Machine Learning Approaches for Assessing Risk Factors of Adrenal Insufficiency in Patients Undergoing Immune Checkpoint Inhibitor Therapy
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Patients and Data Collection
4.2. Statistical Analysis and Machine Learning Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rodriguez, F. Are PD-1 and PD-L1 Checkpoint Inhibitors as Good as We Thought? Available online: https://labiotech.eu/features/pd-1-pd-l1-checkpoint-inhibitors? (accessed on 15 December 2021).
- Webster, R.M. The immune checkpoint inhibitors: Where are we now? Nat. Rev. Drug Discov. 2014, 13, 883–884. [Google Scholar] [CrossRef] [PubMed]
- Haslam, A.; Prasad, V. Estimation of the Percentage of US Patients With Cancer Who Are Eligible for and Respond to Checkpoint Inhibitor Immunotherapy Drugs. JAMA Netw. Open 2019, 2, e192535. [Google Scholar] [CrossRef] [PubMed]
- König, D.; Läubli, H. Mechanisms of Immune-Related Complications in Cancer Patients Treated with Immune Checkpoint Inhibitors. Pharmacology 2021, 106, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Schachter, J.; Long, G.V.; Arance, A.; Grob, J.J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.; Lotem, M.; et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2015, 372, 2521–2532. [Google Scholar] [CrossRef]
- Brahmer, J.; Reckamp, K.L.; Baas, P.; Crinò, L.; Eberhardt, W.E.; Poddubskaya, E.; Antonia, S.; Pluzanski, A.; Vokes, E.E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 123–135. [Google Scholar] [CrossRef]
- Powles, T.; Durán, I.; van der Heijden, M.S.; Loriot, Y.; Vogelzang, N.J.; De Giorgi, U.; Oudard, S.; Retz, M.M.; Castellano, D.; Bamias, A.; et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): A multicentre, open-label, phase 3 randomised controlled trial. Lancet 2018, 391, 748–757. [Google Scholar] [CrossRef]
- Prete, A.; Salvatori, R. Hypophysitis. In Endotext; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., de Herder, W.W., Dhatariya, K., Dungan, K., Hershman, J.M., Hofland, J., Kalra, S., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Iranzo, P.; Callejo, A.; Assaf, J.D.; Molina, G.; Lopez, D.E.; Garcia-Illescas, D.; Pardo, N.; Navarro, A.; Martinez-Marti, A.; Cedres, S.; et al. Overview of Checkpoint Inhibitors Mechanism of Action: Role of Immune-Related Adverse Events and Their Treatment on Progression of Underlying Cancer. Front. Med. (Lausanne) 2022, 9, 875974. [Google Scholar] [CrossRef]
- Bodis, G.; Toth, V.; Schwarting, A. Role of Human Leukocyte Antigens (HLA) in Autoimmune Diseases. Rheumatol. Ther. 2018, 5, 5–20. [Google Scholar] [CrossRef]
- Rogado, J.; Sánchez-Torres, J.M.; Romero-Laorden, N.; Ballesteros, A.I.; Pacheco-Barcia, V.; Ramos-Leví, A.; Arranz, R.; Lorenzo, A.; Gullón, P.; Donnay, O.; et al. Immune-related adverse events predict the therapeutic efficacy of anti-PD-1 antibodies in cancer patients. Eur. J. Cancer 2019, 109, 21–27. [Google Scholar] [CrossRef]
- Läubli, H.; Koelzer, V.H.; Matter, M.S.; Herzig, P.; Dolder Schlienger, B.; Wiese, M.N.; Lardinois, D.; Mertz, K.D.; Zippelius, A. The T cell repertoire in tumors overlaps with pulmonary inflammatory lesions in patients treated with checkpoint inhibitors. Oncoimmunology 2018, 7, e1386362. [Google Scholar] [CrossRef]
- Jeung, H.-C.; Oh, S.E.; Kim, J.H. Immune-related adverse events: Overview and management strategies for the use of immune checkpoint inhibitors. J. Rheumatic Dis. 2019, 26, 221–234. [Google Scholar] [CrossRef]
- Komiya, K.; Nakamura, T.; Abe, T.; Ogusu, S.; Nakashima, C.; Takahashi, K.; Kimura, S.; Sueoka-Aragane, N. Discontinuation due to immune-related adverse events is a possible predictive factor for immune checkpoint inhibitors in patients with non-small cell lung cancer. Thorac. Cancer 2019, 10, 1798–1804. [Google Scholar] [CrossRef]
- Martins, F.; Sofiya, L.; Sykiotis, G.P.; Lamine, F.; Maillard, M.; Fraga, M.; Shabafrouz, K.; Ribi, C.; Cairoli, A.; Guex-Crosier, Y.; et al. Adverse effects of immune-checkpoint inhibitors: Epidemiology, management and surveillance. Nat. Rev. Clin. Oncol. 2019, 16, 563–580. [Google Scholar] [CrossRef]
- Grouthier, V.; Lebrun-Vignes, B.; Moey, M.; Johnson, D.B.; Moslehi, J.J.; Salem, J.E.; Bachelot, A. Immune Checkpoint Inhibitor-Associated Primary Adrenal Insufficiency: WHO VigiBase Report Analysis. Oncologist 2020, 25, 696–701. [Google Scholar] [CrossRef]
- Hobbs, K.B.; Yackzan, S. Adrenal Insufficiency: Immune Checkpoint Inhibitors and Immune-Related Adverse Event Management. Clin. J. Oncol. Nurs. 2020, 24, 240–243. [Google Scholar] [CrossRef]
- Shi, Y.; Shen, M.; Zheng, X.; Yang, T. Immune Checkpoint Inhibitor-Induced Adrenalitis and Primary Adrenal Insufficiency: Systematic Review and Optimal Management. Endocr. Pract. 2021, 27, 165–169. [Google Scholar] [CrossRef]
- Islam, M.R.; Kabir, M.A.; Ahmed, A.; Kamal, A.R.M.; Wang, H.; Ulhaq, A. Depression detection from social network data using machine learning techniques. Health Inf. Sci. Syst. 2018, 6, 8. [Google Scholar] [CrossRef]
- Praveen, S.P.; Srinivasu, P.N.; Shafi, J.; Wozniak, M.; Ijaz, M.F. ResNet-32 and FastAI for diagnoses of ductal carcinoma from 2D tissue slides. Sci. Rep. 2022, 12, 20804. [Google Scholar] [CrossRef]
- Wiesweg, M.; Mairinger, F.; Reis, H.; Goetz, M.; Walter, R.F.H.; Hager, T.; Metzenmacher, M.; Eberhardt, W.E.E.; McCutcheon, A.; Köster, J.; et al. Machine learning-based predictors for immune checkpoint inhibitor therapy of non-small-cell lung cancer. Ann. Oncol. 2019, 30, 655–657. [Google Scholar] [CrossRef]
- Polano, M.; Chierici, M.; Dal Bo, M.; Gentilini, D.; Di Cintio, F.; Baboci, L.; Gibbs, D.L.; Furlanello, C.; Toffoli, G. A Pan-Cancer Approach to Predict Responsiveness to Immune Checkpoint Inhibitors by Machine Learning. Cancers 2019, 11, 1562. [Google Scholar] [CrossRef]
- Pisters, R.; Lane, D.A.; Nieuwlaat, R.; de Vos, C.B.; Crijns, H.J.; Lip, G.Y. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: The Euro Heart Survey. Chest 2010, 138, 1093–1100. [Google Scholar] [CrossRef] [PubMed]
- Peterson, D.; Geison, E. Pharmacist Interventions to Reduce Modifiable Bleeding Risk Factors Using HAS-BLED in Patients Taking Warfarin. Fed. Pract. 2017, 34, S16–s20. [Google Scholar] [PubMed]
- Yang, S. The Receiver Operating Characteristic (ROC) Curve. Available online: https://pulmonarychronicles.com/index.php/pulmonarychronicles/article/view/391/848 (accessed on 26 May 2023).
- Lau, J.Y.-W. The Value of Risk Scores to Predict Clinical Outcomes in Patients with Variceal and Non-Variceal Upper Gastrointestinal Bleeding. Clin. Endosc. 2021, 54, 145–146. [Google Scholar] [CrossRef] [PubMed]
- Ahmadzadeh, M.; Johnson, L.A.; Heemskerk, B.; Wunderlich, J.R.; Dudley, M.E.; White, D.E.; Rosenberg, S.A. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood 2009, 114, 1537–1544. [Google Scholar] [CrossRef]
- Staron, M.M.; Gray, S.M.; Marshall, H.D.; Parish, I.A.; Chen, J.H.; Perry, C.J.; Cui, G.; Li, M.O.; Kaech, S.M. The transcription factor FoxO1 sustains expression of the inhibitory receptor PD-1 and survival of antiviral CD8(+) T cells during chronic infection. Immunity 2014, 41, 802–814. [Google Scholar] [CrossRef]
- Ohaegbulam, K.C.; Assal, A.; Lazar-Molnar, E.; Yao, Y.; Zang, X. Human cancer immunotherapy with antibodies to the PD-1 and PD-L1 pathway. Trends Mol. Med. 2015, 21, 24–33. [Google Scholar] [CrossRef]
- Dong, P.; Xiong, Y.; Yue, J.; Hanley, S.J.B.; Watari, H. Tumor-Intrinsic PD-L1 Signaling in Cancer Initiation, Development and Treatment: Beyond Immune Evasion. Front. Oncol. 2018, 8, 386. [Google Scholar] [CrossRef]
- Keir, M.E.; Butte, M.J.; Freeman, G.J.; Sharpe, A.H. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 2008, 26, 677–704. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef]
- Herbst, R.S.; Giaccone, G.; de Marinis, F.; Reinmuth, N.; Vergnenegre, A.; Barrios, C.H.; Morise, M.; Felip, E.; Andric, Z.; Geater, S.; et al. Atezolizumab for First-Line Treatment of PD-L1-Selected Patients with NSCLC. N. Engl. J. Med. 2020, 383, 1328–1339. [Google Scholar] [CrossRef]
- De Velasco, G.; Je, Y.; Bossé, D.; Awad, M.M.; Ott, P.A.; Moreira, R.B.; Schutz, F.; Bellmunt, J.; Sonpavde, G.P.; Hodi, F.S.; et al. Comprehensive Meta-analysis of Key Immune-Related Adverse Events from CTLA-4 and PD-1/PD-L1 Inhibitors in Cancer Patients. Cancer Immunol. Res. 2017, 5, 312–318. [Google Scholar] [CrossRef]
- Barroso-Sousa, R.; Barry, W.T.; Garrido-Castro, A.C.; Hodi, F.S.; Min, L.; Krop, I.E.; Tolaney, S.M. Incidence of Endocrine Dysfunction Following the Use of Different Immune Checkpoint Inhibitor Regimens: A Systematic Review and Meta-analysis. JAMA Oncol. 2018, 4, 173–182. [Google Scholar] [CrossRef]
- Lee, D.J.; Lee, H.J., Jr.; Farmer, J.R.; Reynolds, K.L. Mechanisms Driving Immune-Related Adverse Events in Cancer Patients Treated with Immune Checkpoint Inhibitors. Curr. Cardiol. Rep. 2021, 23, 98. [Google Scholar] [CrossRef]
- Wang, D.Y.; Salem, J.E.; Cohen, J.V.; Chandra, S.; Menzer, C.; Ye, F.; Zhao, S.; Das, S.; Beckermann, K.E.; Ha, L.; et al. Fatal Toxic Effects Associated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis. JAMA Oncol. 2018, 4, 1721–1728. [Google Scholar] [CrossRef]
- Ribas, A.; Wolchok, J.; Robert, C.; Kefford, K.; Hamid, O.; Daud, A.; Hwu, W.; Weber, J.; Joshua, A.; Gangadhar, T. Updated clinical efficacy of the anti-PD-1 monoclonal antibody pembrolizumab (pembro, MK-3475) in 411 patients (pts) with melanoma (MEL). Pigment. Cell Melanoma Res. 2014, 27, 1222. [Google Scholar]
- Xing, P.; Zhang, F.; Wang, G.; Xu, Y.; Li, C.; Wang, S.; Guo, Y.; Cai, S.; Wang, Y.; Li, J. Incidence rates of immune-related adverse events and their correlation with response in advanced solid tumours treated with NIVO or NIVO+IPI: A systematic review and meta-analysis. J. Immunother. Cancer 2019, 7, 341. [Google Scholar] [CrossRef]
- Cui, K.; Wang, Z.; Zhang, Q.; Zhang, X. Immune checkpoint inhibitors and adrenal insufficiency: A large-sample case series study. Ann. Transl. Med. 2022, 10, 251. [Google Scholar] [CrossRef]
- Peng, K.; Chen, K.; Teply, B.A.; Yee, G.C.; Farazi, P.A.; Lyden, E.R. Impact of Proton Pump Inhibitor Use on the Effectiveness of Immune Checkpoint Inhibitors in Advanced Cancer Patients. Ann. Pharmacother. 2022, 56, 377–386. [Google Scholar] [CrossRef]
- Raghavan, R.; Shawar, S. Mechanisms of Drug-Induced Interstitial Nephritis. Adv. Chronic Kidney Dis. 2017, 24, 64–71. [Google Scholar] [CrossRef]
- Shirali, A.C.; Perazella, M.A.; Gettinger, S. Association of Acute Interstitial Nephritis With Programmed Cell Death 1 Inhibitor Therapy in Lung Cancer Patients. Am. J. Kidney Dis. 2016, 68, 287–291. [Google Scholar] [CrossRef]
- Hopkins, A.M.; Rowland, A.; Kichenadasse, G.; Wiese, M.D.; Gurney, H.; McKinnon, R.A.; Karapetis, C.S.; Sorich, M.J. Predicting response and toxicity to immune checkpoint inhibitors using routinely available blood and clinical markers. Br. J. Cancer 2017, 117, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Sonino, N.; Fava, G.A.; Fallo, F.; Franceschetto, A.; Belluardo, P.; Boscaro, M. Effect of the serotonin antagonists ritanserin and ketanserin in Cushing’s disease. Pituitary 2000, 3, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Cohrs, S.; Röher, C.; Jordan, W.; Meier, A.; Huether, G.; Wuttke, W.; Rüther, E.; Rodenbeck, A. The atypical antipsychotics olanzapine and quetiapine, but not haloperidol, reduce ACTH and cortisol secretion in healthy subjects. Psychopharmacology 2006, 185, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Wetzel, H.; Wiesner, J.; Hiemke, C.; Benkert, O. Acute antagonism of dopamine D2-like receptors by amisulpride: Effects on hormone secretion in healthy volunteers. J. Psychiatr. Res. 1994, 28, 461–473. [Google Scholar] [CrossRef] [PubMed]
- Richelson, E.; Souder, T. Binding of antipsychotic drugs to human brain receptors focus on newer generation compounds. Life Sci. 2000, 68, 29–39. [Google Scholar] [CrossRef]
- Allolio, B.; Deuss, U.; Kaulen, D.; Winkelmann, W. Effect of meclastine, a selective H1 receptor antagonist, upon ACTH release. Clin. Endocrinol. 1983, 19, 239–245. [Google Scholar] [CrossRef]
- Laakmann, G.; Wittmann, M.; Schoen, H.W.; Zygan, K.; Weiss, A.; Meissner, R.; Mueller, O.A.; Stalla, G.K. Effects of receptor blockers (methysergide, propranolol, phentolamine, yohimbine and prazosin) on desimipramine-induced pituitary hormone stimulation in humans--III. Hypothalamo-pituitary-adrenocortical axis. Psychoneuroendocrinology 1986, 11, 475–489. [Google Scholar] [CrossRef]
- al-Damluji, S. Adrenergic control of the secretion of anterior pituitary hormones. Baillieres Clin. Endocrinol. Metab. 1993, 7, 355–392. [Google Scholar] [CrossRef]
- Perez, D.M.; Papay, R.S.; Shi, T. alpha1-Adrenergic receptor stimulates interleukin-6 expression and secretion through both mRNA stability and transcriptional regulation: Involvement of p38 mitogen-activated protein kinase and nuclear factor-kappaB. Mol. Pharmacol. 2009, 76, 144–152. [Google Scholar] [CrossRef]
- Ricci, A.; Bronzetti, E.; Conterno, A.; Greco, S.; Mulatero, P.; Schena, M.; Schiavone, D.; Tayebati, S.K.; Veglio, F.; Amenta, F. alpha1-adrenergic receptor subtypes in human peripheral blood lymphocytes. Hypertension 1999, 33, 708–712. [Google Scholar] [CrossRef]
- Grisanti, L.A.; Perez, D.M.; Porter, J.E. Modulation of immune cell function by α(1)-adrenergic receptor activation. Curr. Top. Membr. 2011, 67, 113–138. [Google Scholar] [CrossRef]
- Stiglic, G.; Kocbek, S.; Pernek, I.; Kokol, P. Comprehensive decision tree models in bioinformatics. PLoS ONE 2012, 7, e33812. [Google Scholar] [CrossRef]
- Tang, J.; Tian, Y.; Liu, X.; Li, D.; Lv, J.; Kou, G. Improved multi-view privileged support vector machine. Neural Netw. 2018, 106, 96–109. [Google Scholar] [CrossRef]
- Gong, C.; Zhou, M.; Hu, Y.; Ren, Z.; Ren, J.; Yao, M. Elastic net-based identification of GAMT as potential diagnostic marker for early-stage gastric cancer. Biochem. Biophys. Res. Commun. 2022, 591, 7–12. [Google Scholar] [CrossRef]
- Common Terminology Criteria for Adverse Events (CTCAE), Version 5.0. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf (accessed on 10 October 2021).
| Complication | No Complication | p-Value | ||
|---|---|---|---|---|
| (n = 14) | (n = 186) | |||
| Sex | 0.500 | |||
| Male | 10 (71.4) | 148 (79.6) | ||
| Female | 4 (28.6) | 38 (20.4) | ||
| Age ≥ 65 years | 0.570 | |||
| Yes | 10 (71.4) | 111 (59.7) | ||
| No | 4 (28.6) | 75 (40.3) | ||
| Body mass index ≥ 25 kg/m2 | 0.770 | |||
| Yes | 6 (46.2) | 69 (39.7) | ||
| No | 7 (53.8) | 105 (60.3) | ||
| Smoking history | 1.000 | |||
| Yes | 2 (14.3) | 37 (19.9) | ||
| No | 12 (85.7) | 149 (80.1) | ||
| Alcohol history | 1.000 | |||
| Yes | 1 (7.1) | 16 (8.6) | ||
| No | 13 (92.9) | 170 (91.4) | ||
| Comorbidities | ||||
| Angina | 1.000 | |||
| Yes | 0 (0) | 1 (0.5) | ||
| No | 14 (100) | 185 (99.5) | ||
| Asthma | 0.140 | |||
| Yes | 1 (7.1) | 1 (0.5) | ||
| No | 13 (92.9) | 185 (99.5) | ||
| Bronchiolitis | 0.140 | |||
| Yes | 1 (7.1) | 1 (0.5) | ||
| No | 13 (92.9) | 185 (99.5) | ||
| Buerger’s disease | 1.000 | |||
| Yes | 0 (0) | 1 (0.5) | ||
| No | 14 (100) | 185 (99.5) | ||
| COPD | 0.130 | |||
| Yes | 3 (21.4) | 16 (8.6) | ||
| No | 11 (78.6) | 170 (91.4) | ||
| Crohn’s disease | 1.000 | |||
| Yes | 0 (0) | 1 (0.5) | ||
| No | 14 (100) | 185 (99.5) | ||
| Diabetes mellitus | 0.760 | |||
| Yes | 4 (28.6) | 47 (25.3) | ||
| No | 10 (71.4) | 139 (74.7) | ||
| Gout | 1.000 | |||
| Yes | 0 (0) | 4 (2.2) | ||
| No | 14 (100) | 182 (97.8) | ||
| Heart Disease | 0.050 | |||
| Yes | 3 (21.4) | 10 (5.4) | ||
| No | 11 (78.6) | 176 (94.6) | ||
| Hepatitis B | 1.000 | |||
| Yes | 0 (0) | 4 (2.2) | ||
| No | 14 (100) | 182 (97.8) | ||
| Hepatitis C | 1.000 | |||
| Yes | 0 (0) | 2 (1.1) | ||
| No | 14 (100) | 184 (98.9) | ||
| Human immunodeficiency virus | 1.000 | |||
| Yes | 0 (0) | 2 (1.1) | ||
| No | 14 (100) | 184 (98.9) | ||
| Hyperlipidemia | 0.370 | |||
| Yes | 0 (0) | 21 (11.3) | ||
| No | 14 (100) | 165 (88.7) | ||
| Hypertension | 0.390 | |||
| Yes | 7 (50) | 66 (35.5) | ||
| No | 7 (50) | 120 (64.5) | ||
| Hypo-, hyperthyroidism | 1.000 | |||
| Yes | 1 (7.1) | 15 (8.1) | ||
| No | 13 (92.9) | 171 (91.9) | ||
| Myasthenia gravis | 1.000 | |||
| Yes | 0 (0) | 1 (0.5) | ||
| No | 14 (100) | 185 (99.5) | ||
| Myocardial infarction | 1.000 | |||
| Yes | 0 (0) | 4 (2.2) | ||
| No | 14 (100) | 182 (97.8) | ||
| Osteoporosis | 1.000 | |||
| Yes | 0 (0) | 2 (1.1) | ||
| No | 14 (100) | 184 (98.9) | ||
| Parkinson’s disease | 1.000 | |||
| Yes | 0 (0) | 1 (0.5) | ||
| No | 14 (100) | 185 (99.5) | ||
| Renal failure | 1.000 | |||
| Yes | 0 (0) | 5 (2.7) | ||
| No | 14 (100) | 181 (97.3) | ||
| Tuberculosis | 1.000 | |||
| Yes | 0 (0) | 1 (0.5) | ||
| No | 14 (100) | 185 (99.5) | ||
| Co-medications | ||||
| 5-HT₃ Antagonists | 0.630 | |||
| Yes | 2 (14.3) | 17 (9.1) | ||
| No | 12 (85.7) | 169 (90.9) | ||
| 5-HT₄ agonists | 1.000 | |||
| Yes | 0 (0) | 3 (1.6) | ||
| No | 14 (100) | 183 (98.4) | ||
| 5α-Reductase inhibitors | 0.230 | |||
| Yes | 2 (14.3) | 11 (5.9) | ||
| No | 12 (85.7) | 175 (94.1) | ||
| ACE inhibitors/ARBs | 1.000 | |||
| Yes | 1 (7.1) | 14 (7.5) | ||
| No | 13 (92.9) | 172 (92.5) | ||
| Antibiotics | 0.210 | |||
| Yes | 3 (21.4) | 20 (10.8) | ||
| No | 11 (78.6) | 166 (89.2) | ||
| Anticoagulants | 0.100 | |||
| Yes | 4 (28.6) | 23 (12.4) | ||
| No | 10 (71.4) | 163 (87.6) | ||
| Antiepileptics | 1.000 | |||
| Yes | 0 (0) | 1 (0.5) | ||
| No | 14 (100) | 185 (99.5) | ||
| Antihistamines | 0.610 | |||
| Yes | 0 (0) | 15 (8.1) | ||
| No | 14 (100) | 171 (91.9) | ||
| Antipsychotics | 0.030 | |||
| Yes | 2 (14.3) | 2 (1.1) | ||
| No | 12 (85.7) | 184 (98.9) | ||
| Antiviral | 1.000 | |||
| Yes | 0 (0) | 3 (1.6) | ||
| No | 14 (100) | 183 (98.4) | ||
| Aspirin | 0.250 | |||
| Yes | 1 (7.1) | 3 (1.6) | ||
| No | 13 (92.9) | 183 (98.4) | ||
| Benzodiazepines | 0.060 | |||
| Yes | 4 (28.6) | 19 (10.2) | ||
| No | 10 (71.4) | 167 (89.8) | ||
| D2 antagonists | 1.000 | |||
| Yes | 0 (0) | 2 (1.1) | ||
| No | 14 (100) | 184 (98.9) | ||
| Diuretics | 0.230 | |||
| Yes | 2 (14.3) | 11 (5.9) | ||
| No | 12 (85.7) | 175 (94.1) | ||
| Dopamine | 1.000 | |||
| Yes | 0 (0) | 3 (1.6) | ||
| No | 14 (100) | 183 (98.4) | ||
| Metformin | 0.690 | |||
| Yes | 2 (14.3) | 23 (12.4) | ||
| No | 12 (85.7) | 163 (87.6) | ||
| NSAIDs | 0.470 | |||
| Yes | 3 (21.4) | 29 (15.6) | ||
| No | 11 (78.6) | 157 (84.4) | ||
| Opioids | 1.000 | |||
| Yes | 10 (71.4) | 128 (68.8) | ||
| No | 4 (28.6) | 58 (31.2) | ||
| P2Y12 inhibitors | 0.590 | |||
| Yes | 1 (7.1) | 11 (5.9) | ||
| No | 13 (92.9) | 175 (94.1) | ||
| PPIs | 0.020 | |||
| Yes | 9 (64.3) | 60 (32.3) | ||
| No | 5 (35.7) | 126 (67.7) | ||
| SSRIs/SNRIs | 0.400 | |||
| Yes | 1 (7.1) | 6 (3.2) | ||
| No | 13 (92.9) | 180 (96.8) | ||
| Statins | 0.470 | |||
| Yes | 1 (7.1) | 33 (17.7) | ||
| No | 13 (92.9) | 153 (82.3) | ||
| Thyroid-related medications | 1.000 | |||
| Yes | 1 (7.1) | 12 (6.5) | ||
| No | 13 (92.9) | 174 (93.5) | ||
| Tricyclic antidepressant | 1.000 | |||
| Yes | 0 (0) | 1 (0.5) | ||
| No | 14 (100) | 185 (99.5) | ||
| Zolpidem | 1.000 | |||
| Yes | 0 (0) | 3 (1.6) | ||
| No | 14 (100) | 183 (98.4) | ||
| α-blockers | 0.010 | |||
| Yes | 5 (35.7) | 16 (8.6) | ||
| No | 9 (64.3) | 170 (91.4) | ||
| β-blockers | 0.450 | |||
| Yes | 1 (7.1) | 7 (3.8) | ||
| No | 13 (92.9) | 179 (96.2) | ||
| Cancer type | 0.860 | |||
| Bladder cancer | 1 (7.1) | 15 (8.1) | ||
| Colon cancer | 0 (0) | 3 (1.6) | ||
| Hepatocellular cancer | 0 (0) | 12 (6.5) | ||
| Lung cancer | 10 (71.4) | 94 (50.5) | ||
| Pancreatic cancer | 0 (0) | 2 (1.1) | ||
| Rectal cancer | 0 (0) | 11 (5.9) | ||
| Stomach | 1 (7.1) | 8 (4.3) | ||
| Others | 2 (14.3) | 40 (21.5) | ||
| Cancer stage | 0.290 | |||
| 2 | 0 (0) | 1 (0.5) | ||
| 3 | 2 (14.3) | 11 (6) | ||
| 4 | 12 (85.7) | 171 (93.4) | ||
| ECOGPS | 0.160 | |||
| 0 | 0 (0) | 1 (0.5) | ||
| 1 | 9 (64.3) | 155 (84.2) | ||
| 2 | 3 (21.4) | 16 (8.7) | ||
| 3 | 2 (14.3) | 12 (6.5) |
| Characteristics | Unadjusted OR (95% CI) | Adjusted OR (95% CI) | Risk Score (Pt) |
|---|---|---|---|
| Female | 1.16 (0.463–5.240) | 3.47 (0.860–13.982) | 1 |
| Age ≥ 65 years | 1.69 (0.511–5.586) | 3.08 (0.675–14.085) | 1 |
| Antipsychotics | 15.33 (1.984–118.526) * | 8.36 (0.805–86.816) | 3 |
| PPIs | 3.78 (1.214–11.768) * | 4.46 (1.306–15.247) * | 1 |
| α-blockers | 5.90 (1.765–19.743) * | 6.03 (1.552–23.446) * | 2 |
| AUROC (95% CI) | AUPRC (95% CI) | |
|---|---|---|
| Logistic regression | 0.75 (0.605–0.896) | 0.48 (0.300–0.654) |
| Elastic net | 0.76 (0.637–0.891) | 0.51 (0.323–0.702) |
| Random forest | 0.69 (0.559–0.827) | 0.49 (0.314–0.676) |
| SVM (linear) | 0.53 (0.304–0.745) | 0.36 (0.209–0.514) |
| SVM (radial) | 0.64 (0.459–0.830) | 0.47 (0.282–0.654) |
| Method | Hyperparameter | |
|---|---|---|
| Model Specification and Search Grids | Selected Values | |
| Elastic net | λ: 100 equally spaced values in logarithmic scale between 10−4 and 0 | λ: 0.129155 |
| α: 0, 0.2, 0.4, 0.6, 0.8, 1 | α: 0 | |
| Random forest | mtry: 1–5 | mtry: 2 |
| SVM with linear kernel | C: 0, 0.001, 0.005, 0.01, 0.05, 0.1, 0.25, 0.5, 0.75, 1, 1.25, 1.5, 1.75, 2, 5 | C: 0.5 |
| SVM with radial kernel | Sigma: 2−15, 2−13, 2−11, 2−9, 2−7, 2−5, 2−3, 2−1, 2, 23 | Sigma: 3.051758 × 10−5 |
| C: 2−5, 2−3, 2−1, 2, 23, 25, 27, 29, 211, 213, 215 | C: 0.03125 | |
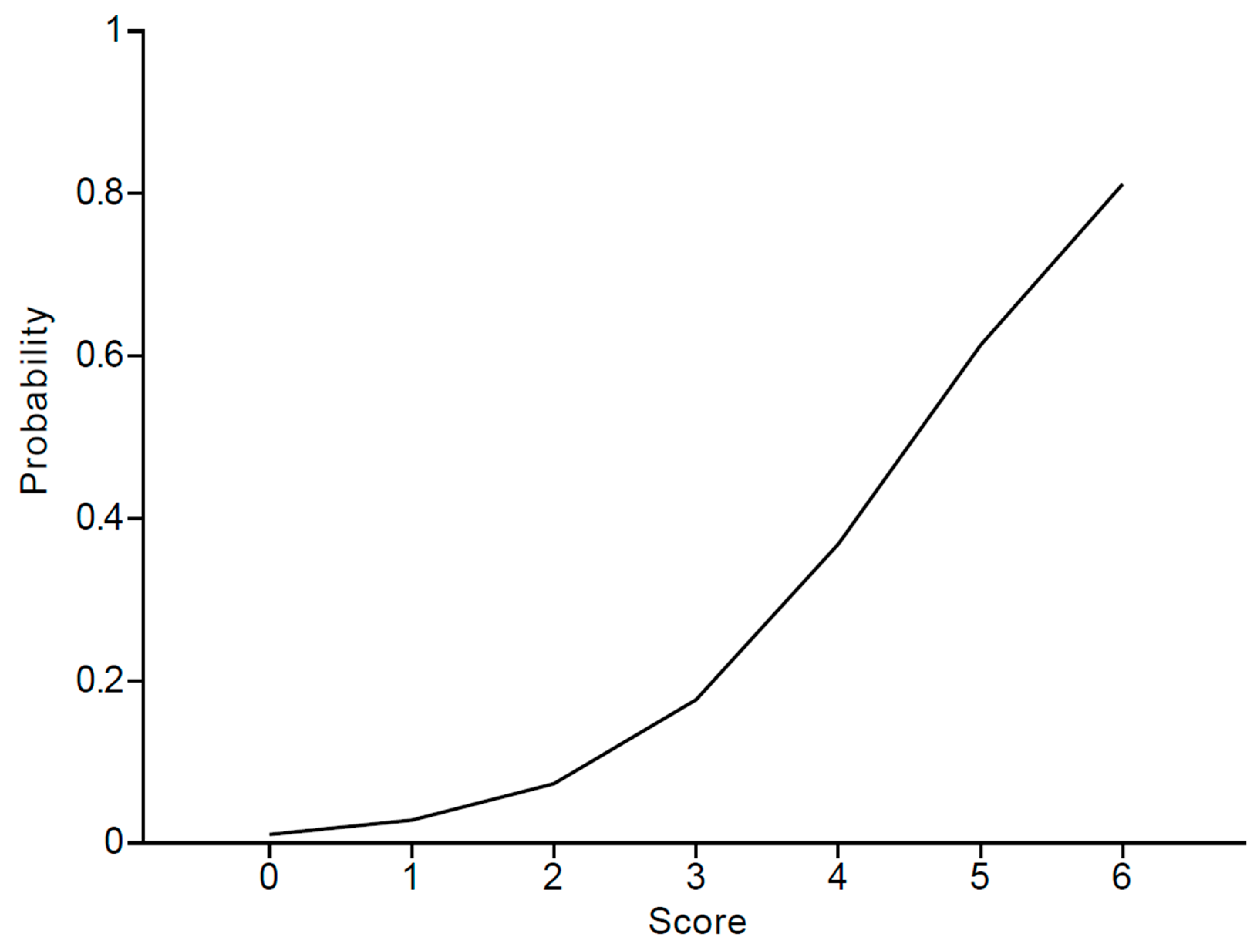
| Score | 0 | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|---|
| Risk probability (%) | 1.1 | 2.8 | 7.3 | 17.6 | 36.8 | 61.3 | 81.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, W.; Cho, Y.A.; Min, K.H.; Kim, D.-C.; Lee, K.-E. Machine Learning Approaches for Assessing Risk Factors of Adrenal Insufficiency in Patients Undergoing Immune Checkpoint Inhibitor Therapy. Pharmaceuticals 2023, 16, 1097. https://doi.org/10.3390/ph16081097
Kim W, Cho YA, Min KH, Kim D-C, Lee K-E. Machine Learning Approaches for Assessing Risk Factors of Adrenal Insufficiency in Patients Undergoing Immune Checkpoint Inhibitor Therapy. Pharmaceuticals. 2023; 16(8):1097. https://doi.org/10.3390/ph16081097
Chicago/Turabian StyleKim, Woorim, Young Ah Cho, Kyung Hyun Min, Dong-Chul Kim, and Kyung-Eun Lee. 2023. "Machine Learning Approaches for Assessing Risk Factors of Adrenal Insufficiency in Patients Undergoing Immune Checkpoint Inhibitor Therapy" Pharmaceuticals 16, no. 8: 1097. https://doi.org/10.3390/ph16081097
APA StyleKim, W., Cho, Y. A., Min, K. H., Kim, D.-C., & Lee, K.-E. (2023). Machine Learning Approaches for Assessing Risk Factors of Adrenal Insufficiency in Patients Undergoing Immune Checkpoint Inhibitor Therapy. Pharmaceuticals, 16(8), 1097. https://doi.org/10.3390/ph16081097





