Natural Compounds of Fungal Origin with Antimicrobial Activity—Potential Cosmetics Applications
Abstract
:1. Introduction
2. Compounds of Fungal Origin with Antimicrobial Activity
2.1. Antibacterial Activity of Substances of Fungal Origin
2.1.1. Compounds of Fungal Origin with Antibacterial Activity
Key Antibacterial Compounds of Fungal Origin
- Penicillin
- Cephalosporin
- Fusidans
Selected Compounds of Fungal Origin from the Group of Isoprenoids, Peptides, and Acetylene Derivatives
- Isoprenoids
- Peptides
- Acetylene derivatives
Other Compounds of Fungal Origin with Antibacterial Activity
2.1.2. Extracts of Fungal Origin with Antibacterial Activity
2.2. Antifungal Activity of Substances of Fungal Origin
2.2.1. Compounds of Fungal Origin with Antifungal Activity
2.2.2. Extracts of Fungal Origin with Antifungal Activity
2.3. Extracts and Chemical Compounds of Fungal Origin with Antiviral Activity
3. Cosmetic Applications of Antimicrobial Fungal-Derived Compounds
3.1. Potential Practical Applications
3.2. Microbiological Factors Causing Skin Diseases
3.3. Selected Compounds of Fungal Origin with Cosmetic Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Deacon, J. Introduction: The Fungi and Fungal Activities. In Fungal Biology; Blackwell Pub: New York, NY, USA, 2006; pp. 1–15. ISBN 978-1-405-13066-0. [Google Scholar]
- Kohlmünzer, S. Farmakognozja. Podręcznik Dla Studentów Farmacji; Wydawnictwa Lekarskie PZWL: Warszawa, Poland, 1998. [Google Scholar]
- Chen, H.P.; Liu, J.K. Secondary Metabolites from Higher Fungi. Prog. Chem. Org. Nat. 2017, 106, 1–201. [Google Scholar]
- Suay, I.; Arenal, F.; Asensio, F.J.; Basilio, A.; Cabello, M.A.; Díez, M.T.; García, J.B.; González del Val, A.; Gorrochategui, J.; Hernández, P. Screening of basidiomycetes for antimicrobial activities. Anton. Leeuw. Int. J. G 2000, 78, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Jakubczyk, D.; Dussart, F. Selected fungal natural products with antimicrobial properties. Molecules 2020, 25, 911. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, K.; Verma, P. Fungal Metabolites: A Recent Trend and Its Potential Biotechnological Applications. In New and Future Developments in Microbial Biotechnology and Bioengineering: Recent Advances in Application of Fungi and Fungal Metabolites: Current Aspects; Elsevier: Amsterdam, The Netherlands, 2021; pp. 1–14. [Google Scholar]
- Di Hsu, K.; Cheng, K.C. From nutraceutical to clinical trial: Frontiers in Ganoderma development. Appl. Microbiol. Biotechnol. 2018, 102, 9037–9051. [Google Scholar] [CrossRef]
- Shah, R.A.; Hsu, J.I.; Patel, R.R.; Mui, U.N.; Tyring, S.K. Antibiotic resistance in dermatology: The scope of the problem and strategies to address It. J. Am. Acad. Dermatol. 2022, 86, 1337–1345. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, M.; Kumar, V.; Naik, B.; Verma, A.; Saris, P.E.J.; Kumar, V.; Gupta, S. Antifungal metabolites, their novel sources, and targets to combat drug resistance. Front. Microbiol. 2022, 13, 1061603. [Google Scholar] [CrossRef] [PubMed]
- Hyde, K.D.; Bahkali, A.H.; Moslem, M.A. Fungi—An unusual source for cosmetics. Fungal. Divers. 2010, 43, 1–9. [Google Scholar] [CrossRef]
- Wu, Y.; Choi, M.H.; Li, J.; Yang, H.; Shin, H.J. Mushroom cosmetics: The present and future. Cosmetics 2016, 3, 22. [Google Scholar] [CrossRef]
- Bowe, W.P.; Pugliese, S. Cosmetic benefits of natural ingredients. J. Drugs. Dermatol. 2014, 13, 1021–1025. [Google Scholar]
- Sharma, N.; Tapwal, A.; Verma, R.; Kumar, D.; Nepovimova, E.; Kuca, K. Medicinal, nutritional, and nutraceutical potential of Sparassis Crispa s. lat.: A review. IMA Fungus 2022, 13, 8. [Google Scholar] [CrossRef]
- Quack, W.; Anke, T.; Oberwinkler, F.; Giannetti, B.M.; Steglich, W. Antibiotics from Basidiomycetes. V. Merulidial, a new antibiotic from the basidiomycete Merulius tremellosus Fr. J. Antibiot. 1978, 31, 737–741. [Google Scholar] [CrossRef] [PubMed]
- Heim, J.; Anke, T.; Mocek, U.; Steffan, B.; Steglich, W. Antibiotics from Basidiomycetes. XXIX: Pilatin, a new antibiotically active marasmane derivative from cultures of Flagelloscypha pilatii Agerer. J. Antibiot. 1988, 41, 1752–1757. [Google Scholar] [CrossRef] [PubMed]
- Kupka, J.; Anke, T.; Giannetti, B.M.; Steglich, W. Antibiotics from Basidiomycetes. XIV. Isolation and biological characterization of hypnophilin, pleurotellol, and pleurotellic acid from Pleurotellus hypnophilus (Berk.) Sacc. Arch. Microbiol. 1981, 130, 223–227. [Google Scholar] [CrossRef]
- Stärk, A.; Anke, T.; Mocek, U.; Steglich, W.; Kirfel, A.; Will, G. Lentinellic acid, a biologically active protoilludane derivative from Lentinellus species (Basidiomycetes). Z Naturforsch. C J. Biosci. 1988, 43, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Obuchi, T.; Kondoh, H.; Watanabe, N.; Tamai, M.; Omura, S.; Jun-Shan, Y.; Xiao-Tian, L. Armillaric acid, a new antibiotic produced by Armillaria mellea. Planta. Med. 1990, 56, 198–201. [Google Scholar] [CrossRef]
- Tabuchi, A.; Fukushima-Sakuno, E.; Osaki-Oka, K.; Futamura, Y.; Motoyama, T.; Osada, H.; Ishikawa, N.K.; Nagasawa, E.; Tokimoto, K. Productivity and bioactivity of enokipodins A–D of Flammulina rossica and Flammulina velutipes. Biosci. Biotechnol. Biochem. 2020, 84, 876–886. [Google Scholar] [CrossRef] [PubMed]
- Novak, R.; Shlaes, D.M. The pleuromutilin antibiotics: A new class for human use. Curr Opin Investig Drugs 2010, 11, 182–191. [Google Scholar]
- Hofle, G.; Oberwinkler, F. The striatins-new antibiotics from the Basidiomycete Cyathus striatus (Huds. Ex Pers.) Willd. J. Antibiot. 1977, 30, 221–225. [Google Scholar]
- Šiljegović, J.D.; Stojković, D.S.; Nikolić, M.M.; Glamočlija, J.M.; Soković, M.D.; Ćirić, A.M. Antimicrobial activity of aqueous extract of Laetiporus sulphureus (Bull.: Fr.) Murill. Zb Matice. Srp. Prir. Nauk. 2011, 299–305. [Google Scholar] [CrossRef]
- Ma, B.; Ren, W.; Zhou, Y.; Ma, J.; Ruan, Y.; Wen, C.N. Triterpenoids from the spores of Ganoderma lucidum. N. Am. J. Med. Sci. 2011, 3, 495–498. [Google Scholar] [CrossRef]
- Mothana, R.A.A.; Jansen, R.; Jülich, W.D.; Lindequist, U. Ganomycins A and B, new antimicrobial farnesyl hydroquinones from the Basidiomycete Ganoderma pfeifferi. J. Nat. Prod. 2000, 63, 416–418. [Google Scholar] [CrossRef] [PubMed]
- Anke, T.; Kupka, J.; Schramm, G.; Steglich, W. Antibiotics from Basidiomycetes. X. Scorodonin, a new antibacterial and antifungal metabolite from Marasmius scorodonius (Fr.) Fr. J. Antibiot. 1980, 33, 463–467. [Google Scholar] [CrossRef]
- Clardy, J.; Fischbach, M.A.; Currie, C.R. The natural history of antibiotics. Curr. Biol. 2009, 19, R437–R441. [Google Scholar] [CrossRef] [PubMed]
- Hutchings, M.; Truman, A.; Wilkinson, B. Antibiotics: Past, present and future. Curr. Opin. Microbiol. 2019, 51, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Dutta, B.; Lahiri, D.; Nag, M.; Ghosh, S.; Dey, A.; Ray, R.R. Fungi in pharmaceuticals and production of antibiotics. In Applied Mycology: Entrepreneurship with Fungi; Springer International Publishing: Cham, Germany, 2022; pp. 233–257. [Google Scholar]
- Conrado, R.; Gomes, T.C.; Roque, G.S.C.; De Souza, A.O. Overview of bioactive fungal secondary metabolites: Cytotoxic and antimicrobial compounds. Antibiotics 2022, 11, 1604. [Google Scholar] [CrossRef] [PubMed]
- Bush, K.; Bradford, P.A. β-lactams and β-lactamase inhibitors: An overview. Cold Spring Harb. Perspect. Med. 2016, 6, a025247. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.L. The penicillins: A review and update. J. Midwifery Womens Health 2002, 47, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Bo, G. Giuseppe Brotzu and the discovery of cephalosporins. Clin. Microbiol. Infect. 2000, 6, 6–8. [Google Scholar] [CrossRef]
- Fernandez, J.; Jimenez-Rodriguez, T.W.; Blanca-Lopez, N. Classifying Cephalosporins: From generation to cross-reactivity. Curr. Opin. Allergy Clin. Immunol. 2021, 21, 346–354. [Google Scholar] [CrossRef]
- Khan, D.A.; Banerji, A.; Bernstein, J.A.; Bilgicer, B.; Blumenthal, K.; Castells, M.; Ein, D.; Lang, D.M.; Phillips, E. Cephalosporin allergy: Current understanding and future challenges. J. Allergy Clin. Immunol. Pract. 2019, 7, 2105–2114. [Google Scholar] [CrossRef]
- Harrison, C.J.; Bratcher, D. Cephalosporins: A review. Pediatr. Rev. 2008, 29, 264–273. [Google Scholar] [CrossRef]
- Cordes, M.G. Fusidic Acid. In xPharm: The Comprehensive Pharmacology Reference; Elsevier: Amsterdam, The Netherlands, 2007; pp. 1–4. [Google Scholar]
- Schöfer, H.; Simonsen, L. Fusidic acid in dermatology: An updated review. Eur. J. Dermatol. 2010, 20, 006–015. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Ji, W.; Zhang, D.; Zhu, Y.; Bi, Y. Bioactivities and structure-activity relationships of fusidic acid derivatives: A review. Front. Pharmacol. 2021, 12, 759220. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, P. Fusidic Acid: A bacterial elongation factor inhibitor for the oral treatment of acute and chronic staphylococcal infections. Cold Spring Harb. Perspect. Med. 2016, 6, a025437. [Google Scholar] [CrossRef] [PubMed]
- Levy, D.; Bluzat, A.; Seigneuret, M.; Rigaud, J.L. Alkali cation transport through liposomes by the antimicrobial fusafungine and its constitutive enniatins. Biochem. Pharmacol. 1995, 50, 2105–2107. [Google Scholar] [CrossRef] [PubMed]
- Lund, V.J.; Grouin, J.M.; Eccles, R.; Bouter, C.; Chabolle, F. Efficacy of fusafungine in acute rhinopharyngitis: A pooled analysis. Rhinology 2004, 42, 207–212. [Google Scholar] [PubMed]
- Tschen, J.S.M.; Chen, L.L.; Hsieh, S.T.; Wu, T.S. Isolation and phytotoxic effects of helvolic acid from plant pathogenic fungus Sarocladium Oryzae. Bot. Bull. Acad. Sin. 1997, 38, 251–256. [Google Scholar]
- Savidov, N.; Gloriozova, T.A.; Poroikov, V.V.; Dembitsky, V.M. Highly oxygenated isoprenoid lipids derived from fungi and fungal endophytes: Origin and biological activities. Steroids 2018, 140, 114–124. [Google Scholar] [CrossRef]
- Ninkuu, V.; Zhang, L.; Yan, J.; Fu, Z.; Yang, T.; Zeng, H. Biochemistry of terpenes and recent advances in plant protection. Int. J. Mol. Sci. 2021, 22, 5710. [Google Scholar] [CrossRef]
- Anke, H.; Sterner, O.; Steglich, W. Structure-activity relationships for unsaturated dialdehydes. Mutagenic, antimicrobial, cytotoxic, and phytotoxic activities of merulidial derivatives. J. Antibiot. 1989, 42, 738–744. [Google Scholar] [CrossRef]
- Mehta, G.; Murthy, A.S.K. The first total synthesis of the novel triquinane natural products pleurotellol and pleurotellic acid. Tetrahedron. Lett. 2003, 44, 5243–5246. [Google Scholar] [CrossRef]
- Cadelis, M.M.; Copp, B.R.; Wiles, S. A Review of fungal protoilludane sesquiterpenoid natural products. Antibiotics 2020, 9, 928. [Google Scholar] [CrossRef] [PubMed]
- Midland, S.L.; Izac, R.R.; Wing, R.M.; Zaki, A.I.; Munnecke, D.E.; Sims, J.J. Melleolide, a new antibiotic from Armillaria mellea. Tetrahedron. Lett. 1982, 23, 2515–2518. [Google Scholar] [CrossRef]
- Ishikawa, N.K.; Fukushi, Y.; Yamaji, K.; Tahara, S.; Takahashi, K. Antimicrobial cuparene-type sesquiterpenes, enokipodins c and d, from a mycelial culture of Flammulina velutipes. J. Nat. Prod. 2001, 64, 932–934. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.T.; Winkler, A.L.; Schwan, W.R.; Volk, T.J.; Rott, M.A.; Monte, A. Antibacterial Compounds from Mushrooms I: A Lanostane-type triterpene and prenylphenol derivatives from Jahnoporus hirtus and Albatrellus flettii and their activities against Bacillus cereus and Enterococcus faecalis. Planta. Med. 2010, 76, 182–185. [Google Scholar] [CrossRef] [PubMed]
- Mygind, P.H.; Fischer, R.L.; Schnorr, K.M.; Hansen, M.T.; Sönksen, C.P.; Ludvigsen, S.; Raventós, D.; Buskov, S.; Christensen, B.; De Maria, L. Plectasin is a peptide antibiotic with therapeutic potential from a saprophytic fungus. Nature 2005, 437, 975–980. [Google Scholar] [CrossRef] [PubMed]
- Brinch, K.S.; Sandberg, A.; Baudoux, P.; Van Bambeke, F.; Tulkens, P.M.; Frimodt-Møller, N.; Høiby, N.; Kristensen, H.H. Plectasin shows intracellular activity against Staphylococcus aureus in Human THP-1 monocytes and in a mouse peritonitis model. Antimicrob. Agents Chemother. 2009, 53, 4801–4808. [Google Scholar] [CrossRef] [PubMed]
- Raap, J.; Erkelens, K.; Ogrel, A.; Skladnev, D.A.; Brückner, H. Fungal biosynthesis of non-ribosomal peptide antibiotics and α, α-dialkylated amino acid constituents. J. Pept. Sci. 2005, 11, 331–338. [Google Scholar] [CrossRef]
- Rossi, C.; Eid, M.; Rippa, S.; Castano, S.; Desbat, B.; Chopineau, J.; Béven, L. Exploring the membrane mechanism of the bioactive peptaibol ampullosporin a using lipid monolayers and supported biomimetic membranes. J. Biophys. 2010, 2010, 179641. [Google Scholar]
- Lee, S.J.; Yeo, W.H.; Yun, B.S.; Yoo, I.D. Isolation and sequence analysis of new peptaibol, boletusin, from Boletus spp. J. Pept. Sci. 1999, 5, 374–378. [Google Scholar] [CrossRef]
- Isaac, C.E.; Jones, A.; Pickard, M.A. Production of cyclosporins by Tylypocladium niveum strains. Antimicrob. Agents Chemother. 1990, 34, 121–127. [Google Scholar] [CrossRef]
- Jonsson, M. The Toxicity of Fusarium Mycotoxins Enniatin and Moniliformin; University of Helsinki: Helsinki, Finland, 2017; ISBN 978-952-225-164-0. [Google Scholar]
- McMorris, T.C.; Turner, W.B. Fungal metabolites. Mycologia 1972, 64, 464. [Google Scholar] [CrossRef]
- Ngai, P.H.K.; Ng, T.B. A ribonuclease with antimicrobial, antimitogenic and antiproliferative activities from the edible mushroom Pleurotus sajor-caju. Peptides 2004, 25, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Garcia, P.; De Oliveira, A.; Batista, R. Occurrence, biological activities and synthesis of kaurane diterpenes and their glycosides. Molecules 2007, 12, 455–483. [Google Scholar] [CrossRef] [PubMed]
- Aqueveque, P.; Becerra, J.; Palfner, G.; Silva, M.; Alarcon, J.; Anke, T.; Sterner, O. Antimicrobial activity of metabolites from mycelial cultures of Chilean Basidiomycetes. J. Chil. Chem. Soc. 2006, 51, 1057–1060. [Google Scholar] [CrossRef]
- Sandargo, B.; Thongbai, B.; Praditya, D.; Steinmann, E.; Stadler, M.; Surup, F. Antiviral 4-hydroxypleurogrisein and antimicrobial pleurotin derivatives from cultures of the nematophagous Basidiomycete Hohenbuehelia grisea. Molecules 2018, 23, 2697. [Google Scholar] [CrossRef] [PubMed]
- Bender, S.; Dumitrache-Anghel, C.N.; Backhaus, J.; Christie, G.; Cross, R.F.; Lonergan, G.T.; Baker, W.L. A case for caution in assessing the antibiotic activity of extracts of culinary-medicinal shiitake mushroom [Lentinus edodes (Berk.) Singer] (Agaricomycetideae). Int. J. Med. Mushrooms. 2003, 5, 6. [Google Scholar] [CrossRef]
- Palmieri, F.; Estoppey, A.; House, G.L.; Lohberger, A.; Bindschedler, S.; Chain, P.S.G.; Junier, P. Oxalic Acid, a Molecule at the Crossroads of Bacterial-Fungal Interactions. Adv. Appl. Microbiol. 2019, 106, 49–77. [Google Scholar] [PubMed]
- Quang, D.N.; Bach, D.D.; Hashimoto, T.; Asakawa, Y. Chemical constituents of the vietnamese inedible mushroom Xylaria intracolorata. Nat. Prod. Res. 2006, 20, 317–321. [Google Scholar] [CrossRef]
- Beattie, K.D.; Rouf, R.; Gander, L.; May, T.W.; Ratkowsky, D.; Donner, C.D.; Gill, M.; Grice, I.D.; Tiralongo, E. Antibacterial metabolites from australian macrofungi from the genus Cortinarius. Phytochemistry 2010, 71, 948–955. [Google Scholar] [CrossRef]
- Schwan, W.R.; Dunek, C.; Gebhardt, M.; Engelbrecht, K.; Klett, T.; Monte, A.; Toce, J.; Rott, M.; Volk, T.J.; LiPuma, J.J. Screening a mushroom extract library for activity against Acinetobacter baumannii and Burkholderia cepacia and the identification of a compound with anti-burkholderia activity. Ann. Clin. Microbiol. Antimicrob. 2010, 9, 4. [Google Scholar] [CrossRef] [PubMed]
- No, H.K.; Park, N.Y.; Lee, S.H.; Meyers, S.P. Antibacterial activity of chitosans and chitosan oligomers with different molecular weights. Int. J. Food Microbiol. 2002, 74, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Coma, V.; Deschamps, A.; Martial-Gros, A. Bioactive packaging materials from edible chitosan polymer—Antimicrobial activity assessment on dairy-related contaminants. J. Food Sci. 2003, 68, 2788–2792. [Google Scholar] [CrossRef]
- Kumar, M. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Jeihanipour, A.; Keikhosro, K.; Taherzadeh, M. Antimicrobial Properties of Fungal Chitosan. Res. J. Biol. Sci. 2007, 2, 239–243. [Google Scholar]
- Hosseinnejad, M.; Jafari, S.M. Evaluation of different factors affecting antimicrobial properties of chitosan. Int. J. Biol. Macromol. 2016, 85, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Paterson, R.R.M. Ganoderma—A therapeutic fungal biofactory. Phytochemistry 2006, 67, 1985–2001. [Google Scholar] [CrossRef]
- Quereshi, S.; Pandey, A.K.; Sandhu, S.S. Evaluation of antibacterial activity of different Ganoderma lucidum extracts. PJSR 2010, 3, 9–13. [Google Scholar]
- Yoon, S.Y.; Eo, S.K.; Kim, Y.S.; Lee, C.K.; Han, S.S. Antimicrobial activity of Ganoderma lucidum extract alone and in combination with some antibiotics. Arch. Pharm. Res. 1994, 17, 438–442. [Google Scholar] [CrossRef]
- Yamaç, M.; Bilgili, F. Antimicrobial activities of fruit bodies and/or mycelial cultures of some mushroom isolates. Pharm. Biol. 2006, 44, 660–667. [Google Scholar] [CrossRef]
- Barros, L.; Venturini, B.A.; Baptista, P.; Estevinho, L.M.; Ferreira, I.C.F.R. Chemical composition and biological properties of Portuguese wild mushrooms: A comprehensive study. J. Agric. Food Chem. 2008, 56, 3856–3862. [Google Scholar] [CrossRef] [PubMed]
- Turkoglu, A.; Duru, M.E.; Mercan, N.; Kivrak, I.; Gezer, K. Antioxidant and antimicrobial activities of Laetiporus sulphureus (Bull.) Murrill. Food Chem. 2006, 101, 267–273. [Google Scholar] [CrossRef]
- Hirasawa, M.; Shouji, N.; Neta, T.; Fukushima, K.; Takada, K. Three kinds of antibacterial substances from Lentinus edodes (Berk.) Sing. (Shiitake, an Edible Mushroom). Int. J. Antimicrob. Agents 1999, 11, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Signoretto, C.; Burlacchini, G.; Marchi, A.; Grillenzoni, M.; Cavalleri, G.; Ciric, L.; Lingström, P.; Pezzati, E.; Daglia, M.; Zaura, E. Testing a low molecular mass fraction of a mushroom (Lentinus edodes) extract formulated as an oral rinse in a cohort of volunteers. J. Biomed. Biotechnol. 2011, 2011, 857987. [Google Scholar] [CrossRef]
- Barros, L.; Baptista, P.; Estevinho, L.M.; Ferreira, I.C.F.R. Bioactive properties of the medicinal mushroom Leucopaxillus giganteus mycelium obtained in the presence of different nitrogen sources. Food Chem. 2007, 105, 179–186. [Google Scholar] [CrossRef]
- Sheena, N.; Ajith, T.A.; Mathew, A.; Janardhanan, K.K. Antibacterial activity of three macrofungi, Ganoderma lucidum, Navesporus floccosa and Phellinus rimosus occurring in South India. Pharm. Biol. 2003, 41, 564–567. [Google Scholar] [CrossRef]
- Kalyoncu, F.; Oskay, M.; Sağlam, H.; Erdoğan, T.F.; Tamer, A.U. Antimicrobial and antioxidant activities of mycelia of 10 wild mushroom species. J. Med. Food 2010, 13, 415–419. [Google Scholar] [CrossRef]
- Klaus, A.; Kozarski, M.; Vunduk, J.; Todorovic, N.; Jakovljevic, D.; Zizak, Z.; Pavlovic, V.; Levic, S.; Niksic, M.; Van Griensven, L.J.L.D. Biological potential of extracts of the wild edible basidiomycete mushroom Grifola frondosa. Food Res. Int. 2015, 67, 272–283. [Google Scholar] [CrossRef]
- Rosa, L.H.; Gomes Machado, K.M.; Jacob, C.; Capelari, M.; Rosa, C.A.; Zani, C.L. Screening of Brazilian Basidiomycetes for antimicrobial activity. Mem. Inst. Oswaldo. Cruz. 2003, 98, 967–974. [Google Scholar] [CrossRef]
- Tambekar, D.H.; Sonar, T.P.; Khodke, M.V.; Khante, B.S. The novel antibacterials from two edible mushrooms: Agaricus bisporus and Pleurotus sajor -caju. Int. J. Pharmacol. 2006, 2, 584–587. [Google Scholar]
- Ozen, T.; Darcan, C.; Aktop, O.; Turkekul, I. Screening of antioxidant, antimicrobial activities and chemical contents of edible mushrooms wildly grown in the Black Sea region of Turkey. Comb. Chem. High Throughput. Screen. 2011, 14, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Dulger, B.; Ergul, C.C.; Gucin, F. Antimicrobial activity of the macrofungus Lepista nuda. Fitoterapia 2002, 73, 695–697. [Google Scholar] [CrossRef] [PubMed]
- Alves, M.; Ferreira, I.F.R.; Dias, J.; Teixeira, V.; Martins, A.; Pintado, M. A review on antimicrobial activity of mushroom (Basidiomycetes) extracts and isolated compounds. Planta. Med. 2012, 78, 1707–1718. [Google Scholar] [CrossRef] [PubMed]
- Petrović, J.; Stojković, D.; Reis, F.S.; Barros, L.; Glamočlija, J.; Ćirić, A.; Ferreira, I.C.F.R.; Soković, M. Study on chemical, bioactive and food preserving properties of Laetiporus sulphureus (Bull.: Fr.) Murr. Food Funct. 2014, 5, 1441–1451. [Google Scholar] [CrossRef] [PubMed]
- Hleba, L. Antimicrobial activity of crude methanolic extracts from Ganoderma lucidum and Trametes versicolor. Anim. Sci. Biotechnol. 2014, 47, 89–93. [Google Scholar]
- Kontoyiannis, D.P.; Lewis, R.E. Antifungal drug resistance of pathogenic fungi. Lancet 2002, 359, 1135–1144. [Google Scholar] [CrossRef] [PubMed]
- Kuklev, D.V.; Domb, A.J.; Dembitsky, V.W. Bioactive acetylenic metabolites. Phytomedicine 2013, 20, 1145–1159. [Google Scholar] [CrossRef]
- Develoux, M. Griseofulvin. Ann. Dermatol. Venereol. 2001, 128, 1317–1325. [Google Scholar]
- Muralidhar, R.; Gummadi, S.N.; Dasu, V.V.; Panda, T. Statistical analysis on some critical parameters affecting the formation of protoplasts from the mycelium of Penicillium griseofulvum. Biochem. Eng. J. 2003, 3, 229–235. [Google Scholar] [CrossRef]
- Ngai, P.H.K.; Zhao, Z.; Ng, T.B. Agrocybin, an antifungal peptide from the edible mushroom Agrocybe cylindracea. Peptides 2005, 26, 191–196. [Google Scholar] [CrossRef]
- Wong, J.H.; Ng, T.B.; Wang, H.; Sze, S.C.W.; Zhang, K.Y.; Li, Q.; Lu, X. Cordymin, an antifungal peptide from the medicinal fungus Cordyceps militaris. Phytomedicine 2011, 18, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.Q.; Shao, H.J.; Zhu, H.J.; Liu, J.K. Activity in Vitro and in Vivo against Plant Pathogenic Fungi of Grifolin Isolated from the Basidiomycete Albatrellus Dispansus. Z. Naturforsch. C. J. Biosci. 2005, 60, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.J.; Li, L.L.; Deng, Q.P.; Yu, X.F.; Yang, L.F.; Luo, F.J.; Xiao, L.B.; Chen, X.Y.; Ye, M.; Liu, J.K. Grifolin, a potent antitumour natural product upregulates death-associated protein kinase 1 DAPK1 via P53 in nasopharyngeal carcinoma cells. Eur. J. Cancer 2011, 47, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Alves, M.; Ferreira, I.; Dias, J.; Teixeira, V.; Martins, A.; Pintado, M. A Review on antifungal activity of mushroom (Basidiomycetes) extracts and isolated compounds. Curr. Top Med. Chem. 2013, 13, 2648–2659. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.Q.; Wang, F.; Bian, X.Y.; Liu, J.K. Rufuslactone, a new antifungal sesquiterpene from the fruiting bodies of the Basidiomycete Lactarius rufus. J. Antibiot. 2005, 58, 456–459. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, H.; Ng, T.B. Isolation of trichogin, an antifungal protein from fresh fruiting bodies of the edible mushroom Tricholoma giganteum. Peptides 2005, 26, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, S.; Kawai, K.; Yamada, S.; Sawai, Y. Isolation of oospolactone as antifungal principle of Gloeophyllum sepiarium. Agric. Biol. Chem. 2014, 40, 811–812. [Google Scholar]
- Ngai, P.H.K.; Ng, T.B. Lentin, a novel and potent antifungal protein from shitake mushroom with inhibitory effects on activity of human immunodeficiency virus-1 reverse transcriptase and proliferation of leukemia cells. Life Sci. 2003, 73, 3363–3374. [Google Scholar] [CrossRef]
- Gilardoni, G.; Clericuzio, M.; Tosi, S.; Zanoni, G.; Vidari, G. Antifungal acylcyclopentenediones from fruiting bodies of Hygrophorus chrysodon. J. Nat. Prod. 2007, 70, 137–139. [Google Scholar] [CrossRef]
- Smania, E.F.; Delle Monache, F.; Smania, A.; Yunes, R.A.; Cuneo, R.S. Antifungal activity of sterols and triterpenes isolated from Ganoderma annulare. Fitoterapia 2003, 74, 375–377. [Google Scholar] [CrossRef]
- Wang, H.; Ng, T.B. Eryngin, a novel antifungal peptide from fruiting bodies of the edible mushroom Pleurotus eryngii. Peptides 2004, 25, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Min, B.; Kim, S.; Oh, Y.L.; Kong, W.S.; Park, H.; Cho, H.; Jang, K.Y.; Kim, J.G.; Choi, I.G. Genomic discovery of the hypsin gene and biosynthetic pathways for terpenoids in Hypsizygus marmoreus. BMC Genom. 2018, 19, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Steglich, W.; Schramm, G. The strobilurins—New antifungal antibiotics from the Basidiomycete Strobilurus tenacellus. J. Antibiot. 1977, 30, 806–810. [Google Scholar]
- Galappaththi, M.C.A.; Patabendige, N.M.; Premarathne, B.M.; Hapuarachchi, K.K.; Tibpromma, S.; Dai, D.Q.; Suwannarach, N.; Rapior, S.; Karunarathna, S.C. A Review of Ganoderma triterpenoids and their bioactivities. Biomolecules 2022, 13, 24. [Google Scholar] [CrossRef]
- Sultan, S. The antifungal activity of aqueous and alcoholic extract of mushroom (Agaricus Bisporus) against Aspergillus flavus. In Proceedings of the 1st International Multi-Disciplinary Conference Theme: Sustainable Development and Smart Planning, IMDC-SDSP, Online, 28–30 June 2020. [Google Scholar]
- Sangdee, A.; Sangdee, K.; Buranrat, B.; Thammawat, S. Effects of mycelial extract and crude protein of the medicinal mushroom, Ophiocordyceps sobolifera, on the pathogenic fungus, Candida albicans. Trop. Pharm. Res. 2018, 17, 2449–2454. [Google Scholar] [CrossRef]
- Öztürk, M.; Duru, M.E.; Kivrak, Ş.; Mercan-Doĝan, N.; Türkoglu, A.; Özler, M.A. In vitro antioxidant, anticholinesterase and antimicrobial activity studies on three Agaricus species with fatty acid compositions and iron contents: A comparative study on the three most edible mushrooms. Food Chem.Toxicol. 2011, 49, 1353–1360. [Google Scholar] [CrossRef] [PubMed]
- Pârvu, M.; Andrei, A.Ş.; Roşca-Casian, O. Antifungal activity of Laetiporus sulphureus mushroom extract. Contrib. Bot. 2010, 45, 2015. [Google Scholar]
- Sułkowska-Ziaja, K.; Muszyńska, B.; Gawalska, A.; Sałaciak, K. Laetiporus sulphureus—Chemical composition and medicinal value. Acta. Sci. Pol. Hortorum. Cultus. 2018, 17, 87–96. [Google Scholar] [CrossRef]
- Barros, L.; Baptista, P.; Estevinho, L.; Ferreira, I. Effect of fruiting body maturity stage on chemical composition and antimicrobial activity of Lactarius sp. mushrooms. J. Agric. Food Chem. 2007, 55, 8766–8771. [Google Scholar] [CrossRef]
- Linnakoski, R.; Reshamwala, D.; Veteli, P.; Cortina-Escribano, M.; Vanhanen, H.; Marjomäki, V. Antiviral agents from fungi: Diversity, mechanisms and potential applications. Front. Microbiol. 2018, 9, 2325. [Google Scholar] [CrossRef]
- Mothana, R.A.A.; Awadh Ali, N.A.; Jansen, R.; Wegner, U.; Mentel, R.; Lindequist, U. Antiviral lanostanoid triterpenes from the fungus Ganoderma pfeifferi. Fitoterapia 2003, 74, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.A.A.; Mothana, R.A.A.; Lesnau, A.; Pilgrim, H.; Lindequist, U. Antiviral activity of Inonotus hispidus. Fitoterapia 2003, 74, 483–485. [Google Scholar]
- Tochikura, T.S.; Nakashima, H.; Hirose, K.; Yamamoto, N. A biological response modifier, PSK, inhibits human immunodeficiency virus infection in vitro. Biochem. Biophys. Res. Commun. 1987, 148, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Cardozo, F.T.; Camelini, C.M.; Mascarello, A.; Rossi, M.J.; Nunes, R.J.; Barardi, C.R. Antiherpetic activity of a sulfated polysaccharide from Agaricus brasiliensis mycelia. Antiviral. Res. 2011, 92, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Yan, N.; He, F.; Piraino, F.F.; Xiang, H.; Chen, J.; Wang, Y. Antiviral activity of a cloned peptide rc28 isolated from the higher basidiomycetes mushroom Rozites caperata in a mouse model of HSV-1 keratitis. Int. J. Med. Mushrooms 2015, 17, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Vlasenko, V.A.; Ilyicheva, T.N.; Teplyakova, T.V.; Svyatchenko, S.V.; Asbaganov, S.V.; Zmitrovich, I.V. Antiviral activity of total polysaccharide fraction of water and ethanol extracts of Pleurotus pulmonarius against the influenza A virus. Curr. Res. Environ. Appl. Mycol. 2020, 10, 224–235. [Google Scholar] [CrossRef]
- Ohta, Y.; Lee, J.B.; Hayashi, K.; Fujita, A.; Dong, K.P.; Hayashi, T. In vivo anti-influenza virus activity of an immunomodulatory acidic polysaccharide isolated from Cordyceps militaris grown on germinated soybeans. J. Agric. Food Chem. 2007, 55, 10194–10199. [Google Scholar] [CrossRef]
- Mlinaric, A.; Kac, J.; Pohleven, F. Screening of selected wood-damaging fungi for the HIV-1 reverse transcriptase inhibitors. Acta. Pharm. 2005, 55, 69–79. [Google Scholar]
- Faccin, L.C.; Benati, F.; Rincão, V.P.; Mantovani, M.S.; Soares, S.A.; Gonzaga, M.L. Antiviral activity of aqueous and ethanol extracts and of an isolated polysaccharide from Agaricus brasiliensis against poliovirus type 1. Lett. Appl. Microbiol. 2007, 45, 24–28. [Google Scholar] [CrossRef]
- Lee, S.M.; Kim, S.M.; Lee, Y.H.; Kim, W.J.; Park, J.K.; Park, Y.I. Macromolecules isolated from Phellinus pini fruiting body: Chemical characterization and antiviral activity. Macromol. Res. 2010, 18, 602–609. [Google Scholar] [CrossRef]
- Hwang, B.S.; Lee, I.K.; Choi, H.J.; Yun, B.S. Anti-influenza activities of polyphenols from the medicinal mushroom Phellinus baumii. Bioorg. Med. Chem. Lett. 2015, 25, 3256–3260. [Google Scholar] [CrossRef]
- Seo, D.J.; Choi, C. Antiviral bioactive compounds of mushrooms and their antiviral mechanisms: A review. Viruses 2021, 23, 350. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Li, X.; Hao, C.; Zeng, P.; Zhang, M.; Liu, Y. Grifola frondosa polysaccharide: A review of antitumor and other biological activity studies in China. Discov. Med. 2018, 25, 159–176. [Google Scholar]
- Gu, C.Q.; Li, J.; Chao, F.H. Inhibition of hepatitis B virus by D-fraction from Grifola frondosa: Synergistic effect of combination with interferon-alpha in HepG2 2.2.15. Antiviral. Res. 2006, 72, 162–165. [Google Scholar] [CrossRef]
- Ren, G.; Xu, L.; Lu, T.; Yin, J. Structural characterization and antiviral activity of lentinan from Lentinus edodes mycelia against infectious hematopoietic necrosis virus. Int. J. Biol. Macromol. 2018, 115, 1202–1210. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, F.; Anderson, D.; Najafzadeh, M. The antiviral, anti-inflammatory effects of natural medicinal herbs and mushrooms and SARS-CoV-2 infection. Nutrients 2020, 12, 2573. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Chen, H.; Dong, P.; Lu, X. Anti-inflammatory and anticancer activities of extracts and compounds from the mushroom Inonotus obliquus. Food Chem. 2013, 139, 503–508. [Google Scholar] [CrossRef]
- Shibnev, V.A.; Mishin, D.V.; Garaev, T.M.; Finogenova, N.P.; Botikov, A.G.; Deryabin, P.G. Antiviral activity of inonotus obliquus fungus extract towards infection caused by hepatitis C virus in cell cultures. Bull. Exp. Biol. Med. 2011, 151, 612–614. [Google Scholar] [CrossRef]
- Ershova, E.I.; Tikhonova, O.V.; Lur’e, L.M.; Efremenkova, O.V.; Kamzolkina, O.V.; Dudnik, I.V. Antimicrobial activity of Laetiporus sulphureus strains grown in submerged culture. Antibiot. Khimioter. 2003, 48, 18–22. [Google Scholar]
- Yang, S.S.; Cheng, M.J.; Chan, H.Y.; Hsieh, S.Y.; Wu, H.C.; Yuan, G.F. New secondary metabolites from an endophytic fungus in Porodaedalea pini. Rec. Nat. Prod. 2017, 11, 251–257. [Google Scholar]
- Taofiq, O.; González-Paramás, A.M.; Martins, A.; Barreiro, M.F.; Ferreira, I.C.F.R. Mushrooms extracts and compounds in cosmetics, cosmeceuticals and nutricosmetics—A review. Ind. Crops. Prod. 2016, 90, 38–48. [Google Scholar] [CrossRef]
- Taofiq, O.; Heleno, S.A.; Calhelha, R.C.; Alves, M.J.; Barros, L.; Barreiro, M.F.; González-Paramás, A.M.; Ferreira, I.C.F.R. Development of mushroom-based cosmeceutical formulations with anti-inflammatory, anti-tyrosinase, antioxidant, and antibacterial properties. Molecules 2016, 21, 1372. [Google Scholar] [CrossRef] [PubMed]
- Eiamthaworn, K.; Kaewkod, T.; Bovonsombut, S.; Tragoolpua, Y. Efficacy of Cordyceps militaris extracts against some skin pathogenic bacteria and antioxidant activity. J. Fungi. 2022, 8, 327. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Joshi, S.R. Industrial Perspectives of Fungi. In Industrial Microbiology and Biotechnology; Verma, P., Ed.; Springer: Singapore, 2022. [Google Scholar]
- Shankar, A.; Sharma, K.K. Fungal secondary metabolites in food and pharmaceuticals in the era of multi-omics. Appl. Microbiol. Biotechnol. 2022, 106, 3465–3488. [Google Scholar] [CrossRef]
- Turner, N.A.; Sharma-Kuinkel, B.K.; Maskarinec, S.A.; Eichenberger, E.M.; Shah, P.P.; Carugati, M. Methicillin-resistant Staphylococcus aureus: An overview of basic and clinical research. Nat. Rev. Microbiol. 2019, 17, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Avire, N.J.; Whiley, H.; Ross, K.A. Review of Streptococcus pyogenes: Public health risk factors, prevention and control. Pathogens 2021, 10, 248. [Google Scholar] [CrossRef] [PubMed]
- Platsidaki, E.; Dessinioti, C. Recent advances in understanding Propionibacterium acnes (Cutibacterium acnes) in acne. F1000Research 2018, 7, 1953. [Google Scholar] [CrossRef]
- Wang, R.; Huang, C.; Zhang, Y.; Li, R. Invasive dermatophyte infection: A systematic review. Mycoses 2021, 64, 340–348. [Google Scholar] [CrossRef]
- Ifuku, S. Chitin and Chitosan Nanofibers: Preparation and Chemical Modifications. Molecules 2014, 19, 18367–18380. [Google Scholar] [CrossRef]
- Park, B.K.; Kim, M.M. Applications of chitin and its derivatives in biological medicine. Int. J. Mol. Sci. 2010, 11, 5152–5164. [Google Scholar] [CrossRef]
- Vasylchenko, O.A.; Abramova, M.V. Comparative analysis of sources for chitosan obtaining. Problems Environ. Biotechnol. 2015, 1, 1–20. [Google Scholar] [CrossRef]
- Mattila, P.; Könkö, K.; Eurola, M.; Pihlava, J.M.; Astola, J.; Vahteristo, L. Contents of vitamins, mineral elements, and some phenolic compounds in cultivated mushrooms. J. Agric. Food Chem. 2001, 49, 2343–2348. [Google Scholar] [CrossRef] [PubMed]
- Fiume, M.M.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C. Safety assessment of tocopherols and tocotrienols as used in cosmetics. Int. J. Toxicol. 2018, 37, 61S–94S. [Google Scholar] [CrossRef]
- Sharif, S.; Rashid, S.; Atta, A.; Irshad, A.; Riaz, M.; Shahid, M. Phenolics, tocopherols and fatty acid profiling of wild and commercial mushrooms from Pakistan. J. Biol. Regul. Homeost. Agents. 2018, 32, 863–867. [Google Scholar] [PubMed]
- Chang, T.S. An updated review of tyrosinase inhibitors. Int. J. Mol. Sci. 2009, 10, 2440–2475. [Google Scholar] [CrossRef] [PubMed]
- Noh, J.M.; Kwak, S.Y.; Seo, H.S.; Seo, J.H.; Kim, B.G.; Lee, Y.S. Kojic acid-amino acid conjugates as tyrosinase inhibitors. Bioorg. Med. Chem. Lett. 2009, 19, 5586–5589. [Google Scholar] [CrossRef]
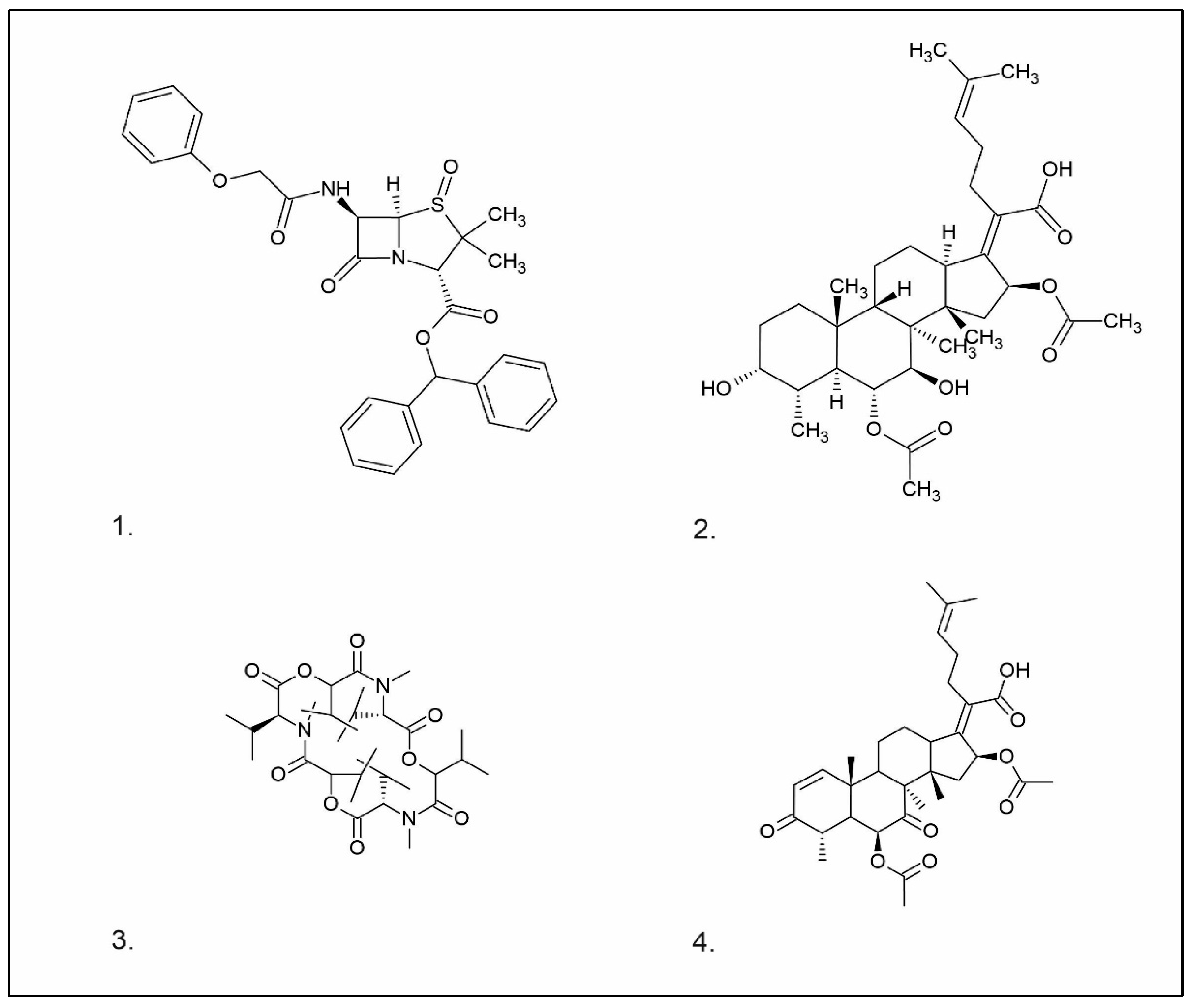

| Group of Compounds | Compound | Species | Chemical Formula | Reference |
|---|---|---|---|---|
| Benzoic acid derivative | Sparassol | Sparassis crispa | 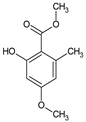 | [13] |
| Sesquiterpenes (C15) | Merulidial | Merulius tremellosus | 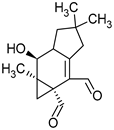 | [14] |
| Pilatin | Flagelloscypha pilatii | 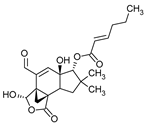 | [15] | |
| Hypnophilin | Pleurotellus hypnophilus | 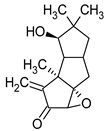 | [16] | |
| Pleurotellol | Pleurotellus hypnophilus | 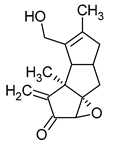 | [16] | |
| Lentinellic acid | Lentinellus omphalodes Lentinellus ursinus | 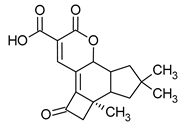 | [17] | |
| Armillaric acid | Armillaria mellea | 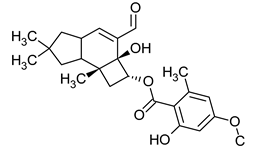 | [18] | |
| Enokipodin A | Flammulina velutipes | 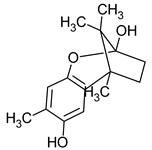 | [19] | |
| Coriolin | Coriolus consors | 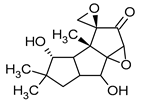 | [3] | |
| Diterpenes (C20) | Pleuromutilin | Clitopilus passeckerianus | 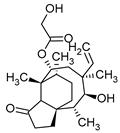 | [20] |
| Striatin A | Cyathus striatus | 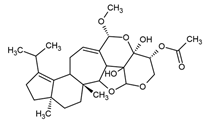 | [21] | |
| Triterpenes (C30) | Sulphurenic acid | Laetiporus sulphureus | 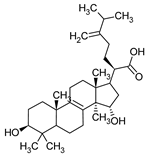 | [22] |
| Ganodermanontriol | Ganoderma lucidum | 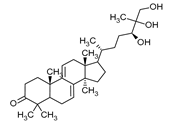 | [23] | |
| Meroterpenoids (C40) | Ganomycin A | Ganoderma pfeiferii | 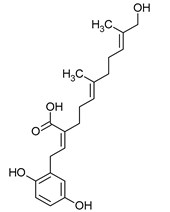 | [24] |
| Acetylene derivatives | Scorodonin | Marasmius scorodonius | 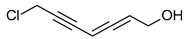 | [25] |
| Sterols | Ganoderiol | Ganoderma lucidum | 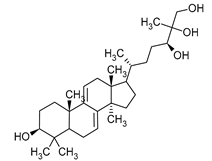 | [23] |
| Species | Extract | Bacteria | References |
|---|---|---|---|
| Aspergillus giganteus Aspergillus nidulans Aspergillus oryzae Aspergillus parasiticus Aspergillus persicinum Aspergillus flavus Penicilium baculatum Penicilium chrysogenum Penicillium turbatum Penicillum chrysogenum | Penicillins | Gram-positive bacteria Diplococcus spp. Enterococcus spp. Staphylococcus spp. Streptococcus spp. Gram-negative bacterial Clostridium spp. Enterobacteriaceae spp. | [30,31] |
| Cephalosporium acremonium | Cephalosporins | Gram-positive bacteria: Diplococcus spp. Enterococcus spp. Staphylococcus spp. Streptococcus spp. Gram-negative bacteria: Clostridium spp. Enterobacteriaceae spp. | [32,33,34,36,38,39] |
| Calcarisporium arbuscula Fusidium coccineum Isaria kogana Mucor ramannianus | Fusidans | Gram-positive bacteria | [36,37,38,39] |
| Fusarium lateritium | Fusafungine | Streptococcus pyogenes Streptococcus pneumoniae Staphylococcus epidermidis Moraxella catarrhalis Legionella pneumophila Mycoplasma pneumoniae | [40,41] |
| Aspergillus fumigatus Cephalosporium caeruleus Emericellopsis terricola Sarocladium oryzae | Fumigacin (helvolic acid) | Gram-negative bacteria | [42] |
| Merulius tremellosus | Merulidial | Gram-positive bacteria: Arthrobacter citreus Bacillus brevis Bacillus subtilis Corynebacterium insidiosum Sarcina lutea Streptomyces viridochrontogenes Gram-negative bacteria: Proteus vulgaris | [14] |
| Flagelloscypha pilati | Pilatin | Salmonella typhimurum | [15] |
| Pleurotellus hypnophillus | Hypnophilin Pleurotellol Pleurotellic acid | Bacillus brevis Salmonella typhimurium | [16] |
| Lentinellus omphalodes Lentinellus ursinus | Lentinellic acid | Bacillus brevis Aerobacter aerogenes Corynebacterium insidosum | [17] |
| Laetiporus sulphureus | Sulphurenic acid Eburicoic acid | Gram-positive bacteria | [22] |
| Clitopilus passeckerianus | Pleuromutilin | Mycoplasma spp. Brachyspira hyodysenteriae Brachyspira pilosicoli | [20] |
| Cyathus striatus | Striatins A, B, C | Arthrobacter citreus Bacillus brevis Bacillus subtilis Escherichia coli Leuconostoc mesenteroides Mycobacterium phlei Nocardia brasiliensis Proteus vulgaris Pseudomonas fluorescens Sarcina lutea Staphylococcus aureus Streptomyces viridochromogenes | [21] |
| Armillaria mellea | Armillaric acid | Gram-positive bacteria | [18] |
| Flammulina velutipes | Enokipodin | Bacillus subtilis Staphylococcus aureus | [49] |
| Jahnoporus hirtus | (24Z)-3,11-Dioxolanosta-8,24-dien-26-oic acid | Bacillus cereus Enterococcus faecalis | [50] |
| Ganoderma pfeifferi | Ganomycin A | Bacillus subtilis Micrococcus flavus Staphylococcus aureus | [24] |
| Pseudoplectania nigrella | Plectasin | Bacillus cereus Bacillus thuringiensi Corynebacterium diphtheriae Corynebacterium jeikeium Enterococcus faecalis Enterococcus faecium Staphylococcus aureus Staphylococcus epidermidis | [51,52] |
| Clitocybe nebularis | Nebularine | Mycobacterium tuberculosis | [58] |
| Pleurotus sajor–caju | Ribonuclease | Pseudomonas aeruginosa Staphylococcus aureus | [59] |
| Gymnophilus spectabilis | Hepta-4,6-diyn-3-ol 7-Chloro-hepta-4,6-diyn-3-ol | Gram-positive/Gram-negative | [61] |
| Hohenbuehelia grisea | Pleurotin | Gram-positive bacteria | [62] |
| Albatrellus flettii | Confluentin Grifolin Neogrifolin | Bacillus cereus Enterococcus faecalis | [50] |
| Lentinula edodes | Oxalic acid | Bacillus cereus Staphylococcus aureus Streptococcus faecalis | [63] |
| Cortinarius basirubencens | Austrocortilutein Austrocortilutein Austrocortirubin Torosachryson | Staphylococcus aureus | [66] |
| Boletus spp. | Boletusin Chrysospermin | Bacillus subtilis Corynebacterium lilium Staphylococcus aureus | [55] |
| Pycnoporus sanguineus | Phenoxazin-3-one | Staphylococcus aureus Streptococcus spp. | [67] |
| Ganoderma pfeifferi | Terpenes | Escherichia coli Proteus mirabilis Serratia marcescens | [24] |
| Xylaria intracolarata | Cloratin A | Escherichia coli Klebsiella pneumonia Pseudomonas aeruginosa Salmonella enteritidis | [65] |
| Leucopaxillus albissimus | Chinoline | Achromobacter xyloxidans Acinetobacter baumannii Burkholderia cenocepacia Burkholderia loccose Burkholderia multivorans Cytophaga johnsonae Pseudomonas aeruginosa | [67] |
| Species | Extract | Bacteria | References |
|---|---|---|---|
| Ganoderma lucidum | Acetone extract Aqueous extract Ethanol extract Methanol extract | Bacillus subtilis Escherichia coli Klebsiella pneumoniae Pseudomonas aeruginosa Salmonella typhimurium Staphylococcus aureus | [74] |
| Ganoderma lucidum | Acetone extract | Bacillus subtilis Klebsiella oxytoca | [75] |
| Hygrophorus agathosmus | Chloroform extract | Bacillus subtilis Enterobacter aerogenes Escherichia coli Pseudomonas aeruginosa Salmonella typhimurium Staphylococcus aureus Staphylococcus epidermidis | [76] |
| Suillus collitinus | Dichloromethanol extract | Bacillus subtilis Staphylococcus epidermidis | [76] |
| Hypholoma fasciculare | Methanol extract | Bacillus cereus Bacillus subtilis Staphylococcus aureus | [77] |
| Laetiporus sulphureus | Ethanol extract | Bacillus cereus Bacillus subtilis Micrococcus flavus Micrococcus luteus | [78] |
| Lentinula edodes | Chloroform extract Acetate-ethyl extract | Actinomyces spp. Lactobacillus spp. Porphyromonas spp. Prevotella spp. Streptococcus spp. | [79] |
| Leucopaxillus giganteus | Methanol extract (mycelial cultures) | Bacillus cereus Bacillus subtilis Staphylococcus aureus | [81] |
| Navesporus loccose Phellinus rimosus | Methanol extract | Bacillus subtilis Staphylococcus aureus | [82] |
| Pleurotus ostreatus Meripilus giganteus | Ethanol extract | Sarcina lutea | [83] |
| Trametes versicolor | Methanol extract | Gram-positive bacteria | [91] |
| Grifola frondosa | Ethanol extracts/polysaccharides | Bacilluscereus | [84] |
| Gloeoporus thelephoroides Hexagonia hydnoides Phellinus spp. | Acetate-ethyl extract | Bacillus cereus | [85] |
| Nothopanus hygrophanus | Acetate-ethyl extract | Listeria monocytogenes Staphylococcus aureus | [85] |
| Agaricus bisporus Pleurotus sajor–caju | Aqueous extract Ethanol extract Methanol extract Xylene extract | Enterobacter aerogenes Escherichia coli 390 Escherichia coli 739 Klebsiella pneumoniae Pseudomonas aeruginosa | [86] |
| Hydnum repandum | Methanol extract | Pseudomonas aeruginosa | [87] |
| Lepista nuda | Methanol extract | Escherichia coli Pseudomonas aeruginosa | [88] |
| Suillus collitinus | Dichloromethane extract | Bacillus subtilis Candida albicans Enterobacter aerogenes Escherichia coli Salmonella typhimurium Staphylococcus aureus Staphylococcus epidermidis | [76] |
| Hygrophorus agathosmus | Chloroform extract | Bacillus subtilis Enterobacter aerogenes Salmonella typhimurium Staphylococcus aureus Staphylococcus epidermidis | [89] |
| Laetiporus sulphureus | Ethanol extract Aqueous extract | Bacillus subtilis Bacillus cereus Micrococcus luteus Micrococcus flavus Klebsiella pneumoniea Listeria monocytogenes | [78,90] |
| Species | Chemical Compound/Extract | Fungal Pathogen | Reference |
|---|---|---|---|
| Trichaptum biforme | Biforminic acid Biformin | Aspergillus niger | [93] |
| Penicillium spp. | Griseofulvin | Epidermophyton Microsporum Trichophyton | [94] |
| Agrocybe cylindracea | Agrocybin | Fusarium oxysporum Mycosphaerella arachidicola | [96] |
| Cordyceps militaris | Cordymin | Bipolaris maydis Candida albicans Mycosphaerella arachidicola Rhizoctonia solani | [97] |
| Lactarius rufus | Rufuslactone | Alternaria alternata Alternaria brassicae Botrytis cinerea Fusarium graminearum | [101] |
| Flammulina velutipes | Enokipodins F, G, I | Aspergillus fumigatus | [49] |
| Tricholoma giganteum | Trichogin | Fusarium oxysporum Mycosphaerella arachidicola Physalospora piricola | [102] |
| Gloeophyllum sepiarium | Oospolactone | Alternaria spp. Fusarium spp. Giberella spp. Penicilim spp. Aspergillus spp. | [103] |
| Lentinula edodes | Lentin | Mycosphaerella | [104] |
| Pleurotus eryngii | Eryngin | Fusarium oxysporum Mycosphaerella arachidicola | [107] |
| Hypsizigus marmoreus | Hypsin | Botrytis cinerea Fusarium oxysporum Mycosphaerella arachidicola Physalospora piricola | [108] |
| Strobilurus tenacellus | Strobilurins | Aspergillus panamensis Candida albicans Paecilomyces varioti Penicillium notatum Rhodotorula glutinis | [109] |
| Hygrophorus chrysodon | Chrysotriones A, B | Fusarium verticillioides | [105] |
| Ganoderma annulare | 5-Ergost-7-en-3-ol 5-Ergosta-7,22-dien-3-ol 5,8-Epidioxy-5,8-ergosta-6,22-die -3-ol Applanoxidic acids A, C, F, G, H | Microsporum canis Trichophyton mentagrophytes | [106] |
| Ganoderma lucidum | Ganodermin | Botrytis cinerea Fusarium oxysporum Physalospora paricola | [110] |
| Pleurotus sajor–caju | Ribonuclease | Fusarium oxysporum Mycosphaerella arachidicola | [59] |
| Crepidotus fulvifibrillosus | Strobilurins | Alternaria porri Aspergillus ochraceus Candida albicans Cladosporium cladosporioides Curvularia lunata Epicoccum purpurascens Mucor miehei Nematospora coryli Neurospora crassa Paecilomyces varioti Penicillium islandicum Penicilium notatum Phoma clematidina Phytophthora infestans | [109] |
| Species | Extract | Fungal Pathogen | Reference |
|---|---|---|---|
| Albatrellus dispansus | Ethanol extrat | Erysiphe graminis Sclerotinina sclerotiorum Fusarium graminearum | [98] |
| Agaricus bisporus | Aqueous extract Ethanol extract | Aspergillus flavus | [111] |
| Ophiocordyceps sobolifera | Ethanol extract | Candida albicans | [112] |
| Agaricus bisporus Agaricus bitorquis Agaricus sylvicola | Methanol extract | Candida albicans Candida tropicalis | [113] |
| Hygrophorus agathosmus | Chloroform extract | Saccharomyces cerevisae | [76] |
| Suillus collitinus | Dichloromethane extract | Candida albicans Saccharomyces cerevisae | [76] |
| Ganoderma lucidum | Ethanol extract | Aspergillus fumigatus Aspergillus versicolor Aspergillus ochraceus Aspergillus niger Trichoderma viride Penicillium funiculosum Penicillium ochrochloron Penicillium verrucosum | [75] |
| Laetiporus sulphureus | Ethanol extract Aqueous-ethanol extract | Candida albicans Aspergillus niger Botrytis cinerea Fusarium oxysporum, Penicillium gladioli Sclerotinia sclerotiorum | [78] |
| Lactarius camphoratus | Methanol extract | Candida albicans | [116] |
| Lentinula edodes | Chloroform extract | Candida albicans | [63] |
| Lepista nuda | Methanol extract | Candida albicans | [88] |
| Trametes versicolor | Methanol extract | Aspergillus fumigatus | [91] |
| Species | Chemical Compound/Extract | Virus Type | Reference |
|---|---|---|---|
| Tricholoma giganteum | Trichogin | HIV-1 | [102] |
| Ganoderma lucidum | Ganoderiol Ganodermanontriol Ganodermic acid | HIV-1 | [110] |
| Ganoderma pfeiferi | Ganodermadiol | H1N1 | [118] |
| Inonotus hispidus | Phenolic compounds | H1N1and B | [119] |
| Trametes versicolor | PSK complex | Cytomegalovirus HIV | [120] |
| Agaricus brasiliensis | Aqueous extracts | HSV | [126] |
| Rozites caperata | Aqueous extracts | HSV | [122] |
| Ganoderma pfeifferi | Aqueous extracts | HSV | [118] |
| Pleurotus pulmonarius | Ethanol extract | (H1N1pdm) | [123] |
| Cordyceps militaris | Polyssaccharide acidic fraction | H1N1 | [124] |
| Laetiporus sulphureus | Aqueosus-methanol extract | HIV | [136] |
| Agaricus brasiliensis | Polysaccharides | PV-1 | [121] |
| Porodaedalea pini | EP-AV1 polysaccaride EP-AV2 polysaccaride | HSV-1 CVB3 | [137] |
| Phellinus baumii | Hispidin Hypholomine B Inoscavin A Davallialactone Phelligridin D | H1N1, H5N1, H3N2 | [128] |
| Agaricus bisporus Pleurotus ostreatus | Laccase enzyme Tyrosinase enzyme | HCV | [129] |
| Grifola frondosa | Β-glucan | HBV | [131] |
| Lentinula edodes | Lentinan | IHNV | [132] |
| Inonotus obliquus | Polysaccharide fraction Aqueous extract | COVID-19 HCV | [133] [135] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sułkowska-Ziaja, K.; Trepa, M.; Olechowska-Jarząb, A.; Nowak, P.; Ziaja, M.; Kała, K.; Muszyńska, B. Natural Compounds of Fungal Origin with Antimicrobial Activity—Potential Cosmetics Applications. Pharmaceuticals 2023, 16, 1200. https://doi.org/10.3390/ph16091200
Sułkowska-Ziaja K, Trepa M, Olechowska-Jarząb A, Nowak P, Ziaja M, Kała K, Muszyńska B. Natural Compounds of Fungal Origin with Antimicrobial Activity—Potential Cosmetics Applications. Pharmaceuticals. 2023; 16(9):1200. https://doi.org/10.3390/ph16091200
Chicago/Turabian StyleSułkowska-Ziaja, Katarzyna, Monika Trepa, Aldona Olechowska-Jarząb, Paweł Nowak, Marek Ziaja, Katarzyna Kała, and Bożena Muszyńska. 2023. "Natural Compounds of Fungal Origin with Antimicrobial Activity—Potential Cosmetics Applications" Pharmaceuticals 16, no. 9: 1200. https://doi.org/10.3390/ph16091200
APA StyleSułkowska-Ziaja, K., Trepa, M., Olechowska-Jarząb, A., Nowak, P., Ziaja, M., Kała, K., & Muszyńska, B. (2023). Natural Compounds of Fungal Origin with Antimicrobial Activity—Potential Cosmetics Applications. Pharmaceuticals, 16(9), 1200. https://doi.org/10.3390/ph16091200






