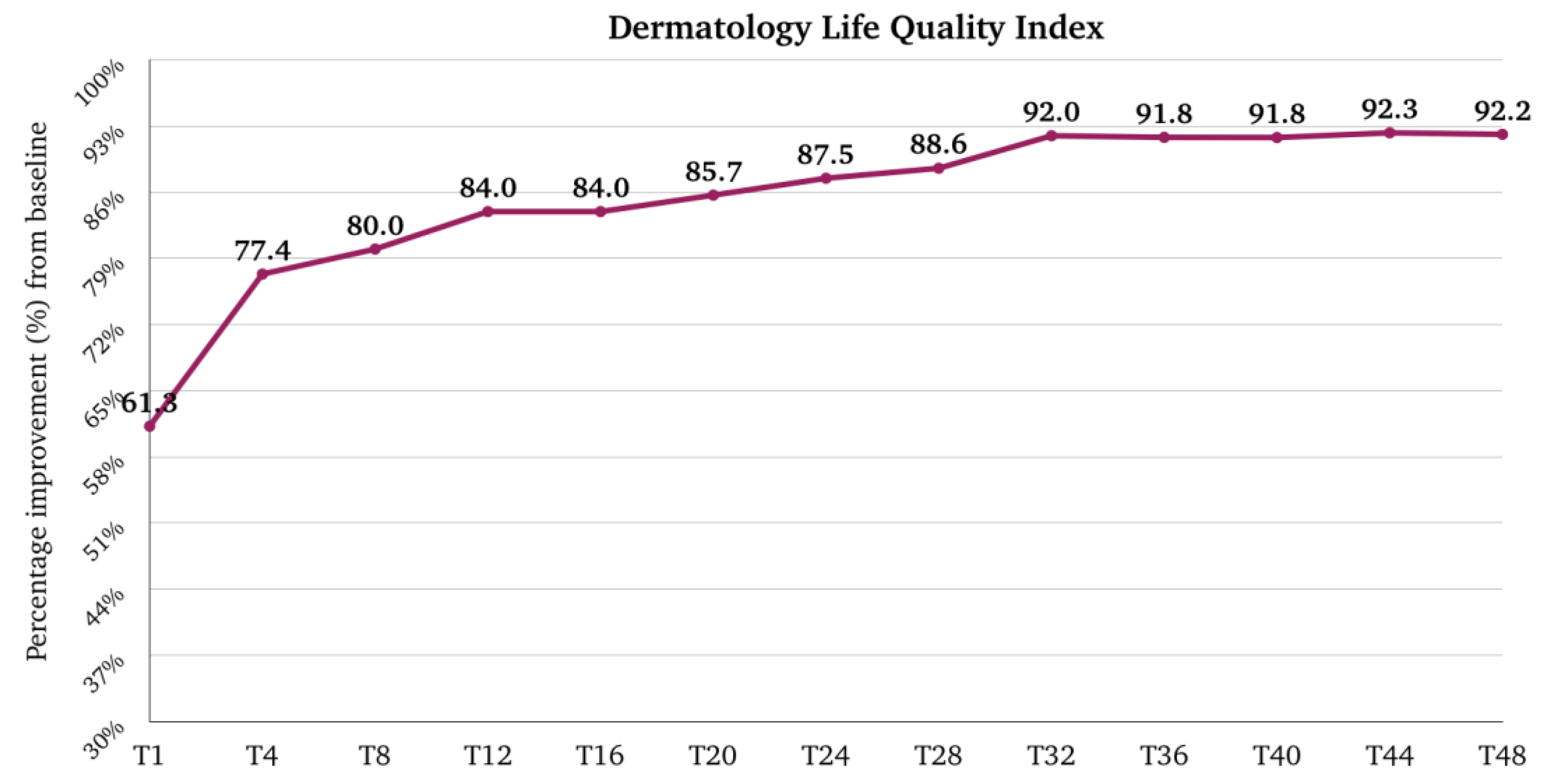
2.5. Dermatology Life Quality Index (DLQI)
2.6. Correlation Analysis
2.7. Regression Analysis
2.8. Safety and Tolerability
3. Discussion
3.1. Descriptive Analysis
3.2. Correlation Analysis
3.3. Regression Analyses
3.4. Strengths and Limitations
4. Materials and Methods
4.1. Population
4.2. Data Collection
4.3. Objectives
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sroka-Tomaszewska, J.; Trzeciak, M. Molecular Mechanisms of Atopic Dermatitis Pathogenesis. Int. J. Mol. Sci. 2021, 22, 4130. [Google Scholar] [CrossRef] [PubMed]
- Weidinger, S.; Beck, L.A.; Bieber, T.; Kabashima, K.; Irvine, A.D. Atopic dermatitis. Nat. Rev. Dis. Primers 2018, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Bylund, S.; Kobyletzki, L.B.; Svalstedt, M.; Svensson, Å. Prevalence and Incidence of Atopic Dermatitis: A Systematic Review. Acta Derm. Venereol. 2020, 100, adv00160. [Google Scholar] [CrossRef] [PubMed]
- Chrostowska-Plak, D.; Reich, A.; Szepietowski, J.C. Relationship between itch and psychological status of patients with atopic dermatitis. J. Eur. Acad. Dermatol. Venereol. 2013, 27, e239–e242. [Google Scholar] [CrossRef]
- Silverberg, J.I.; Garg, N.K.; Paller, A.S.; Fishbein, A.B.; Zee, P.C. Sleep disturbances in adults with eczema are associated with impaired overall health: A US population-based study. J. Investig. Dermatol. 2015, 135, 56–66. [Google Scholar] [CrossRef]
- Sibbald, C.; Drucker, A.M. Patient Burden of Atopic Dermatitis. Dermatol. Clin. 2017, 35, 303–316. [Google Scholar] [CrossRef]
- Drucker, A.M.; Wang, A.R.; Qureshi, A.A. Research Gaps in Quality of Life and Economic Burden of Atopic Dermatitis: The National Eczema Association Burden of Disease Audit. JAMA Dermatol. 2016, 152, 873–874. [Google Scholar] [CrossRef]
- Wollenberg, A.; Barbarot, S.; Bieber, T.; Christen-Zaech, S.; Deleuran, M.; Fink-Wagner, A.; Gieler, U.; Girolomoni, G.; Lau, S.; Muraro, A.; et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: Part I. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 657–682. [Google Scholar] [CrossRef]
- Wollenberg, A.; Barbarot, S.; Bieber, T.; Christen-Zaech, S.; Deleuran, M.; Fink-Wagner, A.; Gieler, U.; Girolomoni, G.; Lau, S.; Muraro, A.; et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: Part II. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 850–878. [Google Scholar] [CrossRef]
- Barrett, A.; Hahn-Pedersen, J.; Kragh, N.; Evans, E.; Gnanasakthy, A. Patient-Reported Outcome Measures in Atopic Dermatitis and Chronic Hand Eczema in Adults. Patient 2019, 12, 445–459. [Google Scholar] [CrossRef]
- Cork, M.J.; Eckert, L.; Simpson, E.L.; Armstrong, A.; Barbarot, S.; Puig, L.; Girolomoni, G.; de Bruin-Weller, M.; Wollenberg, A.; Kataoka, Y.; et al. Dupilumab improves patient-reported symptoms of atopic dermatitis, symptoms of anxiety and depression, and health-related quality of life in moderate-to-severe atopic dermatitis: Analysis of pooled data from the randomized trials SOLO 1 and SOLO 2. J. Dermatol. Treat. 2020, 31, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Simpson, E.L.; Bieber, T.; Guttman-Yassky, E.; Beck, L.A.; Blauvelt, A.; Cork, M.J.; Silverberg, J.I.; Deleuran, M.; Kataoka, Y.; Lacour, J.P.; et al. Two Phase 3 Trials of Dupilumab versus Placebo in Atopic Dermatitis. N. Engl. J. Med. 2016, 375, 2335–2348. [Google Scholar] [CrossRef]
- Blauvelt, A.; de Bruin-Weller, M.; Gooderham, M.; Cather, J.C.; Weisman, J.; Pariser, D.; Simpson, E.L.; Papp, K.A.; Hong, H.C.; Rubel, D.; et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): A 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet 2017, 389, 2287–2303. [Google Scholar] [CrossRef]
- Kimball, A.B.; Delevry, D.; Yang, M.; Chuang, C.C.; Wang, Z.; Bégo-Le-Bagousse, G.; Martins, B.; Wu, E.; Shumel, B.; Wang, J.; et al. Long-Term Effectiveness of Dupilumab in Patients with Atopic Dermatitis: Results up to 3 Years from the RELIEVE-AD Study. Dermatol. Ther. 2023, 13, 2107–2120. [Google Scholar] [CrossRef] [PubMed]
- Beck, L.A.; Deleuran, M.; Bissonnette, R.; de Bruin-Weller, M.; Galus, R.; Nakahara, T.; Seo, S.J.; Khokhar, F.A.; Vakil, J.; Xiao, J.; et al. Dupilumab Provides Acceptable Safety and Sustained Efficacy for up to 4 Years in an Open-Label Study of Adults with Moderate-to-Severe Atopic Dermatitis. Am. J. Clin. Dermatol. 2022, 23, 393–408. [Google Scholar] [CrossRef] [PubMed]
- Beck, L.A.; Thaçi, D.; Deleuran, M.; Blauvelt, A.; Bissonnette, R.; de Bruin-Weller, M.; Hide, M.; Sher, L.; Hussain, I.; Chen, Z.; et al. Dupilumab Provides Favorable Safety and Sustained Efficacy for up to 3 Years in an Open-Label Study of Adults with Moderate-to-Severe Atopic Dermatitis. Am. J. Clin. Dermatol. 2020, 21, 567–577. [Google Scholar] [CrossRef]
- Ortoncelli, M.; Macagno, N.; Mastorino, L.; Gelato, F.; Richiardi, I.; Cavaliere, G.; Quaglino, P.; Ribero, S. Long-Term Efficacy and Safety of Dupilumab in Patients with Atopic Dermatitis: A Single-Centre Retrospective Study. Cosmetics 2023, 10, 153. [Google Scholar] [CrossRef]
- Miniotti, M.; Ribero, S.; Mastorino, L.; Ortoncelli, M.; Gelato, F.; Bailon, M.; Trioni, J.; Stefan, B.; Quaglino, P.; Leombruni, P. Long-term psychological outcome of patients with moderate-to-severe atopic dermatitis continuously treated with Dupilumab: Data up to 3 years. Exp. Dermatol. 2023, 32, 852–858. [Google Scholar] [CrossRef]
- Silvestre Salvador, J.F.; Romero-Pérez, D.; Encabo-Durán, B. Atopic Dermatitis in Adults: A Diagnostic Challenge. J. Investig. Allergol. Clin. Immunol. 2017, 27, 78–88. [Google Scholar] [CrossRef]
- Hanifin, J.M.; Baghoomian, W.; Grinich, E.; Leshem, Y.A.; Jacobson, M.; Simpson, E.L. The Eczema Area and Severity Index-A Practical Guide. Dermatitis 2022, 33, 187–192. [Google Scholar] [CrossRef]
- Reich, A.; Heisig, M.; Phan, N.Q.; Taneda, K.; Takamori, K.; Takeuchi, S.; Furue, M.; Blome, C.; Augustin, M.; Ständer, S.; et al. Visual analogue scale: Evaluation of the instrument for the assessment of pruritus. Acta Derm. Venereol. 2012, 92, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Charman, C.R.; Venn, A.J.; Ravenscroft, J.C.; Williams, H.C. Translating Patient-Oriented Eczema Measure (POEM) scores into clinical practice by suggesting severity strata derived using anchor-based methods. Br. J. Dermatol. 2013, 169, 1326–1332. [Google Scholar] [CrossRef] [PubMed]
- Pariser, D.M.; Simpson, E.L.; Gadkari, A.; Bieber, T.; Margolis, D.J.; Brown, M.; Nelson, L.; Mahajan, P.; Reaney, M.; Guillemin, I.; et al. Evaluating patient-perceived control of atopic dermatitis: Design, validation, and scoring of the Atopic Dermatitis Control Tool (ADCT). Curr. Med. Res. Opin. 2020, 36, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Hongbo, Y.; Thomas, C.L.; Harrison, M.A.; Salek, M.S.; Finlay, A.Y. Translating the science of quality of life into practice: What do dermatology life quality index scores mean? J. Investig. Dermatol. 2005, 125, 659–664. [Google Scholar] [CrossRef]







| Patients (n) | 126 |
| Age, mean ± DS | 39.89 ± 16.6 |
| Sex, n (%) | |
| 75 (59.5) |
| 51 (40.5) |
| Onset age, median (Q1–Q3) | 1.0 (0.0–20.0) |
| AD onset pattern, n (%) | |
| 63 (50.0) |
| 25 (19.8) |
| 38 (30.2) |
| Atopic comorbidities, n (%) | |
| 97 (77.0) |
| 76 (60.3) |
| 54 (42.9) |
| 1 (0.8) |
| 1 (2.4) |
| 27 (21.4) |
| 1 (0.8) |
| AD phenotype, n (%) | |
| 54 (42.9) |
| 27 (21.4) |
| 19 (15.1) |
| 7 (5.6) |
| 13 (10.3) |
| 6 (4.8) |
| Concomitant contact allergy, n (%) | 28 (22.2) |
| Positive family history for atopy, n (%) | 57 (45.2) |
| Other comorbidities, n (%) | |
| 3 (2.4) |
| 2 (1.6) |
| 17 (13.5) |
| 5 (4.0) |
| 5 (4.0) |
| 4 (3.2) |
| 3 (2.4) |
| Previous systemic treatment, n (%) | |
| 108 (85.7) |
| 17 (13.5) |
| 5 (4.0) |
| 1 (0.8) |
| 1 (0.8) |
| 0 (0.0) |
| 0 (0.0) |
| Involvement of specific site at baseline, n (%) | |
| 115 (91.3) |
| 110 (87.3) |
| 51 (40.5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barei, F.; Zussino, M.; Tavecchio, S.; Angileri, L.; Rizzo, A.; Calzari, P.; Marzano, A.V.; Ferrucci, S. Assessment of Patient-Reported Outcomes at 48 Months of Treatment with Dupilumab for Severe Atopic Dermatitis: A Single-Center Real-Life Experience with 126 Patients. Pharmaceuticals 2024, 17, 117. https://doi.org/10.3390/ph17010117
Barei F, Zussino M, Tavecchio S, Angileri L, Rizzo A, Calzari P, Marzano AV, Ferrucci S. Assessment of Patient-Reported Outcomes at 48 Months of Treatment with Dupilumab for Severe Atopic Dermatitis: A Single-Center Real-Life Experience with 126 Patients. Pharmaceuticals. 2024; 17(1):117. https://doi.org/10.3390/ph17010117
Chicago/Turabian StyleBarei, Francesca, Martina Zussino, Simona Tavecchio, Luisa Angileri, Arianna Rizzo, Paolo Calzari, Angelo V. Marzano, and Silvia Ferrucci. 2024. "Assessment of Patient-Reported Outcomes at 48 Months of Treatment with Dupilumab for Severe Atopic Dermatitis: A Single-Center Real-Life Experience with 126 Patients" Pharmaceuticals 17, no. 1: 117. https://doi.org/10.3390/ph17010117
APA StyleBarei, F., Zussino, M., Tavecchio, S., Angileri, L., Rizzo, A., Calzari, P., Marzano, A. V., & Ferrucci, S. (2024). Assessment of Patient-Reported Outcomes at 48 Months of Treatment with Dupilumab for Severe Atopic Dermatitis: A Single-Center Real-Life Experience with 126 Patients. Pharmaceuticals, 17(1), 117. https://doi.org/10.3390/ph17010117







