Medicinal Characteristics of Withania somnifera L. in Colorectal Cancer Management
Abstract
1. Introduction
1.1. Botanical Description and the Biodiversity of Withania somnifera (L.) Plant Species
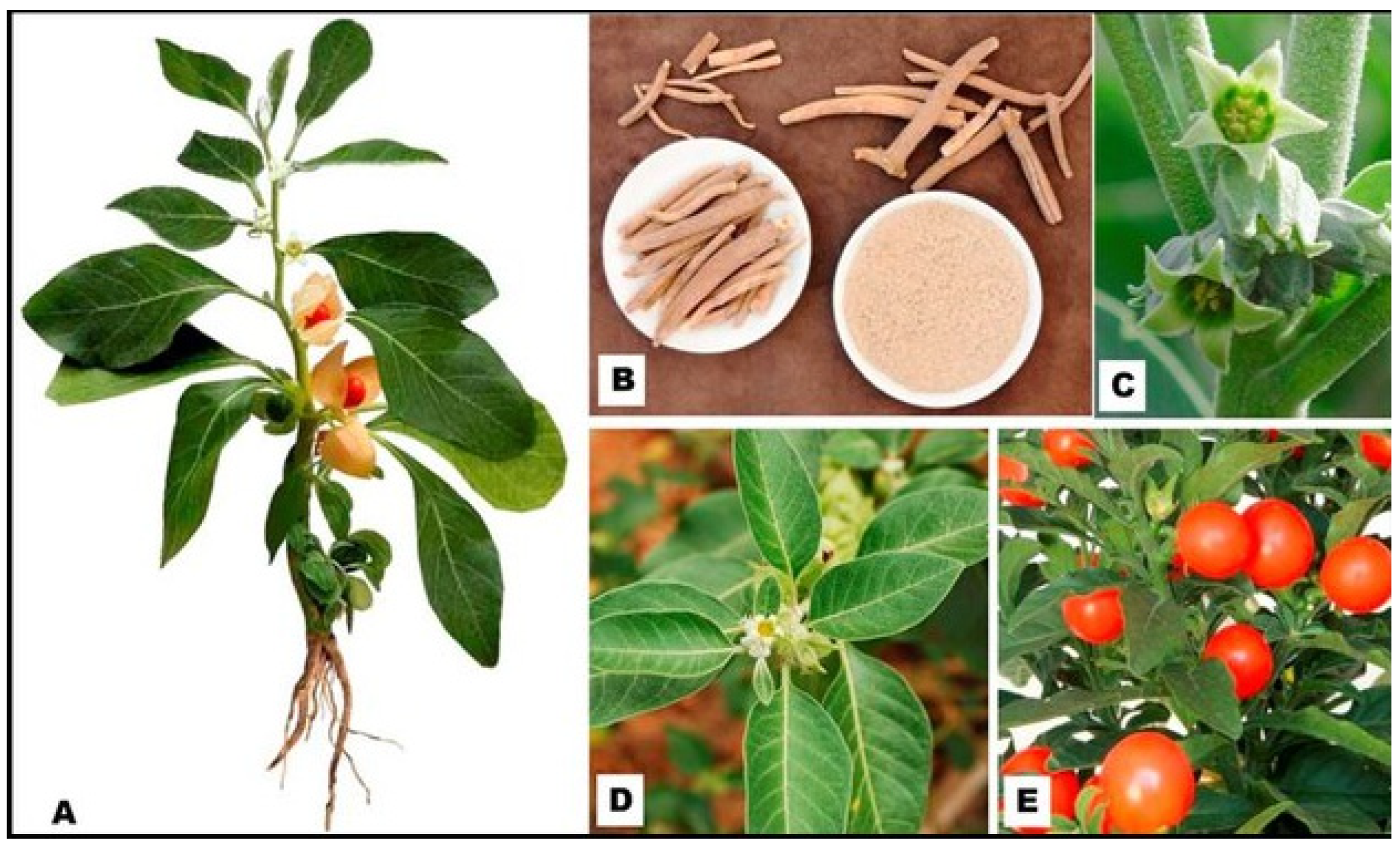
1.2. The Global Incidence of Colon Cancer (CRC) and Its Risk Factors
1.3. Future Strategies for Long-Term CRC Management and Contemporary Treatments
2. Methods
2.1. Applied Study Design and Electronic Databases
2.2. Review Question and the Screening Criterion
2.3. Effective Search Strategy
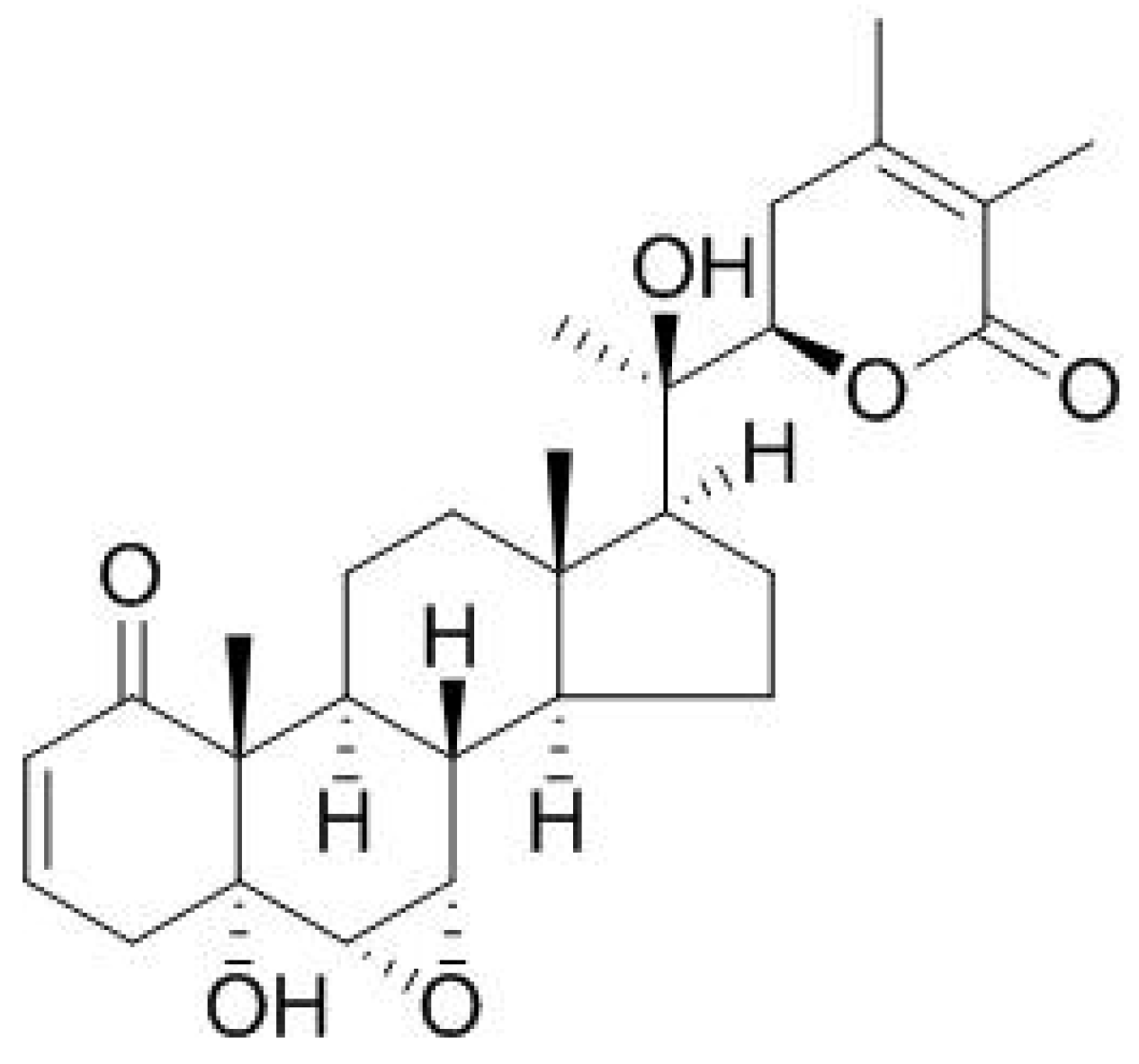
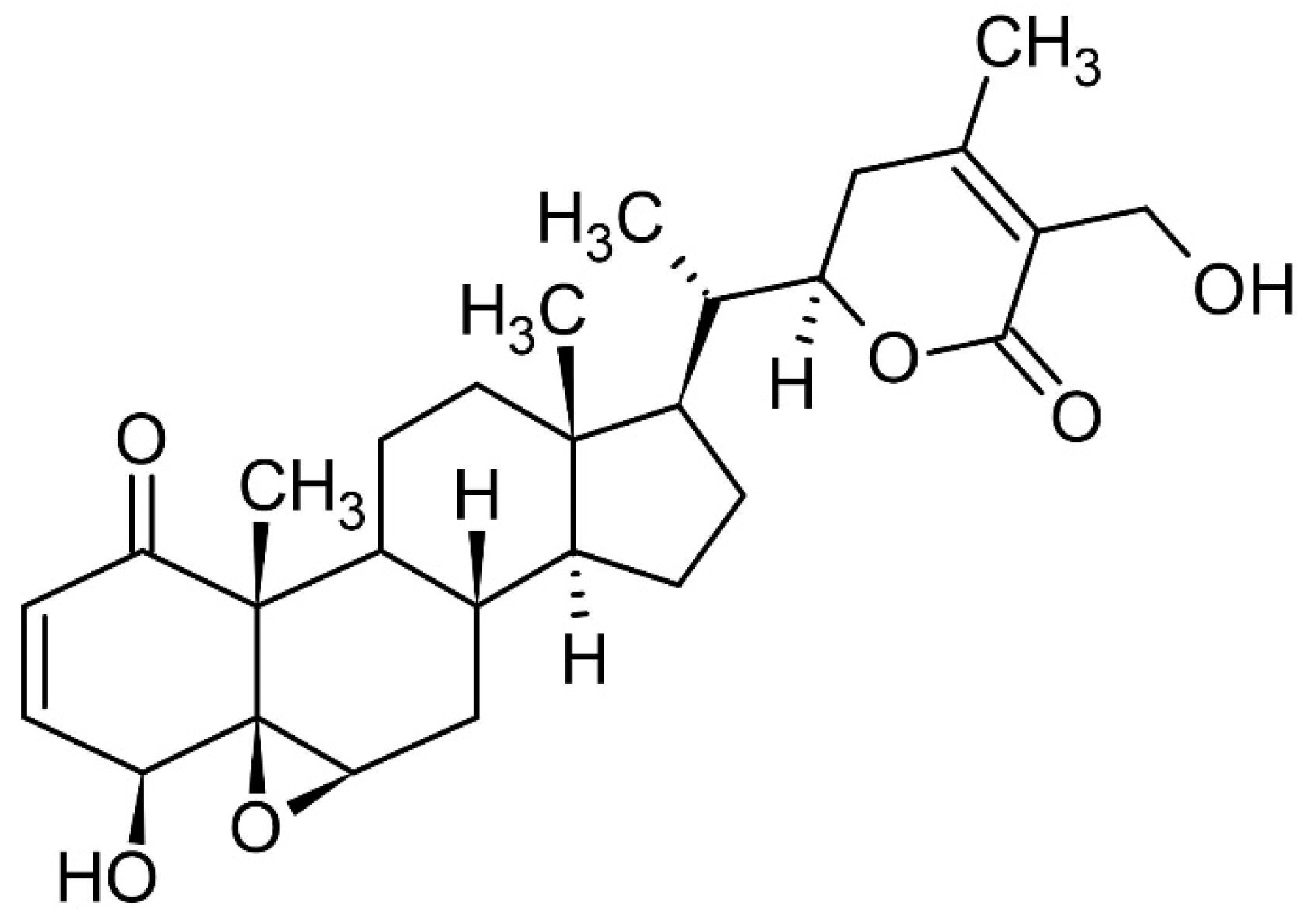
3. Bioactive Compounds Present in W. somnifera L.
3.1. Mechanisms of Carcinogenesis and the Inhibitive Potential Role of W. somnifera L. in CRC Growth and Proliferation
3.2. Cytotoxicity and Safety Properties of Withania somnifera L.
3.3. Significance of Targeted Upregulated Apoptotic Activity in CRC Management
3.4. Antiangiogenic and Antimigratory Potential of W. somnifera L.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mirjalili, M.H.; Moyano, E.; Bonfill, M.; Cusido, R.M.; Palazón, J. Steroidal lactones from Withania somnifera, an ancient plant for novel medicine. Molecules 2009, 14, 2373–2393. [Google Scholar] [CrossRef] [PubMed]
- Gaurav, H.; Yadav, D.; Maurya, A.; Yadav, H.; Yadav, R.; Shukla, A.C.; Sharma, M.; Gupta, V.K.; Palazon, J. Biodiversity, Biochemical Profiling, and Pharmaco-Commercial Applications of Withania somnifera: A Review. Molecules 2023, 28, 1208. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Maqsood, M.; Saeed, R.A.; Alam, A.; Sahar, A.; Kieliszek, M.; Miecznikowski, A.; Muzammil, H.S.; Aadil, R.M. Phytochemistry, food application, and therapeutic potential of the medicinal plant (Withania coagulans): A review. Molecules 2021, 26, 6881. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.; Qazi, G.; Ganju, R.; El-Tamer, M.; Singh, J.; Saxena, A.; Bedi, Y.; Taneja, S.; Bhat, H. Medicinal Plants and Cancer Chemoprevention. Curr. Drug Metab. 2008, 9, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Meena, L.K.; Meena, A.K.; Gupta, J.; Patel, M.; Khan, S.Y.; Kumar, S.; Gupta, A.K. Ashwagandha (Withania somnifera L.). In Medicinal Plants in India: Importance and Cultivation; Jaya publishing house: Delhi, India, 2020. [Google Scholar]
- Dontha, S.; Kamurthy, H.; Mantripragada, B. Phytochemical and pharmacological profile of Ixora: A review. Int. J. Pharm. Sci. Res. 2015, 6, 567–584. [Google Scholar]
- Verma, S.K.; Kumar, A. Therapeutic uses of Withania somnifera (Ashwagandha) with a note on withanolides and its pharmacological actions. Asian J. Pharm. Clin. Res. 2011, 4, 1–4. [Google Scholar]
- Rajeswara Rao, B.R.; Rajput, D.K.; Nagaraju, G.; Adinarayana, G. Opportunities and Challenges in the Cultivation of Ashwagandha {Withania somnifera (L.) Dunal}. J. Pharmacogn. 2012, 3, 88–91. [Google Scholar]
- Tewari, D.; Chander, V.; Dhyani, A.; Sahu, S.; Gupta, P.; Patni, P.; Kalick, L.S.; Bishayee, A. Withania somnifera (L.) Dunal: Phytochemistry, structure-activity relationship, and anticancer potential. Phytomedicine 2022, 98, 153949. [Google Scholar] [CrossRef]
- Sengupta, P.; Agarwal, A.; Pogrebetskaya, M.; Roychoudhury, S.; Durairajanayagam, D.; Henkel, R. Role of Withania somnifera (Ashwagandha) in the management of male infertility. Reprod. Biomed. Online 2018, 36, 311–326. [Google Scholar] [CrossRef]
- Macharia, J.M.; Zhang, L.; Mwangi, R.W.; Rozmann, N.; Kaposztas, Z.; Varjas, T.; Sugár, M.; Alfatafta, H.; Pintér, M.; Bence, R.L. Are chemical compounds in medical mushrooms potent against colorectal cancer carcinogenesis and antimicrobial growth? Cancer Cell Int. 2022, 22, 379. [Google Scholar] [CrossRef] [PubMed]
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global Cancer Statistics. Gut 2017, 66, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Macharia, J.M.; Mwangi, R.W.; Rozmann, N.; Wagara, I.N.; Kaposztas, Z.; Varjas, T.; Mathenge, J.; Bence, R.L. A systematic review of selected plants and their metabolites with anticolorectal cancer effects. Phytomedicine Plus 2022, 2, 100332. [Google Scholar] [CrossRef]
- Iqbal, J.; Abbasi, B.A.; Mahmood, T.; Kanwal, S.; Ali, B.; Shah, S.A.; Khalil, A.T. Plant-derived anticancer agents: A green anticancer approach. Asian Pac. J. Trop. Biomed. 2017, 7, 1129–1150. [Google Scholar] [CrossRef]
- Macharia, J.M.; Kaposztas, Z.; Varjas, T.; Budán, F.; Zand, A.; Bodnar, I.; Bence, R.L. Targeted lactate dehydrogenase genes silencing in probiotic lactic acid bacteria: A possible paradigm shift in colorectal cancer treatment? Biomed. Pharmacother. 2023, 160, 114371. [Google Scholar] [CrossRef]
- Macharia, J.M.; Ngure, V.; Emődy, B.; Király, B.; Káposztás, Z.; Rozmann, N.; Erdélyi, A.; Raposa, B. Pharmacotherapeutic Potential of Aloe secundiflora against Colorectal Cancer Growth and Proliferation. Pharmaceutics 2023, 15, 1558. [Google Scholar] [CrossRef]
- Hayes, C.; Donohoe, C.L.; Davern, M.; Donlon, N.E. The oncogenic and clinical implications of lactate induced immunosuppression in the tumour microenvironment. Cancer Lett. 2021, 500, 75–86. [Google Scholar] [CrossRef]
- Niu, D.; Wu, Y.; Lei, Z.; Zhang, M.; Xie, Z.; Tang, S. Lactic acid, a driver of tumor-stroma interactions. Int. Immunopharmacol. 2022, 106, 108597. [Google Scholar] [CrossRef]
- Schmoll, H.J.; Van Cutsem, E.; Stein, A.; Valentini, V.; Glimelius, B.; Haustermans, K.; Nordlinger, B.; Van de Velde, C.J.; Balmana, J.; Regula, J.; et al. Esmo consensus guidelines for management of patients with colon and rectal cancer. A personalized approach to clinical decision making. Ann. Oncol. 2012, 23, 2479–2516. [Google Scholar] [CrossRef]
- Colussi, D.; Brandi, G.; Bazzoli, F.; Ricciardiello, L. Molecular pathways involved in colorectal cancer: Implications for disease behavior and prevention. Int. J. Mol. Sci. 2013, 14, 16365–16385. [Google Scholar] [CrossRef]
- Carvalho, C.; Marinho, A.; Leal, B.; Bettencourt, A.; Boleixa, D.; Almeida, I.; Farinha, F.; Costa, P.P.; Vasconcelos, C.; Silva, B.M. Association between vitamin D receptor (VDR) gene polymorphisms and systemic lupus erythematosus in Portuguese patients. Lupus 2015, 24, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.H.; Thulasingam, S.; Nagarajan, S. Terpenoids as anti-colon cancer agents—A comprehensive review on its mechanistic perspectives. Eur. J. Pharmacol. 2017, 795, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Munker, S.; Gerken, M.; Fest, P.; Ott, C.; Schnoy, E.; Fichtner-Feigl, S.; Wiggermann, P.; Vogelhuber, M.; Herr, W.; Stroszczynski, C.; et al. Chemotherapy for metastatic colon cancer: No effect on survival when the dose is reduced due to side effects. BMC Cancer 2018, 18, 455. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.; Patel, D.K. Medicinal importance, pharmacological activities, and analytical aspects of aloin: A concise report. J. Acute Dis. 2013, 2, 262–269. [Google Scholar] [CrossRef]
- HO, R.; DN, K. In vitro Antifungal activity of leaf extracts from Aloe secundiflora, Bulbine frutescens, Vernonia lasiopus and Tagetes minuta against Candida albicans. Med. Aromat. Plants 2016, 5, 2–4. [Google Scholar] [CrossRef]
- Macharia, J.M.; Mwangi, R.W.; Rozmann, N.; Zsolt, K.; Varjas, T.; Uchechukwu, P.O.; Wagara, I.N.; Raposa, B.L. Medicinal plants with anti-colorectal cancer bioactive compounds: Potential game-changers in colorectal cancer management. Biomed. Pharmacother. 2022, 153, 113383. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 105906. [Google Scholar] [CrossRef]
- Mwangi, R.W.; Macharia, J.M.; Wagara, I.N.; Bence, R.L. The antioxidant potential of different edible and medicinal mushrooms. Biomed. Pharmacother. 2022, 147, 112621. [Google Scholar] [CrossRef]
- Mwangi, R.W.; Macharia, J.M.; Wagara, I.N.; Bence, R.L. The medicinal properties of Cassia fistula L: A review. Biomed. Pharmacother. 2021, 144, 112240. [Google Scholar] [CrossRef]
- Lau, F.; Kuziemsky, C. Handbook of eHealth Evaluation: An Evidence-Based Approach; University of Victoria: Victoria, BC, Canada, 2016. [Google Scholar]
- Khan, K.S.; Kunz, R.; Kleijnen, J.; Antes, G. Five steps to conducting a systematic review. J. R. Soc. Med. 2003, 96, 118–121. [Google Scholar] [CrossRef]
- Dhanani, T.; Shah, S.; Gajbhiye, N.A.; Kumar, S. Effect of extraction methods on yield, phytochemical constituents and antioxidant activity of Withania somnifera. Arab. J. Chem. 2017, 10, S1193–S1199. [Google Scholar] [CrossRef]
- Kaul, S.C.; Wadhwa, R. Science of Ashwagandha: Preventive and Therapeutic Potentials. Sci. Ashwagandha Prev. Ther. Potentials 2017, 1–508. [Google Scholar] [CrossRef]
- Govindappa, P.K.; Gautam, V.; Tripathi, S.M.; Sahni, Y.P.; Raghavendra, H.L.S. Effect of Withania somnifera on gentamicin induced renal lesions in rats. Rev. Bras. Farmacogn. 2019, 29, 234–240. [Google Scholar] [CrossRef]
- Misra, L.; Mishra, P.; Pandey, A.; Sangwan, R.S.; Sangwan, N.S.; Tuli, R. Withanolides from Withania somnifera roots. Phytochemistry 2008, 69, 1000–1004. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, M.K.; Panda, P.; Sethi, K.K.; Jana, S. Metabolite Profiling in Withania somnifera Roots Hydroalcoholic Extract Using LC/MS, GC/MS and NMR Spectroscopy. Chem. Biodivers. 2017, 14, e1600280. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Duggal, S.; Singh, H.; Singh, J.; Katekhaye, S. Withanolides: Phytoconstituents with significant pharmacological activities. Int. J. Green Pharm. 2010, 4, 229–237. [Google Scholar] [CrossRef]
- Tetali, S.D.; Acharya, S.; Ankari, A.B.; Nanakram, V.; Raghavendra, A.S. Metabolomics of Withania somnifera (L.) Dunal: Advances and applications. J. Ethnopharmacol. 2021, 267, 113469. [Google Scholar] [CrossRef]
- Pandey, V.; Ansari, W.A.; Misra, P.; Atri, N. Withania somnifera: Advances and implementation of molecular and tissue culture techniques to enhance its application. Front. Plant Sci. 2017, 8, 1–11. [Google Scholar] [CrossRef]
- Pant, C.; Mishra, A.; Tomar, H.; Singhal, M.; Shaheen, G.; Kumar, A.; Gaurav, N. Some Potential and Effective Important Medicinal Plants for Cancer Treatment. Int. J. Sci. Eng. Res. 2021, 12, 975–984. [Google Scholar]
- Saleem, S.; Muhammad, G.; Hussain, M.A.; Altaf, M.; Abbas Bukhari, S.N. Withania somnifera L.: Insights into the phytochemical profile, therapeutic potential, clinical trials, and future prospective. Iran. J. Basic Med. Sci. 2020, 23, 1501–1526. [Google Scholar] [CrossRef]
- Winters, M. Ancient medicine, modern use: Withania somnifera and its potential role in integrative oncology. Altern. Med. Rev. 2006, 11, 269–277. [Google Scholar] [PubMed]
- Opiyo, S.A.; Manguro, L.O.A.; Okinda-Owuor, P.; Ateka, E.M.; Lemmen, P. 7α-Acetylugandensolide and antimicrobial properties of Warburgia ugandensis extracts and isolates against sweet potato pathogens. Phytochem. Lett. 2011, 4, 161–165. [Google Scholar] [CrossRef]
- Mariita, R.M.; Orodho, J.A.; Okemo, P.O.; Kirimuhuzya, C.; Otieno, J.N.; Magadula, J.J. Methanolic extracts of Aloe secundiflora Engl. inhibits in vitro growth of tuberculosis and diarrhea-causing bacteria. Pharmacogn. Res. 2011, 3, 95–99. [Google Scholar] [CrossRef]
- Chatterjee, S.; Srivastava, S.; Khalid, A.; Singh, N.; Sangwan, R.S.; Sidhu, O.P.; Roy, R.; Khetrapal, C.L.; Tuli, R. Comprehensive metabolic fingerprinting of Withania somnifera leaf and root extracts. Phytochemistry 2010, 71, 1085–1094. [Google Scholar] [CrossRef]
- Johri, S.; Jamwal, U.; Rasool, S.; Kumar, A.; Verma, V.; Qazi, G.N. Purification and characterization of peroxidases from Withania somnifera (AGB 002) and their ability to oxidize IAA. Plant Sci. 2005, 169, 1014–1021. [Google Scholar] [CrossRef]
- Bhatia, A.; Bharti, S.K.; Tewari, S.K.; Sidhu, O.P.; Roy, R. Metabolic profiling for studying chemotype variations in Withania somnifera (L.) Dunal fruits using GC-MS and NMR spectroscopy. Phytochemistry 2013, 93, 105–115. [Google Scholar] [CrossRef]
- Kherde, S.D.; Parmar, K.M.; Tawar, M.G.; Prasad, S.K.; Itankar, P.R. Study on impact of different climatic zones on physicochemical and phytochemical profile of Withania somnifera (L.) dunal. Indian J. Tradit. Knowl. 2020, 19, 486–493. [Google Scholar] [CrossRef]
- Baghel, G.; Naqvi, Q.A.; Snehi, S.K.; Khan, M.S.; Raj, S.K. Molecular identification of three isolates of Jatropha mosaic India virus associated with mosaic disease of Withania somnifera in India. Arch. Phytopathol. Plant Prot. 2012, 45, 2114–2119. [Google Scholar] [CrossRef]
- Widodo, N.; Takagi, Y.; Shrestha, B.G.; Ishii, T.; Kaul, S.C.; Wadhwa, R. Selective killing of cancer cells by leaf extract of Ashwagandha: Components, activity and pathway analyses. Cancer Lett. 2008, 262, 37–47. [Google Scholar] [CrossRef]
- Paul, S.; Chakraborty, S.; Anand, U.; Dey, S.; Nandy, S.; Ghorai, M.; Saha, S.C.; Patil, M.T.; Kandimalla, R.; Proćków, J.; et al. Withania somnifera (L.) Dunal (Ashwagandha): A comprehensive review on ethnopharmacology, pharmacotherapeutics, biomedicinal and toxicological aspects. Biomed. Pharmacother. 2021, 143, 112175. [Google Scholar] [CrossRef]
- Chandrasekaran, B.; Pal, D.; Kolluru, V.; Tyagi, A.; Baby, B.; Dahiya, N.R.; Youssef, K.; Alatassi, H.; Ankem, M.K.; Sharma, A.K.; et al. The chemopreventive effect of withaferin A on spontaneous and inflammation-associated colon carcinogenesis models. Carcinogenesis 2018, 39, 1537–1547. [Google Scholar] [CrossRef]
- Koduru, S.; Kumar, R.; Srinivasan, S.; Evers, M.B.; Damodaran, C. Notch-1 inhibition by withaferin-A: A therapeutic target against colon carcinogenesis. Mol. Cancer Ther. 2010, 9, 202–210. [Google Scholar] [CrossRef]
- Das, T.; Roy, K.S.; Chakrabarti, T.; Mukhopadhyay, S.; Roychoudhury, S. Withaferin A modulates the Spindle Assembly Checkpoint by degradation of Mad2-Cdc20 complex in colorectal cancer cell lines. Biochem. Pharmacol. 2014, 91, 31–39. [Google Scholar] [CrossRef]
- Choi, B.Y.; Kim, B.-W. Withaferin-A Inhibits Colon Cancer Cell Growth by Blocking STAT3 Transcriptional Activity. J. Cancer Prev. 2015, 20, 185–192. [Google Scholar] [CrossRef]
- Alnuqaydan, A.M.; Rah, B.; Almutary, A.G.; Chauhan, S.S. Synergistic antitumor effect of 5-fluorouracil and withaferin-A induces endoplasmic reticulum stress-mediated autophagy and apoptosis in colorectal cancer cells. Am. J. Cancer Res. 2020, 10, 799–815. [Google Scholar] [PubMed]
- Mwitari, P.; Mutuku, N.; Keter, L.; Gathirwa, J.; Kimani, F.; Kirira, P.; Wachira, S. Immune Modulating and Antiproliferative Potential of Withania somnifera Crude and Pre-purified Fractions on Selected Cancerous and Normal Cells. J. Complement. Altern. Med. Res. 2018, 5, 1–11. [Google Scholar] [CrossRef]
- Jayaprakasam, B.; Zhang, Y.; Seeram, N.P.; Nair, M.G. Growth inhibition of human tumor cell lines by withanolides from Withania somnifera leaves. Life Sci. 2003, 74, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.H.; Piao, X.L.; Kim, J.M.; Kwon, S.W.; Park, J.H. Inhibition of cholinesterase and amyloid-&bgr; aggregation by resveratrol oligomers from Vitis amurensis. Phyther. Res. 2008, 22, 544–549. [Google Scholar]
- Ahmed, W.; Mofed, D.; Zekri, A.R.; El-Sayed, N.; Rahouma, M.; Sabet, S. Antioxidant activity and apoptotic induction as mechanisms of action of Withania somnifera (Ashwagandha) against a hepatocellular carcinoma cell line. J. Int. Med. Res. 2018, 46, 1358–1369. [Google Scholar] [CrossRef]
- Wang, H.C.; Tsai, Y.L.; Wu, Y.C.; Chang, F.R.; Liu, M.H.; Chen, W.Y.; Wu, C.C. Withanolides-induced breast cancer cell death is correlated with their ability to inhibit heat protein 90. PLoS ONE 2012, 7, e37764. [Google Scholar] [CrossRef]
- Gao, R.; Shah, N.; Lee, J.S.; Katiyar, S.P.; Li, L.; Oh, E.; Sundar, D.; Yun, C.O.; Wadhwa, R.; Kaul, S.C. Withanone-rich combination of Ashwagandha withanolides restricts metastasis and angiogenesis through hnRNP-K. Mol. Cancer Ther. 2014, 13, 2930–2940. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.B.; Rao, N.J.; Hingorani, L.L. Safety assessment of Withania somnifera extract standardized for Withaferin A: Acute and sub-acute toxicity study. J. Ayurveda Integr. Med. 2016, 7, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Prabu, P.C.; Panchapakesan, S.; Raj, C.D. Acute and sub-acute oral toxicity assessment of the hydroalcoholic extract of Withania somnifera roots in wistar rats. Phyther. Res. 2013, 27, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- Prabu, P.C.; Panchapakesan, S. Prenatal developmental toxicity evaluation of Withania somnifera root extract in Wistar rats. Drug Chem. Toxicol. 2015, 38, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Baig, M.W.; Nasir, B.; Waseem, D.; Majid, M.; Khan, M.Z.I.; Haq, I.U. Withametelin: A biologically active withanolide in cancer, inflammation, pain and depression. Saudi Pharm. J. 2020, 28, 1526–1537. [Google Scholar] [CrossRef] [PubMed]
- Sajida; Prabhu, A. Anti-angiogenic, apoptotic and matrix metalloproteinase inhibitory activity of Withania somnifera (ashwagandha) on lung adenocarcinoma cells. Phytomedicine 2021, 90, 153639. [Google Scholar] [CrossRef]
- Srivastava, A.N.; Ahmad, R.; Khan, M.A. Evaluation and comparison of the in vitro cytotoxic activity of Withania somnifera methanolic and ethanolic extracts against MDA-MB-231 and vero cell lines. Sci. Pharm. 2016, 84, 41–59. [Google Scholar] [CrossRef]
- Yadav, D.K.; Kumar, S.; Saloni; Singh, H.; Kim, M.H.; Sharma, P.; Misra, S.; Khan, F. Molecular docking, QSAR and ADMET studies of withanolide analogs against breast cancer. Drug Des. Devel. Ther. 2017, 11, 1859–1870. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Banerjee, S.; Biswas, S.; Das, B.; Kar, A.; Katiyar, C.K. Withania somnifera (L.) Dunal—Modern perspectives of an ancient Rasayana from Ayurveda. J. Ethnopharmacol. 2021, 264, 113157. [Google Scholar] [CrossRef]
- Raff, M. Cell suicide for beginners. Nature 1998, 396, 119–122. [Google Scholar] [CrossRef]
- Thornberry, N.A.; Lazebnik, Y. Caspases: Enemies within. Science 1998, 281, 1312–1316. [Google Scholar] [CrossRef] [PubMed]
- Sjöström, J.; Bergh, J. How apoptosis is regulated, and what goes wrong in cancer. Br. Med. J. 2001, 322, 1538–1539. [Google Scholar] [CrossRef] [PubMed]
- Wong, B.C.Y.; Zhu, G.H.; Lam, S.K. Aspirin induced apoptosis in gastric cancer cells. Biomed. Pharmacother. 1999, 53, 315–318. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.; Connelly, A.; Keku, T.O.; Mountcastle, S.B.; Galanko, J.; Woosley, J.T.; Schliebe, B.; Lund, P.K.; Sandler, R.S. Nonsteroidal anti-inflammatory drugs, apoptosis, and colorectal adenomas. Gastroenterology 2002, 123, 1770–1777. [Google Scholar] [CrossRef]
- Shen, X.G.; Wang, C.; Li, Y.; Wang, L.; Zhou, B.; Xu, B.; Jiang, X.; Zhou, Z.G.; Sun, X.F. Downregulation of caspase-9 is a frequent event in patients with stage II colorectal cancer and correlates with poor clinical outcome. Color. Dis. 2010, 12, 1213–1218. [Google Scholar] [CrossRef]
- Xu, B.; Zhou, Z.G.; Li, Y.; Wang, L.; Yang, L.; Zhou, B.; Liu, H.Y.; Song, J.M.; Zeng, Y.J.; Wang, R.; et al. Clinicopathological significance of caspase-8 and caspase-10 expression in rectal cancer. Oncology 2008, 74, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, H.; Takada, Y.; Shishodia, S.; Jayaprakasam, B.; Nair, M.G.; Aggarwal, B.B. Withanolides potentiate apoptosis, inhibit invasion, and abolish osteoclastogenesis through suppression of nuclear factor-ΚB (NF-ΚB) activation and NF-ΚB-regulated gene expression. Mol. Cancer Ther. 2006, 5, 1434–1445. [Google Scholar] [CrossRef]
- Dar, P.A.; Mir, S.A.; Bhat, J.A.; Hamid, A.; Singh, L.R.; Malik, F.; Dar, T.A. An anti-cancerous protein fraction from Withania somnifera induces ROS-dependent mitochondria-mediated apoptosis in human MDA-MB-231 breast cancer cells. Int. J. Biol. Macromol. 2019, 135, 77–87. [Google Scholar] [CrossRef]
- Cheraghi, O.; Dehghan, G.; Mahdavi, M.; Rahbarghazi, R.; Rezabakhsh, A.; Charoudeh, H.N.; Iranshahi, M.; Montazersaheb, S. Potent anti-angiogenic and cytotoxic effect of conferone on human colorectal adenocarcinoma HT-29 cells. Phytomedicine 2016, 23, 398–405. [Google Scholar] [CrossRef]
- Rah, B.; Amin, H.; Yousuf, K.; Khan, S.; Jamwal, G.; Mukherjee, D.; Goswami, A. A Novel MMP-2 Inhibitor 3-azidowithaferin A (3-azidoWA) Abrogates Cancer Cell Invasion and Angiogenesis by Modulating Extracellular Par-4. PLoS ONE 2012, 7, e44039. [Google Scholar] [CrossRef]
- Widodo, N.; Kaur, K.; Shrestha, B.G.; Takagi, Y.; Ishii, T.; Wadhwa, R.; Kaul, S.C. Selective killing of cancer cells by leaf extract of ashwagandha: Identification of a tumor-inhibitory factor and the first molecular insights to its effect. Clin. Cancer Res. 2007, 13, 2298–2306. [Google Scholar] [CrossRef] [PubMed]

| Plant Part/Organ. | Bioactive Compounds | Pharmacological Activity | References |
|---|---|---|---|
| Roots | Withanolide A, withaferin A, withanolide sulfoxide, withanoside IV and VI, withacoagin, dihydrowithanolide D, ixocarpalactone A, glucopyranosyl moieties, glycosides, withasomidienone, 5,7 α-Epoxy-6α, 20α-dihydroxy-1-oxowitha-2, 24-dienolide, ß-sitosterol, ß-sitosterol glucoside, stigmasterol glucoside, Viscosa lactone B, 16ß-acetoxy-17(20)-ene, 6a-hydroxy-5,7a-epoxy, 27 hydroxy withanone, hydroxy, 17-deoxy withaferin A, deoxy withastromonolide, physagulin, benzyl alcohol, 2-phenyl ethanol, benzoic acid, p-hydroxy, phenyl acetic acid, asparagine, choline, palmitic acid, oleic acid, linoleic acid, porphyrine | Anti-inflammatory, memory enhancement, cerebellar ataxia, antiperoxidative, cardiotonic, and antioxidative abilities; also enhances fertility | [2], [32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47] |
| Leaves | Withanolides, withaferin, polyphenols, saponins, alkaloids, steroids, steroidal lactones, flavonoids, glycosides, 27 hydroxy withanone, hydroxy, 17-deoxy withaferin A, deoxy withastromonolide, physagulin, ß-sitosterol, ß-sitosterol glucoside, 2-hydroxy propanol, 2-hydroxy propanoic acid, 1-octanol, benzoic acid, butanedioic acid, phenyl acetic acid, p-hydroxy, phenyl ethano, p-hydroxy benzoic acid, alanine, aspartate, asparagine, choline, palmitic acid, oleic acid, linoleic acid, porphyrine, pheophytin, sterol, TAG, vanillic acid, p-coumaric acid, syringic acid, gallic acid, physagulin, and trigonelline | Antistress, antianxiety, anticarcinogenic activity, antimicrobial, antioxidative ability, anthelmintic, and anti-inflammatory potency | [2] [32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47] |
| Fruits and flowers | Chamase, condensed tannins, peroxidases, proteolytic enzyme, cystine, flavonoids, glutamic acid, amino acids, aspartic acid, alanine glycine, hydroxyproline, isopsoralen, psoralen, proline, tyrosine, and valine | Antimicrobial activity, management of respiratory illness | [2,46,47] |
| Stems | Alkaloids and its derivatives (ashwagandhine, isopelletierine, pseudotropine, [3]-tigloyloxtropine, tropeltigloate, isopelletierine, hygrine, mesoanaferine, choline, somniferine, withanine, withananine, hentriacontane, visamine, withasomnine, somniferinine, somninine, nicotine, cuscohygrine, pseudotropin, anahygrine, anaferine, tropine), glycosides and its derivatives (withanosides I–VII, withanamide), flavonoids (quercetin, 7-hydroxyflavone, kaempferol), and phenolics and their derivatives (coumaric acid, caffeic acid, chlorogenic acid, gallic acid, ferulic acid, catechin) | Used to treat tumors, nocturnal leg cramps, coronary heart diseases, diarrhea, and psychiatric palpitation; improves blood cholesterol levels; antimicrobial; relaxant; antispasmodic; sedative; muscle relaxant; diuretic; strengthens capillary walls, osteoporosis | [2,46,47,48,49] |
| Biological Mechanism | Bioactive Constituents | References |
|---|---|---|
| Downregulation of COX-2 (↓COX-2) | 27-Desoxy-24, 25-dihydrowithaneferin A, 27-O-glucopyranosylviscosalactone B, 4,16-dihydroxy-5 h, 6h-epoxyphysagulin D, diacetylwithaferin A, physagulin D (1→6)-h-D-glucopyranosyl-(1→4)-h-D- glucopyranoside, viscosalactone B, withaferin A, withanolide sulfoxide, withanoside IV | [52,58,59] |
| Downregulation of NF-κB and PI3K/Akt (↓NF-κB, and PI3K/Akt) | Withaferin A, withanolide sulfoxide | [52,53,54] |
| Downregulation of Bcl-2 (↓Bcl-2) | Withaferin A | [52,53,54] |
| Upregulation of apoptosis (↑apoptosis) | Withaferin A, withanolide D, withanone, 4β-hydroxywithanolide E, | [52,60,61] |
| Upregulation of apoptotic caspase-3 gene (↑caspase-3) | Withaferin A | [52,60] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macharia, J.M.; Káposztás, Z.; Bence, R.L. Medicinal Characteristics of Withania somnifera L. in Colorectal Cancer Management. Pharmaceuticals 2023, 16, 915. https://doi.org/10.3390/ph16070915
Macharia JM, Káposztás Z, Bence RL. Medicinal Characteristics of Withania somnifera L. in Colorectal Cancer Management. Pharmaceuticals. 2023; 16(7):915. https://doi.org/10.3390/ph16070915
Chicago/Turabian StyleMacharia, John M., Zsolt Káposztás, and Raposa L. Bence. 2023. "Medicinal Characteristics of Withania somnifera L. in Colorectal Cancer Management" Pharmaceuticals 16, no. 7: 915. https://doi.org/10.3390/ph16070915
APA StyleMacharia, J. M., Káposztás, Z., & Bence, R. L. (2023). Medicinal Characteristics of Withania somnifera L. in Colorectal Cancer Management. Pharmaceuticals, 16(7), 915. https://doi.org/10.3390/ph16070915








