Inhalation of Pelargonium graveolens Essential Oil Alleviates Pain and Related Anxiety and Stress in Patients with Lumbar Spinal Stenosis and Moderate to Severe Pain
Abstract
:1. Introduction
2. Results
2.1. Chemical Profile of Geranium Essential Oil
2.2. Baseline Characteristics of LSS Patients as a Function of Patient-Reported Pain Severity
2.3. Effects of Geranium Essential Oil Inhalation on Anxiety-VAS, Stress-VAS, and Depression-VAS Scores as a Function of Pain Severity
2.4. Pain-Related Variables at Baseline and after Geranium Essential Oil or Placebo Control Inhalation
3. Discussion
4. Materials and Methods
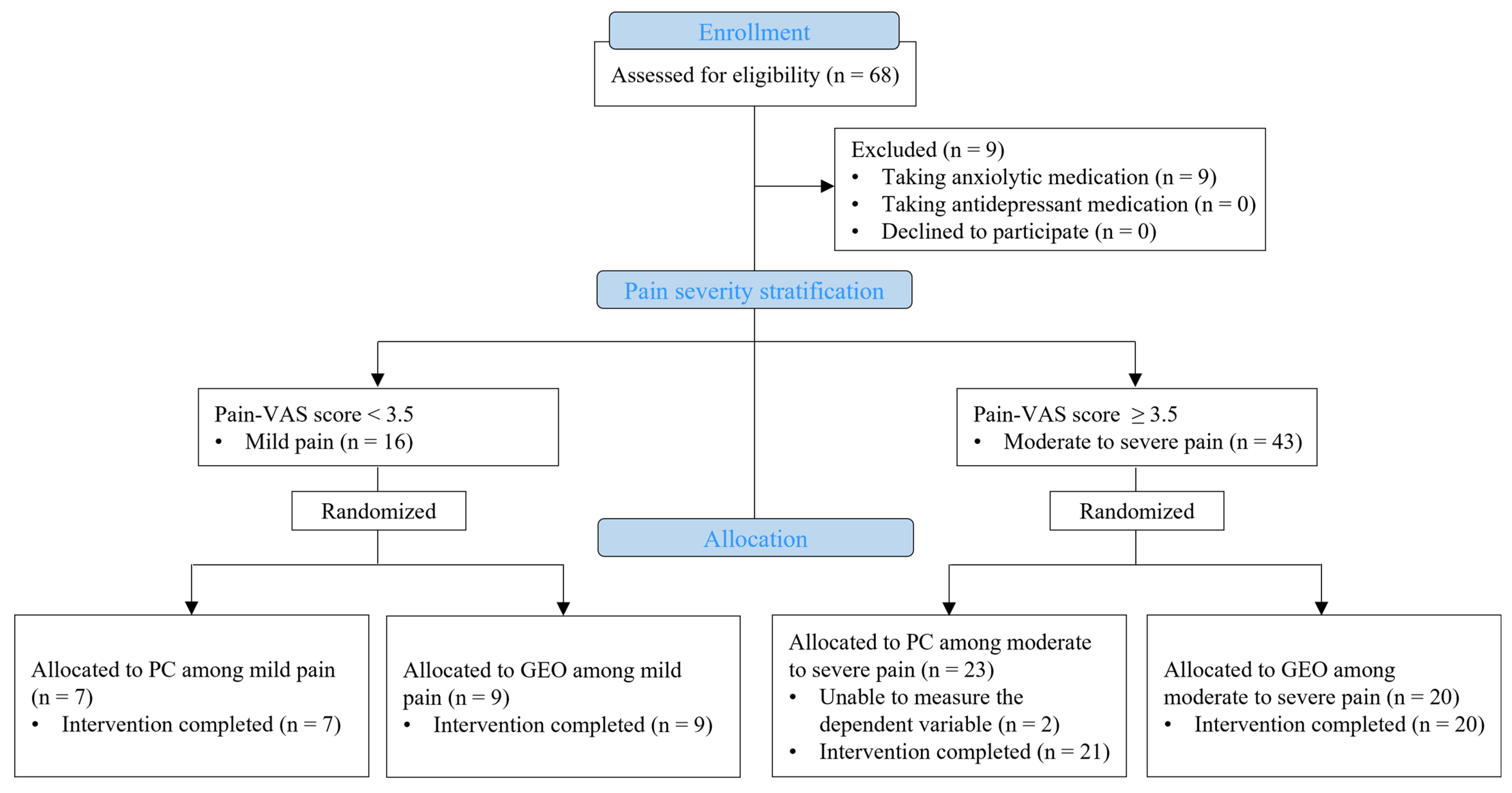
4.1. Participants and Study Design
4.2. Randomization and Masking
4.3. GC/MS Spectrometry Profiling of Geranium Essential Oil
4.4. Classification Based on Self-Reported Pain Severity
4.5. Intervention
4.6. Outcome Measures
4.7. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ravindra, V.M.; Senglaub, S.S.; Rattani, A.; Dewan, M.C.; Härtl, R.; Bisson, E.; Park, K.B.; Shrime, M.G. Degenerative Lumbar Spine Disease: Estimating Global Incidence and Worldwide Volume. Glob. Spine J. 2018, 8, 784–794. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Chung, C.K.; Kim, C.H.; Kwon, J.W. Health Care Burden of Spinal Diseases in the Republic of Korea: Analysis of a Nationwide Database from 2012 through 2016. Neurospine 2018, 15, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Özdemir, E.; Paker, N.; Bugdayci, D.; Tekdos, D.D. Quality of life and related factors in degenerative lumbar spinal stenosis: A controlled study. J. Back Musculoskelet. Rehabil. 2015, 28, 749–753. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.H.; Lee, M.Y.; Jung, S.W.; Lee, S.Y. Does spinal stenosis correlate with MRI findings and pain, psychologic factor and quality of life? Korean J. Anesthesiol. 2015, 68, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Katz, J.N.; Zimmerman, Z.E.; Mass, H.; Makhni, M.C. Diagnosis and Management of Lumbar Spinal Stenosis: A Review. JAMA 2022, 327, 1688–1699. [Google Scholar] [CrossRef] [PubMed]
- Raja, S.N.; Carr, D.B.; Cohen, M.; Finnerup, N.B.; Flor, H.; Gibson, S.; Keefe, F.J.; Mogil, J.S.; Ringkamp, M.; Sluka, K.A.; et al. The revised International Association for the Study of Pain definition of pain: Concepts, challenges, and compromises. Pain 2020, 161, 1976–1982. [Google Scholar] [CrossRef]
- Heikkinen, J.; Honkanen, R.; Williams, L.; Leung, J.; Rauma, P.; Quirk, S.; Koivumaa-Honkanen, H. Depressive disorders, anxiety disorders and subjective mental health in common musculoskeletal diseases: A review. Maturitas 2019, 127, 18–25. [Google Scholar] [CrossRef]
- Pomara, N.; Lee, S.H.; Bruno, D.; Silber, T.; Greenblatt, D.J.; Petkova, E.; Sidtis, J.J. Adverse performance effects of acute lorazepam administration in elderly long-term users: Pharmacokinetic and clinical predictors. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2015, 56, 129–135. [Google Scholar] [CrossRef]
- Minetama, M.; Kawakami, M.; Teraguchi, M.; Kagotani, R.; Mera, Y.; Sumiya, T.; Nakagawa, M.; Yamamoto, Y.; Matsuo, S.; Koike, Y.; et al. Supervised physical therapy vs. home exercise for patients with lumbar spinal stenosis: A randomized controlled trial. Spine J. 2019, 19, 1310–1318. [Google Scholar] [CrossRef]
- Violante, F.S.; Mattioli, S.; Bonfiglioli, R. Low-back pain. Handb. Clin. Neurol. 2015, 131, 397–410. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, C.Z.; Sawadogo, R.; Tan, T.; Yuan, C.S. Effects of Herbal Medicines on Pain Management. Am. J. Chin. Med. 2020, 48, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaeichehr, M.; Mortazavi, H. The Effectiveness of Aromatherapy in the Management of Labor Pain and Anxiety: A Systematic Review. Ethiop. J. Health Sci. 2020, 30, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Liao, H.E.; Change-Lee, S.N.; Yen, Y.Y. Initial and Continuous Effects of Essential Oil Therapy in Relieving Knee Pain among Older Adults with Osteoarthritis. Altern. Ther. Health Med. 2022, 28, 10–17. [Google Scholar] [PubMed]
- Borgonetti, V.; López, V.; Galeotti, N. Ylang-ylang (Cananga odorata (Lam.) Hook. f. & Thomson) essential oil reduced neuropathic-pain and associated anxiety symptoms in mice. J. Ethnopharmacol. 2022, 294, 115362. [Google Scholar] [CrossRef] [PubMed]
- Seol, G.H.; Jung, M.H. Effect of Bergamot Essential Oil-Inhalation on Chronic Pain after Surgery for Lumbar Spinal Stenosis. J. Korean Biol. Nurs. Sci. 2011, 13, 156–163. [Google Scholar]
- Fekri, N.; El Amir, D.; Owis, A.; AbouZid, S. Studies on essential oil from rose-scented geranium, Pelargonium graveolens L’Hérit. (Geraniaceae). Nat. Prod. Res. 2021, 35, 2593–2597. [Google Scholar] [CrossRef] [PubMed]
- Rao, B.R. Chemical composition and uses of Indian rose-scented Geranium (Pelargonium species) essential oil-A review. J. Essent. Oil Bear. Plants 2009, 12, 381–394. [Google Scholar] [CrossRef]
- Gazerani, A.; Sarchahi, Z.; Hosseini, S.S.; Abavisani, M. The effect of inhalation aromatherapy of geranium on pain and physiological indices after appendectomy: A double-blind randomized clinical trial. Int. J. Surg. Open 2021, 28, 44–49. [Google Scholar] [CrossRef]
- Shirzadegan, R.; Gholami, M.; Hasanvand, S.; Birjandi, M.; Beiranvand, A. Effects of geranium aroma on anxiety among patients with acute myocardial infarction: A triple-blind randomized clinical trial. Complement. Ther. Clin. Pract. 2017, 29, 201–206. [Google Scholar] [CrossRef]
- Karimi, N.; Hasanvand, S.; Beiranvand, A.; Gholami, M.; Birjandi, M. The effect of Aromatherapy with Pelargonium graveolens (P. graveolens) on the fatigue and sleep quality of critical care nurses during the COVID-19 pandemic: A randomized controlled trial. Explore 2023, in press. [Google Scholar] [CrossRef]
- Rungqu, P.; Oyedeji, O.; Gondwe, M.; Oyedeji, A. Chemical Composition, Analgesic and Anti-Inflammatory Activity of Pelargonium peltatum Essential Oils from Eastern Cape, South Africa. Molecules 2023, 28, 5294. [Google Scholar] [CrossRef] [PubMed]
- Lizarraga-Valderrama, L.R. Effects of essential oils on central nervous system: Focus on mental health. Phytother. Res. 2021, 35, 657–679. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Kawakami, M.; Minetama, M.; Nakagawa, M.; Teraguchi, M.; Kagotani, R.; Mera, Y.; Sumiya, T.; Matsuo, S.; Kitano, T.; et al. Psychological Predictors of Satisfaction after Lumbar Surgery for Lumbar Spinal Stenosis. Asian Spine J. 2022, 16, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Minetama, M.; Kawakami, M.; Teraguchi, M.; Kagotani, R.; Mera, Y.; Sumiya, T.; Nakagawa, M.; Yamamoto, Y.; Matsuo, S.; Sakon, N.; et al. Associations between psychological factors and daily step count in patients with lumbar spinal stenosis. Physiother. Theory Pract. 2022, 38, 1519–1527. [Google Scholar] [CrossRef] [PubMed]
- Houle, M.; Bonneau, J.D.; Marchand, A.A.; Descarreaux, M. Physical and Psychological Factors Associated with Walking Capacity in Patients with Lumbar Spinal Stenosis with Neurogenic Claudication: A Systematic Scoping Review. Front. Neurol. 2021, 12, 720662. [Google Scholar] [CrossRef] [PubMed]
- Leeuw, M.; Goossens, M.E.; Linton, S.J.; Crombez, G.; Boersma, K.; Vlaeyen, J.W. The fear-avoidance model of musculoskeletal pain: Current state of scientific evidence. J. Behav. Med. 2007, 30, 77–94. [Google Scholar] [CrossRef] [PubMed]
- Halicka, M.; Duarte, R.; Catherall, S.; Maden, M.; Coetsee, M.; Wilby, M.; Brown, C. Predictors of Pain and Disability Outcomes Following Spinal Surgery for Chronic Low Back and Radicular Pain: A Systematic Review. Clin. J. Pain 2022, 38, 368–380. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.B.; Rahali, F.Z.; Nehme, R.; Falleh, H.; Jemaa, M.B.; Sellami, I.H.; Ksouri, R.; Bouhallab, S.; Ceciliani, F.; Abdennebi-Najar, L.; et al. Anti-inflammatory activity of essential oils from Tunisian aromatic and medicinal plants and their major constituents in THP-1 macrophages. Food Res. Int. 2023, 167, 112678. [Google Scholar] [CrossRef]
- Su, Y.W.; Chao, S.H.; Lee, M.H.; Ou, T.Y.; Tsai, Y.C. Inhibitory effects of citronellol and geraniol on nitric oxide and prostaglandin E2production in macrophages. Planta Med. 2010, 76, 1666–1671. [Google Scholar] [CrossRef]
- Galea, C. Perspectives on the use of geranium essential oil: Pelargonium graveolens and pelargonium roseum, in dental medicine. Rom. J. Med. Dent. Educ. 2023, 12, 23–34. [Google Scholar]
- Enthoven, W.T.; Roelofs, P.D.; Deyo, R.A.; van Tulder, M.W.; Koes, B.W. Non-steroidal anti-inflammatory drugs for chronic low back pain. Cochrane Database Syst. Rev. 2016, 2, CD012087. [Google Scholar] [CrossRef] [PubMed]
- Bampidis, V.; Azimonti, G.; Bastos, M.L.; Christensen, H.; Durjava, M.; Kouba, M.; López-Alonso, M.; López Puente, S.; Marcon, F.; Mayo, B.; et al. Safety and efficacy of a feed additive consisting of an essential oil from the herbaceous parts of Pelargonium graveolens L’Hér. (geranium rose oil) for all animal species (FEFANA asbl). EFSA J. 2023, 21, e08161. [Google Scholar] [CrossRef] [PubMed]
- Abouhosseini Tabari, M.; Hajizadeh Moghaddam, A.; Maggi, F.; Benelli, G. Anxiolytic and antidepressant activities of Pelargonium roseum essential oil on Swiss albino mice: Possible involvement of serotonergic transmission. Phytother. Res. 2018, 32, 1014–1022. [Google Scholar] [CrossRef] [PubMed]
- Tesfaye, S.; Sloan, G.; Petrie, J.; White, D.; Bradburn, M.; Julious, S.; Rajbhandari, S.; Sharma, S.; Rayman, G.; Gouni, R.; et al. Comparison of amitriptyline supplemented with pregabalin, pregabalin supplemented with amitriptyline, and duloxetine supplemented with pregabalin for the treatment of diabetic peripheral neuropathic pain (OPTION-DM): A multicentre, double-blind, randomised crossover trial. Lancet 2022, 400, 680–690. [Google Scholar] [CrossRef] [PubMed]
- Konno, S.; Oda, N.; Ochiai, T.; Alev, L. Randomized, Double-blind, Placebo-controlled Phase III Trial of Duloxetine Monotherapy in Japanese Patients with Chronic Low Back Pain. Spine 2016, 41, 1709–1717. [Google Scholar] [CrossRef]
- Skljarevski, V.; Ossanna, M.; Liu-Seifert, H.; Zhang, Q.; Chappell, A.; Iyengar, S.; Detke, M.; Backonja, M. A double-blind, randomized trial of duloxetine versus placebo in the management of chronic low back pain. Eur. J. Neurol. 2009, 16, 1041–1048. [Google Scholar] [CrossRef]
- Park, S.Y.; An, H.S.; Moon, S.H.; Lee, H.M.; Suh, S.W.; Chen, D.; Jeon, J.H. Neuropathic Pain Components in Patients with Lumbar Spinal Stenosis. Yonsei Med. J. 2015, 56, 1044–1050. [Google Scholar] [CrossRef]
- Haddadi, K.; Asadian, L.; Isazade, A. Effects of Nasal Calcitonin vs. Oral Gabapentin on Pain and Symptoms of Lumbar Spinal Stenosis: A Clinical Trial Study. Clin. Med. Insights Arthritis Musculoskelet. Disord. 2016, 9, 133–138. [Google Scholar] [CrossRef]
- Bussières, A.; Cancelliere, C.; Ammendolia, C.; Comer, C.M.; Zoubi, F.A.; Châtillon, C.E.; Chernish, G.; Cox, J.M.; Gliedt, J.A.; Haskett, D.; et al. Non-Surgical Interventions for Lumbar Spinal Stenosis Leading to Neurogenic Claudication: A Clinical Practice Guideline. J. Pain 2021, 22, 1015–1039. [Google Scholar] [CrossRef]
- Lee, J.Y.; Choi, H.Y.; Park, C.S.; Jang, C.; Lee, K.T.; Lee, J.Y.; Youn, I.; Yune, T.Y. Inhibition of COX-2 alleviates lumbar spinal stenosis-induced chronic mechanical allodynia in rats. Int. Immunopharmacol. 2019, 75, 105738. [Google Scholar] [CrossRef]
- Nikaido, T.; Takatsuna, H.; Tabata, S.; Shiosakai, K.; Nakatani, T.; Konno, S.I. Efficacy and Safety of Add-on Mirogabalin to NSAIDs in Lumbar Spinal Stenosis with Peripheral Neuropathic Pain: A Randomized, Open-Label Study. Pain Ther. 2022, 11, 1195–1214. [Google Scholar] [CrossRef] [PubMed]
- Sairyo, K.; Biyani, A.; Goel, V.K.; Leaman, D.W.; Booth, R., Jr.; Thomas, J.; Ebraheim, N.A.; Cowgill, I.A.; Mohan, S.E. Lumbar ligamentum flavum hypertrophy is due to accumulation of inflammation-related scar tissue. Spine 2007, 32, E340–E347. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.Y.; Li, P.; Ao, X.; Qian, L.; Peng, Y.X.; Chu, J.; Jiang, T.; Lian, Z.N.; Zhang, Z.M.; Wang, L. Characterization of a Novel Model of Lumbar Ligamentum Flavum Hypertrophy in Bipedal Standing Mice. Orthop. Surg. 2021, 13, 2457–2467. [Google Scholar] [CrossRef] [PubMed]
- Seol, G.H.; Kang, P.; Lee, H.S.; Seol, G.H. Antioxidant activity of linalool in patients with carpal tunnel syndrome. BMC Neurol. 2016, 16, 17. [Google Scholar] [CrossRef] [PubMed]
- Wendin, K.; Pálsdóttir, A.M.; Spendrup, S.; Mårtensson, L. Odor Perception and Descriptions of Rose-Scented Geranium Pelargonium graveolens ‘Dr. Westerlund’-Sensory and Chemical Analyses. Molecules 2023, 28, 4511. [Google Scholar] [CrossRef]
- Boonstra, A.M.; Schiphorst Preuper, H.R.; Balk, G.A.; Stewart, R.E. Cut-off points for mild, moderate, and severe pain on the visual analogue scale for pain in patients with chronic musculoskeletal pain. Pain 2014, 155, 2545–2550. [Google Scholar] [CrossRef]
| No | RT (min) | Compound | Peak Area | % Area | No | RT (min) | Compound | Peak Area | % Area |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 34.576 | Citronellol | 994,299,533 | 26.43 | 13 | 17.831 | cis-Rose oxide | 66,694,494 | 1.77 |
| 2 | 37.328 | Geraniol | 470,930,376 | 12.52 | 14 | 32.995 | Citral | 66,694,494 | 1.77 |
| 3 | 29.079 | Citronellyl formate | 330,124,024 | 8.78 | 15 | 24.492 | (−)-β-Bourbonene | 66,141,115 | 1.76 |
| 4 | 45.323 | γ-Eudesmol | 317,871,828 | 8.45 | 16 | 35.677 | Citronellyl butyrate | 55,400,776 | 1.47 |
| 5 | 23.441 | Isomenthone | 283,628,500 | 7.54 | 17 | 31.873 | Germacrene D | 53,899,475 | 1.43 |
| 6 | 26.264 | Linalool | 210,448,432 | 5.59 | 18 | 48.110 | 2-Phenylethyl tiglate | 53,393,219 | 1.42 |
| 7 | 32.212 | Geranyl formate | 141,407,662 | 3.76 | 19 | 36.326 | Geranyl propionate | 42,799,625 | 1.14 |
| 8 | 22.284 | l-Menthone | 111,436,296 | 2.96 | 20 | 49.084 | β-Eudesmol | 37,278,965 | 0.99 |
| 9 | 45.951 | Geranyl tiglate | 98,880,842 | 2.63 | 21 | 38.175 | α-Agarofuran | 31,336,257 | 0.83 |
| 10 | 33.765 | (−)-β-Cadinene | 92,330,860 | 2.45 | 22 | 36.488 | Calamenene | 30,803,550 | 0.82 |
| 11 | 38.909 | Geranyl butyrate | 83,822,233 | 2.23 | 23 | 34.082 | Geranyl acetate | 29,143,116 | 0.77 |
| 12 | 27.625 | Caryophyllene | 67,260,209 | 1.79 | 24 | 5.271 | α-Pinene | 26,062,379 | 0.69 |
| Variable | Mild Pain (n = 16) | Moderate to Severe Pain (n = 41) | p-Value |
|---|---|---|---|
| Pain-VAS, cm | 2.34 ± 0.69 | 6.10 ± 1.76 | >0.001 *** |
| Gender, women, n (%) | 10 (62.50) | 32 (78.05) | 0.317 a |
| Age, yr | 64.44 ± 8.76 | 66.27 ± 7.41 | 0.539 |
| BMI, kg/m2 | 25.49 ± 2.53 | 25.40 ± 3.39 | 0.118 |
| Cigarette smoker, n (%) | 2 (12.50) | 6 (14.63) | 1.000 a |
| Alcohol drinker, n (%) | 4 (25) | 8 (19.51) | 0.723 a |
| Medications for pain control | |||
| NSAIDs, n (%) | 12 (75.00) | 28 (68.29) | 0.753 a |
| Opioids, n (%) | 3 (18.75) | 14 (34.15) | 0.342 a |
| Pregabalin, n (%) | 5 (31.25) | 11 (26.83) | 0.752 a |
| Psychological status | |||
| GAD-7, point | 5.38 ± 4.95 | 8.49 ± 5.42 | 0.035 * |
| PHQ-9, point | 7.31 ± 4.81 | 9.85 ± 6.30 | 0.155 |
| Anxiety-VAS, cm | 1.38 ± 1.51 | 3.98 ± 2.85 | 0.001 ** |
| Stress-VAS, cm | 2.13 ± 2.22 | 4.73 ± 3.10 | 0.004 ** |
| Depression-VAS, cm | 1.89 ± 1.85 | 3.96 ± 2.70 | 0.005 ** |
| Hypertension, n (%) | 9 (56.25) | 23 (56.10) | 1.000 |
| sBP, mmHg | 130.50 ± 11.78 | 130.12 ± 12.03 | 0.880 |
| dBP, mmHg | 73.63 ± 5.58 | 74.66 ± 11.52 | 0.852 |
| HR, bpm/min | 78.50 ± 14.29 | 78.83 ± 11.81 | 0.763 |
| Serum biochemical parameters | |||
| WBC count, μL | 7181.25 ± 2293.82 | 6648.78 ± 1739.41 | 0.384 |
| CRP, mg/dL | 1.09 ± 1.23 | 1.25 ± 1.27 | 0.267 |
| Total Ca2+, mg/dL | 10.06 ± 0.60 | 9.74 ± 0.44 | 0.061 |
| Variable | Mild Pain | Moderate to Severe Pain | ||||
|---|---|---|---|---|---|---|
| PC (n = 7) | GEO (n = 9) | p-Value | PC (n = 21) | GEO (n = 20) | p-Value | |
| Pain-VAS, cm | 2.47 ± 0.72 | 2.23 ± 0.69 | 0.470 | 5.59 ± 1.28 | 6.65 ± 2.05 | 0.105 |
| Gender, women, n (%) | 5 (71.43) | 5 (55.56) | 0.633 a | 14 (66.67) | 18 (90.00) | 0.130 |
| Age, yr | 67.57 ± 6.85 | 62.00 ± 9.66 | 0.185 | 66.33 ± 6.13 | 66.89 ± 6.01 | 0.354 |
| BMI, kg/m2 | 25.82 ± 3.01 | 25.23 ± 2.25 | 0.560 | 61.16 ± 3.24 | 24.63 ± 3.33 | 0.705 |
| Cigarette smoker, n (%) | 1 (14.29) | 1 (11.11) | 1.000 a | 5 (23.81) | 2 (10.00) | 0.663 a |
| Alcohol drinker, n (%) | 1 (14.29) | 3 (33.33) | 0.585 a | 4 (19.05) | 3 (15.00) | 0.697 a |
| Medications for pain control | ||||||
| NSAIDs, n (%) | 5 (71.43) | 7 (77.78) | 1.000 | 14 (66.67) | 14 (70.00) | 1.000 |
| Opioids, n (%) | 1 (14.29) | 2 (22.22) | 1.000 a | 7 (33.33) | 7 (35.00) | 1.000 |
| Pregabalin, n (%) | 3(42.86) | 2 (22.22) | 0.596 a | 6 (28.57) | 5 (25.00) | 1.000 |
| Psychological status | ||||||
| GAD-7, point | 5.71 ± 6.50 | 5.11 ± 3.70 | 0.142 | 8.81 ± 4.41 | 8.15 ± 6.41 | 0.214 |
| PHQ-9, point | 7.43 ± 6.00 | 7.22 ± 4.06 | 0.351 | 9.10 ± 3.96 | 10.65 ± 8.11 | 0.794 |
| Anxiety-VAS, cm | 1.63 ± 1.79 | 1.19 ± 1.33 | 0.681 | 4.02 ± 2.00 | 3.95 ± 3.59 | 0.434 |
| Stress-VAS, cm | 1.64 ± 1.88 | 2.50 ± 2.50 | 0.758 | 4.01 ± 1.81 | 5.48 ± 3.95 | 0.303 |
| Depression-VAS, cm | 1.84 ± 1.60 | 1.93 ± 2.12 | 0.918 | 4.20 ± 2.01 | 3.71 ± 3.30 | 0.434 |
| Hypertension, n (%) | 5 (71.43) | 4 (44.44) | 0.358 a | 10 (47.62) | 12 (60.00) | 0.756 |
| sBP, mmHg | 135.14 ± 11.22 | 126.89 ± 11.50 | 0.152 | 128.76 ± 10.49 | 131.55 ± 13.58 | 0.896 |
| dBP, mmHg | 72.14 ± 6.44 | 74.78 ± 4.89 | 0.408 | 74.43 ± 10.76 | 74.90 ± 12.54 | 0.990 |
| HR, bpm/min | 80.86 ± 13.73 | 76.67 ± 15.26 | 0.606 | 78.10 ± 11.21 | 79.60 ± 11.35 | 0.583 |
| Serum biochemical parameters | ||||||
| WBC count, μL | 5585.71 ± 1579.48 | 8422.22 ± 2008.59 | 0.120 | 6609.52 ± 1738.65 | 6690.00 ± 1784.41 | 0.774 |
| CRP, mg/dL | 1.40 ± 1.64 | 0.85 ± 0.82 | 0.606 | 1.19 ± 1.06 | 1.31 ± 1.50 | 0.845 |
| Total Ca2+,mg/dL | 10.14 ± 0.66 | 10.00 ± 0.59 | 0.536 | 9.82 ± 0.41 | 9.67 ± 0.47 | 0.260 |
| Variable | Mild Pain | Moderate To Severe Pain | ||||
|---|---|---|---|---|---|---|
| PC (n = 7) | GEO (n = 9) | p-Value | PC (n = 21) | GEO (n = 20) | p-Value | |
| Anxiety VAS, cm | ||||||
| Pre | 1.63 ± 1.79 | 1.19 ± 1.33 | 4.02 ± 2.00 | 3.95 ± 3.59 | ||
| Post | 1.54 ± 1.44 | 0.76 ± 1.38 | 4.31 ± 1.82 | 2.87 ± 3.30 | ||
| Mean difference | −0.09 ± 0.43 | −0.043 ± 1.16 | 0.681 | 0.29 ± 1.26 | −1.08 ± 1.65 | 0.002 ** |
| p-value | 0.786 | 0.271 | 0.288 | 0.002 ** | ||
| Stress VAS, cm | ||||||
| Pre | 1.64 ± 1.88 | 2.50 ± 2.50 | 4.01 ± 1.81 | 5.48 ± 3.95 | ||
| Post | 1.40 ± 1.53 | 0.88 ± 1.43 | 3.92 ± 1.70 | 3.41 ± 3.61 | ||
| Mean difference | −0.24 ± 0.43 | −1.62 ± 2.51 | 0.408 | −0.09 ± 1.35 | −2.08 ± 2.21 | 0.003 ** |
| p-value | 0.039 * | 0.043 * | 0.823 | >0.001 *** | ||
| Depression VAS, cm | ||||||
| Pre | 1.84 ± 1.60 | 1.93 ± 2.12 | 4.20 ± 2.01 | 3.71 ± 3.30 | ||
| Post | 1.81 ± 1.67 | 0.77 ± 1.19 | 4.16 ± 1.71 | 3.09 ± 3.33 | ||
| Mean difference | −0.03 ± 0.51 | −1.17 ± 2.44 | 0.606 | −0.05 ± 1.60 | −0.62 ± 1.35 | 0.283 |
| p-value | 0.893 | 0.176 | 0.687 | 0.030 * | ||
| Variable | PC (n = 28) | GEO (n = 29) | p-Value |
|---|---|---|---|
| Medications for pain control | |||
| NSAIDs, n (%) | 19 (67.86) | 21 (71.41) | 0.707 |
| Opioids, n (%) | 8 (28.57) | 9 (31.03) | 0.839 |
| Pregabalin, n (%) | 9 (32.14) | 7 (24.14) | 0.501 |
| Pain VAS, cm | |||
| Pre | 4.81 ± 1.79 | 5.28 ± 2.70 | 0.632 |
| Post | 4.42 ± 1.82 | 3.87 ± 2.89 | |
| Mean difference | −0.39 ± 0.80 | −1.41 ± 1.90 | 0.003 ** |
| p-value | 0.300 | <0.001 *** | |
| Anxiety VAS, cm | |||
| Pre | 3.42 ± 2.18 | 3.09 ± 3.31 | 0.167 |
| Post | 3.62 ± 2.10 | 2.21 ± 2.98 | |
| Mean difference | 0.20 ±1.11 | −0.88 ± 1.52 | 0.004 * |
| p-value | 0.401 | 0.001 ** | |
| Stress VAS, cm | |||
| Pre | 3.42 ± 2.07 | 4.56 ± 3.79 | 0.518 |
| Post | 3.29 ± 1.98 | 2.62 ± 3.29 | |
| Mean difference | −0.13 ± 1.19 | −1.93 ± 2.27 | 0.001 ** |
| p-value | 0.443 | <0.001 *** | |
| Depression VAS, cm | |||
| Pre | 3.61 ± 2.15 | 3.16 ± 3.06 | 0.240 |
| Post | 3.57 ± 1.96 | 2.37 ± 3.02 | |
| Mean difference | −0.04 ± 1.40 | −0.79 ± 1.74 | 0.165 |
| p-value | 0.700 | 0.006 ** | |
| sBP | |||
| Pre | 130.36 ± 10.84 | 130.10 ± 12.95 | 0.836 |
| Post | 128.04 ± 11.26 | 124.86 ± 14.32 | |
| Mean difference | −2.32 ± 5.14 | −5.24 ± 7.50 | 0.099 |
| p-value | 0.012 * | 0.002 ** | |
| dBP | |||
| Pre | 73.86 ± 9.80 | 74.86 ± 10.66 | 0.755 |
| Post | 75.36 ± 10.65 | 72.55 ± 11.78 | |
| Mean difference | 1.50 ± 4.42 | 2.31 ± 8.12 | 0.019 * |
| p-value | 0.084 | 0.155 | |
| HR, bpm/min | |||
| Pre | 78.79 ± 12.59 | 78.69 ± 12.48 | 0.905 |
| Post | 78.57 ± 11.99 | 77.03 ± 11.27 | |
| Mean difference | −0.21 ± 4.75 | −1.66 ± 6.60 | 0.350 |
| p-value | 0.799 | 0.074 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, E.; Cho, Y.; Lee, J.-M.; Seol, G.H. Inhalation of Pelargonium graveolens Essential Oil Alleviates Pain and Related Anxiety and Stress in Patients with Lumbar Spinal Stenosis and Moderate to Severe Pain. Pharmaceuticals 2024, 17, 1. https://doi.org/10.3390/ph17010001
Seo E, Cho Y, Lee J-M, Seol GH. Inhalation of Pelargonium graveolens Essential Oil Alleviates Pain and Related Anxiety and Stress in Patients with Lumbar Spinal Stenosis and Moderate to Severe Pain. Pharmaceuticals. 2024; 17(1):1. https://doi.org/10.3390/ph17010001
Chicago/Turabian StyleSeo, Eunhye, Yoonah Cho, Jeong-Min Lee, and Geun Hee Seol. 2024. "Inhalation of Pelargonium graveolens Essential Oil Alleviates Pain and Related Anxiety and Stress in Patients with Lumbar Spinal Stenosis and Moderate to Severe Pain" Pharmaceuticals 17, no. 1: 1. https://doi.org/10.3390/ph17010001
APA StyleSeo, E., Cho, Y., Lee, J.-M., & Seol, G. H. (2024). Inhalation of Pelargonium graveolens Essential Oil Alleviates Pain and Related Anxiety and Stress in Patients with Lumbar Spinal Stenosis and Moderate to Severe Pain. Pharmaceuticals, 17(1), 1. https://doi.org/10.3390/ph17010001






