Calcium Electrochemotherapy for Tumor Eradication and the Potential of High-Frequency Nanosecond Protocols
Abstract
1. Introduction
2. Results
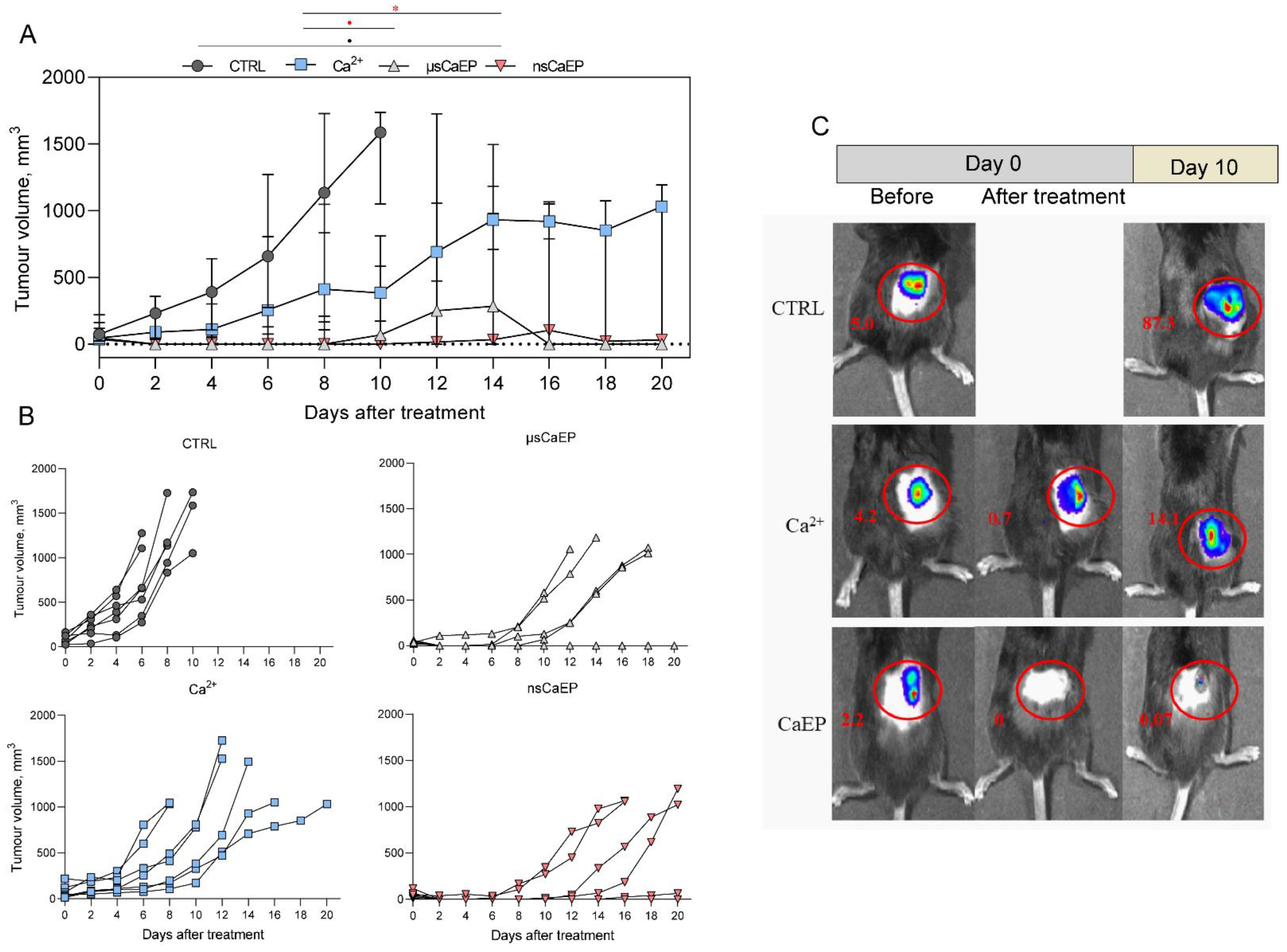
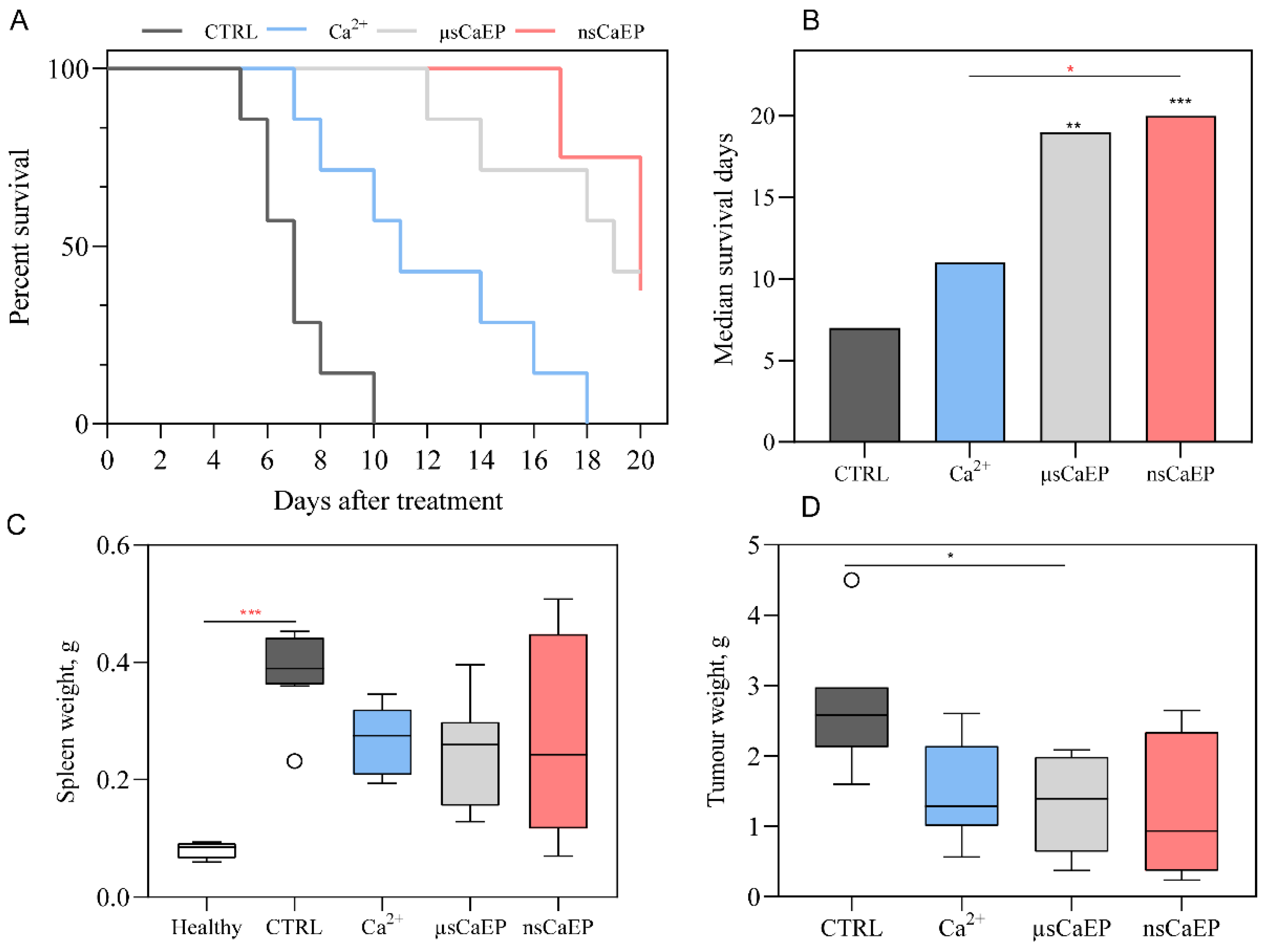
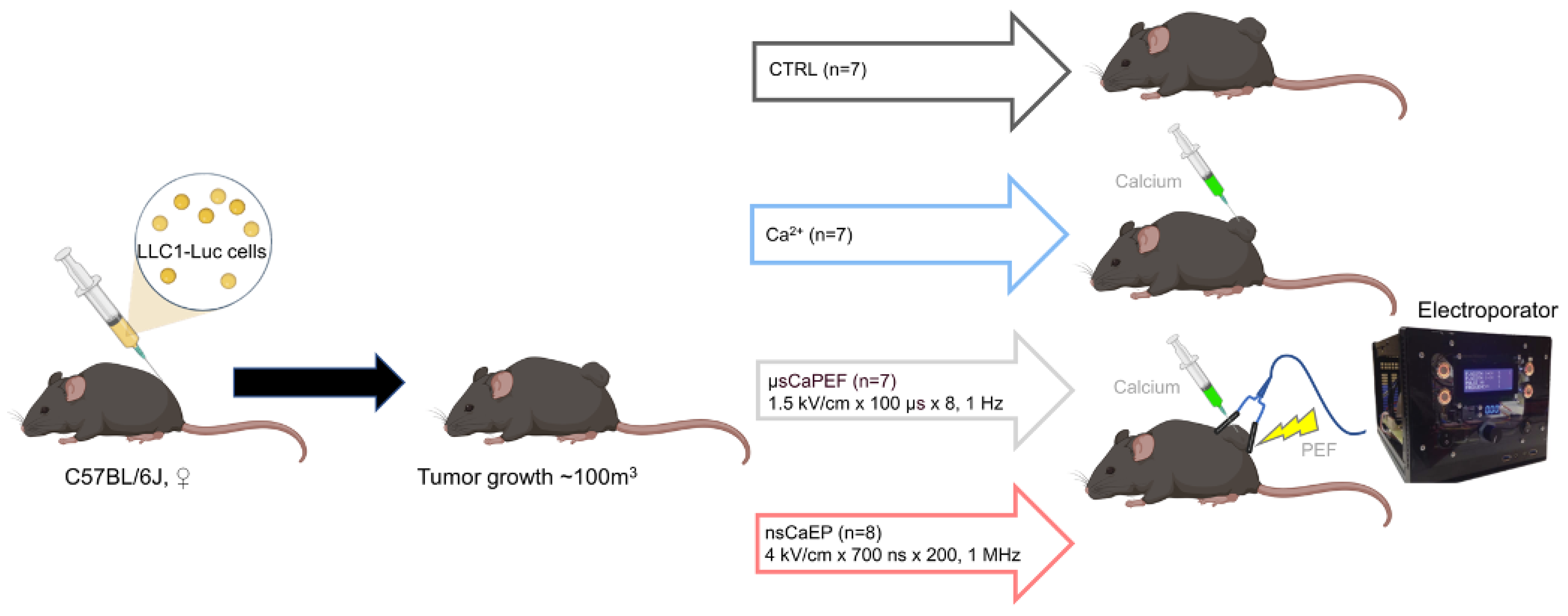
2.1. Tumor Reduction and Increased Survival by Different Modalities of Calcium Electroporation
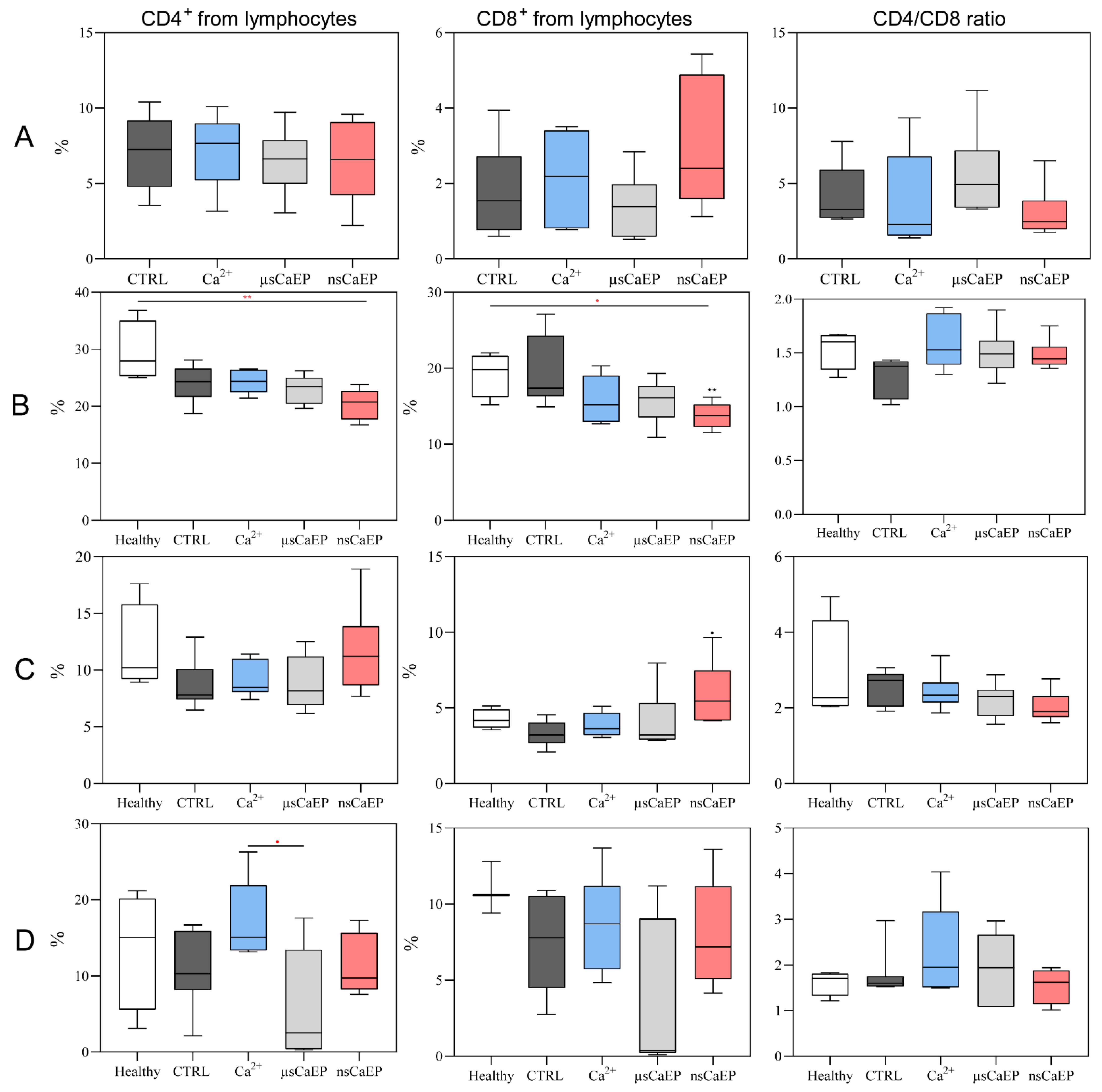
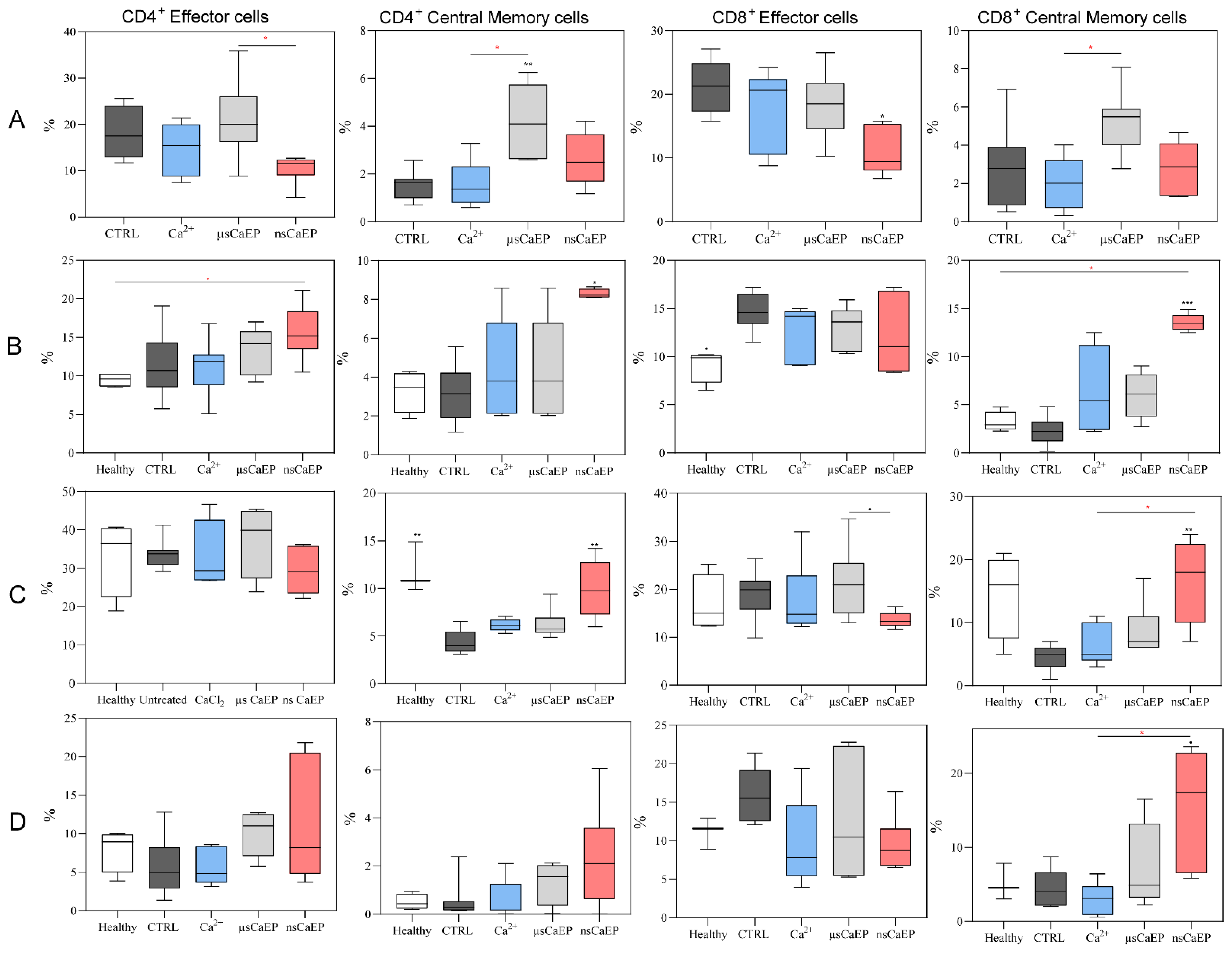
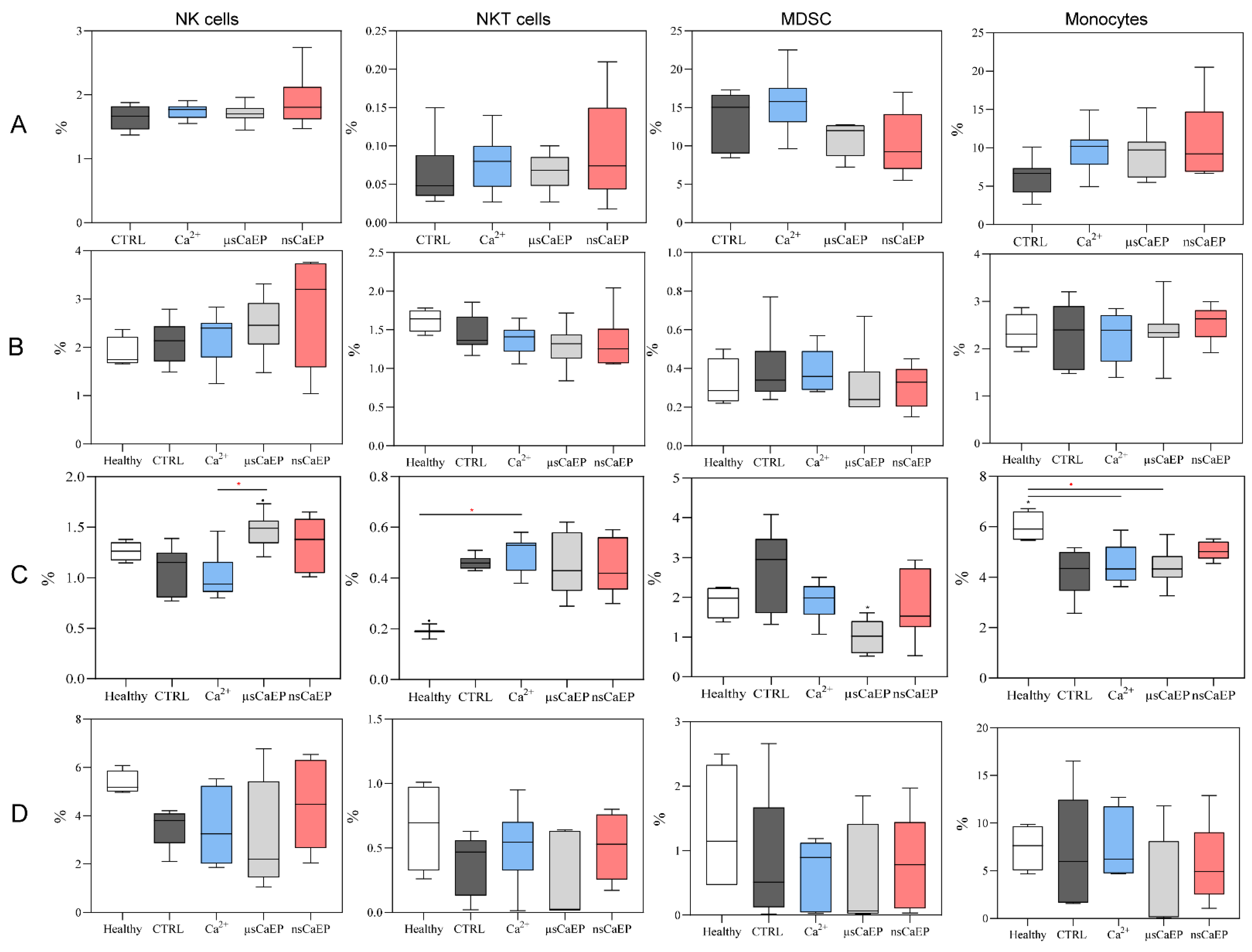
2.2. Immune Cell Subsets in Spleen, Lymph Nodes, Blood, and Tumors
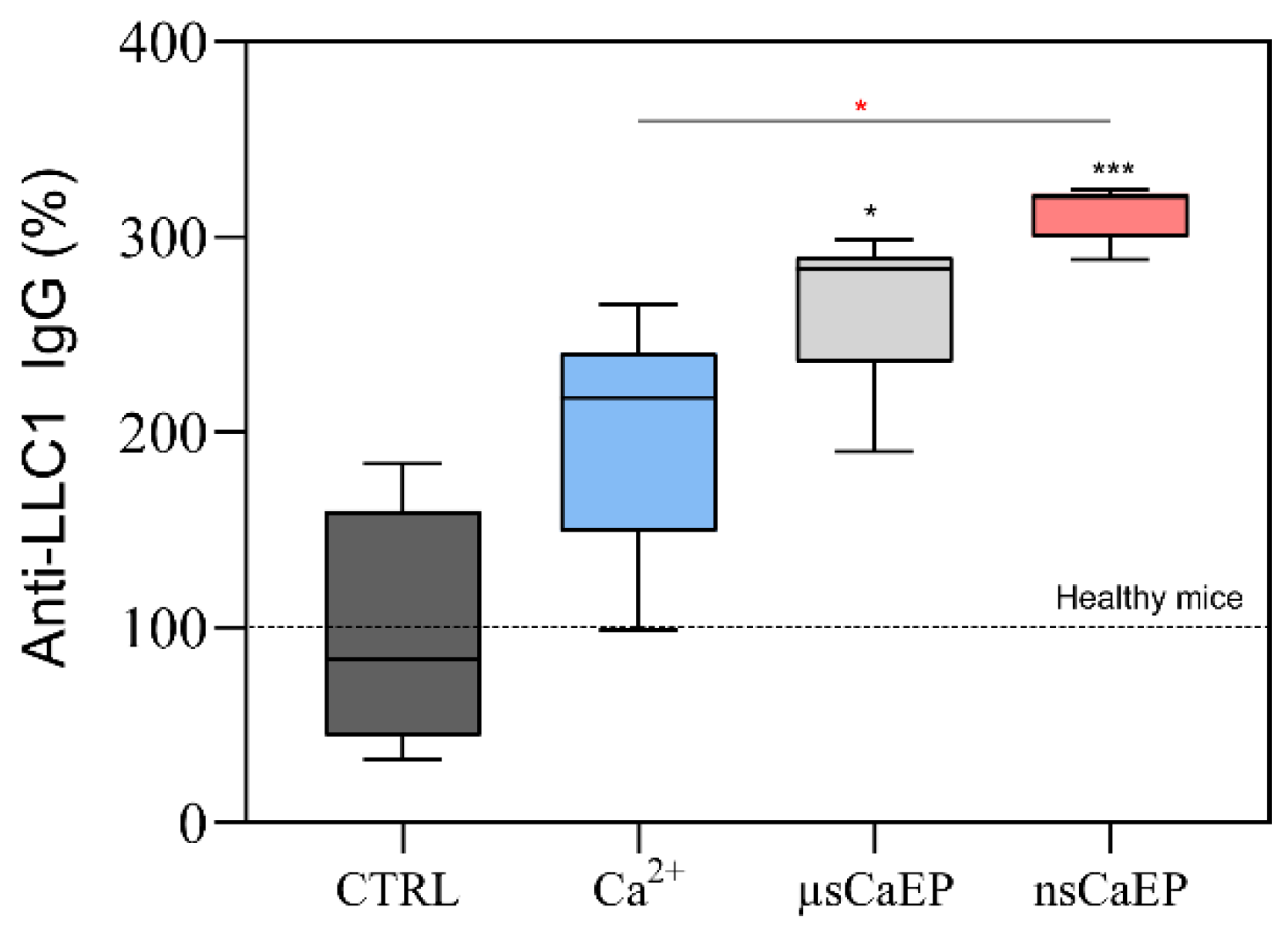
2.3. Calcium Electroporation Stimulates Humoral Immune Response
3. Discussion
4. Materials and Methods
4.1. Electroporation Setup and Parameters
4.2. Cells
4.3. Animals and Tumor Model
4.4. Tissue Preparation
4.5. Flow Cytometry
4.6. Antitumor Antibodies Determination
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Weiderpass, E.; Soerjomataram, I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer 2021, 127, 3029–3030. [Google Scholar] [CrossRef]
- Bidram, E.; Esmaeili, Y.; Ranji-Burachaloo, H.; Al-Zaubai, N.; Zarrabi, A.; Stewart, A.; Dunstan, D.E. A concise review on cancer treatment methods and delivery systems. J. Drug Deliv. Sci. Technol. 2019, 54, 101350. [Google Scholar] [CrossRef]
- Debela, D.T.; Muzazu, S.G.Y.; Heraro, K.D.; Ndalama, M.T.; Mesele, B.W.; Haile, D.C.; Kitui, S.K.; Manyazewal, T. New approaches and procedures for cancer treatment: Current perspectives. SAGE Open Med. 2021, 9, 20503121211034366. [Google Scholar] [CrossRef]
- Cemazar, M.; Sersa, G. Recent Advances in Electrochemotherapy. Bioelectricity 2019, 1, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Falk, H.; Forde, P.F.; Bay, M.L.; Mangalanathan, U.M.; Hojman, P.; Soden, D.M.; Gehl, J. Calcium electroporation induces tumor eradication, long-lasting immunity and cytokine responses in the CT26 colon cancer mouse model. Oncoimmunology 2017, 6, e1301332. [Google Scholar] [CrossRef] [PubMed]
- Frandsen, S.K.; Vissing, M.; Gehl, J. A comprehensive review of calcium electroporation —A novel cancer treatment modality. Cancers 2020, 12, 290. [Google Scholar] [CrossRef] [PubMed]
- Probst, U.; Fuhrmann, I.; Beyer, L.; Wiggermann, P. Electrochemotherapy as a new modality in interventional oncology: A review. Technol. Cancer Res. Treat. 2018, 17, 1533033818785329. [Google Scholar] [CrossRef]
- Pucihar, G.; Krmelj, J.; Reberšek, M.; Napotnik, T.B.; Miklavčič, D. Equivalent pulse parameters for electroporation. IEEE Trans. Biomed. Eng. 2011, 58, 3279–3288. [Google Scholar] [CrossRef]
- Novickij, V.; Rembiałkowska, N.; Szlasa, W.; Kulbacka, J. Does the shape of the electric pulse matter in electroporation? Front. Oncol. 2022, 12, 958128. [Google Scholar] [CrossRef]
- Łapińska, Z.; Szwedowicz, U.; Choromańska, A.; Saczko, J. Electroporation and Electrochemotherapy in Gynecological and Breast Cancer Treatment. Molecules 2022, 27, 2476. [Google Scholar] [CrossRef]
- Marty, M.; Sersa, G.; Garbay, J.R.; Gehl, J.; Collins, C.G.; Snoj, M.; Billard, V.; Geertsen, P.F.; Larkin, J.O.; Miklavcic, D.; et al. Electrochemotherapy—An easy, highly effective and safe treatment of cutaneous and subcutaneous metastases: Results of ESOPE (European Standard Operating Procedures of Electrochemotherapy) study. Eur. J. Cancer Suppl. 2006, 4, 3–13. [Google Scholar] [CrossRef]
- Frandsen, S.K.; Gissel, H.; Hojman, P.; Tramm, T.; Eriksen, J.; Gehl, J. Direct therapeutic applications of calcium electroporation to effectively induce tumor necrosis. Cancer Res. 2012, 72, 1336–1341. [Google Scholar] [CrossRef] [PubMed]
- Frandsen, S.K.; Krüger, M.B.; Mangalanathan, U.M.; Tramm, T.; Mahmood, F.; Novak, I.; Gehl, J. Normal and malignant cells exhibit differential responses to calcium electroporation. Cancer Res. 2017, 77, 4389–4401. [Google Scholar] [CrossRef] [PubMed]
- Tremble, L.F.; Heffron, C.C.B.B.; Forde, P.F. The effect of calcium electroporation on viability, phenotype and function of melanoma conditioned macrophages. Sci. Rep. 2020, 10, 20645. [Google Scholar] [CrossRef]
- Frandsen, S.K.; Gibot, L.; Madi, M.; Gehl, J.; Rols, M.P. Calcium electroporation: Evidence for differential effects in normal and malignant cell lines, evaluated in a 3D spheroid model. PLoS ONE 2015, 10, e0144028. [Google Scholar] [CrossRef]
- Szewczyk, A.; Gehl, J.; Daczewska, M.; Saczko, J.; Frandsen, S.K.; Kulbacka, J. Calcium electroporation for treatment of sarcoma in preclinical studies. Oncotarget 2018, 9, 11604–11618. [Google Scholar] [CrossRef]
- Ball, C.; Thomson, K.R.; Kavnoudias, H. Irreversible electroporation: A new challenge in “out of operating theater” anesthesia. Anesth. Analg. 2010, 110, 1305–1309. [Google Scholar] [CrossRef]
- Fusco, R.; Di Bernardo, E.; D’Alessio, V.; Salati, S.; Cadossi, M. Reduction of muscle contraction and pain in electroporation-based treatments: An overview. World J. Clin. Oncol. 2021, 12, 367–381. [Google Scholar] [CrossRef]
- Novickij, V.; Baleviciute, A.; Malysko, V.; Zelvys, A.; Radzeviciute, E.; Kos, B.; Zinkeviciene, A.; Miklavcic, D.; Novickij, J.; Girkontaite, I. Effects of Time Delay Between Unipolar Pulses in High Frequency Nano-Electrochemotherapy. IEEE Trans. Biomed. Eng. 2022, 69, 1726–1732. [Google Scholar] [CrossRef]
- Vižintin, A.; Marković, S.; Ščančar, J.; Miklavčič, D. Electroporation with nanosecond pulses and bleomycin or cisplatin results in efficient cell kill and low metal release from electrodes. Bioelectrochemistry 2021, 140, 107798. [Google Scholar] [CrossRef]
- Radzevičiūtė, E.; Malyško-Ptašinskė, V.; Kulbacka, J.; Rembiałkowska, N.; Novickij, J.; Girkontaitė, I.; Novickij, V. Nanosecond electrochemotherapy using bleomycin or doxorubicin: Influence of pulse amplitude, duration and burst frequency. Bioelectrochemistry 2022, 148, 108251. [Google Scholar] [CrossRef] [PubMed]
- Vizintin, A.; Markovic, S.; Scancar, J.; Kladnik, J.; Turel, I.; Miklavcic, D. Nanosecond Electric Pulses are Equally Effective in Electrochemotherapy with Cisplatin as Microsecond Pulses. Radiol. Oncol. 2022, 56, 326. [Google Scholar] [CrossRef] [PubMed]
- Kiełbik, A.; Szlasa, W.; Novickij, V.; Szewczyk, A.; Maciejewska, M.; Saczko, J.; Kulbacka, J. Effects of high-frequency nanosecond pulses on prostate cancer cells. Sci. Rep. 2021, 11, 15835. [Google Scholar] [CrossRef]
- Novickij, V.; Malyško, V.; Želvys, A.; Balevičiūte, A.; Zinkevičiene, A.; Novickij, J.; Girkontaite, I. Electrochemotherapy using doxorubicin and nanosecond electric field pulses: A pilot in vivo study. Molecules 2020, 25, 4601. [Google Scholar] [CrossRef]
- Tunikowska, J.; Antończyk, A.; Rembiałkowska, N.; Jóźwiak, Ł.; Novickij, V.; Kulbacka, J. The First Application of Nanoelectrochemotherapy in Feline Oral Malignant Melanoma Treatment—Case Study. Animals 2020, 10, 556. [Google Scholar] [CrossRef]
- Novickij, V.; Česna, R.; Perminaite, E.; Zinkevičiene, A.; Characiejus, D.; Novickij, J.; Šatkauskas, S.; Ruzgys, P.; Girkontaite, I. Antitumor response and immunomodulatory effects of sub-microsecond irreversible electroporation and its combination with calcium electroporation. Cancers 2019, 11, 1763. [Google Scholar] [CrossRef]
- Balevičiūtė, A.; Radzevičiūtė, E.; Želvys, A.; Malyško-Ptašinskė, V.; Novickij, J.; Zinkevičienė, A.; Kašėta, V.; Novickij, V.; Girkontaitė, I. High-Frequency Nanosecond Bleomycin Electrochemotherapy and its Effects on Changes in the Immune System and Survival. Cancers 2022, 14, 6254. [Google Scholar] [CrossRef]
- Miklavčič, D.; Pucihar, G.; Pavlovec, M.; Ribarič, S.; Mali, M.; MačEk-Lebar, A.; Petkovšek, M.; Nastran, J.; Kranjc, S.; Čemažar, M.; et al. The effect of high frequency electric pulses on muscle contractions and antitumor efficiency in vivo for a potential use in clinical electrochemotherapy. Bioelectrochemistry 2005, 65, 121–128. [Google Scholar] [CrossRef]
- Guo, S.; Burcus, N.I.; Hornef, J.; Jing, Y.; Jiang, C.; Heller, R.; Beebe, S.J. Nano-Pulse Stimulation for the Treatment of Pancreatic Cancer and the Changes in Immune Profile. Cancers 2018, 10, 217. [Google Scholar] [CrossRef] [PubMed]
- Cornelis, F.H.; Cindrič, H.; Kos, B.; Fujimori, M.; Petre, E.N.; Miklavčič, D.; Solomon, S.B.; Srimathveeravalli, G. Peri-Tumoral Metallic Implants Reduces the Efficacy of Irreversible Electroporation for the Ablation of Colorectal Liver Metastases. Cardiovasc. Intervent. Radiol. 2020, 43, 84. [Google Scholar] [CrossRef]
- Cvetkoska, A.; Maček-Lebar, A.; Trdina, P.; Miklavčič, D.; Reberšek, M. Muscle contractions and pain sensation accompanying high-frequency electroporation pulses. Sci. Rep. 2022, 12, 8019. [Google Scholar] [CrossRef] [PubMed]
- Pliquett, U.; Nuccitelli, R. Measurement and simulation of Joule heating during treatment of B-16 melanoma tumors in mice with nanosecond pulsed electric fields. Bioelectrochemistry 2014, 100, 62–68. [Google Scholar] [CrossRef]
- Frandsen, S.K.; Hansen, H.F.; Gehl, J. New drugs for electrochemotherapy with emphasis on calcium electroporation. Handb. Electroporat. 2017, 3, 1637–1650. [Google Scholar] [CrossRef]
- Frandsen, S.K.; Gissel, H.; Hojman, P.; Eriksen, J.; Gehl, J. Calcium electroporation in three cell lines: A comparison of bleomycin and calcium, calcium compounds, and pulsing conditions. Biochim. Biophys. Acta—Gen. Subj. 2014, 1840, 1204–1208. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, M.M.; Tsimpaki, T.; Berchner-Pfannschmidt, U.; Bechrakis, N.E.; Seitz, B.; Fiorentzis, M. Calcium Electroporation Reduces Viability and Proliferation Capacity of Four Uveal Melanoma Cell Lines in 2D and 3D Cultures. Cancers 2022, 14, 2889. [Google Scholar] [CrossRef]
- Lisec, B.; Markelc, B.; Ursic Valentinuzzi, K.; Sersa, G.; Cemazar, M. The effectiveness of calcium electroporation combined with gene electrotransfer of a plasmid encoding IL-12 is tumor type-dependent. Front. Immunol. 2023, 14, 2694. [Google Scholar] [CrossRef]
- Staresinic, B.; Jesenko, T.; Kamensek, U.; Krog Frandsen, S.; Sersa, G.; Gehl, J.; Cemazar, M. Effect of calcium electroporation on tumour vasculature. Sci. Rep. 2018, 8, 9412. [Google Scholar] [CrossRef] [PubMed]
- Hansen, E.L.; Sozer, E.B.; Romeo, S.; Frandsen, S.K.; Vernier, P.T.; Gehl, J. Correction: Dose-Dependent ATP Depletion and Cancer Cell Death following Calcium Electroporation, Relative Effect of Calcium Concentration and Electric Field Strength. PLoS ONE 2015, 10, e0122973. [Google Scholar] [CrossRef]
- Zhivotovsky, B.; Orrenius, S. Calcium and cell death mechanisms: A perspective from the cell death community. Cell Calcium 2011, 50, 211–221. [Google Scholar] [CrossRef]
- Ghibelli, L.; Cerella, C.; Diederich, M. The Dual Role of Calcium as Messenger and Stressor in Cell Damage, Death, and Survival. Int. J. Cell Biol. 2010, 2010, 546163. [Google Scholar] [CrossRef]
- Nuccitelli, R.; Lui, K.; Kreis, M.; Athos, B.; Nuccitelli, P. Nanosecond pulsed electric field stimulation of reactive oxygen species in human pancreatic cancer cells is Ca2+-dependent. Biochem. Biophys. Res. Commun. 2013, 435, 580–585. [Google Scholar] [CrossRef]
- Garg, A.D.; Nowis, D.; Golab, J.; Vandenabeele, P.; Krysko, D.V.; Agostinis, P. Immunogenic cell death, DAMPs and anticancer therapeutics: An emerging amalgamation. Biochim. Biophys. Acta—Rev. Cancer 2010, 1805, 53–71. [Google Scholar] [CrossRef] [PubMed]
- Calvet, C.Y.; Famin, D.; André, F.M.; Mir, L.M. Electrochemotherapy with bleomycin induces hallmarks of immunogenic cell death in murine colon cancer cells. Oncoimmunology 2014, 3, e28131. [Google Scholar] [CrossRef] [PubMed]
- Falk, H.; Matthiessen, L.W.; Wooler, G.; Gehl, J. Calcium electroporation for treatment of cutaneous metastases; a randomized double-blinded phase II study, comparing the effect of calcium electroporation with electrochemotherapy. Acta Oncol. 2017, 57, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Falk, H.; Lambaa, S.; Johannesen, H.H.; Wooler, G.; Venzo, A.; Gehl, J. Electrochemotherapy and calcium electroporation inducing a systemic immune response with local and distant remission of tumors in a patient with malignant melanoma—A case report. Acta Oncol. 2017, 56, 1126–1131. [Google Scholar] [CrossRef]
- Semenov, I.; Casciola, M.; Ibey, B.L.; Xiao, S.; Pakhomov, A.G. Electropermeabilization of cells by closely spaced paired nanosecond-range pulses. Bioelectrochemistry 2018, 121, 135–141. [Google Scholar] [CrossRef]
- Novickij, V.; Ruzgys, P.; Grainys, A.; Šatkauskas, S. High frequency electroporation efficiency is under control of membrane capacitive charging and voltage potential relaxation. Bioelectrochemistry 2018, 119, 92–97. [Google Scholar] [CrossRef]
- Radzevičiūtė, E.; Malyško-Ptašinskė, V.; Novickij, J.; Novickij, V.; Girkontaitė, I. Transfection by Electroporation of Cancer and Primary Cells Using Nanosecond and Microsecond Electric Fields. Pharmaceutics 2022, 14, 1239. [Google Scholar] [CrossRef]
- Silaeva, Y.Y.; Kalinina, A.A.; Khromykh, L.M.; Kazansky, D.B. Functional properties of CD8+ T cells under lymphopenic conditions. bioRxiv 2018. [Google Scholar] [CrossRef]
- Bhonsle, S.P.; Arena, C.B.; Sweeney, D.C.; Davalos, R.V. Mitigation of impedance changes due to electroporation therapy using bursts of high-frequency bipolar pulses. Biomed. Eng. Online 2015, 14 (Suppl. 3), S3. [Google Scholar] [CrossRef]
- Pakhomov, A.G.; Grigoryev, S.; Semenov, I.; Casciola, M.; Jiang, C.; Xiao, S. The second phase of bipolar, nanosecond-range electric pulses determines the electroporation efficiency. Bioelectrochemistry 2018, 122, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Li, Y.; Zhang, S.; Kong, G.; Li, Z. Association between cellular immune response and spleen weight in mice with hepatocellular carcinoma. Oncol. Lett. 2021, 22, 625. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Wang, Z.; Lei, K.; Liao, J.; Peng, Z.; Lin, M.; Liang, P.; Yu, J.; Peng, S.; Chen, S.; et al. Irreversible electroporation induces CD8+ T cell immune response against post-ablation hepatocellular carcinoma growth. Cancer Lett. 2021, 503, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Jing, Y.; Burcus, N.I.; Lassiter, B.P.; Tanaz, R.; Heller, R.; Beebe, S.J. Nano-pulse stimulation induces potent immune responses, eradicating local breast cancer while reducing distant metastases. Int. J. Cancer 2018, 142, 629–640. [Google Scholar] [CrossRef]
- Chen, R.; Sain, N.M.; Harlow, K.T.; Chen, Y.J.; Shires, P.K.; Heller, R.; Beebe, S.J. A protective effect after clearance of orthotopic rat hepatocellular carcinoma by nanosecond pulsed electric fields. Eur. J. Cancer 2014, 50, 2705–2713. [Google Scholar] [CrossRef]
- He, C.; Huang, X.; Zhang, Y.; Lin, X.; Li, S. T-cell activation and immune memory enhancement induced by irreversible electroporation in pancreatic cancer. Clin. Transl. Med. 2020, 10, e39. [Google Scholar] [CrossRef]
- Liu, Q.; Sun, Z.; Chen, L. Memory T cells: Strategies for optimizing tumor immunotherapy. Protein Cell 2020, 11, 549–564. [Google Scholar] [CrossRef]
- Ugel, S.; Canegrave, S.; De Sanctis, F.; Bronte, V. Monocytes in the Tumor Microenvironment. Annu. Rev. Pathol. 2021, 16, 93–122. [Google Scholar] [CrossRef]
- Novickij, V.; Grainys, A.; Butkus, P.; Tolvaišienė, S.; Švedienė, J.; Paškevičius, A.; Novickij, J. High-frequency submicrosecond electroporator. Biotechnol. Biotechnol. Equip. 2016, 30, 607–613. [Google Scholar] [CrossRef]
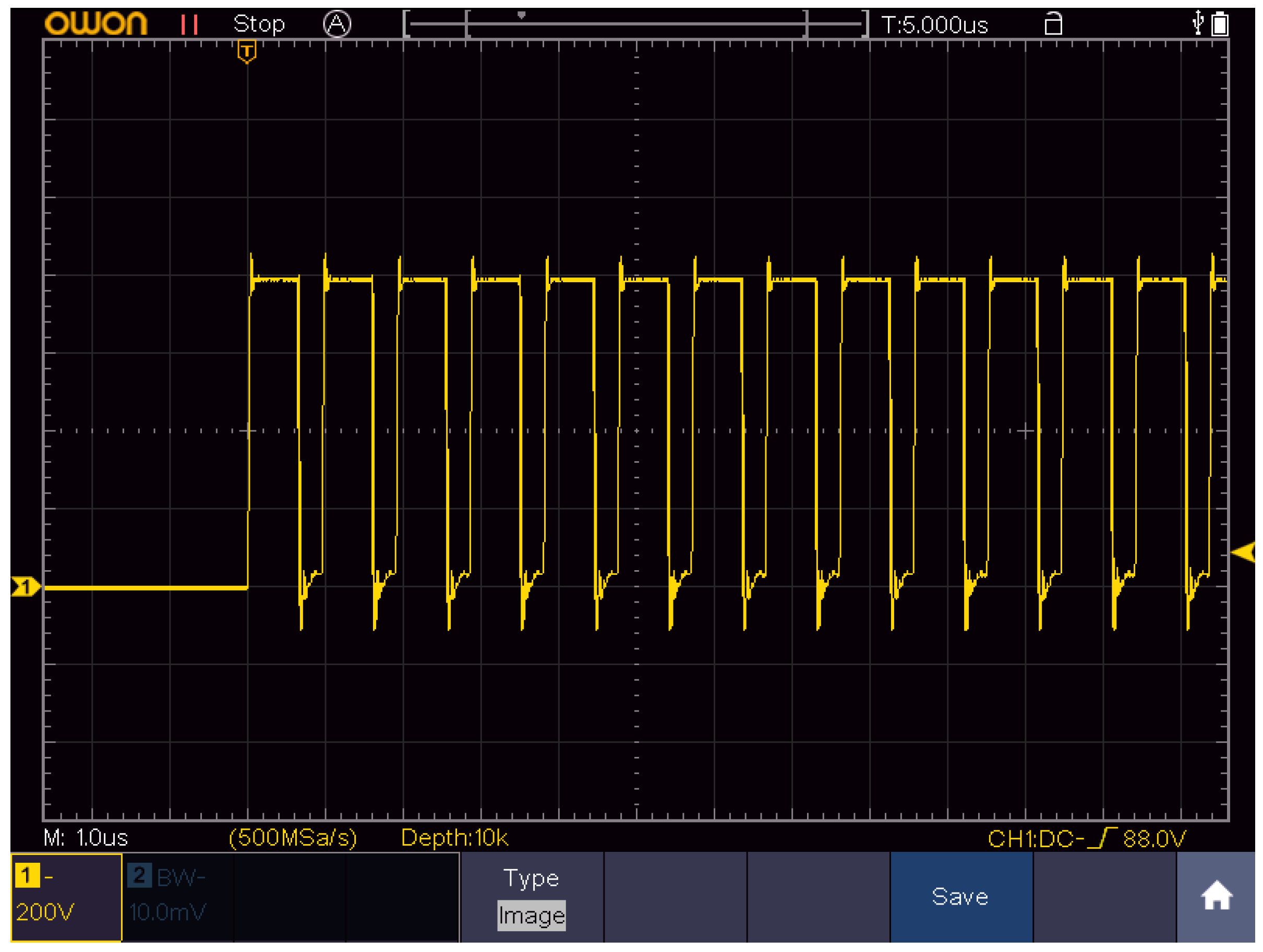
| Protocol | Pulse Parameters | Current (A) | Energy (J) |
|---|---|---|---|
| nsCaEP | 4 kV/cm × 700 ns × 200, 1 MHz | 3.4 | 0.38 |
| μsCaEP | 1.5 kV/cm × 100 μs × 8, 1 Hz | 1.2 | 0.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radzevičiūtė-Valčiukė, E.; Želvys, A.; Mickevičiūtė, E.; Gečaitė, J.; Zinkevičienė, A.; Malyško-Ptašinskė, V.; Kašėta, V.; Novickij, J.; Ivaškienė, T.; Novickij, V. Calcium Electrochemotherapy for Tumor Eradication and the Potential of High-Frequency Nanosecond Protocols. Pharmaceuticals 2023, 16, 1083. https://doi.org/10.3390/ph16081083
Radzevičiūtė-Valčiukė E, Želvys A, Mickevičiūtė E, Gečaitė J, Zinkevičienė A, Malyško-Ptašinskė V, Kašėta V, Novickij J, Ivaškienė T, Novickij V. Calcium Electrochemotherapy for Tumor Eradication and the Potential of High-Frequency Nanosecond Protocols. Pharmaceuticals. 2023; 16(8):1083. https://doi.org/10.3390/ph16081083
Chicago/Turabian StyleRadzevičiūtė-Valčiukė, Eivina, Augustinas Želvys, Eglė Mickevičiūtė, Jovita Gečaitė, Auksė Zinkevičienė, Veronika Malyško-Ptašinskė, Vytautas Kašėta, Jurij Novickij, Tatjana Ivaškienė, and Vitalij Novickij. 2023. "Calcium Electrochemotherapy for Tumor Eradication and the Potential of High-Frequency Nanosecond Protocols" Pharmaceuticals 16, no. 8: 1083. https://doi.org/10.3390/ph16081083
APA StyleRadzevičiūtė-Valčiukė, E., Želvys, A., Mickevičiūtė, E., Gečaitė, J., Zinkevičienė, A., Malyško-Ptašinskė, V., Kašėta, V., Novickij, J., Ivaškienė, T., & Novickij, V. (2023). Calcium Electrochemotherapy for Tumor Eradication and the Potential of High-Frequency Nanosecond Protocols. Pharmaceuticals, 16(8), 1083. https://doi.org/10.3390/ph16081083









