Postoperative Nausea and Vomiting following Endoscopic Sinus Surgery under the Guidance of Adequacy of Anesthesia or Pupillometry with Intravenous Propofol/Remifentanil
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
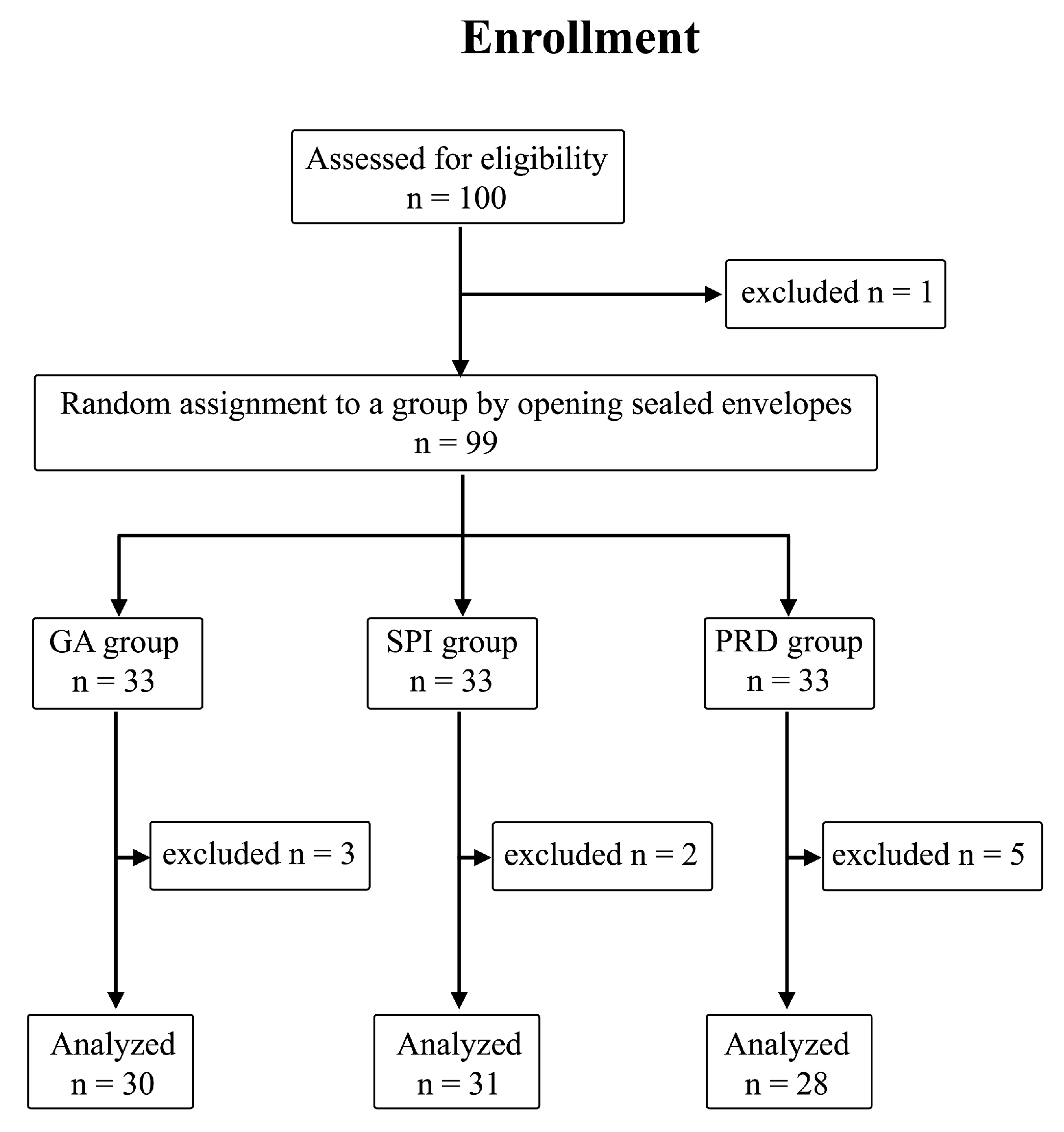
4.1. Patients
4.2. Anesthesia Technique
4.2.1. Stage 1
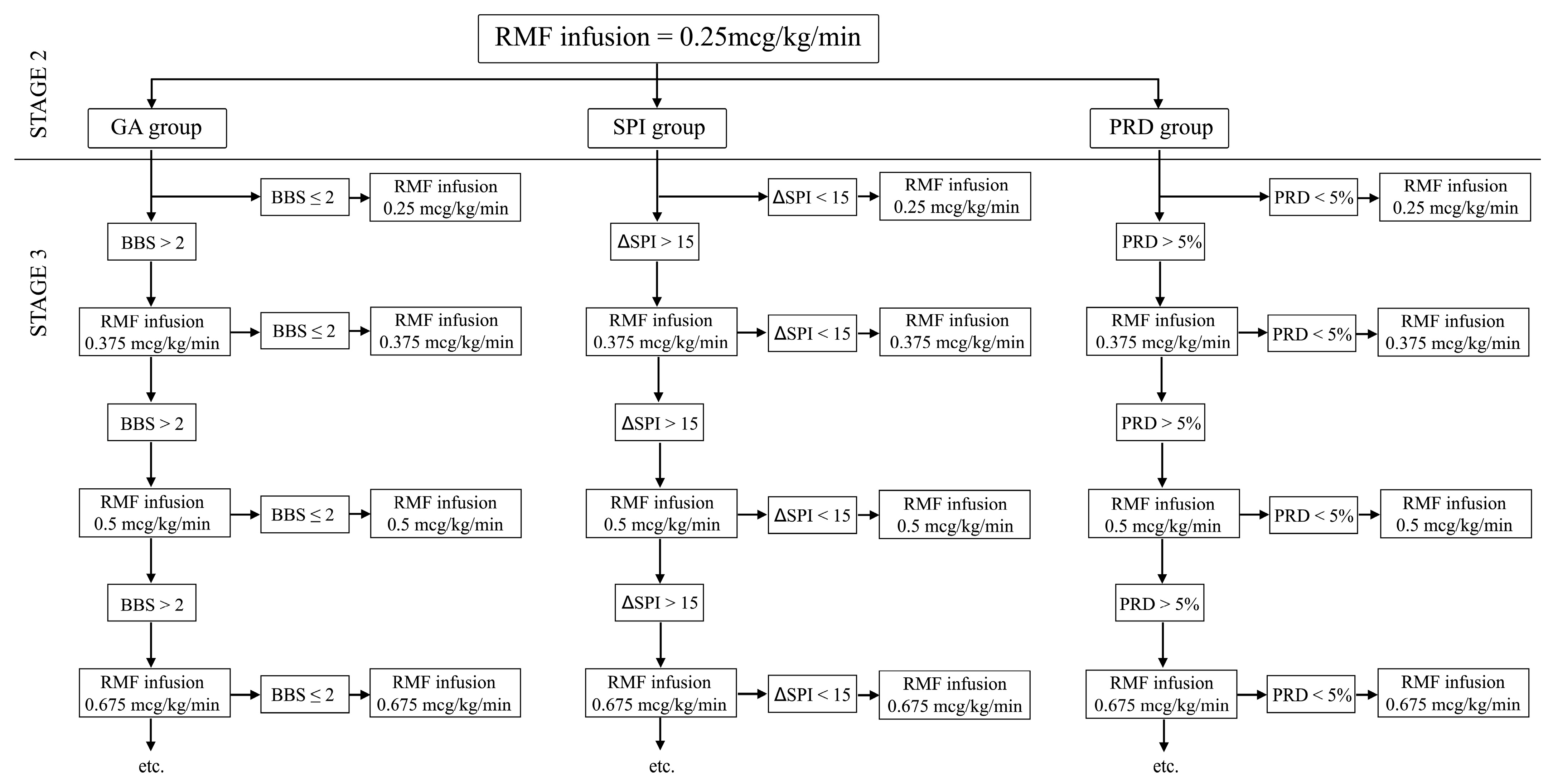
4.2.2. Stage 2
4.2.3. Stage 3 Intraoperatively
4.2.4. SPI Group
4.2.5. PRD Group
4.2.6. GA Group
4.3. ESS Technique and Surgical Considerations
4.4. Postoperative Observation at the Post-Anesthesia Care Unit (PACU) and Department of Laryngology (DoL)
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Data | Total | GA Group | SPI Group | PRD Group | p | |
|---|---|---|---|---|---|---|
| n = 89 (100%) | n = 30 (33.7%) | n = 31 (34.8%) | n = 28 (31.5%) | |||
| age | (years) | 50.2 ± 14.6 | 49 ± 15.4 51 (26) | 47.7 ± 13.9 46 (21) | 54.1 ± 14.4 59.5 (23) | 0.22 |
| gender | Male | 56 (62.9) | 20 (66.7) | 18 (58.1) | 18 (64.3) | 0.77 |
| Female | 33 (37.1) | 10 (33.3) | 13 (41.9) | 10 (35.7) | ||
| height | (cm) | 171.4 ± 9 171 (12) | 173.5 ± 9.3 175 (9) | 170.9 ± 8.7 170 (12) | 169.6 ± 9.1 170 (12) | 0.26 |
| weight | (kg) | 78.2 ± 14.6 79 (20) | 80.3 ± 11.5 81 (18) | 78.3 ± 14.7 79 (25) | 75.9 ± 17.3 74 (23.5) | 0.52 |
| BMI | (kg/m2) | 26.5 ± 3.9 26.6 (5.2) | 26.8 ± 3.9 26.9 (4.4) | 26.6 ± 3.6 27 (4.6) | 26.1 ± 4.2 25.2 (5.2) | 0.82 |
| BMI | norm | 29 (32.6) | 9 (30) | 7 (22.6) | 13 (46.4) | 0.38 |
| overweight | 43 (48.3) | 15 (50) | 18 (58.1) | 10 (35.7) | ||
| obese | 17 (19.1) | 6 (20) | 6 (19.4) | 5 (17.9) | ||
| ASA | I | 25 (29.8) | 9 (31) | 11 (37.9) | 5 (19.2) | 0.31 |
| II | 45 (53.6) | 18 (62.1) | 14 (48.3) | 13 (50) | 0.52 | |
| III | 14 (16.7) | 2 (6.9) | 4 (13.8) | 8 (30.8) | 0.05 | |
| LM CT scale mean | LM < 12 | 50 (56.2) | 12 (40) | 19 (61.3) | 19 (67.9) | 0.08 |
| LM ≥ 12 | 39 (43.8) | 18 (60) | 12 (38.7) | 9 (32.1) | ||
| primary ESS/revision | 74 (84.1) | 25 (86.2) | 26 (83.9) | 23 (82.1) | 0.91 | |
| Samter’s triad | 7 (8.3) | 5 (17.2) | 0 (0) | 2 (7.7) | 0.05 | |
| asthma | 17 (20.2) | 10 (34.5) | 4 (13.8) | 3 (11.5) | 0.06 | |
| arterial hypertension | 31 (36.9) | 10 (34.5) | 10 (34.5) | 11 (42.3) | 0.79 | |
| coronary artery disease | 7 (8.3) | 2 (6.9) | 1 (3.4) | 4 (15.4) | 0.27 | |
| Intraoperative Parameters | Total | GA Group | SPI Group | PRD Group | p |
|---|---|---|---|---|---|
| n = 89 (100%) | n = 30 (33.7%) | n = 31 (34.8%) | n = 28 (31.5%) | ||
| Total intraoperative blood loss (mL) | 207.5 ± 154.3 170 (200) | 283.3 ± 193.5 220 (300) | 165.2 ± 100.2 150 (150) | 173.1 ± 128.6 135 (189) | 0.04 GA vs. SPI |
| Length of operation (min) | 74.1 ± 32.3 73 (35) | 82.6 ± 33.1 85 (40) | 75.8 ± 34.2 70 (39) | 63.1 ± 26.7 65.5 (35) | 0.05 GA vs. PRD |
| Total propofol consumption (mg) | 666 ± 269.5 620 (340) | 762.3 ± 273.2 750 (350) | 665.8 ± 237.8 650 (400) | 562.9 ± 269.1 520 (285) | 0.008 GA vs. PRD |
| Max speed of propofol infusion (mcg/kg/min) | 99.10 ± 41.62 86.81 (43.51) | 110.40 ± 48.12 102.04 (46.90) | 94.04 ± 31.70 83.33 (35.46) | 92.80 ± 39.50 78.44 (45.02) | 0.15 |
| Min speed of propofol infusion (mcg/kg/min) | 65.05 ± 24.25 59.52 (36) | 63.27 ± 22.46 59.52 (28.33) | 65.26 ± 21.54 63.64 (31.75) | 66.67 ± 29.01 58.17 (44.93) | 0.88 |
| Mean speed of propofol infusion (mcg/kg/min) | 80.33 ± 27.37 73.53 (35.28) | 85.73 ± 26.91 83.33 (30.15) | 76.14 ± 23.28 77.80 (31.59) | 79.25 ± 31.62 68.56 (36.86) | 0.37 |
| Total RMF consumption (mg) | 1.6 ± 1.2 1.5 (1.1) | 1.7 ± 1.1 1.5 (1) | 1.8 ± 0.9 1.8 (0.9) | 1.3 ± 1.4 1 (0.7) | 0.005 SPI vs. PRD |
| Max speed of RMF infusion (mcg/kg/min) | 0.38 ± 0.16 0.38 (0.25) | 0.42 ± 0.21 0.38 (0.25) | 0.4 ± 0.12 0.38 (0.25) | 0.33 ± 0.15 0.25 (0.13) | 0.02 GA vs. PRD |
| Min speed of RMF infusion (mcg/kg/min) | 0.22 ± 0.06 0.25 (0.13) | 0.21 ± 0.06 0.25 (0.13) | 0.25 ± 0.05 0.25 (0) | 0.21 ± 0.07 0.25 (0.13) | 0.03 |
| Mean speed of RMF infusion (mcg/kg/min) | 0.31 ± 0.12 0.28 (0.12) | 0.32 ± 0.14 0.28 (0.16) | 0.33 ± 0.09 0.34 (0.16) | 0.27 ± 0.12 0.25 (0.1) | 0.007 SPI vs. PRD |
| Max values of BBS | 2.7 ± 0.7 3 (1) | 2.9 ± 0.7 3 (1) | 2.5 ± 0.6 3 (1) | 2.6 ± 0.7 3 (1) | 0.2 |
| Min values of BBS | 1.7 ± 0.5 2 (1) | 1.8 ± 0.4 2 (0) | 1.7 ± 0.5 2 (1) | 1.6 ± 0.5 2 (1) | 0.09 |
| Mean values of BBS | 2 ± 0.4 2.1 (0.3) | 2.1 ± 0.5 2.2 (0.4) | 1.9 ± 0.5 2 (0.5) | 2 ± 0.3 2 (0.2) | 0.07 |
| Mean time of BBS > 2 | 12.7 ± 15.3 5 (20) | 16.3 ± 16.8 15 (30) | 11.8 ± 14.9 5 (25) | 9.6 ± 13.7 5 (15) | 0.23 |
| Mean number of incidences of BBS > 2 | 1.07 ± 1.1 1 (2) | 1.4 ± 1.3 1 (2) | 0.9 ± 0.9 1 (1) | 0.89 ± 1 1 (1.5) | 0.19 |
| Fluid challenge (mL) | 1540.34 ± 381 1500 (500) | 1534.48 ± 446.83 1500 (500) | 1546.77 ± 372.59 1500 (500) | 1539.29 ± 326.13 1500 (275) | 0.93 |
References
- Veiga-Gil, L.; Pueyo, J.; López-Olaondo, L. Postoperative nausea and vomiting: Physiopathology, risk factors, prophylaxis and treatment. Rev. Esp. Anestesiol. Reanim. 2017, 64, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Ziemann-Gimmel, P.; Goldfarb, A.A.; Koppman, J.; Marema, R.T. Opioid-free total intravenous anaesthesia reduces postoperative nausea and vomiting in bariatric surgery beyond triple prophylaxis. Br. J. Anaesth. 2014, 112, 906–911. [Google Scholar] [CrossRef] [PubMed]
- Kienbaum, P.; Schaefer, M.S.; Weibel, S.; Schlesinger, T.; Meybohm, P.; Eberhart, L.H.; Kranke, P. Update on PONV-What is new in prophylaxis and treatment of postoperative nausea and vomiting? Summary of recent consensus recommendations and Cochrane reviews on prophylaxis and treatment of postoperative nausea and vomiting. Anaesthesist 2022, 71, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Ding, P.; Zheng, D.-Y.; Pu, J.; Yang, L.-Y.; Zhou, Y.-Y.; Li, D.-J.; Chen, W.; Li, Y.-H. Wearable transcutaneous electrical acupoint stimulation bracelet for prevention of postoperative nausea and vomiting in patients undergoing hysteroscopic surgery: A randomised controlled trial. Br. J. Anaesth. 2022, 129, e85–e87. [Google Scholar] [CrossRef] [PubMed]
- Rogobete, A.F.; Bedreag, O.H.; Papurica, M.; Popovici, S.E.; Bratu, L.M.; Rata, A.; Barsac, C.R.; Maghiar, A.; Garofil, D.N.; Negrea, M.; et al. Multiparametric Monitoring of Hypnosis and Nociception-Antinociception Balance during General Anesthesia—A New Era in Patient Safety Standards and Healthcare Management. Medicina 2021, 57, 132. [Google Scholar] [CrossRef]
- Stasiowski, M.J.; Szumera, I.; Wardas, P.; Król, S.; Żak, J.; Missir, A.; Pluta, A.; Niewiadomska, E.; Krawczyk, L.; Jałowiecki, P.; et al. Adequacy of Anesthesia and Pupillometry for Endoscopic Sinus Surgery. J. Clin. Med. 2021, 10, 4683. [Google Scholar] [CrossRef]
- Birkenbeuel, J.L.; Warner, D.C.; Abiri, A.; Brown, N.J.; Nguyen, E.S.; Lee, A.; Goshtasbi, K.; Boladian, L.A.; Hsu, Z.; Bitner, B.F.; et al. Predictors of Postoperative Nausea and Vomiting After Endoscopic Skull Base Surgery. Laryngoscope 2022, 132, 761–768. [Google Scholar] [CrossRef]
- Lin, D.; Dalgorf, D.; Witterick, I.J. Predictors of unexpected hospital admissions after outpatient endoscopic sinus surgery: Retrospective review. J. Otolaryngol. Head Neck Surg. 2008, 37, 309–311. [Google Scholar]
- Khalil, S.; Philbrook, L.; Rabb, M.; Wells, L.; Aves, T.; Villanueva, G.; Amhan, M.; Chuang, A.Z.; Lemak, N.A. Ondansetron/promethazine combination or promethazine alone reduces nausea and vomiting after middle ear surgery. J. Clin. Anesth. 1999, 11, 596–600. [Google Scholar] [CrossRef]
- Thaler, E.R.; Gottschalk, A.; Samaranayake, R.; Lanza, D.C.; Kennedy, D.W. Anesthesia in endoscopic sinus surgery. Am. J. Rhinol. 1997, 11, 409–413. [Google Scholar] [CrossRef]
- Kim, D.H.; Kang, H.; Hwang, S.H. The Effect of Sphenopalatine Block on the Postoperative Pain of Endoscopic Sinus Surgery: A Meta-analysis. Otolaryngol. Head Neck Surg. 2019, 160, 223–231. [Google Scholar] [CrossRef] [PubMed]
- DeMaria, S.; Govindaraj, S.; Chinosorvatana, N.; Kang, S.; Levine, A.I. Bilateral sphenopalatine ganglion blockade improves postoperative analgesia after endoscopic sinus surgery. Am. J. Rhinol. Allergy 2012, 26, e23–e27. [Google Scholar] [CrossRef] [PubMed]
- Korkut, A.Y.; Erkalp, K.; Erden, V.; Teker, A.M.; Demirel, A.; Gedikli, O.; Saidoglu, L. Effect of pharyngeal packing during nasal surgery on postoperative nausea and vomiting. Otolaryngol. Head Neck Surg. 2010, 143, 831–836. [Google Scholar] [CrossRef] [PubMed]
- Green, R.; Konuthula, N.; Sobrero, M.; Saini, A.; Parasher, A.; Pool, C.; Levine, A.I.; DeMaria, S.; Tufts, R.; Govindaraj, S.; et al. Use of pharyngeal packs in functional endoscopic sinus surgery: A randomized controlled trial. Laryngoscope 2017, 127, 2460–2465. [Google Scholar] [CrossRef]
- Piltcher, O.; Lavinsky, M.; Lavinsky, J.; de Oliveira Basso, P.R. Effectiveness of hypopharyngeal packing during nasal and sinus surgery in the prevention of PONV. Otolaryngol. Head Neck Surg. 2007, 137, 552–554. [Google Scholar] [CrossRef]
- Akkaya, A.; Tekelioglu, U.Y.; Demirhan, A.; Bilgi, M.; Yildiz, I.; Apuhan, T.; Kocoglu, H. Comparison of the effects of magnesium sulphate and dexmedetomidine on surgical vision quality in endoscopic sinus surgery: Randomized clinical study. Rev. Bras. Anestesiol. 2014, 64, 406–412. [Google Scholar] [CrossRef]
- Goksu, S.; Arik, H.; Demiryurek, S.; Mumbuc, S.; Oner, U.; Demiryurek, A.T. Effects of dexmedetomidine infusion in patients undergoing functional endoscopic sinus surgery under local anaesthesia. Eur. J. Anaesthesiol. 2008, 25, 22–28. [Google Scholar] [CrossRef]
- Guven, D.G.; Demiraran, Y.; Sezen, G.; Kepek, O.; Iskender, A. Evaluation of outcomes in patients given dexmedetomidine in functional endoscopic sinus surgery. Ann. Otol. Rhinol. Laryngol. 2011, 120, 586–592. [Google Scholar] [CrossRef]
- Liu, T.; Gu, Y.; Chen, K.; Shen, X. Quality of recovery in patients undergoing endoscopic sinus surgery after general anesthesia: Total intravenous anesthesia vs. desflurane anesthesia. Int. Forum Allergy Rhinol. 2019, 9, 248–254. [Google Scholar] [CrossRef]
- Heller, J.A.; DeMaria, S.; Govindaraj, S.; Lin, H.-M.; Fischer, G.W.; Evans, A.; Weiner, M.M. Cerebral oximetry monitoring during sinus endoscopy. Laryngoscope 2015, 125, E127–E131. [Google Scholar] [CrossRef]
- Ming, J.-L.; Kuo, B.I.-T.; Lin, J.-G.; Lin, L.-C. The efficacy of acupressure to prevent nausea and vomiting in post-operative patients. J. Adv. Nurs. 2002, 39, 343–351. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. FDA Drug Safety Communication: New Information Regarding QT Prolongation with Ondansetron (Zofran). Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-new-information-regarding-qt-prolongation-ondansetron-zofran (accessed on 29 June 2021).
- Doggrell, S.A.; Hancox, J.C. Cardiac safety concerns for ondansetron, an antiemetic commonly used for nausea linked to cancer treatment and following anaesthesia. Expert. Opin. Drug Saf. 2013, 12, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Pluta, A.; Stasiowski, M.J.; Lyssek-Boroń, A.; Król, S.; Krawczyk, L.; Niewiadomska, E.; Żak, J.; Kawka, M.; Dobrowolski, D.; Grabarek, B.O.; et al. Adverse Events during Vitrectomy under Adequacy of Anesthesia–An Additional Report. J. Clin. Med. 2021, 10, 4172. [Google Scholar] [CrossRef] [PubMed]
- Abubaker, A.K.; Al-Qudah, M.A. The Role of Endoscopic Sphenopalatine Ganglion Block on Nausea and Vomiting After Sinus Surgery. Am. J. Rhinol. Allergy 2018, 32, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Hozumi, J.; Egi, M.; Sugita, S.; Sato, T. Dose of intraoperative remifentanil administration is independently associated with increase in the risk of postoperative nausea and vomiting in elective mastectomy under general anesthesia. J. Clin. Anesth. 2016, 34, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Cui, Y.; Cao, L. Opioid-Free Anesthesia Working Group The effect of opioid-free anaesthesia on the quality of recovery after endoscopic sinus surgery: A multicentre randomised controlled trial. Eur. J. Anaesthesiol. 2023, 40, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Ilies, C.; Gruenewald, M.; Ludwigs, J.; Thee, C.; Höcker, J.; Hanss, R.; Steinfath, M.; Bein, B. Evaluation of the surgical stress index during spinal and general anaesthesia. Br. J. Anaesth. 2010, 105, 533–537. [Google Scholar] [CrossRef]
- Gruenewald, M.; Meybohm, P.; Ilies, C.; Höcker, J.; Hanss, R.; Scholz, J.; Bein, B. Influence of different remifentanil concentrations on the performance of the surgical stress index to detect a standardized painful stimulus during sevoflurane anaesthesia. Br. J. Anaesth. 2009, 103, 586–593. [Google Scholar] [CrossRef]
- Sabourdin, N.; Barrois, J.; Louvet, N.; Rigouzzo, A.; Guye, M.-L.; Dadure, C.; Constant, I. Pupillometry-guided Intraoperative Remifentanil Administration versus Standard Practice Influences Opioid Use: A Randomized Study. Anesthesiology 2017, 127, 284–292. [Google Scholar] [CrossRef]
- Khan, Z.; Bollu, P.C. Horner Syndrome. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Mitsuoka, K.; Kikutani, T.; Sato, I. Morphological relationship between the superior cervical ganglion and cervical nerves in Japanese cadaver donors. Brain Behav. 2016, 7, e00619. [Google Scholar] [CrossRef]
- Larson, M.D.; Behrends, M. Portable infrared pupillometry: A review. Anesth. Analg. 2015, 120, 1242–1253. [Google Scholar] [CrossRef] [PubMed]
- Stasiowski, M.J.; Pluta, A.; Lyssek-Boroń, A.; Niewiadomska, E.; Krawczyk, L.; Dobrowolski, D.; Grabarek, B.O.; Kawka, M.; Rejdak, R.; Szumera, I.; et al. Adequacy of Anaesthesia for Nociception Detection during Vitreoretinal Surgery. Life 2023, 13, 505. [Google Scholar] [CrossRef]
- Laporta, M.L.; O’Brien, E.K.; Stokken, J.K.; Choby, G.; Sprung, J.; Weingarten, T.N. Anesthesia Management and Postanesthetic Recovery Following Endoscopic Sinus Surgery. Laryngoscope 2021, 131, E815–E820. [Google Scholar] [CrossRef] [PubMed]
- Borgeat, A.; Stirnemann, H.R. Antiemetic effect of propofol. Anaesthesist 1998, 47, 918–924. [Google Scholar] [CrossRef] [PubMed]
- Misiołek, H.; Cettler, M.; Woroń, J.; Wordliczek, J.; Dobrogowski, J.; Mayzner-Zawadzka, E. The 2014 guidelines for post-operative pain management. Anaesthesiol. Intensive Ther. 2014, 46, 221–244. [Google Scholar] [CrossRef] [PubMed]
- Kemppainen, T.; Kokki, H.; Tuomilehto, H.; Seppä, J.; Nuutinen, J. Acetaminophen is highly effective in pain treatment after endoscopic sinus surgery. Laryngoscope 2006, 116, 2125–2128. [Google Scholar] [CrossRef] [PubMed]
- Jóźwiak-Bebenista, M.; Nowak, J.Z. Paracetamol: Mechanism of action, applications and safety concern. Acta Pol. Pharm. 2014, 71, 11–23. [Google Scholar] [PubMed]
- Lee, D.J.; Grose, E.; Brenna, C.T.A.; Philteos, J.; Lightfoot, D.; Kirubalingam, K.; Chan, Y.; Palmer, J.N.; Adappa, N.D.; Lee, J.M. The benefits and risks of non-steroidal anti-inflammatory drugs for postoperative analgesia in sinonasal surgery: A systematic review and meta-analysis. Int. Forum Allergy Rhinol. 2023, 13, 1738–1757. [Google Scholar] [CrossRef]
- Chu, C.-C.; Hsing, C.-H.; Shieh, J.-P.; Chien, C.-C.; Ho, C.-M.; Wang, J.-J. The cellular mechanisms of the antiemetic action of dexamethasone and related glucocorticoids against vomiting. Eur. J. Pharmacol. 2014, 722, 48–54. [Google Scholar] [CrossRef]
- Reibaldi, M.; Fallico, M.; Longo, A.; Avitabile, T.; Astuto, M.; Murabito, P.; Minardi, C.; Bonfiglio, V.; Boscia, F.; Furino, C.; et al. Efficacy of Three Different Prophylactic Treatments for Postoperative Nausea and Vomiting after Vitrectomy: A Randomized Clinical Trial. J. Clin. Med. 2019, 8, 391. [Google Scholar] [CrossRef]
- Gan, T.J.; Belani, K.G.; Bergese, S.; Chung, F.; Diemunsch, P.; Habib, A.S.; Jin, Z.; Kovac, A.L.; Meyer, T.A.; Urman, R.D.; et al. Fourth Consensus Guidelines for the Management of Postoperative Nausea and Vomiting. Anesth. Analg. 2020, 131, 411–448. [Google Scholar] [CrossRef] [PubMed]
- Stasiowski, M.J.; Pluta, A.; Lyssek-Boroń, A.; Król, S.; Krawczyk, L.; Niewiadomska, E.; Żak, J.; Kawka, M.; Dobrowolski, D.; Grabarek, B.O.; et al. Adverse Events during Vitreoretinal Surgery under Adequacy of Anesthesia Guidance—Risk Factor Analysis. Pharmaceuticals 2022, 15, 237. [Google Scholar] [CrossRef] [PubMed]
- Ledowski, T.; Burke, J.; Hruby, J. Surgical pleth index: Prediction of postoperative pain and influence of arousal. Br. J. Anaesth. 2016, 117, 371–374. [Google Scholar] [CrossRef] [PubMed]
- Stasiowski, M.; Missir, A.; Pluta, A.; Szumera, I.; Stasiak, M.; Szopa, W.; Błaszczyk, B.; Możdżyński, B.; Majchrzak, K.; Tymowski, M.; et al. Influence of infiltration anaesthesia on perioperative outcomes following lumbar discectomy under surgical pleth index-guided general anaesthesia: A preliminary report from a randomised controlled prospective trial. Adv. Med. Sci. 2020, 65, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Stasiowski, M.J.; Starzewska, M.; Niewiadomska, E.; Król, S.; Marczak, K.; Żak, J.; Pluta, A.; Eszyk, J.; Grabarek, B.O.; Szumera, I.; et al. Adequacy of Anesthesia Guidance for Colonoscopy Procedures. Pharmaceuticals 2021, 14, 464. [Google Scholar] [CrossRef] [PubMed]
- Stasiowski, M.J.; Marciniak, R.; Duława, A.; Krawczyk, L.; Jałowiecki, P. Epileptiform EEG patterns during different techniques of induction of general anaesthesia with sevoflurane and propofol: A randomised trial. Anaesthesiol. Intensive Ther. 2019, 51, 21–34. [Google Scholar] [CrossRef]
- Guerci, P.; Jay, G.; Arnout, C.; Herbain, D.; Baka, N.; Poirel, O.; Novy, E.; Bouaziz, H.; Vial, F. Effects of pupillary reflex dilation-guided opioid administration on remifentanil and morphine consumption during laparoscopic surgery: A randomised controlled trial. Eur. J. Anaesthesiol. 2021, 38, 975–984. [Google Scholar] [CrossRef]
- Chung, Y.-J.; An, S.-Y.; Yeon, J.-Y.; Shim, W.S.; Mo, J.-H. Effect of a Chitosan Gel on Hemostasis and Prevention of Adhesion After Endoscopic Sinus Surgery. Clin. Exp. Otorhinolaryngol. 2016, 9, 143. [Google Scholar] [CrossRef]
- Nair, S.; Collins, M.; Hung, P.; Rees, G.; Close, D.; Wormald, P.-J. The effect of beta-blocker premedication on the surgical field during endoscopic sinus surgery. Laryngoscope 2004, 114, 1042–1046. [Google Scholar] [CrossRef]
- Apfel, C.C.; Läärä, E.; Koivuranta, M.; Greim, C.A.; Roewer, N. A simplified risk score for predicting postoperative nausea and vomiting: Conclusions from cross-validations between two centers. Anesthesiology 1999, 91, 693–700. [Google Scholar] [CrossRef]

| Intraoperative Data | Total n = 89 (100%) | GA Group n = 30 (33.7%) | SPI Group n = 31 (34.8%) | PRD Group n = 28 (31.5%) | p |
|---|---|---|---|---|---|
| Overall PONV (yes/no) (%) | 7/82 7.9% | 1/29 3.3% | 2/29 6.5% | 4/24 14.3% | 0.282 |
| Post-Anesthesia Care Unit (PACU) | |||||
| PONV in PACU (yes/no) (%) | 4/85 4.5% | 0/30 0% | 1/30 3.2% | 3/25 10.7% | 0.132 |
| Nausea in PACU (yes/no) (%) | 4/85 4.5% | 0/30 0% | 1/30 3.2% | 3/25 10.7% | 0.132 |
| Vomiting in PACU (yes/no) (%) | 4/84 4.5% | 0/30 0% | 1/30 3.2% | 3/25 10.7% | 0.132 |
| Department of Laryngology (DoL) | |||||
| PONV in DoL (yes/no) (%) | 3/86 3.4% | 1/29 3.3% | 1/30 3.2% | 1/27 3.6% | 0.997 |
| Nausea in DoL (yes/no) (%) | 3/86 3.4% | 1/29 3.3% | 1/30 3.2% | 1/27 3.6% | 0.997 |
| Vomiting in DoL (yes/no) (%) | 2/86 2.3% | 1/29 3.3% | 1/30 3.2% | 1/27 3.6% | 0.997 |
| Overall medication with antiemetic properties in DoL | 44/45 49.4% | 10/20 33.3% | 19/12 61.3% | 15/13 53.6% | 0.080 |
| Paracetamol in DoL | 37/52 41.6% | 7/23 23.3% | 17/14 54.8% | 13/15 46.4% | 0.036 |
| PONV incidence both in PACU and DoL | |||||
| PONV in PACU and DoL (yes/no) (%) | 0/89 0% | 0/30 0% | 0/31 0% | 0/28 0% | - |
| Nausea in PACU and DoL (yes/no) (%) | 0/89 0% | 0/30 0% | 0/31 0% | 0/28 0% | - |
| Vomiting in PACU and DoL (yes/no) (%) | 0/89 0% | 0/30 0% | 0/31 0% | 0/28 0% | - |
| Number of patients with Apfel score 0 and PONV | 0 | 0 | 0 | 0 | - |
| Number of patients with Apfel score 1 and PONV | 3 | 0 | 1 | 2 | - |
| Number of patients with Apfel score 2 and PONV | 2 | 1 | 0 | 1 | - |
| Number of patients with Apfel score 3 and PONV | 2 | 0 | 1 | 1 | - |
| Data | Total | GA Group | SPI Group | PRD Group | p |
|---|---|---|---|---|---|
| n = 89 (100%) | n = 30 (33.7%) | n = 31 (34.8%) | n = 28 (31.5%) | ||
| Apfel (%) | 26.4 ± 10.8 21.0 (18.0) | 25.3 ± 9.7 21.0 (18.0) | 28.2 ± 11.9 21.0 (18.0) | 25.5 ± 10.9 21.0 ± (4.5) | 0.542 |
| Gender Female/Male | 33/56 37.1%/62.9% | 10/20 33.3%/66.7% | 13/18 41.9%/58.1% | 10/18 35.7%/64.3% | 0.773 |
| Motion sickness Yes/No | 0/89 0%/100% | 0/30 0%/100% | 0/31 0%/100% | 0/28 0%/100% | - |
| History of PONV Yes/No | 4/85 4.5%/95.5% | 1/29 3.3%/96.7% | 2/29 6.5%/93.5% | 1/27 3.6%/96.4% | - |
| Number of patients with Apfel score 0 | 8 | 3 | 3 | 2 | 0.297 |
| Number of patients with Apfel score 1 | 52 | 18 | 15 | 19 | |
| Number of patients with Apfel score 2 | 27 | 9 | 12 | 6 | |
| Number of patients with Apfel score 3 | 2 | 0 | 1 | 1 | |
| Number of patients with Apfel score 4 | 0 | 0 | 0 | 0 | - |
| Amplitude | PRD < 5% | 5% ≤ PRD < 12% | 12% ≤ PRD < 20% | PRD ≥ 20% |
|---|---|---|---|---|
| Sensitivity | Zero | Weak | High | Very high |
| Grade | Description |
|---|---|
| 0 | No bleeding |
| 1 | Slight bleeding—suctioning not required |
| 2 | Slight bleeding—suctioning occasionally required |
| 3 | Slight bleeding—suctioning frequently required; bleeding threatens the surgical field a few seconds after suction removal |
| 4 | Moderate bleeding—suctioning frequently required; bleeding threatens the surgical field directly after suction removal |
| 5 | Severe bleeding—suctioning constantly required; bleeding appears faster than can be removed by suction |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stasiowski, M.J.; Zmarzły, N.; Grabarek, B.O.; Gąsiorek, J. Postoperative Nausea and Vomiting following Endoscopic Sinus Surgery under the Guidance of Adequacy of Anesthesia or Pupillometry with Intravenous Propofol/Remifentanil. Pharmaceuticals 2024, 17, 2. https://doi.org/10.3390/ph17010002
Stasiowski MJ, Zmarzły N, Grabarek BO, Gąsiorek J. Postoperative Nausea and Vomiting following Endoscopic Sinus Surgery under the Guidance of Adequacy of Anesthesia or Pupillometry with Intravenous Propofol/Remifentanil. Pharmaceuticals. 2024; 17(1):2. https://doi.org/10.3390/ph17010002
Chicago/Turabian StyleStasiowski, Michał J., Nikola Zmarzły, Beniamin Oskar Grabarek, and Jakub Gąsiorek. 2024. "Postoperative Nausea and Vomiting following Endoscopic Sinus Surgery under the Guidance of Adequacy of Anesthesia or Pupillometry with Intravenous Propofol/Remifentanil" Pharmaceuticals 17, no. 1: 2. https://doi.org/10.3390/ph17010002
APA StyleStasiowski, M. J., Zmarzły, N., Grabarek, B. O., & Gąsiorek, J. (2024). Postoperative Nausea and Vomiting following Endoscopic Sinus Surgery under the Guidance of Adequacy of Anesthesia or Pupillometry with Intravenous Propofol/Remifentanil. Pharmaceuticals, 17(1), 2. https://doi.org/10.3390/ph17010002






