Antiarrhythmic Sotalol, Occlusion/Occlusion-like Syndrome in Rats, and Stable Gastric Pentadecapeptide BPC 157 Therapy
Abstract
1. Introduction
2. Results
2.1. A Perilous Syndrome Occurred Peripherally and Centrally
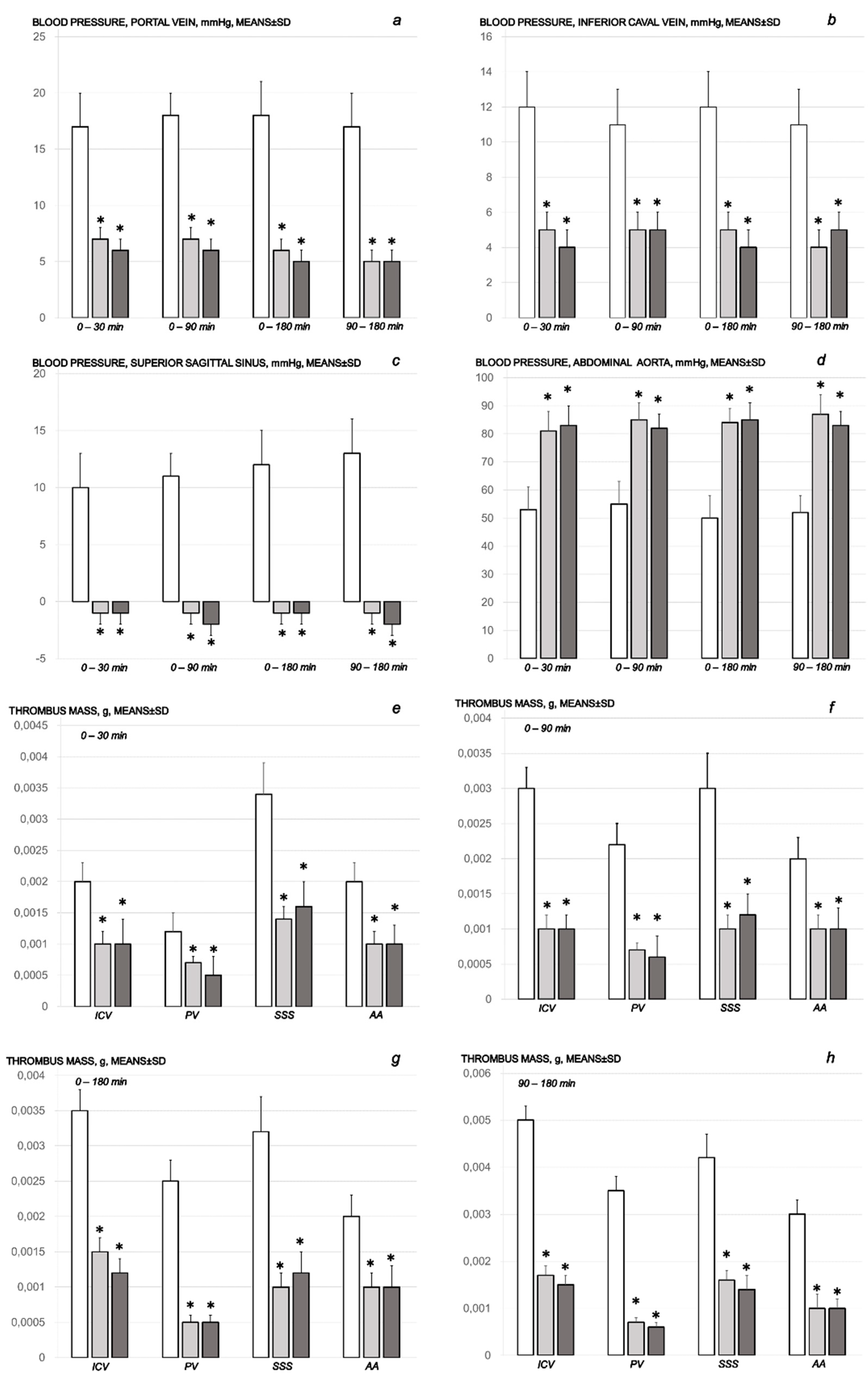
2.1.1. Blood Pressure Disturbances
2.1.2. Thrombosis
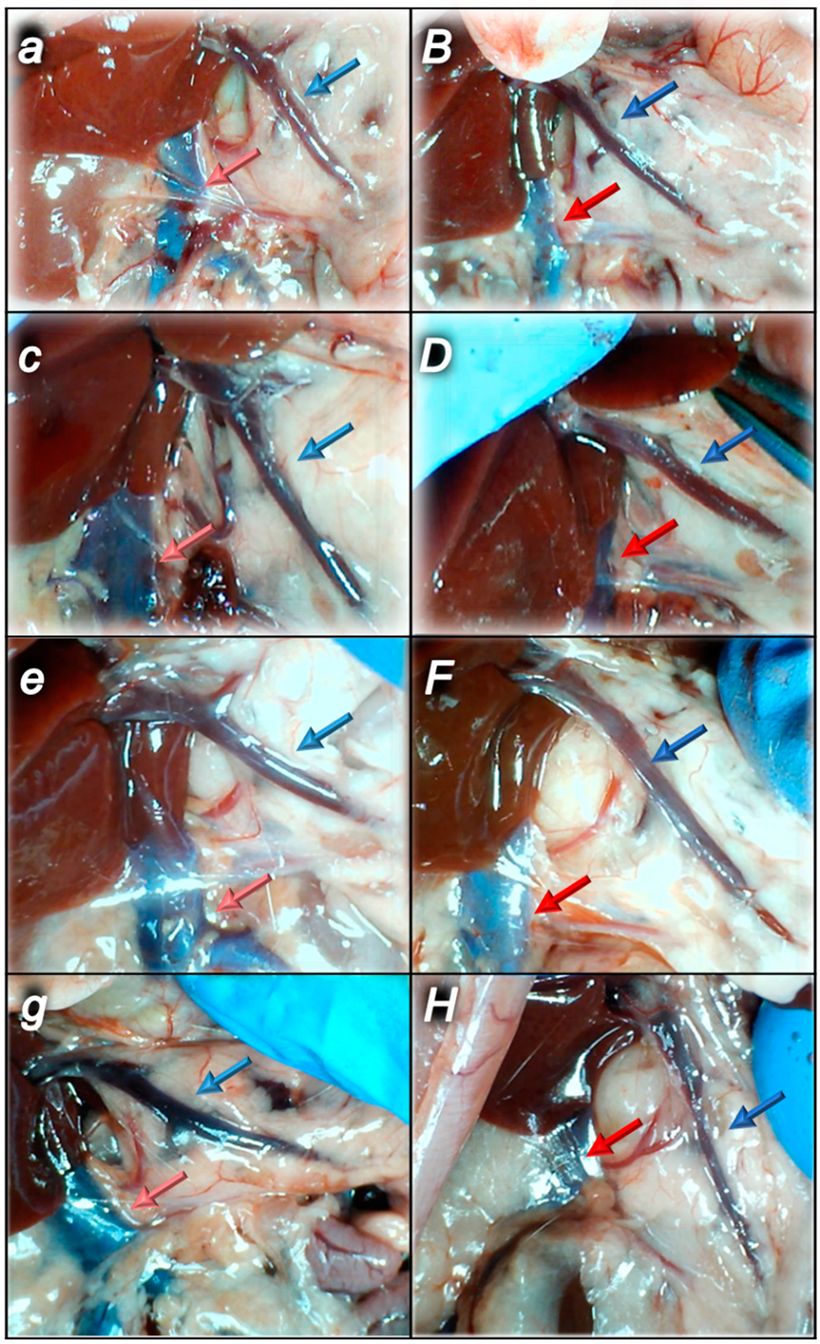
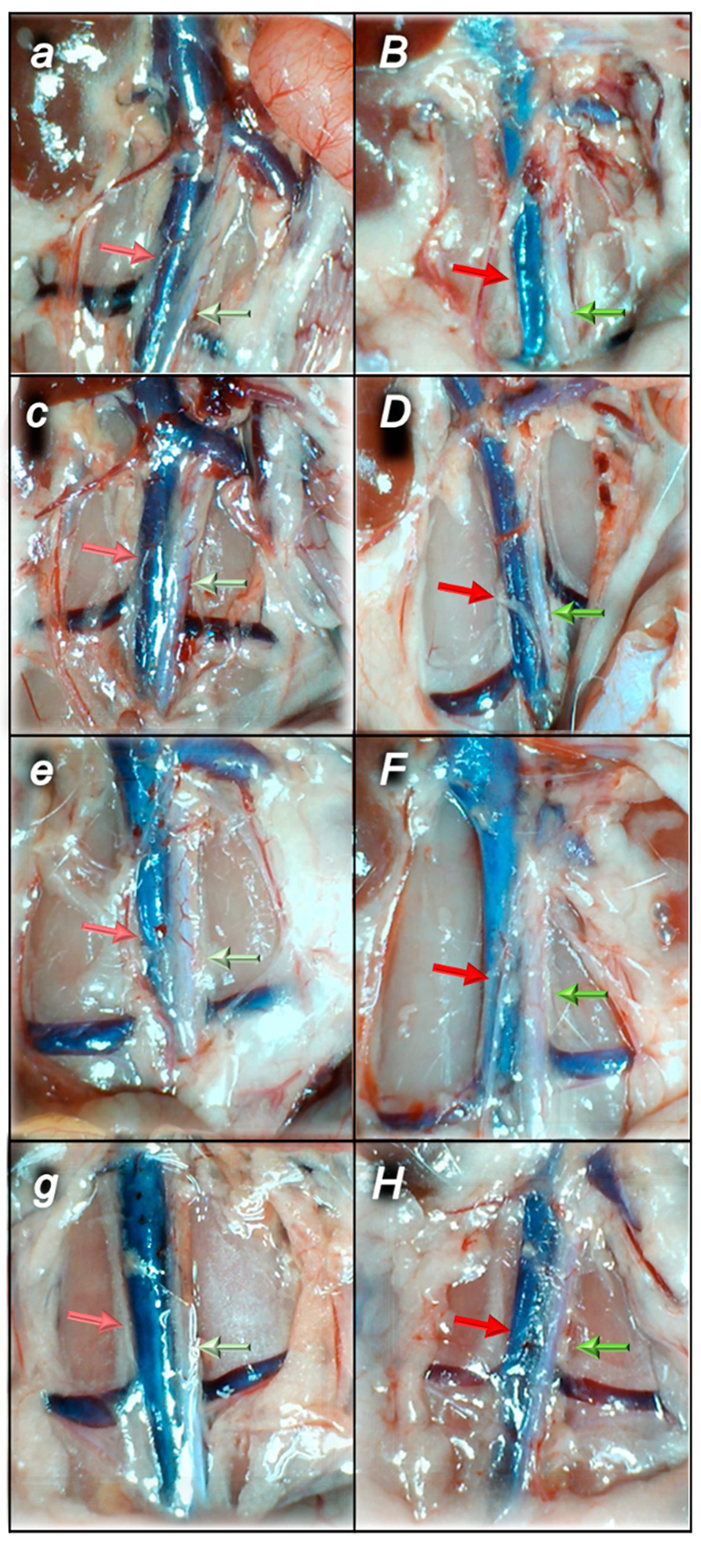
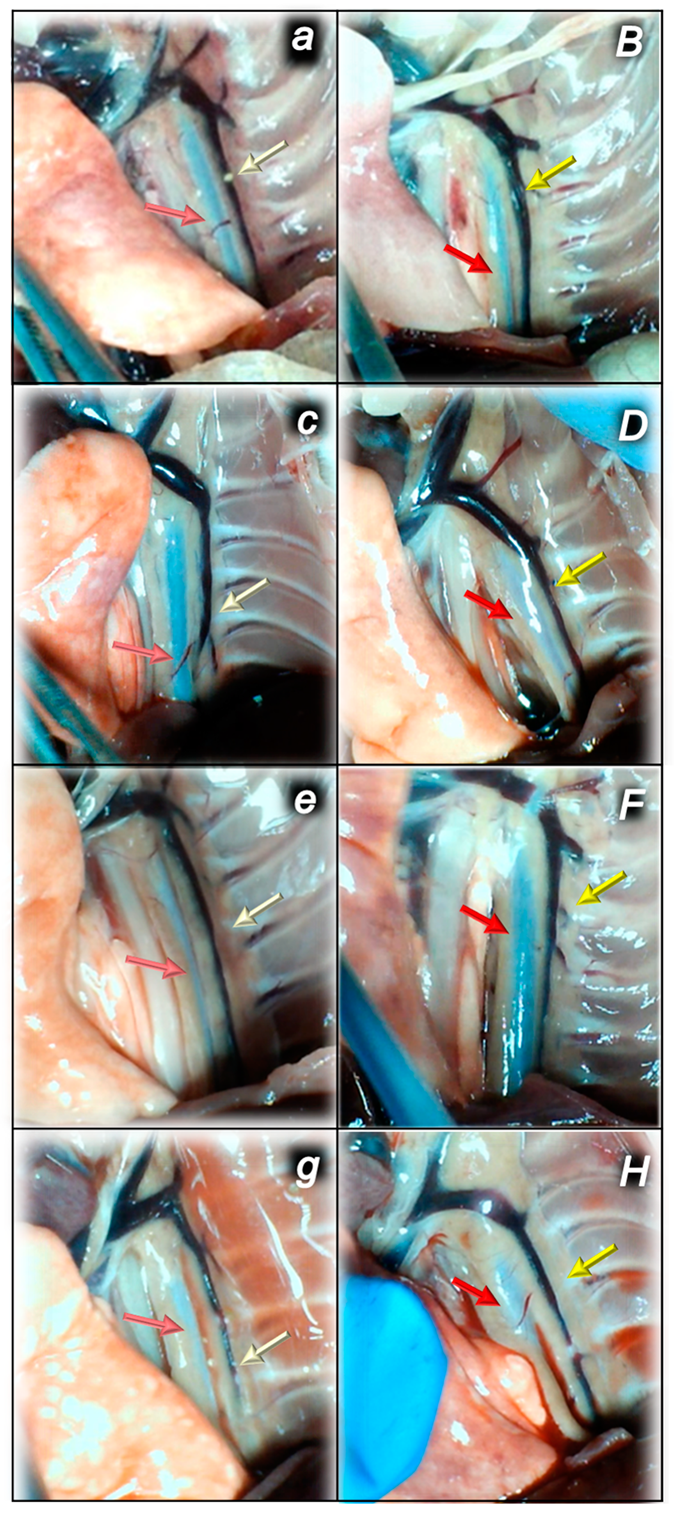
2.1.3. Collateral Pathways, Blood Vessels, and Brain Gross Presentation
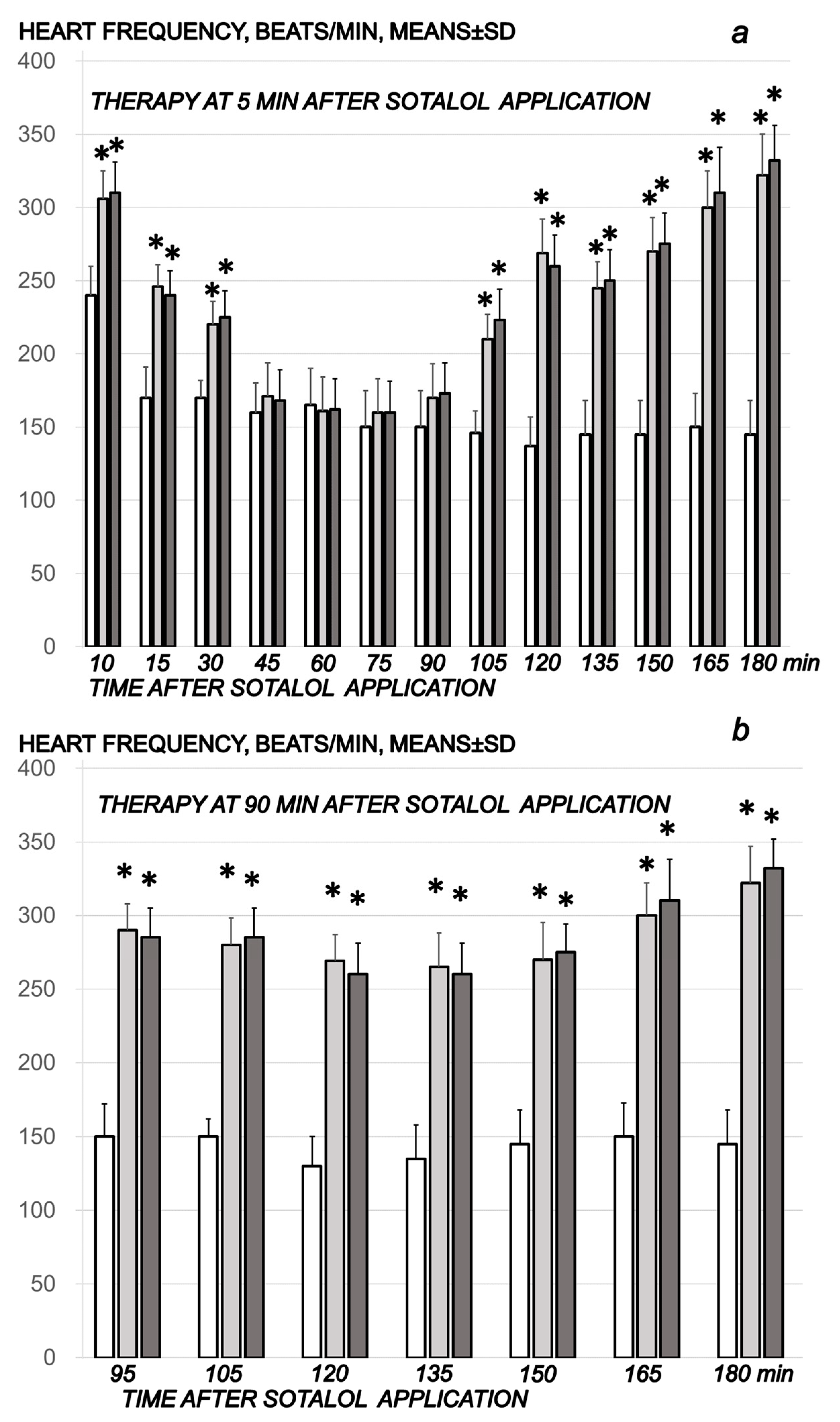
2.1.4. Heart and ECG Disturbances
2.2. A Perilous Syndrome Occurred Peripherally
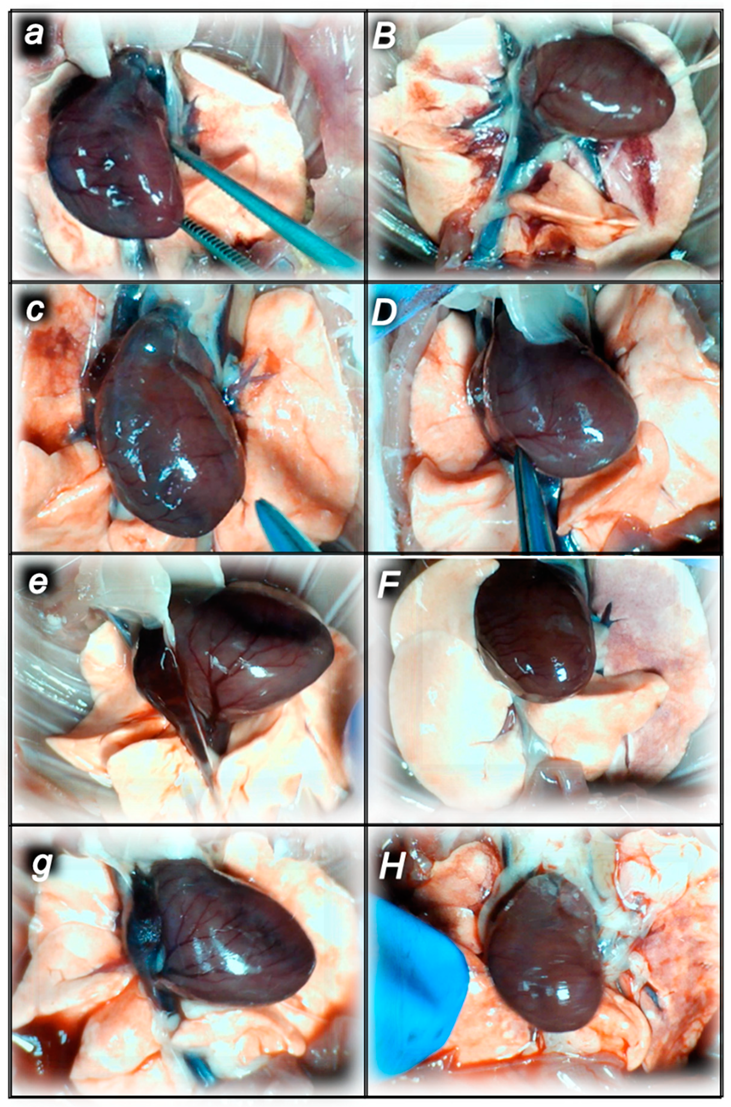
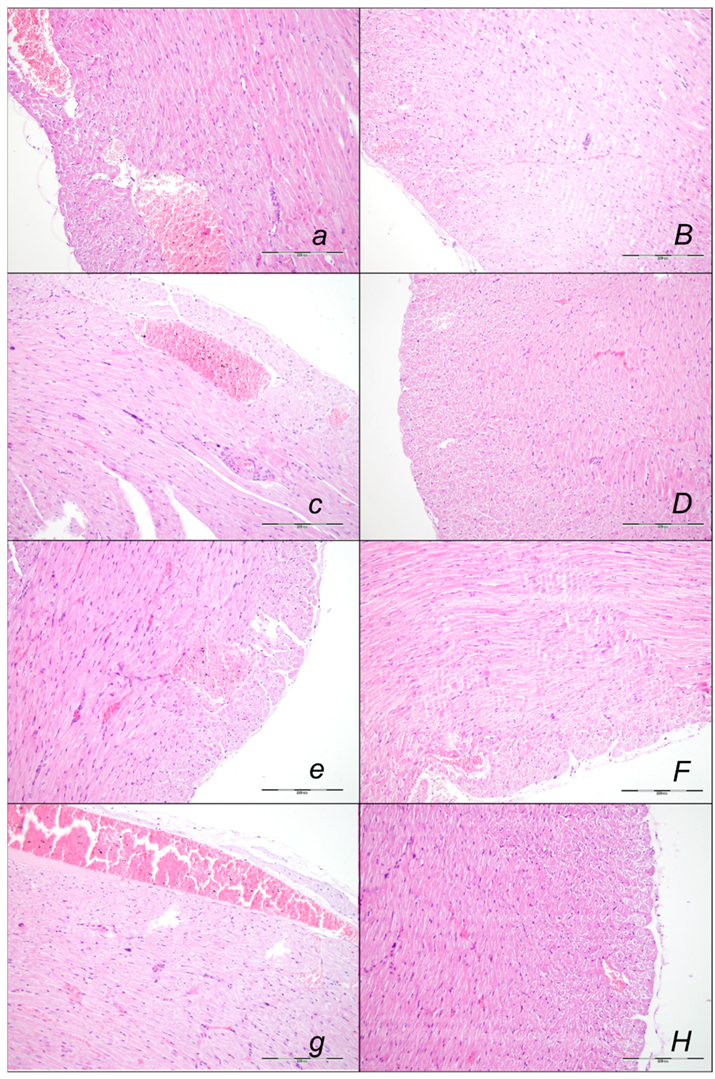
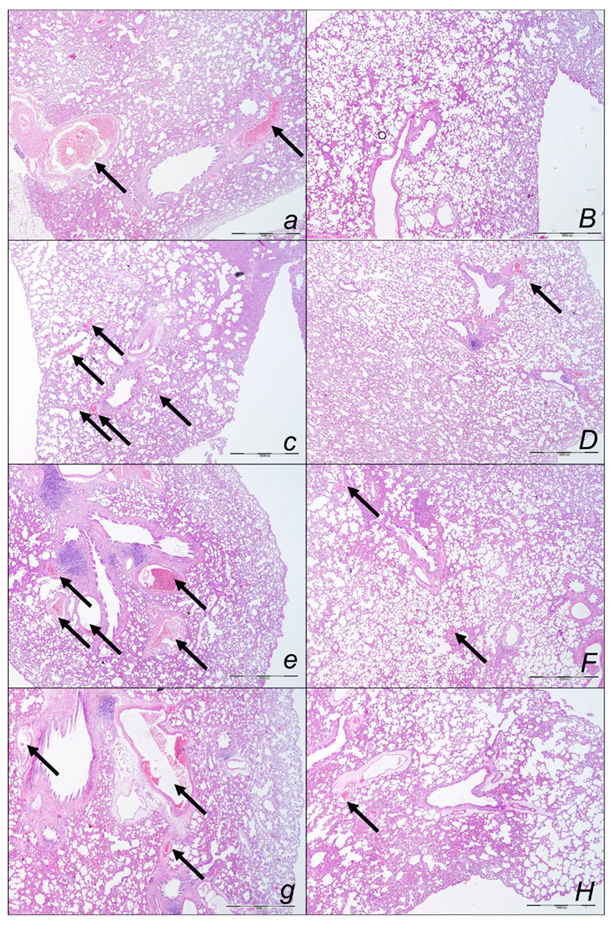
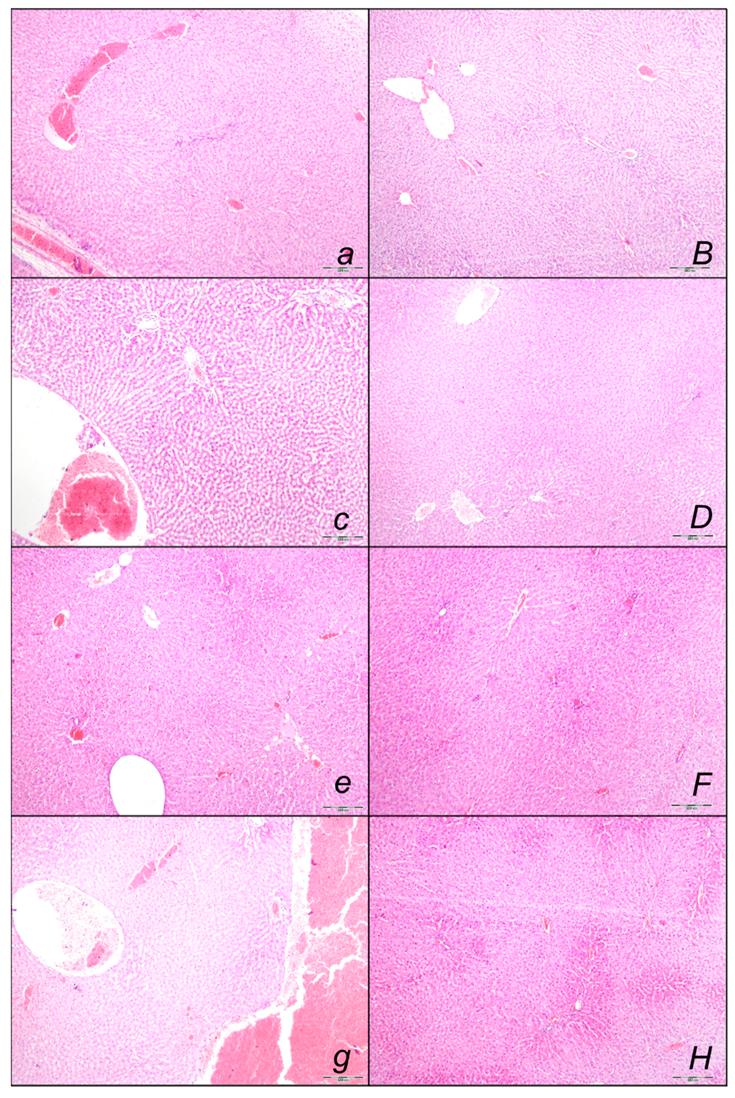
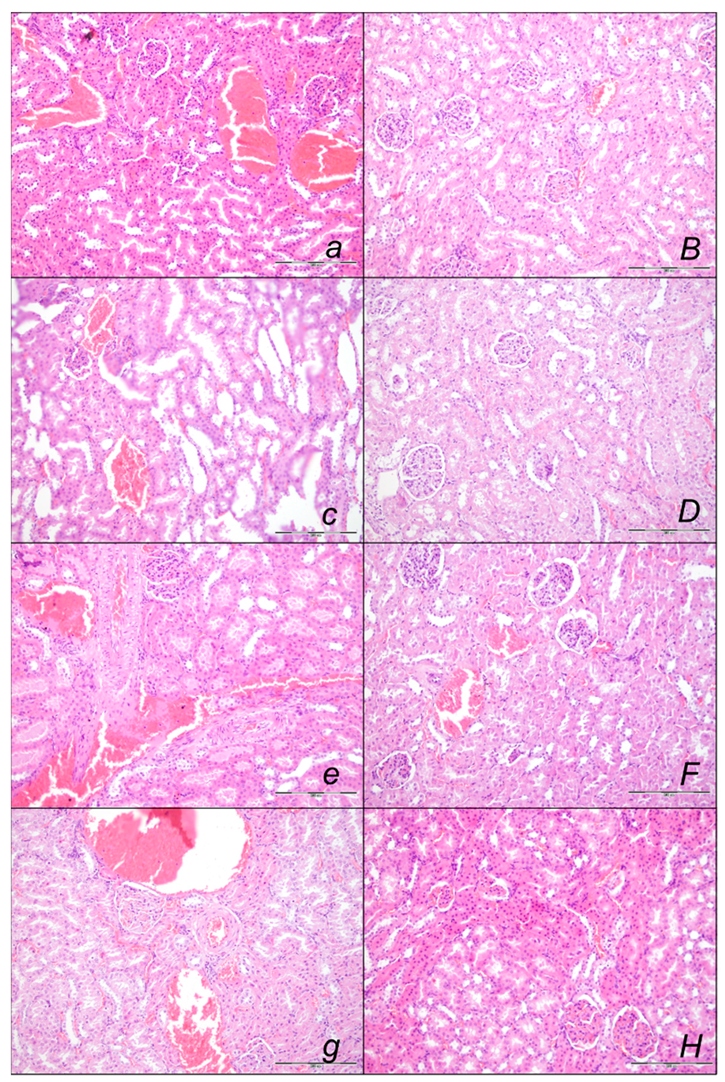
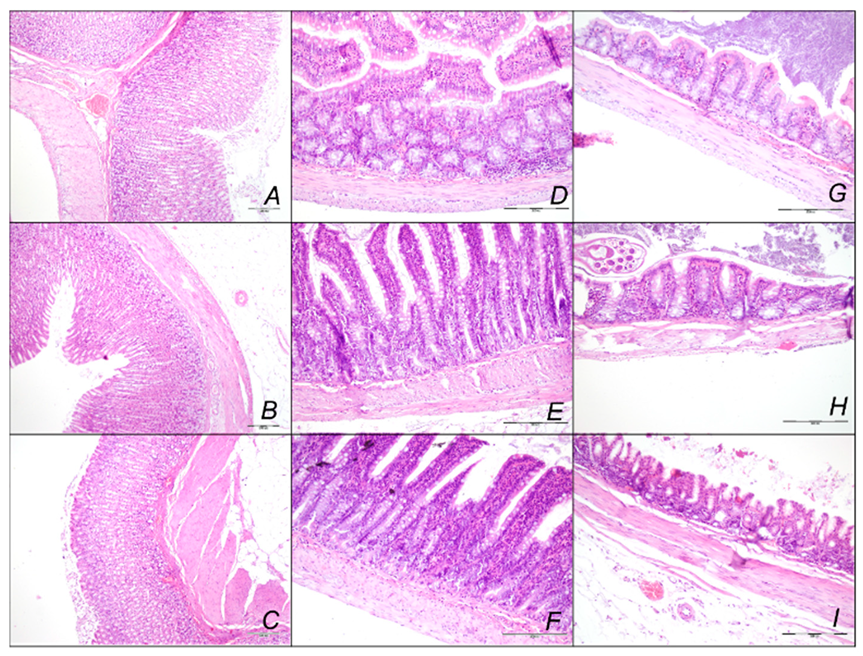
2.2.1. Heart Lung, Liver, Kidney, and Gastrointestinal Lesions
Heart Lesions
Lung Lesions
Liver Lesions
Kidney Lesions
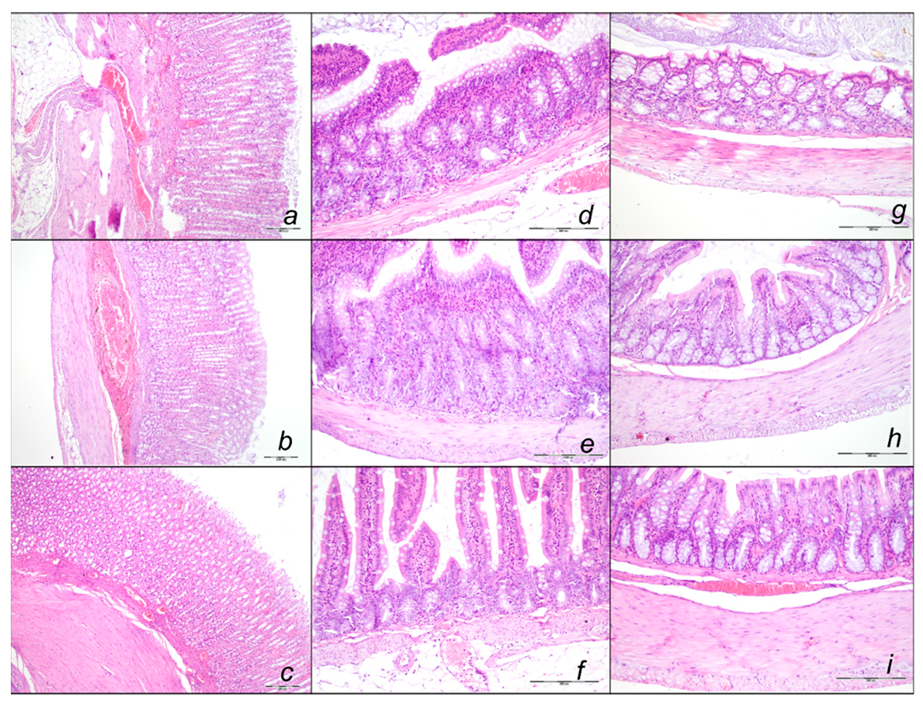
Stomach, Small Intestine, and Colon Lesions
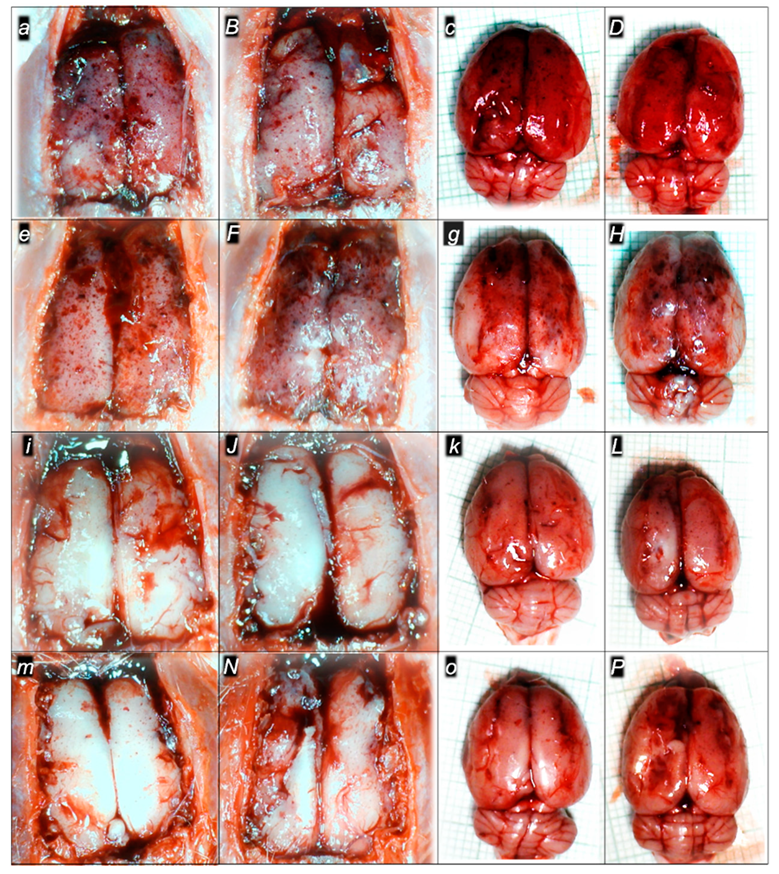
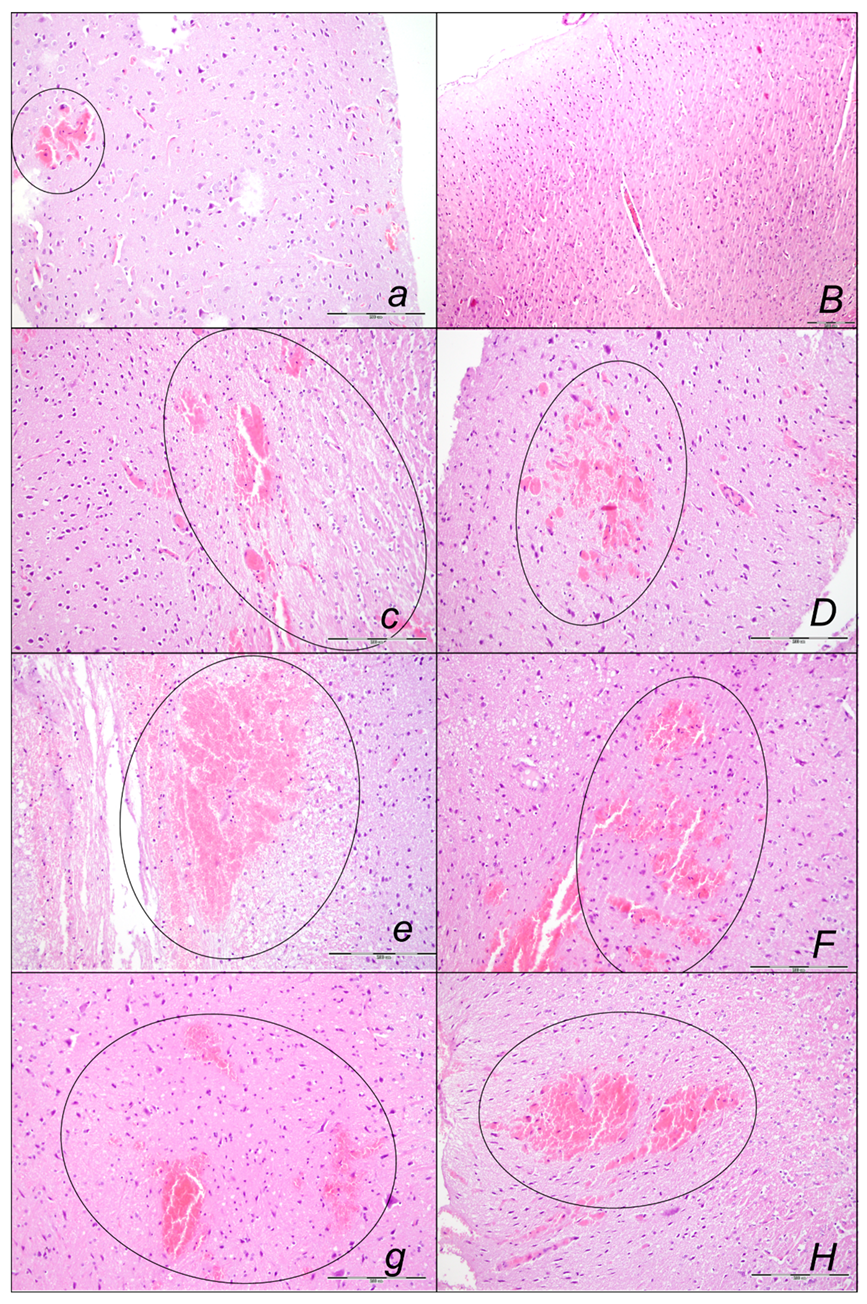
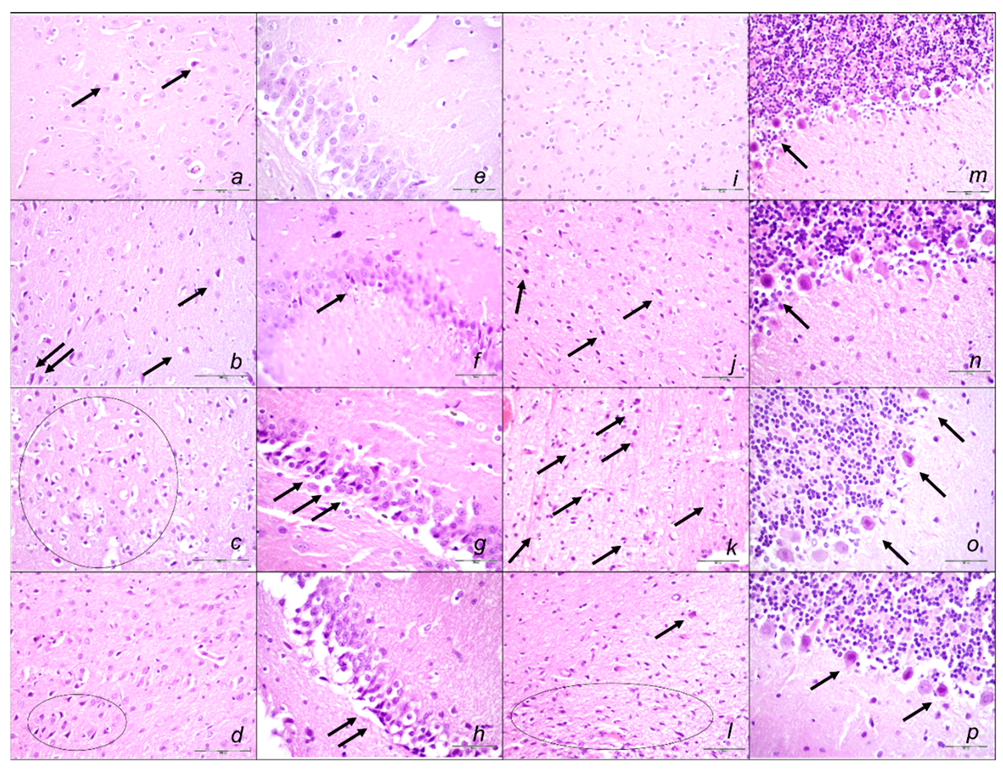
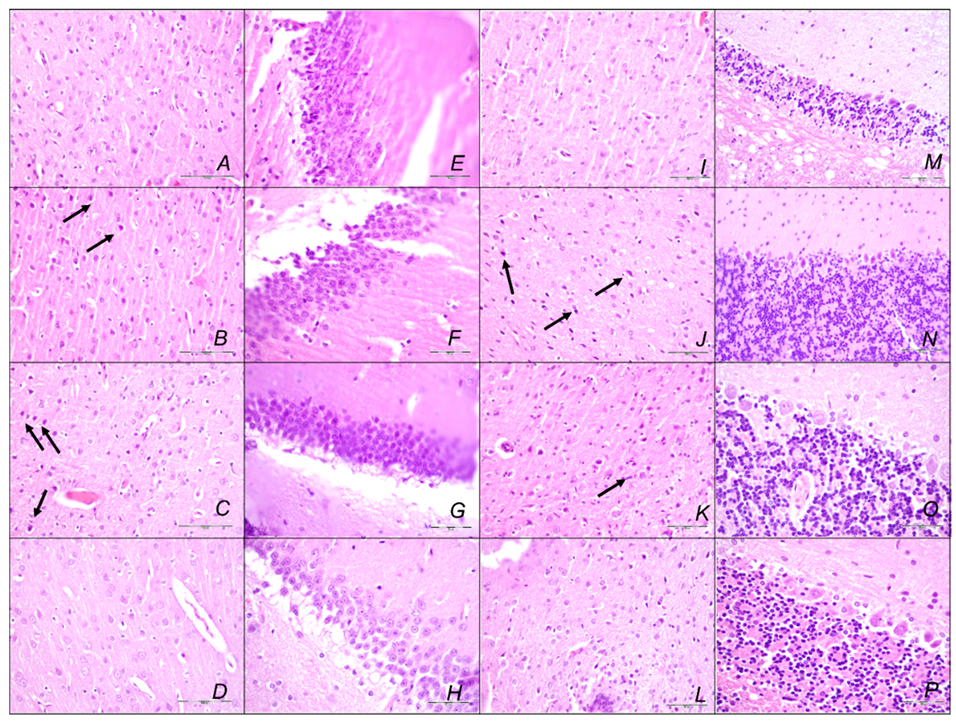
2.3. A Perilous Syndrome Occurred Centrally
Brain Lesions of Cerebral and Cerebellar Cortex, Hypothalamus, and Hippocampus
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Drugs
4.3. Experimental Protocol
4.4. Superior Sagittal Sinus, Portal, and Caval Vein, and Abdominal Aorta Pressure Recording
4.5. ECG Recording
4.6. Thrombus Assessment
4.7. Brain Volume, Heart, and Vessel Volume Presentation
4.8. Gross Assessment of Gastrointestinal Lesions
4.9. Microscopy
4.9.1. Brain Histology
4.9.2. Lung Histology
4.9.3. Renal, Liver, and Heart Histology
4.9.4. Gastrointestinal Histology
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Samanta, R.; Thiagalingam, A.; Turner, C.; Lakkireddy, D.J.; Kovoor, P. The use of intravenous sotalol in cardiac arrhythmias. Heart Lung Circ. 2018, 27, 1318–1326. [Google Scholar] [CrossRef]
- Semasinghe Bandaralage, S.P.; Nirthanan, S.; Niranjan, S. Does sotalol still have a role in the management of arrhythmias? Am. J. Ther. 2019, 26, e161–e169. [Google Scholar] [CrossRef]
- Proietti, R.; Russo, V.; AlTurki, A. Anti-arrhythmic therapy in patients with non-ischemic cardiomyopathy. Pharmacol. Res. 2019, 143, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Sikiric, P.; Gojkovic, S.; Knezevic, M.; Tepes, M.; Strbe, S.; Vukojevic, J.; Duzel, A.; Kralj, T.; Krezic, I.; Zizek, H.; et al. Stable gastric pentadecapeptide BPC 157: Prompt particular activation of the collateral pathways. Curr. Med. Chem. 2022, 30, 1568–1573. [Google Scholar] [CrossRef] [PubMed]
- Sikiric, P.; Udovicic, M.; Barisic, I.; Balenovic, D.; Zivanovic Posilovic, G.; Strinic, D.; Uzun, S.; Sikiric, S.; Krezic, I.; Zizek, H.; et al. Stable gastric pentadecapeptide BPC 157 as useful cytoprotective peptide therapy in the heart disturbances, myocardial infarction, heart failure, pulmonary hypertension, arrhythmias and thrombosis presentation. Biomedicines 2022, 10, 2696. [Google Scholar] [CrossRef]
- Staresinic, M.; Japjec, M.; Vranes, H.; Prtoric, A.; Zizek, H.; Krezic, I.; Gojkovic, S.; Smoday, I.M.; Oroz, K.; Staresinic, E.; et al. Stable gastric pentadecapeptide BPC 157 and striated, smooth, and heart muscle. Biomedicines 2022, 10, 3221. [Google Scholar] [CrossRef] [PubMed]
- Sikiric, P.; Gojkovic, S.; Krezic, I.; Smoday, I.M.; Kalogjera, L.; Zizek, H.; Oroz, K.; Vranes, H.; Vukovic, V.; Labidi, M.; et al. Stable gastric pentadecapeptide BPC 157 may recover brain–gut axis and gut–brain axis function. Pharmaceuticals 2023, 16, 676. [Google Scholar] [CrossRef]
- Vukojevic, J.; Siroglavic, M.; Kasnik, K.; Kralj, T.; Stancic, D.; Kokot, A.; Kolaric, D.; Drmic, D.; Sever, A.Z.; Barisic, I.; et al. Rat inferior caval vein (ICV) ligature and particular new insights with the stable gastric pentadecapeptide BPC 157. Vascul. Pharmacol. 2018, 106, 54–66. [Google Scholar] [CrossRef]
- Kolovrat, M.; Gojkovic, S.; Krezic, I.; Malekinusic, D.; Vrdoljak, B.; Kasnik Kovac, K.; Kralj, T.; Drmic, D.; Barisic, I.; Horvat Pavlov, K.; et al. Pentadecapeptide BPC 157 resolves Pringle maneuver in rats, both ischemia and reperfusion. World J. Hepatol. 2020, 12, 184–206. [Google Scholar] [CrossRef]
- Gojkovic, S.; Krezic, I.; Vrdoljak, B.; Malekinusic, D.; Barisic, I.; Petrovic, A.; Horvat Pavlov, K.; Kolovrat, M.; Duzel, A.; Knezevic, M.; et al. Pentadecapeptide BPC 157 resolves suprahepatic occlusion of the inferior caval vein, Budd-Chiari syndrome model in rats. World J. Gastrointest. Pathophysiol. 2020, 11, 1–19. [Google Scholar] [CrossRef]
- Knezevic, M.; Gojkovic, S.; Krezic, I.; Zizek, H.; Vranes, H.; Malekinusic, D.; Vrdoljak, B.; Knezevic, T.; Pavlov, K.H.; Drmic, D.; et al. Complex syndrome of the complete occlusion of the end of the superior mesenteric vein, opposed with the stable gastric pentadecapeptide BPC 157 in rats. Biomedicines 2021, 9, 1029. [Google Scholar] [CrossRef] [PubMed]
- Knezevic, M.; Gojkovic, S.; Krezic, I.; Zizek, H.; Malekinusic, D.; Vrdoljak, B.; Knezevic, T.; Vranes, H.; Drmic, D.; Staroveski, M.; et al. Occluded superior mesenteric artery and vein. Therapy with the stable gastric pentadecapeptide BPC 157. Biomedicines 2021, 9, 792. [Google Scholar] [CrossRef] [PubMed]
- Knezevic, M.; Gojkovic, S.; Krezic, I.; Zizek, H.; Malekinusic, D.; Vrdoljak, B.; Vranes, H.; Knezevic, T.; Barisic, I.; Horvat Pavlov, K.; et al. Occlusion of the superior mesenteric artery in rats reversed by collateral pathways activation: Gastric pentadecapeptide BPC 157 therapy counteracts multiple organ dysfunction syndrome; intracranial, portal and caval hypertension; and aortal hypotension. Biomedicines 2021, 9, 609. [Google Scholar] [CrossRef] [PubMed]
- Kralj, T.; Kokot, A.; Zlatar, M.; Masnec, S.; Kasnik Kovac, K.; Milkovic Perisa, M.; Batelja Vuletic, L.; Giljanovic, A.; Strbe, S.; Sikiric, S.; et al. Stable gastric pentadecapeptide BPC 157 therapy of rat glaucoma. Biomedicines 2021, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Vukojevic, J.; Vrdoljak, B.; Malekinusic, D.; Siroglavic, M.; Milavic, M.; Kolenc, D.; Boban Blagaic, A.; Bateljam, L.; Drmic, D.; Seiwerth, S.; et al. The effect of pentadecapeptide BPC 157 on hippocampal ischemia/reperfusion injuries in rats. Brain Behav. 2020, 10, e01726. [Google Scholar] [CrossRef]
- Gojkovic, S.; Krezic, I.; Vranes, H.; Zizek, H.; Drmic, D.; Pavlov, K.H.; Petrovic, A.; Batelja, L.; Milavic, M.; Sikiric, S.; et al. BPC 157 therapy and the permanent occlusion of the superior sagittal sinus in rat. Vascular recruitment. Biomedicines 2021, 9, 744. [Google Scholar] [CrossRef]
- Gojkovic, S.; Krezic, I.; Vranes, H.; Zizek, H.; Drmic, D.; Batelja Vuletic, L.; Milavic, M.; Sikiric, S.; Stilinovic, I.; Simeon, P.; et al. Robert’s intragastric alcohol-induced gastric lesion model as an escalated general peripheral and central syndrome, counteracted by the stable gastric pentadecapeptide BPC 157. Biomedicines 2021, 9, 1300. [Google Scholar] [CrossRef]
- Strbe, S.; Gojkovic, S.; Krezic, I.; Zizek, H.; Vranes, H.; Barisic, I.; Strinic, D.; Orct, T.; Vukojevic, J.; Ilic, S.; et al. Over-dose lithium toxicity as an occlusive-like syndrome in rats and gastric pentadecapeptide BPC 157. Biomedicines 2021, 9, 1506. [Google Scholar] [CrossRef]
- Strbe, S.; Smoday, I.M.; Krezic, I.; Kalogjera, L.; Vukovic, V.; Zizek, H.; Gojkovic, S.; Vranes, H.; Barisic, I.; Sikiric, S.; et al. Innate vascular failure by application of neuroleptics, amphetamine, and domperidone rapidly induced severe occlusion/occlusion-like syndromes in rats and stable gastric pentadecapeptide BPC 157 as therapy. Pharmaceuticals 2023, 16, 788. [Google Scholar] [CrossRef]
- Smoday, I.M.; Petrovic, I.; Kalogjera, L.; Vranes, H.; Zizek, H.; Krezic, I.; Gojkovic, S.; Skorak, I.; Hriberski, K.; Brizic, I.; et al. Therapy effect of the stable gastric pentadecapeptide BPC 157 on acute pancreatitis as vascular failure-induced severe peripheral and central syndrome in rats. Biomedicines 2022, 10, 1299. [Google Scholar] [CrossRef]
- Tepes, M.; Gojkovic, S.; Krezic, I.; Zizek, H.; Madzar, Z.; Santak, G.; Batelja, L.; Milavic, M.; Sikiric, S.; Kocman, I.; et al. Stable gastric pentadecapeptide BPC 157 therapy for primary abdominal compartment syndrome in rats. Front. Pharmacol. 2021, 12, 718147. [Google Scholar] [CrossRef] [PubMed]
- Barisic, I.; Balenovic, D.; Udovicic, M.; Bardak, D.; Strinic, D.; Vlainic, J.; Vranes, H.; Smoday, I.M.; Krezic, I.; Milavic, M.; et al. Stable gastric pentadecapeptide BPC 157 may counteract myocardial infarction induced by isoprenaline in rats. Biomedicines 2022, 10, 265. [Google Scholar] [CrossRef] [PubMed]
- Robert, A. Cytoprotection by prostaglandins. Gastroenterology 1979, 77, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Szabo, S.; Trier, J.S.; Brown, A.; Schnoor, J. Early vascular injury and increased vascular permeability in gastric mucosal injury caused by ethanol in the rat. Gastroenterology 1985, 88, 228–236. [Google Scholar] [CrossRef]
- Park, J.M.; Lee, H.J.; Sikiric, P.; Hahm, K.B. BPC 157 rescued NSAID-cytotoxicity via stabilizing intestinal permeability and enhancing cytoprotection. Curr. Pharm. Des. 2020, 26, 2971–2981. [Google Scholar] [CrossRef]
- Belosic Halle, Z.; Vlainic, J.; Drmic, D.; Strinic, D.; Luetic, K.; Sucic, M.; Medvidovic-Grubisic, M.; Pavelic Turudic, T.; Petrovic, I.; Seiwerth, S.; et al. Class side effects: Decreased pressure in the lower oesophageal and the pyloric sphincters after the administration of dopamine antagonists, neuroleptics, anti-emetics, L-NAME, pentadecapeptide BPC 157 and L-arginine. Inflammopharmacology 2017, 25, 511–522. [Google Scholar] [CrossRef]
- Luetic, K.; Sucic, M.; Vlainic, J.; Halle, Z.B.; Strinic, D.; Vidovic, T.; Luetic, F.; Marusic, M.; Gulic, S.; Pavelic, T.T.; et al. Cyclophosphamide induced stomach and duodenal lesions as a NO-system disturbance in rats: L-NAME, L-arginine, stable gastric pentadecapeptide BPC 157. Inflammopharmacology 2017, 25, 255–264. [Google Scholar] [CrossRef]
- Sucic, M.; Luetic, K.; Jandric, I.; Drmic, D.; Sever, A.Z.; Vuletic, L.B.; Halle, Z.B.; Strinic, D.; Kokot, A.; Seiwerth, R.S.; et al. Therapy of the rat hemorrhagic cystitis induced by cyclophosphamide. Stable gastric pentadecapeptide BPC 157, L-arginine, L-NAME. Eur. J. Pharmacol. 2019, 861, 172593. [Google Scholar] [CrossRef]
- Sever, A.Z.; Sever, M.; Vidovic, T.; Lojo, N.; Kolenc, D.; Vuletic, L.B.; Drmic, D.; Kokot, A.; Zoricic, I.; Coric, M.; et al. Stable gastric pentadecapeptide BPC 157 in the therapy of the rats with bile duct ligation. Eur. J. Pharmacol. 2019, 847, 130–142. [Google Scholar] [CrossRef]
- Japjec, M.; Horvat Pavlov, K.; Petrovic, A.; Staresinic, M.; Sebecic, B.; Buljan, M.; Vranes, H.; Giljanovic, A.; Drmic, D.; Japjec, M.; et al. Stable Gastric Pentadecapeptide BPC 157 as a therapy for the disable myotendinous junctions in rats. Biomedicines 2021, 9, 1547. [Google Scholar] [CrossRef]
- Sikiric, P.; Drmic, D.; Boban Blagaic, A.; Tvrdeic, A.; Krezic, I.; Gojkovic, S.; Zizek, H.; Sikiric, S.; Strbe, S.; Smoday, I.M.; et al. Stable gastric pentadecapeptide BPC 157 and NO-system. In Nitric Oxide: From Research to Therapeutics; Ray, A., Gulati, K., Eds.; Advances in Biochemistry in Health and Disease 22; Springer Nature Switzerland AG: Cham, Switzerland, 2023; pp. 349–376. [Google Scholar] [CrossRef]
- Sikiric, P.; Seiwerth, S.; Rucman, R.; Turkovic, B.; Rokotov, D.S.; Brcic, L.; Sever, M.; Klicek, R.; Radic, B.; Drmic, D.; et al. Stable gastric pentadecapeptide BPC 157-NO-system relation. Curr. Pharm. Des. 2014, 20, 1126–1135. [Google Scholar] [CrossRef]
- Sikiric, P.; Seiwerth, S.; Grabarevic, Z.; Rucman, R.; Petek, M.; Jagic, V.; Turkovic, B.; Rotkvic, I.; Mise, S.; Zoricic, I.; et al. The influence of a novel pentadecapeptide, BPC 157, on N(G)-nitro-L-arginine methylester and L-arginine effects on stomach mucosa integrity and blood pressure. Eur. J. Pharmacol. 1997, 332, 23–33. [Google Scholar] [CrossRef]
- Turkovic, B.; Sikiric, P.; Seiwerth, S.; Mise, S.; Anic, T.; Petek, M. Stable gastric pentadecapeptide BPC 157 studied for inflammatory bowel disease (PLD-116, PL14736, Pliva) induces nitric oxide synthesis. Gastroenterology 2004, 126, 287. [Google Scholar]
- Stupnisek, M.; Kokot, A.; Drmic, D.; Hrelec Patrlj, M.; Zenko Sever, A.; Kolenc, D.; Radic, B.; Suran, J.; Bojic, D.; Vcev, A.; et al. Pentadecapeptide BPC 157 reduces bleeding and thrombocytopenia after amputation in rats treated with heparin, warfarin, L-NAME and L-arginine. PLoS ONE 2015, 10, e0123454. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.J.; Liu, H.T.; Wang, C.N.; Huang, H.Y.; Lin, Y.; Ko, Y.S.; Wang, J.S.; Chang, V.H.; Pang, J.S. Therapeutic potential of pro-angiogenic BPC157 is associated with VEGFR2 activation and up-regulation. J. Mol. Med. 2017, 95, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.J.; Lee, C.H.; Chueh, H.Y.; Chang, G.J.; Huang, H.Y.; Lin, Y.; Pang, J.S. Modulatory effects of BPC 157 on vasomotor tone and the activation of Src-Caveolin-1-endothelial nitric oxide synthase pathway. Sci. Rep. 2020, 10, 17078. [Google Scholar] [CrossRef] [PubMed]
- Stupnisek, M.; Franjic, S.; Drmic, D.; Hrelec, M.; Kolenc, D.; Radic, B.; Bojic, D.; Vcev, A.; Seiwerth, S.; Sikiric, P. Pentadecapeptide BPC 157 reduces bleeding time and thrombocytopenia after amputation in rats treated with heparin, warfarin or aspirin. Thromb. Res. 2012, 129, 652–659. [Google Scholar] [CrossRef]
- Konosic, S.; Petricevic, M.; Ivancan, V.; Konosic, L.; Goluza, E.; Krtalic, B.; Drmic, D.; Stupnisek, M.; Seiwerth, S.; Sikiric, P. Intragastric application of aspirin, clopidogrel, cilostazol, and BPC 157 in rats: Platelet aggregation and blood clot. Oxid. Med. Cell Longev. 2019, 2019, 9084643. [Google Scholar] [CrossRef]
- Huang, T.; Zhang, K.; Sun, L.; Xue, X.; Zhang, C.; Shu, Z.; Mu, N.; Gu, J.; Zhang, W.; Wang, Y.; et al. Body protective compound-157 enhances alkali-burn wound healing in vivo and promotes proliferation, migration, and angiogenesis in vitro. Drug Des. Devel. Ther. 2015, 9, 2485–2499. [Google Scholar] [CrossRef]
- Huang, B.S.; Huang, S.C.; Chen, F.H.; Chang, Y.; Mei, H.F.; Huang, H.Y.; Chen, W.Y.; Pang, J.S. Pentadecapeptide BPC 157 efficiently reduces radiation-induced liver injury and lipid accumulation through Kruppel-like factor 4 upregulation both in vivo and in vitro. Life Sci. 2022, 310, 121072. [Google Scholar] [CrossRef]
- Chang, C.H.; Tsai, W.C.; Lin, M.S.; Hsu, Y.H.; Pang, J.H.S. The promoting effect of pentadecapeptide BPC 157 on tendon healing involves tendon outgrowth, cell survival, and cell migration. J. Appl. Physiol. 2011, 110, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.H.; Tsai, W.C.; Hsu, Y.H.; Pang, J.H.S. Pentadecapeptide BPC 157 enhances the growth hormone receptor expression in tendon fibroblasts. Molecules 2014, 19, 19066–19077. [Google Scholar] [CrossRef]
- Wang, X.Y.; Qu, M.; Duan, R.; Shi, D.; Jin, L.; Gao, J.; Wood, J.D.; Li, J.; Wang, G.D. Cytoprotective mechanism of the novel gastric peptide BPC157 in gastrointestinal tract and cultured enteric neurons and glial cells. Neurosci. Bull. 2019, 35, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Tkalcevic, V.I.; Cuzic, S.; Brajsa, K.; Mildner, B.; Bokulic, A.; Situm, K.; Perovic, D.; Glojnaric, I.; Parnham, M.J. Enhancement by PL 14736 of granulation and collagen organization in healing wounds and the potential role of egr-1 expression. Eur. J. Pharmacol. 2007, 570, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.A.; Han, Y.M.; An, J.M.; Park, Y.J.; Sikiric, P.; Kim, D.H.; Kwon, K.A.; Kim, Y.J.; Yang, D.; Tchah, H.; et al. BPC157 as potential agent rescuing from cancer cachexia. Curr. Pharm. Des. 2018, 24, 1947–1956. [Google Scholar] [CrossRef]
- Wu, H.; Wei, M.; Li, N.; Lu, Q.; Shrestha, S.M.; Tan, J.; Zhang, Z.; Wu, G.; Shi, R. Clopidogrel-induced gastric injury in rats is attenuated by stable gastric pentadecapeptide BPC 157. Drug Des. Devel. Ther. 2020, 14, 5599–5610. [Google Scholar] [CrossRef]
- Waldo, A.L.; Camm, A.J.; de Ruyter, H.; Friedman, P.L.; MacNeil, D.J.; Pitt, B.; Pratt, C.M.; Schwartz, P.J.; Veltri, E.P.; SWORD Investigators. Preliminary mortality results from the survival with oral D-sotalol (SWORD) trial. J. Am. Coll. Cardiol. 1995, 25 (Suppl. S1), 15A. [Google Scholar]
- Mackin, C.; DeWitt, E.S.; Black, K.J.; Tang, X.; Polizzotti, B.D.; van den Bosch, S.J.; Alexander, M.E.; Kheir, J.N. Intravenous amiodarone and sotalol impair contractility and cardiac output, but procainamide does not: A Langendorff study. J. Cardiovasc. Pharmacol. Ther. 2019, 24, 288–297. [Google Scholar] [CrossRef]
- Özgür Barış, V.; Dinçsoy, B.; Gedikli, E.; Erdemb, A. Empagliflozin significantly attenuates sotalol-induced QTc prolongation in rats. Kardiol. Pol. 2021, 79, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Antonaccio, M.J.; Gomoll, A. Pharmacologic basis of the antiarrhythmic and hemodynamic effects of sotalol. Am. J. Cardiol. 1993, 72, 27A–37A. [Google Scholar] [CrossRef] [PubMed]
- Levy, J.V. Comparative action of propranolol and sotalol (MJ-1999) on myocardial oxygen consumption in dogs: Hemodynamic correlates. Proc. Soc. Exp. Biol. Med. 1970, 133, 114–119. [Google Scholar] [CrossRef]
- Sharma, P.L. Mechanism of action of a new adrenergic beta-receptor antagonist MJ 1999 in the prevention of halothane-adrenaline-induced and ouabain-induced ventricular tachycardia in the dog. Indian J. Med. Res. 1967, 55, 1357–1365. [Google Scholar] [PubMed]
- Somani, P.; Lum, B.K. Blockade of epinephrine- and ouabain-induced cardiac arrhythmias in the dog heart-lung preparation. J. Pharmacol. Exp. Ther. 1966, 152, 235–242. [Google Scholar]
- Schmid, J.R.; Hanna, C. A comparison of the antiarrhythmic actions of two new synthetic compounds, iproveratril and MJ 1999, with quinidine and pronethalol. J. Pharmacol. Exp. Ther. 1967, 156, 331–338. [Google Scholar] [PubMed]
- Somani, P.; Fleming, J.G.; Chan, G.K.; Lum, B.K. Antagonism of epinephrine-induced cardiac arrhythmias by 4-(2-isopropylamino-1-hydroxyethyl)-methanesulfonanilide (MJ 1999). J. Pharmacol. Exp. Ther. 1966, 151, 32–37. [Google Scholar] [PubMed]
- Bouvier, M.; de Champlain, J. Effects of acute and chronic administration of sotalol on the blood pressure and sympathoadrenal activity of anesthetized deoxycorticosterone acetate-salt hypertensive rats. Can. J. Physiol. Pharmacol. 1986, 64, 1164–1169. [Google Scholar] [CrossRef]
- Hohnloser, S.H.; Meinertz, T.; Stubbs, P.; Crijns, H.J.; Blanc, J.J.; Rizzon, P.; Cheuvart, B. Efficacy and safety of d-sotalol, a pure class III antiarrhythmic compound, in patients with symptomatic complex ventricular ectopy. Results of a multicenter, randomized, double-blind, placebo-controlled dose-finding study. The d-Sotalol PVC Study Group. Circulation 1995, 92, 1517–1525. [Google Scholar] [CrossRef]
- Korbut, R.; Swies, J.; Marcinkiewicz, E.; Gryglewski, R.J. Thrombolytic activity of beta-adrenolytic drug, sotalol. J. Physiol. Pharmacol. 1998, 49, 51–60. [Google Scholar]
- Peller, M.; Ozierański, K.; Balsam, P.; Grabowski, M.; Filipiak, K.J.; Opolski, G. Influence of beta-blockers on endothelial function: A meta-analysis of randomized controlled trials. Cardiol. J. 2015, 22, 708–716. [Google Scholar] [CrossRef]
- Mohammed, B.T.; Alchalabi, M.; Laskova, A.; Sun, C.; Lodhi, O.; Gerais, Y.; Abdelrahman Alkhidir, A.G. Case report of sotalol induced IgA vasculitis. Curr. Rheumatol. Rev. 2023, 19, 113–119. [Google Scholar] [CrossRef]
- Pelleg, T.; Kadakia, R.S.; Siddiqui, F.; Biscardi, F.; Ie, S. Sotalol induced diffuse alveolar hemorrhage. Am. J. Resp. Crit. Care Med. 2023, 207, A5569. [Google Scholar]
- Balenovic, D.; Bencic, M.L.; Udovicic, M.; Simonji, K.; Hanzevacki, J.S.; Barisic, I.; Kranjcevic, S.; Prkacin, I.; Coric, V.; Brcic, L.; et al. Inhibition of methyldigoxin-induced arrhythmias by pentadecapeptide BPC 157: A relation with NO-system. Regul. Pept. 2009, 156, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Barisic, I.; Balenovic, D.; Klicek, R.; Radic, B.; Nikitovic, B.; Drmic, D.; Udovicic, M.; Strinic, D.; Bardak, D.; Berkopic, L.; et al. Mortal hyperkalemia disturbances in rats are NO-system related. The life saving effect of pentadecapeptide BPC 157. Regul. Pept. 2013, 181, 50–66. [Google Scholar] [CrossRef]
- Balenovic, D.; Barisic, I.; Prkacin, I.; Horvat, I.; Udovicic, M.; Uzun, S.; Strinic, D.; Pevec, D.; Drmic, D.; Radic, B.; et al. Mortal furosemide-hypokalemia-disturbances in rats NO-system related shorten survival by L-NAME. Therapy benefit with BPC 157 peptide more than with L-arginine. J. Clin. Exp. Cardiolog. 2012, 3, 201. [Google Scholar] [CrossRef]
- Stambolija, V.; Stambolija, T.P.; Holjevac, J.K.; Murselovic, T.; Radonic, J.; Duzel, V.; Duplancic, B.; Uzun, S.; Zivanovic-Posilovic, G.; Kolenc, D.; et al. BPC 157: The counteraction of succinylcholine, hyperkalemia, and arrhythmias. Eur. J. Pharmacol. 2016, 781, 83–91. [Google Scholar] [CrossRef]
- Zivanovic-Posilovic, G.; Balenovic, D.; Barisic, I.; Strinic, D.; Stambolija, V.; Udovicic, M.; Uzun, S.; Drmic, D.; Vlainic, J.; Bencic, M.L.; et al. Stable gastric pentadecapeptide BPC 157 and bupivacaine. Eur. J. Pharmacol. 2016, 793, 56–65. [Google Scholar] [CrossRef]
- Lozic, M.; Stambolija, V.; Krezic, I.; Dugandzic, A.; Zivanovic-Posilovic, G.; Gojkovic, S.; Kovacevic, J.; Vrdoljak, L.; Mirkovic, I.; Kokot, A.; et al. In relation to NO-system, stable pentadecapeptide BPC 157 counteracts lidocaine-Induced adverse effects in rats and depolarisation in vitro. Emerg. Med. Int. 2020, 2020, 6805354. [Google Scholar] [CrossRef] [PubMed]
- Strinic, D.; Belosic Halle, Z.; Luetic, K.; Nedic, A.; Petrovic, I.; Sucic, M.; Zivanovic Posilovic, G.; Balenovic, D.; Strbe, S.; Udovicic, M.; et al. BPC 157 counteracts QTc prolongation induced by haloperidol, fluphenazine, clozapine, olanzapine, quetiapine, sulpiride, and metoclopramide in rats. Life Sci. 2017, 186, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Lovric-Bencic, M.; Sikiric, P.; Hanzevacki, J.S.; Seiwerth, S.; Rogic, D.; Kusec, V.; Aralica, G.; Konjevoda, P.; Batelja, L.; Blagaic, A.B. Doxorubicine-congestive heart failure-increased big endothelin-1 plasma concentration: Reversal by amlodipine, losartan, and gastric pentadecapeptide BPC157 in rat and mouse. J. Pharmacol. Sci. 2004, 95, 19–26. [Google Scholar] [CrossRef]
- Gamulin, O.; Oroz, K.; Coric, L.; Krajacic, M.; Skrabic, M.; Dretar, V.; Strbe, S.; Talapko, J.; Juzbasic, M.; Krezic, I.; et al. Fourier transform infrared spectroscopy reveals molecular changes in blood vessels of rats treated with pentadecapeptide BPC 157. Biomedicines 2022, 10, 3130. [Google Scholar] [CrossRef]
- Seiwerth, S.; Milavic, M.; Vukojevic, J.; Gojkovic, S.; Krezic, I.; Vuletic, L.B.; Pavlov, K.H.; Petrovic, A.; Sikiric, S.; Vranes, H.; et al. Stable gastric pentadecapeptide BPC 157 and wound healing. Front. Pharmacol. 2021, 12, 627533. [Google Scholar] [CrossRef]
- Sikiric, P.; Seiwerth, S.; Rucman, R.; Turkovic, B.; Rokotov, D.S.; Brcic, L.; Sever, M.; Klicek, R.; Radic, B.; Drmic, D.; et al. Toxicity by NSAIDs. Counteraction by stable gastric pentadecapeptide BPC 157. Curr. Pharm. Des. 2013, 19, 76–83. [Google Scholar] [PubMed]
- Mayama, T.; Matsumura, K.; Lin, H.; Ogawa, K.; Imanaga, I. Remodelling of cardiac gap junction connexin 43 and arrhythmogenesis. Exp. Clin. Cardiol. 2007, 12, 67–76. [Google Scholar] [PubMed]
- Aberg, G.; Dzedin, T.; Lundholm, L.; Olsson, L.; Svedmyr, N. A comparative study of some cardiovascular effects of sotalol (MJ 1999) and propranolol. Life Sci. 1969, 8, 353–365. [Google Scholar] [CrossRef]
- Stanton, H.C.; Kirchgessner, T.; Parmenter, K. Cardiovascular pharmacology of two new beta-adrenergic receptor antagonists. J. Pharmacol. Exp. Ther. 1965, 149, 174–182. [Google Scholar]
- Lish, P.M.; Weikel, J.H.; Dungan, K.W. Pharmacological and toxicological properties of two new beta-adrenergic receptor antagonists. J. Pharmacol. Exp. Ther. 1965, 149, 161–173. [Google Scholar]
- Farmer, J.B.; Levy, G.P. A comparison of some cardiovascular properties of propranolol, MJ 1999 and quinidine in relation to their effects in hypertensive animals. Br. J. Pharmacol. 1968, 34, 116–126. [Google Scholar] [CrossRef]
- Lish, P.M.; Shelanski, M.V.; LaBudde, J.A.; Williams, W.R. Inhibition of cardiac chronotropic action of isoproterenol by sotalol (MJ 1999) in rat, dog and man. Curr. Ther. Res. Clin. Exp. 1967, 9, 311–324. [Google Scholar] [PubMed]
- Hermansen, K. Antibrillatory effect of some beta-adrenergic receptor blocking agents determined by a new test procedure in mice. Acta Pharmacol. Toxicol. 1970, 28, 17–27. [Google Scholar] [CrossRef]
- Kloner, R.A.; Dow, J.S.; Bhandari, A. First direct comparison of the late sodium current blocker ranolazine to established antiarrhythmic agents in an ischemia/reperfusion model. J. Cardiovasc. Pharmacol. Ther. 2011, 16, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Mittelstadt, S.W.; Maynard, A.E.; Benn, D.R.; Lowe, E.R.; Kostreva, D.R. Effects of azimilide and d,l-sotalol on the heart rate and blood pressure response to isoproterenol in anesthetized rats. Cardiovasc. Drugs Ther. 1997, 11, 591–598. [Google Scholar] [CrossRef]
- Weeke, P.; Delaney, J.; Mosley, J.D.; Wells, Q.; Van Driest, S.; Norris, K.; Kucera, G.; Stubblefield, T.; Roden, D.M. QT variability during initial exposure to sotalol: Experience based on a large electronic medical record. Europace 2013, 15, 1791–1797. [Google Scholar] [CrossRef] [PubMed]
- Fitton, A.; Sorkin, E.M. Sotalol. An updated review of its pharmacological properties and therapeutic use in cardiac arrhythmias. Drugs 1993, 46, 678–719. [Google Scholar] [CrossRef] [PubMed]
- Nappi, J.M.; McCollam, P.L. Sotalol: A breakthrough antiarrhythmic? Ann. Pharmacother. 1993, 27, 1359–1368. [Google Scholar] [CrossRef]
- Guidance for Industry, Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers, Pharmacology and Toxicology. U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER). July 2005. Available online: https://www.fda.gov/cder/guidance/index.htm (accessed on 4 May 2023).
- Somani, P.; Watson, D.L. Antiarrhythmic activity of the dextro- and levorotatory isomers of 4-(2-isopropylamino-1-hydroxyethyl) methanesulfonanilide (MJ 1999). J. Pharmacol. Exp. Ther. 1968, 164, 317–325. [Google Scholar]
- Leatham, E.W.; Holt, D.W.; McKenna, W.J. Class III antiarrhythmics in overdose. Presenting features and management principles. Drug Saf. 1993, 9, 450–462. [Google Scholar] [CrossRef]
- Gustavsson, C.G.; Vinge, E.; Norlander, B.O.; Pantev, E. Pharmacokinetic evaluation of a case of massive sotalol intoxication. Ann. Pharmacother. 1997, 31, 856–859. [Google Scholar] [CrossRef] [PubMed]
- Kassotis, J.; Sauberman, R.B.; Cabo, C.; Wit, A.L.; Coromilas, J. Beta receptor blockade potentiates the antiarrhythmic actions of d-sotalol on reentrant ventricular tachycardia in a canine model of myocardial infarction. J. Cardiovasc. Electrophysiol. 2003, 14, 1233–1244. [Google Scholar] [CrossRef]
- Huang, J.L.; Jing, X.; Tian, X.; Qin, M.C.; Xu, Z.H.; Wu, D.P.; Zhong, Z.G. Neuroprotective properties of Panax notoginseng saponins via preventing oxidative stress injury in SAMP8 mice. Evid. Based Complement. Alternat. Med. 2017, 2017, 8713561. [Google Scholar] [CrossRef]
- Bauer, M.; Deigendesch, N.; Wittig, H.; Scheurer, E.; Lenz, C. Tissue sample analysis for post mortem determination of brain edema. Forensic Sci. Int. 2021, 323, 110808. [Google Scholar] [CrossRef]
- Chui, C.J.; McArdle, A.H.; Brown, R.; Scott, H.; Gurd, F. Intestinal mucosal lesion in low-flow states. Arch. Surg. 1970, 101, 478–483. [Google Scholar] [CrossRef] [PubMed]
- Lane, J.S.; Todd, K.E.; Lewis, M.P.; Gloor, B.; Ashley, S.W.; Reber, H.A.; McFadden, D.W.; Chandler, C.F. Interleukin-10 reduces the systemic inflammatory response in a murine model of intestinal ischemia/reperfusion. Surgery 1997, 122, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Seiwerth, S.; Rucman, R.; Turkovic, B.; Sever, M.; Klicek, R.; Radic, B.; Drmic, D.; Stupnisek, M.; Misic, M.; Vuletic, L.B.; et al. BPC 157 and standard angiogenic growth factors. Gastrointestinal tract healing, lessons from tendon, ligament, muscle and bone healing. Curr. Pharm. Des. 2018, 24, 1972–1989. [Google Scholar] [CrossRef] [PubMed]
- Grabarevic, Z.; Tisljar, M.; Artukovic, B.; Bratulic, M.; Dzaja, P.; Seiwerth, S.; Sikiric, P.; Peric, J.; Geres, D.; Kos, J. The influence of BPC 157 on nitric oxide agonist and antagonist induced lesions in broiler chicken. J. Physiol. Paris 1997, 91, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Tlak Gajger, I.; Ribaric, J.; Smodis Skerl, M.; Vlainic, J.; Sikiric, P. Stable gastric pentadecapeptide BPC 157 in honeybee (Apis mellifera) therapy, to control Nosema ceranae invasions in apiary conditions. J. Vet. Pharmacol. Ther. 2018, 41, 614–621. [Google Scholar] [CrossRef]
- Tlak Gajger, I.; Smodis Skerl, M.I.; Sostaric, P.; Suran, J.; Sikiric, P.; Vlainic, J. Physiological and immunological status of adult honeybees (Apis mellifera) fed sugar syrup supplemented with pentadecapeptide BPC 157. Biology 2021, 10, 891. [Google Scholar] [CrossRef]
- Xu, C.; Sun, L.; Ren, F.; Huang, P.; Tian, Z.; Cui, J.; Zhang, W.; Wang, S.; Zhang, K.; He, L.; et al. Preclinical safety evaluation of body protective compound-157, a potential drug for treating various wounds. Regul. Toxicol. Pharmacol. 2020, 114, 104665. [Google Scholar] [CrossRef]
| Relative Volume (Control/Treated) (%) of the Brain, Heart, Azygos Vein, in Rats at 30 min and 90 min, and 180 min following Sotalol Application | ||||
|---|---|---|---|---|
| Periods Assessed in Relation to the Medication Application | 0–15 min | 0–90 min | 0–180 min | 90–180 min |
| Relative volume (control/treated) (%) of the brain, in vivo, Means ± SD | ||||
| BPC 157 10 μg/kg | 111 ± 3 * | 111 ± 3 * | 116 ± 3 * | 110 ± 3 * |
| BPC 157 10 ng/kg | 112 ± 3 * | 112 ± 3 * | 114 ± 3 * | 111 ± 3 * |
| Relative volume (control/treated) (%) of the heart, Means ± SD | ||||
| BPC 157 10 μg/kg | 135 ± 5 * | 139 ± 7 * | 146 ± 6 * | 139 ± 7 * |
| BPC 157 10 ng/kg | 132 ± 6 * | 142 ± 5 * | 144 ± 5 * | 141 ± 6 * |
| Relative volume (control/treated) (%) of the inferior caval vein, Means ± SD | ||||
| BPC 157 10 μg/kg | 155 ± 8 * | 149 ± 9 * | 156 ± 6 * | 199 ± 10 * |
| BPC 157 10 ng/kg | 151 ± 9 * | 155 ± 8 * | 159 ± 9 * | 191 ± 11 * |
| Relative volume (control/treated) (%) of the superior mesenteric vein, Means ± SD | ||||
| BPC 157 10 μg/kg | 225 ± 8 * | 229 ± 9 * | 216 ± 9 * | 289 ± 10 * |
| BPC 157 10 ng/kg | 222 ± 9 * | 227 ± 8 * | 224 ± 9 * | 281 ± 8 * |
| Relative volume (control/treated) (%) of the azygos vein, Means ± SD | ||||
| BPC 157 10 μg/kg | 35 ± 6 * | 69 ± 7 * | 66 ± 5 * | 68 ± 7 * |
| BPC 157 10 ng/kg | 32 ± 7 * | 67 ± 8 * | 62 ± 9 * | 61 ± 68 * |
| Relative volume (control/treated) (%) of the abdominal aorta, Means ± SD | ||||
| BPC 157 10 μg/kg | 32 ± 6 * | 66 ± 7 * | 76 ± 8 * | 43 ± 7 * |
| BPC 157 10 ng/kg | 35 ± 7 * | 63 ± 8 * | 72 ± 9 * | 41 ± 6 * |
| Relative volume (control/treated) (%) of the brain, ex vivo, Means ± SD | ||||
| BPC 157 10 μg/kg | 114 ± 3 * | 110 ± 2 * | 116 ± 3 * | 111 ± 3 * |
| BPC 157 10 ng/kg | 113 ± 3 * | 111 ± 3 * | 115 ± 3 * | 112 ± 3 * |
| Microscopic Presentation of the Lesions in the Heart, Lung, Liver, Kidney, Stomach, Small Intestine, and Large Intestine, and Gross Lesions Presentation in the Stomach in Rats at 15 min and 90 min, and 180 min following Sotalol Application | ||||
|---|---|---|---|---|
| Periods Assessed in Relation to the Medication Application | 0–15 min | 0–90 min | 0–180 min | 90–180 min |
| Heart (scored 0–3, Min/Med/Max) | ||||
| Saline 5 mL/kg (control) | 3/3/3 | 3/3/3 | 3/3/3 | 3/3/3 |
| BPC 157 10 μg/kg | 0/0/0 * | 0/0/0 * | 0/1/1 * | 0/0/0 * |
| BPC 157 10 ng/kg | 0/0/0 * | 0/0/0 * | 0/1/1 * | 0/0/0 * |
| Lung (scored 0–3, Min/Med/Max) | ||||
| Saline 5 mL/kg (control) | 3/3/3 | 3/3/3 | 3/3/3 | 3/3/3 |
| BPC 157 10 μg/kg | 0/0/0 * | 1/1/1 * | 1/1/1 * | 1/1/1 * |
| BPC 157 10 ng/kg | 0/0/0 * | 1/1/1 * | 1/1/1 * | 1/1/1 * |
| Liver (scored 0–3, Min/Med/Max) | ||||
| Saline 5 mL/kg (control) | 3/3/3 | 3/3/3 | 3/3/3 | 3/3/3 |
| BPC 157 10 μg/kg | 0/0/0 * | 0/0/0 * | 0/1/1 * | 0/0/0 * |
| BPC 157 10 ng/kg | 0/0/0 * | 0/0/0 * | 0/1/1 * | 0/0/0 * |
| Kidney (scored 0–3, Min/Med/Max) | ||||
| Saline 5 mL/kg (control) | 3/3/3 | 3/3/3 | 3/3/3 | 3/3/3 |
| BPC 157 10 μg/kg | 0/0/0 * | 0/0/0 * | 0/1/1 * | 0/0/0 * |
| BPC 157 10 ng/kg | 0/0/0 * | 0/0/0 * | 0/1/1 * | 0/0/0 * |
| Stomach (sum of longest diameters, mm, means ± SD) | ||||
| Saline 5 mL/kg (control) | 5 ± 1 | 6 ± 1 | 5 ± 1 | 5 ± 1 |
| BPC 157 10 μg/kg | 0 ± 0 * | 0 ± 0 * | 0 ± 0 * | 0 ± 0 * |
| BPC 157 10 ng/kg | 0 ± 0 * | 0 ± 0 * | 0 ± 0 * | 0 ± 0 * |
| Stomach (scored 0–15, Min/Med/Max) | ||||
| Saline 5 mL/kg (control) | 5/5/5 | 5/5/5 | 5/5/5 | 5/5/5 |
| BPC 157 10 μg/kg | 1/1/1 * | 1/1/1 * | 1/1/1 * | 1/1/1 * |
| BPC 157 10 ng/kg | 1/1/1 * | 1/1/1 * | 1/1/1 * | 1/1/1 * |
| Small intestine (scored 0–15, Min/Med/Max) | ||||
| Saline 5 mL/kg (control) | 5/5/5 | 5/5/5 | 5/5/5 | 5/5/5 |
| BPC 157 10 μg/kg | 0/0/0 * | 0/0/0 * | 0/0/0 * | 0/0/0 * |
| BPC 157 10 ng/kg | 0/0/0 * | 0/0/0 * | 0/0/0 * | 0/0/0 * |
| Large intestine (scored 0–15, Min/Med/Max) | ||||
| Saline 5 mL/kg (control) | 5/5/5 | 5/5/5 | 5/5/5 | 5/5/5 |
| BPC 157 10 μg/kg | 0/0/0 * | 0/0/0 * | 0/0/0 * | 0/0/0 * |
| BPC 157 10 ng/kg | 0/0/0 * | 0/0/0 * | 0/0/0 * | 0/0/0 * |
| Microscopic Presentation of the Brain Lesions in the Cerebrum, Cerebellum, Hippocampus, and Hypothalamus at 15 min and 30 min, and 180 min following Sotalol Application | ||||
|---|---|---|---|---|
| Periods Assessed in Relation to the Medication Application | 0–15 min | 0–90 min | 0–180 min | 90–180 min |
| Cerebrum (scored 0–8, Min/Med/Max) # | ||||
| Saline 5 mL/kg (control) | 1/1/1 | 1/2/2 | 2/2/2 | 2/2/2 |
| BPC 157 10 μg/kg | 0/0/0 * | 0/1/1 * | 0/1/1 * | 0/0/0 * |
| BPC 157 10 ng/kg | 0/0/0 * | 0/1/1 * | 0/1/1 * | 0/0/0 * |
| Neuronal damage in the karyopyknotic areas, %, Means ± SD (10 HPF, 400×) | ||||
| Saline 5 mL/kg (control) | 10 ± 10 | 21 ± 10 | 32 ± 10 | 29 ± 10 |
| BPC 157 10 μg/kg | 0 ± 0 * | 5 ± 5 * | 5 ± 5 * | 0 ± 0 * |
| BPC 157 10 ng/kg | 0 ± 0 * | 5 ± 5 * | 5 ± 5 * | 0 ± 0 * |
| Hemorrhage (% of total area), Means ± SD | ||||
| Saline 5 mL/kg (control) | 5 ± 2 | 10 ± 4 | 15 ± 4 | 15 ± 3 |
| BPC 157 10 μg/kg | 0 ± 0 * | 5 ± 1 * | 10 ± 2 * | 10 ± 2 * |
| BPC 157 10 ng/kg | 0 ± 0 * | 5 ± 1 * | 10 ± 2 * | 9 ± 2 * |
| Edema (scored 0–3, Min/Med/Max) | ||||
| Saline 5 mL/kg (control) | 3/3/3 | 3/3/3 | 3/3/3 | 3/3/3 |
| BPC 157 10 μg/kg | 1/1/1 * | 1/1/1 * | 1/1/1 * | 1/1/1 * |
| BPC 157 10 ng/kg | 1/1/1 * | 1/1/1 * | 1/1/1 * | 1/1/1 * |
| Cerebellum (scored 0–8, Min/Med/Max) * | ||||
| Saline 5 mL/kg (control) | 0/1/1 | 0/1/1 | 0/1/1 | 0/1/1 |
| BPC 157 10 μg/kg | 0/0/0 * | 0/0/0 * | 0/0/0 * | 0/0/0 * |
| BPC 157 10 ng/kg | 0/0/0 * | 0/0/0 * | 0/0/0 * | 0/0/0 * |
| Neuronal damage in the karyopyknotic areas, %, Means ± SD (10 HPF, 400×) | ||||
| Saline 5 mL/kg (control) | 9 ± 5 | 11 ± 5 | 34 ± 10 | 32 ± 10 |
| BPC 157 10 μg/kg | 0 ± 0 * | 0 ± 0 * | 0 ± 0 * | 0 ± 0 * |
| BPC 157 10 ng/kg | 0 ± 0 * | 0 ± 0 * | 0 ± 0 * | 0 ± 0 * |
| Hemorrhage (% of total area), Means ± SD | ||||
| Saline 5 mL/kg (control) | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| BPC 157 10 μg/kg | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| BPC 157 10 ng/kg | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| Edema (scored 0–3, Min/Med/Max) | ||||
| Saline 5 mL/kg (control) | 3/3/3 | 3/3/3 | 3/3/3 | 3/3/3 |
| BPC 157 10 μg/kg | 1/1/1 * | 1/1/1 * | 1/1/1 * | 1/1/1 * |
| BPC 157 10 ng/kg | 1/1/1 * | 1/1/1 * | 1/1/1 * | 1/1/1 * |
| Hippocampus (scored 0–8, Min/Med/Max) * | ||||
| Saline 5 mL/kg (control) | 0/0/0 | 0/0/0 | 1/1/1 | 1/1/1 |
| BPC 157 10 μg/kg | 0/0/0 | 0/0/0 | 0/0/0 | 0/0/0 |
| BPC 157 10 ng/kg | 0/0/0 | 0/0/0 | 0/0/0 | 0/0/0 |
| Neuronal damage in the karyopyknotic areas, %, Means ± SD (10 HPF, 400×) | ||||
| Saline 5 mL/kg (control) | 0 ± 0 | 10 ± 5 | 36 ± 5 | 33 ± 5 |
| BPC 157 10 μg/kg | 0 ± 0 | 0 ± 0 * | 0 ± 0 * | 0 ± 0 * |
| BPC 157 10 ng/kg | 0 ± 0 | 0 ± 0 * | 0 ± 0 * | 0 ± 0 * |
| Hemorrhage (% of total area), Means ± SD | ||||
| Saline 5 mL/kg (control) | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| BPC 157 10 μg/kg | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| BPC 157 10 ng/kg | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| Edema (scored 0–3, Min/Med/Max) | ||||
| Saline 5 mL/kg (control) | 2/2/2 | 3/3/3 | 3/3/3 | 3/3/3 |
| BPC 157 10 μg/kg | 1/1/1 * | 1/1/1 * | 1/1/1 * | 1/1/1 * |
| BPC 157 10 ng/kg | 1/1/1 * | 1/1/1 * | 1/1/1 * | 1/1/1 * |
| Hypothalamus (scored 0–8, Min/Med/Max) * | ||||
| Saline 5 mL/kg (control) | 0/0/0 | 0/2/2 | 2/2/2 | 2/3/3 |
| BPC 157 10 μg/kg | 0/0/0 | 0/1/1 * | 0/1/1 * | 0/0/0 * |
| BPC 157 10 ng/kg | 0/0/0 | 0/1/1 * | 0/1/1 * | 0/0/0 * |
| Neuronal damage in the karyopyknotic areas, %, Means ± SD (10 HPF, 400×) | ||||
| Saline 5 mL/kg (control) | 0 ± 0 | 13 ± 5 | 42 ± 5 | 39 ± 5 |
| BPC 157 10 μg/kg | 0 ± 0 | 5 ± 2 * | 10 ± 5 * | 0 ± 0 * |
| BPC 157 10 ng/kg | 0 ± 0 | 6 ± 2 * | 11 ± 5 * | 0 ± 0 * |
| Hemorrhage (% of total area), Means ± SD | ||||
| Saline 5 mL/kg (control) | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| BPC 157 10 μg/kg | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| BPC 157 10 ng/kg | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| Edema (scored 0–3, Min/Med/Max) | ||||
| Saline 5 mL/kg (control) | 2/2/2 | 3/3/3 | 3/3/3 | 3/3/3 |
| BPC 157 10 μg/kg | 1/1/1 * | 1/1/1 * | 1/1/1 * | 1/1/1 * |
| BPC 157 10 ng/kg | 1/1/1 * | 1/1/1 * | 1/1/1 * | 1/1/1 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Premuzic Mestrovic, I.; Smoday, I.M.; Kalogjera, L.; Krezic, I.; Zizek, H.; Vranes, H.; Vukovic, V.; Oroz, K.; Skorak, I.; Brizic, I.; et al. Antiarrhythmic Sotalol, Occlusion/Occlusion-like Syndrome in Rats, and Stable Gastric Pentadecapeptide BPC 157 Therapy. Pharmaceuticals 2023, 16, 977. https://doi.org/10.3390/ph16070977
Premuzic Mestrovic I, Smoday IM, Kalogjera L, Krezic I, Zizek H, Vranes H, Vukovic V, Oroz K, Skorak I, Brizic I, et al. Antiarrhythmic Sotalol, Occlusion/Occlusion-like Syndrome in Rats, and Stable Gastric Pentadecapeptide BPC 157 Therapy. Pharmaceuticals. 2023; 16(7):977. https://doi.org/10.3390/ph16070977
Chicago/Turabian StylePremuzic Mestrovic, Ivica, Ivan Maria Smoday, Luka Kalogjera, Ivan Krezic, Helena Zizek, Hrvoje Vranes, Vlasta Vukovic, Katarina Oroz, Ivan Skorak, Ivan Brizic, and et al. 2023. "Antiarrhythmic Sotalol, Occlusion/Occlusion-like Syndrome in Rats, and Stable Gastric Pentadecapeptide BPC 157 Therapy" Pharmaceuticals 16, no. 7: 977. https://doi.org/10.3390/ph16070977
APA StylePremuzic Mestrovic, I., Smoday, I. M., Kalogjera, L., Krezic, I., Zizek, H., Vranes, H., Vukovic, V., Oroz, K., Skorak, I., Brizic, I., Hriberski, K., Novosel, L., Kavelj, I., Barisic, I., Beketic Oreskovic, L., Zubcic, S., Strbe, S., Mestrovic, T., Pavic, P., ... Sikiric, P. (2023). Antiarrhythmic Sotalol, Occlusion/Occlusion-like Syndrome in Rats, and Stable Gastric Pentadecapeptide BPC 157 Therapy. Pharmaceuticals, 16(7), 977. https://doi.org/10.3390/ph16070977







