Multidirectional Effects of Terpenoids from Sorbus intermedia (EHRH.) PERS Fruits in Cellular Model of Benign Prostate Hyperplasia
Abstract
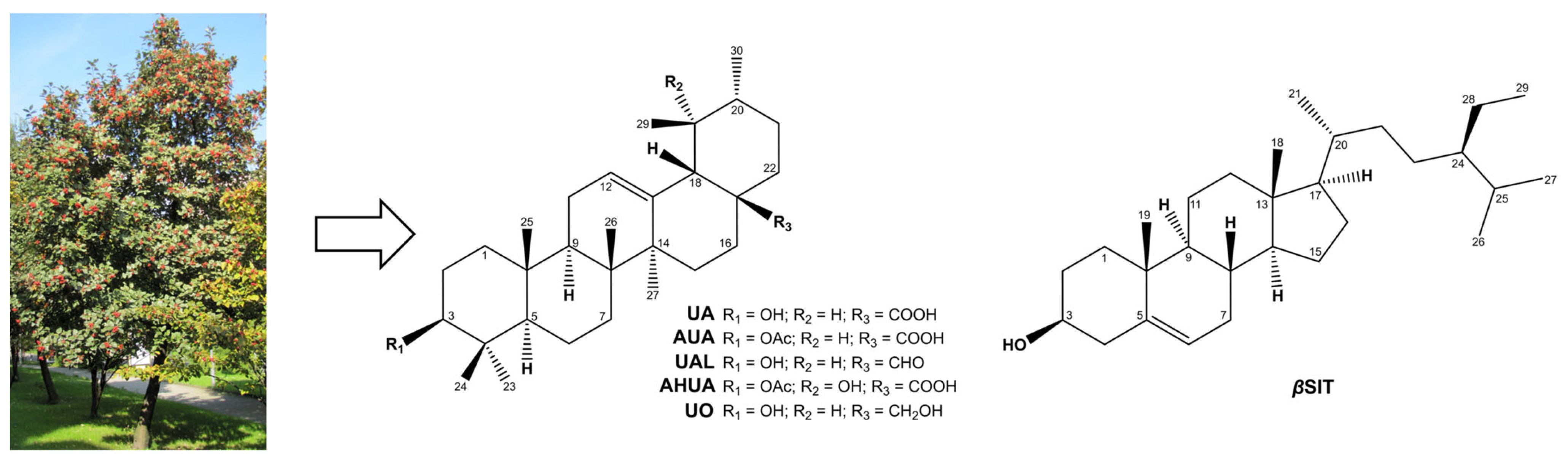
1. Introduction
2. Results and Discussion
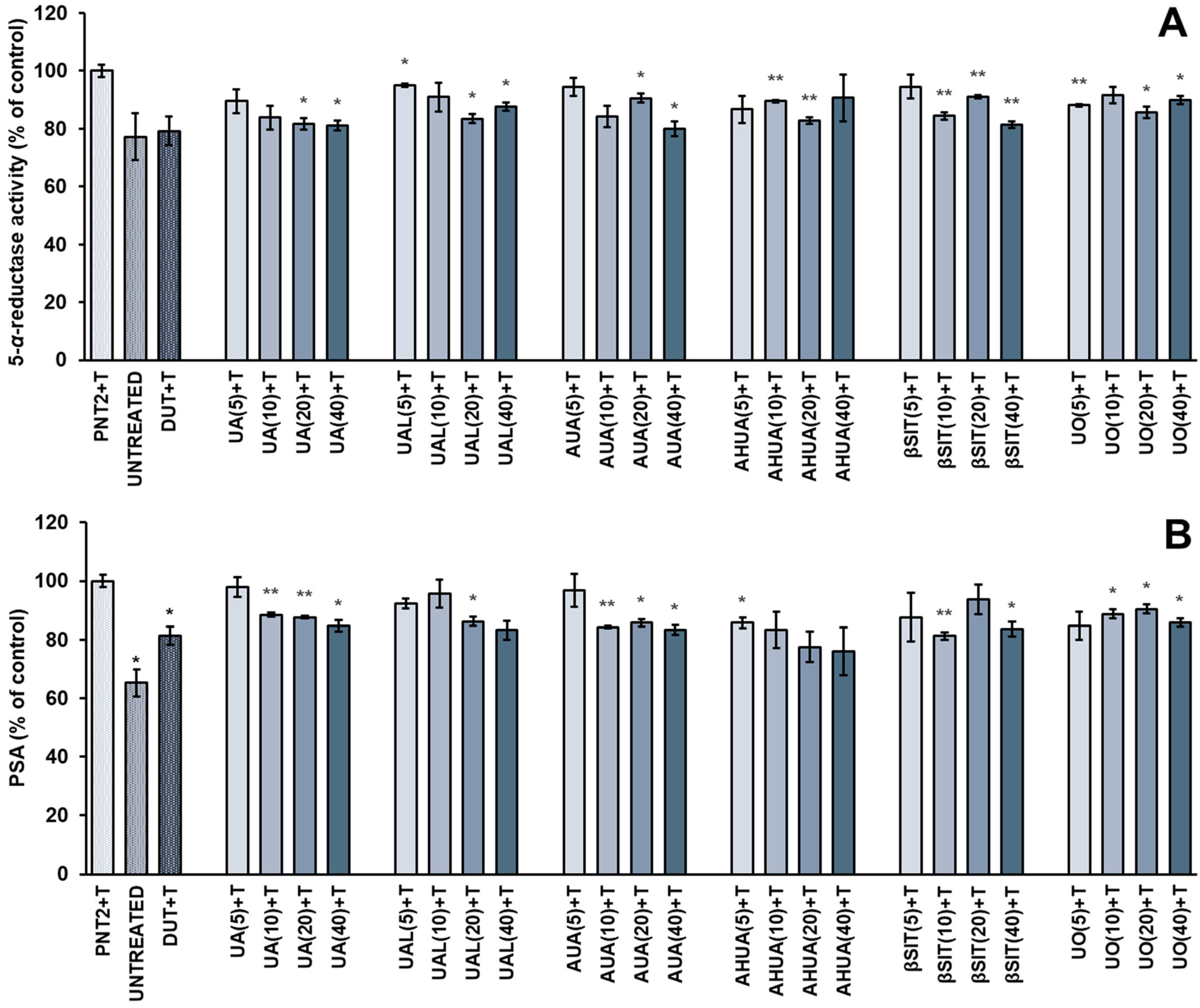
2.1. Antiandrogenic Effects of S. intermedia Terpenoids on Testosterone-Stimulated PNT2 Cells
2.2. Antiproliferative Activities of S. intermedia Terpenoids
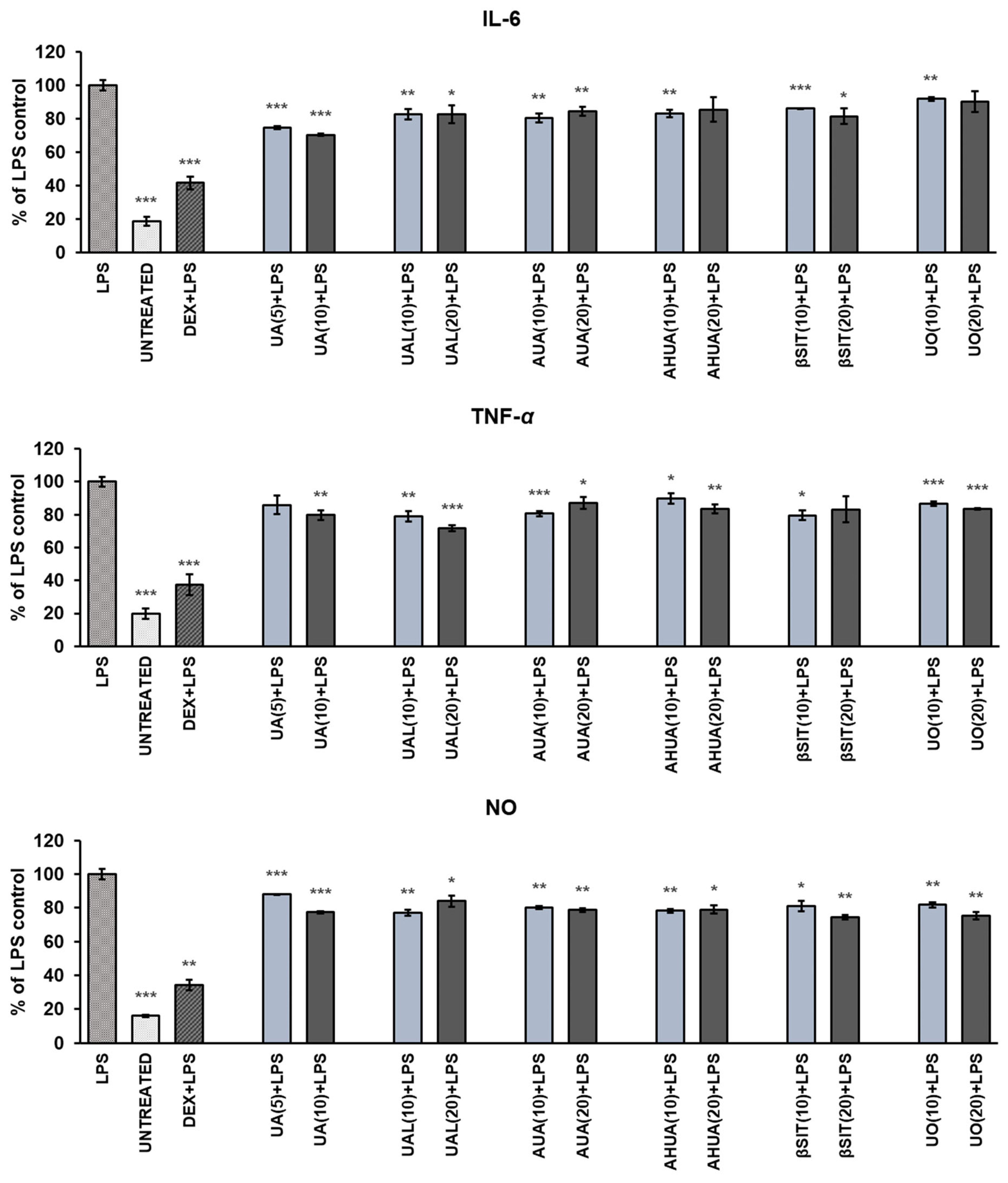
2.3. Anti-Inflammatory Activities of S. intermedia Terpenoids
2.3.1. Inhibition of Albumin Heat-Induced Denaturation
2.3.2. The Effects of S. intermedia Terpenoids on the Release of Pro-Inflammatory Mediators in LPS-Stimulated RAW 264.7 Macrophages
2.3.3. The Effects of S. intermedia Terpenoids on Hyaluronidase Activity
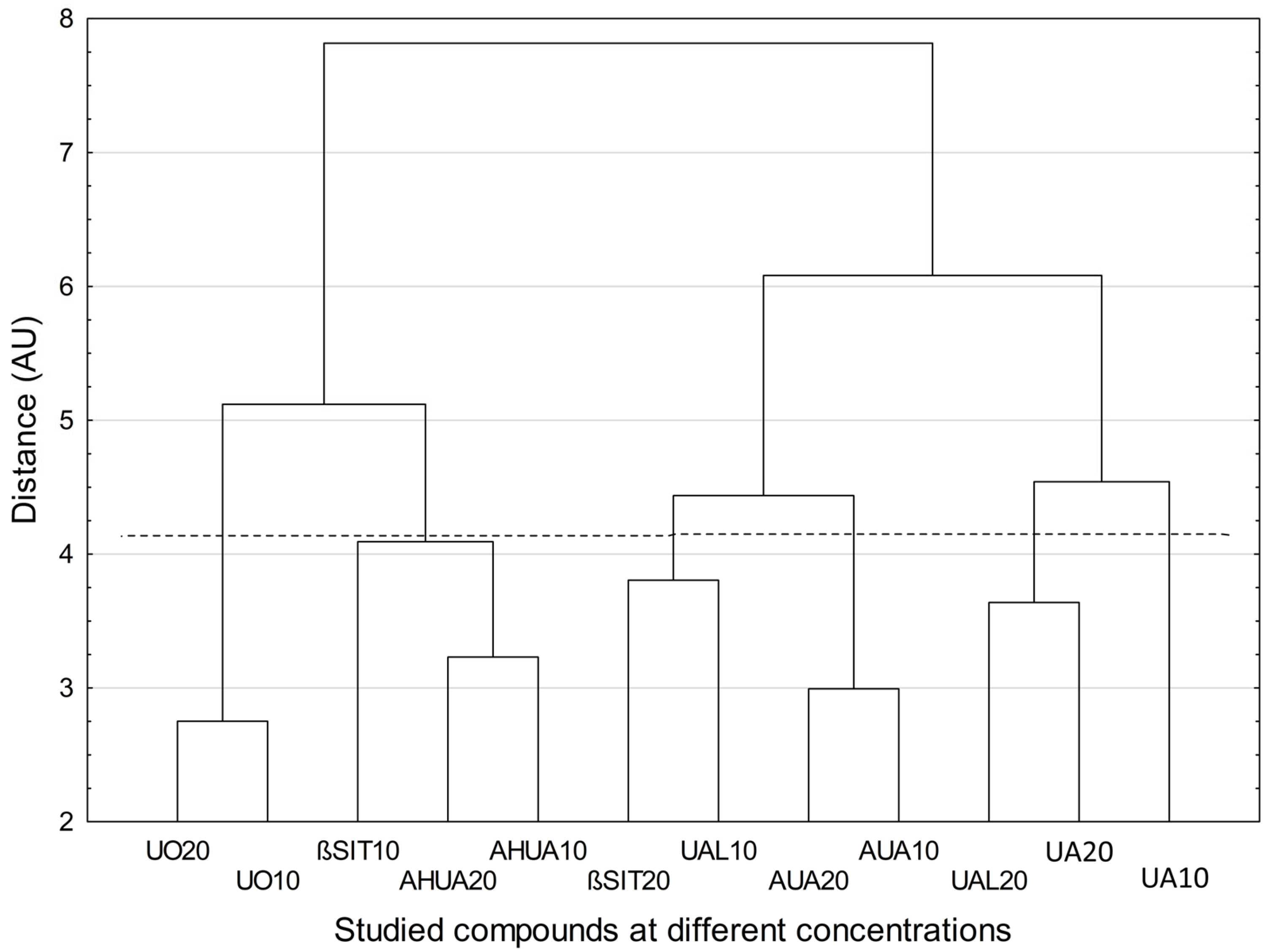
2.4. Chemometric Analysis
3. Materials and Methods
3.1. Reagents and Instruments
3.2. Cell Culture Conditions
3.3. Determination of PSA and 5-α-reductase
3.4. Proliferation Assay
3.5. Inhibition of Albumin Denaturation
- AS—absorbance of the tested substance;
- APc—absorbance of the product control solution;
- AC—absorbance in the absence of inhibitor;
- AB—absorbance of blind control.
3.6. Determination of NO, IL-6 and TNF-α Release
3.7. Anti-Hyaluronidase Assay
- AI—absorbance of enzyme + substrate (control I);
- AII—absorbance in the absence of enzyme (control II);
- As—absorbance of sample solution;
- APc—absorbance of the product control solution;
- AB—absorbance of a blank control of experiment.
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Devlin, C.M.; Simms, M.S.; Maitland, N.J. Benign prostatic hyperplasia—What do we know? BJU Int. 2020, 127, 389–399. [Google Scholar] [CrossRef]
- Csikós, E.; Horváth, A.; Ács, K.; Papp, N.; Balázs, V.L.; Dolenc, M.S.; Kenda, M.; Glavač, N.K.; Nagy, M.; Protti, M.; et al. Treatment of Benign Prostatic Hyperplasia by Natural Drugs. Molecules 2021, 26, 7141. [Google Scholar] [CrossRef] [PubMed]
- Allkanjari, O.; Vitalone, A. What do we know about phytotherapy of benign prostatic hyperplasia? Life Sci. 2015, 126, 42–56. [Google Scholar] [CrossRef]
- La Vignera, S.; Condorelli, R.A.; Russo, G.I.; Morgia, G.; Calogero, A.E. Endocrine control of benign prostatic hyperplasia. Andrology 2016, 4, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Chughtai, B.; Forde, J.C.; Thomas, D.D.M.; Laor, L.; Hossack, T.; Woo, H.H.; Te, A.E.; Kaplan, S.A. Benign prostatic hyperplasia. Nat. Rev. Dis. Prim. 2016, 2, 16031. [Google Scholar] [CrossRef] [PubMed]
- Tsunemori, H.; Sugimoto, M. Effects of inflammatory prostatitis on the development and progression of benign prostatic hyperplasia: A literature review. Int. J. Urol. 2021, 28, 1086–1092. [Google Scholar] [CrossRef]
- Cao, D.; Sun, R.; Peng, L.; Li, J.; Huang, Y.; Chen, Z.; Chen, B.; Li, J.; Ai, J.; Yang, L.; et al. Immune Cell Proinflammatory Microenvironment and Androgen-Related Metabolic Regulation During Benign Prostatic Hyperplasia in Aging. Front. Immunol. 2022, 13, 842008. [Google Scholar] [CrossRef] [PubMed]
- Madersbacher, S.; Alivizatos, G.; Nordling, J.; Sanz, C.R.; Emberton, M.; de la Rosette, J.J. EAU 2004 Guidelines on Assessment, Therapy and Follow-Up of Men with Lower Urinary Tract Symptoms Suggestive of Benign Prostatic Obstruction (BPH Guidelines). Eur. Urol. 2004, 46, 547–554. [Google Scholar] [CrossRef]
- De La Rosette, J.J.; Alivizatos, G.; Madersbacher, S.; Perachino, M.; Thomas, D.; Desgrandchamps, F.; De Wildt, M. EAU Guidelines on Benign Prostatic Hyperplasia (BPH). Eur. Urol. 2001, 40, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.H.; Chan, E.M.C.; Lai, Y.K. The global burden of lower urinary tract symptoms suggestive of benign prostatic hyperplasia: A systematic review and meta-analysis. Sci. Rep. 2017, 7, 7984. [Google Scholar] [CrossRef] [PubMed]
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef]
- Shin, I.-S.; Lee, M.-Y.; Jung, D.-Y.; Seo, C.-S.; Ha, H.-K.; Shin, H.-K. Ursolic acid reduces prostate size and dihydrotestosterone level in a rat model of benign prostatic hyperplasia. Food Chem. Toxicol. 2012, 50, 884–888. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Tamura, S.; Kurashiki, K.; Shimizu, K.; Noda, K.; Konishi, F.; Kumamoto, S.; Kondo, R. Anti-Androgen Effects of Extracts and Compounds from Ganoderma lucidum. Chem. Biodivers. 2009, 6, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Kurashiki, K.; Shimizu, K.; Kondo, R. Structure–activity relationship for inhibition of 5α-reductase by triterpenoids isolated from Ganoderma lucidum. Bioorganic Med. Chem. 2006, 14, 8654–8660. [Google Scholar] [CrossRef] [PubMed]
- Nizomov, S.A.; Sorokina, I.V.; Zhukova, N.A.; Borisov, S.A.; Tolstikova, T.G.; Semenov, D.E.; Bakarev, M.A. Prostatotropic Action of Glycyrrhizic Acid Disodium Salt in Benign Prostatic Hyperplasia Models. Bull. Exp. Biol. Med. 2020, 169, 114–118. [Google Scholar] [CrossRef]
- Smith, D.K.; Dds, S.L.H.; Wang, J.; Kallifatidis, G.; Morera, D.S.; Jordan, A.R.; Terris, M.K.; Klaassen, Z.; Bollag, R.; Lokeshwar, V.B.; et al. Promotion of epithelial hyperplasia by interleukin-8—CXCR axis in human prostate. Prostate 2020, 80, 938–949. [Google Scholar] [CrossRef]
- Cheon, S.-Y.; Jin, B.-R.; Kim, H.-J.; An, H.-J. Oleanolic Acid Ameliorates Benign Prostatic Hyperplasia by Regulating PCNA-Dependent Cell Cycle Progression In Vivo and In Vitro. J. Nat. Prod. 2020, 83, 1183–1189. [Google Scholar] [CrossRef]
- Sołtys, A.; Galanty, A.; Zagrodzki, P.; Grabowska, K.; Malarz, J.; Podolak, I. Sorbus intermedia (EHRH.) PERS. fruits as a novel source of biologically active triterpenoids—Comparative studies of ursolic acid derivatives with cytotoxic potential. Biomed. Pharmacother. 2022, 154, 113592. [Google Scholar] [CrossRef] [PubMed]
- Sołtys, A.; Galanty, A.; Zagrodzki, P.; Podolak, I. Relationship between Maturity Stage, Triterpenoid Content and Cytotoxicity of Sorbus intermedia (EHRH.) PERS. Fruits—A Chemometric Approach. Chem. Biodivers. 2021, 18, e2100552. [Google Scholar] [CrossRef] [PubMed]
- Roehrborn, C.G. Pathology of benign prostatic hyperplasia. Int. J. Impot. Res. 2008, 20, S11–S18. [Google Scholar] [CrossRef] [PubMed]
- Love, H.D.; Booton, S.E.; Boone, B.E.; Breyer, J.P.; Koyama, T.; Revelo, M.P.; Shappell, S.B.; Smith, J.R.; Hayward, S.W. Androgen Regulated Genes in Human Prostate Xenografts in Mice: Relation to BPH and Prostate Cancer. PLoS ONE 2009, 4, e8384. [Google Scholar] [CrossRef] [PubMed]
- Wilt, T.J.; Ishani, A.; MacDonald, R.; Stark, G.; Mulrow, C.D.; Lau, J. Beta-sitosterols for benign prostatic hyperplasia. Cochrane Database Syst. Rev. 1999, 2011, CD001043. [Google Scholar] [CrossRef] [PubMed]
- Wilt, T.J.; Macdonald, R.; Ishani, A. β-sitosterol for the treatment of benign prostatic hyperplasia. BJU Int. 1999, 83, 976–983. [Google Scholar] [CrossRef] [PubMed]
- Cabezal, M.; Bratoeff, E.; Heuze, I.; Ramírez, E.; Sánchez, M.; Flores, E. Effect of Beta-Sitosterol as Inhibitor of 5 Al-pha-Reductase in Hamster Prostate. Proc. West. Pharmacol. Soc. 2003, 46, 153–155. [Google Scholar]
- Cicero, A.F.; Allkanjari, O.; Busetto, G.M.; Cai, T.; Larganà, G.; Magri, V.; Perletti, G.; Della Cuna, F.S.R.; Russo, G.I.; Stamatiou, K.; et al. Nutraceutical treatment and prevention of benign prostatic hyperplasia and prostate cancer. Arch. Ital. Urol. Androl. 2019, 91, 139–152. [Google Scholar] [CrossRef]
- Azizi, A.; Mumin, N.H.; Shafqat, N. Phytochemicals with Anti 5-alpha-reductase Activity: A Prospective for Prostate Cancer Treatment. F1000Research 2021, 10, 221. [Google Scholar] [CrossRef]
- Sudeep, H.V.; Thomas, J.V.; Shyamprasad, K. A double blind, placebo-controlled randomized comparative study on the efficacy of phytosterol-enriched and conventional saw palmetto oil in mitigating benign prostate hyperplasia and androgen deficiency. BMC Urol. 2020, 20, 86. [Google Scholar] [CrossRef]
- Kassi, E.; Papoutsi, Z.; Pratsinis, H.; Aligiannis, N.; Manoussakis, M.; Moutsatsou, P. Ursolic acid, a naturally occurring triterpenoid, demonstrates anticancer activity on human prostate cancer cells. J. Cancer Res. Clin. Oncol. 2007, 133, 493–500. [Google Scholar] [CrossRef]
- Mu, D.; Zhou, G.; Li, J.; Su, B.; Guo, H. Ursolic acid activates the apoptosis of prostate cancer via ROCK/PTEN mediated mitochondrial translocation of cofilin-1. Oncol. Lett. 2017, 15, 3202–3206. [Google Scholar] [CrossRef]
- Choi, Y.H.; Baek, J.H.; Yoo, M.A.; Chung, H.Y.; Kim, N.D.; Kim, K.W. Induction of apoptosis by ursolic acid through activation of caspases and down-regulation of c-IAPs in human prostate epithelial cells. Int. J. Oncol. 2000, 17, 565–571. [Google Scholar] [CrossRef]
- Shin, S.W.; Kim, S.Y.; Park, J.-W. Autophagy inhibition enhances ursolic acid-induced apoptosis in PC3 cells. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2012, 1823, 451–457. [Google Scholar] [CrossRef]
- Harmand, P.-O.; Duval, R.; Liagre, B.; Jayat-Vignoles, C.; Beneytout, J.-L.; Delage, C.; Simon, A. Ursolic acid induces apoptosis through caspase-3 activation and cell cycle arrest in HaCat cells. Int. J. Oncol. 2003, 23, 105–112. [Google Scholar] [CrossRef]
- Es-Saady, D.; Simon, A.; Ollier, M.; Maurizis, J.C.; Chulia, A.J.; Delage, C. Inhibitory effect of ursolic acid on B16 proliferation through cell cycle arrest. Cancer Lett. 1996, 106, 193–197. [Google Scholar] [CrossRef]
- Weng, H.; Tan, Z.-J.; Hu, Y.-P.; Shu, Y.-J.; Bao, R.-F.; Jiang, L.; Wu, X.-S.; Li, M.-L.; Ding, Q.; Wang, X.-A.; et al. Ursolic acid induces cell cycle arrest and apoptosis of gallbladder carcinoma cells. Cancer Cell Int. 2014, 14, 96. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Tang, S.; Tao, Q.; Ming, T.; Lei, J.; Liang, Y.; Peng, Y.; Wang, M.; Liu, M.; Yang, H.; et al. Ursolic Acid Suppresses Colorectal Cancer by Down-Regulation of Wnt/β-Catenin Signaling Pathway Activity. J. Agric. Food Chem. 2023, 71, 3981–3993. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Zhang, Y.; Zhang, H.; Xia, L. Molecular Mechanism of β-Sitosterol and its Derivatives in Tumor Progression. Front. Oncol. 2022, 12, 926975. [Google Scholar] [CrossRef] [PubMed]
- Awad, A.B.; Burr, A.T.; Fink, C.S. Effect of resveratrol and β-sitosterol in combination on reactive oxygen species and prostaglandin release by PC-3 cells. Prostaglandins Leukot. Essent. Fat. Acids 2005, 72, 219–226. [Google Scholar] [CrossRef] [PubMed]
- AlQathama, A.; Shao, L.; Bader, A.; Khondkar, P.; Gibbons, S.; Prieto, J.M. Differential Anti-Proliferative and Anti-Migratory Activities of Ursolic Acid, 3-O-Acetylursolic Acid and Their Combination Treatments with Quercetin on Melanoma Cells. Biomolecules 2020, 10, 894. [Google Scholar] [CrossRef]
- Bonel-Pérez, G.C.; Pérez-Jiménez, A.; Gris-Cárdenas, I.; Parra-Pérez, A.M.; Lupiáñez, J.A.; Reyes-Zurita, F.J.; Siles, E.; Csuk, R.; Peragón, J.; Rufino-Palomares, E.E. Antiproliferative and Pro-Apoptotic Effect of Uvaol in Human Hepatocarcinoma HepG2 Cells by Affecting G0/G1 Cell Cycle Arrest, ROS Production and AKT/PI3K Signaling Pathway. Molecules 2020, 25, 4254. [Google Scholar] [CrossRef]
- Allouche, Y.; Warleta, F.; Campos, M.; Sánchez-Quesada, C.; Uceda, M.; Beltrán, G.; Gaforio, J.J. Antioxidant, Antiproliferative, and Pro-apoptotic Capacities of Pentacyclic Triterpenes Found in the Skin of Olives on MCF-7 Human Breast Cancer Cells and Their Effects on DNA Damage. J. Agric. Food Chem. 2011, 59, 121–130. [Google Scholar] [CrossRef]
- Amico, V.; Barresi, V.; Condorelli, D.; Spatafora, C.; Tringali, C. Antiproliferative Terpenoids from Almond Hulls (Prunus dulcis): Identification and Structure−Activity Relationships. J. Agric. Food Chem. 2006, 54, 810–814. [Google Scholar] [CrossRef]
- Gandaglia, G.; Briganti, A.; Gontero, P.; Mondaini, N.; Novara, G.; Salonia, A.; Sciarra, A.; Montorsi, F. The role of chronic prostatic inflammation in the pathogenesis and progression of benign prostatic hyperplasia (BPH). BJU Int. 2013, 112, 432–441. [Google Scholar] [CrossRef]
- Robert, G.; Descazeaud, A.; Nicolaïew, N.; Terry, S.; Sirab, N.; Vacherot, F.; Maillé, P.; Allory, Y.; de la Taille, A. Inflammation in benign prostatic hyperplasia: A 282 patients’ immunohistochemical analysis. Prostate 2009, 69, 1774–1780. [Google Scholar] [CrossRef]
- Vickman, R.E.; Aaron-Brooks, L.; Zhang, R.; Lanman, N.A.; Lapin, B.; Gil, V.; Greenberg, M.; Sasaki, T.; Cresswell, G.M.; Broman, M.M.; et al. TNF is a potential therapeutic target to suppress prostatic inflammation and hyperplasia in autoimmune disease. Nat. Commun. 2022, 13, 2133. [Google Scholar] [CrossRef]
- Vickman, R.E.; Franco, O.E.; Hayward, S.W. Could TNF-antagonists be a novel treatment strategy for BPH patients? Cell Stress 2022, 6, 65–67. [Google Scholar] [CrossRef]
- Zhou, R.-Y.; Tong, Y.; Guo, Y.-J.; Zhang, Q.; Bi, H.-X.; Kai, K. Combined treatment with dihydrotestosterone and lipopolysaccharide modulates prostate homeostasis by upregulating TNF-α from M1 macrophages and promotes proliferation of prostate stromal cells. Asian J. Androl. 2022, 24, 513–520. [Google Scholar] [CrossRef]
- Du, S.-Y.; Huang, H.-F.; Li, X.-Q.; Zhai, L.-X.; Zhu, Q.-C.; Zheng, K.; Song, X.; Xu, C.-S.; Li, C.-Y.; Li, Y.; et al. Anti-inflammatory properties of uvaol on DSS-induced colitis and LPS-stimulated macrophages. Chin. Med. 2020, 15, 43. [Google Scholar] [CrossRef]
- Wang, J.; Jin, M.; Jin, C.; Ye, C.; Zhou, Y.; Wang, R.; Cui, H.; Zhou, W.; Li, G. A new pentacyclic triterpenoid from the leaves of Rhododendron dauricum L. with inhibition of NO production in LPS-induced RAW 264.7 cells. Nat. Prod. Res. 2019, 34, 3313–3319. [Google Scholar] [CrossRef]
- Yang, Z.-G.; Matsuzaki, K.; Takamatsu, S.; Kitanaka, S. Inhibitory Effects of Constituents from Morus alba var. multicaulis on Differentiation of 3T3-L1 Cells and Nitric Oxide Production in RAW264.7 Cells. Molecules 2011, 16, 6010–6022. [Google Scholar] [CrossRef]
- Jiang, X.; Shen, P.; Zhou, J.; Ge, H.; Raj, R.; Wang, W.; Yu, B.; Zhang, J. Microbial transformation and inhibitory effect assessment of uvaol derivates against LPS and HMGB1 induced NO production in RAW264.7 macrophages. Bioorganic Med. Chem. Lett. 2022, 58, 128523. [Google Scholar] [CrossRef]
- Hu, T.; He, X.-W.; Jiang, J.-G. Functional Analyses on Antioxidant, Anti-inflammatory, and Antiproliferative Effects of Extracts and Compounds from Ilex latifolia Thunb., a Chinese Bitter Tea. J. Agric. Food Chem. 2014, 62, 8608–8615. [Google Scholar] [CrossRef]
- Kim, M.-H.; Kim, J.N.; Han, S.N.; Kim, H.-K. Ursolic acid isolated from guava leaves inhibits inflammatory mediators and reactive oxygen species in LPS-stimulated macrophages. Immunopharmacol. Immunotoxicol. 2015, 37, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.-X.; Wink, M. Evidence for Anti-Inflammatory Activity of Isoliquiritigenin, 18β Glycyrrhetinic Acid, Ursolic Acid, and the Traditional Chinese Medicine Plants Glycyrrhiza glabra and Eriobotrya japonica, at the Molecular Level. Medicines 2019, 6, 55. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Choi, J.K.; Jeong, N.-H.; Yoo, J.; Ha, Y.S.; Lee, B.; Choi, H.; Park, P.-H.; Shin, T.-Y.; Kwon, T.K.; et al. Anti-inflammatory effects of ursolic acid-3-acetate on human synovial fibroblasts and a murine model of rheumatoid arthritis. Int. Immunopharmacol. 2017, 49, 118–125. [Google Scholar] [CrossRef]
- Ding, Y.; Nguyen, H.T.; Kim, S.I.; Kim, H.W.; Kim, Y.H. The regulation of inflammatory cytokine secretion in macrophage cell line by the chemical constituents of Rhus sylvestris. Bioorganic Med. Chem. Lett. 2009, 19, 3607–3610. [Google Scholar] [CrossRef]
- Theocharis, A.D.; Skandalis, S.S.; Gialeli, C.; Karamanos, N.K. Extracellular matrix structure. Adv. Drug Deliv. Rev. 2016, 97, 4–27. [Google Scholar] [CrossRef]
- Karamanos, N.K.; Theocharis, A.D.; Piperigkou, Z.; Manou, D.; Passi, A.; Skandalis, S.S.; Vynios, D.H.; Orian-Rousseau, V.; Ricard-Blum, S.; Schmelzer, C.E.; et al. A guide to the composition and functions of the extracellular matrix. FEBS J. 2021, 288, 6850–6912. [Google Scholar] [CrossRef]
- Girish, K.S.; Kemparaju, K.; Nagaraju, S.; Vishwanath, B.S. Hyaluronidase Inhibitors: A Biological and Therapeutic Perspective. Curr. Med. Chem. 2009, 16, 2261–2288. [Google Scholar] [CrossRef]
- Kovar, J.L.; Johnson, M.A.; Volcheck, W.M.; Chen, J.; Simpson, M.A. Hyaluronidase Expression Induces Prostate Tumor Metastasis in an Orthotopic Mouse Model. Am. J. Pathol. 2006, 169, 1415–1426. [Google Scholar] [CrossRef]
- Benitez, A.; Yates, T.J.; Lopez, L.E.; Cerwinka, W.H.; Bakkar, A.; Lokeshwar, V.B. Targeting Hyaluronidase for Cancer Therapy: Antitumor Activity of Sulfated Hyaluronic Acid in Prostate Cancer Cells. Cancer Res. 2011, 71, 4085–4095. [Google Scholar] [CrossRef] [PubMed]
- Khammee, T.; Rujitanapanich, S.; Chunhakant, S.; Jaratrungtawee, A.; Kuno, M. In Vitro and in Silico Evaluations of Chemical Constituents from the Rhizomes of Aglaonema simplex (Blume) Blume as Hyaluronidase Inhibitor. Thai J. Sci. Technol. 2020, 9, 269–277. [Google Scholar] [CrossRef]
- Süntar, I.; Akkol, E.K.; Keles, H.; Yesilada, E.; Sarker, S.D.; Baykal, T. Comparative evaluation of traditional prescriptions from Cichorium intybus L. for wound healing: Stepwise isolation of an active component by in vivo bioassay and its mode of activity. J. Ethnopharmacol. 2012, 143, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Chaiyana, W.; Anuchapreeda, S.; Punyoyai, C.; Neimkhum, W.; Lee, K.-H.; Lin, W.-C.; Lue, S.-C.; Viernstein, H.; Mueller, M. Ocimum sanctum Linn. as a natural source of skin anti-ageing compounds. Ind. Crops Prod. 2018, 127, 217–224. [Google Scholar] [CrossRef]
- Neimkhum, W.; Anuchapreeda, S.; Lin, W.-C.; Lue, S.-C.; Lee, K.-H.; Chaiyana, W. Effects of Carissa carandas Linn. Fruit, Pulp, Leaf, and Seed on Oxidation, Inflammation, Tyrosinase, Matrix Metalloproteinase, Elastase, and Hyaluronidase Inhibition. Antioxidants 2021, 10, 1345. [Google Scholar] [CrossRef]
- Nema, N.K.; Maity, N.; Sarkar, B.K.; Mukherjee, P.K. Matrix metalloproteinase, hyaluronidase and elastase inhibitory potential of standardized extract of Centella asiatica. Pharm. Biol. 2013, 51, 1182–1187. [Google Scholar] [CrossRef]
- Michel, P.; Owczarek, A.; Matczak, M.; Kosno, M.; Szymański, P.; Mikiciuk-Olasik, E.; Kilanowicz, A.; Wesołowski, W.; Olszewska, M.A. Metabolite Profiling of Eastern Teaberry (Gaultheria procumbens L.) Lipophilic Leaf Extracts with Hyaluronidase and Lipoxygenase Inhibitory Activity. Molecules 2017, 22, 412. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, N.H.; Thomas, N.F.; Sivasothy, Y.; Lee, V.S.; Liew, S.Y.; Noorbatcha, I.A.; Awang, K. Hyaluronidase Inhibitory Activity of Pentacylic Triterpenoids from Prismatomeris tetrandra (Roxb.) K. Schum: Isolation, Synthesis and QSAR Study. Int. J. Mol. Sci. 2016, 17, 143. [Google Scholar] [CrossRef]
- Nakayama, A.; Ide, H.; Lu, Y.; Takei, A.; Fukuda, K.; Osaka, A.; Arai, G.; Horie, S.; Okada, H.; Saito, K. Effects of Curcumin Combined With the 5-alpha Reductase Inhibitor Dutasteride on LNCaP Prostate Cancer Cells. In Vivo 2021, 35, 1443–1450. [Google Scholar] [CrossRef]
- Galanty, A.; Zagrodzki, P.; Gdula-Argasińska, J.; Grabowska, K.; Koczurkiewicz-Adamczyk, P.; Wróbel-Biedrawa, D.; Podolak, I.; Pękala, E.; Paśko, P. A Comparative Survey of Anti-Melanoma and Anti-Inflammatory Potential of Usnic Acid Enantiomers—A Comprehensive In Vitro Approach. Pharmaceuticals 2021, 14, 945. [Google Scholar] [CrossRef]
- Grabowska, K.; Wróbel, D.; Żmudzki, P.; Podolak, I. Anti-inflammatory activity of saponins from roots of Impatiens parviflora DC. Nat. Prod. Res. 2020, 34, 1581–1585. [Google Scholar] [CrossRef]

| Concentration | Albumin Denaturation Inhibition (%) | ||
|---|---|---|---|
| (µg/mL) | AHUA | UO | DS |
| 5 | NE | NE | 34.29 ± 2.07 |
| 10 | 19.61 ± 6.76 | 9.35 ± 2.50 | 52.45 ± 2.84 |
| 25 | 28.85 ± 2.50 * | 11.17 ± 1.69 * | 81.06 ± 3.04 |
| 50 | 31.46 ± 3.07 * | 18.35 ± 5.37 | 95.75 ± 1.71 |
| 100 | 30.63 ± 3.34 * | 28.49 ± 3.82 * | 99.06 ± 0.26 |
| 250 | 22.79 ± 5.00 | 31.98 ± 2.34 * | 99.76 ± 0.26 |
| 500 | 28.67 ± 2.72 * | 30.78 ± 1.56 * | 100.00 ± 0.00 |
| 750 | 30.24 ± 2.72 * | 29.30 ± 1.28 * | 100.00 ± 0.00 |
| 1000 | 32.81 ± 4.75 * | 30.37 ± 3.36 * | 100.00 ± 0.00 |
| IC50 | >Cmax | >Cmax | 8.61 |
| Concentration (µg/mL) | Hyaluronidase Inhibition (%) | ||||||
|---|---|---|---|---|---|---|---|
| UA | UAL | AUA | AHUA | βSIT | UO | QUERCETIN | |
| 10 | 0.00 ± 0.00 | NE | 7.87 ± 0.95 * | 0.00 ± 0.00 | 3.72 ± 0.78 * | 0.00 ± 0.00 | 0.61 ± 0.53 |
| 25 | 2.52 ± 2.18 | 0.00 ± 0.00 | 11.70 ± 1.55 * | 0.17 ± 0.30 | 12.09 ± 0.28 * | 0.00 ± 0.00 | 1.17 ± 0.41 |
| 50 | 3.23 ± 1.71 | 1.62 ± 0.35 | 16.76 ± 4.36 | 0.35 ± 0.30 | 20.53 ± 0.75 * | 0.00 ± 0.00 | 1.18 ± 0.95 |
| 100 | 11.65 ± 0.92 * | 3.91 ± 0.56 | 19.27 ± 4.85 | 2.32 ± 1.88 | 23.32 ± 0.75 * | 0.00 ± 0.00 | 4.36 ± 0.88 |
| 250 | 60.31 ± 0.88 * | 15.45 ± 1.67 | 30.57 ± 0.68 * | 18.54 ± 3.44 | 25.79 ± 0.45 * | 0.52 ± 0.52 | 18.08 ± 1.00 |
| 500 | 82.38 ± 3.77 * | 78.79 ± 1.63 * | 45.63 ± 1.37 * | 42.07 ± 1.72 | 27.78 ± 3.83 | 4.01 ± 1.14 | 38.84 ± 1.33 |
| 750 | 85.35 ± 2.30 | NE | 52.13 ± 4.94 | 93.41 ± 2.75 | 30.56 ± 3.89 | 13.71 ± 1.53 | 87.27 ± 1.16 |
| 1000 | 96.01 ± 3.54 | 96.25 ± 0.86 | 56.73 ± 4.26 | 99.22 ± 0.68 * | 36.75 ± 4.61 | 35.13 ± 4.85 | 90.94 ± 1.72 |
| IC50 | 225.75 | 369.77 | 705.74 | 519.87 | >Cmax | >Cmax | 517.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sołtys, A.; Galanty, A.; Grabowska, K.; Paśko, P.; Zagrodzki, P.; Podolak, I. Multidirectional Effects of Terpenoids from Sorbus intermedia (EHRH.) PERS Fruits in Cellular Model of Benign Prostate Hyperplasia. Pharmaceuticals 2023, 16, 965. https://doi.org/10.3390/ph16070965
Sołtys A, Galanty A, Grabowska K, Paśko P, Zagrodzki P, Podolak I. Multidirectional Effects of Terpenoids from Sorbus intermedia (EHRH.) PERS Fruits in Cellular Model of Benign Prostate Hyperplasia. Pharmaceuticals. 2023; 16(7):965. https://doi.org/10.3390/ph16070965
Chicago/Turabian StyleSołtys, Agnieszka, Agnieszka Galanty, Karolina Grabowska, Paweł Paśko, Paweł Zagrodzki, and Irma Podolak. 2023. "Multidirectional Effects of Terpenoids from Sorbus intermedia (EHRH.) PERS Fruits in Cellular Model of Benign Prostate Hyperplasia" Pharmaceuticals 16, no. 7: 965. https://doi.org/10.3390/ph16070965
APA StyleSołtys, A., Galanty, A., Grabowska, K., Paśko, P., Zagrodzki, P., & Podolak, I. (2023). Multidirectional Effects of Terpenoids from Sorbus intermedia (EHRH.) PERS Fruits in Cellular Model of Benign Prostate Hyperplasia. Pharmaceuticals, 16(7), 965. https://doi.org/10.3390/ph16070965





