A Concise Review of the Recent Structural Explorations of Chromones as MAO-B Inhibitors: Update from 2017 to 2023
Abstract
:1. Introduction
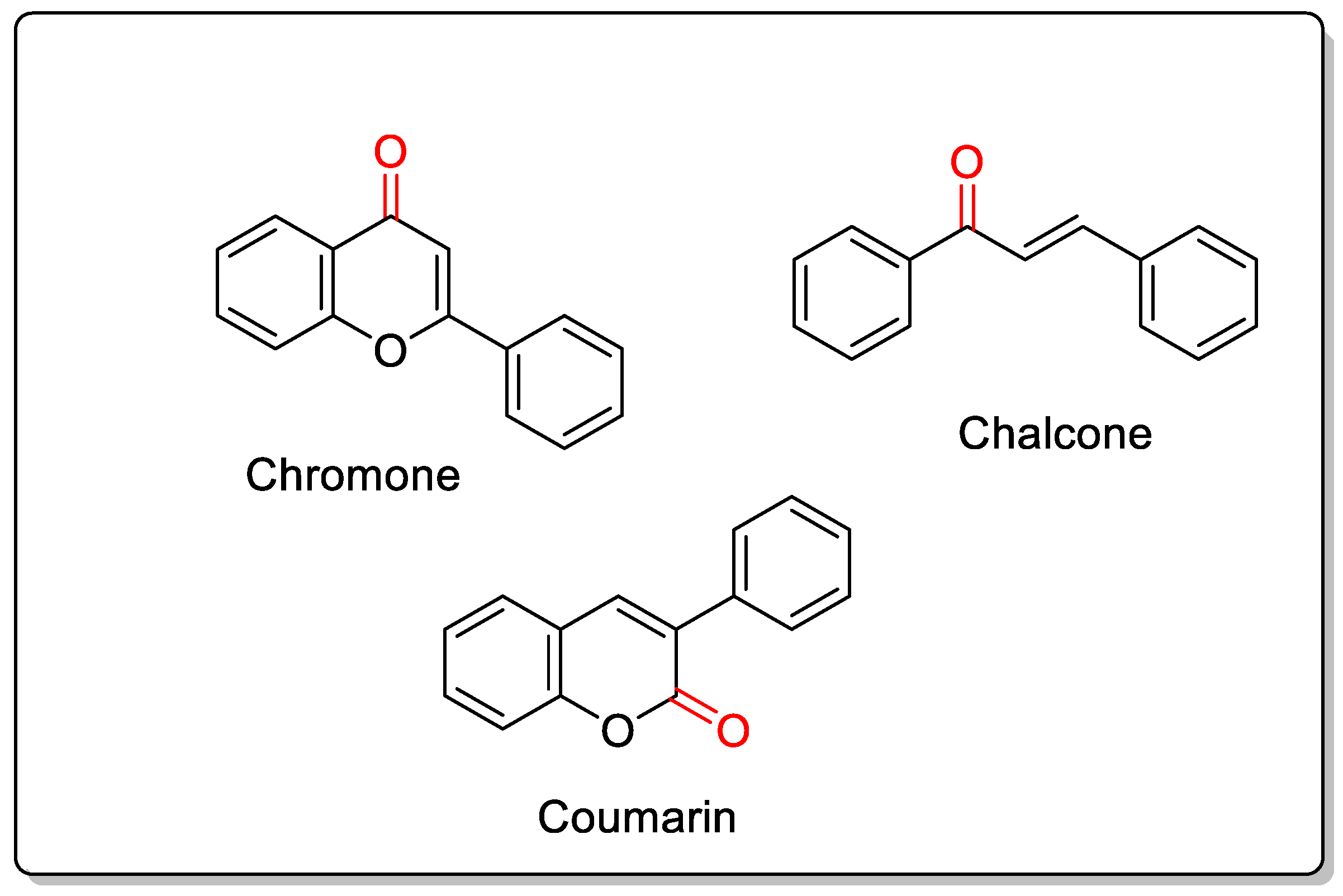
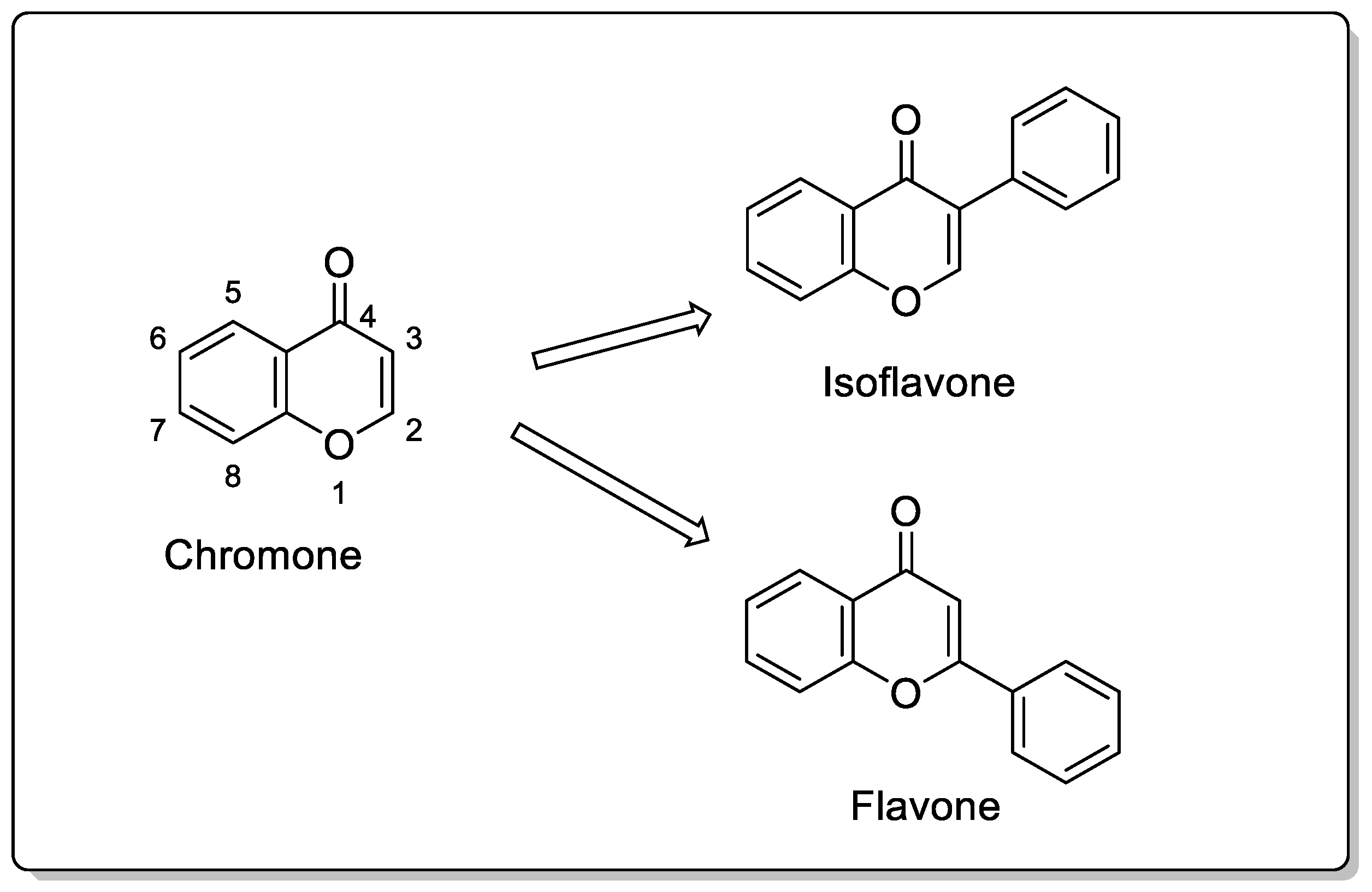
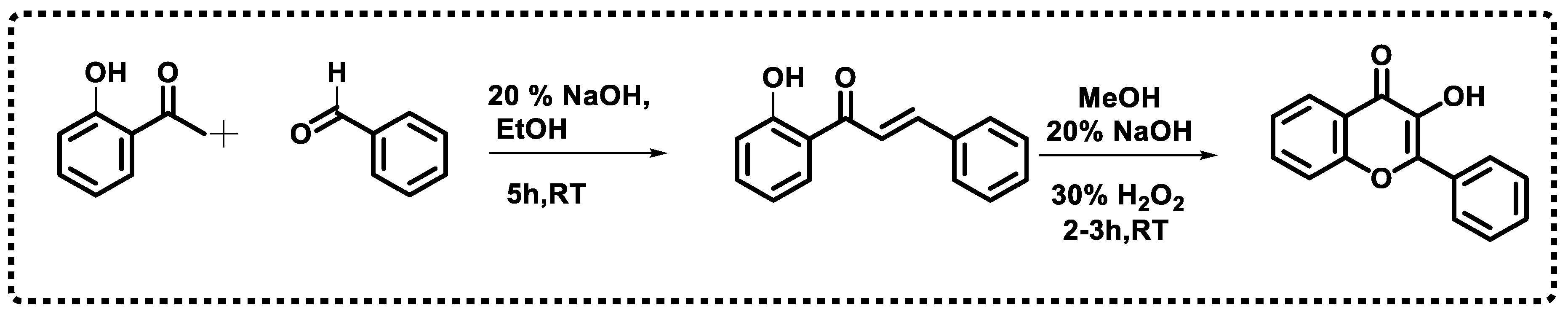
2. Chemistry of Chromones
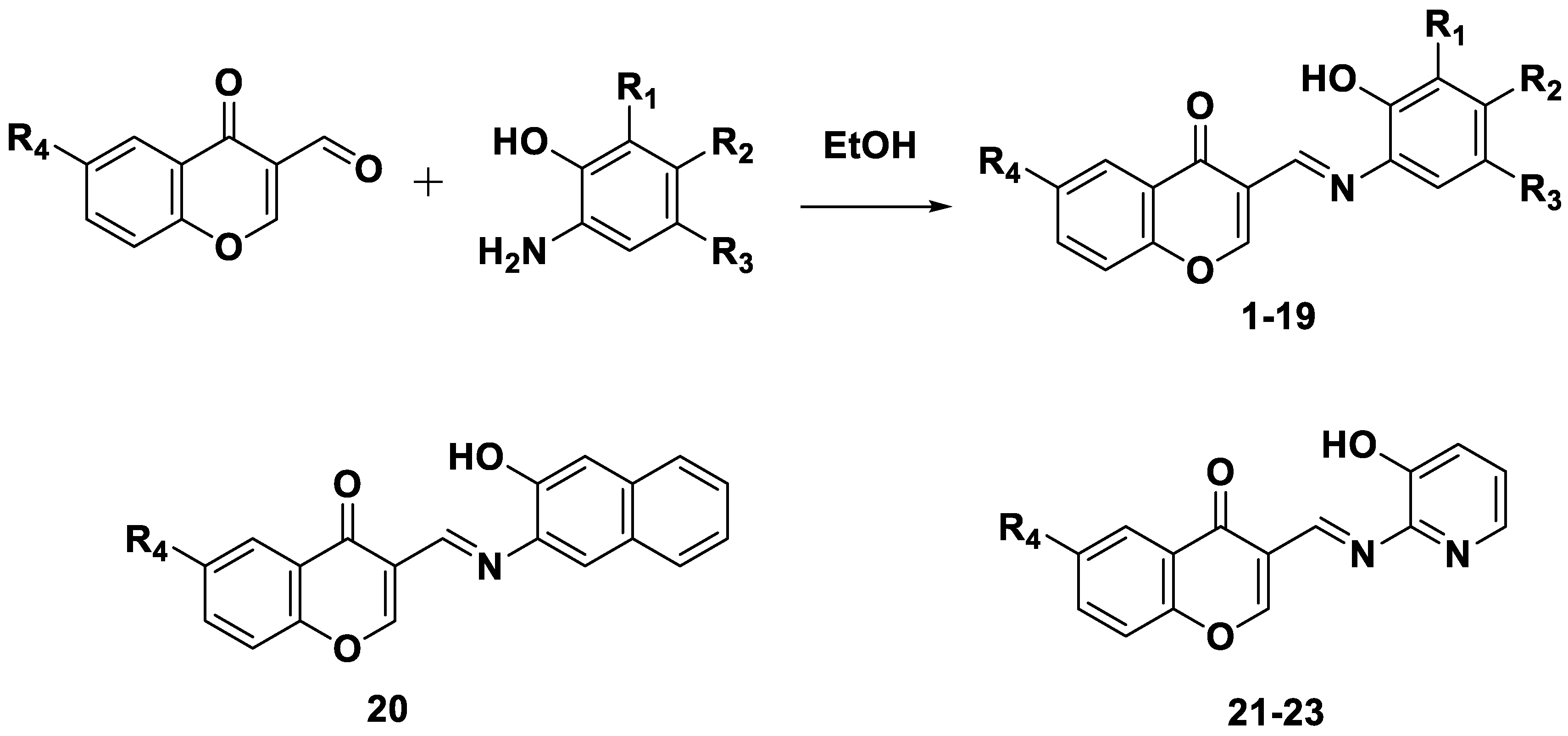
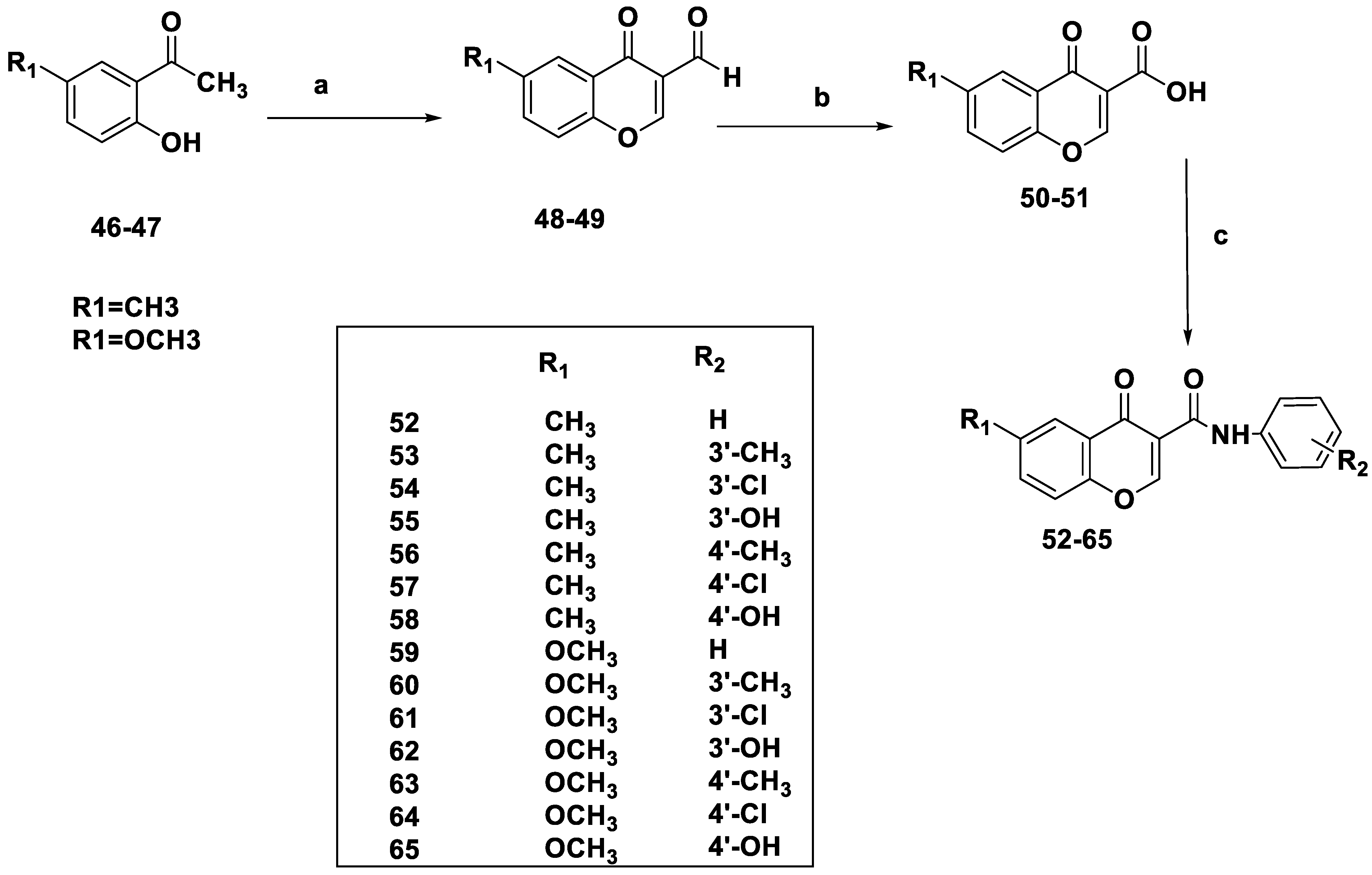
3. SAR Studies of Chromone as MAO-B Inhibitors
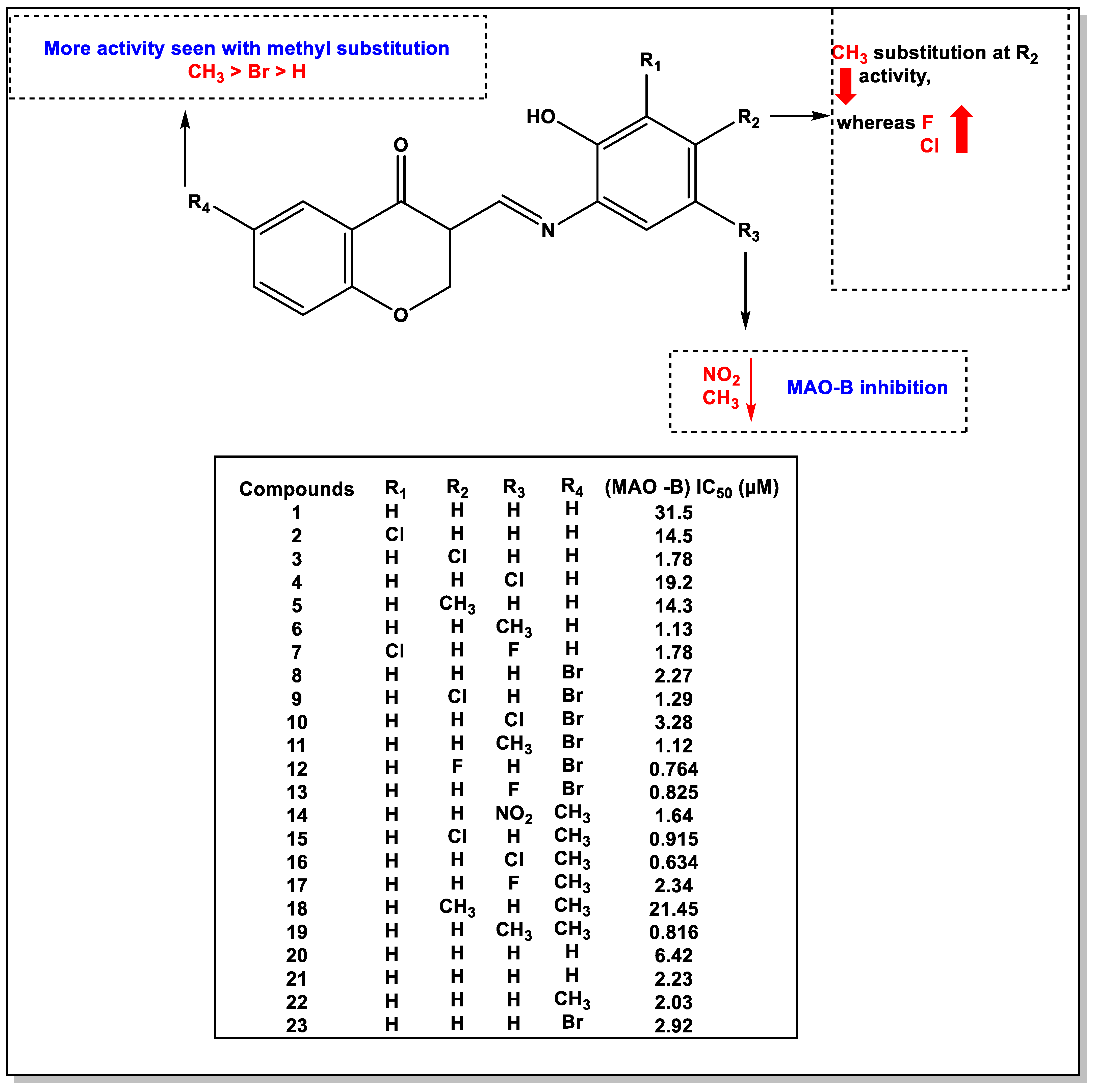
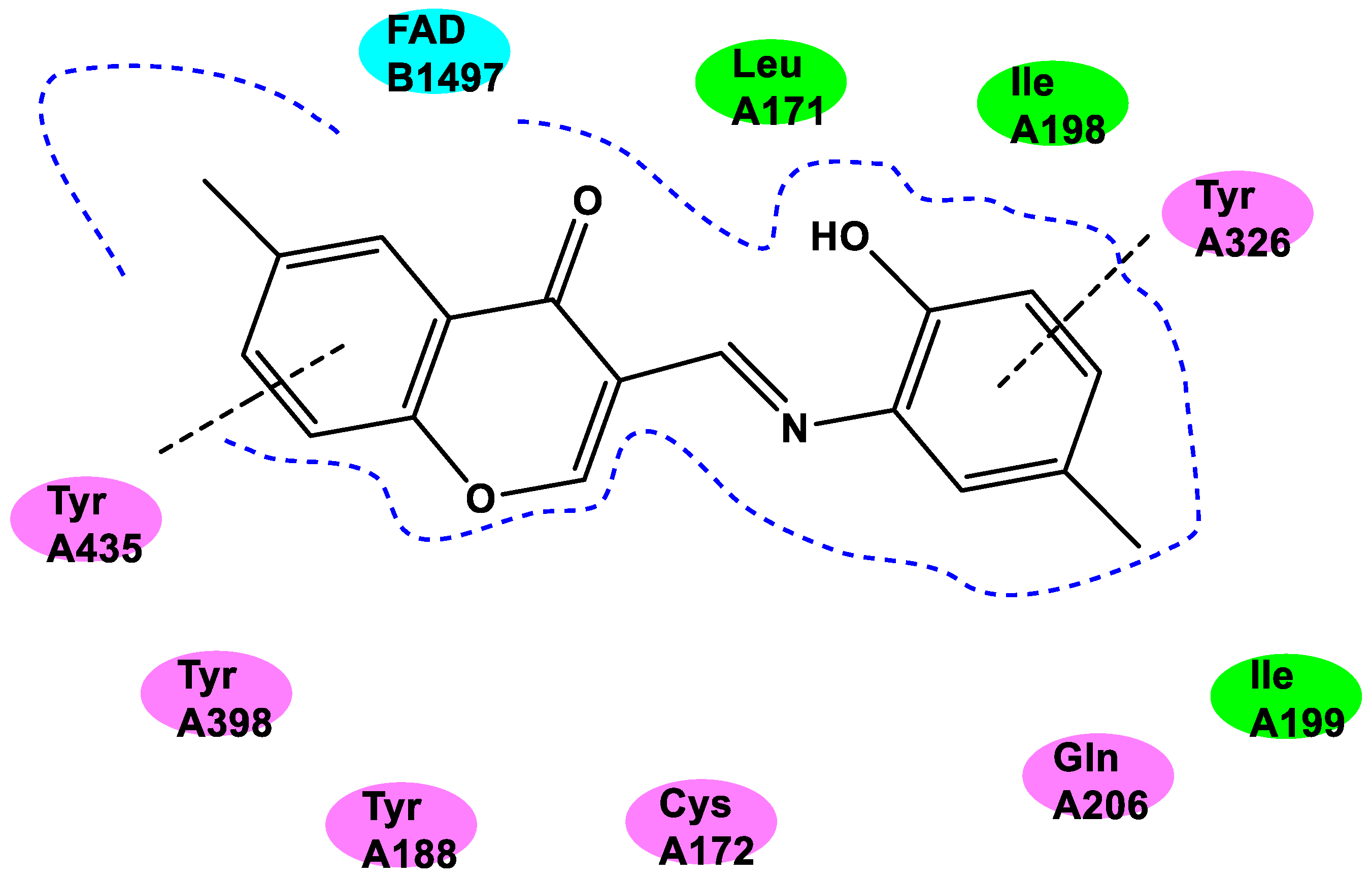
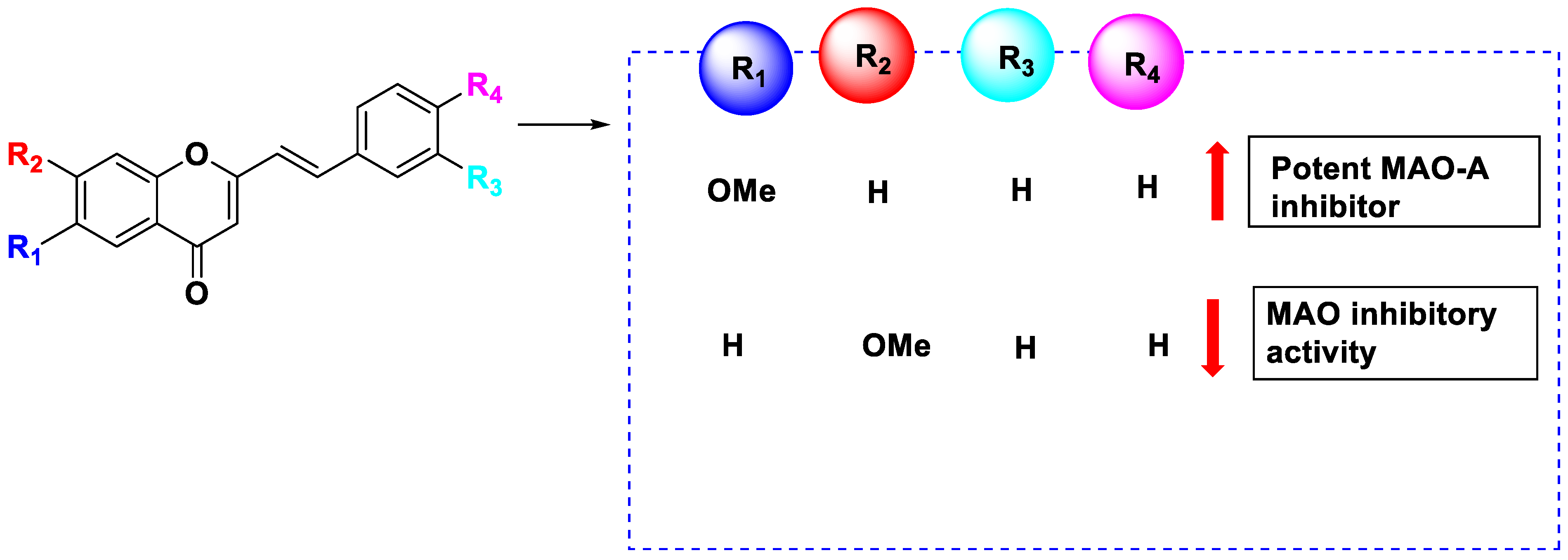
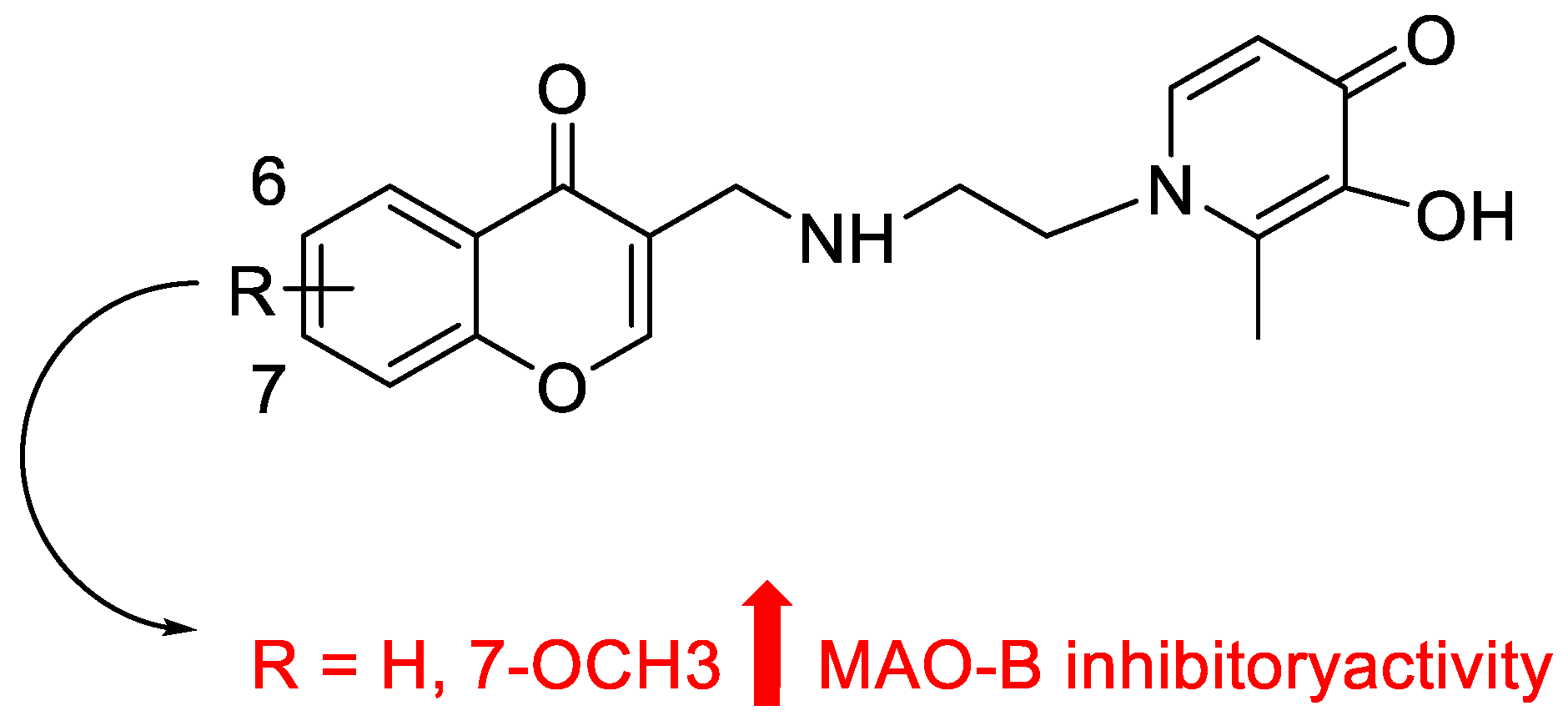
4. The Role of 3-Styryl Chromones’ Substituents in Inhibiting MAO-B
5. Conclusions
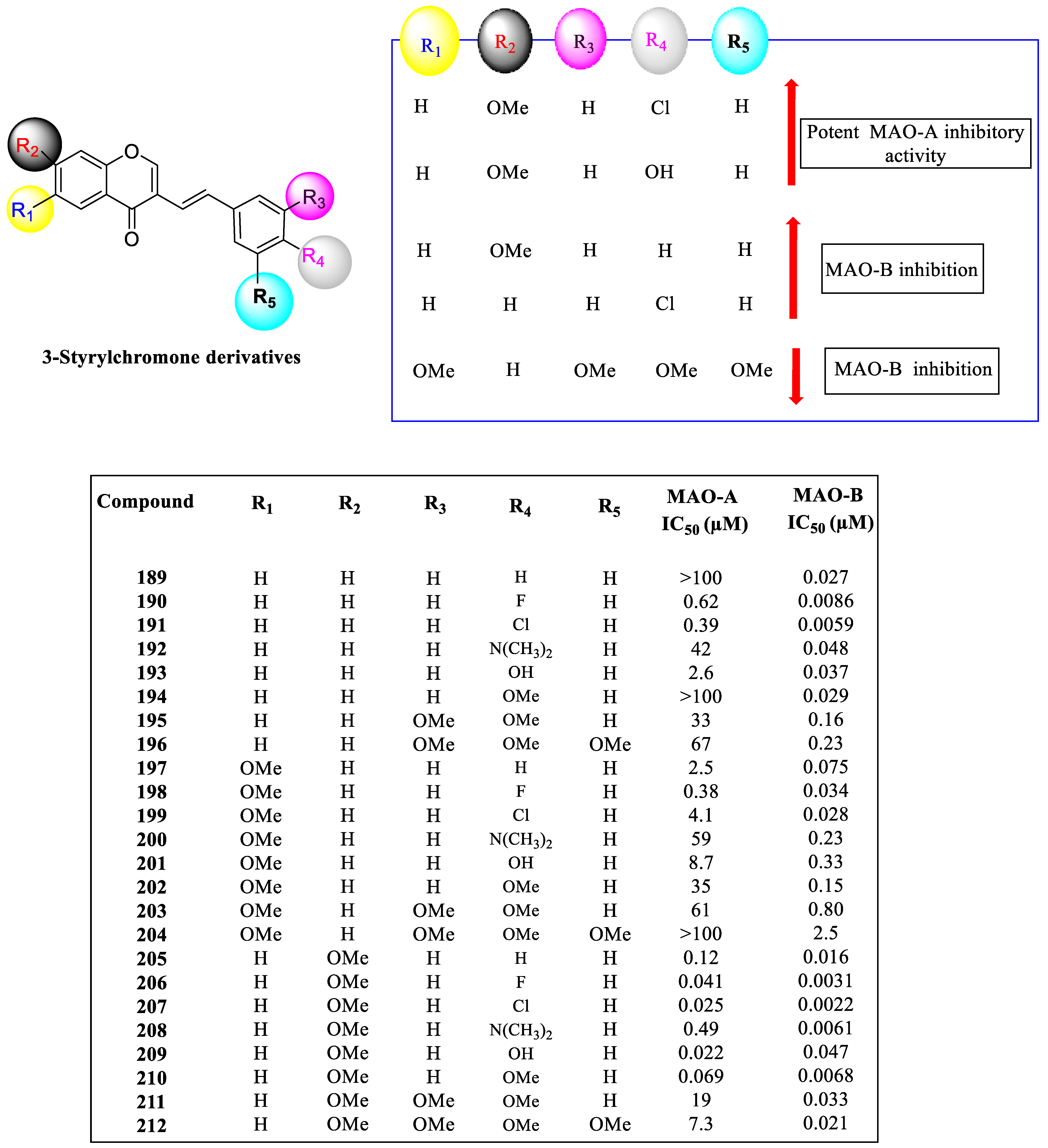
- The methyl group at the R4 position of chromone was shown to be a beneficial substitution.
- The R4 position of the chromone with a bromine group demonstrated a large increase in MAO inhibition and fluorine substitution at R2 of the phenyl ring.
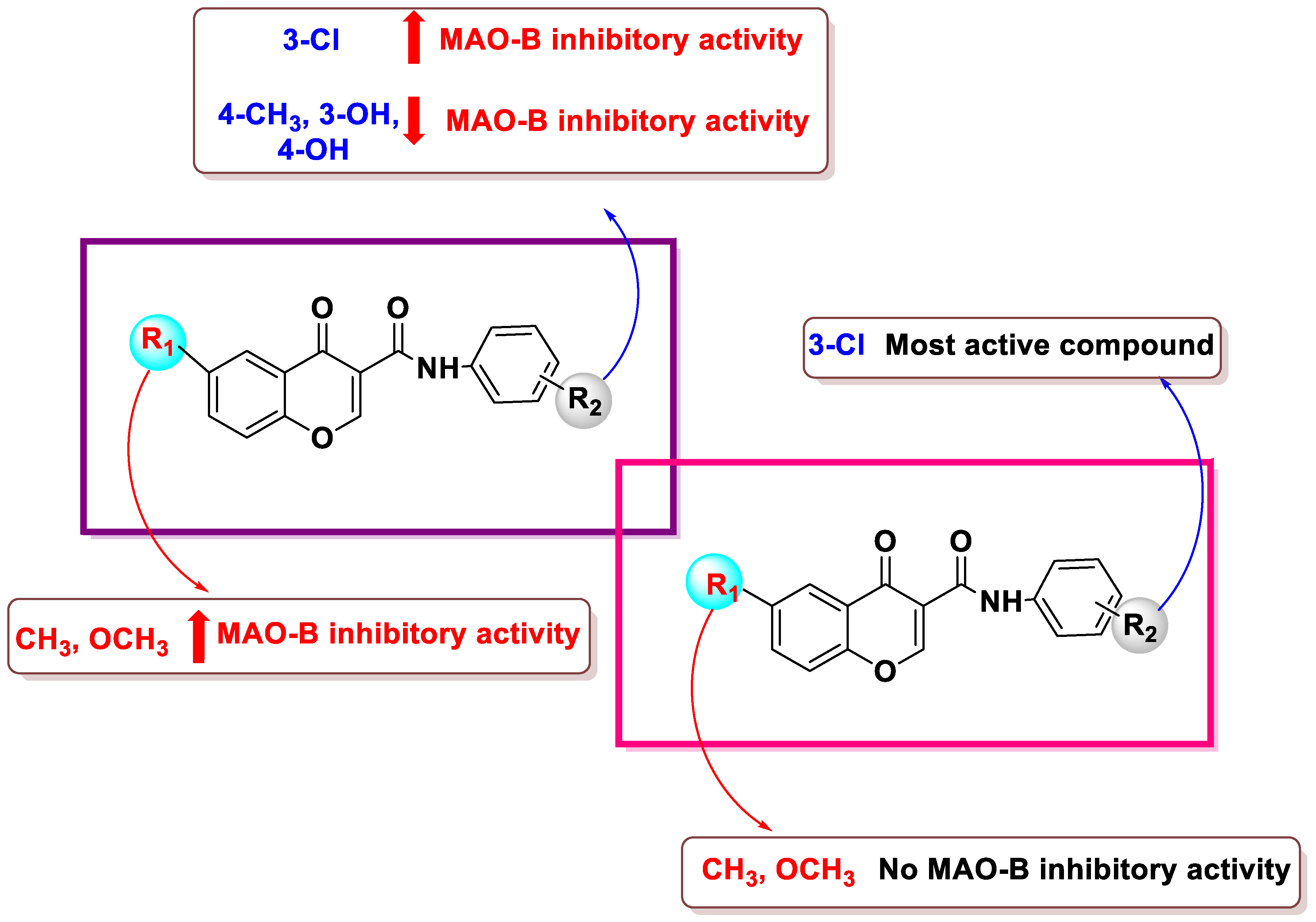
- NO2 and CH3 groups in R3 decreased MAO activity, whereas the electronegative halogens chlorine and fluorine caused a marked increase in activity.
- Electronegative groups, such as Cl and F substitutions at the para position of styryl chromones, demonstrated higher MAO-B inhibition, as seen in the cases of compounds 207 and 206.
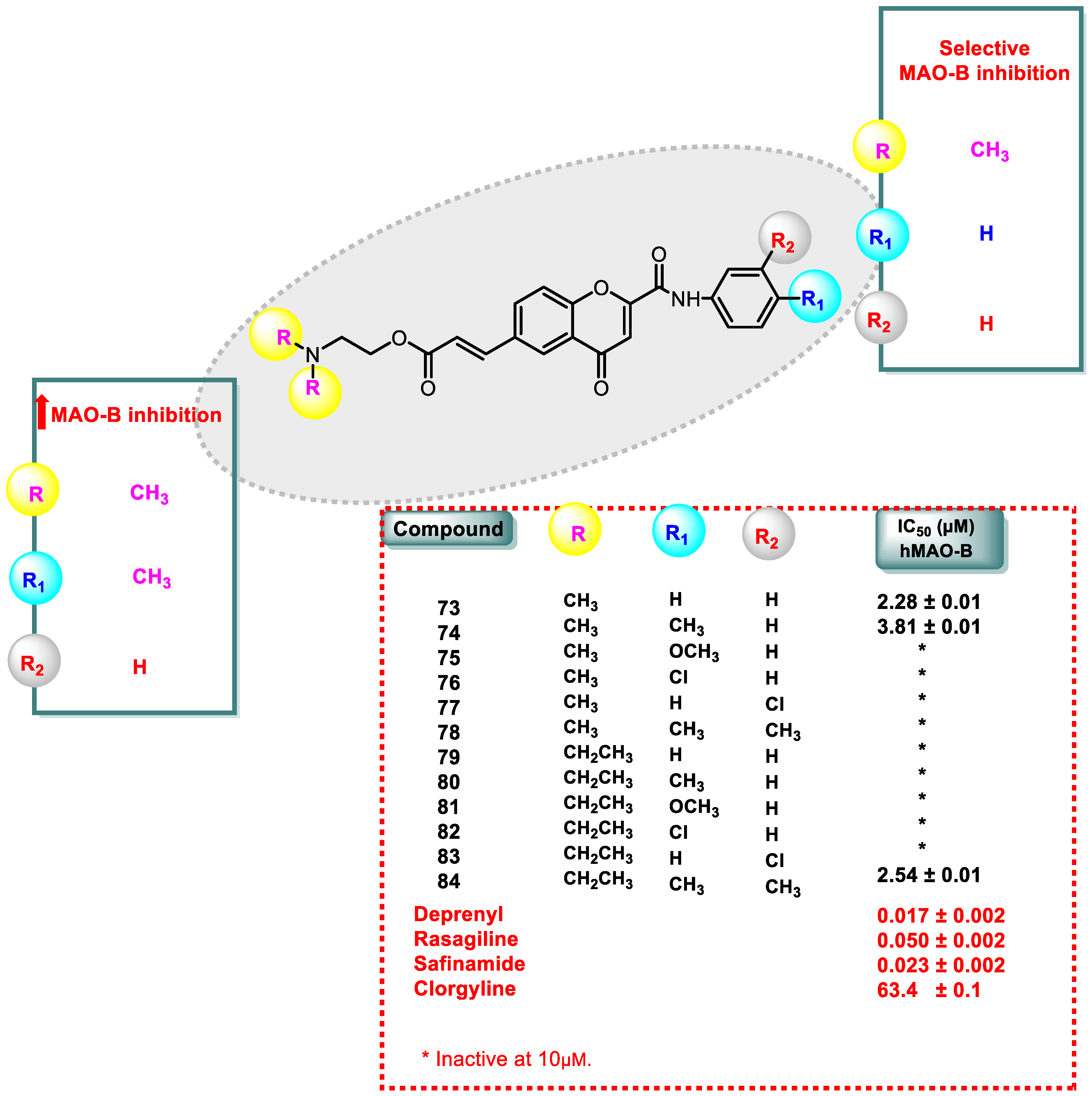
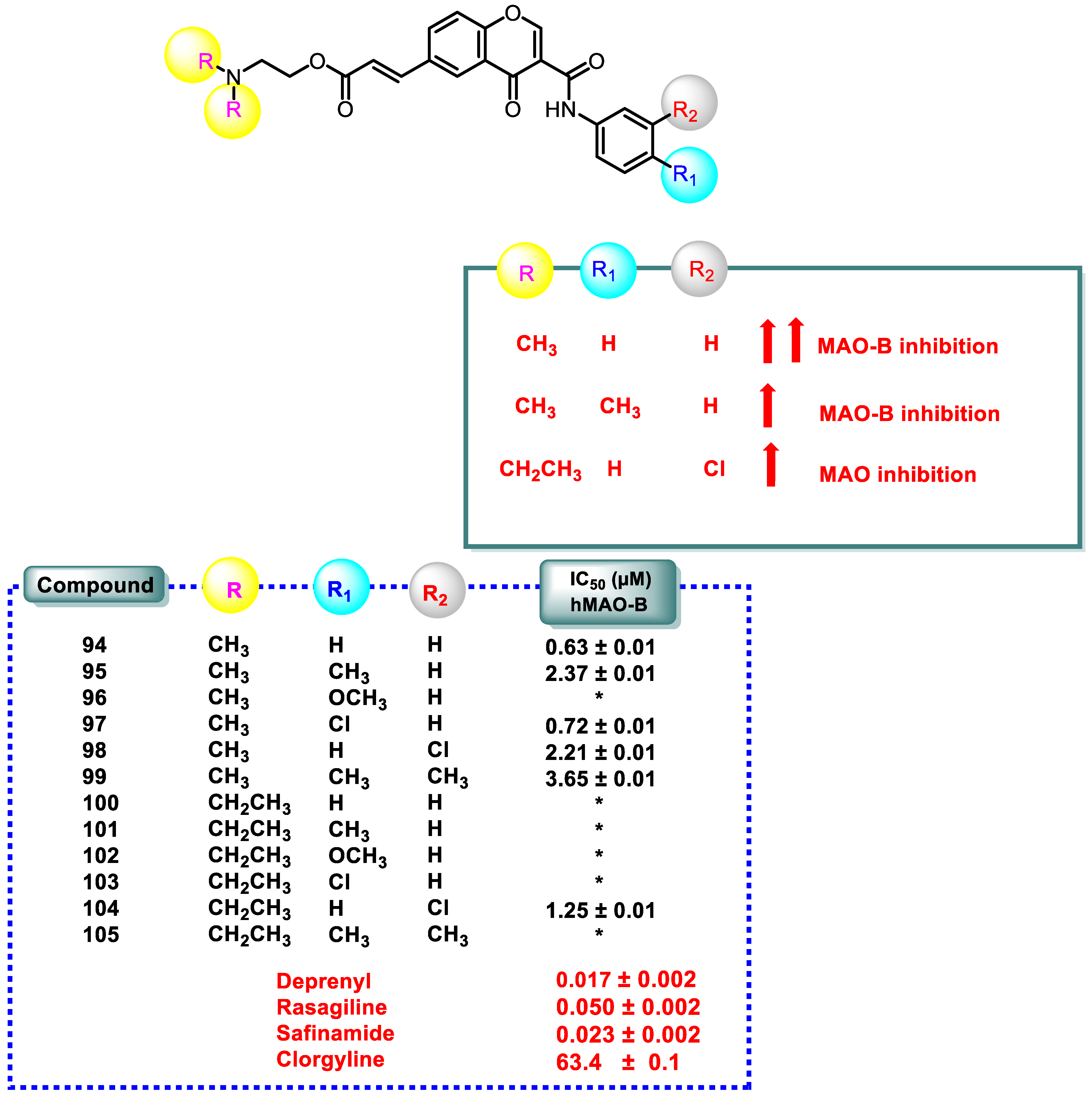
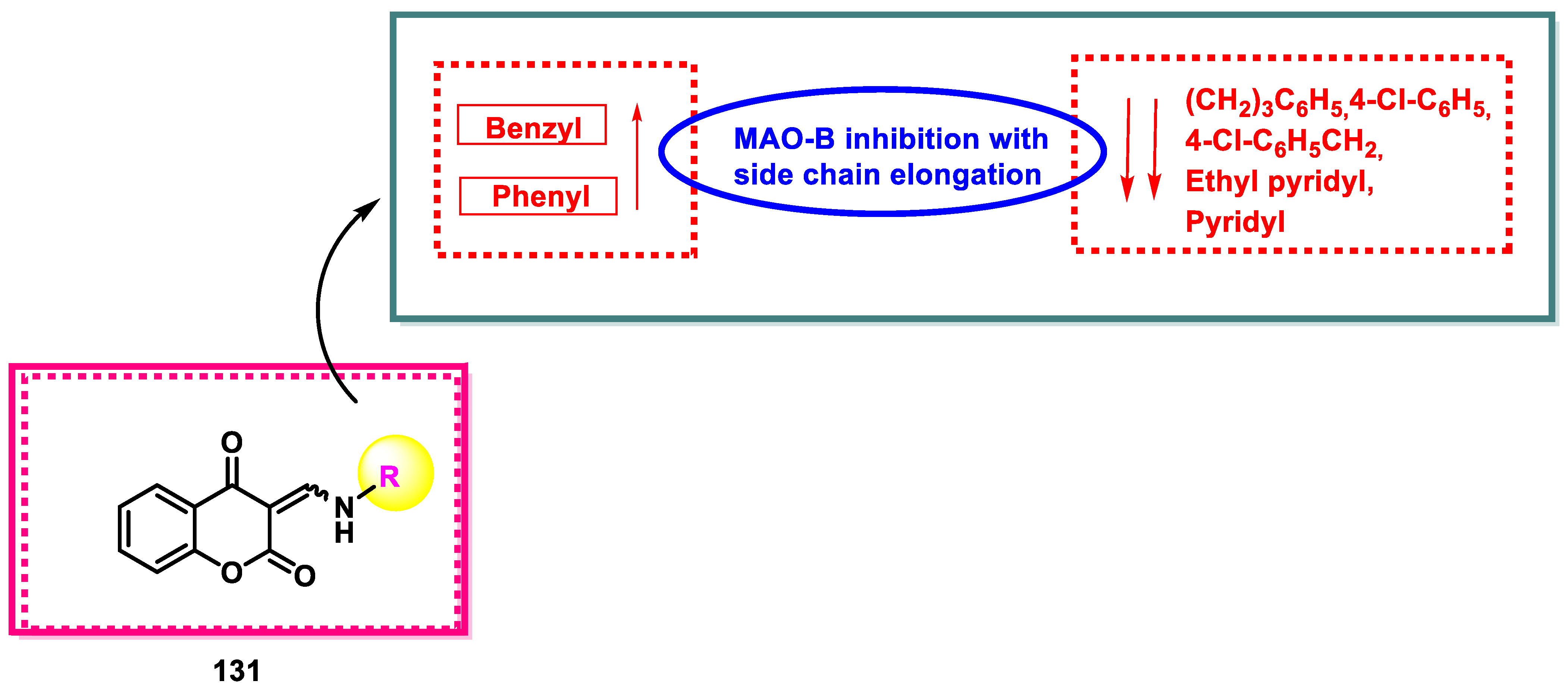
- The amino chromone derivatives exhibited more powerful MAO-B inhibition than the ester derivatives 126 and 131. As demonstrated for compounds 131 and 132, phenyl-to-benzyl chain elongation increased MAO-B inhibition, but further chain elongation diminished activity, as seen for compound 135. The inhibition of MAO-B was reduced by compounds containing pyridyl side chains.
- Derivatives with meta substituents on the exocyclic ring showed increased potency.
- In the future perspective of view, researchers could modify and extend the alkyl chains at the R1 and R5 positions of the chromone ring to develop potent MAO-B inhibitors. The introduction of heterocyclic-based amide on the C-3 position of chromone was not explored so far for the development of MAO-B inhibition. This information will be beneficial for discovering and creating a novel category of powerful and specific MAO-B blockers based on chromones.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fonseca, A.; Reis, J.; Silva, T.; Matos, M.J.; Bagetta, D.; Ortuso, F.; Alcaro, S.; Uriarte, E.; Borges, F. Coumarin versus Chromone Monoamine Oxidase B Inhibitors: Quo Vadis? J. Med. Chem. 2017, 60, 7206–7212. [Google Scholar] [CrossRef] [PubMed]
- Castellani, R.J.; Rolston, R.K.; Smith, M.A. Alzheimer Disease. Disease-a-Month 2010, 56, 484–546. [Google Scholar] [CrossRef] [PubMed]
- de Lau, L.M.; Breteler, M.M. Epidemiology of Parkinson’s Disease. Lancet Neurol. 2006, 5, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Townsend, M. When Will Alzheimer’s Disease Be Cured? A Pharmaceutical Perspective. J. Alzheimer’s Dis. 2011, 24, 43–52. [Google Scholar] [CrossRef]
- Mohsin, N.U.A.; Irfan, M.; Hassan, S.U.; Saleem, U. Current Strategies in Development of New Chromone Derivatives with Diversified Pharmacological Activities: A Review. Pharm. Chem. J. 2020, 54, 241–257. [Google Scholar] [CrossRef]
- Madhav, H.; Jameel, E.; Rehan, M.; Hoda, N. Recent Advancements in Chromone as a Privileged Scaffold towards the Development of Small Molecules for Neurodegenerative Therapeutics. RSC Med. Chem. 2022, 13, 258–279. [Google Scholar] [CrossRef]
- Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.G.; Croteau, D.L.; Bohr, V.A. Ageing as a Risk Factor for Neurodegenerative Disease. Nat. Rev. Neurol. 2019, 15, 565–581. [Google Scholar] [CrossRef]
- Fernandopulle, M.S.; Lippincott-Schwartz, J.; Ward, M.E. RNA Transport and Local Translation in Neurodevelopmental and Neurodegenerative Disease. Nat. Neurosci. 2021, 24, 622–632. [Google Scholar] [CrossRef]
- Fu, H.; Hardy, J.; Duff, K.E. Selective Vulnerability in Neurodegenerative Diseases. Nat. Neurosci. 2018, 21, 1350–1358. [Google Scholar] [CrossRef]
- D’Souza, G.X.; Rose, S.E.; Knupp, A.; Nicholson, D.A.; Keene, C.D.; Young, J.E. The Application of in vitro-derived Human Neurons in Neurodegenerative Disease Modeling. J. Neurosci. Res. 2021, 99, 124–140. [Google Scholar] [CrossRef]
- Schikowski, T.; Altuğ, H. The Role of Air Pollution in Cognitive Impairment and Decline. Neurochem. Int. 2020, 136, 104708. [Google Scholar] [CrossRef] [PubMed]
- Durães, F.; Pinto, M.; Sousa, E. Old Drugs as New Treatments for Neurodegenerative Diseases. Pharmaceuticals 2018, 11, 44. [Google Scholar] [CrossRef] [PubMed]
- Jenner, P. Functional Models of Parkinson’s Disease: A Valuable Tool in the Development of Novel Therapies. Ann. Neurol. 2009, 64, S16–S29. [Google Scholar] [CrossRef] [PubMed]
- Rowinska-Zyrek, M.; Salerno, M.; Kozlowski, H. Neurodegenerative Diseases—Understanding Their Molecular Bases and Progress in the Development of Potential Treatments. Coord. Chem. Rev. 2015, 284, 298–312. [Google Scholar] [CrossRef]
- Adan, G.; Mitchell, J.W.; Ziso, B.; Larner, A.J. Diagnosis and Management of Seizures in Neurodegenerative Diseases. Curr. Treat. Options Neurol. 2021, 23, 1. [Google Scholar] [CrossRef]
- Ebadi, M.; Brown-Borg, H.; Ren, J.; Sharma, S.; Shavali, S.; El ReFaey, H.; Carlson, E. Therapeutic Efficacy of Selegiline in Neurodegenerative Disorders and Neurological Diseases. Curr. Drug Targets 2006, 7, 1513–1529. [Google Scholar] [CrossRef]
- Dhiman, P.; Malik, N.; Sobarzo-Sánchez, E.; Uriarte, E.; Khatkar, A. Quercetin and Related Chromenone Derivatives as Monoamine Oxidase Inhibitors: Targeting Neurological and Mental Disorders. Molecules 2019, 24, 418. [Google Scholar] [CrossRef]
- Kalgutkar, A.S.; Dalvie, D.K.; Castagnoli, N.; Taylor, T.J. Interactions of Nitrogen-Containing Xenobiotics with Monoamine Oxidase (MAO) Isozymes A and B: SAR Studies on MAO Substrates and Inhibitors. Chem. Res. Toxicol. 2001, 14, 1139–1162. [Google Scholar] [CrossRef]
- Shih, J.C.; Chen, K.; Ridd, M.J. Monoamine Oxidase: From Genes to Behavior. Annu. Rev. Neurosci. 1999, 22, 197–217. [Google Scholar] [CrossRef]
- Prah, A.; Purg, M.; Stare, J.; Vianello, R.; Mavri, J. How Monoamine Oxidase A Decomposes Serotonin: An Empirical Valence Bond Simulation of the Reactive Step. J. Phys. Chem. B 2020, 124, 8259–8265. [Google Scholar] [CrossRef]
- Bortolato, M.; Chen, K.; Shih, J.C. Monoamine Oxidase Inactivation: From Pathophysiology to Therapeutics. Adv. Drug Deliv. Rev. 2008, 60, 1527–1533. [Google Scholar] [CrossRef] [PubMed]
- Carradori, S.; Silvestri, R. New Frontiers in Selective Human MAO-B Inhibitors. J. Med. Chem. 2015, 58, 6717–6732. [Google Scholar] [CrossRef] [PubMed]
- Benny, F.; Kumar, S.; Jayan, J.; Abdelgawad, M.A.; Ghoneim, M.M.; Kumar, A.; Manoharan, A.; Susan, R.; Sudevan, S.T.; Mathew, B. Review of Β-carboline and Its Derivatives as Selective MAO-A Inhibitors. Arch. Pharm. 2023, 356, e2300091. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.; Rathitharan, G.; Meyer, J.H.; Furukawa, Y.; Ang, L.-C.; Boileau, I.; Guttman, M.; Hornykiewicz, O.; Kish, S.J. Brain Monoamine Oxidase B and A in Human Parkinsonian Dopamine Deficiency Disorders. Brain 2017, 140, 2460–2474. [Google Scholar] [CrossRef] [PubMed]
- Mathew, B.; Mathew, G.E.; Petzer, J.P.; Petzer, A. Structural Exploration of Synthetic Chromones as Selective MAO-B Inhibitors: A Mini Review. Comb. Chem. High Throughput Screen. 2017, 20, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Saura, J.; Luque, J.M.; Cesura, A.M.; Da Prada, M.; Chan-Palay, V.; Huber, G.; Löffler, J.; Richards, J.G. Increased Monoamine Oxidase b Activity in Plaque-Associated Astrocytes of Alzheimer Brains Revealed by Quantitative Enzyme Radioautography. Neuroscience 1994, 62, 15–30. [Google Scholar] [CrossRef]
- Cesura, A.M.; Pletscher, A. The New Generation of Monoamine Oxidase Inhibitors. In Progress in Drug Research/Fortschritte der Arzneimittelforschung/Progrès Des Recherches Pharmaceutiques; Birkhäuser: Basel, Switzerland, 1992; pp. 171–297. [Google Scholar]
- Amrein, R.; Martin, J.R.; Cameron, A.M. Moclobemide in Patients with Dementia and Depression. Adv. Neurol. 1999, 80, 509–519. [Google Scholar]
- Li, F.; Wu, J.-J.; Wang, J.; Yang, X.-L.; Cai, P.; Liu, Q.-H.; Kong, L.-Y.; Wang, X.-B. Synthesis and Pharmacological Evaluation of Novel Chromone Derivatives as Balanced Multifunctional Agents against Alzheimer’s Disease. Bioorganic Med. Chem. 2017, 25, 3815–3826. [Google Scholar] [CrossRef]
- Youdim, M.B.H.; Lavie, L. Selective MAO-A and B Inhibitors, Radical Scavengers and Nitric Oxide Synthase Inhibitors in Parkinson’s Desease. Life Sci. 1994, 55, 2077–2082. [Google Scholar] [CrossRef]
- Sayre, L.M.; Perry, G.; Smith, M.A. Oxidative Stress and Neurotoxicity. Chem. Res. Toxicol. 2008, 21, 172–188. [Google Scholar] [CrossRef]
- Ramsay, R.R. Inhibitor Design for Monoamine Oxidases. Curr. Pharm. Des. 2013, 19, 2529–2539. [Google Scholar] [CrossRef] [PubMed]
- Knudsen Gerber, D.S. Selegiline and Rasagiline: Twins or Distant Cousins? Guidelines. Consult. Pharm. 2011, 26, 48–51. [Google Scholar] [CrossRef] [PubMed]
- Fariello, R.G. Safinamide. Neurotherapeutics 2007, 4, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Binda, C.; Wang, J.; Pisani, L.; Caccia, C.; Carotti, A.; Salvati, P.; Edmondson, D.E.; Mattevi, A. Structures of Human Monoamine Oxidase B Complexes with Selective Noncovalent Inhibitors: Safinamide and Coumarin Analogs. J. Med. Chem. 2007, 50, 5848–5852. [Google Scholar] [CrossRef] [PubMed]
- Chimenti, F.; Secci, D.; Bolasco, A.; Chimenti, P.; Bizzarri, B.; Granese, A.; Carradori, S.; Yáñez, M.; Orallo, F.; Ortuso, F.; et al. Synthesis, Molecular Modeling, and Selective Inhibitory Activity against Human Monoamine Oxidases of 3-Carboxamido-7-Substituted Coumarins. J. Med. Chem. 2009, 52, 1935–1942. [Google Scholar] [CrossRef]
- Delogu, G.; Picciau, C.; Ferino, G.; Quezada, E.; Podda, G.; Uriarte, E.; Viña, D. Synthesis, Human Monoamine Oxidase Inhibitory Activity and Molecular Docking Studies of 3-Heteroarylcoumarin Derivatives. Eur. J. Med. Chem. 2011, 46, 1147–1152. [Google Scholar] [CrossRef]
- Secci, D.; Carradori, S.; Bolasco, A.; Chimenti, P.; Yáñez, M.; Ortuso, F.; Alcaro, S. Synthesis and Selective Human Monoamine Oxidase Inhibition of 3-Carbonyl, 3-Acyl, and 3-Carboxyhydrazido Coumarin Derivatives. Eur. J. Med. Chem. 2011, 46, 4846–4852. [Google Scholar] [CrossRef]
- Chimenti, F.; Fioravanti, R.; Bolasco, A.; Chimenti, P.; Secci, D.; Rossi, F.; Yáñez, M.; Orallo, F.; Ortuso, F.; Alcaro, S. Chalcones: A Valid Scaffold for Monoamine Oxidases Inhibitors. J. Med. Chem. 2009, 52, 2818–2824. [Google Scholar] [CrossRef]
- Silva, C.F.M.; Pinto, D.C.G.A.; Silva, A.M.S. Chromones: A Promising Ring System for New Anti-Inflammatory Drugs. ChemMedChem 2016, 11, 2252–2260. [Google Scholar] [CrossRef]
- Silva, C.F.M.; Batista, V.F.; Pinto, D.C.G.A.; Silva, A.M.S. Challenges with Chromone as a Privileged Scaffold in Drug Discovery. Expert. Opin. Drug Discov. 2018, 13, 795–798. [Google Scholar] [CrossRef]
- Horton, D.A.; Bourne, G.T.; Smythe, M.L. The Combinatorial Synthesis of Bicyclic Privileged Structures or Privileged Substructures. Chem. Rev. 2003, 103, 893–930. [Google Scholar] [CrossRef] [PubMed]
- Roy, T.; Boateng, S.T.; Banang-Mbeumi, S.; Singh, P.K.; Basnet, P.; Chamcheu, R.-C.N.; Ladu, F.; Chauvin, I.; Spiegelman, V.S.; Hill, R.A.; et al. Synthesis, Inverse Docking-Assisted Identification and in Vitro Biological Characterization of Flavonol-Based Analogs of Fisetin as c-Kit, CDK2 and MTOR Inhibitors against Melanoma and Non-Melanoma Skin Cancers. Bioorganic Chem. 2021, 107, 104595. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, A.K.; Kaur, S.; Mahata, P.K.; Biswas, D.; Pramanik, B.N.; Chan, T.M. Synthesis and Properties of 3-Acyl-γ-Pyrones, a Novel Class of Flavones and Chromones. Tetrahedron Lett. 2005, 46, 4119–4121. [Google Scholar] [CrossRef]
- Brühlmann, C.; Ooms, F.; Carrupt, P.-A.; Testa, B.; Catto, M.; Leonetti, F.; Altomare, C.; Carotti, A. Coumarins Derivatives as Dual Inhibitors of Acetylcholinesterase and Monoamine Oxidase. J. Med. Chem. 2001, 44, 3195–3198. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Bachiller, M.I.; Pérez, C.; Monjas, L.; Rademann, J.; Rodríguez-Franco, M.I. New Tacrine–4-Oxo-4 H-Chromene Hybrids as Multifunctional Agents for the Treatment of Alzheimer’s Disease, with Cholinergic, Antioxidant, and β-Amyloid-Reducing Properties. J. Med. Chem. 2012, 55, 1303–1317. [Google Scholar] [CrossRef]
- Erickson, R.H.; Natalie, K.J.; Bock, W.; Lu, Z.; Farzin, F.; Sherrill, R.G.; Meloni, D.J.; Patch, R.J.; Rzesotarski, W.J. (Aminoalkoxy)Chromones. Selective Sigma Receptor Ligands. J. Med. Chem. 1992, 35, 1526–1535. [Google Scholar] [CrossRef]
- Gaspar, A.; Reis, J.; Matos, M.J.; Uriarte, E.; Borges, F. In Search for New Chemical Entities as Adenosine Receptor Ligands: Development of Agents Based on Benzo-γ-Pyrone Skeleton. Eur. J. Med. Chem. 2012, 54, 914–918. [Google Scholar] [CrossRef]
- Gaspar, A.; Reis, J.; Kachler, S.; Paoletta, S.; Uriarte, E.; Klotz, K.-N.; Moro, S.; Borges, F. Discovery of Novel A3 Adenosine Receptor Ligands Based on Chromone Scaffold. Biochem. Pharmacol. 2012, 84, 21–29. [Google Scholar] [CrossRef]
- Cagide, F.; Gaspar, A.; Reis, J.; Chavarria, D.; Vilar, S.; Hripcsak, G.; Uriarte, E.; Kachler, S.; Klotz, K.N.; Borges, F. Navigating in Chromone Chemical Space: Discovery of Novel and Distinct A 3 Adenosine Receptor Ligands. RSC Adv. 2015, 5, 78572–78585. [Google Scholar] [CrossRef]
- Andrews, S.P.; Mason, J.S.; Hurrell, E.; Congreve, M. Structure-Based Drug Design of Chromone Antagonists of the Adenosine A 2A Receptor. Med. Chem. Commun. 2014, 5, 571–575. [Google Scholar] [CrossRef]
- Cagide, F.; Reis, J.; Gaspar, A.; Chavarria, D.; Kachler, S.; Klotz, K.N.; Gomes, L.R.; Low, J.N.; Vilar, S.; Hripcsak, G.; et al. Discovery of the First A 1 Adenosine Receptor Ligand Based on the Chromone Scaffold. RSC Adv. 2016, 6, 46972–46976. [Google Scholar] [CrossRef]
- Reis, J.; Cagide, F.; Valencia, M.E.; Teixeira, J.; Bagetta, D.; Pérez, C.; Uriarte, E.; Oliveira, P.J.; Ortuso, F.; Alcaro, S.; et al. Multi-Target-Directed Ligands for Alzheimer’s Disease: Discovery of Chromone-Based Monoamine Oxidase/Cholinesterase Inhibitors. Eur. J. Med. Chem. 2018, 158, 781–800. [Google Scholar] [CrossRef] [PubMed]
- Mpitimpiti, A.N.; Petzer, J.P.; Petzer, A.; Jordaan, J.H.L.; Lourens, A.C.U. Synthesis and Evaluation of Chromone Derivatives as Inhibitors of Monoamine Oxidase. Mol. Divers. 2019, 23, 897–913. [Google Scholar] [CrossRef] [PubMed]
- Legoabe, L.J.; Petzer, A.; Petzer, J.P. Selected Chromone Derivatives as Inhibitors of Monoamine Oxidase. Bioorganic Med. Chem. Lett. 2012, 22, 5480–5484. [Google Scholar] [CrossRef]
- Alcaro, S.; Gaspar, A.; Ortuso, F.; Milhazes, N.; Orallo, F.; Uriarte, E.; Yáñez, M.; Borges, F. Chromone-2- and -3-Carboxylic Acids Inhibit Differently Monoamine Oxidases A and B. Bioorganic Med. Chem. Lett. 2010, 20, 2709–2712. [Google Scholar] [CrossRef]
- Gaspar, A.; Silva, T.; Yáñez, M.; Vina, D.; Orallo, F.; Ortuso, F.; Uriarte, E.; Alcaro, S.; Borges, F. Chromone, a Privileged Scaffold for the Development of Monoamine Oxidase Inhibitors. J. Med. Chem. 2011, 54, 5165–5173. [Google Scholar] [CrossRef]
- Gaspar, A.; Reis, J.; Fonseca, A.; Milhazes, N.; Viña, D.; Uriarte, E.; Borges, F. Chromone 3-Phenylcarboxamides as Potent and Selective MAO-B Inhibitors. Bioorganic Med. Chem. Lett. 2011, 21, 707–709. [Google Scholar] [CrossRef]
- Cagide, F.; Silva, T.; Reis, J.; Gaspar, A.; Borges, F.; Gomes, L.R.; Low, J.N. Discovery of Two New Classes of Potent Monoamine Oxidase-B Inhibitors by Tricky Chemistry. Chem. Commun. 2015, 51, 2832–2835. [Google Scholar] [CrossRef]
- Torres, P.H.; Sodero, A.C.; Jofily, P.; Silva, F.P., Jr. Key topics in molecular docking for drug design. Int. J. Mol. Sci. 2019, 20, 4574. [Google Scholar] [CrossRef]
- Takao, K.; Endo, S.; Nagai, J.; Kamauchi, H.; Takemura, Y.; Uesawa, Y.; Sugita, Y. 2-Styrylchromone Derivatives as Potent and Selective Monoamine Oxidase B Inhibitors. Bioorganic Chem. 2019, 92, 103285. [Google Scholar] [CrossRef]
- Wang, X.-B.; Yin, F.-C.; Huang, M.; Jiang, N.; Lan, J.-S.; Kong, L.-Y. Chromone and Donepezil Hybrids as New Multipotent Cholinesterase and Monoamine Oxidase Inhibitors for the Potential Treatment of Alzheimer’s Disease. RSC Med. Chem. 2020, 11, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Takao, K.; Sakatsume, T.; Kamauchi, H.; Sugita, Y. Syntheses and Evaluation of 2- or 3-(N-cyclicamino) Chromone Derivatives as Monoamine Oxidase Inhibitors. Chem. Pharm. Bull. 2020, 68, 1082–1089. [Google Scholar] [CrossRef]
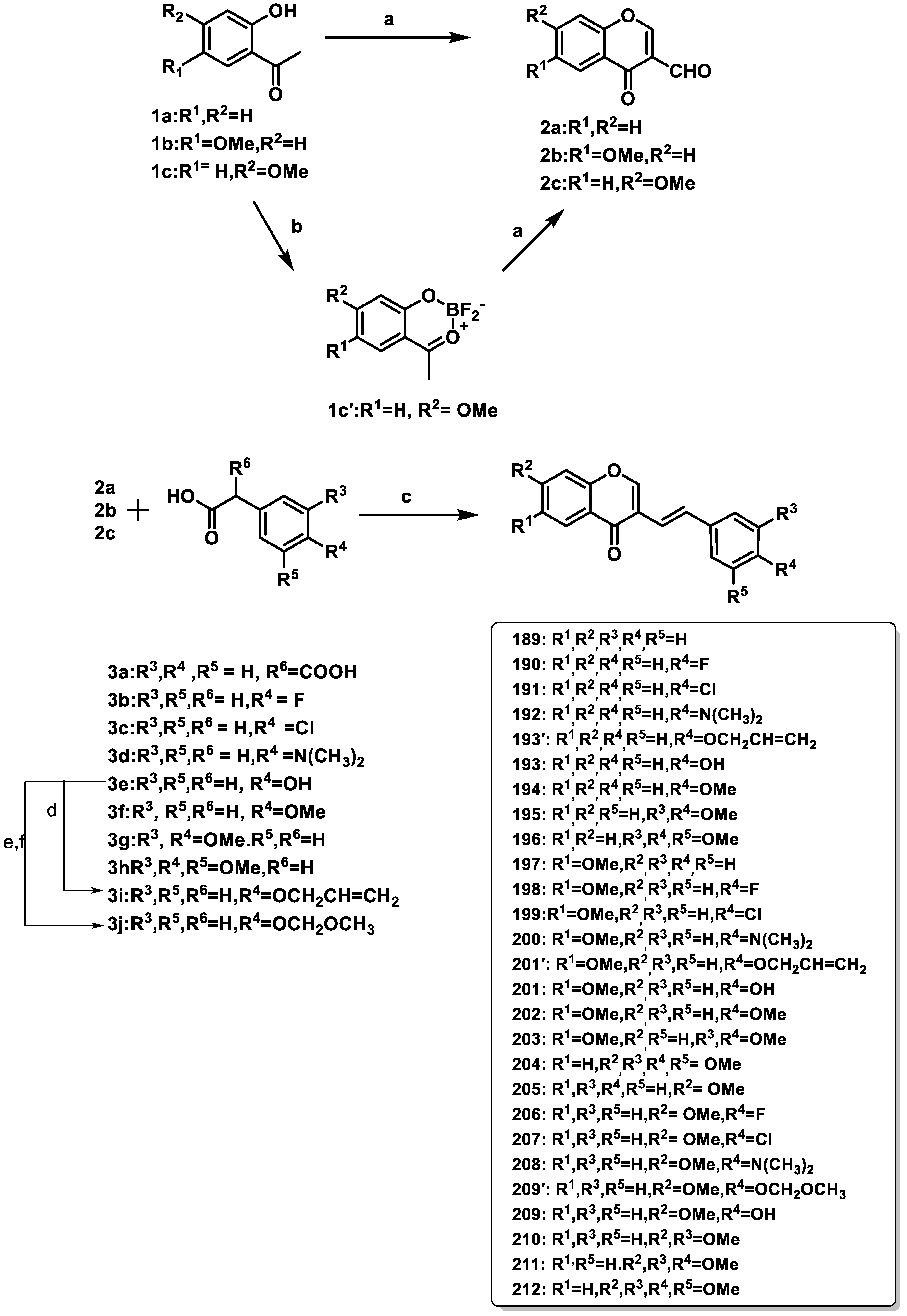
- Takao, K.; Takemura, Y.; Nagai, J.; Kamauchi, H.; Hoshi, K.; Mabashi, R.; Uesawa, Y.; Sugita, Y. Synthesis and Biological Evaluation of 3-Styrylchromone Derivatives as Selective Monoamine Oxidase B Inhibitors. Bioorganic Med. Chem. 2021, 42, 116255. [Google Scholar] [CrossRef] [PubMed]
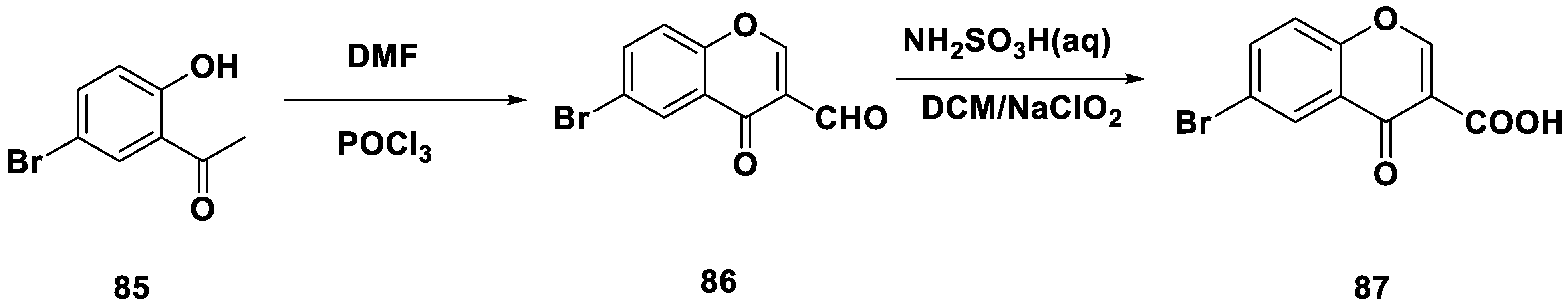
- Zhang, C.; Zhang, Y.; Lv, Y.; Guo, J.; Gao, B.; Lu, Y.; Zang, A.; Zhu, X.; Zhou, T.; Xie, Y. Chromone-Based Monoamine Oxidase B Inhibitor with Potential Iron-Chelating Activity for the Treatment of Alzheimer’s Disease. J. Enzyme Inhib. Med. Chem. 2023, 38, 100–117. [Google Scholar] [CrossRef] [PubMed]



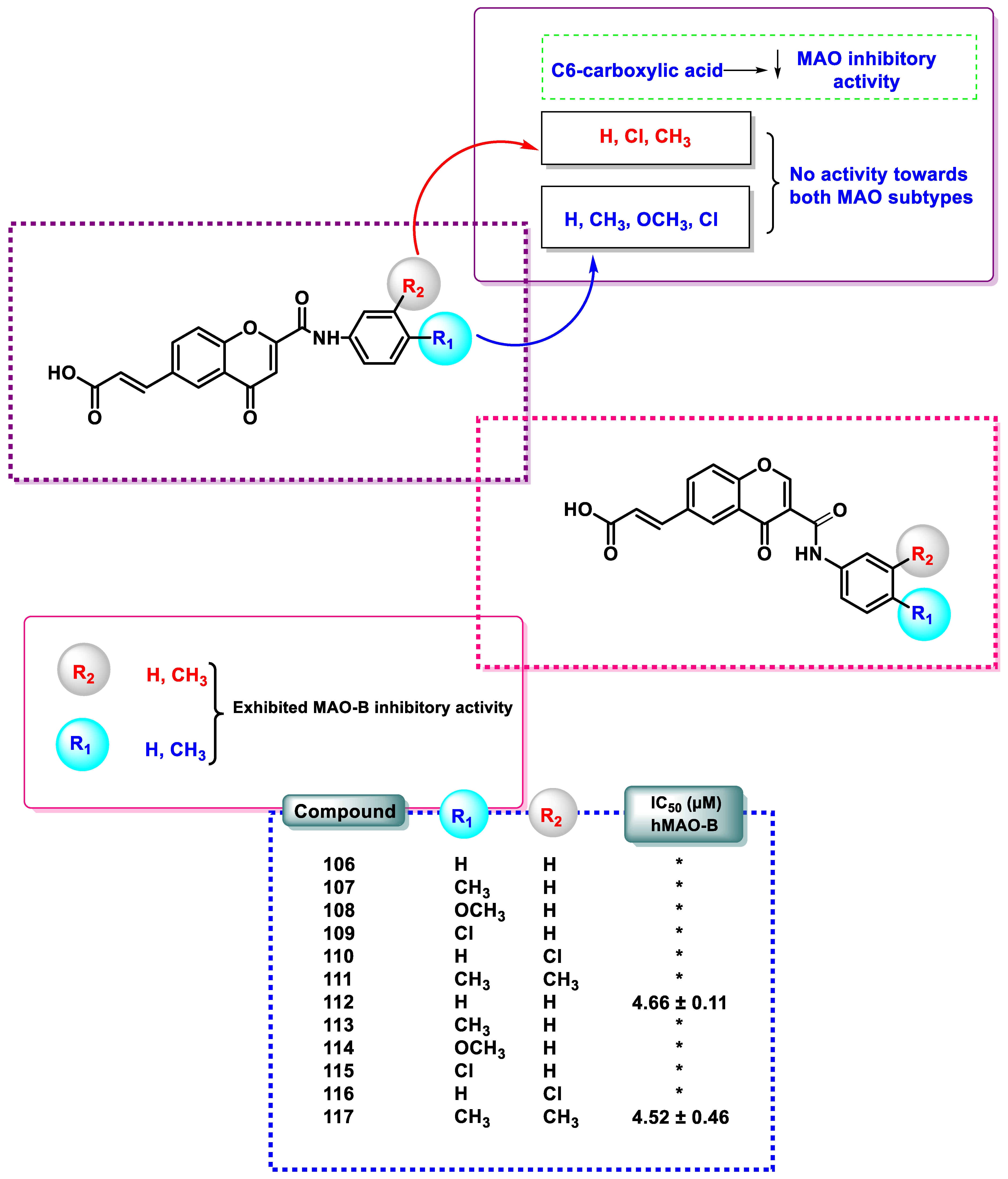
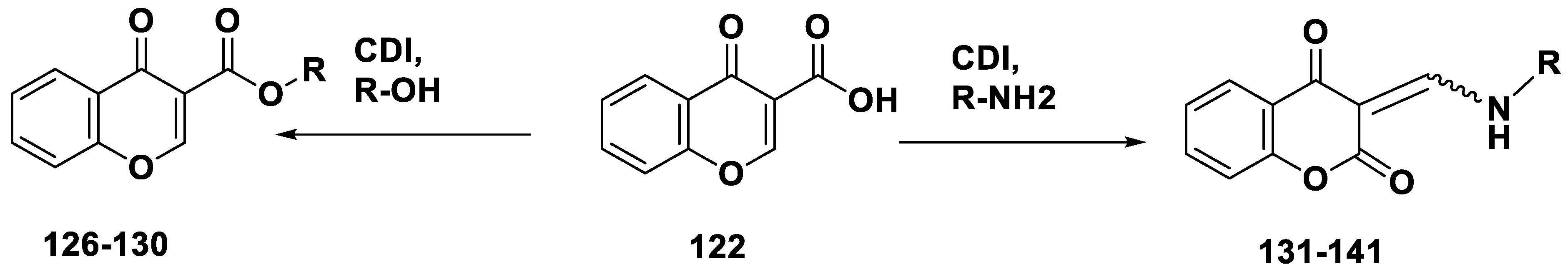
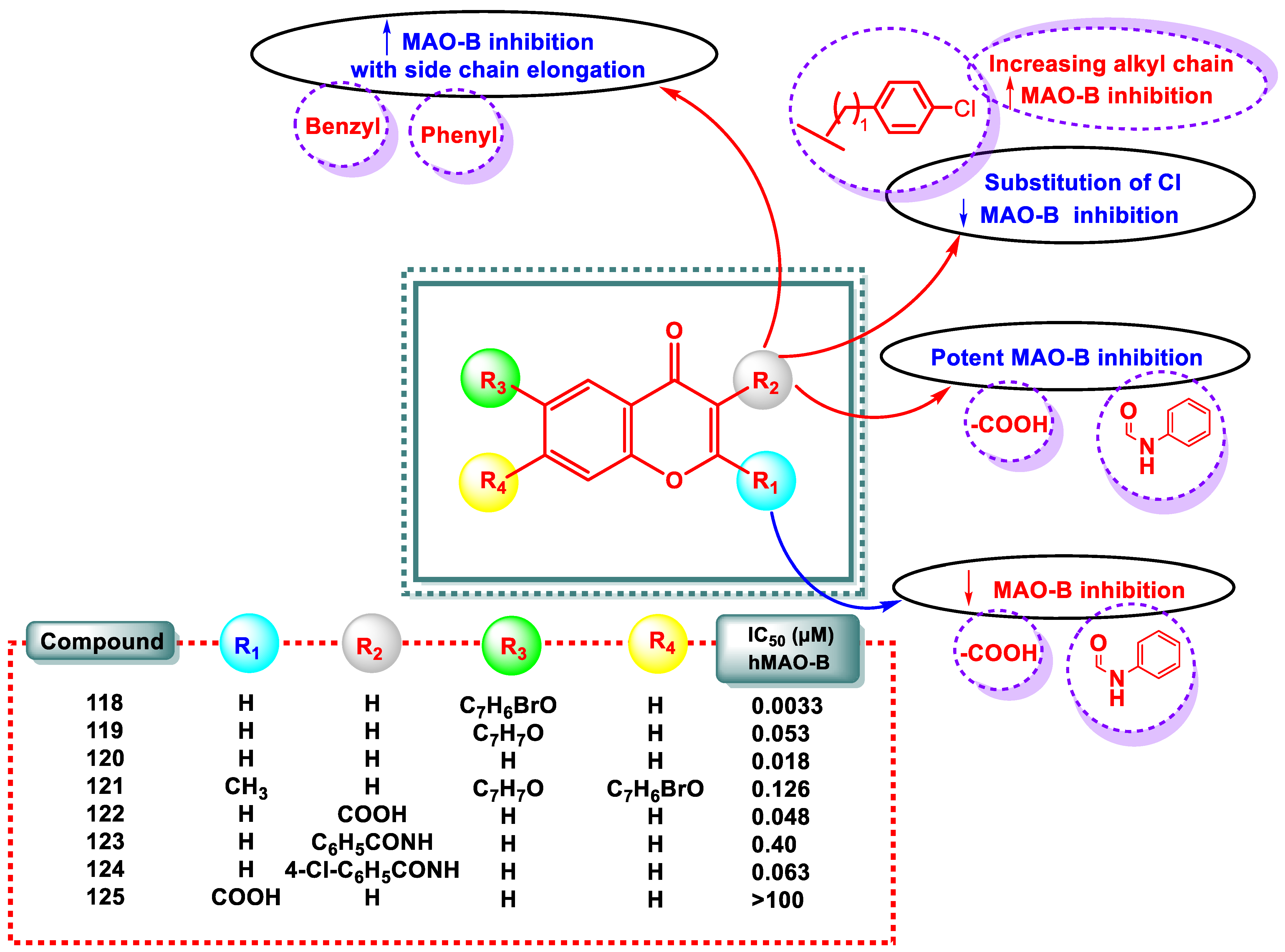
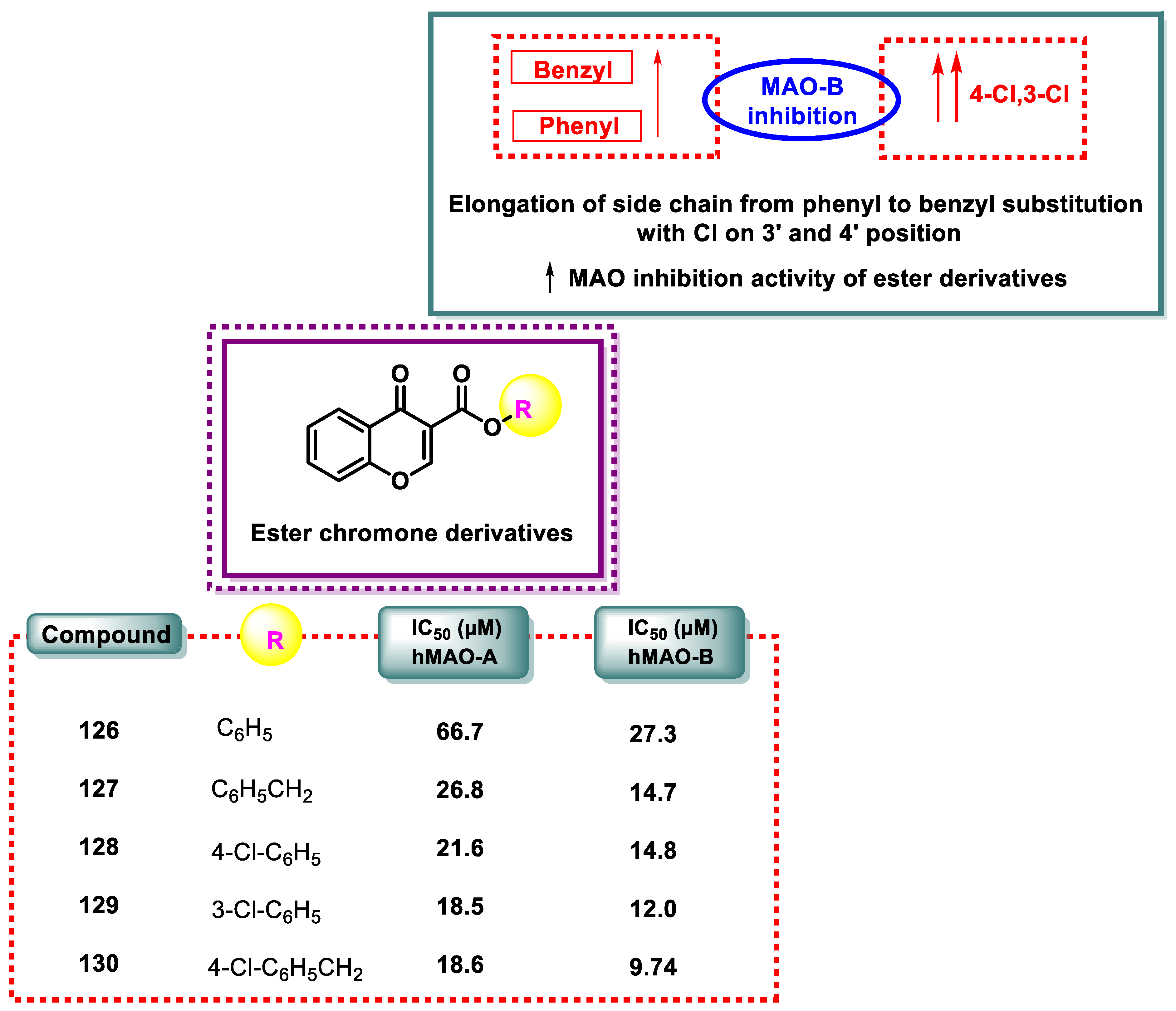

| Compound | IC50 (μM) hMAO-B | Compound | IC50 (μM) hMAO-B |
|---|---|---|---|
| 32 | 15.32 ± 1.02 | 52 | 21.35 ± 1.10 |
| 33 | 7.52 ± 1.05 | 53 | 17.10 ± 1.17 |
| 34 | 5.07 ± 1.25 | 54 | 4.20 ± 1.08 |
| 35 | 45.40 ± 1.30 | 55 | 78.22 ± 1.30 |
| 36 | 13.90 ± 1.30 | 56 | 151.6 ± 5.14 |
| 37 | 11.08 ± 1.20 | 57 | 45.42 ± 2.32 |
| 38 | 621.70 ± 1.8 | 58 | 512.6 ± 2.81 |
| 39 | 5.95 ± 1.28 | 59 | 41.8 ± 2.2 |
| 40 | 47.24 ± 1.12 | 60 | 21.80 ± 1.21 |
| 41 | 9.03 ± 1.07 | 61 | 3.94 ± 1.08 |
| 42 | 228.6 ± 1.26 | 62 | 113.5 ± 1.10 |
| 43 | 19.43 ± 1.19 | 63 | 210.8 ± 8.1 |
| 44 | 18.90 ± 1.01 | 64 | 10.31 ± 1.55 |
| 45 | * | 65 | 674.2 ± 1.72 |
| Deprenyl | 16.73 ± 1.48 | Safinamide | 23.07 ± 2.07 |
| Rasagiline | 49.66 ± 2.26 | Clorgyline | * |
| Code | R | IC50 (µM) | |
|---|---|---|---|
| MAO-A | MAO-B | ||
| 132 | C6H5 | 79.6 | 0.947 |
| 133 | C6H5CH2 | 77.9 | 0.638 |
| 134 | C6H5(CH2)2 | 101 | 0.897 |
| 135 | C6H5(CH2)3 | 312 | 1.43 |
| 136 | C6H5(CH2)4 | 155 | 142 |
| 137 | 4Cl-C6H5 | 72.1 | 3.08 |
| 138 | 4-Cl-C6H5cH2 | 288 | NI |
| 139 | 3-Cl-C6H5(CH2)2 | NI | NI |
| 140 | C5H4N | 73.1 | 38.7 |
| 141 | C5H4N(CH2)2 | 41.6 | 16.66 |
| Compound | R1 | R2 | R3 | R4 | TC50 (µM) MAO-A | IC50 (µM) MAO-B |
|---|---|---|---|---|---|---|
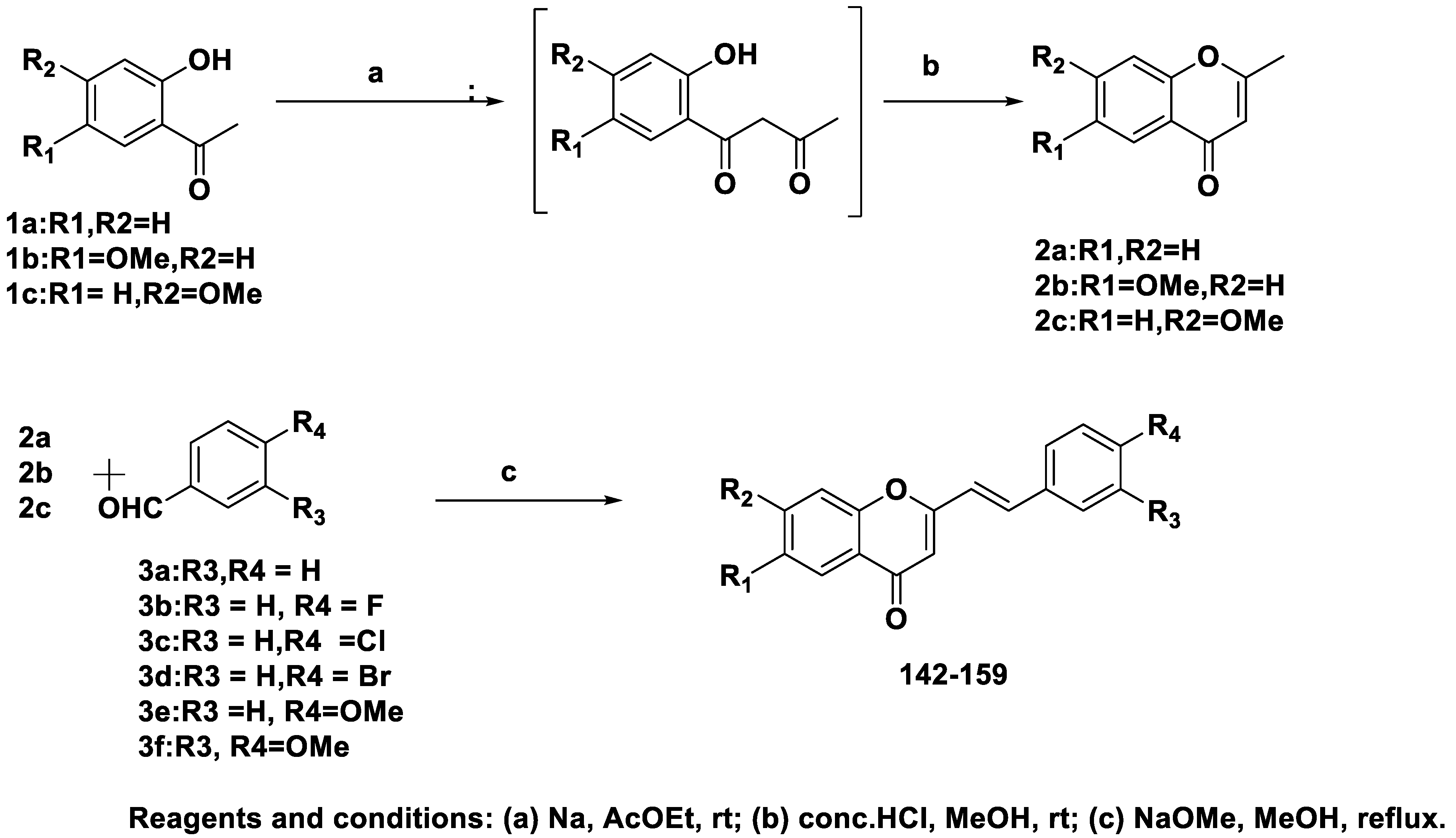
| 142 | H | H | H | H | 0.95 | 0.24 |
| 143 | H | H | H | F | 0.59 | 0.17 |
| 144 | H | H | H | Cl | 0.29 | 0.079 |
| 145 | H | H | H | Br | 0.33 | 0.069 |
| 146 | H | H | H | OMe | 2.3 | 0.049 |
| 147 | H | H | OMe | OMe | 25 | 2.8 |
| 148 | OMe | H | H | H | 0.20 | 0.18 |
| 149 | OMe | H | H | F | 0.12 | 0.064 |
| 150 | OMe | H | H | Cl | 26 | 0.017 |
| 151 | OMe | H | H | Br | 0.53 | 0.024 |
| 152 | OMe | H | H | OMe | 0.21 | 0.19 |
| 153 | OMe | H | OMe | OMe | 21 | 0.68 |
| 154 | H | OMe | H | H | 26 | 0.22 |
| 155 | H | OMe | H | F | 35 | 0.12 |
| 156 | H | OMe | H | Cl | >100 | 0.27 |
| 157 | H | OMe | H | Br | >100 | 0.45 |
| 158 | H | OMe | H | OMe | 72 | 1.4 |
| 159 | H | OMe | OMe | OMe | >100 | 10 |
| hMAO-A | hMAO-B | ||
|---|---|---|---|
| Compound | R | IC50 (µM) | IC50 (µM) |
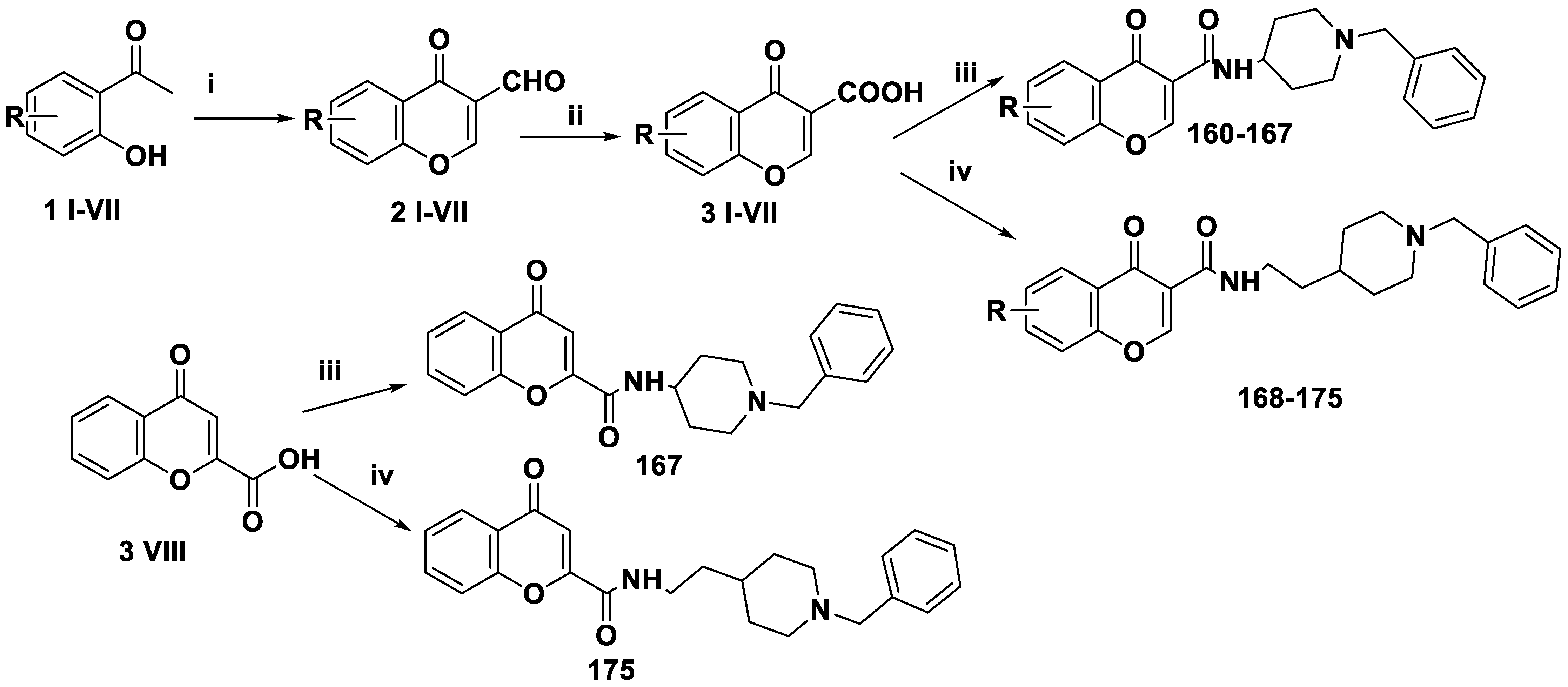
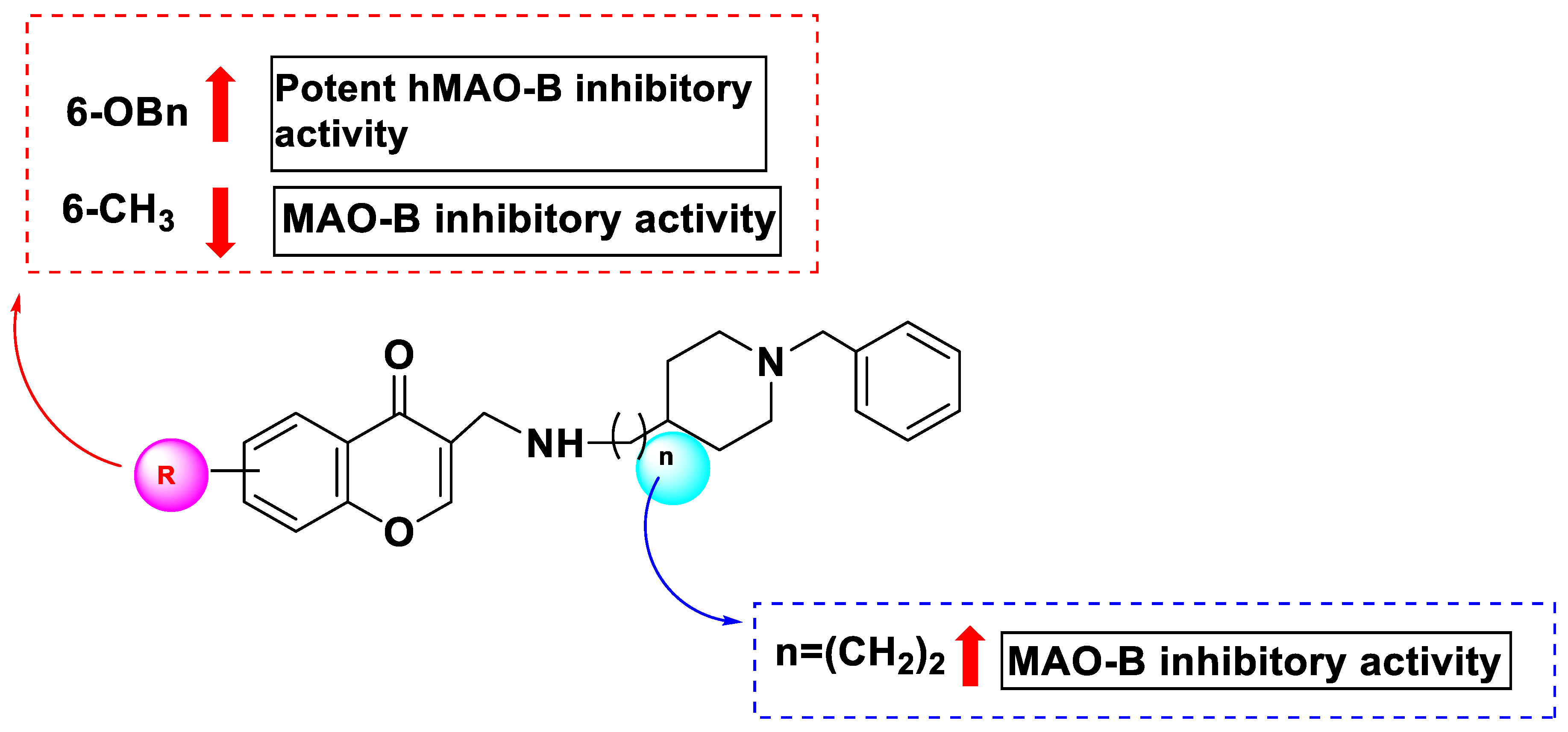
| 160 | H | 63.8 | 17.68 |
| 161 | 6-OCH3 | 7.5 | 46.27 |
| 162 | 6-OBn | 11.6 | 0.035 |
| 163 | 6 cH3 | 48.1 | 19.46 |
| 164 | 6-Br | 35.5 | 22.82 |
| 165 | 7-OCH3 | 37.4 | 28.64 |
| 166 | 7-Br | 31.6 | 19.73 |
| 167 | H | 29.7 | 33.29 |
| 168 | H | 68.6 | 35.29 |
| 169 | 6-OCH3 | 72.4 | 11.23 |
| 170 | 6-OBn | 67.2 | 0.272 |
| 171 | 6-CH3 | 30.6 | 52.45 |
| 172 | 6-Br | 48.2 | 26.13 |
| 173 | 7-OCH3 | 32.4 | 30.93 |
| 174 | 7-Br | 30.3 | 29.46 |
| 175 | H | 29.5 | 20.03 |
| Compound | R1 | R2 | IC50 (µM) | |
|---|---|---|---|---|
| MAO-A | MAO-B | |||
| 2-(N-Cyclic amino)chromone | ||||
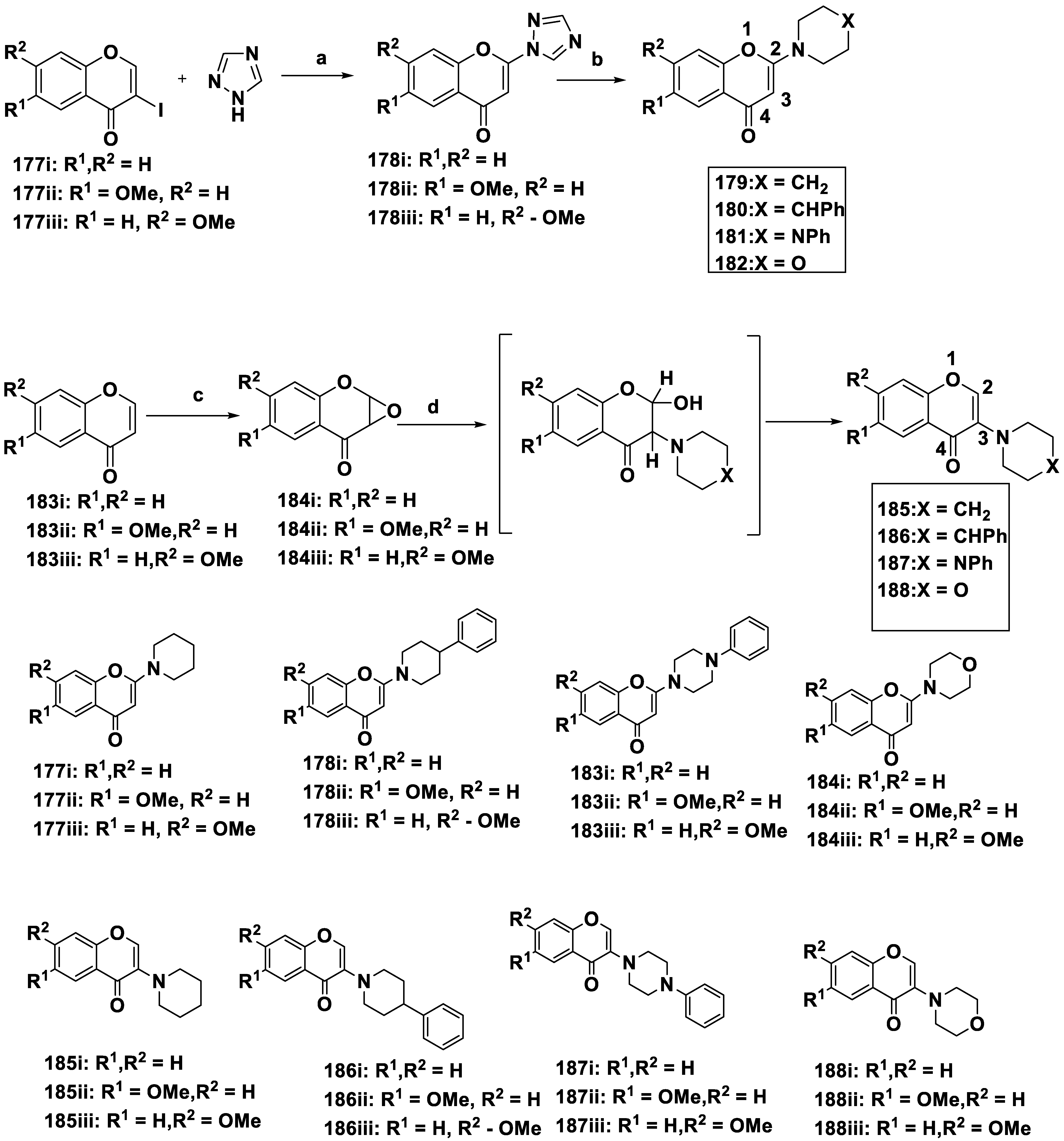
| 177i | H | H | 38 | 59 |
| 177ii | OMe | H | 16 | 27 |
| 177iii | H | OMe | 57 | 36 |
| 178i | H | H | 31 | 22 |
| 178ii | OMe | H | 4.1 | 5.6 |
| 178iii | H | OMe | 8.8 | 7.4 |
| 183i | H | H | 38 | 22 |
| 183ii | OMe | H | 2.6 | 2.8 |
| 183iii | H | OMe | 59 | 12 |
| 184i | H | H | 66 | 58 |
| 184ii | OMe | H | 36 | 40 |
| 184iii | H | OMe | 34 | 31 |
| 3-(N-Cyclic amino)chromone | ||||
| 185i | H | H | 23 | 2.0 |
| 185ii | OMe | H | 23 | 0.99 |
| 185iii | H | OMe | 18 | 14 |
| 186i | H | H | >100 | 1.5 |
| 186ii | OMe | H | >100 | >100 |
| 186iii | H | OMe | >100 | 0.25 |
| 187i | H | H | >100 | 0.72 |
| 187ii | OMe | H | >100 | >100 |
| 187iii | H | OMe | >100 | 0.015 |
| 188i | H | H | 57 | 23 |
| 188ii | OMe | H | 34 | 8.0 |
| 18Siii | H | OMe | 25 | 7.3 |
| Compound | R | IC50 (μM) hMAO-B |
|---|---|---|
| 213 | H | 0.0672 |
| 214 | 7-methyl | 0.0827 |
| 215 | 6-methyl | 0.0863 |
| 216 | 7-methoxy | 0.0670 |
| 217 | 6-methoxy | 0.0886 |
| Pargyline | 0.1113 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ipe, R.S.; Kumar, S.; Benny, F.; Jayan, J.; Manoharan, A.; Sudevan, S.T.; George, G.; Gahtori, P.; Kim, H.; Mathew, B. A Concise Review of the Recent Structural Explorations of Chromones as MAO-B Inhibitors: Update from 2017 to 2023. Pharmaceuticals 2023, 16, 1310. https://doi.org/10.3390/ph16091310
Ipe RS, Kumar S, Benny F, Jayan J, Manoharan A, Sudevan ST, George G, Gahtori P, Kim H, Mathew B. A Concise Review of the Recent Structural Explorations of Chromones as MAO-B Inhibitors: Update from 2017 to 2023. Pharmaceuticals. 2023; 16(9):1310. https://doi.org/10.3390/ph16091310
Chicago/Turabian StyleIpe, Reshma Susan, Sunil Kumar, Feba Benny, Jayalakshmi Jayan, Amritha Manoharan, Sachitra Thazhathuveedu Sudevan, Ginson George, Prashant Gahtori, Hoon Kim, and Bijo Mathew. 2023. "A Concise Review of the Recent Structural Explorations of Chromones as MAO-B Inhibitors: Update from 2017 to 2023" Pharmaceuticals 16, no. 9: 1310. https://doi.org/10.3390/ph16091310
APA StyleIpe, R. S., Kumar, S., Benny, F., Jayan, J., Manoharan, A., Sudevan, S. T., George, G., Gahtori, P., Kim, H., & Mathew, B. (2023). A Concise Review of the Recent Structural Explorations of Chromones as MAO-B Inhibitors: Update from 2017 to 2023. Pharmaceuticals, 16(9), 1310. https://doi.org/10.3390/ph16091310








