Stability Profile and Clinical Evaluation of an Innovative Hydrogel Containing Polymeric Micelles as Drug Delivery Systems with Oregano Essential Oil against Fibroepithelial Polyps
Abstract
1. Introduction
2. Results
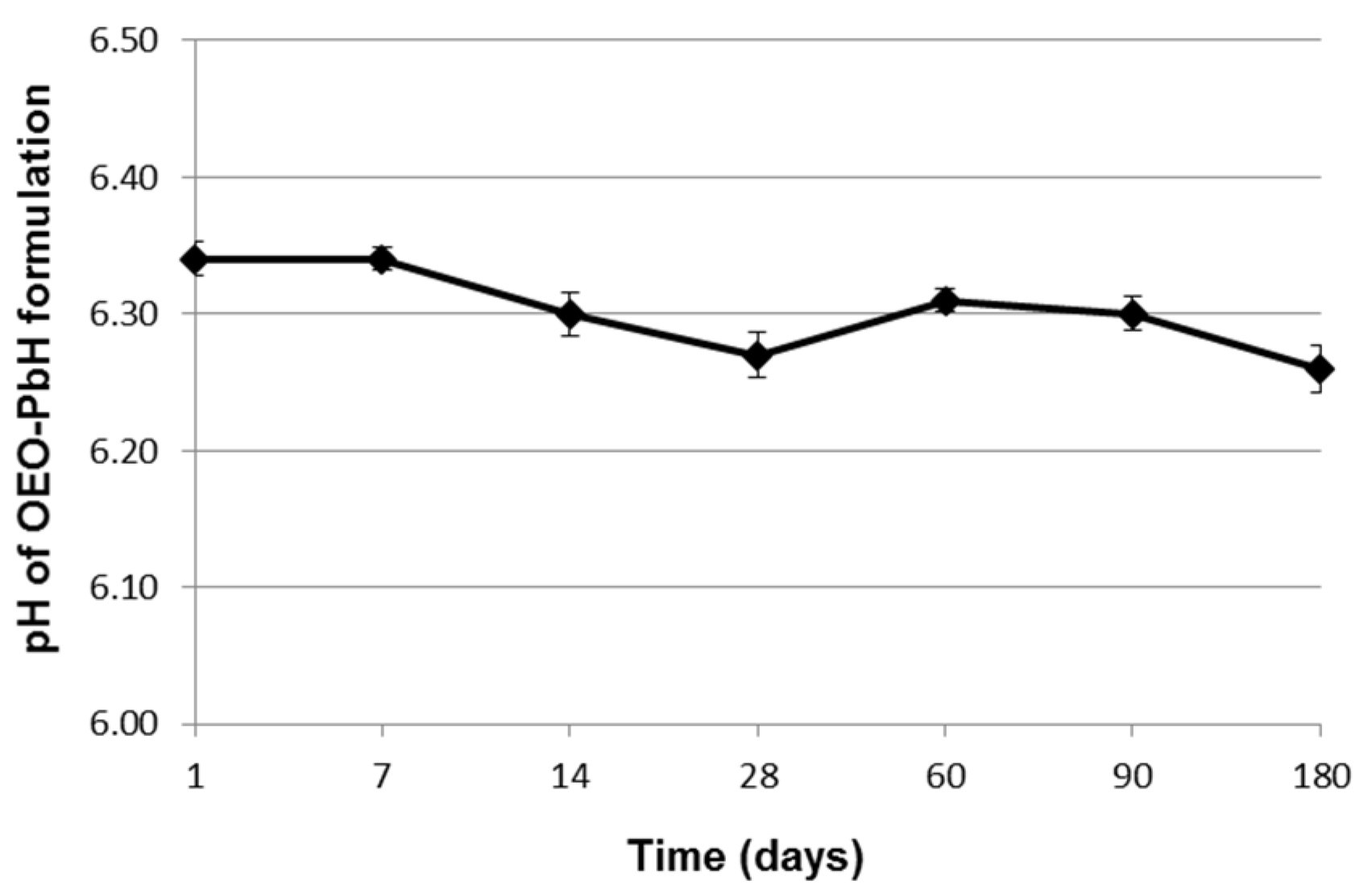
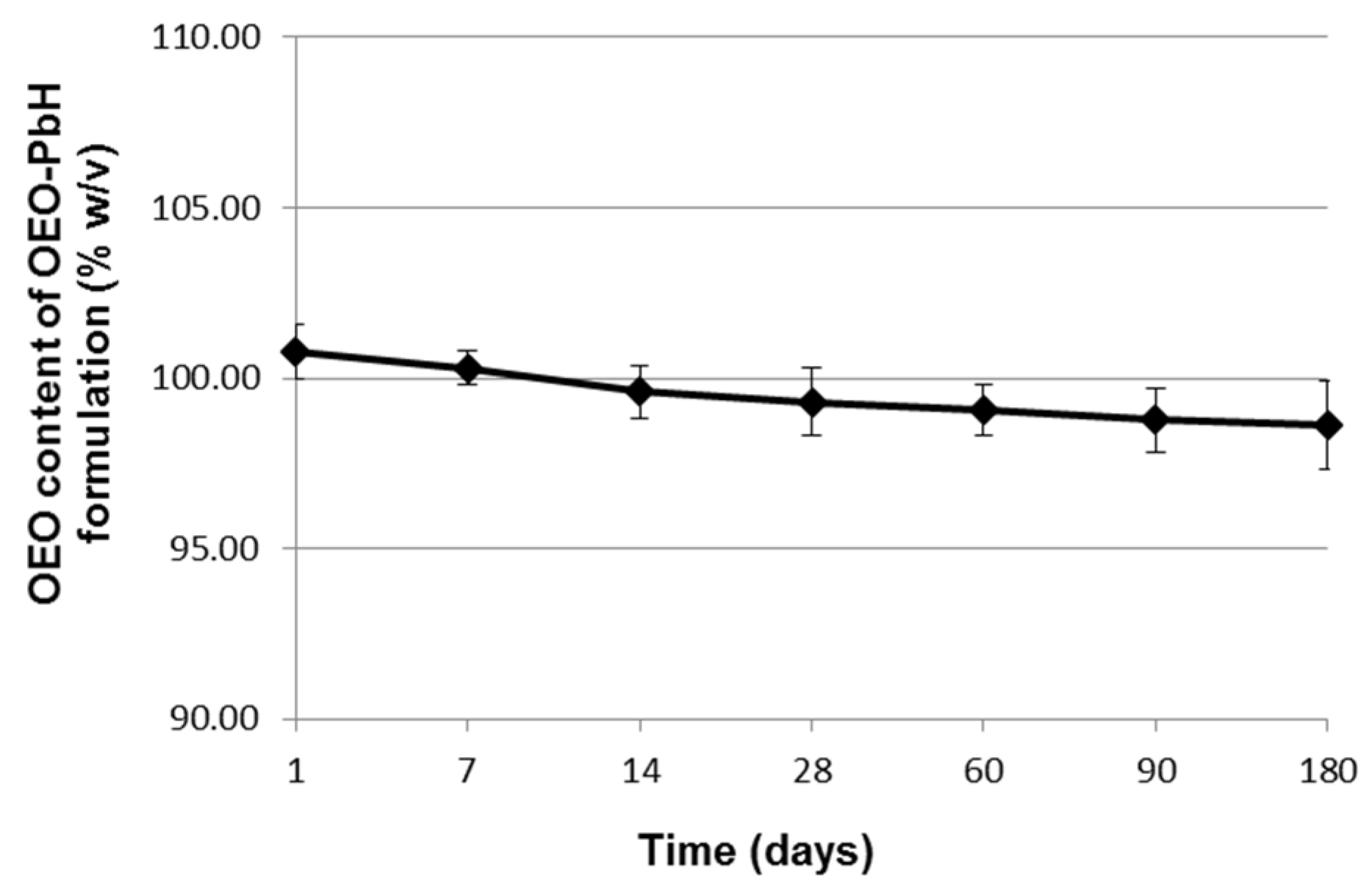
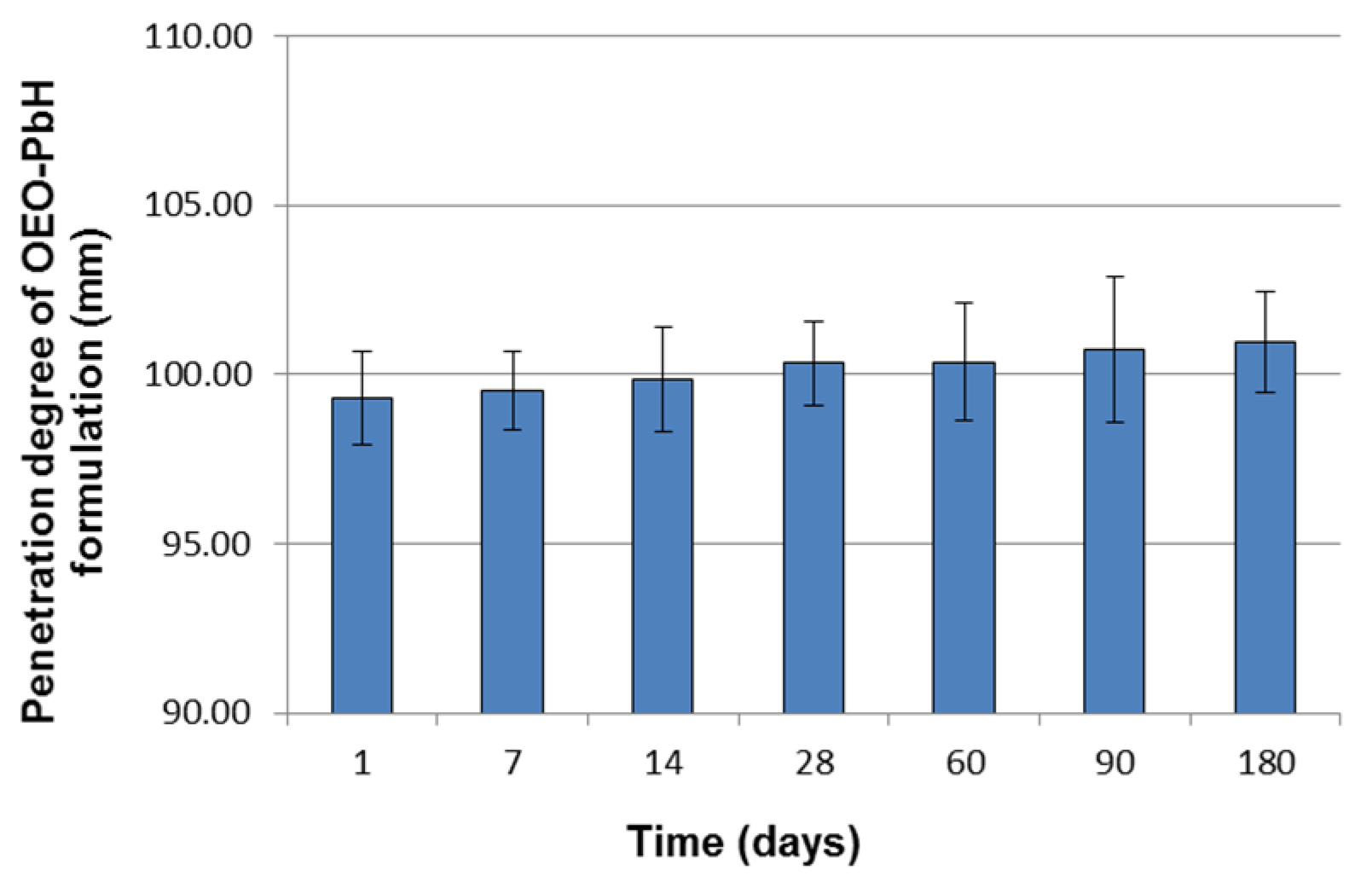
2.1. Long-Term Stability Evaluation of the Experimental OEO-PbH Formulation
2.2. Microbiological Assessment of OEO-PbH
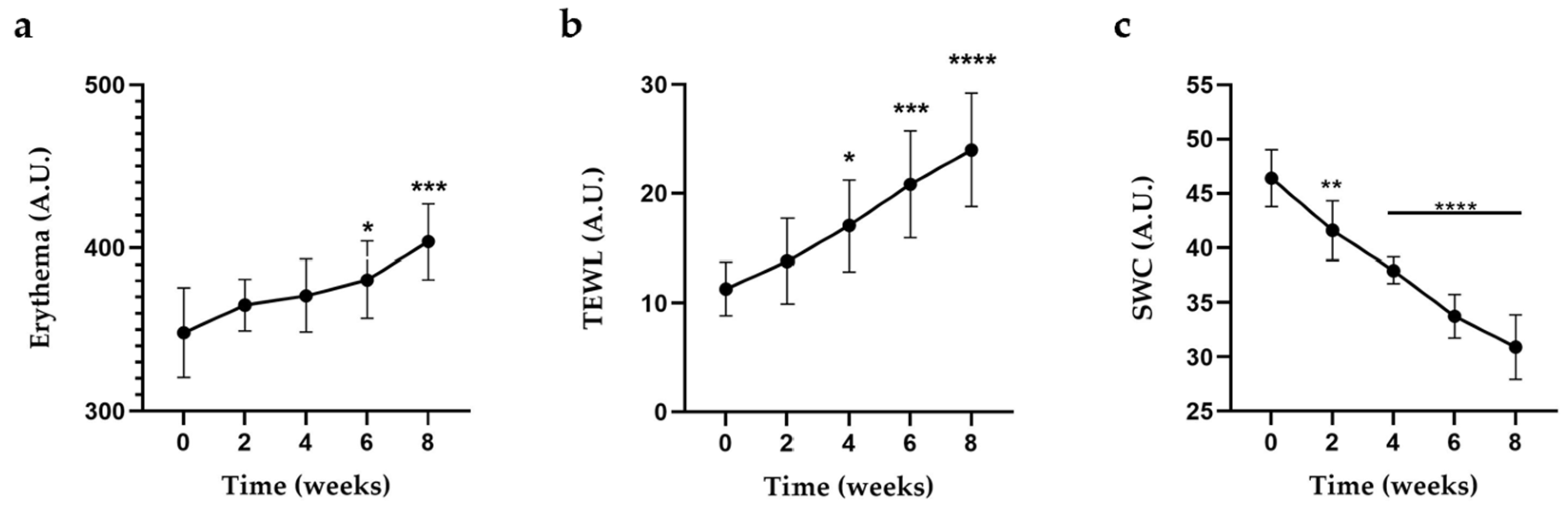
2.3. Erythema Index, TEWL, and -SWC Assessment
2.4. Histopathological Examination
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of Poloxamer-Based Binary Hydrogel Loaded with OEO
4.3. Quality Control Tests for OEO-PbH
4.3.1. Long-Term Stability Evaluation of OEO-PbH
4.3.2. Sterility Testing
4.3.3. Preservative Efficacy Test (PET)
4.4. Study Design
4.5. Skin Parameter Measurements
4.6. Histological Processing
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| A.U. | Arbitrary units |
| CAM | Chorioallantoic membrane |
| CFU | Colony-forming units |
| COS | Columbia agar +5% sheep blood |
| EO | Essential oil |
| EtOH | Ethanol |
| FP | Fibroepithelial polyp |
| H&E | Hematoxylin and eosin |
| HaCaT | Human keratinocyte cell line |
| HDL | High-density lipoprotein |
| IGF-1 | Insulin-like growth factor-1 |
| IL-6 | Interleukin-6 |
| IL-10 | Interleukin-10 |
| IL-23 | Interleukin-23 |
| OEO | Origanum vulgare L. essential oil |
| OEO-PbH | Origanum vulgare L. essential oil-poloxamer-based hydrogel |
| PET | Preservative efficacy test |
| SD | Standard deviation |
| SWC | Skin surface water content |
| TEWL | Transepidermal water loss |
| TNF-α | Tumor necrosis factor |
| UV-VIS | Ultraviolet-visible |
References
- Cichoń, M.A.; Elbe-Bürger, A. Epidermal/Dermal Separation Techniques and Analysis of Cell Populations in Human Skin Sheets. J. Investig. Dermatol. 2023, 143, 11–17. [Google Scholar] [CrossRef]
- Bonifant, H.; Holloway, S. A Review of the Effects of Ageing on Skin Integrity and Wound Healing. Br. J. Community Nurs. 2019, 24, S28–S33. [Google Scholar] [CrossRef] [PubMed]
- Menon, G.K.; Cleary, G.W.; Lane, M.E. The Structure and Function of the Stratum Corneum. Int. J. Pharm. 2012, 435, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Nilforoushzadeh, M.A.; Reza, H.; Ashtiani, A.; Jaffary, F.; Jahangiri, F.; Nikkhah, N.; Mahmoudbeyk, M.; Fard, M.; Ansari, Z.; Zare, S. Dermal Fibroblast Cells: Biology and Function in Skin Regeneration. J. Ski. Stem Cell 2017, 4, 1–5. [Google Scholar] [CrossRef]
- Schwartz, J.; Friedman, A.J. Exogenous Factors in Skin Barrier Repair. J. Drugs Dermatol. 2016, 15, 1289–1294. [Google Scholar]
- Schmid-Wendtner, M.H.; Korting, H.C. The PH of the Skin Surface and Its Impact on the Barrier Function. Ski. Pharmacol. Physiol. 2006, 19, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Elias, P.M.; Wakefield, J.S. Skin Barrier Function. In Nutrition for Healthy Skin: Strategies for Clinical and Cosmetic Practice; Krutmann, J., Humbert, P., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 35–48. [Google Scholar] [CrossRef]
- Pandey, A.; Sonthalia, S. Skin Tags; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Fredman, G.; Qiu, Y.; Ardigò, M.; Mogensen, M. Skin Tags Imaged by Reflectance Confocal Microscopy, Optical Coherence Tomography and Multispectral Optoacoustic Tomography at the Bedside. Ski. Res. Technol. 2021, 27, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Barbato, M.T.; Criado, P.R.; da Silva, A.K.; Averbeck, E.; Guerine, M.B.; de Sá, N.B. Association of Acanthosis Nigricans and Skin Tags with Insulin Resistance. An. Bras. Dermatol. 2012, 87, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Sari, R.; Akman, A.; Alpsoy, E.; Balci, M.K. The Metabolic Profile in Patients with Skin Tags. Clin. Exp. Med. 2010, 10, 193–197. [Google Scholar] [CrossRef] [PubMed]
- El Safoury, O.; Rashid, L.; Ibrahim, M. A Study of Androgen and Estrogen Receptors α, β in Skin Tags. Indian J. Dermatol. 2010, 55, 20–24. [Google Scholar] [CrossRef]
- Farag, A.G.A.; Allah, A.M.K.A.; El-Rebey, H.S.; Ibraheem, K.I.M.; Mohamed, A.S.E.D.; Labeeb, A.Z.; Elgazzar, A.E.; Haggag, M.M. Role of Insulin-like Growth Factor-1 in Skin Tags: A Clinical, Genetic and Immunohistochemical Study in a Sample of Egyptian Patients. Clin. Cosmet. Investig. Dermatol. 2019, 12, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Bustan, R.S.; Wasim, D.; Yderstræde, K.B.; Bygum, A. Specific Skin Signs as a Cutaneous Marker of Diabetes Mellitus and the Prediabetic State—A Systematic Review. Dan. Med. J. 2017, 64, A5316. [Google Scholar] [PubMed]
- Tripathy, T.; Singh, B.S.; Kar, B.R. Association of Skin Tags with Metabolic Syndrome and Its Components: A Case-Control Study from Eastern India. Indian Dermatol. Online J. 2019, 10, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, M.A.; Abdel Fattah, N.S.A.; Sayed, Y.A.A.; Saad, A.A. Assessment of Serum Leptin, Insulin Resistance and Metabolic Syndrome in Patients with Skin Tags. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 1552–1557. [Google Scholar] [CrossRef]
- Antunes, A.; Rossel, B.; Adriaens, E. Efficacy Evaluation of the Pixie® Skin Tag Cryogenic Device on Skin Tags in a Prospective, Single-Blinded, Randomized, Comparative Clinical Trial. Dermatol. Ther. 2021, 11, 995–1007. [Google Scholar] [CrossRef]
- Sharmeen, J.B.; Mahomoodally, F.M.; Zengin, G.; Maggi, F. Essential Oils as Natural Sources of Fragrance Compounds for Cosmetics and Cosmeceuticals. Molecules 2021, 26, 666. [Google Scholar] [CrossRef]
- Cunha, C.; Ribeiro, H.M.; Rodrigues, M.; Araujo, A.R.T.S. Essential Oils Used in Dermocosmetics: Review about Its Biological Activities. J. Cosmet. Dermatol. 2022, 21, 513–529. [Google Scholar] [CrossRef]
- Draelos, Z.D. Cosmeceuticals: What’s Real, What’s Not. Dermatol. Clin. 2019, 37, 107–115. [Google Scholar] [CrossRef]
- Amer, M.; Maged, M. Cosmeceuticals versus Pharmaceuticals. Clin. Dermatol. 2009, 27, 428–430. [Google Scholar] [CrossRef] [PubMed]
- Reszko, A.E.; Berson, D.; Lupo, M.P. Cosmeceuticals: Practical Applications. Dermatol. Clin. 2009, 27, 401–416. [Google Scholar] [CrossRef]
- Fierascu, R.C.; Fierascu, I.; Baroi, A.M.; Ortan, A. Selected Aspects Related to Medicinal and Aromatic Plants as Alternative Sources of Bioactive Compounds. Int. J. Mol. Sci. 2021, 22, 1521. [Google Scholar] [CrossRef] [PubMed]
- Cragg, G.M.; Newman, D.J. Natural Products: A Continuing Source of Novel Drug Leads. Biochim. Biophys. Acta—Gen. Subj. 2013, 1830, 3670–3695. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.K.; Kumar, P.; Saxena, M.J.; Maurya, P. Distribution of Aromatic Plants in the World and Their Properties; Elsevier Inc.: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Diniz do Nascimento, L.; Moraes, A.; Costa, K.; Galucio, J.; Taube, P.; Costa, C.; Cruz, J.; Andrade, E.; Faria, L. Bioactive Natural Compounds and Antioxidant Activity of Essential Oils from Spice Plants: New Findings and Potential Applications. Biomolecules 2020, 10, 988. [Google Scholar] [CrossRef]
- Akhtar, M.; Swamy, M.; Sinniah, U. Natural Bio-Active Compunds Volume 1: Production and Applications; Springer: Singapore, 2019; pp. 1–608. [Google Scholar] [CrossRef]
- Gupta, V.; Mohapatra, S.; Mishra, H.; Farooq, U.; Kumar, K.; Iqbal, Z. Nanotechnology in Cosmetics and Cosmeceuticals—A Review. Gels 2022, 8, 173. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, I.T.; Estevinho, B.N.; Santos, L. Application of Microencapsulated Essential Oils in Cosmetic and Personal Healthcare Products—A Review. Int. J. Cosmet. Sci. 2016, 38, 109–119. [Google Scholar] [CrossRef]
- Neves, A.; Marto, J.; Duarte, A.; Gonçalves, L.M.; Pinto, P.; Figueiredo, A.C.; Ribeiro, H.M. Characterization of Portuguese Thymbra capitata, Thymus caespititius and Myrtus communis Essential Oils in Topical Formulations. Flavour Fragr. J. 2017, 32, 392–402. [Google Scholar] [CrossRef]
- Bogdan, M.; Endres, L.; Pasca, B.; Tit, D.M.; Uivarosan, D.; Copolovici, D.M.; Aleya, L.; Bungau, S. Study on the Stability and Compatibility of the Cosmetic Products with Lavandula angustifolia Oil Kept in PPH Polypropylene Homopolymer Plastic Containers. Mater. Plast. 2019, 56, 133–137. [Google Scholar] [CrossRef]
- Herman, A.; Herman, A.P.; Domagalska, B.W.; Młynarczyk, A. Essential Oils and Herbal Extracts as Antimicrobial Agents in Cosmetic Emulsion. Indian J. Microbiol. 2013, 53, 232–237. [Google Scholar] [CrossRef]
- Muyima, N.Y.O.; Zulu, G.; Bhengu, T.; Popplewell, D. The Potential Application of Some Novel Essential Oils as Natural Cosmetic Preservatives in a Aqueous Cream Formulation. Flavour Fragr. J. 2002, 17, 258–266. [Google Scholar] [CrossRef]
- Dreger, M.; Wielgus, K. Application of Essential Oils as Natural Cosmetic Preservatives. Herba Pol. 2013, 59, 142–156. [Google Scholar] [CrossRef]
- Nardoni, S.; Mugnaini, L.; Pistelli, L.; Leonardi, M.; Sanna, V.; Perrucci, S.; Pisseri, F.; Mancianti, F. Clinical and Mycological Evaluation of an Herbal Antifungal Formulation in Canine Malassezia Dermatitis. J. Mycol. Med. 2014, 24, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Yadav, N.P.; Rai, V.K.; Mishra, N.; Sinha, P.; Bawankule, D.U.; Pal, A.; Tripathi, A.K.; Chanotiya, C.S. A Novel Approach for Development and Characterization of Effective Mosquito Repellent Cream Formulation Containing Citronella Oil. BioMed Res. Int. 2014, 2014, 786084. [Google Scholar] [CrossRef]
- Mamood, S.N.H.; Hidayatulfathi, O.; Budin, S.B.; Ahmad Rohi, G.; Zulfakar, M.H. The Formulation of the Essential Oil of Piper aduncum Linnaeus (Piperales: Piperaceae) Increases Its Efficacy as an Insect Repellent. Bull. Entomol. Res. 2017, 107, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.K.; Mishra, A.; Verma, A.; Chattopadhyay, P. Effects of Calendula Essential Oil-Based Cream on Biochemical Parameters of Skin of Albino Rats against Ultraviolet B Radiation. Sci. Pharm. 2012, 80, 669–683. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.; Narkowicz, C.K.; Jacobson, G.A.; Peterson, G.M. Safety and Efficacy of Kunzea Oil-Containing Formulations for the Management of Psoriasis: A Randomized, Controlled Trial. J. Clin. Pharm. Ther. 2015, 40, 566–572. [Google Scholar] [CrossRef]
- Srivilai, J.; Phimnuan, P.; Jaisabai, J.; Luangtoomma, N.; Waranuch, N.; Khorana, N.; Wisuitiprot, W.; Scholfield, C.N.; Champachaisri, K.; Ingkaninan, K. Curcuma aeruginosa Roxb. Essential Oil Slows Hair-Growth and Lightens Skin in Axillae; a Randomised, Double Blinded Trial. Phytomedicine 2017, 25, 29–38. [Google Scholar] [CrossRef]
- Mazzarello, V.; Gavini, E.; Rassu, G.; Donadu, M.G.; Usai, D.; Piu, G.; Pomponi, V.; Sucato, F.; Zanetti, S.; Montesu, M.A. Clinical Assessment of New Topical Cream Containing Two Essential Oils Combined with Tretinoin in the Treatment of Acne. Clin. Cosmet. Investig. Dermatol. 2020, 13, 233–239. [Google Scholar] [CrossRef]
- Süntar, I.; Akkol, E.K.; Keleş, H.; Oktem, A.; Başer, K.H.C.; Yeşilada, E. A Novel Wound Healing Ointment: A Formulation of Hypericum perforatum Oil and Sage and Oregano Essential Oils Based on Traditional Turkish Knowledge. J. Ethnopharmacol. 2011, 134, 89–96. [Google Scholar] [CrossRef]
- Verma, R.S.; Padalia, R.C.; Chauhan, A.; Verma, R.K.; Yadav, A.K.; Singh, H.P. Chemical Diversity in Indian Oregano (Origanum vulgare L.). Chem. Biodivers. 2010, 7, 2054–2064. [Google Scholar] [CrossRef]
- Węglarz, Z.; Kosakowska, O.; Przybył, J.L.; Pióro-Jabrucka, E.; Baczek, K. The Quality of Greek Oregano (O. vulgare L. subsp. Hirtum (Link) Ietswaart) and Common Oregano (O. vulgare L. Subsp. vulgare) Cultivated in the Temperate Climate of Central Europe. Foods 2020, 9, 1671. [Google Scholar] [CrossRef]
- Asdal, A.; Galambosi, B.; Bjorn, G.; Olsson, K.; Pihlik, U.; Radušiene, J.; Porvaldsdottir, E.; Wedelsback; Žukauska, I. Spice- and Medicinal Plants in the Nordic and Baltic Countries. Conservation of Genetic Resources; Nordic Gene Bank: Alnarp, Sweden, 2006. [Google Scholar]
- Orchard, A.; Van Vuuren, S. Commercial Essential Oils as Potential Antimicrobials to Treat Skin Diseases. Evidence-based Complement. Altern. Med. 2017, 2017, 4517971. [Google Scholar] [CrossRef] [PubMed]
- Bora, L.; Avram, S.; Pavel, I.Z.; Muntean, D.; Liga, S.; Buda, V.; Gurgus, D.; Danciu, C. An Up-To-Date Review Regarding Cutaneous Benefits of Origanum vulgare L. Essential Oil. Antibiotics 2022, 11, 549. [Google Scholar] [CrossRef] [PubMed]
- Lombrea, A.; Antal, D.; Ardelean, F.; Avram, S.; Pavel, I.Z.; Vlaia, L.; Mut, A.M.; Diaconeasa, Z.; Dehelean, C.A.; Soica, C.; et al. A Recent Insight Regarding the Phytochemistry and Bioactivity of Origanum vulgare L. Essential Oil. Int. J. Mol. Sci. 2020, 21, 9653. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.; Villa, C. Poloxamer Hydrogels for Biomedical Applications. Pharmaceutics 2019, 11, 671. [Google Scholar] [CrossRef]
- Avram, Ș.; Bora, L.; Vlaia, L.L.; Muț, A.M.; Olteanu, G.-E.; Olariu, I.; Magyari-Pavel, I.Z.; Minda, D.; Diaconeasa, Z.; Sfirloaga, P.; et al. Cutaneous Polymeric-Micelles-Based Hydrogel Containing Origanum vulgare L. Essential Oil: In Vitro Release and Permeation, Angiogenesis, and Safety Profile In Ovo. Pharmaceuticals 2023, 16, 940. [Google Scholar] [CrossRef]
- Bora, L.; Burkard, T.; Juan, M.H.S.; Radeke, H.H.; Muț, A.M.; Vlaia, L.L.; Magyari-Pavel, I.Z.; Diaconeasa, Z.; Socaci, S.; Borcan, F.; et al. Phytochemical Characterization and Biological Evaluation of Origanum vulgare L. Essential Oil Formulated as Polymeric Micelles Drug Delivery Systems. Pharmaceutics 2022, 14, 2413. [Google Scholar] [CrossRef]
- Acosta, N.; Sánchez, E.; Calderón, L.; Cordoba-Diaz, M.; Cordoba-Diaz, D.; Dom, S.; Heras, A. Physical Stability Studies of Semi-Solid Formulations from Natural Compounds Loaded with Chitosan Microspheres. Mar. Drugs 2015, 13, 5901–5919. [Google Scholar] [CrossRef]
- Nnamani, P.O.; Kenechukwu, F.C.; Anugwolu, C.L.; Attama, A.A. Evaluation of Hydrogels Based on Poloxamer 407 and Polyacrylic Acids for Enhanced Topical Activity of Gentamicin against Susceptible Infections. Trop. J. Pharm. Res. 2014, 13, 1385–1391. [Google Scholar] [CrossRef]
- González-González, O.; Ramirez, I.O.; Ramirez, B.I.; O’Connell, P.; Ballesteros, M.P.; Torrado, J.J.; Serrano, D.R. Drug Stability: ICH versus Accelerated Predictive Stability Studies. Pharmaceutics 2022, 14, 2324. [Google Scholar] [CrossRef]
- ICH Q1A (R2) Stability Testing of New Drug Substances and Drug Products–Step 5. Available online: https://www.ema.europa.eu/en/ich-q1a-r2-stability-testing-new-drug-substances-drug-products-scientific-guideline (accessed on 29 May 2023).
- Chen, I.C.; Su, C.Y.; Chen, P.Y.; Hoang, T.C.; Tsou, Y.S.; Fang, H.W. Investigation and Characterization of Factors Affecting Rheological Properties of Poloxamer-Based Thermo-Sensitive Hydrogel. Polymers 2022, 14, 5353. [Google Scholar] [CrossRef]
- Owen, S.C.; Chan, D.P.Y.; Shoichet, M.S. Polymeric Micelle Stability. Nano Today 2012, 7, 53–65. [Google Scholar] [CrossRef]
- Lukić, M.; Pantelić, I.; Savić, S.D. Towards Optimal Ph of the Skin and Topical Formulations: From the Current State of the Art to Tailored Products. Cosmetics 2021, 8, 69. [Google Scholar] [CrossRef]
- Bajaj, S.; Singla, D.; Sakhuja, N. Stability Testing of Pharmaceutical Products. J. Appl. Pharm. Sci. 2012, 2, 129–138. [Google Scholar] [CrossRef]
- Siska, B.; Snejdrova, E.; Machac, I.; Dolecek, P.; Martiska, J. Contribution to the Rheological Testing of Pharmaceutical Semisolids. Pharm. Dev. Technol. 2019, 24, 80–88. [Google Scholar] [CrossRef]
- Contri, R.V.; Frank, L.A.; Kaiser, M.; Pohlmann, A.R.; Guterres, S.S. The Use of Nanoencapsulation to Decrease Human Skin Irritation Caused by Capsaicinoids. Int. J. Nanomed. 2014, 9, 951–962. [Google Scholar] [CrossRef]
- Omeragic, E.; Dedic, M.; Elezovic, A.; Becic, E.; Imamovic, B.; Kladar, N.; Niksic, H. Application of Direct Peptide Reactivity Assay for Assessing the Skin Sensitization Potential of Essential Oils. Sci. Rep. 2022, 12, 7470. [Google Scholar] [CrossRef] [PubMed]
- Parra, A.; Jarak, I.; Santos, A.; Veiga, F.; Figueiras, A. Polymeric Micelles: A Promising Pathway for Dermal Drug Delivery. Materials 2021, 14, 7278. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Kim, M.Y.; Lee, P.K.; Kim, H.O.; Park, Y.M. Evaluation of Natural Change of Skin Function in Split-Thickness Skin Grafts by Noninvasive Bioengineering Methods. Dermatol. Surg. 2006, 32, 1358–1363. [Google Scholar] [CrossRef]
- Fluhr, J.W.; Kuss, O.; Diepgen, T.; Lazzerini, S.; Pelosi, A.; Gloor, M.; Berardesca, E. Testing for Irritation with a Multifactorial Approach: Comparison of Eight Non-Invasive Measuring Techniques on Five Different Irritation Types. Br. J. Dermatol. 2001, 145, 696–703. [Google Scholar] [CrossRef]
- Kołodziejczak, A.M.; Rotsztejn, H. Mexametric and Cutometric Assessment of the Signs of Aging of the Skin Area around the Eyes after the Use of Non-Ablative Fractional Laser, Non-Ablative Radiofrequency and Intense Pulsed Light. Dermatol. Ther. 2017, 30, e12470. [Google Scholar] [CrossRef]
- Shamrikova, V.A.; Sorokina, E.D.; Dubrovskaya, E.V.; Krakhaleva, Y.A.; Kurniavkina, E.A.; Krinitsyna, Y.M.; Yakubovich, A.I.; Sergeeva, I.G. Mexametric Assessment of Melanin Level in Children’s Skin. Acta Biomed. Sci. 2020, 5, 12–16. [Google Scholar] [CrossRef]
- Abdlaty, R.; Hayward, J.; Farrell, T.; Fang, Q. Skin Erythema and Pigmentation: A Review of Optical Assessment Techniques. Photodiagn. Photodyn. Ther. 2021, 33, 102127. [Google Scholar] [CrossRef] [PubMed]
- Matias, A.R.; Ferreira, M.; Costa, P.; Neto, P. Skin Colour, Skin Redness and Melanin Biometric Measurements: Comparison Study between Antera 3D, Mexameter and Colorimeter. Ski. Res. Technol. 2015, 21, 346–362. [Google Scholar] [CrossRef] [PubMed]
- Masaki, H.; Yamashita, Y.; Kyotani, D.; Honda, T.; Takano, K.; Tamura, T.; Mizutani, T.; Okano, Y. Correlations between Skin Hydration Parameters and Corneocyte-Derived Parameters to Characterize Skin Conditions. J. Cosmet. Dermatol. 2019, 18, 308–314. [Google Scholar] [CrossRef]
- Ezerskaia, A.; Pereira, S.F.; Urbach, H.P.; Verhagen, R.; Varghese, B. Quantitative and Simultaneous Non-Invasive Measurement of Skin Hydration and Sebum Levels. Biomed. Opt. Express 2016, 7, 2311–2320. [Google Scholar] [CrossRef]
- O’goshi, K.; Serup, J. Inter-Instrumental Variation of Skin Capacitance Measured with the Corneometer. Ski. Res. Technol. 2005, 11, 107–109. [Google Scholar] [CrossRef]
- Kikuchi, K.; Asano, M.; Tagami, H.; Kato, M.; Aiba, S. Comparison of the Measuring Efficacy of Transepidermal Water Loss of a Reasonably Priced, Portable Closed- chamber System Device H4500 with That of Rather Expensive, Conventional Devices Such as Tewameter® and Vapometer®. Ski. Res. Technol. 2017, 23, 597–601. [Google Scholar] [CrossRef]
- Gardien, K.L.M.; Baas, D.C.; De Vet, H.C.W.; Middelkoop, E. Transepidermal Water Loss Measured with the Tewameter TM300 in Burn Scars. Burns 2016, 42, 1455–1462. [Google Scholar] [CrossRef]
- Machková, L.; Švadlák, D.; Dolečková, I. A Comprehensive In Vivo Study of Caucasian Facial Skin Parameters on 442 Women. Arch. Dermatol. Res. 2018, 310, 691–699. [Google Scholar] [CrossRef]
- Firooz, A.; Sadr, B.; Babakoohi, S.; Sarraf-Yazdy, M.; Fanian, F.; Kazerouni-Timsar, A.; Nassiri-Kashani, M.; Naghizadeh, M.M.; Dowlati, Y. Variation of Biophysical Parameters of the Skin with Age, Gender, and Body Region. Sci. World J. 2012, 2012, 386936. [Google Scholar] [CrossRef]
- Proks, M.; Borcan, F.; Cheveresan, A.; Pinzaru, I.; Guta, B.A.; Coricovac, D.; Paunescu, V.; Lazureanu, V. Study on the Release and Bioevaluations of Green Silver Nanoparticles Entrapped Inside Polymer-Based Nanovesicles. Mater. Plast 2018, 55, 696–699. [Google Scholar] [CrossRef]
- Lichterfeld-Kottner, A.; Lahmann, N.; Blume-Peytavi, U.; Mueller-Werdan, U.; Kottner, J. Dry Skin in Home Care: A Representative Prevalence Study. J. Tissue Viability 2018, 27, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Ha, N.G.; Lee, S.H.; Lee, E.H.; Chang, M.; Yoo, J.; Lee, W.J. Safety and Efficacy of a New Hydrogel Based on Hyaluronic Acid as Cosmeceutical for Xerosis. J. Cosmet. Dermatol. 2022, 21, 6840–6849. [Google Scholar] [CrossRef] [PubMed]
- Lueangarun, S.; Soktepy, B.; Tempark, T. Efficacy of Anti-Inflammatory Moisturizer vs Hydrophilic Cream in Elderly Patients with Moderate to Severe Xerosis: A Split Site, Triple-Blinded, Randomized, Controlled Trial. J. Cosmet. Dermatol. 2020, 19, 1432–1438. [Google Scholar] [CrossRef]
- Park, J.S.; Saeidian, A.H.; Youssefian, L.; Hsu, S.; Vahidnezhad, H.; Uitto, J. Acquired Ichthyosis, Asteatotic Dermatitis or Xerosis? An Update on Pathoetiology and Drug-Induced Associations. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 47–56. [Google Scholar] [CrossRef]
- Augustin, M.; Wilsmann-Theis, D.; Körber, A.; Kerscher, M.; Itschert, G.; Dippel, M.; Staubach, P. Diagnosis and Treatment of Xerosis Cutis—A Position Paper. JDDG—J. Ger. Soc. Dermatol. 2019, 17, 3–33. [Google Scholar] [CrossRef]
- Schmolka, I.R. Artificial Skin I. Preparation and Properties of Pluronic F-127 Gels for Treatment of Burns. J. Biomed. Mater. Res. 1972, 6, 571–582. [Google Scholar] [CrossRef]
- Directorate for the Quality of Medicines & Healthcare of the Council of Europe. Potentiometric Determination of PH. In European Pharmacopeia; EDQM Council of Europe: Strasbourg, France, 2021. [Google Scholar]
- Directorate for the Quality of Medicines & Healthcare of the Council of Europe. Measurement of Consistency by Penetrometry. In European Pharmacopeia; EDQM Council of Europe: Strasbourg, France, 2020; p. 337. [Google Scholar]
- Garg, A.; Aggarwal, D.; Garg, S.; Singla, A. Spreading of Semisolids Formulation: An Update. Pharm. Technol. 2002, 9, 84–105. [Google Scholar]
- Directorate for the Quality of Medicines & Healthcare of the Council of Europe. Sterility. In European Pharmacopeia; EDQM Council of Europe: Strasbourg, France, 2020; p. 189. [Google Scholar]
- World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA—J. Am. Med. Assoc. 2013, 310, 2191–2194. [CrossRef]
- Simu, G.M.; Coricovac, D.; Cseh, L.; Soica, C.; Borcan, F.; Aionescu, D.; Andoni, M.; Dragos, D.; Dehelean, C. Assessment of Skin Injuries Induced by Organic and Inorganic Phases of the Cosorb Process by Means of Non Invasives Techniques. Rev. Chim. 2016, 67, 291–296. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bora, L.; Iftode, A.; Muț, A.M.; Vlaia, L.L.; Olteanu, G.-E.; Muntean, D.; Dehelean, C.A.; Buda, V.; Coneac, G.H.; Danciu, C. Stability Profile and Clinical Evaluation of an Innovative Hydrogel Containing Polymeric Micelles as Drug Delivery Systems with Oregano Essential Oil against Fibroepithelial Polyps. Pharmaceuticals 2023, 16, 980. https://doi.org/10.3390/ph16070980
Bora L, Iftode A, Muț AM, Vlaia LL, Olteanu G-E, Muntean D, Dehelean CA, Buda V, Coneac GH, Danciu C. Stability Profile and Clinical Evaluation of an Innovative Hydrogel Containing Polymeric Micelles as Drug Delivery Systems with Oregano Essential Oil against Fibroepithelial Polyps. Pharmaceuticals. 2023; 16(7):980. https://doi.org/10.3390/ph16070980
Chicago/Turabian StyleBora, Larisa, Andrada Iftode, Ana Maria Muț, Lavinia Lia Vlaia, Gheorghe-Emilian Olteanu, Delia Muntean, Cristina Adriana Dehelean, Valentina Buda, Georgeta Hermina Coneac, and Corina Danciu. 2023. "Stability Profile and Clinical Evaluation of an Innovative Hydrogel Containing Polymeric Micelles as Drug Delivery Systems with Oregano Essential Oil against Fibroepithelial Polyps" Pharmaceuticals 16, no. 7: 980. https://doi.org/10.3390/ph16070980
APA StyleBora, L., Iftode, A., Muț, A. M., Vlaia, L. L., Olteanu, G.-E., Muntean, D., Dehelean, C. A., Buda, V., Coneac, G. H., & Danciu, C. (2023). Stability Profile and Clinical Evaluation of an Innovative Hydrogel Containing Polymeric Micelles as Drug Delivery Systems with Oregano Essential Oil against Fibroepithelial Polyps. Pharmaceuticals, 16(7), 980. https://doi.org/10.3390/ph16070980












