Study of Symptom Severity and Adherence to Therapy of Myelofibrosis Patients Treated with Ruxolitinib
Abstract
1. Introduction
2. Results
2.1. Demographics, Severity of Symptoms, and Adherence to Therapy
2.2. Statistical Analysis
3. Discussion
4. Materials and Methods
4.1. Design of the Study
4.2. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tefferi, A. Primary myelofibrosis: 2019 update on diagnosis, risk-stratification, and management. Am. J. Hematol. 2018, 93, 1551–1560. [Google Scholar] [CrossRef] [PubMed]
- Mesa, R.; Miller, C.B.; Thyne, M.; Mangan, J.; Goldberger, S.; Fazal, S.; Ma, X.; Wilson, W.; Paranagama, D.C.; Dubinski, D.G.; et al. Myeloproliferative neoplasms (MPNs) have a significant impact on patients’ overall health and productivity: The MPN Landmark survey. BMC Cancer 2016, 16, 167. [Google Scholar] [CrossRef] [PubMed]
- United States Food and Drug Administration. JAKAFI (Ruxolitinib) Label; FDA: Silver Spring, MD, USA, 2011. [Google Scholar]
- EMA. European Public Assessment Report. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/inrebic (accessed on 24 May 2023).
- EMA. European Assessment Report. Available online: https://www.ema.europa.eu/en/documents/product-information/jakavi-epar-product-information_bg.pdf (accessed on 24 May 2023).
- Wernig, G.; Kharas, M.G.; Okabe, R.; Moore, S.A.; Leeman, D.S.; Cullen, D.E.; Gozo, M.; McDowell, E.P.; Levine, R.L.; Doukas, J.; et al. Efficacy of TG101348, a selective JAK2 inhibitor, in treatment of a murine model of JAK2V617F-induced polycythemia vera. Cancer Cell 2008, 13, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Masarova, L.; Bose, P.; Pemmaraju, N.; Daver, N.G.; Sasaki, K.; Chifotides, H.T.; Zhou, L.; Kantarjian, H.M.; Estrov, Z.; Verstovsek, S. Improved survival of patients with myelofibrosis in the last decade: Single-center experience. Cancer 2022, 128, 1658–1665. [Google Scholar] [CrossRef] [PubMed]
- Ostojic, A.; Vrhovac, R.; Verstovsek, S. Ruxolitinib: A new JAK1/2 inhibitor that offers promising options for treatment of myelofibrosis. Future Oncol. 2011, 7, 1035–1043. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.P.; Keating, G.M. Ruxolitinib: In the treatment of myelofibrosis. Drugs 2012, 72, 2117–2127. [Google Scholar] [CrossRef] [PubMed]
- Shilling, A.D.; Nedza, F.M.; Emm, T.; Diamond, S.; McKeever, E.; Punwani, N.; Williams, W.; Arvanitis, A.; Galya, L.G.; Li, M.; et al. Metabolism, excretion, and pharmacokinetics of [14C]INCB018424, a selective Janus tyrosine kinase 1/2 inhibitor, in humans. Drug Metab Dispos. 2010, 38, 2023–2031. [Google Scholar] [CrossRef] [PubMed]
- Mughal, T.I.; Girnius, S.; Rosen, S.T.; Kumar, S.; Wiestner, A.; Abdel-Wahab, O.; Kiladjian, J.J.; Wilson, W.H.; Van Etten, R.A. Emerging therapeutic paradigms to target the dysregulated Janus ki-nase/signal transducer and activator of transcription pathway in hematological malignancies. Leuk. Lymphoma 2014, 55, 1968–1979. [Google Scholar] [CrossRef] [PubMed]
- Ajayi, S.; Becker, H.; Reinhardt, H.; Engelhardt, M.; Zeiser, R.; von Bubnoff, N.; Wasch, R. Ruxolitinib. Recent Results Cancer Res. 2018, 212, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Guglielmelli, P.; Palandri, F.; Selleri, C.; Cilloni, D.; Mendicino, F.; Mazza, P.; Pastore, D.; Palumbo, G.A.; Siragusa, S.; Pavone, V.; et al. Adherence to Treatment in Myelofibrosis Patients: Preliminary Results from Italian Romei Observational Study. Blood 2019, 134, 4179. [Google Scholar] [CrossRef]
- World Health Organization. Adherence to Long-Term Therapies: Evidence for Action; World Health Organization: Geneva, Switzerland, 2003; Available online: https://apps.who.int/iris/handle/10665/42682 (accessed on 24 May 2023).
- Tremblay, D.; Mascarenhas, J. Next Generation Therapeutics for the Treatment of Myelofi-brosis. Cells 2021, 10, 1034. [Google Scholar] [CrossRef] [PubMed]
- Breccia, M.; Baratè, C.; Benevolo, G.; Bonifacio, M.; Elli, E.M.; Guglielmelli, P.; Maffioli, M.; Malato, A.; Mendicino, F.; Palumbo, G.A.; et al. Tracing the decision-making process for myelofibrosis: Diagnosis, stratification, and management of ruxolitinib therapy in real-word practice. Ann. Hematol. 2020, 99, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Nurgat, Z.; Lawrence, M. Management of Myeloproliferative Neoplasms (MPNs). J. Oncol. Pharm. Pract. 2022, 28, 1400–1410. [Google Scholar] [CrossRef] [PubMed]
- Morisky, D.E.; Ang, A.; Krousel-Wood, M.; Ward, H.J. Predictive validity of a medication adherence measure in an outpatient setting. J. Clin. Hypertens. 2008, 10, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Krousel-Wood, M.A.; Islam, T.; Webber, L.S.; Re, R.N.; Morisky, D.E.; Muntner, P. New medication adherence scale versus pharmacy fill rates in seniors with hypertension. Am. J. Manag. Care 2009, 15, 59–66. [Google Scholar] [PubMed]
- Morisky, D.E.; DiMatteo, M.R. Improving the measurement of self-reported medication nonadherence. J. Clin. Epidemiol. 2011, 64, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Guglielmelli, P.; Palandri, F.; Selleri, C.; Cilloni, D.; Mendicino, F.; Mazza, P.; Pastore, D.; Palumbo, G.A.; Santoro, M.; Pavone, V.; et al. ROMEI study group. Adherence to ruxolitinib, an oral JAK1/2 inhibitor, in patients with myelofibrosis: Interim analysis from an Italian, prospective cohort study (ROMEI). Leuk. Lymphoma 2022, 63, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Palandri, F.; Elli, E.M.; Iurlo, A.; Auteri, G.; Trawinska, M.M.; Bonifacio, M.; Mendicino, F.; Latagliata, R.; Mazzoni, C.; Biondo, M.; et al. Ruxolitinib Adherence in Myelofibrosis and Polycythemia Vera: The “RAMP” Multicenter Prospective Study. Blood 2022, 140 (Suppl. S1), 9726–9728. [Google Scholar] [CrossRef]
- Mesa, R.; Verstovsek, S.; Kiladjian, J.J.; Griesshammer, M.; Masszi, T.; Durrant, S.; Passamonti, F.; Harrison, C.N.; Pane, F.; Zachee, P.; et al. Changes in quality of life and disease-related symptoms in patients with polycythemia vera receiving ruxolitinib or standard therapy. Eur. J. Haematol. 2016, 97, 192–200. [Google Scholar] [CrossRef] [PubMed]
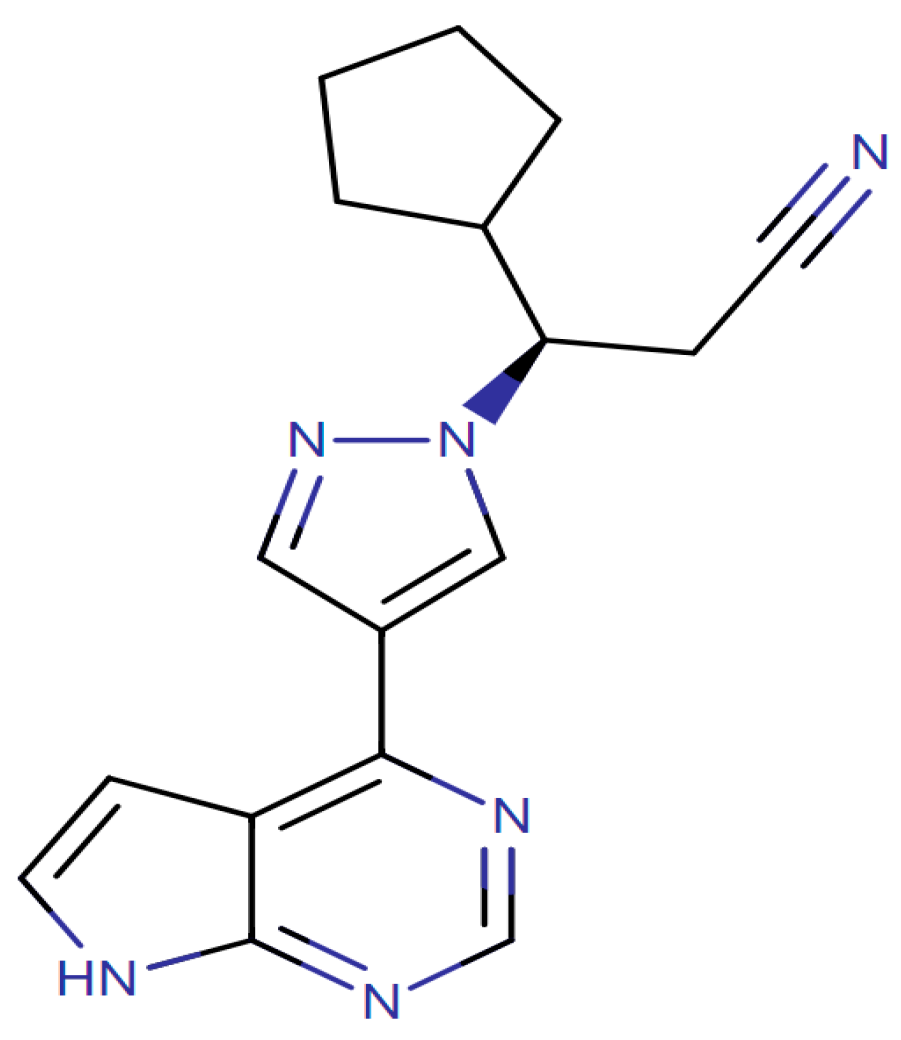

| Characteristic | N | Average (SD) |
|---|---|---|
| Total number | 21 | |
| Female | 10 | 48% |
| Male | 11 | 52% |
| Age at the moment of observation | from 39 to 76 years | 61.2 (8.4) |
| Age at diagnosis | from 34 to 70 years | 58.49 (9.13) |
| Time from diagnosis of the disease | from 0.7 to 8 years | 3.16 (1.88) |
| Type of myelofibrosis | ||
| PMF | 10 | 48% |
| Post-PV MF | 9 | 42% |
| Post-ET MF | 2 | 10% |
| Risk group | ||
| Intermediate-1 risk | 5 | 24% |
| Intermediate-2 risk | 5 | 24% |
| Unknown | 2 | 10% |
| High risk | 3 | 14% |
| Low risk | 6 | 28% |
| JAK2 V617F mutation status | ||
| Homozygote mutation | 6 | 28% |
| Heterozygote mutation | 8 | 38% |
| Negative for mutation | 4 | 20% |
| No information | 3 | 14% |
| Ruxolitinib dose | ||
| 5 mg 2 × daily | 3 | 14% |
| 10 mg 2 × daily | 6 | 30% |
| 15 mg 2 × daily | 8 | 38% |
| 20 mg 2 × daily | 3 | 14% |
| Indicator | Second Measurement–Average (SD) | Third Measurement–Average (SD) | |
|---|---|---|---|
| Leukocytes (G/L) | 14.38 (7.9) | 20.02 (16.16) | 18.57 (3.26) |
| Hemoglobin (g/L) | 106.92 (24.2) | 117.82 (23.44) | 110.38 (17.13) |
| Thrombocytes (G/L) | 328.3 (150.1) | 354.27 (165.26) | 260.75 (93.25) |
| LDH (U/L) | 688.38 (337.67) | 751.9 (547.34) | 626.25 (414.63) |
| Spleen size (cm) | 21.8 (2.88) | 19.5 (3) | 19.33 (2.2) |
| Instrument | 1st Inquiry Average (SD) | 2nd Inquiry Average (SD) | 3rd Inquiry Average (SD) | 4th Inquiry Average (SD) | 5th Inquiry Average (SD) | 6th Inquiry Average (SD) |
|---|---|---|---|---|---|---|
| MPN-SAF TSS | 26.7 (11.06) | 28.8 (12.2) | 26.09 (13.9) | 20.4 (12.8) | 18.3 (12.9) | 14.4 (8.36) |
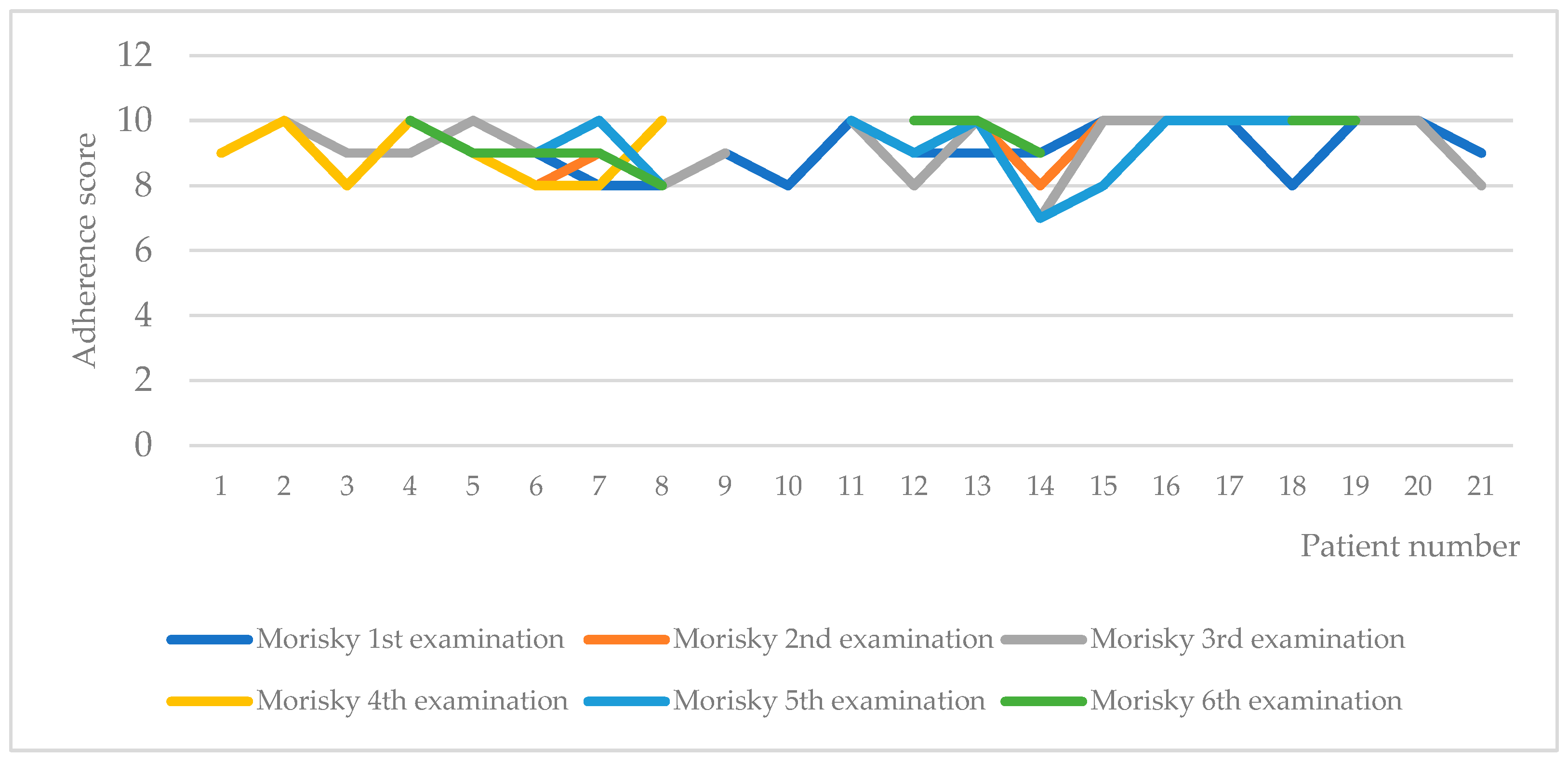
| Morisky | 9.1 (0.7) | 8.8 (1.3) | 9.5 (0.9) | 9.1 (0.8) | 9.3 (0.8) | 9.3 (0.7) |
| Variable | GENDER = 1 | GENDER = 2 | |||
|---|---|---|---|---|---|
| Median | Average Rank | Median | Average Rank | P | |
| MOR | 9.0000 | 12.0500 | 9.0000 | 10.0455 | 0.4300 |
| MPN_SAF_TSS | 25.5000 | 11.3000 | 18.5000 | 9.7000 | 0.5446 |
| Disease Duration (DD) | Morisky 3rd observation | MPN_SAF_TSS_3rd observation | |
| DD | Correlation coefficient Significance Level P n | −0.153 0.5208 20 | 0.555 0.0137 19 |
| Disease duration | Morisky 4th observation | MPN_SAF_TSS_4th observation | |
| DD | Correlation coefficient Significance Level P n | −0.397 0.1024 18 | 0.527 0.0298 17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stoeva, V.; Petrova, G.; Mitov, K.; Tachkov, K. Study of Symptom Severity and Adherence to Therapy of Myelofibrosis Patients Treated with Ruxolitinib. Pharmaceuticals 2023, 16, 976. https://doi.org/10.3390/ph16070976
Stoeva V, Petrova G, Mitov K, Tachkov K. Study of Symptom Severity and Adherence to Therapy of Myelofibrosis Patients Treated with Ruxolitinib. Pharmaceuticals. 2023; 16(7):976. https://doi.org/10.3390/ph16070976
Chicago/Turabian StyleStoeva, Vera, Guenka Petrova, Konstantin Mitov, and Konstantin Tachkov. 2023. "Study of Symptom Severity and Adherence to Therapy of Myelofibrosis Patients Treated with Ruxolitinib" Pharmaceuticals 16, no. 7: 976. https://doi.org/10.3390/ph16070976
APA StyleStoeva, V., Petrova, G., Mitov, K., & Tachkov, K. (2023). Study of Symptom Severity and Adherence to Therapy of Myelofibrosis Patients Treated with Ruxolitinib. Pharmaceuticals, 16(7), 976. https://doi.org/10.3390/ph16070976







