Bioprospecting of Five Ocimum sp. Cultivars from Croatia: New Potential for Dietary and Dermatological Application with Embryotoxicity Tests
Abstract
1. Introduction
2. Results
2.1. Chemical Composition of Headspace Volatiles of Five Basil Cultivars
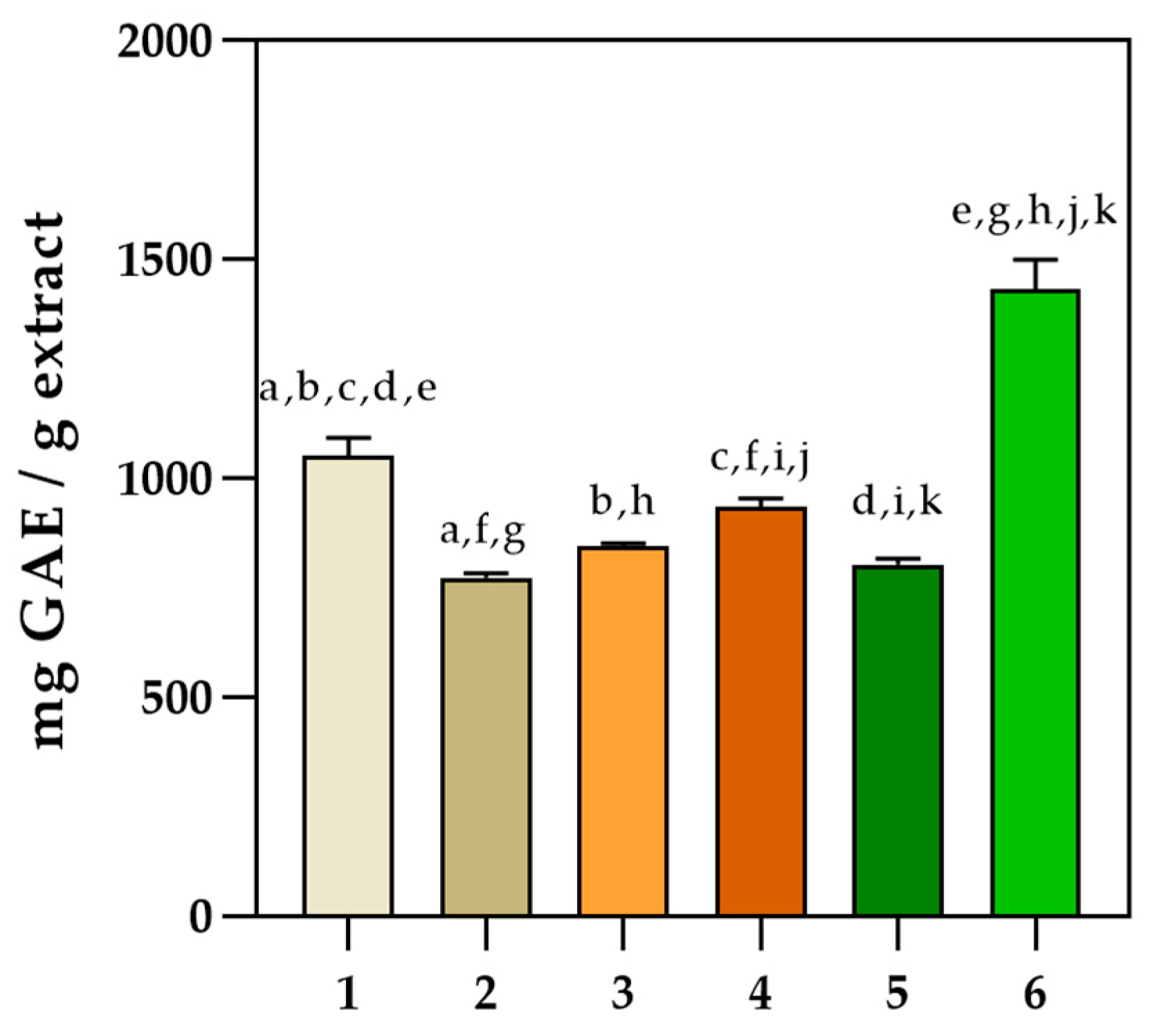
2.2. Determination of Phenolic Profile of the Five Basil Cultivars
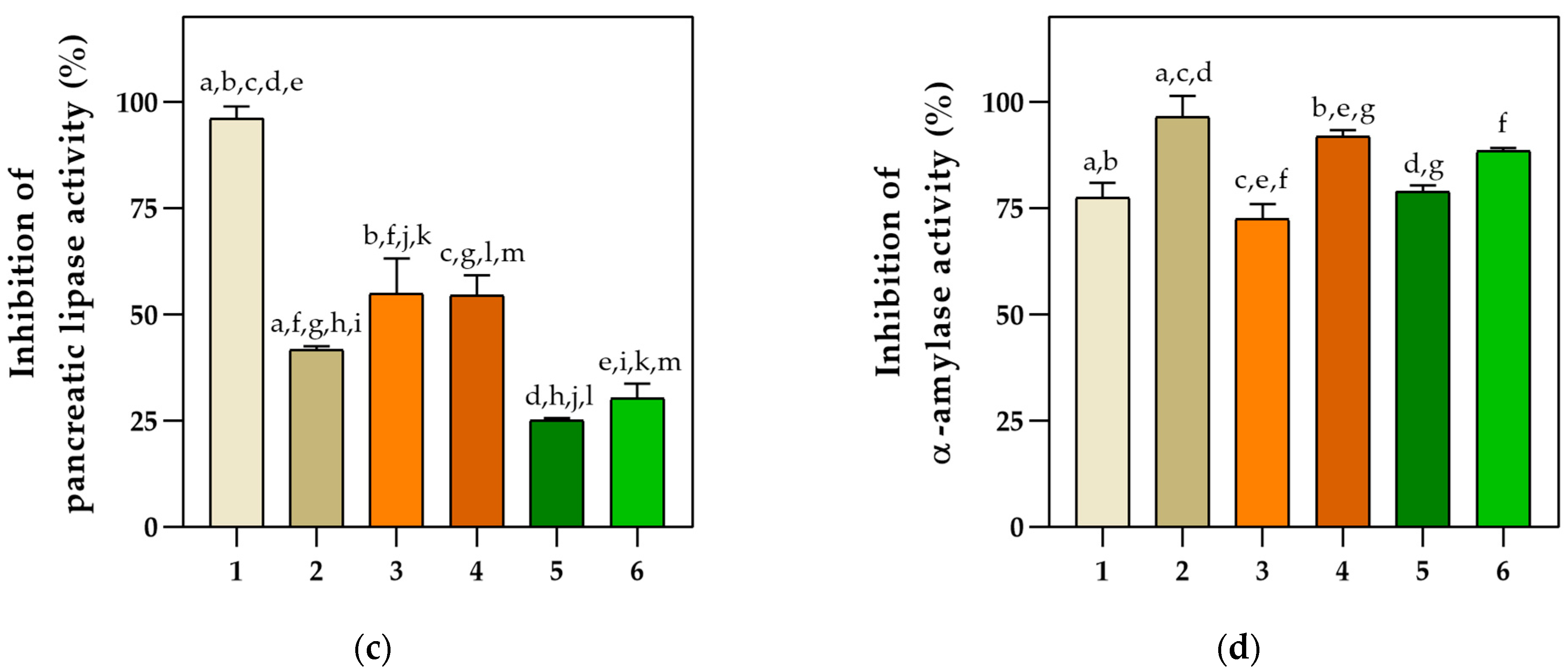
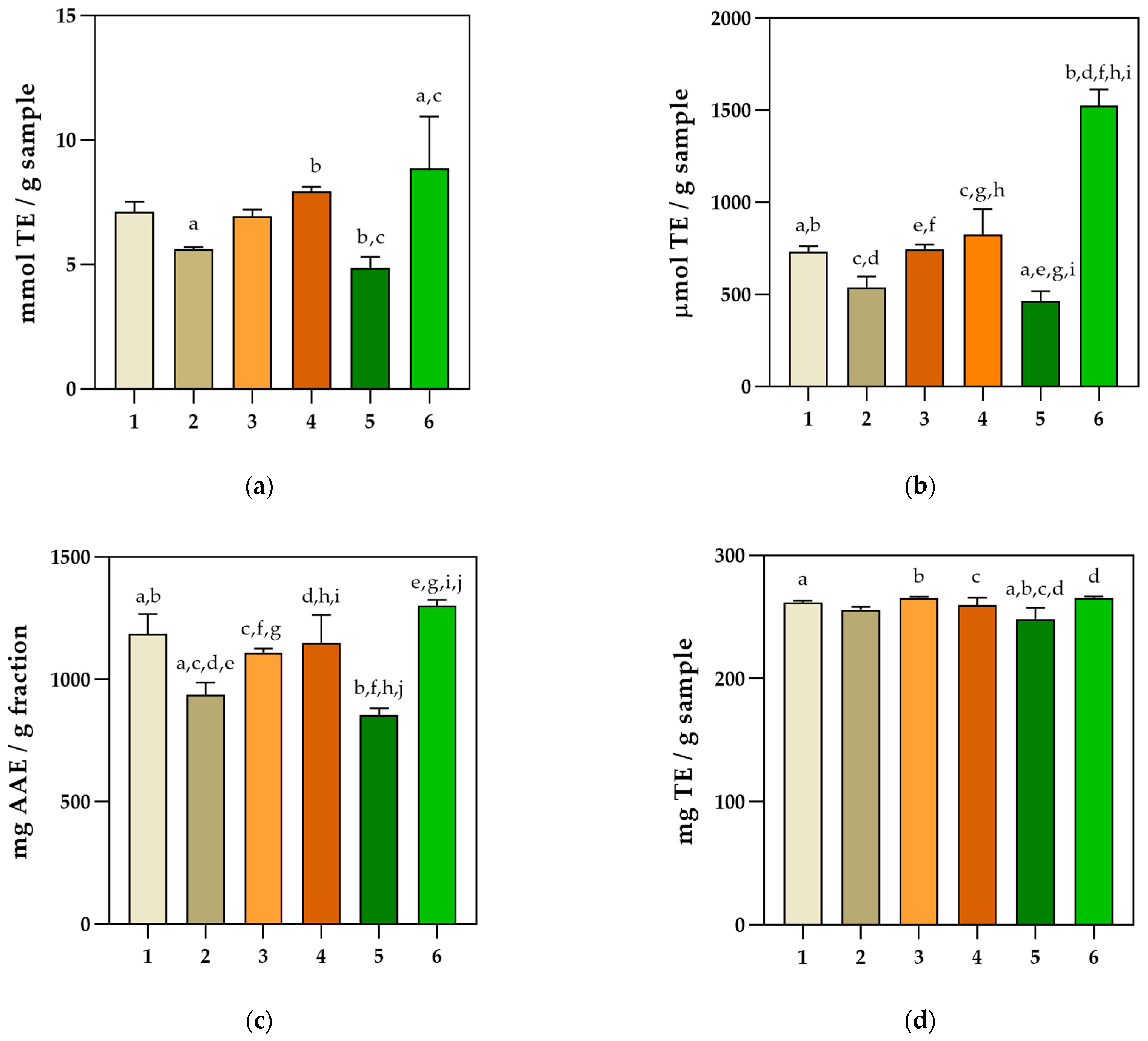
2.3. Anticollagenase, Neuroprotective, Antidiabetic, and Antilipidemic Properties
2.4. Antimicrobial Activity of Basil Extracts
2.5. Antioxidant Activity Determination
2.6. Embryotoxicity Testing
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Chemicals
4.3. Preparation of Extracts
4.4. Headspace Solid-Phase Microextraction (HS-SPME)
4.5. Gas Chromatography–Mass Spectrometry Analysis of VOCs
4.6. Determination of Phenols Using HPLC
4.7. Determination of Basil Extracts’ Inhibition Potential against Enzymatic Activities Using Spectrophotometric Assays
4.7.1. Determination of Collagenase Inhibition by Basil Extracts Using a Spectrophotometric Assay
4.7.2. Determination of Acetylcholinesterase Inhibition by Basil Extracts Using a Spectrophotometric Assay
4.7.3. Determination of Pancreatic Lipase Inhibition by Basil Extracts Using a Spectrophotometric Assay
4.7.4. Determination of α-Amylase Inhibition by Basil Extracts Using a Spectrophotometric Assay
4.8. Determination of Antimicrobial Activity
4.9. In Vitro Antioxidant Activity Determination
4.10. Zebrafish Embryotoxicity Test
4.11. Statistical Data Processing
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siddiqui, B.S.; Bhatti, H.A.; Begum, S.; Perwaiz, S. Evaluation of the antimycobacterium activity of the constituents from Ocimum basilicum against Mycobacterium tuberculosis. J. Ethnopharmacol. 2012, 144, 220–222. [Google Scholar] [CrossRef] [PubMed]
- Egata, D.F. Benefit and use of sweet basil (Ocimum basilicum L.) in Ethiopia: A review. J. Nutr. Food Process. 2021, 4, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Shahrajabian, M.H.; Sun, W.; Cheng, Q. Chemical components and pharmacological benefits of basil (Ocimum basilicum): A review. Int. J. Food Prop. 2020, 23, 1961–1970. [Google Scholar] [CrossRef]
- Machado, D.G.; Bettio, L.E.; Cunha, M.P.; Santos, R.S.; Pizzolatti, M.G.; Brighente, I.M.C.; Rodriguez, A.L. Antidepressant-like effect of rutin isolated from the ethanolic extract from Schinus molle L. in mice: Evidence for the involvement of the serotonergic and noradrenergic systems. Eur. J. Pharmacol. 2008, 587, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Deb Mandal, M.; Kumar Pal, N. Enhancing chloramphenicol and trimethoprim in vitro activity by Ocimum sanctum Linn. (Lamiaceae) leaf extract against Salmonella enterica serovar Typhi. Asian Pac. J. Trop. Med. 2012, 5, 220–224. [Google Scholar] [CrossRef]
- Labra, M.; Miele, M.; Ledda, B.; Grassi, F.; Mazzei, M.; Sala, F. Morphological characterization, essential oil composition and DNA genotyping of Ocimum basilicum L. cultivars, Plant Sci. 2004, 167, 725–731. [Google Scholar] [CrossRef]
- Waldstein, A. Mexican migrant ethnopharmacology: Pharmacopoeia, classification of medicines and explanations of efficacy. J. Ethnopharmacol. 2006, 108, 299–310. [Google Scholar] [CrossRef]
- Sembulingam, K.; Sembulingam, P.; Namasivayam, A. Effect of Ocimum sanctum Linn on the changes in central cholinergic system induced by acute noise stress. J. Ethnopharmacol. 2005, 96, 477–482. [Google Scholar] [CrossRef]
- Samson, J.; Sheeladevi, R.; Ravindran, R. Oxidative stress in brain and antioxidant activity of Ocimum sanctum in noise exposure. Neurotoxicology 2007, 28, 679–685. [Google Scholar] [CrossRef]
- Avetisyan, A.; Markosian, A.; Petrosyan, M.; Sahakyan, N.; Babayan, A.; Aloyan, S.; Trchounian, A. Chemical composition and some biological activities of the essential oils from basil Ocimum different cultivars. BMC Complement. Altern. Med. 2017, 17, 60. [Google Scholar] [CrossRef]
- Sęczyk, Ł.; Ozdemir, F.A.; Kołodziej, B. In vitro bioaccessibility and activity of basil (Ocimum basilicum L.) phytochemicals as affected by cultivar and postharvest preservation method—Convection drying, freezing, and freeze-drying. Food Chem. 2022, 382, 132363. [Google Scholar] [CrossRef]
- Jayasinghe, C.; Goto, N.; Aoki, T.; Wada, S. Phenolics composition and antioxidant activity of sweet basil (Ocimum basilicum L.). J. Agric. Food Chem. 2003, 51, 4442–4449. [Google Scholar] [CrossRef]
- Nadeem, H.R.; Akhtar, S.; Sestili, P.; Ismail, T.; Neugart, S.; Qamar, M.; Esatbeyoglu, T. Toxicity, antioxidant activity, and phytochemicals of basil (Ocimum basilicum L.) leaves cultivated in southern Punjab, Pakistan. Foods 2022, 11, 1239. [Google Scholar] [CrossRef]
- Yibeltal, G.; Yusuf, Z.; Desta, M. Physicochemical properties, antioxidant and antimicrobial activities of Ethiopian sweet basil (Ocimum basilicum L.) Leaf and Flower Oil Extracts. Recent Adv. Anti-Infect. Drug Discov. 2022, 17, 131–138. [Google Scholar] [CrossRef]
- Perna, S.; Alawadhi, H.; Riva, A.; Allegrini, P.; Petrangolini, G.; Gasparri, C.; Alalwan, T.A.; Rondanelli, M. In vitro and in vivo anticancer activity of basil (Ocimum spp.): Current insights and future prospects. Cancers 2022, 14, 2375. [Google Scholar] [CrossRef]
- Rezzoug, M.; Bakchiche, B.; Gherib, A.; Roberta, A.; Guido, F.; Kilincarslan, O.; Mammadov, R.; Bardaweel, S.K. Chemical composition and bioactivity of essential oils and ethanolic extracts of Ocimum basilicum L. and Thymus algeriensis Boiss. & Reut. from the Algerian Saharan Atlas. BMC Complement. Altern. Med. 2019, 19, 146. [Google Scholar] [CrossRef]
- Majdi, C.; Pereira, C.; Dias, M.I.; Calhelha, R.C.; Alves, M.J.; Rhourri-Frih, B.; Charrouf, Z.; Barros, L.; Amaral, J.S.; Ferreira, I.C.F.R. Phytochemical characterization and bioactive properties of cinnamon basil (Ocimum basilicum cv. ‘Cinnamon’) and lemon basil (Ocimum × citriodorum). Antioxidants 2020, 9, 369. [Google Scholar] [CrossRef]
- Carović-Stanko, K.; Orlić, S.; Politeo, O.; Strikić, F.; Kolak, I.; Miloš, M.; Satovic, Z. Composition and antibacterial activities of essential oils of seven Ocimum taxa. Food Chem. 2010, 119, 196–201. [Google Scholar] [CrossRef]
- Kačániová, M.; Galovičová, L.; Borotová, P.; Vukovic, N.L.; Vukic, M.; Kunová, S.; Hanus, P.; Bakay, L.; Zagrobelna, E.; Kluz, M.; et al. Assessment of Ocimum basilicum rssential oil anti-insect activity and antimicrobial protection in fruit and vegetable quality. Plants 2022, 11, 1030. [Google Scholar] [CrossRef]
- Opalchenova, G.; Obreshkova, D. Comparative studies on the activity of basil—An essential oil from Ocimum basilicum L.—Against multidrug resistant clinical isolates of the genera Staphylococcus, Enterococcus and Pseudomonas by using different test methods. J. Microbiol. Methods 2003, 54, 105–110. [Google Scholar] [CrossRef]
- Galgano, M.; Capozza, P.; Pellegrini, F.; Cordisco, M.; Sposato, A.; Sblano, S.; Camero, M.; Lanave, G.; Fracchiolla, G.; Corrente, M.; et al. Antimicrobial activity of essential oils evaluated in vitro against Escherichia coli and Staphylococcus aureus. Antibiotics 2022, 11, 979. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, C.; Chen, X.; Cui, H.; Lin, L. The interference mechanism of basil essential oil on the cell membrane barrier and respiratory metabolism of Listeria monocytogenes. Front. Microbiol. 2022, 13, 855905. [Google Scholar] [CrossRef] [PubMed]
- Hiltunen, R.; Holm, Y. Basil: The Genus Ocimum; Harwood Academic Publishers: Amsterdam, The Nethelands, 1999. [Google Scholar]
- Cikoš, A.-M.; Flanjak, I.; Bojanić, K.; Babić, S.; Čižmek, L.; Čož-Rakovac, R.; Jokić, S.; Jerković, I. Bioprospecting of coralline red alga Amphiroa rigida J.V. Lamouroux: Volatiles, fatty acids and pigments. Molecules 2021, 26, 520. [Google Scholar] [CrossRef] [PubMed]
- Utakod, N.; Laosripaiboon, W.; Chunhachart, O.; Issakul, K. The efficiency and the correlation between testing methods on antimicrobial and antioxidant activities of selected medicinal essential oils. Int. Food Res. J. 2017, 24, 2616–2624. [Google Scholar]
- Marotti, M.; Piccaglia, R.; Giovanelli, E. Differences in essential oil composition of basil (Ocimum basilicum L.) Italian cultivars related to morphological characteristics. J. Agric. Food Chem. 1996, 44, 3926–3929. [Google Scholar] [CrossRef]
- Varga, F.; Carović-Stanko, K.; Ristić, M.; Grdiša, M.; Liber, Z.; Šatović, Z. Morphological and biochemical intraspecific characterization of Ocimum basilicum L. Ind. Crops. Prod. 2017, 109, 611–618. [Google Scholar] [CrossRef]
- Juškevičienė, D.; Radzevičius, A.; Viškelis, P.; Maročkienė, N.; Karklelienė, R. Estimation of morphological features and essential oil content of basils (Ocimum basilicum L.) grown under different conditions. Plants 2022, 11, 1896. [Google Scholar] [CrossRef]
- Grayer Renée, J.; Kite Geoffrey, C.; Goldstone Fiona, J.; Bryan Sarah, E.; Paton, A.; Putievsky, E. Infraspecific taxonomy and essential oil chemotypes in sweet basil, Ocimum basilicum. Phytochemistry 1996, 43, 1033–1039. [Google Scholar] [CrossRef]
- Vernin, G.; Metzger, J.; Fraisse, D. Analysis of basil oils by GC-MS data bank. Perfum. Flavor. 1984, 9, 71–86. [Google Scholar]
- Wogiatzi, E.; Papachatzis, A.; Kalorizou, H.; Chouliara, A.; Chouliaras, N. Evaluation of essential oil yield and chemical components of selected basil cultivars. Biotechnol. Biotechnol. Equip. 2011, 25, 2525–2527. [Google Scholar] [CrossRef]
- Simon, J.E.; Quinn, J.; Murray, R.G. Basil: A source of essential oils. In Advances in New Crops; Janick, J., Simon, J.E., Eds.; Timber Press: Portland, OR, USA, 1990; pp. 484–489. [Google Scholar]
- Paulus, D.; Valmorbida, R.; Ramos, C.E.P. Productivity and chemical composition of the essential oil of Ocimum x citriodorum Vis. according to ontogenetic and diurnal variation. J. Appl. Res. Med. Aromat. Plants 2019, 12, 59–65. [Google Scholar] [CrossRef]
- Tangpao, T.; Chung, H.H.; Sommano, S.R. Aromatic profiles of essential oils from five commonly used Thai basils. Foods 2018, 7, 175. [Google Scholar] [CrossRef]
- Maggio, A.; Roscigno, G.; Bruno, M.; De Falco, E.; Senatore, F. Essential-oil variability in a collection of Ocimum basilicum L. (Basil) cultivars. Chem. Biodivers. 2016, 13, 1357–1367. [Google Scholar] [CrossRef]
- Hussain, A.I.; Anwar, F.; Hussain Sherazi, S.T.; Przybylski, R. Chemical composition, antioxidant and antimicrobial activities of basil (Ocimum basilicum) essential oils depends on seasonal variations. Food Chem. 2008, 108, 986–995. [Google Scholar] [CrossRef]
- Sakkas, H.; Papadopoulou, C. Antimicrobial activity of basil, oregano, and thyme essential oils. J. Microbiol. Biotechnol. 2017, 27, 429–438. [Google Scholar] [CrossRef]
- Klimánková, E.; Holadová, K.; Hajšlová, J.; Čajka, T.; Poustka, J.; Koudela, M. Aroma profiles of five basil (Ocimum basilicum L.) cultivars grown under conventional and organic conditions. Food Chem. 2008, 107, 464–472. [Google Scholar] [CrossRef]
- Javanmardi, J.; Khalighi, A.; Kashi, A.; Bais, H.P.; Vivanco, J.M. Chemical characterization of basil (Ocimum basilicum L.) found in local accessions and used in traditional medicines in Iran. J. Agric. Food Chem. 2002, 50, 5878–5883. [Google Scholar] [CrossRef]
- Kivilompolo, M.; Hyötyläinen, T. Comprehensive two-dimensional liquid chromatography in analysis of Lamiaceae herbs: Characterisation and quantification of antioxidant phenolic acids. J. Chromatogr. A 2007, 1145, 155–164. [Google Scholar] [CrossRef]
- Prinsi, B.; Morgutti, S.; Negrini, N.; Faoro, F.; Espen, L. Insight into composition of bioactive phenolic compounds in leaves and flowers of green and purple basil. Plants 2020, 9, 22. [Google Scholar] [CrossRef]
- Pandey, A.K.; Singh, P.; Tripathi, N.N. Chemistry and bioactivities of essential oils of some Ocimum species: An overview. Asian Pac. J. Trop. Biomed. 2014, 4, 682–694. [Google Scholar] [CrossRef]
- Siriwardhana, N.; Lee, K.W.; Kim, S.H.; Ha, J.W.; Jeon, Y.J. Antioxidant activity of Hizikia fusiformis on reactive oxygen species scavenging and lipid peroxidation inhibition. Food Sci. Technol. Int. 2003, 9, 339–346. [Google Scholar] [CrossRef]
- Javanmardi, J.; Stushnoff, C.; Locke, E.; Vivanco, J.M. Antioxidant activity and total phenolic content of Iranian Ocimum accessions. Food Chem. 2003, 83, 547–550. [Google Scholar] [CrossRef]
- Cižmek, L.; Bavcon Kralj, M.; Čož-Rakovac, R.; Mazur, D.; Ul’yanovskii, N.; Likon, M.; Trebše, P. Supercritical carbon dioxide extraction of four medicinal mediterranean plants: Investigation of chemical composition and antioxidant activity. Molecules 2021, 26, 5697. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.T.; Nguyen, N.Q.; Thi, N.Q.N.; Thi, C.Q.N.; Truc, T.T.; Nghi, P.T.B. Studies on chemical, polyphenol content, flavonoid content, and antioxidant activity of sweet basil leaves (Ocimum basilicum L.). IOP Conf. Ser. Mater. Sci. Eng. 2021, 1092, 012083. [Google Scholar] [CrossRef]
- Singh, V.; Kahol, A.; Singh, I.P.; Saraf, I.; Shri, R. Evaluation of anti-amnesic effect of extracts of selected Ocimum species using in-vitro and in-vivo models. J. Ethnopharmacol. 2016, 193, 490–499. [Google Scholar] [CrossRef]
- Žlabur, J.Š.; Opačić, N.; Žutić, I.; Voća, S.; Poštek, M.; Radman, S.; Benko, B.; Uher, S.F. Valorization of nutritional potential and specialized metabolites of basil cultivars depending on cultivation method. Agronomy 2021, 11, 1048. [Google Scholar] [CrossRef]
- Abdulkhaleq, L.A.; Assi, M.A.; Noor, M.H.M.; Abdullah, R.; Saad, M.Z.; Taufiq-Yap, Y.H. Therapeutic uses of epicatechin in diabetes and cancer. Vet. World 2017, 10, 869–872. [Google Scholar] [CrossRef]
- Shanak, S.; Bassalat, N.; Albzoor, R.; Kadan, S.; Zaid, H. In vitro and in silico evaluation for the inhibitory action of O. basilicum methanol extract on α -glucosidase and α-amylase. Evid. Complement. Altern. Med. 2021, 2021, 5515775. [Google Scholar] [CrossRef]
- Ugbogu, O.C.; Emmanuel, O.; Agi, G.O.; Ibe, C.; Ekweogu, C.N.; Ude, V.C.; Uche, M.E.; Nnanna, R.O.; Ugbogu, E.A. A review on the traditional uses, phytochemistry, and pharmacological activities of clove basil (Ocimum gratissimum L.). Heliyon 2021, 7, e08404. [Google Scholar] [CrossRef]
- Wang, M.; Cantrell, C.L.; Mathews, S.T.; Paudel, P.; Lee, J.; Mentreddy, S.R. Agronomy, chemical analysis, and antidiabetic activity of basil (Ocimum species). ACS Food Sci. Technol. 2022, 2, 1243–1256. [Google Scholar] [CrossRef]
- Mastaneh, M.; Ahmad, M.; Taher, N.; Mehrdad, H. Antioxidant effect of Ocimum basilicum cv. dark opal (Lamiaceae) phenolics. Orient. J. Chem. 2014, 30, 1965–1969. [Google Scholar] [CrossRef]
- Widjaja, S.S.; Rusdiana; Savira, M. Glucose lowering effect of basil leaves in diabetic rats. Open Access Maced. J. Med. Sci. 2019, 7, 1415–1417. [Google Scholar] [CrossRef]
- El-Nahal, D.M.; Thabet, H.A.; Syed-Ahmed, E.F. Study the impact of sweet basil extracts (Ocimum basilicum) to reduce blood cholesterol. Egypt. J. Nutr. Health 2012, 7, 1–18. [Google Scholar] [CrossRef]
- Antonopoulou, I.; Sapountzaki, E.; Rova, U.; Christakopoulos, P. Ferulic acid from plant biomass: A phytochemical with promising antiviral properties. Front Nutr. 2022, 8, 777576. [Google Scholar] [CrossRef]
- Ou, S.; Kwok, K.C. Ferulic acid: Pharmaceutical functions, preparation and applications in foods. J. Sci. Food Agric. 2004, 84, 1261–1269. [Google Scholar] [CrossRef]
- Seyed, M.A.; Ayesha, S.; Azmi, N.; Al-RTabae, F.M.; Al-Alwy, A.I.; Al-Zahrani, O.R.; Hawsawi, Y. The neuroprotective attribution of Ocimum basilicum: A review on the prevention and management of neurodegenerative disorders. Futur. J. Pharm. Sci. 2021, 7, 139. [Google Scholar] [CrossRef]
- Fernandes, F.; Pereira, E.; Ćirić, A.; Soković, M.; Calhelha, R.C.; Barros, L.; Ferreira, I.C.F.R. Ocimum basilicum var. purpurascens leaves (red rubin basil): A source of bioactive compounds and natural pigments for the food industry. Food Funct. 2019, 10, 3161–3171. [Google Scholar] [CrossRef]
- Nadia, S.N.; Hafiza, Y.; Norlelawati, A.; Nadia, Y.H. Antibacterial properties of Ocimum spp. (Ocimum basilicum L. and Ocimum basilicum var. Purpurascens) against selected bacteria. East Afr. Sch. J. Agric. Life Sci. 2021, 4, 194–200. [Google Scholar] [CrossRef]
- Capparucci, F.; de Benedetto, G.; Natale, S.; Pecoraro, R.; Iaria, C.; Marino, F. Evaluation of anaesthetic effect of commercial basil Ocimum basilicum on zebrafish (Danio rerio) embryos. Fishes 2022, 7, 318. [Google Scholar] [CrossRef]
- De Vera, J.S.; De Castro, M.E.; Dulay, R.M. Phytochemical screening and teratogenic effect of lyophilized water extracts from Ocimum sanctum L. (Holy Basil) and Tamarindus indica L. (Tamarind) leaves in Danio rerio embryos. Der. Pharma Chem. 2016, 8, 86–91. [Google Scholar]
- Veeren, B.; Ghaddar, B.; Bringart, M.; Khazaal, S.; Gonthier, M.-P.; Meilhac, O.; Diotel, N.; Bascands, J.-L. Phenolic profile of herbal infusion and polyphenol-rich extract from leaves of the medicinal plant antirhea borbonica: Toxicity assay determination in zebrafish embryos and larvae. Molecules 2020, 25, 4482. [Google Scholar] [CrossRef] [PubMed]
- Šatović, Z.; Carović-Stanko, K.; Grdiša, M.; Jug-Dujaković, M.; Kolak, I.; Liber, Z. Conservation of Medicinal and Aromatic Plants in Croatia; Springer: Dordrecht, The Netherlands, 2012; pp. 261–269. [Google Scholar]
- Paton, A.; Putievsky, E. Taxonomic problems and cytotaxonomic relationships between and within varieties of Ocimum basilicum and related species (Labiatae). Kew Bull. 1996, 51, 509–524. [Google Scholar] [CrossRef]
- Radman, S.; Čagalj, M.; Šimat, V.; Jerković, I. Seasonal variability of volatilome from Dictyota dichotoma. Molecules 2022, 27, 3012. [Google Scholar] [CrossRef] [PubMed]
- Mathew, M.; Subramanian, S. In vitro screening for anti-cholinesterase and antioxidant activity of methanolic extracts of ayurvedic medicinal plants used for cognitive disorders. PLoS ONE 2014, 9, e86804. [Google Scholar] [CrossRef]
- Bustanji, Y.; Issa, A.; Mohammad, M.; Hudaib, M.; Tawah, K.; Alkhatib, H.; Almasri, I.; Al-Khalidi, B. Inhibition of hormone sensitive lipase and pancreatic lipase by Rosmarinus officinalis extract and selected phenolic constituents. J. Med. Plants Res. 2010, 4, 2235–2242. [Google Scholar]
- Mohammad, M.; Aiedeh, K.M.; Alkhatib, H.S.; Tawaha, K.; Alkhalidi, B.; Al-Masri, I.M.; Hamed, S.; Bustanji, Y. A Comparative enzymatic inhibition assay as a surrogate indicator of pharmaceuticals and potency equivalence of two orlistat formulations. Jordan J. Pharm. Sci. 2010, 3, 69–86. [Google Scholar]
- Yang, X.W.; Huang, M.Z.; Jin, Y.S.; Sun, L.N.; Song, Y.; Chen, H.S. Phenolics from Bidens bipinnata and their amylase inhibitory properties. Fitoterapia 2012, 83, 1169–1175. [Google Scholar] [CrossRef]
- Zengin, G. A study on in vitro enzyme inhibitory properties of Asphodeline anatolica: New sources of natural inhibitors for public health problems. Ind. Crops. Prod. 2016, 83, 39–43. [Google Scholar] [CrossRef]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef]
- Radman, S.; Čižmek, L.; Babić, S.; Cikoš, A.-M.; Čož-Rakovac, R.; Jokić, S.; Jerković, I. Bioprospecting of less-polar fractions of Ericaria crinita and Ericaria amentacea: Developmental toxicity and antioxidant activity. Mar. Drugs 2022, 20, 57. [Google Scholar] [CrossRef]
- Matijević, G.; Babić, S.; Maršavelski, A.; Stipaničev, D.; Repec, S.; Čoz-Rakovac, R.; Klobučar, G. Estimating risk of cardiovascular pharmaceuticals in freshwaters using zebrafish embryotoxicity test—Statins threat revealed. Chemosphere 2023, 313, 137574. [Google Scholar] [CrossRef]
- Babić, S.; Čizmek, L.; Maršavelski, A.; Malev, O.; Pflieger, M.; Strunjak-Perović, I.; Topić Popović, N.; Čoz-Rakovac, R.; Trebše, P. Utilization of the zebrafish model to unravel the harmful effects of biomass burning during Amazonian wildfires. Sci. Rep. 2021, 11, 2527. [Google Scholar] [CrossRef]

| Compound | Retention Index | Genovese Basil | Dark Opal Basil I | Dark Opal Basil II | Cinnamon Basil | Purple Basil | Lemon Basil | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | II | I | II | I | II | I | II | I | II | I | II | ||
| (Z)-Hex-3-en-1-ol | <900 | - | - | - | - | - | - | 0.47 | 0.81 | - | - | 0.28 | 0.30 |
| α-Pinene | 945 | - | - | - | - | 0.13 | 0.12 | 0.15 | - | - | - | - | - |
| Benzaldehyde | 971 | 0.19 | 0.32 | - | - | 0.07 | - | - | - | - | - | - | - |
| Sabinene | 982 | - | - | - | - | 0.16 | 0.13 | - | - | - | - | - | - |
| β-Pinene | 986 | 0.30 | - | - | - | 0.35 | 0.28 | - | - | - | - | - | - |
| 6-Methylhept-5-en-2-one | 992 | - | - | - | - | - | - | 0.60 | 1.18 | - | - | 0.49 | 0.65 |
| β-Myrcene | 996 | 0.36 | 0.25 | 0.26 | - | 0.70 | 0.42 | - | - | 0.15 | - | - | - |
| Limonene | 1037 | 0.20 | - | 0.18 | - | 0.16 | 0.16 | 0.28 | 0.21 | 0.54 | 0.50 | - | - |
| 1,8-Cineole | 1040 | 2.91 | 1.98 | 1.73 | 1.38 | 4.12 | 3.86 | 0.28 | 0.20 | - | - | - | - |
| 5-Methyl-5-vinyldihydrofuran-2(3H)-one (Lavender lactone) | 1049 | - | - | - | - | 0.08 | - | - | - | - | - | - | - |
| (E)-β-Ocimene | 1055 | 0.25 | - | - | - | - | - | 0.31 | 0.27 | 0.32 | 0.23 | - | - |
| Benzyl alcohol | 1043 | 0.53 | 0.40 | 1.08 | 1.50 | 0.21 | 0.59 | 0.54 | 0.59 | 0.60 | 0.77 | 0.41 | 0.38 |
| trans-Linalool oxide | 1079 | - | - | - | - | - | - | 0.61 | 0.36 | - | - | - | - |
| Fenchone | 1094 | - | - | - | - | - | - | 1.11 | 1.05 | - | - | - | - |
| Linalool | 1104 | 40.82 | 47.83 | 45.77 | 54.60 | 60.43 | 65.20 | 8.22 | 9.16 | 24.75 | 28.82 | 0.35 | 0.21 |
| 6-Methyl-3,5-heptadien-2-one | 1109 | - | - | - | - | - | - | 0.25 | 0.75 | - | - | - | - |
| Hexyl propanoate | 1110 | - | - | - | - | - | - | - | - | 0.05 | - | - | - |
| 2-Phenylethanol | 1120 | 0.58 | 0.96 | - | - | - | - | - | - | - | - | - | - |
| Camphor | 1152 | - | - | 0.83 | 0.77 | - | - | - | - | 0.10 | 0.05 | - | - |
| 3,7-Dimethylocta-3,6-dienal | 1188 | - | - | - | - | - | - | 0.70 | 0.48 | - | - | 1.22 | 1.25 |
| α-Terpineol | 1196 | 0.77 | 0.75 | 0.63 | 0.95 | 0.49 | 0.50 | 1.06 | 1.28 | 0.24 | - | - | - |
| Estragole | 1219 | - | - | - | - | 0.15 | - | 0.52 | 0.67 | 35.28 | 33.70 | 0.15 | - |
| Octyl acetate | 1217 | 0.32 | 0.10 | - | - | - | - | 1.07 | 1.12 | 0.17 | 0.15 | 0.94 | 0.56 |
| Fenchyl acetate | 1225 | - | - | - | - | 0.28 | 0.23 | - | - | - | - | - | - |
| Nerol | 1235 | - | - | - | - | - | - | 1.62 | 1.71 | - | - | 2.45 | 1.59 |
| Neral | 1248 | - | - | - | - | - | - | 13.12 | 12.13 | - | - | 20.57 | 25.54 |
| Geraniol | 1262 | 0.38 | 0.30 | 0.14 | 0.10 | 0.96 | 0.77 | 0.35 | 0.36 | - | - | 1.29 | 0.75 |
| Citral | 1276 | - | - | - | - | - | - | 24.26 | 22.77 | - | - | 36.27 | 41.47 |
| Bornyl acetate | 1290 | 1.22 | 1.07 | 0.99 | 0.95 | - | - | - | - | - | - | - | - |
| Eugenol | 1364 | 21.16 | 18.26 | 21.14 | 22.53 | 1.64 | 1.13 | - | - | 11.00 | 11.17 | - | - |
| α-Copaene | 1381 | 0.38 | 0.36 | - | - | 0.36 | 0.32 | 1.74 | 1.40 | 0.32 | 0.28 | 1.11 | 0.88 |
| Sativene | 1393 | 0.24 | - | - | - | 0.60 | 0.23 | 0.16 | - | 0.17 | 0.37 | 0.28 | |
| β-Elemene | 1396 | 2.26 | 2.66 | 2.40 | 1.92 | 4.78 | 4.36 | - | - | 3.23 | 2.88 | - | - |
| cis-α-Bergamotene | 1432 | 3.46 | 2.44 | 3.04 | 2.80 | - | - | - | - | - | - | - | - |
| trans-Caryophyllene | 1424 | - | - | - | - | 1.59 | 1.49 | 4.52 | 4.64 | 1.51 | 1.43 | 5.23 | 4.00 |
| α-Humulene | 1459 | 0.54 | 0.53 | 0.48 | 0.05 | 0.51 | 0.45 | 0.79 | 0.78 | 0.92 | 0.78 | - | - |
| trans-α-Bergamotene | 1441 | 3.46 | 2.44 | - | - | 1.24 | 1.15 | 6.07 | 6.01 | - | - | 3.10 | 2.51 |
| α-Guaiene | 1444 | 1.01 | 1.16 | 1.11 | 1.01 | 2.19 | 2.02 | 0.49 | 0.55 | 1.40 | 1.31 | - | - |
| Bicyclosesquiphellandrene | 1648 | - | - | - | - | - | - | - | - | 0.92 | 0.24 | 1.08 | 0.88 |
| (Z)-β-Farnesene | 1463 | - | - | - | - | 0.16 | - | - | - | - | - | 0.67 | 0.48 |
| Germacrene D | 1485 | 3.79 | 4.21 | 1.48 | 1.10 | 3.94 | 3.41 | 0.92 | 0.93 | 4.62 | 4.03 | 2.15 | 1.09 |
| β-Eudesmene | 1490 | - | - | - | - | - | - | 1.11 | 1.07 | - | - | - | - |
| Bicyclogermacrene | 1501 | 1.04 | 0.92 | 0.67 | 0.10 | 2.90 | 2.48 | - | - | 1.50 | 1.15 | - | - |
| δ-Guaiene | 1510 | 2.15 | 2.51 | 2.22 | 1.98 | 4.75 | 4.31 | - | - | 3.10 | 2.98 | - | - |
| β-Bisabolene | 1513 | - | - | - | - | - | - | 0.73 | 0.64 | - | - | 0.37 | 0.30 |
| γ-Cadinene | 1518 | 3.78 | 3.55 | 3.50 | 3.00 | 1.58 | 1.47 | - | - | 1.91 | 1.79 | - | - |
| cis-Calamenene | 1528 | 0.52 | - | 0.60 | - | 0.35 | 0.26 | - | - | 0.33 | - | - | - |
| Dihydroactinidiolide | 1534 | - | - | 0.18 | - | - | - | - | - | 0.18 | 0.23 | 0.18 | 0.20 |
| (E)-α-Bisabolene | 1549 | - | - | - | - | - | - | 7.69 | 8.23 | - | - | 6.38 | 5.26 |
| Caryophyllene oxide | 1586 | - | - | - | - | - | - | 5.36 | 5.17 | - | - | 1.93 | 1.47 |
| t-Cadinol | 1647 | 2.80 | 3.80 | 4.55 | 3.82 | 0.90 | 0.97 | - | -- | 1.93 | 1.67 | - | - |
| Compound | RT | Area Percentage (%) | |||||
|---|---|---|---|---|---|---|---|
| Genovese Basil | Dark Opal Basil I | Dark Opal Basil II | Cinnamon Basil | Purple Basil | Lemon Basil | ||
| Hydroxyphenylethanol | 25.142 | - | 0.524 | - | - | - | - |
| Chlorogenic acid | 27.208 | - | - | 0.405 | 0.406 | - | - |
| Epicatechin | 29.250 | 0.343 | 0.500 | 0.241 | - | 0.447 | |
| Vanillic acid | 30.383 | - | - | 3.054 | 3.439 | 3.451 | - |
| Caffeic acid | 30.625 | 3.522 | 3.185 | - | - | - | 2.744 |
| Coumaric acid | 36.108 | - | 1.039 | 2.677 | - | 0.552 | - |
| Ferulic acid | 37.158 | - | 16.842 | - | 1.027 | 1.444 | - |
| Rosmarinic acid | 40.025 | 13.422 | - | 17.096 | 21.001 | 18.117 | 24.826 |
| Rutin | 40.725 | 3.077 | - | 3.406 | 3.789 | 3.740 | 2.018 |
| Diosmin | 42.292 | 3.473 | 2.488 | 2.085 | 1.208 | 0.784 | 1.049 |
| Myricetin | 43.567 | - | 2.036 | 1.139 | 0.762 | 0.839 | 1.373 |
| Naringenin | 48.200 | 0.420 | 0.527 | 1.143 | 0.801 | 0.660 | 0.593 |
| Quercetin | 48.575 | 0.678 | 0.333 | - | 0.576 | 1.194 | 0.510 |
| Luteolin | 50.383 | 0.222 | 0.464 | 0.254 | 1.354 | 1.909 | - |
| Thymol | 58.658 | 0.120 | 0.089 | - | 0.331 | - | 0.242 |
| Amentoflavone | 59.067 | 0.017 | 0.021 | 0.020 | 0.089 | 0.221 | 0.067 |
| Totals | 25.293 | 28.048 | 31.279 | 35.025 | 32.911 | 33.869 | |
| S. aureus | B. subtilis | E. coli | |
|---|---|---|---|
| Purple basil | MIC 150 MBC 300 | MIC 300 | Partial inhibition 300 |
| Dark Opal basil I | MIC 150 MBC 300 | MIC 300 | Partial inhibition 300 |
| Correlation Coefficient (r) | DPPH | ABTS | FRAP | ORAC | Folin–Ciocalteu |
|---|---|---|---|---|---|
| DPPH | - | ||||
| ABTS | 0.88 * | - | |||
| FRAP | 0.97 * | 0.83 * | - | ||
| ORAC | 0.85 * | 0.69 | 0.88 * | - | |
| Folin–Ciocalteu | 0.84 * | 0.59 | 0.82 * | 0.95 * | - |
| Sample Number | Sample | Mass (g) | Volume (mL) |
|---|---|---|---|
| 1 | Purple basil | 15.51 | 200 |
| 2 | Cinnamon basil | 9.75 | 200 |
| 3 | Dark Opal basil I | 14.45 | 250 |
| 4 | Lemon basil | 10.97 | 183 |
| 5 | Genovese basil | 17.10 | 250 |
| 6 | Dark Opal basil II | 13.29 | 200 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baković, M.; Perković, L.; Matijević, G.; Martić, A.; Vujović, T.; Ekić, S.; Fumić, M.; Jurić, S.; Čož-Rakovac, R.; Roje, M.; et al. Bioprospecting of Five Ocimum sp. Cultivars from Croatia: New Potential for Dietary and Dermatological Application with Embryotoxicity Tests. Pharmaceuticals 2023, 16, 981. https://doi.org/10.3390/ph16070981
Baković M, Perković L, Matijević G, Martić A, Vujović T, Ekić S, Fumić M, Jurić S, Čož-Rakovac R, Roje M, et al. Bioprospecting of Five Ocimum sp. Cultivars from Croatia: New Potential for Dietary and Dermatological Application with Embryotoxicity Tests. Pharmaceuticals. 2023; 16(7):981. https://doi.org/10.3390/ph16070981
Chicago/Turabian StyleBaković, Marija, Lucija Perković, Gabrijela Matijević, Ana Martić, Tamara Vujović, Sara Ekić, Monika Fumić, Sara Jurić, Rozelindra Čož-Rakovac, Marin Roje, and et al. 2023. "Bioprospecting of Five Ocimum sp. Cultivars from Croatia: New Potential for Dietary and Dermatological Application with Embryotoxicity Tests" Pharmaceuticals 16, no. 7: 981. https://doi.org/10.3390/ph16070981
APA StyleBaković, M., Perković, L., Matijević, G., Martić, A., Vujović, T., Ekić, S., Fumić, M., Jurić, S., Čož-Rakovac, R., Roje, M., Jokić, S., & Jerković, I. (2023). Bioprospecting of Five Ocimum sp. Cultivars from Croatia: New Potential for Dietary and Dermatological Application with Embryotoxicity Tests. Pharmaceuticals, 16(7), 981. https://doi.org/10.3390/ph16070981










