Role of Galectin in Cardiovascular Conditions including Cirrhotic Cardiomyopathy
Abstract
1. Introduction
2. Galectin in Various Cardiovascular Diseases
3. Galectin-3 and Cardiac Inflammation
4. Galectin-3 and Organ Remodeling/Fibrosis
5. Galectin Pathophysiology in Noncirrhotic Cardiovascular Conditions
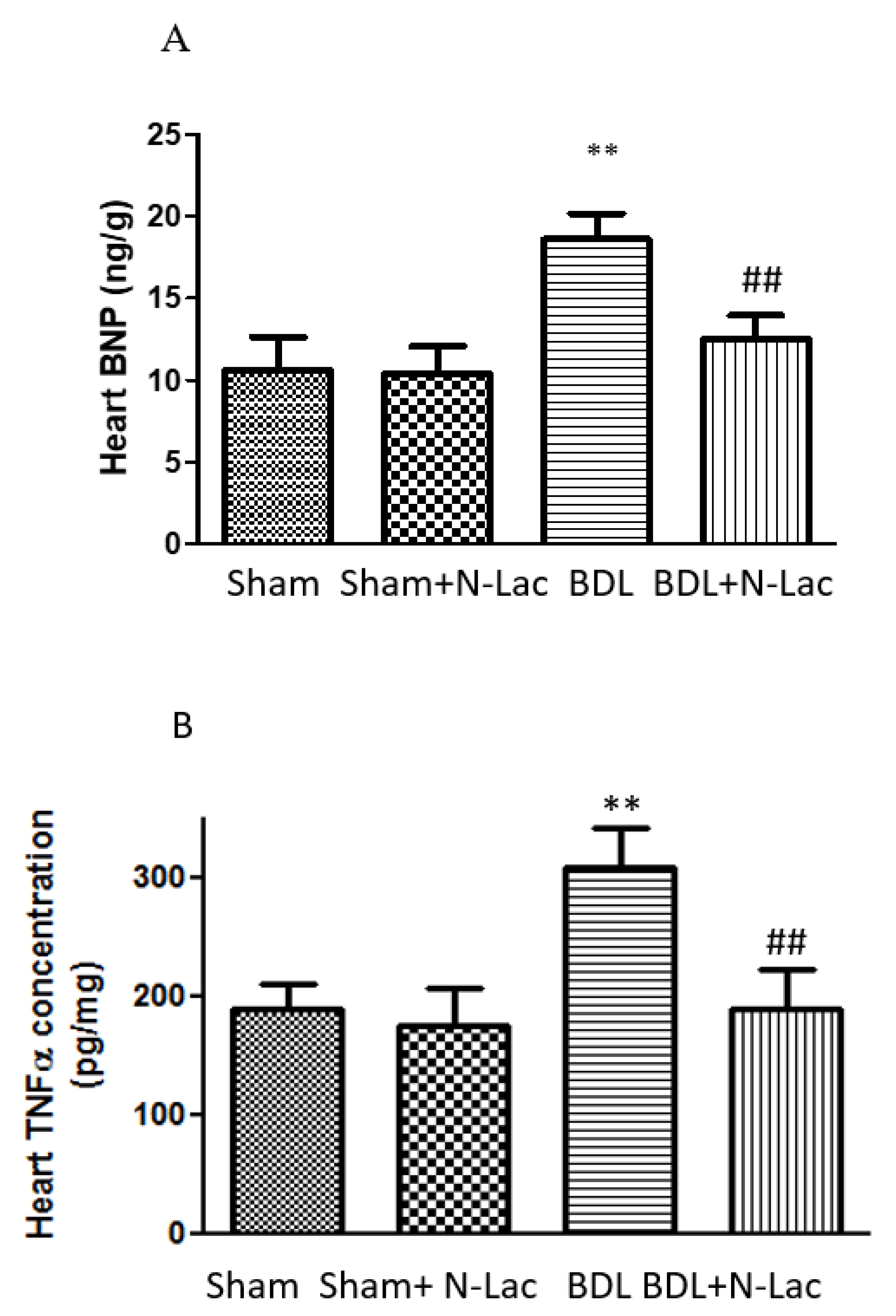
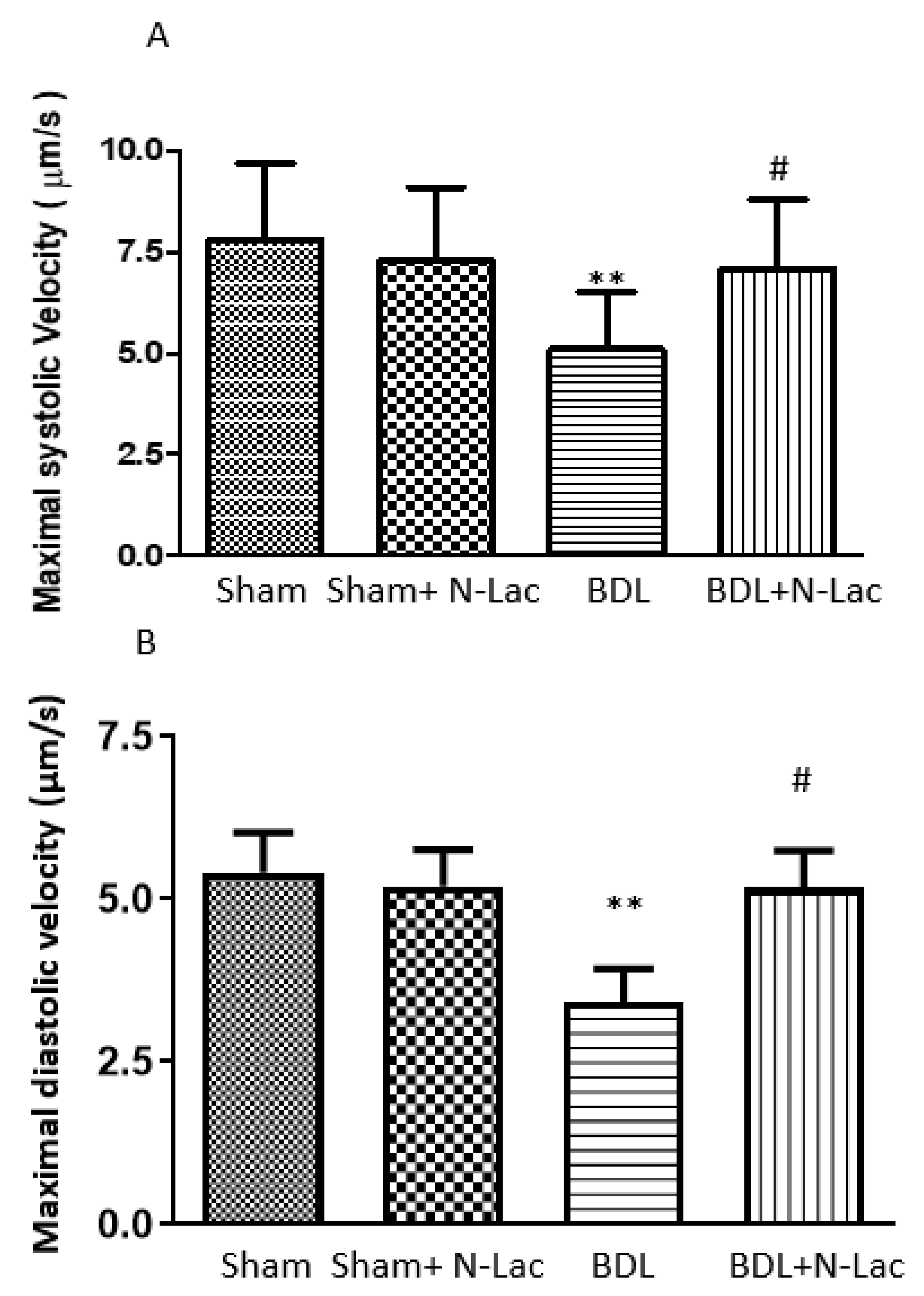
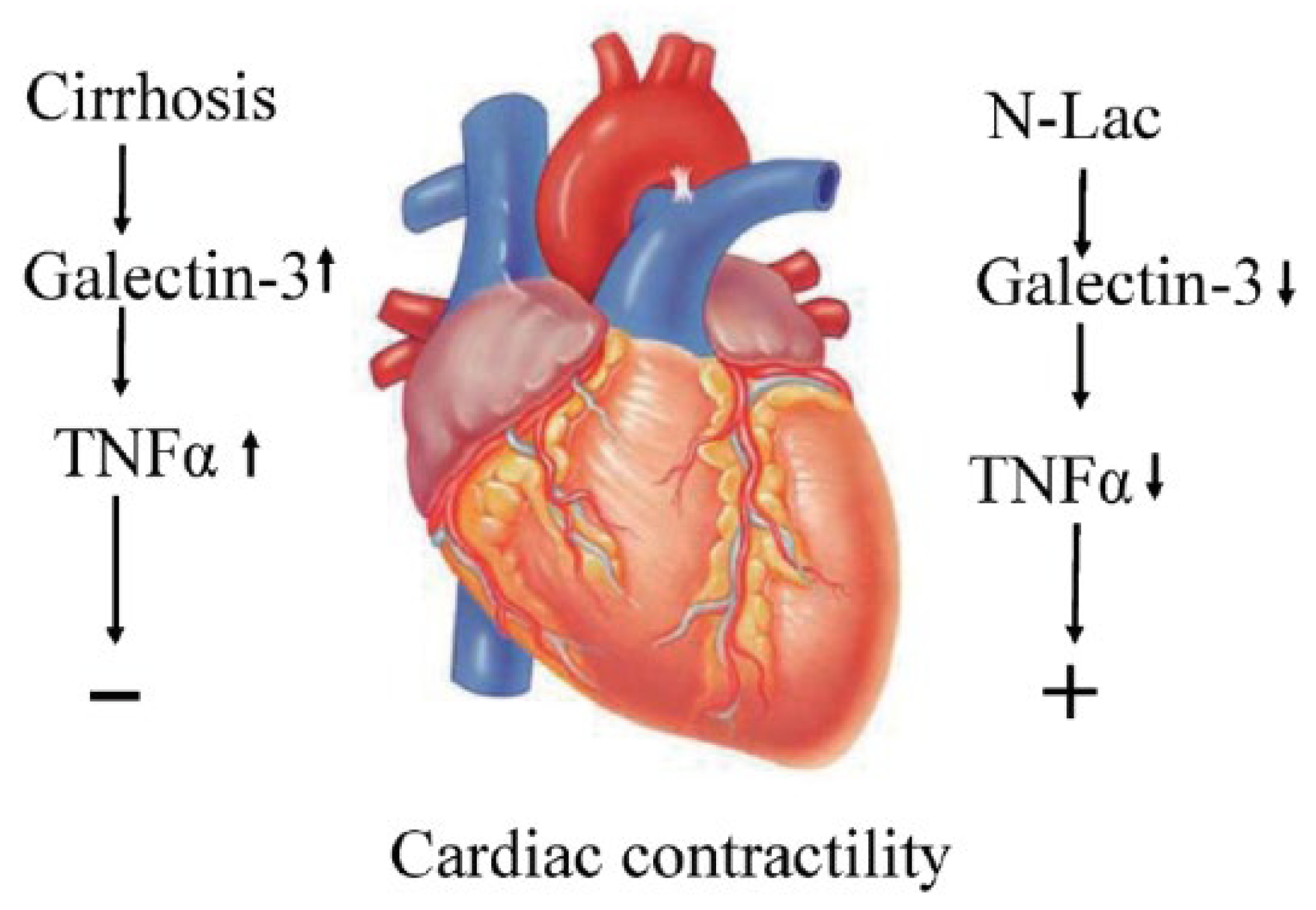
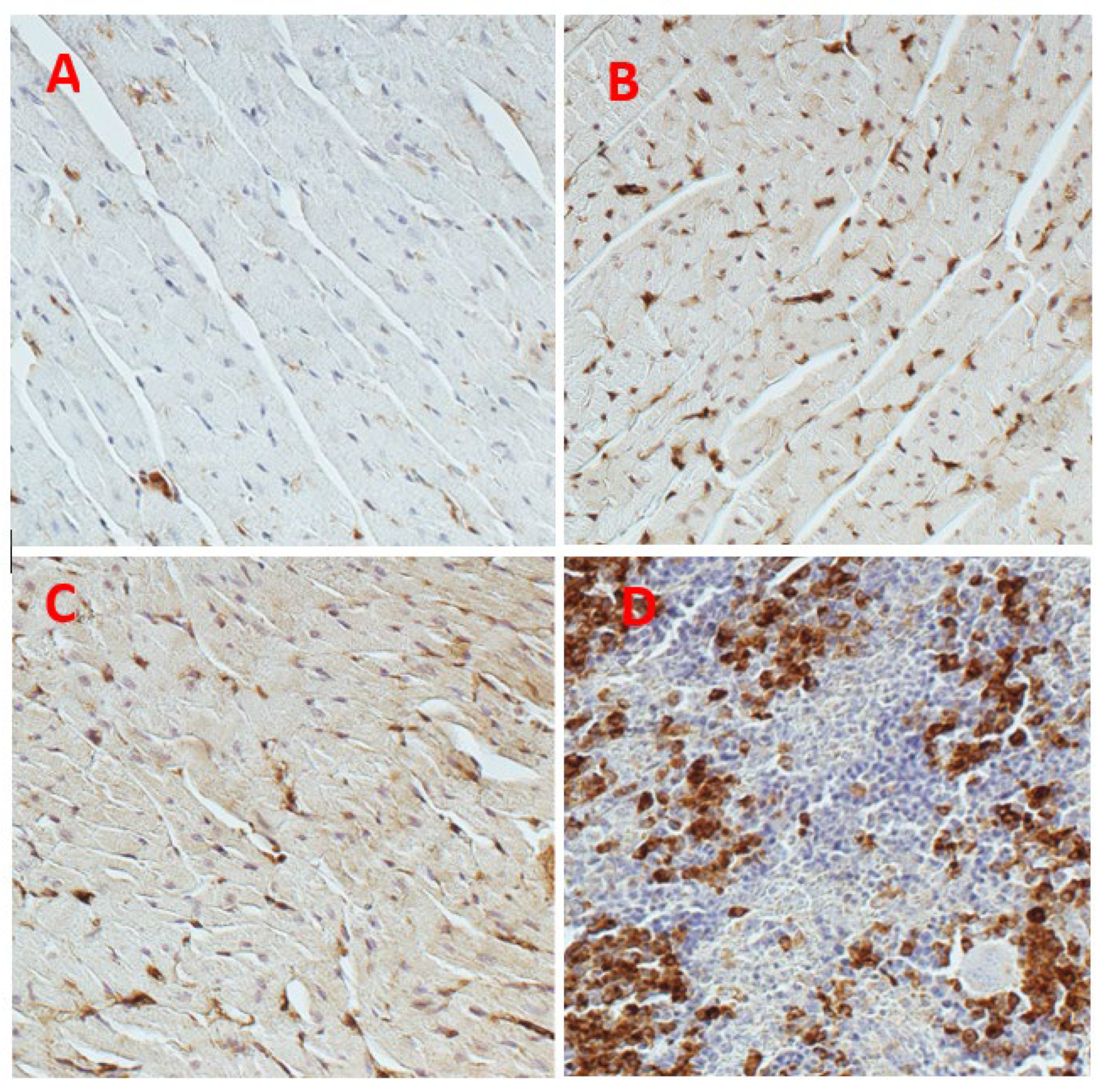
6. Galectin in Cirrhotic Cardiomyopathy
7. Galectin-3 Inhibitor to Treat Cirrhotic Cardiomyopathy
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, S.S. Cardiac abnormalities in liver cirrhosis. West. J. Med. 1989, 151, 530–535. [Google Scholar]
- Ma, Z.; Lee, S.S. Cirrhotic cardiomyopathy: Getting to the heart of the matter. Hepatology 1996, 24, 451–459. [Google Scholar] [CrossRef]
- Chahal, D.; Liu, H.; Shamatutu, C.; Sidhu, H.; Lee, S.S.; Marquez, V. Review article: Comprehensive analysis of cirrhotic cardiomyopathy. Aliment. Pharmacol. Ther. 2021, 53, 985–998. [Google Scholar] [PubMed]
- Liu, H.; Nguyen, H.H.; Yoon, K.T.; Lee, S.S. Pathogenic Mechanisms Underlying Cirrhotic Cardiomyopathy. Front. Netw. Physiol. 2022, 2, 849253. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.T.; Liu, H.; Lee, S.S. Cirrhotic Cardiomyopathy. Curr. Gastroenterol. Rep. 2020, 22, 45. [Google Scholar] [CrossRef]
- Lee, W.; Vandenberk, B.; Raj, S.R.; Lee, S.S. Prolonged QT Interval in Cirrhosis: Twisting Time? Gut Liver 2022, 16, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.T.; Liu, H.; Lee, S.S. β-blockers in advanced cirrhosis: More friend than enemy. Clin. Mol. Hepatol. 2021, 27, 425–436. [Google Scholar] [CrossRef]
- Dumic, J.; Dabelic, S.; Flogel, M. Galectin-3: An open-ended story. Biochim. Biophys. Acta 2006, 1760, 616–635. [Google Scholar] [CrossRef] [PubMed]
- Raz, A.; Carmi, P.; Raz, T.; Hogan, V.; Mohamed, A.; Wolman, S.R. Molecular cloning and chromosomal mapping of a human galactoside-binding protein. Cancer Res. 1991, 51, 2173–2178. [Google Scholar]
- Krzeslak, A.; Lipinska, A. Galectin-3 as a multifunctional protein. Cell. Mol. Biol. Lett. 2004, 9, 305–328. [Google Scholar]
- Ponikowska, B.; Iwanek, G.; Zdanowicz, A.; Urban, S.; Zymlinski, R.; Ponikowski, P.; Biegus, J. Biomarkers of Myocardial Injury and Remodeling in Heart Failure. J. Pers. Med. 2022, 12, 799. [Google Scholar] [CrossRef]
- Dong, R.; Zhang, M.; Hu, Q.; Zheng, S.; Soh, A.; Zheng, Y.; Yuan, H. Galectin-3 as a novel biomarker for disease diagnosis and a target for therapy (Review). Int. J. Mol. Med. 2018, 41, 599–614. [Google Scholar] [CrossRef] [PubMed]
- Al-Salam, S.; Kandhan, K.; Sudhadevi, M.; Yasin, J.; Tariq, S. Early Doxorubicin Myocardial Injury: Inflammatory, Oxidative Stress, and Apoptotic Role of Galectin-3. Int. J. Mol. Sci. 2022, 23, 12479. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Huai, W.; Ye, X.; Pan, Y.; Yang, X.; Chen, M.; Ma, Q.B.; Gao, Y.; Zhang, Y. Circulating plasma galectin-3 predicts new-onset atrial fibrillation in patients after acute myocardial infarction during hospitalization. BMC Cardiovasc. Disord. 2022, 22, 392. [Google Scholar] [CrossRef] [PubMed]
- Tsigkou, V.; Siasos, G.; Oikonomou, E.; Zaromitidou, M.; Mourouzis, K.; Dimitropoulos, S.; Bletsa, E.; Gouliopoulos, N.; Stampouloglou, P.K.; Panoilia, M.E.; et al. The prognostic role of galectin-3 and endothelial function in patients with heart failure. Cardiol. J. 2022. [Google Scholar] [CrossRef]
- Chaves, A.T.; Oliveira, A.L.G.; Guimaraes, N.S.; Magalhaes, I.C.; Menezes, C.; Rocha, M. Galectin-3 and fibrosis intensity in Chronic Chagas Cardiomyopathy: A systematic review. Rev. Inst. Med. Trop. Sao Paulo 2022, 64, e45. [Google Scholar] [CrossRef]
- Hrynchyshyn, N.; Jourdain, P.; Desnos, M.; Diebold, B.; Funck, F. Galectin-3: A new biomarker for the diagnosis, analysis and prognosis of acute and chronic heart failure. Arch. Cardiovasc. Dis. 2013, 106, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Gaskari, S.A.; Liu, H.; D’Mello, C.; Kunos, G.; Lee, S.S. Blunted cardiac response to hemorrhage in cirrhotic rats is mediated by local macrophage-released endocannabinoids. J. Hepatol. 2015, 62, 1272–1277. [Google Scholar] [CrossRef]
- Schroeder, J.T.; Adeosun, A.A.; Bieneman, A.P. Epithelial Cell-Associated Galectin-3 Activates Human Dendritic Cell Subtypes for Pro-Inflammatory Cytokines. Front. Immunol. 2020, 11, 524826. [Google Scholar] [CrossRef]
- Yoon, K.T.; Liu, H.; Zhang, J.; Han, S.; Lee, S.S. Galectin-3 inhibits cardiac contractility via a tumor necrosis factor alpha-dependent mechanism in cirrhotic rats. Clin. Mol. Hepatol. 2022, 28, 232–241. [Google Scholar] [CrossRef]
- Liu, L.; Liu, H.; Nam, S.W.; Lee, S.S. Protective effects of erythropoietin on cirrhotic cardiomyopathy in rats. Dig. Liver Dis. 2012, 44, 1012–1017. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.Y.; Liu, H.; Nam, S.W.; Kunos, G.; Lee, S.S. Mechanisms of TNFalpha-induced cardiac dysfunction in cholestatic bile duct-ligated mice: Interaction between TNFalpha and endocannabinoids. J. Hepatol. 2010, 53, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ma, Z.; Lee, S.S. Contribution of nitric oxide to the pathogenesis of cirrhotic cardiomyopathy in bile duct-ligated rats. Gastroenterology 2000, 118, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Sharma, U.C.; Pokharel, S.; van Brakel, T.J.; van Berlo, J.H.; Cleutjens, J.P.; Schroen, B.; Andre, S.; Crijns, H.J.; Gabius, H.J.; Maessen, J.; et al. Galectin-3 marks activated macrophages in failure-prone hypertrophied hearts and contributes to cardiac dysfunction. Circulation 2004, 110, 3121–3128. [Google Scholar] [CrossRef]
- Glenn, T.K.; Honar, H.; Liu, H.; ter Keurs, H.E.; Lee, S.S. Role of cardiac myofilament proteins titin and collagen in the pathogenesis of diastolic dysfunction in cirrhotic rats. J. Hepatol. 2011, 55, 1249–1255. [Google Scholar] [CrossRef]
- Trippel, T.D.; Mende, M.; Dungen, H.D.; Hashemi, D.; Petutschnigg, J.; Nolte, K.; Herrmann-Lingen, C.; Binder, L.; Hasenfuss, G.; Pieske, B.; et al. The diagnostic and prognostic value of galectin-3 in patients at risk for heart failure with preserved ejection fraction: Results from the DIAST-CHF study. ESC Heart Fail. 2021, 8, 829–841. [Google Scholar] [CrossRef]
- Reifenberg, K.; Lehr, H.A.; Torzewski, M.; Steige, G.; Wiese, E.; Kupper, I.; Becker, C.; Ott, S.; Nusser, P.; Yamamura, K.; et al. Interferon-gamma induces chronic active myocarditis and cardiomyopathy in transgenic mice. Am. J. Pathol. 2007, 171, 463–472. [Google Scholar] [CrossRef]
- Thandavarayan, R.A.; Watanabe, K.; Ma, M.; Veeraveedu, P.T.; Gurusamy, N.; Palaniyandi, S.S.; Zhang, S.; Muslin, A.J.; Kodama, M.; Aizawa, Y. 14-3-3 protein regulates Ask1 signaling and protects against diabetic cardiomyopathy. Biochem. Pharmacol. 2008, 75, 1797–1806. [Google Scholar] [CrossRef]
- Sharma, U.; Rhaleb, N.E.; Pokharel, S.; Harding, P.; Rasoul, S.; Peng, H.; Carretero, O.A. Novel anti-inflammatory mechanisms of N-Acetyl-Ser-Asp-Lys-Pro in hypertension-induced target organ damage. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H1226–H1232. [Google Scholar] [CrossRef]
- Frunza, O.; Russo, I.; Saxena, A.; Shinde, A.V.; Humeres, C.; Hanif, W.; Rai, V.; Su, Y.; Frangogiannis, N.G. Myocardial Galectin-3 Expression Is Associated with Remodeling of the Pressure-Overloaded Heart and May Delay the Hypertrophic Response without Affecting Survival, Dysfunction, and Cardiac Fibrosis. Am. J. Pathol. 2016, 186, 1114–1127. [Google Scholar] [CrossRef]
- Nguyen, M.N.; Su, Y.; Vizi, D.; Fang, L.; Ellims, A.H.; Zhao, W.B.; Kiriazis, H.; Gao, X.M.; Sadoshima, J.; Taylor, A.J.; et al. Mechanisms responsible for increased circulating levels of galectin-3 in cardiomyopathy and heart failure. Sci. Rep. 2018, 8, 8213. [Google Scholar] [CrossRef]
- Humphries, D.C.; Mills, R.; Boz, C.; McHugh, B.J.; Hirani, N.; Rossi, A.G.; Pedersen, A.; Schambye, H.T.; Slack, R.J.; Leffler, H.; et al. Galectin-3 inhibitor GB0139 protects against acute lung injury by inhibiting neutrophil recruitment and activation. Front. Pharmacol. 2022, 13, 949264. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, W.W.; Sun, J.H.; Yang, H.X.; Xu, G.R.; Zhang, Y.; Song, Q.H.; Zhang, C.; Liu, W.Z.; Liu, X.C.; et al. Modified citrus pectin prevents isoproterenol-induced cardiac hypertrophy associated with p38 signalling and TLR4/JAK/STAT3 pathway. Biomed. Pharmacother. 2021, 143, 112178. [Google Scholar] [CrossRef]
- Wu, H.; Guo, J.; Yao, Y.; Xu, S. Polystyrene nanoplastics induced cardiomyocyte apoptosis and myocardial inflammation in carp by promoting ROS production. Fish Shellfish Immunol. 2022, 125, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.R.; Lin, H.M.; Biliran, H.; Raz, A. Cell cycle arrest and inhibition of anoikis by galectin-3 in human breast epithelial cells. Cancer Res. 1999, 59, 4148–4154. [Google Scholar] [PubMed]
- Zong, D.; Liu, X.; Shen, C.; Liu, T.; Ouyang, R. Involvement of Galectin-3 in neurocognitive impairment in obstructive sleep apnea via regulating inflammation and oxidative stress through NLRP3. Sleep Med. 2023, 101, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Tian, M.; Jiang, X.; Liu, Y.; Wang, Y.; Li, Y. Inhibition of galectin-3 ameliorates high-glucose-induced oxidative stress and inflammation in ARPE-19 cells. Cutan. Ocul. Toxicol. 2022, 41, 179–186. [Google Scholar] [CrossRef]
- Miao, R.; Wang, L.; Chen, Z.; Ge, S.; Li, L.; Zhang, K.; Chen, Y.; Guo, W.; Duan, X.; Zhu, M.; et al. Advances in the study of nicotinamide adenine dinucleotide phosphate oxidase in myocardial remodeling. Front. Cardiovasc. Med. 2022, 9, 1000578. [Google Scholar] [CrossRef]
- Zhu, N.; Zhu, L.; Huang, B.; Xiang, W.; Zhao, X. Galectin-3 Inhibition Ameliorates Streptozotocin-Induced Diabetic Cardiomyopathy in Mice. Front. Cardiovasc. Med. 2022, 9, 868372. [Google Scholar] [CrossRef]
- Henderson, N.C.; Mackinnon, A.C.; Farnworth, S.L.; Poirier, F.; Russo, F.P.; Iredale, J.P.; Haslett, C.; Simpson, K.J.; Sethi, T. Galectin-3 regulates myofibroblast activation and hepatic fibrosis. Proc. Natl. Acad. Sci. USA 2006, 103, 5060–5065. [Google Scholar] [CrossRef]
- Ou, S.M.; Tsai, M.T.; Chen, H.Y.; Li, F.A.; Tseng, W.C.; Lee, K.H.; Chang, F.P.; Lin, Y.P.; Yang, R.B.; Tarng, D.C. Identification of Galectin-3 as Potential Biomarkers for Renal Fibrosis by RNA-Sequencing and Clinicopathologic Findings of Kidney Biopsy. Front. Med. 2021, 8, 748225. [Google Scholar] [CrossRef] [PubMed]
- De Boer, R.A.; Voors, A.A.; Muntendam, P.; van Gilst, W.H.; van Veldhuisen, D.J. Galectin-3: A novel mediator of heart failure development and progression. Eur. J. Heart Fail. 2009, 11, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Vlachou, F.; Varela, A.; Stathopoulou, K.; Ntatsoulis, K.; Synolaki, E.; Pratsinis, H.; Kletsas, D.; Sideras, P.; Davos, C.H.; Capetanaki, Y.; et al. Galectin-3 interferes with tissue repair and promotes cardiac dysfunction and comorbidities in a genetic heart failure model. Cell. Mol. Life Sci. 2022, 79, 250. [Google Scholar] [CrossRef] [PubMed]
- Khadeja Bi, A.; Santhosh, V.; Sigamani, K. Levels of Galectin-3 in Chronic Heart Failure: A Case-Control Study. Cureus 2022, 14, e28310. [Google Scholar] [CrossRef]
- Pozder Geb Gehlken, C.; Rogier van der Velde, A.; Meijers, W.C.; Sillje, H.H.W.; Muntendam, P.; Dokter, M.M.; van Gilst, W.H.; Schols, H.A.; de Boer, R.A. Pectins from various sources inhibit galectin-3-related cardiac fibrosis. Curr. Res. Transl. Med. 2022, 70, 103321. [Google Scholar] [CrossRef]
- Yu, L.; Ruifrok, W.P.; Meissner, M.; Bos, E.M.; van Goor, H.; Sanjabi, B.; van der Harst, P.; Pitt, B.; Goldstein, I.J.; Koerts, J.A.; et al. Genetic and pharmacological inhibition of galectin-3 prevents cardiac remodeling by interfering with myocardial fibrogenesis. Circ. Heart Fail. 2013, 6, 107–117. [Google Scholar] [CrossRef]
- Wu, C.K.; Su, M.Y.; Lee, J.K.; Chiang, F.T.; Hwang, J.J.; Lin, J.L.; Chen, J.J.; Liu, F.T.; Tsai, C.T. Galectin-3 level and the severity of cardiac diastolic dysfunction using cellular and animal models and clinical indices. Sci. Rep. 2015, 5, 17007. [Google Scholar] [CrossRef]
- Van Kimmenade, R.R.; Januzzi, J.L.; Ellinor, P.T., Jr.; Sharma, U.C.; Bakker, J.A.; Low, A.F.; Martinez, A.; Crijns, H.J.; MacRae, C.A.; Menheere, P.P.; et al. Utility of amino-terminal pro-brain natriuretic peptide, galectin-3, and apelin for the evaluation of patients with acute heart failure. J. Am. Coll. Cardiol. 2006, 48, 1217–1224. [Google Scholar] [CrossRef]
- Weir, R.A.; Petrie, C.J.; Murphy, C.A.; Clements, S.; Steedman, T.; Miller, A.M.; McInnes, I.B.; Squire, I.B.; Ng, L.L.; Dargie, H.J.; et al. Galectin-3 and cardiac function in survivors of acute myocardial infarction. Circ. Heart Fail. 2013, 6, 492–498. [Google Scholar] [CrossRef]
- Xu, G.R.; Zhang, C.; Yang, H.X.; Sun, J.H.; Zhang, Y.; Yao, T.T.; Li, Y.; Ruan, L.; An, R.; Li, A.Y. Modified citrus pectin ameliorates myocardial fibrosis and inflammation via suppressing galectin-3 and TLR4/MyD88/NF-kappaB signaling pathway. Biomed. Pharmacother. 2020, 126, 110071. [Google Scholar] [CrossRef]
- Liu, Y.H.; D’Ambrosio, M.; Liao, T.D.; Peng, H.; Rhaleb, N.E.; Sharma, U.; Andre, S.; Gabius, H.J.; Carretero, O.A. N-acetyl-seryl-aspartyl-lysyl-proline prevents cardiac remodeling and dysfunction induced by galectin-3, a mammalian adhesion/growth-regulatory lectin. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H404–H412. [Google Scholar] [CrossRef]
- Wanninger, J.; Weigert, J.; Wiest, R.; Bauer, S.; Karrasch, T.; Farkas, S.; Scherer, M.N.; Walter, R.; Weiss, T.S.; Hellerbrand, C.; et al. Systemic and hepatic vein galectin-3 are increased in patients with alcoholic liver cirrhosis and negatively correlate with liver function. Cytokine 2011, 55, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Abu-Elsaad, N.M.; Elkashef, W.F. Modified citrus pectin stops progression of liver fibrosis by inhibiting galectin-3 and inducing apoptosis of stellate cells. Can. J. Physiol. Pharmacol. 2016, 94, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.W.; Liu, H.; Wong, J.Z.; Feng, A.Y.; Chu, G.; Merchant, N.; Lee, S.S. Cardiomyocyte apoptosis contributes to pathogenesis of cirrhotic cardiomyopathy in bile duct-ligated mice. Clin. Sci. 2014, 127, 519–526. [Google Scholar] [CrossRef]
- Vandenberk, B.; Altieri, M.H.; Liu, H.; Raj, S.R.; Lee, S.S. Review article: Diagnosis, pathophysiology and management of atrial fibrillation in cirrhosis and portal hypertension. Aliment. Pharmacol. Ther. 2023, 57, 290–303. [Google Scholar] [CrossRef] [PubMed]
- Gundling, F.; Schmidtler, F.; Zelihic, E.; Seidl, H.; Haller, B.; Ronel, J.; Loffler, N.; Schepp, W. Frequency of cardiac arrhythmia in patients with liver cirrhoses and evaluation of associated factors. Z. Gastroenterol. 2012, 50, 1149–1155. [Google Scholar]
- Erdem, K.; Kurtoglu, E.; Oc, M.; Oc, B.; Ilgenli, T.F.; Unlu, A.; Eryavuz Onmaz, D. The plasma galectin-3 level has high specificity and sensitivity for predicting postoperative atrial fibrillation after coronary artery bypass surgery. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 9072–9078. [Google Scholar]
- Kornej, J.; Schmidl, J.; Ueberham, L.; John, S.; Daneschnejad, S.; Dinov, B.; Hindricks, G.; Adams, V.; Husser, D.; Bollmann, A. Galectin-3 in patients with atrial fibrillation undergoing radiofrequency catheter ablation. PLoS ONE 2015, 10, e0123574. [Google Scholar] [CrossRef]
- Ruan, Z.B.; Gao, R.F.; Wang, F.; Chen, G.C.; Zhu, J.G.; Ren, Y.; Zhu, L. Circulating Galectin-3 and Aldosterone for Predicting Atrial Fibrillation Recurrence after Radiofrequency Catheter Ablation. Cardiovasc. Ther. 2022, 2022, 6993904. [Google Scholar] [CrossRef]
- Lee, K.N.; Kim, D.Y.; Boo, K.Y.; Kim, Y.G.; Roh, S.Y.; Baek, Y.S.; Kim, D.H.; Lee, D.I.; Shim, J.; Choi, J.I.; et al. Therapeutic implications of galectin-3 in patients with atrial fibrillation. Sci. Rep. 2022, 12, 784. [Google Scholar] [CrossRef]
- Altieri, M.H.; Liu, H.; Lee, S.S. Cardiovascular events after liver transplantation: MACE hurts. Rev. Cardiovasc. Med. 2022, 23, 91. [Google Scholar] [CrossRef]
- VanWagner, L.B.; Ning, H.; Whitsett, M.; Levitsky, J.; Uttal, S.; Wilkins, J.T.; Abecassis, M.M.; Ladner, D.P.; Skaro, A.I.; Lloyd-Jones, D.M. A point-based prediction model for cardiovascular risk in orthotopic liver transplantation: The CAR-OLT score. Hepatology 2017, 66, 1968–1979. [Google Scholar] [CrossRef]
- Darrat, Y.H.; Smer, A.; Elayi, C.S.; Morales, G.X.; Alqahtani, F.; Alkhouli, M.; Catanzaro, J.; Shah, J.; Salih, M. Mortality and morbidity in patients with atrial fibrillation and liver cirrhosis. World J. Cardiol. 2020, 12, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Abbas, W.A.; Kasem Ahmed, S.M.; Abdel Aal, A.M.; Mahmoud, A.A.; Abdelmalek, M.O.; Mekky, M.A.; Abozaid, M.A.; Ibrahim, A.K. Galactin-3 and brain natriuretic peptide versus conventional echocardiography in the early detection of cirrhotic cardiomyopathy. Turk. J. Gastroenterol. 2016, 27, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Nobbe, A.M.; McCurdy, H.M. Management of the Adult Patient with Cirrhosis Complicated by Ascites. Crit. Care Nurs. Clin. North. Am. 2022, 34, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yoon, K.T.; Zhang, J.; Lee, S.S. Advances in cirrhotic cardiomyopathy. Curr. Opin. Gastroenterol. 2021, 37, 187–193. [Google Scholar] [CrossRef]
- Limas, C.J.; Guiha, N.H.; Lekagul, O.; Cohn, J.N. Impaired left ventricular function in alcoholic cirrhosis with ascites. Ineffectiveness of ouabain. Circulation 1974, 49, 754–760. [Google Scholar] [CrossRef]
- Luo, H.; Liu, B.; Zhao, L.; He, J.; Li, T.; Zha, L.; Li, X.; Qi, Q.; Liu, Y.; Yu, Z. Galectin-3 mediates pulmonary vascular remodeling in hypoxia-induced pulmonary arterial hypertension. J. Am. Soc. Hypertens. 2017, 11, 673–683.e3. [Google Scholar] [CrossRef]
- Traber, P.G.; Chou, H.; Zomer, E.; Hong, F.; Klyosov, A.; Fiel, M.I.; Friedman, S.L. Regression of fibrosis and reversal of cirrhosis in rats by galectin inhibitors in thioacetamide-induced liver disease. PLoS ONE 2013, 8, e75361. [Google Scholar] [CrossRef]
- Harrison, S.A.; Marri, S.R.; Chalasani, N.; Kohli, R.; Aronstein, W.; Thompson, G.A.; Irish, W.; Miles, M.V.; Xanthakos, S.A.; Lawitz, E.; et al. Randomised clinical study: GR-MD-02, a galectin-3 inhibitor, vs. placebo in patients having non-alcoholic steatohepatitis with advanced fibrosis. Aliment. Pharmacol. Ther. 2016, 44, 1183–1198. [Google Scholar] [CrossRef]
- Chalasani, N.; Abdelmalek, M.F.; Garcia-Tsao, G.; Vuppalanchi, R.; Alkhouri, N.; Rinella, M.; Noureddin, M.; Pyko, M.; Shiffman, M.; Sanyal, A.; et al. Effects of Belapectin, an Inhibitor of Galectin-3, in Patients with Nonalcoholic Steatohepatitis with Cirrhosis and Portal Hypertension. Gastroenterology 2020, 158, 1334–1345.e5. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Hwang, S.-Y.; Lee, S.S. Role of Galectin in Cardiovascular Conditions including Cirrhotic Cardiomyopathy. Pharmaceuticals 2023, 16, 978. https://doi.org/10.3390/ph16070978
Liu H, Hwang S-Y, Lee SS. Role of Galectin in Cardiovascular Conditions including Cirrhotic Cardiomyopathy. Pharmaceuticals. 2023; 16(7):978. https://doi.org/10.3390/ph16070978
Chicago/Turabian StyleLiu, Hongqun, Sang-Youn Hwang, and Samuel S. Lee. 2023. "Role of Galectin in Cardiovascular Conditions including Cirrhotic Cardiomyopathy" Pharmaceuticals 16, no. 7: 978. https://doi.org/10.3390/ph16070978
APA StyleLiu, H., Hwang, S.-Y., & Lee, S. S. (2023). Role of Galectin in Cardiovascular Conditions including Cirrhotic Cardiomyopathy. Pharmaceuticals, 16(7), 978. https://doi.org/10.3390/ph16070978






