CRISPR-Based Gene Editing in Acinetobacter baumannii to Combat Antimicrobial Resistance
Abstract
1. Introduction
2. Clinical Significance of A. baumannii
3. Antimicrobial Resistance Mechanisms in A. baumannii
| Antimicrobial Categories | Resistance Mechanism | Class/Family/Activity | Enzymes/Genes/Proteins | References |
|---|---|---|---|---|
| β-lactams | β-lactamases | Class A | Extended-Spectrum β-lactamases | |
| blaCARB-(4, 10) | [76,87] | |||
| blaCTX-M-(2, 15, 43, 55, 115) | [88,89,90] | |||
| blaPER-(1, 2, 3, 7) | [91,92,93] | |||
| blaSHV-(5, 12, 33) | [57,94,95] | |||
| blaVEB-(1, 3, 7) | [96,97,98] | |||
| blaTEM-(1, 92, 116) | [99,100,101] | |||
| Narrow-Spectrum β-lactamases | ||||
| blaSCO-1 | [102] | |||
| Carbapenem-Hydrolyzing β-lactamases | ||||
| blaGES-(1, 5, 11, 12, 14, 15) | [103,104,105] | |||
| blaKPC-(2, 3, 5, 10) | [55,56,106] | |||
| Class B | blaFIM-1 | [107] | ||
| blaGIM-1 | [108] | |||
| blaIMP-(1, 2, 4, 5, 6, 8, 10, 11, 14, 16, 19, 24) | [103,109,110] | |||
| blaNDM-(1, 2, 3) | [111,112,113] | |||
| blaSIM-1 | [114] | |||
| blaSPM-1 | [115] | |||
| blaVIM-(1, 2, 3, 4, 6, 11) | [116,117] | |||
| Class C | blaAmpC-(69, 70, 71) | [118,119,120] | ||
| blaADC-(11, 25, 30, 56, 76, 152, 196, 222) | [121] | |||
| Class D (Oxacillinases or OXA family) | blaOXA-(21, 37, 128) (Narrow spectrum) | [122] | ||
| blaOXA-23 group, including blaOXA-(27, 49, 73, 102, 103, 105, 133, 134, 146, 165, 171,225, 239) | [104,123,124,125] | |||
| blaOXA-24 group, including blaOXA-(25, 26, 27, 40, 72, 139, 160, 207, 40/24) | [90,126,127] | |||
| blaOXA-48 group, including blaOXA-(48b, 162, 163, 181, 199, 204, 232, 247) | [128,129] | |||
| blaOXA-51 group, including blaOXA-(64, 65, 66, 71, 75, 80, 82, 84, 86, 95, 98, 100, 104, 106, 113, 115) | [69,130,131] | |||
| blaOXA-58 group, including blaOXA-(58, 96, 97, 164) | [9,41,132] | |||
| blaOXA-143 group, including blaOXA-(143, 182, 231) | [133,134,135] | |||
| blaOXA-235 group, including blaOXA-(235, 255) | [136,137] | |||
| Aminoglycosides | Overactive efflux pumps | Resistance nodulation division (RND) | AdeABC, AdeFGH, AdeIJK, AdeR, AdeS | [47,69,138] |
| Reduced membrane permeability | OmpA, Omp25, Omp33, OprB, OprC, OprD, OmpW, CarO | [139,140] | ||
| Genetic mutations | Penicillin-binding protein (PBP) | PBP2, PBP3, PBP6b, ftsI | [47,141] | |
| Overactive efflux pumps | RND | AdeABC, AmvA, AdeE, AdeR | [139,142,143] | |
| Genetic mutations | 16sRNA methylase genes | armA, rmt-(A, B, B1, C, D, E) | [139,141,143] | |
| Enzymatic inactivation | Aminoglycoside modifying enzymes (AME) | AAC, APH, ANT | [144,145,146,147] | |
| Tetracyclines | Ribosomal protection | Dissociation of tetracycline from ribosome | Tet-M, Tet-O | [148] |
| Overactive efflux pumps | RND and Tet pump | Tet-(A, B, C, D, G, H, M, X), AdeABC, AdeIJK | [47,80,144] | |
| Polymyxins | Genetic mutations | Lipid A, biotin | MCR-(1, 4, 4.3), PmrCAB, Lps-(B, D), Lpx-(A, C, D), pldA, PheS, ZndP | [47,149,150,151,152] |
| Macrolides | Overactive efflux pumps | Small multidrug resistance (SMR) pump | AbeS | [153] |
| Fluoroquinolones | Overactive efflux pumps | RND and multidrug and toxic compound extrusion | AdeABC, AbeM | [154,155] |
| Genetic mutations | DNA gyrase, quinolone resistance pentapeptide repeat protein | GyrA, ParC, AAC, Qnr-(A, B, B19, S) | [83,84] |
4. Latest Strategies to Combat Antimicrobial Resistance in Bacteria
5. Clustered Regularly Interspaced Palindromic Repeats/CRISPR-Associated Protein (CRISPR-Cas) System
6. Classification of CRISPR-Cas System
7. Importance of CRISPR-Cas System
7.1. Role of CRISPR-Cas System against AMR Bacteria
- It can be used in target-specific cleavage of infection-causing genes, deploying the desired bacteria while leaving the host’s microbiome unaffected [220,221]. For example, chromosomal genes for cell division and metabolism were removed from the mixed cell cultures of E. coli and S. enterica strains using the Type I CRISPR-Cas system [222];
- It can be applied to cleave drug-resistant genes by killing the pathogenic bacteria but not affecting wild-types [222,223]. Bikard et al. applied the RNA-guided nuclease Cas9 against the virulence genes in Staphylococcus aureus, which resulted in the specific killing of virulent strain without affecting avirulent staphylococci [224];
- It can be engineered to modify or silence resistance genes, causing bacterial mutations where the functionality of resistance genes is halted, while bacterial viability is maintained, known as the re-sensitization process [225,226]. The re-sensitization of E. coli strains using ESBL-encoding plasmids was carried out by Kim et al. [220]. They used plasmids encoding for Cas9 and crRNAs against conserved areas in the ESBL genes to transform strains of E. coli that produce ESBLs. The CRISPR-Cas9 system effectively reduced the resistance in the transformants by targeting specific cleavage of resistant plasmids. The realization of the broad utility of the CRISPR-Cas system in gene editing accelerated the need to search for Cas protein variants with enhanced functions, including higher activity, potential for therapeutic delivery, nucleic acid detection, etc. [227]. Among various Cas proteins, the most frequently used Cas proteins are Cas9, which results in a double-strand break by specifically cleaving the targeted sequence [214]; dCas9, a catalytically “dead or defective” Cas9 protein that contrasts with Cas9 by not showing double-strand nuclease activity, but instead staying attached to the targeted sequence and obstructing the RNA polymerase binding to that specific region, thud hindering the transcription initiation [228]; nSpCas9:rAPOBEC1, a Cas9 protein without nuclease activity attached to a deaminase, resulting in the conversion of cytidine bases into thymine and hence forming a stop codon [229]; and Cas13a, an RNA-specific endonuclease that, when recognized by particular DNA sequence, causes the cleavage of RNA fragments [5]. Cas14 is also attracting scientists’ attention as it is small, has single-stranded (ss) DNA-targeting activity, and does not require protospacer adjacent motif (PAM) sequences to bind, as compared to Cas9 and Cas12 proteins [227,230].
7.2. Recent Studies on the Application of CRISPR-Cas System in AMR Bacteria
| Genus | Bacterial Strains | Gram Staining | Targeted Gene/s | Resulted Modifications/Outcomes | References |
|---|---|---|---|---|---|
| Actinomyces | Gram + | ||||
| Actinomycetes | actIORF1 and actVB | Genome modification and gene inactivation and replacement | [261] | ||
| Acinetobacter | Gram – | ||||
| A. baumannii | blaOXA-23, blaTEM-1D, and blaADC-25 | Genome editing and gene manipulation and deletions | [262] | ||
| A. baumannii AB43 | AbaI | Type I-F CRISPR-Cas system | [263] | ||
| A. baumannii | AdvA and ftsZ | CRISPRi, transposon mutagenesis, and gene editing | [264] | ||
| A. baumannii | gltA and β-lactamase genes | Multiplex PCR and CRISPR-Cas12a | [265] | ||
| A. baumannii AYE | pyrF | Genome editing, gene knock-out, and gene manipulation and deletions | [266] | ||
| Actinoplanes | Gram + | ||||
| Actinoplanes sp. SE50/110 | MelC | Genome editing and gene deletions | [267] | ||
| Bacillus | Gram + | ||||
| B. subtilis | ku and ligD | Genome alteration, DSB, and non-homologous end-joining (NHEJ) repair | [268] | ||
| B. subtilis | uppS | CRISPRi and gene activity of essential genes | [269] | ||
| B. subtilis ATCC 6051a | amyE, aprE, nprE, spoIIAC, and srfC | Genome editing and gene manipulation (up to 50%) | [270] | ||
| B. subtilis 168 | trpc2 | Genome alteration, gene deletions, and point mutations | [271] | ||
| B. smithii | pyrF | Genome modification, gene deletions, and silencing and insertions (90%, 100%, and 20%, respectively) | [272] | ||
| B. smithii ET 138 | ldhL | Genome editing, gene inactivation, and silencing with ThermoCas9 (active @ 55 °C) | [273] | ||
| B. licheniformis | yvmC | Genome editing and gene knock-outs and integration | [274] | ||
| Brucella | Gram – | ||||
| B. melitensis | BE3 | Gene manipulation and 100% base replacement (C-T) | [275] | ||
| Campylobacter | Gram – | ||||
| C. jejuni strains M1Cam and 81–176 | flaA, flab, astA, and flgR, | CRISPRi-based repression | [257] | ||
| C. jejuni strains M1Cam and 81–176 | flaA, flab, and flgR, | CRISPRi-based gene repression | [258] | ||
| Caulobacter | Gram – | ||||
| C. crescentus | ctrA and gcrA | CRISPRi and gene knock-downs | [256] | ||
| Clostridium | Gram + | ||||
| C. acetobutylicum ATCC 824 | upp | Genome editing and gene deletions, substitution, and insertions | [276] | ||
| C. acetobutylicum DSM792 | hprK | Genome editing and gene deletion and modifications | [277] | ||
| C. autoethanogenum | adh and 2,3-bdh | Genome editing and gene deletions | [278] | ||
| C. acetobutylicum ATCC 824 and C. beijerinckii NCIMB 8052 | spoOA | CRISPRi and genome deletion (C. acetobutylicum = 20 bp) (C. beijerinckii = 20–1149 bp) | [279] | ||
| C. beijerinckii | pta | Genome modifications and single-nucleotide modification, deletion, and insertion | [280] | ||
| C. beijerinckii | Amylase gene | CRISPRi and genetic manipulation (up to 97%) | [281] | ||
| C. botulinum | Genome alteration and CRISPR-system presence analysis | [282] | |||
| C. cellulolyticum | afp | Genome editing and gene deletion and integration | [283] | ||
| C. difficile | Multiple genome-editing applications | [284] | |||
| C. difficile JIR8094 | selD | Genome editing and ~20–50% site-specific mutations | [285] | ||
| C. saccharoperbutylacetonicum N1–4 | pta and buk | Genomic modifications, gene deletions (~75%), and butanol production | [286] | ||
| C. pasteurianum | cpa | Genome editing and gene deletion and insertion | [287] | ||
| Corynebacterium | Gram + | ||||
| C. glutamicum | glgC, idsA, gltA, and pyc | CRISPRi | [288] | ||
| C. glutamicum | pyk and ldhA | Base editor at different loci | [289] | ||
| C. glutamicum | ldhA | Genome modification, gene deletion and insertion (~60%), and 80% gene modification | [290] | ||
| C. glutamicum | crtYf | Genome editing and 86–100% successful deletions | [291] | ||
| C. glutamicum | clpX, mepA, and porB | Genome editing, deletion, insertion, and point mutation | [292] | ||
| C. glutamicum ATCC 13032 | argR, gabT, and gabP | Genome editing and gene knock-out for gamma-aminobutyric acid (GABA) over-production | [293] | ||
| C. glutamicum | pgi, pck, and pyk | CRISPRi (~98%) | [294] | ||
| Escherichia | Gram – | ||||
| E. coli | talB, tktA, xylA, and xylB | Genetic manipulation, CRISPR, and enhanced xylose production | [295] | ||
| E. coli | sad1, sdhA, sdhB, sucD, and sucC | CRISPRi | [296] | ||
| E. coli | aroA | Gene replacements and insertions, point mutations, and deletions | [297] | ||
| E. coli | norVW | Programmable DNA looping | [298] | ||
| E. coli | galK, lacZ, and pyrF | Genome editing and simultaneous integration of 03 heterologous genes | [299] | ||
| E. coli | ackA, adhE, ldhA, maeA, and pta | CRISPRi and increased malate production | [300] | ||
| E. coli | lacZ | Genome editing, and gene replacement and insertions | [301] | ||
| E. coli | gltA, cat1, sucD, 4hbd, cat2, bld, and bdh | CRISPRI, gene knock-out and knock-in, and 1,4-butanediol production | [302] | ||
| E. coli | gltA | CRISPRi, genome modification, and n-butanol production | [303] | ||
| E. coli | arcAB and cpxR | CRISPR-dCas9-based gene repression and multiple gene regulation | [304] | ||
| E. coli | soxR | Genome engineering | [250] | ||
| E. coli | sul1 | CRISPRi | [251] | ||
| E. coli | AcrA, AcrB, and TolC | CRISPRi | [252] | ||
| E. coli | luxS | CRISPRi | [253] | ||
| Enterobacter | Gram – | ||||
| E. hormaechei 34978 and E. xiangfangensis 34399 | blaKPC-3 | Genome modifications and gene deletions | [243] | ||
| E. hormaechei 4962 | blaTEM-1 | Genome editing and gene manipulation | [234] | ||
| Enterococcus | Gram + | ||||
| E. faecium E745 | msrC | Genome editing | [305] | ||
| E. faecalis T11 | pCF10 | CRISPR based genome editing | [306] | ||
| E. faecalis V583 | pCF10 | Genome manipulation | [307] | ||
| E. faecalis CK135 and E. faecalis OG1SSp | tetM and ermB | Genome editing | [242] | ||
| E. faecalis | croR and ebpA | CRISPRi and gene inactivation and silencing | [254] | ||
| Klebsiella | Gram – | ||||
| K. pneumoniae Y4 | mgrB | Genome modification and gene inactivation | [308] | ||
| K. pneumoniae Y17 | tetA and ramR | Genome modification and gene inactivation | [308] | ||
| K. pneumoniae Kp97_58 and K. pneumoniae 13001 | blaKPC-2 | Genome modification and gene deletion | [243] | ||
| K. pneumoniae 492110 and K. pneumoniae 5193 | blaOXA-48 and blaOXA-48-like | Genome modification and gene deletion | [243] | ||
| K. pneumoniae 3744 and 5573 | pyrF, fepB, ramA, fosA, and fepB | Genetic manipulation using site-specific base editing | [229] | ||
| K. pneumoniae KPCRE23 | blaKPC-2, blaSHV, and blaCTX-M-65 | Genetic manipulation using site-specific base editing | [229] | ||
| Lactobacilli | Gram + | ||||
| L. casei | LC2W_1326, LC2W_1628, and LC2W_2189 | Genome editing and gene deletions and integrations up to 25–60% | [309] | ||
| L. gassen | CRISPR-Cas activity analysis in multiple strains | [310] | |||
| L. reuteri | Efficient site-specific base alterations 90–100% | [311] | |||
| Mycobacterium | Gram + | ||||
| M. tuberculosis | pknB and sigH | CRISPRi and genetic modifications | [312] | ||
| M. tuberculosis | sigA | CRISPRi and single/multiple targeted genetic modifications | [313] | ||
| M. tuberculosis | Sth1 | CRISPRi and gene inactivation | [314] | ||
| Pseudomonas | Gram – | ||||
| P. aeruginosa PAO1 and P. aeruginosa PAK | rhlB, rhlR, and prtR | [315] | |||
| P. aeruginosa PA154197 | mexB, mexF, mexH, mexR, mexT, and gyrA | [138] | |||
| P. aeruginosa PAO1 and P. aeruginosa PAK | algR, lasR, nalD, rhlB, rhlR, and rsaL | [225] | |||
| P. putida KT2440 | ldhL | CRISPRi-based genome editing | [273] | ||
| P. fluorescens Pf0-1, SBW25, and WH6 | mNG, ftsZ, and mreB | CRISPRi and gene silencing | [255] | ||
| P. aeruginosa, P. putida, and P. fluorescens | ftsZ | CRISPRi-based genome editing | [316] | ||
| Staphylococcus | Gram + | Genome editing and gene inactivation | |||
| S. aureus | agrA, cntA, and esaD | Genome modification and base editing | [317] | ||
| S. aureus RN4220 | ermR and mecA | Genome editing and gene deletions | [318] | ||
| S. aureus | rfp | Genome alteration and gene knock-out, insertion, knock-in, and single-base editing | [319] | ||
| S. aureus CCARM, 3798, 3803, and 3877 | mecA | [237] | |||
| S. aureus 6538-GFP | nuc | [320] | |||
| S. aureus AH1 | mec | Type III-A CRISPR-Cas system for gene editing | [321] | ||
| S. aureus ATCC 29213 | rpoB | Genome modifications and gene deletions | [322] | ||
| S. aureus USA300, USA300-∆mecA and RN4220 | mecA | Genome editing and gene inactivation | [5] | ||
| S. aureus USA300φ and S. aureus RNφ | mecA | Genome editing | [224] | ||
| S. aureus ATCC 6538 | tarH, tarG, and tarO | Genome alteration and gene knock-out | [228] | ||
| S. aureus CTH96 | Nuc | Genome editing and genetic manipulation and deletion | [323] | ||
| Streptomyces | Gram + | ||||
| Streptomyces | Multiple genes | Multiplex gene disruption | [324] | ||
| S. coelicolor | Genome editing and gene knocked-outs | [325] | |||
| S. lividans, S. albus, S. roseosporus, S. venezuelae, and S. viridochromogenes | Biosynthetic gene clusters (BGCs) | Multiple genome editing and gene knock-in and gene insertion | [326] | ||
| S. coelicolor M145 | actI-ORF2 | Genome editing and gene deletion (~900 bp) | [327] | ||
| S. avermitilis | Ac(3)Ⅳ | Genomic disruption using Type I-E CRSIPR-Cas system | [328] | ||
| S. rimosus | zwf2 and devB | Genome editing, gene deletions, point mutations, and oxytetracycline production | [329] | ||
| S. lividans, S. viridochromogenes, and S. albus | sshg_05713 | Multiple genome editing and genome deletion (20 bp–30 kb) | [330] | ||
| S. coelicolor A3(2) | actIORF1 (SCO5087) and actVB (SCO5092) | CRISPRi and gene deletion | [261] | ||
| S. coelicolor | actII-orf4, redD, and glnR | Genome editing and single- and multiple-gene deletions | [331] | ||
| Synechococcus | Gram – | ||||
| S. elongatus UTEX 2973 | nbla | Genome editing and gene deletion | [332] | ||
7.3. CRISPR-Cas System in A. baumannii
7.4. Recent Studies on the Application of the CRISPR-Cas System in A. baumannii
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zohra, T.; Numan, M.; Ikram, A.; Salman, M.; Khan, T.; Din, M.; Salman, M.; Farooq, A.; Amir, A.; Ali, M. Cracking the challenge of antimicrobial drug resistance with CRISPR/Cas9, nanotechnology and other strategies in ESKAPE pathogens. Microorganisms 2021, 9, 954. [Google Scholar] [CrossRef] [PubMed]
- Jansen, K.U.; Knirsch, C.; Anderson, A.S. The role of vaccines in preventing bacterial antimicrobial resistance. Nat. Med. 2018, 24, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.L.; Fajardo, A.; Garmendia, L.; Hernandez, A.; Linares, J.F.; Martínez-Solano, L.; Sánchez, M.B. A global view of antibiotic resistance. FEMS Microbiol. Rev. 2008, 33, 44–65. [Google Scholar] [CrossRef] [PubMed]
- Hutchings, M.I.; Truman, A.W.; Wilkinson, B. Antibiotics: Past, present and future. Curr. Opin. Microbiol. 2019, 51, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Kiga, K.; Tan, X.-E.; Ibarra-Chávez, R.; Watanabe, S.; Aiba, Y.; Sato’o, Y.; Li, F.-Y.; Sasahara, T.; Cui, B.; Kawauchi, M. Development of CRISPR-Cas13a-based antimicrobials capable of sequence-specific killing of target bacteria. Nat. Commun. 2020, 11, 2934. [Google Scholar] [CrossRef]
- Eze, E.C.; Chenia, H.Y.; El Zowalaty, M.E. Acinetobacter baumannii biofilms: Effects of physicochemical factors, virulence, antibiotic resistance determinants, gene regulation, and future antimicrobial treatments. Infect. Drug Resist. 2018, 11, 2277. [Google Scholar] [CrossRef]
- Mahamat, A.; Bertrand, X.; Moreau, B.; Hommel, D.; Couppie, P.; Simonnet, C.; Kallel, H.; Demar, M.; Djossou, F.; Nacher, M. Clinical epidemiology and resistance mechanisms of carbapenem-resistant Acinetobacter baumannii, French Guiana, 2008–2014. Int. J. Antimicrob. Agents 2016, 48, 51–55. [Google Scholar] [CrossRef]
- Diancourt, L.; Passet, V.; Nemec, A.; Dijkshoorn, L.; Brisse, S. The population structure of Acinetobacter baumannii: Expanding multiresistant clones from an ancestral susceptible genetic pool. PLoS ONE 2010, 5, e10034. [Google Scholar] [CrossRef]
- Zarrilli, R.; Pournaras, S.; Giannouli, M.; Tsakris, A. Global evolution of multidrug-resistant Acinetobacter baumannii clonal lineages. Int. J. Antimicrob. Agents 2013, 41, 11–19. [Google Scholar] [CrossRef]
- de la Fuente-Nunez, C.; Torres, M.D.; Mojica, F.J.; Lu, T.K. Next-generation precision antimicrobials: Towards personalized treatment of infectious diseases. Curr. Opin. Microbiol. 2017, 37, 95–102. [Google Scholar] [CrossRef]
- Morris, F.C.; Dexter, C.; Kostoulias, X.; Uddin, M.I.; Peleg, A.Y. The mechanisms of disease caused by Acinetobacter baumannii. Front. Microbiol. 2019, 10, 1601. [Google Scholar] [CrossRef]
- Lim, S.M.S.; Abidin, A.Z.; Liew, S.; Roberts, J.; Sime, F. The global prevalence of multidrug-resistance among Acinetobacter baumannii causing hospital-acquired and ventilator-associated pneumonia and its associated mortality: A systematic review and meta-analysis. J. Infect. 2019, 79, 593–600. [Google Scholar]
- Dexter, C.; Murray, G.L.; Paulsen, I.T.; Peleg, A.Y. Community-acquired Acinetobacter baumannii: Clinical characteristics, epidemiology and pathogenesis. Expert Rev. Anti-Infect. Ther. 2015, 13, 567–573. [Google Scholar] [CrossRef]
- Fournier, P.E.; Richet, H.; Weinstein, R.A. The epidemiology and control of Acinetobacter baumannii in health care facilities. Clin. Infect. Dis. 2006, 42, 692–699. [Google Scholar] [CrossRef]
- Wisplinghoff, H. Pseudomonas spp., Acinetobacter spp. and miscellaneous Gram-negative bacilli. In Infectious Diseases; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1579–1599.e1572. [Google Scholar]
- Metan, G.; Alp, E.; Aygen, B.; Sumerkan, B. Acinetobacter baumannii meningitis in post-neurosurgical patients: Clinical outcome and impact of carbapenem resistance. J. Antimicrob. Chemother. 2007, 60, 197–199. [Google Scholar] [CrossRef]
- Chung, D.R.; Song, J.H.; Kim, S.H.; Thamlikitkul, V.; Huang, S.G.; Wang, H.; So, T.M.k.; Yasin, R.M.; Hsueh, P.R.; Carlos, C.C. High prevalence of multidrug-resistant nonfermenters in hospital-acquired pneumonia in Asia. Am. J. Respir. Crit. Care Med. 2011, 184, 1409–1417. [Google Scholar] [CrossRef]
- Santimaleeworagun, W.; Sumret, W.; Likitmongkonsuk, K.; Noo-in, P.; Cheeaboonkana, P.; Suphannavej, A.; Suphanklang, J.; Saelim, W. Treatment Outcomes and Risk Factors Related to Mortality and Treatment Failure of Patients Infected with Acinetobacter baumannii at a General Hospital. BKK Med. J. 2019, 15, 154. [Google Scholar] [CrossRef]
- Wong, D.; Nielsen, T.B.; Bonomo, R.A.; Pantapalangkoor, P.; Luna, B.; Spellberg, B. Clinical and pathophysiological overview of Acinetobacter infections: A century of challenges. Clin. Microbiol. Rev. 2017, 30, 409–447. [Google Scholar] [CrossRef]
- Rafei, R.; Dabboussi, F.; Hamze, M.; Eveillard, M.; Lemarié, C.; Mallat, H.; Rolain, J.M.; Joly-Guillou, M.L.; Kempf, M. First report of blaNDM-1-producing Acinetobacter baumannii isolated in Lebanon from civilians wounded during the Syrian war. Int. J. Infect. Dis. 2014, 21, 21–23. [Google Scholar] [CrossRef]
- Tao, C.; Kang, M.; Chen, Z.; Xie, Y.; Fan, H.; Qin, L.; Ma, Y. Microbiologic study of the pathogens isolated from wound culture among Wenchuan earthquake survivors. Diagn. Microbiol. Infect. Dis. 2009, 63, 268–270. [Google Scholar] [CrossRef]
- Masuda, N.; Sakagawa, E.; Ohya, S.; Gotoh, N.; Tsujimoto, H.; Nishino, T. Contribution of the MexX-MexY-OprM efflux system to intrinsic resistance in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2000, 44, 2242–2246. [Google Scholar] [CrossRef] [PubMed]
- Randall, C.P.; Mariner, K.R.; Chopra, I.; O’Neill, A.J. The target of daptomycin is absent from Escherichia coli and other gram-negative pathogens. Antimicrob. Agents Chemother. 2013, 57, 637–639. [Google Scholar] [CrossRef] [PubMed]
- Kostyanev, T.; Can, F. The global crisis of antimicrobial resistance. In Antimicrobial Stewardship; Elsevier: Amsterdam, The Netherlands, 2017; pp. 3–12. [Google Scholar]
- Pang, Z.; Raudonis, R.; Glick, B.R.; Lin, T.-J.; Cheng, Z. Antibiotic resistance in Pseudomonas aeruginosa: Mechanisms and alternative therapeutic strategies. Biotechnol. Adv. 2019, 37, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Salimiyan-Rizi, K.; Noghondar, M. Adaptive antibiotic resistance: Overview and perspectives. J. Infect. Dis. Ther. 2018, 6, 363. [Google Scholar] [CrossRef]
- Tenover, F.C. Mechanisms of antimicrobial resistance in bacteria. Am. J. Med. 2006, 119, S3–S10. [Google Scholar] [CrossRef]
- Blair, J.M.; Webber, M.A.; Baylay, A.J.; Ogbolu, D.O.; Piddock, L.J. Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol. 2015, 13, 42–51. [Google Scholar] [CrossRef]
- Ferenci, T.; Phan, K. How porin heterogeneity and trade-offs affect the antibiotic susceptibility of Gram-negative bacteria. Genes 2015, 6, 1113–1124. [Google Scholar] [CrossRef]
- Reygaert, W.C. An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol. 2018, 4, 482. [Google Scholar] [CrossRef]
- Wassef, M.; Abdelhaleim, M.; AbdulRahman, E.; Ghaith, D. The role of OmpK35, OmpK36 porins, and production of β-lactamases on imipenem susceptibility in Klebsiella pneumoniae clinical isolates, Cairo, Egypt. Microb. Drug Resist. 2015, 21, 577–580. [Google Scholar] [CrossRef]
- Sandoval-Motta, S.; Aldana, M. Adaptive resistance to antibiotics in bacteria: A systems biology perspective. Wiley Interdiscip. Rev. Syst. Biol. Med. 2016, 8, 253–267. [Google Scholar] [CrossRef]
- Fernández, L.; Breidenstein, E.B.; Hancock, R.E. Creeping baselines and adaptive resistance to antibiotics. Drug Resist. Updat. 2011, 14, 1–21. [Google Scholar] [CrossRef]
- Kang, I.B.; Seo, K.H. Variation of antibiotic resistance in Salmonella Enteritidis, Escherichia coli O157: H7, and Listeria monocytogenes after exposure to acid, salt, and cold stress. J. Food Saf. 2020, 40, e12804. [Google Scholar] [CrossRef]
- Ayoub Moubareck, C.; Hammoudi Halat, D. Insights into Acinetobacter baumannii: A review of microbiological, virulence, and resistance traits in a threatening nosocomial pathogen. Antibiotics 2020, 9, 119. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, S.; Kim, Y.; Hong, K.; Wie, S.; Park, Y.; Jeong, H.; Kang, M. Carbapenem-resistant Acinetobacter baumannii: Diversity of resistant mechanisms and risk factors for infection. Epidemiol. Infect. 2012, 140, 137–145. [Google Scholar] [CrossRef]
- Vrancianu, C.O.; Gheorghe, I.; Czobor, I.B.; Chifiriuc, M.C. Antibiotic resistance profiles, molecular mechanisms and innovative treatment strategies of Acinetobacter baumannii. Microorganisms 2020, 8, 935. [Google Scholar] [CrossRef]
- Pakharukova, N.; Tuittila, M.; Paavilainen, S.; Malmi, H.; Parilova, O.; Teneberg, S.; Knight, S.D.; Zavialov, A.V. Structural basis for Acinetobacter baumannii biofilm formation. Proc. Natl. Acad. Sci. USA 2018, 115, 5558–5563. [Google Scholar] [CrossRef]
- Yang, C.-H.; Su, P.-W.; Moi, S.-H.; Chuang, L.-Y. Biofilm formation in Acinetobacter baumannii: Genotype-phenotype correlation. Molecules 2019, 24, 1849. [Google Scholar] [CrossRef]
- Esterly, J.S.; Richardson, C.L.; Eltoukhy, N.S.; Qi, C.; Scheetz, M.H. Genetic mechanisms of antimicrobial resistance of Acinetobacter baumannii. Ann. Pharmacother. 2011, 45, 218–228. [Google Scholar] [CrossRef]
- Ravasi, P.; Limansky, A.S.; Rodriguez, R.E.; Viale, A.M.; Mussi, M.A. ISAba825, a functional insertion sequence modulating genomic plasticity and blaOXA-58 expression in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2011, 55, 917–920. [Google Scholar] [CrossRef]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.; Giske, C.; Harbarth, S.; Hindler, J.; Kahlmeter, G.; Olsson-Liljequist, B. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Nordmann, P.; Poirel, L. Epidemiology and diagnostics of carbapenem resistance in gram-negative bacteria. Clin. Infect. Dis. 2019, 69, S521–S528. [Google Scholar] [CrossRef] [PubMed]
- Piperaki, E.-T.; Tzouvelekis, L.; Miriagou, V.; Daikos, G. Carbapenem-resistant Acinetobacter baumannii: In pursuit of an effective treatment. Clin. Microbiol. Infect. 2019, 25, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, S.; Khyriem, A.B.; Bhattacharya, P.; Bhattacharjee, A.; Joshi, S.R. High-level aminoglycoside resistance in Acinetobacter baumannii recovered from Intensive Care Unit patients in Northeastern India. Indian J. Med. Microbiol. 2018, 36, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Basatian-Tashkan, B.; Niakan, M.; Khaledi, M.; Afkhami, H.; Sameni, F.; Bakhti, S.; Mirnejad, R. Antibiotic resistance assessment of Acinetobacter baumannii isolates from Tehran hospitals due to the presence of efflux pumps encoding genes (adeA and adeS genes) by molecular method. BMC Res. Notes 2020, 13, 543. [Google Scholar] [CrossRef]
- Kyriakidis, I.; Vasileiou, E.; Pana, Z.D.; Tragiannidis, A. Acinetobacter baumannii antibiotic resistance mechanisms. Pathogens 2021, 10, 373. [Google Scholar] [CrossRef]
- Martínez-Trejo, A.; Ruiz-Ruiz, J.M.; Gonzalez-Avila, L.U.; Saldaña-Padilla, A.; Hernández-Cortez, C.; Loyola-Cruz, M.A.; Bello-López, J.M.; Castro-Escarpulli, G. Evasion of Antimicrobial Activity in Acinetobacter baumannii by Target Site Modifications: An Effective Resistance Mechanism. Int. J. Mol. Sci. 2022, 23, 6582. [Google Scholar] [CrossRef]
- Araujo Lima, A.V.; da Silva, S.M.; do Nascimento Júnior, J.A.A.; Correia, M.d.S.; Luz, A.C.; Leal-Balbino, T.C.; da Silva, M.V.; Lima, J.L.d.C.; Maciel, M.A.V.; Napoleao, T.H. Occurrence and diversity of intra-and interhospital drug-resistant and biofilm-forming Acinetobacter baumannii and Pseudomonas aeruginosa. Microb. Drug Resist. 2020, 26, 802–814. [Google Scholar] [CrossRef]
- Arhoune, B.; Oumokhtar, B.; Hmami, F.; El Fakir, S.; Moutaouakkil, K.; Chami, F.; Bouharrou, A. Intestinal carriage of antibiotic resistant Acinetobacter baumannii among newborns hospitalized in Moroccan neonatal intensive care unit. PLoS ONE 2019, 14, e0209425. [Google Scholar] [CrossRef]
- D’Onofrio, V.; Conzemius, R.; Varda-Brkić, D.; Bogdan, M.; Grisold, A.; Gyssens, I.C.; Bedenić, B.; Barišić, I. Epidemiology of colistin-resistant, carbapenemase-producing Enterobacteriaceae and Acinetobacter baumannii in Croatia. Infect. Genet. Evol. 2020, 81, 104263. [Google Scholar] [CrossRef]
- Makke, G.; Bitar, I.; Salloum, T.; Panossian, B.; Alousi, S.; Arabaghian, H.; Medvecky, M.; Hrabak, J.; Merheb-Ghoussoub, S.; Tokajian, S. Whole-genome-sequence-based characterization of extensively drug-resistant Acinetobacter baumannii hospital outbreak. mSphere 2020, 5, e00934-19. [Google Scholar] [CrossRef]
- Simo Tchuinte, P.L.; Rabenandrasana, M.A.N.; Kowalewicz, C.; Andrianoelina, V.H.; Rakotondrasoa, A.; Andrianirina, Z.Z.; Enouf, V.; Ratsima, E.H.; Randrianirina, F.; Collard, J.-M. Phenotypic and molecular characterisations of carbapenem-resistant Acinetobacter baumannii strains isolated in Madagascar. Antimicrob. Resist. Infect. Control 2019, 8, 31. [Google Scholar] [CrossRef]
- Tooke, C.L.; Hinchliffe, P.; Bragginton, E.C.; Colenso, C.K.; Hirvonen, V.H.; Takebayashi, Y.; Spencer, J. β-lactamases and β-lactamase inhibitors in the 21st century. J. Mol. Biol. 2019, 431, 3472–3500. [Google Scholar] [CrossRef]
- Caneiras, C.; Calisto, F.; Jorge da Silva, G.; Lito, L.; Melo-Cristino, J.; Duarte, A. First description of colistin and tigecycline-resistant Acinetobacter baumannii producing KPC-3 carbapenemase in Portugal. Antibiotics 2018, 7, 96. [Google Scholar] [CrossRef]
- Martinez, T.; Martinez, I.; Vazquez, G.J.; Aquino, E.E.; Robledo, I.E. Genetic environment of the KPC gene in Acinetobacter baumannii ST2 clone from Puerto Rico and genomic insights into its drug resistance. J. Med. Microbiol. 2016, 65, 784. [Google Scholar] [CrossRef]
- Benamrouche, N.; Lafer, O.; Benmahdi, L.; Benslimani, A.; Amhis, W.; Ammari, H.; Assaous, F.; Azzam, A.; Rahal, K.; Tali Maamar, H. Phenotypic and genotypic characterization of multidrug-resistant Acinetobacter baumannii isolated in Algerian hospitals. J. Infect. Dev. Ctries. 2020, 14, 1395–1401. [Google Scholar] [CrossRef]
- Smiline, A.; Vijayashree, J.; Paramasivam, A. Molecular characterization of plasmid-encoded blaTEM, blaSHV and blaCTX-M among extended spectrum β-lactamases [ESBLs] producing Acinetobacter baumannii. Br. J. Biomed. Sci. 2018, 75, 200–202. [Google Scholar] [CrossRef]
- Jeon, J.H.; Lee, J.H.; Lee, J.J.; Park, K.S.; Karim, A.M.; Lee, C.R.; Jeong, B.C.; Lee, S.H. Structural basis for carbapenem-hydrolyzing mechanisms of carbapenemases conferring antibiotic resistance. Int. J. Mol. Sci. 2015, 16, 9654–9692. [Google Scholar] [CrossRef]
- Queenan, A.M.; Bush, K. Carbapenemases: The versatile β-lactamases. Clin. Microbiol. Rev. 2007, 20, 440–458. [Google Scholar] [CrossRef]
- Giuseppe, C.; Helen, G.; Gian-Maria, R. Metallo-β-lactamases: A last frontier for β-lactams. Lancet Infect. Dis. 2011, 11, 381–393. [Google Scholar]
- Amin, M.; Navidifar, T.; Saleh Shooshtari, F.; Goodarzi, H. Association of the genes encoding metallo-β-lactamase with the presence of integrons among multidrug-resistant clinical isolates of Acinetobacter baumannii. Infect. Drug Resist. 2019, 12, 1171–1180. [Google Scholar] [CrossRef]
- López, C.; Ayala, J.A.; Bonomo, R.A.; González, L.J.; Vila, A.J. Protein determinants of dissemination and host specificity of metallo-β-lactamases. Nat. Commun. 2019, 10, 3617. [Google Scholar] [CrossRef] [PubMed]
- Moulana, Z.; Babazadeh, A.; Eslamdost, Z.; Shokri, M.; Ebrahimpour, S. Phenotypic and genotypic detection of metallo-beta-lactamases in Carbapenem resistant Acinetobacter baumannii. Caspian J. Intern. Med. 2020, 11, 171. [Google Scholar] [PubMed]
- Hamidian, M.; Hall, R.M. Tn6168, a transposon carrying an ISAba1-activated ampC gene and conferring cephalosporin resistance in Acinetobacter baumannii. J. Antimicrob. Chemother. 2014, 69, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Lopes, B.; Amyes, S. Role of ISAba1 and ISAba125 in governing the expression of blaADC in clinically relevant Acinetobacter baumannii strains resistant to cephalosporins. J. Med. Microbiol. 2012, 61, 1103–1108. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-R.; Lee, J.H.; Park, M.; Park, K.S.; Bae, I.K.; Kim, Y.B.; Cha, C.-J.; Jeong, B.C.; Lee, S.H. Biology of Acinetobacter baumannii: Pathogenesis, antibiotic resistance mechanisms, and prospective treatment options. Front. Cell. Infect. Microbiol. 2017, 7, 55. [Google Scholar] [CrossRef] [PubMed]
- Chagas, T.P.G.; Carvalho, K.R.; de Oliveira Santos, I.C.; Carvalho-Assef, A.P.D.A.; Asensi, M.D. Characterization of carbapenem-resistant Acinetobacter baumannii in Brazil (2008–2011): Countrywide spread of OXA-23–producing clones (CC15 and CC79). Diagn. Microbiol. Infect. Dis. 2014, 79, 468–472. [Google Scholar] [CrossRef]
- Evans, B.A.; Amyes, S.G. OXA β-lactamases. Clin. Microbiol. Rev. 2014, 27, 241–263. [Google Scholar] [CrossRef]
- Hu, W.S.; Yao, S.-M.; Fung, C.-P.; Hsieh, Y.-P.; Liu, C.-P.; Lin, J.-F. An OXA-66/OXA-51-like carbapenemase and possibly an efflux pump are associated with resistance to imipenem in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2007, 51, 3844–3852. [Google Scholar] [CrossRef]
- Mammina, C.; Bonura, C.; Aleo, A.; Calà, C.; Caputo, G.; Cataldo, M.; Benedetto, A.D.; Distefano, S.; Fasciana, T.; Labisi, M. Characterization of Acinetobacter baumannii from intensive care units and home care patients in Palermo, Italy. Clin. Microbiol. Infect. 2011, 17, E12–E15. [Google Scholar] [CrossRef]
- Pagano, M.; Martins, A.; Machado, A.; Barin, J.; Barth, A. Carbapenem-susceptible Acinetobacter baumannii carrying the ISAba1 upstream blaOXA-51-like gene in Porto Alegre, southern Brazil. Epidemiol. Infect. 2013, 141, 330–333. [Google Scholar] [CrossRef]
- Pajand, O.; Hojabri, Z.; Nahaei, M.R.; Hajibonabi, F.; Pirzadeh, T.; Aghazadeh, M.; Fasciana, T.; Bonura, C.; Mammina, C. In vitro activities of tetracyclines against different clones of multidrug-resistant Acinetobacter baumannii isolates from two Iranian hospitals. Int. J. Antimicrob. Agents 2014, 43, 476–478. [Google Scholar] [CrossRef]
- Wong, M.H.Y.; Chan, B.K.W.; Chan, E.W.C.; Chen, S. Over-expression of ISAba1-linked intrinsic and exogenously acquired OXA type carbapenem-hydrolyzing-class D-β-lactamase-encoding genes is key mechanism underlying carbapenem resistance in Acinetobacter baumannii. Front. Microbiol. 2019, 10, 2809. [Google Scholar] [CrossRef]
- Xu, C.; Bilya, S.; Xu, W. adeABC efflux gene in Acinetobacter baumannii. New Microbes New Infect. 2019, 30, 100549. [Google Scholar] [CrossRef]
- Ramirez, M.S.; Tolmasky, M.E. Aminoglycoside modifying enzymes. Drug Resist. Updat. 2010, 13, 151–171. [Google Scholar] [CrossRef]
- Foong, W.E.; Wilhelm, J.; Tam, H.-K.; Pos, K.M. Tigecycline efflux in Acinetobacter baumannii is mediated by TetA in synergy with RND-type efflux transporters. J. Antimicrob. Chemother. 2020, 75, 1135–1139. [Google Scholar] [CrossRef]
- He, T.; Wang, R.; Liu, D.; Walsh, T.R.; Zhang, R.; Lv, Y.; Ke, Y.; Ji, Q.; Wei, R.; Liu, Z. Emergence of plasmid-mediated high-level tigecycline resistance genes in animals and humans. Nat. Microbiol. 2019, 4, 1450–1456. [Google Scholar] [CrossRef]
- Li, L.; Hassan, K.A.; Tetu, S.G.; Naidu, V.; Pokhrel, A.; Cain, A.K.; Paulsen, I.T. The transcriptomic signature of tigecycline in Acinetobacter baumannii. Front. Microbiol. 2020, 11, 565438. [Google Scholar] [CrossRef]
- Savari, M.; Ekrami, A.; Shoja, S.; Bahador, A. Plasmid borne carbapenem-hydrolyzing class D β-lactamases (CHDLs) and AdeABC efflux pump conferring carbapenem-tigecycline resistance among Acinetobacter baumannii isolates harboring TnAbaRs. Microb. Pathog. 2017, 104, 310–317. [Google Scholar] [CrossRef]
- Wang, L.; Liu, D.; Lv, Y.; Cui, L.; Li, Y.; Li, T.; Song, H.; Hao, Y.; Shen, J.; Wang, Y. Novel plasmid-mediated tet(X5) gene conferring resistance to tigecycline, eravacycline, and omadacycline in a clinical Acinetobacter baumannii isolate. Antimicrob. Agents Chemother. 2019, 64, e01326-19. [Google Scholar] [CrossRef]
- Cho, Y.J.; Moon, D.C.; Jin, J.S.; Choi, C.H.; Lee, Y.C.; Lee, J.C. Genetic basis of resistance to aminoglycosides in Acinetobacter spp. and spread of armA in Acinetobacter baumannii sequence group 1 in Korean hospitals. Diagn. Microbiol. Infect. Dis. 2009, 64, 185–190. [Google Scholar] [CrossRef]
- Vázquez-López, R.; Solano-Gálvez, S.G.; Juárez Vignon-Whaley, J.J.; Abello Vaamonde, J.A.; Padró Alonzo, L.A.; Rivera Reséndiz, A.; Muleiro Álvarez, M.; Vega López, E.N.; Franyuti-Kelly, G.; Álvarez-Hernández, D.A. Acinetobacter baumannii resistance: A real challenge for clinicians. Antibiotics 2020, 9, 205. [Google Scholar] [CrossRef] [PubMed]
- Zaki, M.E.S.; Abou ElKheir, N.; Mofreh, M. Molecular study of quinolone resistance determining regions of gyrA gene and parC genes in clinical isolates of Acintobacter baumannii resistant to fluoroquinolone. Open Microbiol. J. 2018, 12, 116. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Yang, S.; Jia, M.; Zhao, L.; Hou, C.; You, X.; Zhao, J.; Chen, A. Comparative study between macrolide regulatory proteins MphR (A) and MphR (E) in ligand identification and DNA binding based on the rapid in vitro detection system. Anal. Bioanal. Chem. 2016, 408, 1623–1631. [Google Scholar] [CrossRef] [PubMed]
- Lima, W.G.; Alves, M.C.; Cruz, W.S.; Paiva, M.C. Chromosomally encoded and plasmid-mediated polymyxins resistance in Acinetobacter baumannii: A huge public health threat. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 1009–1019. [Google Scholar] [CrossRef] [PubMed]
- Potron, A.; Poirel, L.; Croizé, J.; Chanteperdrix, V.; Nordmann, P. Genetic and biochemical characterization of the first extended-spectrum Carb-type β-lactamase, RTG-4, from Acinetobacter baumannii. Antimicrob. Agents Chemother. 2009, 53, 3010–3016. [Google Scholar] [CrossRef] [PubMed]
- Freitas, D.Y.; Araújo, S.; Folador, A.R.; Ramos, R.T.; Azevedo, J.S.; Tacão, M.; Silva, A.; Henriques, I.; Baraúna, R.A. Extended spectrum beta-lactamase-producing gram-negative bacteria recovered from an Amazonian lake near the city of Belém, Brazil. Front. Microbiol. 2019, 10, 364. [Google Scholar] [CrossRef]
- Gupta, R.; Malik, A.; Rizvi, M.; Ahmed, M. Presence of metallo-beta-lactamases (MBL), extended-spectrum beta-lactamase (ESBL) & AmpC positive non-fermenting Gram-negative bacilli among Intensive Care Unit patients with special reference to molecular detection of blaCTX-M & blaAmpC genes. Indian J. Med. Res. 2016, 144, 271. [Google Scholar]
- Mayanskiy, N.; Chebotar, I.; Alyabieva, N.; Kryzhanovskaya, O.; Savinova, T.; Turenok, A.; Bocharova, Y.; Lazareva, A.; Polikarpova, S.; Karaseva, O. Emergence of the uncommon clone ST944/ST78 carrying blaOXA-40-like and blaCTX-M-like genes among carbapenem-nonsusceptible Acinetobacter baumannii in Moscow, Russia. Microb. Drug Resist. 2017, 23, 864–870. [Google Scholar] [CrossRef]
- Al-Hassan, L.; El Mahallawy, H.; Amyes, S. First report of bla (PER-3) in Acinetobacter baumannii. Int. J. Antimicrob. Agents 2012, 41, 93–94. [Google Scholar] [CrossRef]
- Aly, M.; Abu Alsoud, N.; Elrobh, M.; Al Johani, S.; Balkhy, H. High prevalence of the PER-1 gene among carbapenem-resistant Acinetobacter baumannii in Riyadh, Saudi Arabia. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 1759–1766. [Google Scholar] [CrossRef]
- Bonnin, R.A.; Nordmann, P.; Potron, A.; Lecuyer, H.; Zahar, J.-R.; Poirel, L. Carbapenem-hydrolyzing GES-type extended-spectrum β-lactamase in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2011, 55, 349–354. [Google Scholar] [CrossRef]
- Alkasaby, N.M.; El Sayed Zaki, M. Molecular study of Acinetobacter baumannii isolates for metallo-β-lactamases and extended-spectrum-β-lactamases genes in intensive care unit, Mansoura University Hospital, Egypt. Int. J. Microbiol. 2017, 2017, 3925868. [Google Scholar] [CrossRef]
- Naas, T.; Namdari, F.; Réglier-Poupet, H.; Poyart, C.; Nordmann, P. Panresistant extended-spectrum β-lactamase SHV-5-producing Acinetobacter baumannii from New York City. J. Antimicrob. Chemother. 2007, 60, 1174–1176. [Google Scholar] [CrossRef]
- Huang, L.-Y.; Chen, T.-L.; Lu, P.-L.; Tsai, C.-A.; Cho, W.-L.; Chang, F.-Y.; Fung, C.-P.; Siu, L. Dissemination of multidrug-resistant, class 1 integron-carrying Acinetobacter baumannii isolates in Taiwan. Clin. Microbiol. Infect. 2008, 14, 1010–1019. [Google Scholar] [CrossRef]
- Poirel, L.; Mugnier, P.D.; Toleman, M.A.; Walsh, T.R.; Rapoport, M.J.; Petroni, A.; Nordmann, P. IS CR2, another vehicle for bla VEB gene acquisition. Antimicrob. Agents Chemother. 2009, 53, 4940–4943. [Google Scholar] [CrossRef]
- Safari, M.; Nejad, A.S.M.; Bahador, A.; Jafari, R.; Alikhani, M.Y. Prevalence of ESBL and MBL encoding genes in Acinetobacter baumannii strains isolated from patients of intensive care units (ICU). Saudi J. Biol. Sci. 2015, 22, 424–429. [Google Scholar] [CrossRef]
- Abdar, M.H.; Taheri-Kalani, M.; Taheri, K.; Emadi, B.; Hasanzadeh, A.; Sedighi, A.; Pirouzi, S.; Sedighi, M. Prevalence of extended-spectrum beta-lactamase genes in Acinetobacter baumannii strains isolated from nosocomial infections in Tehran, Iran. GMS Infect. Dis. 2019, 14, Doc02. [Google Scholar]
- Agoba, E.E.; Govinden, U.; Peer, A.K.C.; Osei Sekyere, J.; Essack, S.Y. ISAba1 regulated OXA-23 carbapenem resistance in Acinetobacter baumannii strains in durban, South Africa. Microb. Drug Resist. 2018, 24, 1289–1295. [Google Scholar] [CrossRef]
- Asgin, N.; Otlu, B.; Cakmakliogullari, E.K.; Celik, B. High prevalence of TEM, VIM, and OXA-2 beta-lactamases and clonal diversity among Acinetobacter baumannii isolates in Turkey. J. Infect. Dev. Ctries. 2019, 13, 794–801. [Google Scholar] [CrossRef]
- Poirel, L.; Corvec, S.; Rapoport, M.; Mugnier, P.; Petroni, A.; Pasteran, F.; Faccone, D.; Galas, M.; Drugeon, H.; Cattoir, V. Identification of the novel narrow-spectrum β-lactamase SCO-1 in Acinetobacter spp. from Argentina. Antimicrob. Agents Chemother. 2007, 51, 2179–2184. [Google Scholar] [CrossRef]
- Al-Agamy, M.H.; Jeannot, K.; El-Mahdy, T.S.; Shibl, A.M.; Kattan, W.; Plésiat, P.; Courvalin, P. First detection of GES-5 carbapenemase-producing Acinetobacter baumannii isolate. Microb. Drug Resist. 2017, 23, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Hammoudi, D.; Moubareck, C.A.; Hakime, N.; Houmani, M.; Barakat, A.; Najjar, Z.; Suleiman, M.; Fayad, N.; Sarraf, R.; Sarkis, D.K. Spread of imipenem-resistant Acinetobacter baumannii co-expressing OXA-23 and GES-11 carbapenemases in Lebanon. Int. J. Infect. Dis. 2015, 36, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Mabrouk, A.; Grosso, F.; Botelho, J.; Achour, W.; Ben Hassen, A.; Peixe, L. GES-14-producing Acinetobacter baumannii isolates in a neonatal intensive care unit in Tunisia are associated with a typical Middle East clone and a transferable plasmid. Antimicrob. Agents Chemother. 2017, 61, e00142-17. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, P.C.S.; Monteiro, A.S.; Marques, S.G.; Monteiro, S.G.; Monteiro-Neto, V.; Coqueiro, M.M.M.; Marques, A.C.G.; de Jesus Gomes Turri, R.; Santos, S.G.; Bomfim, M.R.Q. Phenotypic and molecular detection of the blaKPC gene in clinical isolates from inpatients at hospitals in São Luis, MA, Brazil. BMC Infect. Dis. 2016, 16, 737. [Google Scholar] [CrossRef]
- Pollini, S.; Maradei, S.; Pecile, P.; Olivo, G.; Luzzaro, F.; Docquier, J.-D.; Rossolini, G.M. FIM-1, a new acquired metallo-β-lactamase from a Pseudomonas aeruginosa clinical isolate from Italy. Antimicrob. Agents Chemother. 2013, 57, 410–416. [Google Scholar] [CrossRef]
- Girija, S.A.; Jayaseelan, V.P.; Arumugam, P. Prevalence of VIM-and GIM-producing Acinetobacter baumannii from patients with severe urinary tract infection. Acta Microbiol. Immunol. Hung. 2018, 65, 539–550. [Google Scholar] [CrossRef]
- Cayô, R.; Rodrigues-Costa, F.; Matos, A.P.; Carvalhaes, C.G.; Jové, T.; Gales, A.C. Identification of a new integron harboring blaIMP-10 in carbapenem-resistant Acinetobacter baumannii clinical isolates. Antimicrob. Agents Chemother. 2015, 59, 3687–3689. [Google Scholar] [CrossRef]
- Shakibaie, M.R.; Azizi, O.; Shahcheraghi, F. Insight into stereochemistry of a new IMP allelic variant (IMP-55) metallo-β-lactamase identified in a clinical strain of Acinetobacter baumannii. Infect. Genet. Evol. 2017, 51, 118–126. [Google Scholar] [CrossRef]
- Bonnin, R.; Poirel, L.; Naas, T.; Pirs, M.; Seme, K.; Schrenzel, J.; Nordmann, P. Dissemination of New Delhi metallo-β-lactamase-1-producing Acinetobacter baumannii in Europe. Clin. Microbiol. Infect. 2012, 18, E362–E365. [Google Scholar] [CrossRef]
- Kumar, M. Identification of a novel NDM variant, blaNDM-3, from a multidrug-resistant Acinetobacter baumannii. Infect. Control Hosp. Epidemiol. 2016, 37, 747–748. [Google Scholar] [CrossRef]
- Voulgari, E.; Politi, L.; Pitiriga, V.; Dendrinos, J.; Poulou, A.; Georgiadis, G.; Tsakris, A. First report of an NDM-1 metallo-β-lactamase-producing Acinetobacter baumannii clinical isolate in Greece. Int. J. Antimicrob. Agents 2016, 6, 761–762. [Google Scholar] [CrossRef]
- Gholami, M.; Moshiri, M.; Ahanjan, M.; Salimi Chirani, A.; Hasannejad Bibalan, M.; Asadi, A.; Eshaghi, M.; Pournajaf, A.; Abbasian, S.; Kouhsari, E.; et al. The diversity of class B and class D carbapenemases in clinical Acinetobacter baumannii isolates. Infez. Med. 2018, 26, 329–335. [Google Scholar]
- Toleman, M.A.; Simm, A.M.; Murphy, T.A.; Gales, A.C.; Biedenbach, D.J.; Jones, R.N.; Walsh, T.R. Molecular characterization of SPM-1, a novel metallo-β-lactamase isolated in Latin America: Report from the SENTRY antimicrobial surveillance programme. J. Antimicrob. Chemother. 2002, 50, 673–679. [Google Scholar] [CrossRef]
- Papa, A.; Koulourida, V.; Souliou, E. Molecular epidemiology of carbapenem-resistant Acinetobacter baumannii in a newly established Greek hospital. Microb. Drug Resist. 2009, 15, 257–260. [Google Scholar] [CrossRef]
- Ramadan, R.A.; Gebriel, M.G.; Kadry, H.M.; Mosallem, A. Carbapenem-resistant Acinetobacter baumannii and Pseudomonas aeruginosa: Characterization of carbapenemase genes and E-test evaluation of colistin-based combinations. Infect. Drug Resist. 2018, 11, 1261. [Google Scholar] [CrossRef]
- Jia, H.; Chen, Y.; Wang, J.; Ruan, Z. Genomic characterisation of a clinical Acinetobacter baumannii ST1928 isolate carrying a new ampC allelic variant blaADC-196 gene from China. J. Glob. Antimicrob. Resist. 2019, 19, 43–45. [Google Scholar] [CrossRef]
- Kumburu, H.H.; Sonda, T.; van Zwetselaar, M.; Leekitcharoenphon, P.; Lukjancenko, O.; Mmbaga, B.T.; Alifrangis, M.; Lund, O.; Aarestrup, F.M.; Kibiki, G.S. Using WGS to identify antibiotic resistance genes and predict antimicrobial resistance phenotypes in MDR Acinetobacter baumannii in Tanzania. J. Antimicrob. Chemother. 2019, 74, 1484–1493. [Google Scholar] [CrossRef]
- Uddin, F.; McHugh, T.D.; Roulston, K.; Platt, G.; Khan, T.A.; Sohail, M. Detection of carbapenemases, AmpC and ESBL genes in Acinetobacter isolates from ICUs by DNA microarray. J. Microbiol. Methods 2018, 155, 19–23. [Google Scholar] [CrossRef]
- Hujer, K.M.; Hamza, N.S.; Hujer, A.M.; Perez, F.; Helfand, M.S.; Bethel, C.R.; Thomson, J.M.; Anderson, V.E.; Barlow, M.; Rice, L.B. Identification of a new allelic variant of the Acinetobacter baumannii cephalosporinase, ADC-7 β-lactamase: Defining a unique family of class C enzymes. Antimicrob. Agents Chemother. 2005, 49, 2941–2948. [Google Scholar] [CrossRef]
- Giannouli, M.; Tomasone, F.; Agodi, A.; Vahaboglu, H.; Daoud, Z.; Triassi, M.; Tsakris, A.; Zarrilli, R. Molecular epidemiology of carbapenem-resistant Acinetobacter baumannii strains in intensive care units of multiple Mediterranean hospitals. J. Antimicrob. Chemother. 2009, 63, 828–830. [Google Scholar] [CrossRef]
- Mosqueda, N.; Espinal, P.; Cosgaya, C.; Viota, S.; Plasensia, V.; Álvarez-Lerma, F.; Montero, M.; Gómez, J.; Horcajada, J.P.; Vila, J. Globally expanding carbapenemase finally appears in Spain: Nosocomial outbreak of Acinetobacter baumannii producing plasmid-encoded OXA-23 in Barcelona, Spain. Antimicrob. Agents Chemother. 2013, 57, 5155–5157. [Google Scholar] [CrossRef] [PubMed]
- Santimaleeworagun, W.; Samret, W.; Preechachuawong, P.; Kerdsin, A.; Jitwasinkul, T. Emergence of co-carbapenemase genes, blaOXA23, blaVIM, and blaNDM in carbapenem resistant Acinetobacter baumannii clinical isolates. Southeast Asian J. Trop. Med. Public Health 2016, 47, 1001–1007. [Google Scholar] [PubMed]
- Wibberg, D.; Salto, I.P.; Eikmeyer, F.G.; Maus, I.; Winkler, A.; Nordmann, P.; Pühler, A.; Poirel, L.; Schlüter, A. Complete genome sequencing of Acinetobacter baumannii strain K50 discloses the large conjugative plasmid pK50a encoding carbapenemase OXA-23 and extended-spectrum β-lactamase GES-11. Antimicrob. Agents Chemother. 2018, 62, e00212-18. [Google Scholar] [CrossRef] [PubMed]
- Acosta, J.; Merino, M.; Viedma, E.; Poza, M.; Sanz, F.; Otero, J.R.; Chaves, F.; Bou, G. Multidrug-resistant Acinetobacter baumannii harboring OXA-24 carbapenemase, Spain. Emerg. Infect. Dis. 2011, 17, 1064. [Google Scholar] [CrossRef] [PubMed]
- Merino, M.; Acosta, J.; Poza, M.; Sanz, F.; Beceiro, A.; Chaves, F.; Bou, G. OXA-24 carbapenemase gene flanked by XerC/XerD-like recombination sites in different plasmids from different Acinetobacter species isolated during a nosocomial outbreak. Antimicrob. Agents Chemother. 2010, 54, 2724–2727. [Google Scholar] [CrossRef] [PubMed]
- Oteo, J.; Hernández, J.M.; Espasa, M.; Fleites, A.; Sáez, D.; Bautista, V.; Pérez-Vázquez, M.; Fernández-García, M.D.; Delgado-Iribarren, A.; Sánchez-Romero, I. Emergence of OXA-48-producing Klebsiella pneumoniae and the novel carbapenemases OXA-244 and OXA-245 in Spain. J. Antimicrob. Chemother. 2013, 68, 317–321. [Google Scholar] [CrossRef]
- Potron, A.; Rondinaud, E.; Poirel, L.; Belmonte, O.; Boyer, S.; Camiade, S.; Nordmann, P. Genetic and biochemical characterisation of OXA-232, a carbapenem-hydrolysing class D β-lactamase from Enterobacteriaceae. Int. J. Antimicrob. Agents 2013, 41, 325–329. [Google Scholar] [CrossRef]
- Aly, M.; Tayeb, H.; Al Johani, S.; Alyamani, E.; Aldughaishem, F.; Alabdulkarim, I.; Balkhy, H. Genetic diversity of OXA-51-like genes among multidrug-resistant Acinetobacter baumannii in Riyadh, Saudi Arabia. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 1223–1228. [Google Scholar] [CrossRef]
- Rafei, R.; Pailhoriès, H.; Hamze, M.; Eveillard, M.; Mallat, H.; Dabboussi, F.; Joly-Guillou, M.-L.; Kempf, M. Molecular epidemiology of Acinetobacter baumannii in different hospitals in Tripoli, Lebanon using blaOXA-51-like sequence based typing. BMC Microbiol. 2015, 15, 103. [Google Scholar] [CrossRef]
- Mathlouthi, N.; Ben Lamine, Y.; Somai, R.; Bouhalila-Besbes, S.; Bakour, S.; Rolain, J.-M.; Chouchani, C. Incidence of OXA-23 and OXA-58 carbapenemases coexpressed in clinical isolates of Acinetobacter baumannii in Tunisia. Microb. Drug Resist. 2018, 24, 136–141. [Google Scholar] [CrossRef]
- Gionco, B.; Pelayo, J.S.; Venancio, E.J.; Cayô, R.; Gales, A.C.; Carrara-Marroni, F.E. Detection of OXA-231, a new variant of blaOXA-143, in Acinetobacter baumannii from Brazil: A case report. J. Antimicrob. Chemother. 2012, 67, 2531–2532. [Google Scholar] [CrossRef]
- Mostachio, A.K.; Levin, A.S.; Rizek, C.; Rossi, F.; Zerbini, J.; Costa, S.F. High prevalence of OXA-143 and alteration of outer membrane proteins in carbapenem-resistant Acinetobacter spp. isolates in Brazil. Int. J. Antimicrob. Agents 2012, 39, 396–401. [Google Scholar] [CrossRef]
- Sarikhani, Z.; Nazari, R.; Rostami, M.N. First report of OXA-143-lactamase producing Acinetobacter baumannii in Qom, Iran. Iran. J. Basic Med. Sci. 2017, 20, 1282. [Google Scholar]
- Boyd, D.A.; Mataseje, L.F.; Pelude, L.; Mitchell, R.; Bryce, E.; Roscoe, D.; Embree, J.; Katz, K.; Kibsey, P.; Lavallee, C. Results from the Canadian Nosocomial Infection Surveillance Program for detection of carbapenemase-producing Acinetobacter spp. in Canadian hospitals, 2010–16. J. Antimicrob. Chemother. 2019, 74, 315–320. [Google Scholar] [CrossRef]
- Higgins, P.G.; Pérez-Llarena, F.J.; Zander, E.; Fernández, A.; Bou, G.; Seifert, H. OXA-235, a novel class D β-lactamase involved in resistance to carbapenems in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2013, 57, 2121–2126. [Google Scholar] [CrossRef]
- Xu, Z.; Li, M.; Li, Y.; Cao, H.; Miao, L.; Xu, Z.; Higuchi, Y.; Yamasaki, S.; Nishino, K.; Woo, P.C. Native CRISPR-Cas-mediated genome editing enables dissecting and sensitizing clinical multidrug-resistant P. aeruginosa. Cell Rep. 2019, 29, 1707–1717.e1703. [Google Scholar] [CrossRef]
- Rumbo, C.; Gato, E.; López, M.; Ruiz de Alegría, C.; Fernández-Cuenca, F.; Martínez-Martínez, L.; Vila, J.; Pachón, J.; Cisneros, J.M.; Rodríguez-Baño, J. Contribution of efflux pumps, porins, and β-lactamases to multidrug resistance in clinical isolates of Acinetobacter baumannii. Antimicrob. Agents Chemother. 2013, 57, 5247–5257. [Google Scholar] [CrossRef]
- Smani, Y.; Fàbrega, A.; Roca, I.; Sánchez-Encinales, V.; Vila, J.; Pachón, J. Role of OmpA in the multidrug resistance phenotype of Acinetobacter baumannii. Antimicrob. Agents Chemother. 2014, 58, 1806–1808. [Google Scholar] [CrossRef]
- Mirshekar, M.; Shahcheraghi, F.; Azizi, O.; Solgi, H.; Badmasti, F. Diversity of class 1 integrons, and disruption of carO and dacD by insertion sequences among Acinetobacter baumannii isolates in Tehran, Iran. Microb. Drug Resist. 2018, 24, 359–366. [Google Scholar] [CrossRef]
- Rajamohan, G.; Srinivasan, V.B.; Gebreyes, W.A. Molecular and functional characterization of a novel efflux pump, AmvA, mediating antimicrobial and disinfectant resistance in Acinetobacter baumannii. J. Antimicrob. Chemother. 2010, 65, 1919–1925. [Google Scholar] [CrossRef]
- Sheikhalizadeh, V.; Hasani, A.; Rezaee, M.A.; Rahmati-Yamchi, M.; Hasani, A.; Ghotaslou, R.; Goli, H.R. Comprehensive study to investigate the role of various aminoglycoside resistance mechanisms in clinical isolates of Acinetobacter baumannii. J. Infect. Chemother. 2017, 23, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Costello, S.E.; Gales, A.C.; Morfin-Otero, R.; Jones, R.N.; Castanheira, M. Mechanisms of resistance, clonal expansion, and increasing prevalence of Acinetobacter baumannii strains displaying elevated tigecycline MIC values in Latin America. Microb. Drug Resist. 2016, 22, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Hasani, A.; Sheikhalizadeh, V.; Ahangarzadeh Rezaee, M.; Rahmati-Yamchi, M.; Hasani, A.; Ghotaslou, R.; Goli, H.R. Frequency of aminoglycoside-modifying enzymes and ArmA among different sequence groups of Acinetobacter baumannii in Iran. Microb. Drug Resist. 2016, 22, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.-F.; Liou, M.-L.; Tu, C.-C.; Yeh, H.-W.; Lan, C.-Y. Molecular epidemiology of integron-associated antimicrobial gene cassettes in the clinical isolates of Acinetobacter baumannii from northern Taiwan. Ann. Lab. Med. 2013, 33, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Salimizand, H.; Zomorodi, A.R.; Mansury, D.; Khakshoor, M.; Azizi, O.; Khodaparast, S.; Baseri, Z.; Karami, P.; Zamanlou, S.; Farsiani, H. Diversity of aminoglycoside modifying enzymes and 16S rRNA methylases in Acinetobacter baumannii and Acinetobacter nosocomialis species in Iran; wide distribution of aadA1 and armA. Infect. Genet. Evol. 2018, 66, 195–199. [Google Scholar] [CrossRef]
- Dönhöfer, A.; Franckenberg, S.; Wickles, S.; Berninghausen, O.; Beckmann, R.; Wilson, D.N. Structural basis for TetM-mediated tetracycline resistance. Proc. Natl. Acad. Sci. USA 2012, 109, 16900–16905. [Google Scholar] [CrossRef] [PubMed]
- Bojkovic, J.; Richie, D.L.; Six, D.A.; Rath, C.M.; Sawyer, W.S.; Hu, Q.; Dean, C.R. Characterization of an Acinetobacter baumannii lptD deletion strain: Permeability defects and response to inhibition of lipopolysaccharide and fatty acid biosynthesis. J. Bacteriol. 2016, 198, 731–741. [Google Scholar] [CrossRef]
- Martins-Sorenson, N.; Snesrud, E.; Xavier, D.E.; Cacci, L.C.; Iavarone, A.T.; McGann, P.; Riley, L.W.; Moreira, B.M. A novel plasmid-encoded mcr-4.3 gene in a colistin-resistant Acinetobacter baumannii clinical strain. J. Antimicrob. Chemother. 2020, 75, 60–64. [Google Scholar] [CrossRef]
- Moffatt, J.H.; Harper, M.; Boyce, J.D. Mechanisms of polymyxin resistance. In Polymyxin Antibiotics: From Laboratory Bench to Bedside; Springer: Berlin/Heidelberg, Germany, 2019; pp. 55–71. [Google Scholar]
- Trebosc, V.; Gartenmann, S.; Tötzl, M.; Lucchini, V.; Schellhorn, B.; Pieren, M.; Lociuro, S.; Gitzinger, M.; Tigges, M.; Bumann, D. Dissecting colistin resistance mechanisms in extensively drug-resistant Acinetobacter baumannii clinical isolates. mBio 2019, 10, e01083-19. [Google Scholar] [CrossRef]
- Srinivasan, V.B.; Rajamohan, G.; Gebreyes, W.A. Role of AbeS, a novel efflux pump of the SMR family of transporters, in resistance to antimicrobial agents in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2009, 53, 5312–5316. [Google Scholar] [CrossRef]
- Doi, Y.; Murray, G.L.; Peleg, A.Y. Acinetobacter baumannii: Evolution of antimicrobial resistance—Treatment options. Semin. Respir. Crit. Care Med. 2015, 36, 085–098. [Google Scholar]
- Perez, F.; Hujer, A.M.; Hujer, K.M.; Decker, B.K.; Rather, P.N.; Bonomo, R.A. Global challenge of multidrug-resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 2007, 51, 3471–3484. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.F.; Lan, C.-Y. Antimicrobial resistance in Acinetobacter baumannii: From bench to bedside. World J. Clin. Cases 2014, 2, 787. [Google Scholar] [CrossRef] [PubMed]
- Lucidi, M.; Visaggio, D.; Prencipe, E.; Imperi, F.; Rampioni, G.; Cincotti, G.; Leoni, L.; Visca, P. New shuttle vectors for real-time gene expression analysis in multidrug-resistant Acinetobacter species: In vitro and in vivo responses to environmental stressors. Appl. Environ. Microbiol. 2019, 85, e01334-19. [Google Scholar] [CrossRef] [PubMed]
- Lima, P.G.; Oliveira, J.T.; Amaral, J.L.; Freitas, C.D.; Souza, P.F. Synthetic antimicrobial peptides: Characteristics, design, and potential as alternative molecules to overcome microbial resistance. Life Sci. 2021, 278, 119647. [Google Scholar] [CrossRef]
- Gallagher, L.A. Methods for Tn-Seq analysis in Acinetobacter baumannii. In Acinetobacter baumannii: Methods and Protocols; Humana: New York, NY, USA, 2019; pp. 115–134. [Google Scholar]
- Roy, R.; You, R.I.; Lin, M.-D.; Lin, N.-T. Mutation of the carboxy-terminal processing protease in Acinetobacter baumannii affects motility, leads to loss of membrane integrity, and reduces virulence. Pathogens 2020, 9, 322. [Google Scholar] [CrossRef]
- Sun, B.; Liu, H.; Jiang, Y.; Shao, L.; Yang, S.; Chen, D. New mutations involved in colistin resistance in Acinetobacter baumannii. mSphere 2020, 5, e00895-19. [Google Scholar] [CrossRef]
- Dehbanipour, R.; Ghalavand, Z. Anti-virulence therapeutic strategies against bacterial infections: Recent advances. Germs 2022, 12, 262–275. [Google Scholar] [CrossRef]
- De Silva, P.M.; Patidar, R.; Graham, C.I.; Brassinga, A.K.C.; Kumar, A. A response regulator protein with antar domain, avnr, in Acinetobacter baumannii ATCC 17978 impacts its virulence and amino acid metabolism. Microbiology 2020, 166, 554–566. [Google Scholar] [CrossRef]
- Godeux, A.S.; Svedholm, E.; Lupo, A.; Haenni, M.; Venner, S.; Laaberki, M.-H.; Charpentier, X. Scarless removal of large resistance island AbaR results in antibiotic susceptibility and increased natural transformability in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2020, 64, e00951-20. [Google Scholar] [CrossRef]
- Biswas, I. Genetic tools for manipulating Acinetobacter baumannii genome: An overview. J. Med. Microbiol. 2015, 64, 657–669. [Google Scholar] [CrossRef]
- Fels, U.; Gevaert, K.; Van Damme, P. Bacterial genetic engineering by means of recombineering for reverse genetics. Front. Microbiol. 2020, 11, 548410. [Google Scholar] [CrossRef]
- Gordillo Altamirano, F.L.; Barr, J.J. Phage therapy in the postantibiotic era. Clin. Microbiol. Rev. 2019, 32, e00066-18. [Google Scholar] [CrossRef]
- Kortright, K.E.; Chan, B.K.; Koff, J.L.; Turner, P.E. Phage therapy: A renewed approach to combat antibiotic-resistant bacteria. Cell Host Microbe 2019, 25, 219–232. [Google Scholar] [CrossRef]
- Hetta, H.F.; Ramadan, Y.N.; Al-Harbi, A.I.; Ahmed, E.A.; Battah, B.; Abd Ellah, N.H.; Zanetti, S.; Donadu, M.G. Nanotechnology as a Promising Approach to Combat Multidrug Resistant Bacteria: A Comprehensive Review and Future Perspectives. Biomedicines 2023, 11, 413. [Google Scholar] [CrossRef]
- Makabenta, J.M.V.; Nabawy, A.; Li, C.-H.; Schmidt-Malan, S.; Patel, R.; Rotello, V.M. Nanomaterial-based therapeutics for antibiotic-resistant bacterial infections. Nat. Rev. Microbiol. 2021, 19, 23–36. [Google Scholar] [CrossRef]
- Munir, M.U.; Ahmad, M.M. Nanomaterials aiming to tackle antibiotic-resistant bacteria. Pharmaceutics 2022, 14, 582. [Google Scholar] [CrossRef]
- Egorov, A.; Ulyashova, M.; Rubtsova, M.Y. Bacterial enzymes and antibiotic resistance. Acta Nat. 2018, 10, 33–48. [Google Scholar] [CrossRef]
- Gontijo, A.V.L.; Pereira, S.L.; de Lacerda Bonfante, H. Can drug repurposing be effective against carbapenem-resistant Acinetobacter baumannii? Curr. Microbiol. 2022, 79, 13. [Google Scholar] [CrossRef]
- Koh Jing Jie, A.; Hussein, M.; Rao, G.G.; Li, J.; Velkov, T. Drug Repurposing Approaches towards Defeating Multidrug-Resistant Gram-Negative Pathogens: Novel Polymyxin/Non-Antibiotic Combinations. Pathogens 2022, 11, 1420. [Google Scholar] [CrossRef]
- Liu, Y.; Tong, Z.; Shi, J.; Li, R.; Upton, M.; Wang, Z. Drug repurposing for next-generation combination therapies against multidrug-resistant bacteria. Theranostics 2021, 11, 4910. [Google Scholar] [CrossRef] [PubMed]
- Buchy, P.; Ascioglu, S.; Buisson, Y.; Datta, S.; Nissen, M.; Tambyah, P.A.; Vong, S. Impact of vaccines on antimicrobial resistance. Int. J. Infect. Dis. 2020, 90, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Micoli, F.; Bagnoli, F.; Rappuoli, R.; Serruto, D. The role of vaccines in combatting antimicrobial resistance. Nat. Rev. Microbiol. 2021, 19, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Rosini, R.; Nicchi, S.; Pizza, M.; Rappuoli, R. Vaccines against antimicrobial resistance. Front. Immunol. 2020, 11, 1048. [Google Scholar] [CrossRef]
- Kole, R.; Krainer, A.R.; Altman, S. RNA therapeutics: Beyond RNA interference and antisense oligonucleotides. Nat. Rev. Drug Discov. 2012, 11, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Murugaiyan, J.; Kumar, P.A.; Rao, G.S.; Iskandar, K.; Hawser, S.; Hays, J.P.; Mohsen, Y.; Adukkadukkam, S.; Awuah, W.A.; Jose, R.A.M. Progress in alternative strategies to combat antimicrobial resistance: Focus on antibiotics. Antibiotics 2022, 11, 200. [Google Scholar] [CrossRef] [PubMed]
- Good, L.; Stach, J.E. Synthetic RNA silencing in bacteria–antimicrobial discovery and resistance breaking. Front. Microbiol. 2011, 2, 185. [Google Scholar] [CrossRef]
- Kotil, S.; Jakobsson, E. Rationally designing antisense therapy to keep up with evolving bacterial resistance. PLoS ONE 2019, 14, e0209894. [Google Scholar] [CrossRef]
- Gaj, T.; Sirk, S.J.; Shui, S.-l.; Liu, J. Genome-editing technologies: Principles and applications. Cold Spring Harb. Perspect. Biol. 2016, 8, a023754. [Google Scholar] [CrossRef]
- Li, H.; Yang, Y.; Hong, W.; Huang, M.; Wu, M.; Zhao, X. Applications of genome editing technology in the targeted therapy of human diseases: Mechanisms, advances and prospects. Signal Transduct. Target. Ther. 2020, 5, 1. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, H.; Cheng, C.H. Mutagenesis in Xenopus and zebrafish using TALENs. In TALENs: Methods and Protocols; Humana: New York, NY, USA, 2016; pp. 207–227. [Google Scholar]
- Zhang, H.-X.; Zhang, Y.; Yin, H. Genome editing with mRNA encoding ZFN, TALEN, and Cas9. Mol. Ther. 2019, 27, 735–746. [Google Scholar] [CrossRef]
- Hille, F.; Charpentier, E. CRISPR-Cas: Biology, mechanisms and relevance. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016, 371, 20150496. [Google Scholar] [CrossRef]
- Ishino, Y.; Krupovic, M.; Forterre, P. History of CRISPR-Cas from encounter with a mysterious repeated sequence to genome editing technology. J. Bacteriol. 2018, 200, e00580-17. [Google Scholar] [CrossRef]
- Ibrahim, A.; ÖZSÖZ, M.; Tirah, G.; Gideon, O. Genome engineering using the CRISPR Cas9 system. J. Biomed. Pharm. Sci. 2019, 2, 2. [Google Scholar]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A programmable dual-RNA–guided DNA endonuclease in adaptive bacterial immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef]
- O’Connell, M.R.; Oakes, B.L.; Sternberg, S.H.; East-Seletsky, A.; Kaplan, M.; Doudna, J.A. Programmable RNA recognition and cleavage by CRISPR/Cas9. Nature 2014, 516, 263–266. [Google Scholar] [CrossRef]
- Adli, M. The CRISPR tool kit for genome editing and beyond. Nat. Commun. 2018, 9, 1911. [Google Scholar] [CrossRef]
- Rath, D.; Amlinger, L.; Rath, A.; Lundgren, M. The CRISPR-Cas immune system: Biology, mechanisms and applications. Biochimie 2015, 117, 119–128. [Google Scholar] [CrossRef]
- Palacios Araya, D.; Palmer, K.L.; Duerkop, B.A. CRISPR-based antimicrobials to obstruct antibiotic-resistant and pathogenic bacteria. PLoS Pathog. 2021, 17, e1009672. [Google Scholar] [CrossRef]
- Yeh, T.-K.; Jean, S.-S.; Lee, Y.-L.; Lu, M.-C.; Ko, W.-C.; Lin, H.-J.; Liu, P.-Y.; Hsueh, P.-R. Bacteriophages and phage-delivered CRISPR-Cas system as antibacterial therapy. Int. J. Antimicrob. Agents 2022, 59, 106475. [Google Scholar] [CrossRef]
- Chen, Y.; Wen, R.; Yang, Z.; Chen, Z. Genome editing using CRISPR/Cas9 to treat hereditary hematological disorders. Gene Ther. 2022, 29, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Mani, I. CRISPR-Cas9 for treating hereditary diseases. Prog. Mol. Biol. Transl. Sci. 2021, 181, 165–183. [Google Scholar] [PubMed]
- Fuziwara, C.S.; de Mello, D.C.; Kimura, E.T. Gene Editing with CRISPR/Cas Methodology and Thyroid Cancer: Where Are We? Cancers 2022, 14, 844. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Lee, S.-W. Therapeutic Application of Genome Editing Technologies in Viral Diseases. Int. J. Mol. Sci. 2022, 23, 5399. [Google Scholar] [CrossRef]
- Makarova, K.S.; Wolf, Y.I.; Iranzo, J.; Shmakov, S.A.; Alkhnbashi, O.S.; Brouns, S.J.; Charpentier, E.; Cheng, D.; Haft, D.H.; Horvath, P. Evolutionary classification of CRISPR–Cas systems: A burst of class 2 and derived variants. Nat. Rev. Microbiol. 2020, 18, 67–83. [Google Scholar] [CrossRef]
- Wan, F.; Draz, M.S.; Gu, M.; Yu, W.; Ruan, Z.; Luo, Q. Novel strategy to combat antibiotic resistance: A sight into the combination of CRISPR/Cas9 and nanoparticles. Pharmaceutics 2021, 13, 352. [Google Scholar] [CrossRef]
- Hidalgo-Cantabrana, C.; Goh, Y.J.; Barrangou, R. Characterization and repurposing of type I and type II CRISPR–Cas systems in bacteria. J. Mol. Biol. 2019, 431, 21–33. [Google Scholar] [CrossRef]
- Cady, K.; White, A.; Hammond, J.; Abendroth, M.; Karthikeyan, R.; Lalitha, P.; Zegans, M.; O’Toole, G. Prevalence, conservation and functional analysis of Yersinia and Escherichia CRISPR regions in clinical Pseudomonas aeruginosa isolates. Microbiology 2011, 157, 430. [Google Scholar] [CrossRef]
- Gholizadeh, P.; Köse, Ş.; Dao, S.; Ganbarov, K.; Tanomand, A.; Dal, T.; Aghazadeh, M.; Ghotaslou, R.; Ahangarzadeh Rezaee, M.; Yousefi, B. How CRISPR-Cas system could be used to combat antimicrobial resistance. Infect. Drug Resist. 2020, 13, 1111–1121. [Google Scholar] [CrossRef]
- Zhang, S.; Shen, J.; Li, D.; Cheng, Y. Strategies in the delivery of Cas9 ribonucleoprotein for CRISPR/Cas9 genome editing. Theranostics 2021, 11, 614. [Google Scholar] [CrossRef]
- Aslam, B.; Rasool, M.; Idris, A.; Muzammil, S.; Alvi, R.F.; Khurshid, M.; Rasool, M.H.; Zhang, D.; Ma, Z.; Baloch, Z. CRISPR-Cas system: A potential alternative tool to cope antibiotic resistance. Antimicrob. Resist. Infect. Control 2020, 9, 131. [Google Scholar] [CrossRef]
- Barrangou, R.; Ousterout, D. Repurposing CRISPR-Cas systems as DNA-based smart antimicrobials. Cell Gene Ther. Insights 2017, 3, 63–72. [Google Scholar] [CrossRef]
- Serajian, S.; Ahmadpour, E.; Oliveira, S.M.R.; Pereira, M.d.L.; Heidarzadeh, S. CRISPR-cas technology: Emerging applications in clinical microbiology and infectious diseases. Pharmaceuticals 2021, 14, 1171. [Google Scholar] [CrossRef]
- Duan, C.; Cao, H.; Zhang, L.-H.; Xu, Z. Harnessing the CRISPR-Cas systems to combat antimicrobial resistance. Front. Microbiol. 2021, 12, 716064. [Google Scholar] [CrossRef]
- Gleerup, J.L.; Mogensen, T.H. CRISPR-Cas in diagnostics and therapy of infectious diseases. J. Infect. Dis. 2022, 226, 1867–1876. [Google Scholar] [CrossRef]
- Shim, H. Investigating the genomic background of CRISPR-Cas genomes for CRISPR-based antimicrobials. Evol. Bioinform. 2022, 18, 11769343221103887. [Google Scholar] [CrossRef]
- Shabbir, M.A.B.; Shabbir, M.Z.; Wu, Q.; Mahmood, S.; Sajid, A.; Maan, M.K.; Ahmed, S.; Naveed, U.; Hao, H.; Yuan, Z. CRISPR-cas system: Biological function in microbes and its use to treat antimicrobial resistant pathogens. Ann. Clin. Microbiol. Antimicrob. 2019, 18, 21. [Google Scholar] [CrossRef]
- Ekwebelem, O.C.; Aleke, J.; Ofielu, E.; Nnorom-Dike, O. CRISPR-Cas9 system: A revolutionary tool in the fight against antimicrobial resistance. Infect. Microbes Dis. 2021, 3, 51–56. [Google Scholar] [CrossRef]
- Getahun, Y.A.; Ali, D.A.; Taye, B.W.; Alemayehu, Y.A. Multidrug-Resistant Microbial Therapy Using Antimicrobial Peptides and the CRISPR/Cas9 System. Vet. Med. Res. Rep. 2022, 13, 173–190. [Google Scholar] [CrossRef]
- Greene, A.C. CRISPR-based antibacterials: Transforming bacterial defense into offense. Trends Biotechnol. 2018, 36, 127–130. [Google Scholar] [CrossRef]
- Li, Y.; Peng, N. Endogenous CRISPR-Cas system-based genome editing and antimicrobials: Review and prospects. Front. Microbiol. 2019, 10, 2471. [Google Scholar] [CrossRef] [PubMed]
- Alavi, M.; Rai, M. Antisense RNA, the modified CRISPR-Cas9, and metal/metal oxide nanoparticles to inactivate pathogenic bacteria. Cell. Mol. Biomed. Rep. 2021, 1, 52–59. [Google Scholar] [CrossRef]
- Cañez, C.; Selle, K.; Goh, Y.J.; Barrangou, R. Outcomes and characterization of chromosomal self-targeting by native CRISPR-Cas systems in Streptococcus thermophilus. FEMS Microbiol. Lett. 2019, 366, fnz105. [Google Scholar] [CrossRef] [PubMed]
- Citorik, R.J.; Mimee, M.; Lu, T.K. Sequence-specific antimicrobials using efficiently delivered RNA-guided nucleases. Nat. Biotechnol. 2014, 32, 1141–1145. [Google Scholar] [CrossRef]
- Kim, J.-S.; Cho, D.-H.; Park, M.; Chung, W.-J.; Shin, D.; Ko, K.S.; Kweon, D.-H. CRISPR/Cas9-mediated re-sensitization of antibiotic-resistant Escherichia coli harboring extended-spectrum β-lactamases. J. Microbiol. Biotechnol. 2016, 26, 394–401. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, D.; Wang, X.; Tao, H.; Feng, E.; Zhu, L.; Pan, C.; Wang, B.; Liu, C.; Liu, X. Highly efficient genome engineering in Bacillus anthracis and Bacillus cereus using the CRISPR/Cas9 system. Front. Microbiol. 2019, 10, 1932. [Google Scholar] [CrossRef]
- Gomaa, A.A.; Klumpe, H.E.; Luo, M.L.; Selle, K.; Barrangou, R.; Beisel, C.L. Programmable removal of bacterial strains by use of genome-targeting CRISPR-Cas systems. mBio 2014, 5, e00928-13. [Google Scholar] [CrossRef]
- Liu, Y.; Wan, X.; Wang, B. Engineered CRISPRa enables programmable eukaryote-like gene activation in bacteria. Nat. Commun. 2019, 10, 3693. [Google Scholar] [CrossRef]
- Bikard, D.; Euler, C.W.; Jiang, W.; Nussenzweig, P.M.; Goldberg, G.W.; Duportet, X.; Fischetti, V.A.; Marraffini, L.A. Exploiting CRISPR-Cas nucleases to produce sequence-specific antimicrobials. Nat. Biotechnol. 2014, 32, 1146–1150. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, Y.; Zhang, Y.; Pi, Y.; Gu, T.; Song, L.; Wang, Y.; Ji, Q. CRISPR/Cas9-based genome editing in Pseudomonas aeruginosa and cytidine deaminase-mediated base editing in Pseudomonas species. iScience 2018, 6, 222–231. [Google Scholar] [CrossRef]
- Li, Q.; Sun, B.; Chen, J.; Zhang, Y.; Jiang, Y.; Yang, S. A modified pCas/pTargetF system for CRISPR-Cas9-assisted genome editing in Escherichia coli. Acta Biochim. Biophys. Sin. 2021, 53, 620–627. [Google Scholar] [CrossRef]
- Savage, D.F. Cas14: Big advances from small CRISPR proteins. Biochemistry 2019, 58, 1024–1025. [Google Scholar] [CrossRef]
- Wu, X.; Zha, J.; Koffas, M.A.; Dordick, J.S. Reducing Staphylococcus aureus resistance to lysostaphin using CRISPR-dCas9. Biotechnol. Bioeng. 2019, 116, 3149–3159. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Chen, W.; Song, L.; Zhang, Y.; Shen, Z.; Yu, F.; Li, M.; Ji, Q. CRISPR-Cas9 and CRISPR-assisted cytidine deaminase enable precise and efficient genome editing in Klebsiella pneumoniae. Appl. Environ. Microbiol. 2018, 84, e01834-18. [Google Scholar] [CrossRef]
- Aquino-Jarquin, G. CRISPR-Cas14 is now part of the artillery for gene editing and molecular diagnostic. Nanomed. Nanotechnol. Biol. Med. 2019, 18, 428–431. [Google Scholar] [CrossRef]
- Uribe, R.V.; Rathmer, C.; Jahn, L.J.; Ellabaan, M.M.H.; Li, S.S.; Sommer, M.O.A. Bacterial resistance to CRISPR-Cas antimicrobials. Sci. Rep. 2021, 11, 17267. [Google Scholar] [CrossRef]
- Wu, Y.; Battalapalli, D.; Hakeem, M.J.; Selamneni, V.; Zhang, P.; Draz, M.S.; Ruan, Z. Engineered CRISPR-Cas systems for the detection and control of antibiotic-resistant infections. J. Nanobiotechnol. 2021, 19, 401. [Google Scholar] [CrossRef]
- Song, Z.; Yu, Y.; Bai, X.; Jia, Y.; Tian, J.; Gu, K.; Zhao, M.; Zhou, C.; Zhang, X.; Wang, H. Pathogen-Specific Bactericidal Method Mediated by Conjugative Delivery of CRISPR-Cas13a Targeting Bacterial Endogenous Transcripts. Microbiol. Spectr. 2022, 10, e01300-22. [Google Scholar] [CrossRef]
- Tagliaferri, T.L.; Guimarães, N.R.; Pereira, M.D.P.M.; Vilela, L.F.F.; Horz, H.P.; Dos Santos, S.G.; Mendes, T.A.D.O. Exploring the potential of CRISPR-Cas9 under challenging conditions: Facing high-copy plasmids and counteracting beta-lactam resistance in clinical strains of Enterobacteriaceae. Front. Microbiol. 2020, 11, 578. [Google Scholar] [CrossRef]
- Wongpayak, P.; Meesungnoen, O.; Saejang, S.; Subsoontorn, P. A highly effective and self-transmissible CRISPR antimicrobial for elimination of target plasmids without antibiotic selection. PeerJ 2021, 9, e11996. [Google Scholar] [CrossRef]
- Wang, P.; He, D.; Li, B.; Guo, Y.; Wang, W.; Luo, X.; Zhao, X.; Wang, X. Eliminating mcr-1-harbouring plasmids in clinical isolates using the CRISPR/Cas9 system. J. Antimicrob. Chemother. 2019, 74, 2559–2565. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.K.; Kwon, K.; Ryu, J.S.; Lee, H.N.; Park, C.; Chung, H.J. Nonviral genome editing based on a polymer-derivatized CRISPR nanocomplex for targeting bacterial pathogens and antibiotic resistance. Bioconjug. Chem. 2017, 28, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Wan, P.; Cui, S.; Ma, Z.; Chen, L.; Li, X.; Zhao, R.; Xiong, W.; Zeng, Z. Reversal of mcr-1-mediated colistin resistance in Escherichia coli by CRISPR-Cas9 system. Infect. Drug Resist. 2020, 13, 1171–1178. [Google Scholar] [CrossRef] [PubMed]
- Nath, A.; Bhattacharjee, R.; Nandi, A.; Sinha, A.; Kar, S.; Manoharan, N.; Mitra, S.; Mojumdar, A.; Panda, P.K.; Patro, S. Phage delivered CRISPR-Cas system to combat multidrug-resistant pathogens in gut microbiome. Biomed. Pharmacother. 2022, 151, 113122. [Google Scholar] [CrossRef] [PubMed]
- Neil, K.; Allard, N.; Roy, P.; Grenier, F.; Menendez, A.; Burrus, V.; Rodrigue, S. High-efficiency delivery of CRISPR-Cas9 by engineered probiotics enables precise microbiome editing. Mol. Syst. Biol. 2021, 17, e10335. [Google Scholar] [CrossRef]
- Price, V.J.; McBride, S.W.; Hullahalli, K.; Chatterjee, A.; Duerkop, B.A.; Palmer, K.L. Enterococcus faecalis CRISPR-Cas is a robust barrier to conjugative antibiotic resistance dissemination in the murine intestine. mSphere 2019, 4, e00464-19. [Google Scholar] [CrossRef]
- Rodrigues, M.; McBride, S.W.; Hullahalli, K.; Palmer, K.L.; Duerkop, B.A. Conjugative delivery of CRISPR-Cas9 for the selective depletion of antibiotic-resistant enterococci. Antimicrob. Agents Chemother. 2019, 63, e01454-19. [Google Scholar] [CrossRef]
- Hao, M.; He, Y.; Zhang, H.; Liao, X.-P.; Liu, Y.-H.; Sun, J.; Du, H.; Kreiswirth, B.N.; Chen, L. CRISPR-Cas9-mediated carbapenemase gene and plasmid curing in carbapenem-resistant Enterobacteriaceae. Antimicrob. Agents Chemother. 2020, 64, e00843-20. [Google Scholar] [CrossRef]
- He, Y.-Z.; Kuang, X.; Long, T.-F.; Li, G.; Ren, H.; He, B.; Yan, J.-R.; Liao, X.-P.; Liu, Y.-H.; Chen, L. Re-engineering a mobile-CRISPR/Cas9 system for antimicrobial resistance gene curing and immunization in Escherichia coli. J. Antimicrob. Chemother. 2022, 77, 74–82. [Google Scholar] [CrossRef]
- Mackow, N.A.; Shen, J.; Adnan, M.; Khan, A.S.; Fries, B.C.; Diago-Navarro, E. CRISPR-Cas influences the acquisition of antibiotic resistance in Klebsiella pneumoniae. PLoS ONE 2019, 14, e0225131. [Google Scholar] [CrossRef]
- Yao, S.; Wei, D.; Tang, N.; Song, Y.; Wang, C.; Feng, J.; Zhang, G. Efficient Suppression of Natural Plasmid-Borne Gene Expression in Carbapenem-Resistant Klebsiella pneumoniae Using a Compact CRISPR Interference System. Antimicrob. Agents Chemother. 2022, 66, e00890-22. [Google Scholar] [CrossRef]
- Kang, S.; Kim, J.; Hur, J.K.; Lee, S.-S. CRISPR-based genome editing of clinically important Escherichia coli SE15 isolated from indwelling urinary catheters of patients. J. Med. Microbiol. 2017, 66, 18–25. [Google Scholar] [CrossRef]
- Zuberi, A.; Ahmad, N.; Khan, A.U. CRISPRi induced suppression of fimbriae gene (fimH) of a uropathogenic Escherichia coli: An approach to inhibit microbial biofilms. Front. Immunol. 2017, 8, 1552. [Google Scholar] [CrossRef]
- Boettcher, M.; McManus, M.T. Choosing the right tool for the job: RNAi, TALEN, or CRISPR. Mol. Cell 2015, 58, 575–585. [Google Scholar] [CrossRef]
- Chen, C.; Choudhury, A.; Zhang, S.; Garst, A.D.; Song, X.; Liu, X.; Chen, T.; Gill, R.T.; Wang, Z. Integrating CRISPR-enabled trackable genome engineering and transcriptomic analysis of global regulators for antibiotic resistance selection and identification in Escherichia coli. Msystems 2020, 5, e00232-20. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, P.; Li, L.; Zhao, H.; Shi, L.; Tian, P. Engineering a CRISPR interference system to repress a class 1 integron in Escherichia coli. Antimicrob. Agents Chemother. 2020, 64, e01789-19. [Google Scholar] [CrossRef]
- Wan, X.; Li, Q.; Olsen, R.H.; Meng, H.; Zhang, Z.; Wang, J.; Zheng, H.; Li, L.; Shi, L. Engineering a CRISPR interference system targeting AcrAB-TolC efflux pump to prevent multidrug resistance development in Escherichia coli. J. Antimicrob. Chemother. 2022, 77, 2158–2166. [Google Scholar] [CrossRef]
- Zuberi, A.; Misba, L.; Khan, A.U. CRISPR interference (CRISPRi) inhibition of luxS gene expression in E. coli: An approach to inhibit biofilm. Front. Cell. Infect. Microbiol. 2017, 7, 214. [Google Scholar] [CrossRef]
- Afonina, I.; Ong, J.; Chua, J.; Lu, T.; Kline, K.A. Multiplex CRISPRi system enables the study of stage-specific biofilm genetic requirements in Enterococcus faecalis. mBio 2020, 11, e01101-20. [Google Scholar] [CrossRef]
- Noirot-Gros, M.F.; Forrester, S.; Malato, G.; Larsen, P.E.; Noirot, P. CRISPR interference to interrogate genes that control biofilm formation in Pseudomonas fluorescens. Sci. Rep. 2019, 9, 15954. [Google Scholar] [CrossRef]
- Guzzo, M.; Castro, L.K.; Reisch, C.R.; Guo, M.S.; Laub, M.T. A CRISPR interference system for efficient and rapid gene knockdown in Caulobacter crescentus. mBio 2020, 11, e02415-19. [Google Scholar] [CrossRef] [PubMed]
- Coates, R.; Grant, A.; Floto, A.; Parkhill, J. Development of a CRISPR Interference System in Campylobacter jejuni. Ph.D. Dissertation, University of Cambridge, Cambridge, UK, 2022. [Google Scholar]
- Costigan, R.; Stoakes, E.; Floto, R.A.; Parkhill, J.; Grant, A.J. Development and validation of a CRISPR interference system for gene regulation in Campylobacter jejuni. BMC Microbiol. 2022, 22, 238. [Google Scholar] [CrossRef] [PubMed]
- Dolgin, E. The kill-switch for CRISPR that could make gene-editing safer. Nature 2020, 577, 308–311. [Google Scholar] [CrossRef] [PubMed]
- Kundar, R.; Gokarn, K. CRISPR-Cas System: A Tool to Eliminate Drug-Resistant Gram-Negative Bacteria. Pharmaceuticals 2022, 15, 1498. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Charusanti, P.; Zhang, L.; Weber, T.; Lee, S.Y. CRISPR-Cas9 based engineering of actinomycetal genomes. ACS Synth. Biol. 2015, 4, 1020–1029. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Z.; Chen, Y.; Hua, X.; Yu, Y.; Ji, Q. A highly efficient CRISPR-Cas9-based genome engineering platform in Acinetobacter baumannii to understand the H2O2-sensing mechanism of OxyR. Cell Chem. Biol. 2019, 26, 1732–1742.e1735. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, J.; Sun, X.; Li, M.; Zhang, P.; Zhu, Z.; Jiao, H.; Guo, T.; Li, G. CRISPR-Cas in Acinetobacter baumannii Contributes to Antibiotic Susceptibility by Targeting Endogenous AbaI. Microbiol. Spectr. 2022, 10, e00829-22. [Google Scholar] [CrossRef]
- Bai, J.; Dai, Y.; Farinha, A.; Tang, A.Y.; Syal, S.; Vargas-Cuebas, G.; van Opijnen, T.; Isberg, R.R.; Geisinger, E. Essential gene analysis in Acinetobacter baumannii by high-density transposon mutagenesis and CRISPR interference. J. Bacteriol. 2021, 203, e00565-20. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, Y.; Zhang, L.; Yang, Y.; Yang, S.; Yang, L.; Chen, H.; Liu, C.; Li, J.; Xie, G. Integration of multiplex PCR and CRISPR-Cas allows highly specific detection of multidrug-resistant Acinetobacter baumannii. Sens. Actuators B Chem. 2021, 334, 129600. [Google Scholar] [CrossRef]
- Wu, S.; Xu, R.; Su, M.; Gao, C.; Liu, Y.; Chen, Y.; Luan, G.; Jia, X.; Wang, R. A pyrF-Based Efficient Genetic Manipulation Platform in Acinetobacter baumannii to Explore the Vital DNA Components of Adaptive Immunity for IF CRISPR-Cas. Microbiol. Spectr. 2022, 10, e01957-22. [Google Scholar] [CrossRef]
- Wolf, T.; Gren, T.; Thieme, E.; Wibberg, D.; Zemke, T.; Pühler, A.; Kalinowski, J. Targeted genome editing in the rare actinomycete Actinoplanes sp. SE50/110 by using the CRISPR/Cas9 system. J. Biotechnol. 2016, 231, 122–128. [Google Scholar] [CrossRef]
- Bernheim, A.; Calvo-Villamañán, A.; Basier, C.; Cui, L.; Rocha, E.P.; Touchon, M.; Bikard, D. Inhibition of NHEJ repair by type II-A CRISPR-Cas systems in bacteria. Nat. Commun. 2017, 8, 2094. [Google Scholar] [CrossRef]
- Peters, J.M.; Colavin, A.; Shi, H.; Czarny, T.L.; Larson, M.H.; Wong, S.; Hawkins, J.S.; Lu, C.H.; Koo, B.-M.; Marta, E. A comprehensive, CRISPR-based functional analysis of essential genes in bacteria. Cell 2016, 165, 1493–1506. [Google Scholar] [CrossRef]
- Zhang, K.; Duan, X.; Wu, J. Multigene disruption in undomesticated Bacillus subtilis ATCC 6051a using the CRISPR/Cas9 system. Sci. Rep. 2016, 6, 27943. [Google Scholar] [CrossRef]
- Altenbuchner, J. Editing of the Bacillus subtilis genome by the CRISPR-Cas9 system. Appl. Environ. Microbiol. 2016, 82, 5421–5427. [Google Scholar] [CrossRef]
- Mougiakos, I.; Bosma, E.F.; Weenink, K.; Vossen, E.; Goijvaerts, K.; van der Oost, J.; van Kranenburg, R. Efficient genome editing of a facultative thermophile using mesophilic spCas9. ACS Synth. Biol. 2017, 6, 849–861. [Google Scholar] [CrossRef]
- Mougiakos, I.; Mohanraju, P.; Bosma, E.F.; Vrouwe, V.; Finger Bou, M.; Naduthodi, M.I.; Gussak, A.; Brinkman, R.B.; Van Kranenburg, R.; Van Der Oost, J. Characterizing a thermostable Cas9 for bacterial genome editing and silencing. Nat. Commun. 2017, 8, 1647. [Google Scholar] [CrossRef]
- Li, K.; Cai, D.; Wang, Z.; He, Z.; Chen, S. Development of an efficient genome editing tool in Bacillus licheniformis using CRISPR-Cas9 nickase. Appl. Environ. Microbiol. 2018, 84, e02608-17. [Google Scholar] [CrossRef]
- Zheng, K.; Wang, Y.; Li, N.; Jiang, F.-F.; Wu, C.-X.; Liu, F.; Chen, H.-C.; Liu, Z.-F. Highly efficient base editing in bacteria using a Cas9-cytidine deaminase fusion. Commun. Biol. 2018, 1, 32. [Google Scholar] [CrossRef]
- Wasels, F.; Jean-Marie, J.; Collas, F.; López-Contreras, A.M.; Ferreira, N.L. A two-plasmid inducible CRISPR/Cas9 genome editing tool for Clostridium acetobutylicum. J. Microbiol. Methods 2017, 140, 5–11. [Google Scholar] [CrossRef]
- Bruder, M.R.; Pyne, M.E.; Moo-Young, M.; Chung, D.A.; Chou, C.P. Extending CRISPR-Cas9 technology from genome editing to transcriptional engineering in the genus Clostridium. Appl. Environ. Microbiol. 2016, 82, 6109–6119. [Google Scholar] [CrossRef] [PubMed]
- Nagaraju, S.; Davies, N.K.; Walker, D.J.F.; Köpke, M.; Simpson, S.D. Genome editing of Clostridium autoethanogenum using CRISPR/Cas9. Biotechnol. Biofuels 2016, 9, 219. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Chen, J.; Minton, N.P.; Zhang, Y.; Wen, Z.; Liu, J.; Yang, H.; Zeng, Z.; Ren, X.; Yang, J. CRISPR-based genome editing and expression control systems in Clostridium acetobutylicum and Clostridium beijerinckii. Biotechnol. J. 2016, 11, 961–972. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Z.T.; Seo, S.O.; Lynn, P.; Lu, T.; Jin, Y.S.; Blaschek, H.P. Bacterial genome editing with CRISPR-Cas9: Deletion, integration, single nucleotide modification, and desirable “clean” mutant selection in Clostridium beijerinckii as an example. ACS Synth. Biol. 2016, 5, 721–732. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Z.T.; Seo, S.O.; Lynn, P.; Lu, T.; Jin, Y.S.; Blaschek, H.P. Gene transcription repression in Clostridium beijerinckii using CRISPR-dCas9. Biotechnol. Bioeng. 2016, 113, 2739–2743. [Google Scholar] [CrossRef]
- Negahdaripour, M.; Nezafat, N.; Hajighahramani, N.; Rahmatabadi, S.S.; Ghasemi, Y. Investigating CRISPR-Cas systems in Clostridium botulinum via bioinformatics tools. Infect. Genet. Evol. 2017, 54, 355–373. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Li, Y.; Shi, Z.; Hemme, C.L.; Li, Y.; Zhu, Y.; Van Nostrand, J.D.; He, Z.; Zhou, J. Efficient genome editing in Clostridium cellulolyticum via CRISPR-Cas9 nickase. Appl. Environ. Microbiol. 2015, 81, 4423–4431. [Google Scholar] [CrossRef]
- Hong, W.; Zhang, J.; Cui, G.; Wang, L.; Wang, Y. Multiplexed CRISPR-Cpf1-mediated genome editing in Clostridium difficile toward the understanding of pathogenesis of C. difficile infection. ACS Synth. Biol. 2018, 7, 1588–1600. [Google Scholar] [CrossRef]
- McAllister, K.N.; Bouillaut, L.; Kahn, J.N.; Self, W.T.; Sorg, J.A. Using CRISPR-Cas9-mediated genome editing to generate C. difficile mutants defective in selenoproteins synthesis. Sci. Rep. 2017, 7, 14672. [Google Scholar] [CrossRef]
- Wang, S.; Dong, S.; Wang, P.; Tao, Y.; Wang, Y. Genome editing in Clostridium saccharoperbutylacetonicum N1-4 with the CRISPR-Cas9 system. Appl. Environ. Microbiol. 2017, 83, e00233-17. [Google Scholar] [CrossRef]
- Pyne, M.E.; Bruder, M.; Moo-Young, M.; Chung, D.; Chou, C.P. Harnessing Heterologous and Endogenous CRISPR-Cas Machineries for Efficient Markerless Genome Editing in Clostridium. U.S. Patent 16/098,035, 16 May 2019. [Google Scholar]
- Park, J.; Shin, H.; Lee, S.-M.; Um, Y.; Woo, H.M. RNA-guided single/double gene repressions in Corynebacterium glutamicum using an efficient CRISPR interference and its application to industrial strain. Microb. Cell Factories 2018, 17, 4. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Liu, J.; Guo, Y.; Fan, L.; Ni, X.; Zheng, X.; Wang, M.; Zheng, P.; Sun, J. MACBETH: Multiplex automated Corynebacterium glutamicum base editing method. Metab. Eng. 2018, 47, 200–210. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Lu, Y.; Zheng, P.; Sun, J.; Ma, Y. Development of a CRISPR/Cas9 genome editing toolbox for Corynebacterium glutamicum. Microb. Cell Factories 2017, 16, 205. [Google Scholar] [CrossRef]
- Jiang, Y.; Qian, F.; Yang, J.; Liu, Y.; Dong, F.; Xu, C.; Sun, B.; Chen, B.; Xu, X.; Li, Y. CRISPR-Cpf1 assisted genome editing of Corynebacterium glutamicum. Nat. Commun. 2017, 8, 15179. [Google Scholar] [CrossRef]
- Peng, F.; Wang, X.; Sun, Y.; Dong, G.; Yang, Y.; Liu, X.; Bai, Z. Efficient gene editing in Corynebacterium glutamicum using the CRISPR/Cas9 system. Microb. Cell Factories 2017, 16, 201. [Google Scholar] [CrossRef]
- Cho, J.S.; Choi, K.R.; Prabowo, C.P.S.; Shin, J.H.; Yang, D.; Jang, J.; Lee, S.Y. CRISPR/Cas9-coupled recombineering for metabolic engineering of Corynebacterium glutamicum. Metab. Eng. 2017, 42, 157–167. [Google Scholar] [CrossRef]
- Cleto, S.; Jensen, J.V.; Wendisch, V.F.; Lu, T.K. Corynebacterium glutamicum metabolic engineering with CRISPR interference (CRISPRi). ACS Synth. Biol. 2016, 5, 375–385. [Google Scholar] [CrossRef]
- Zhu, X.; Zhao, D.; Qiu, H.; Fan, F.; Man, S.; Bi, C.; Zhang, X. The CRISPR/Cas9-facilitated multiplex pathway optimization (CFPO) technique and its application to improve the Escherichia coli xylose utilization pathway. Metab. Eng. 2017, 43, 37–45. [Google Scholar] [CrossRef]
- Lv, L.; Ren, Y.-L.; Chen, J.-C.; Wu, Q.; Chen, G.-Q. Application of CRISPRi for prokaryotic metabolic engineering involving multiple genes, a case study: Controllable P (3HB-co-4HB) biosynthesis. Metab. Eng. 2015, 29, 160–168. [Google Scholar] [CrossRef]
- Yan, M.Y.; Yan, H.Q.; Ren, G.-X.; Zhao, J.P.; Guo, X.P.; Sun, Y.-C. CRISPR-Cas12a-assisted recombineering in bacteria. Appl. Environ. Microbiol. 2017, 83, e00947-17. [Google Scholar] [CrossRef]
- Hao, N.; Shearwin, K.E.; Dodd, I.B. Programmable DNA looping using engineered bivalent dCas9 complexes. Nat. Commun. 2017, 8, 1628. [Google Scholar] [CrossRef] [PubMed]
- Ao, X.; Yao, Y.; Li, T.; Yang, T.T.; Dong, X.; Zheng, Z.T.; Chen, G.Q.; Wu, Q.; Guo, Y. A multiplex genome editing method for Escherichia coli based on CRISPR-Cas12a. Front. Microbiol. 2018, 9, 2307. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Wang, S.; Hu, G.; Guo, L.; Chen, X.; Xu, P.; Liu, L. Engineering Escherichia coli for malate production by integrating modular pathway characterization with CRISPRi-guided multiplexed metabolic tuning. Biotechnol. Bioeng. 2018, 115, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.E.; Yeh, I.H.; Sung, L.Y.; Wu, M.Y.; Chao, Y.P.; Ng, I.S.; Hu, Y.C. Enhanced integration of large DNA into E. coli chromosome by CRISPR/Cas9. Biotechnol. Bioeng. 2017, 114, 172–183. [Google Scholar] [CrossRef]
- Wu, M.Y.; Sung, L.Y.; Li, H.; Huang, C.H.; Hu, Y.C. Combining CRISPR and CRISPRi systems for metabolic engineering of E. coli and 1, 4-BDO biosynthesis. ACS Synth. Biol. 2017, 6, 2350–2361. [Google Scholar] [CrossRef] [PubMed]
- Heo, M.J.; Jung, H.M.; Um, J.; Lee, S.W.; Oh, M.K. Controlling citrate synthase expression by CRISPR/Cas9 genome editing for n-butanol production in Escherichia coli. ACS Synth. Biol. 2017, 6, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, J.; Cheng, Q.; Zheng, X.; Zhao, G.; Wang, J. Multiplex gene regulation by CRISPR-ddCpf1. Cell Discov. 2017, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- de Maat, V.; Stege, P.B.; Dedden, M.; Hamer, M.; van Pijkeren, J.-P.; Willems, R.J.; van Schaik, W. CRISPR-Cas9-mediated genome editing in vancomycin-resistant Enterococcus faecium. FEMS Microbiol. Lett. 2019, 366, fnz256. [Google Scholar] [CrossRef]
- Price, V.J.; Huo, W.; Sharifi, A.; Palmer, K.L. CRISPR-Cas and restriction-modification act additively against conjugative antibiotic resistance plasmid transfer in Enterococcus faecalis. mSphere 2016, 1, e00064-16. [Google Scholar] [CrossRef]
- Hullahalli, K.; Rodrigues, M.; Palmer, K.L. Exploiting CRISPR-Cas to manipulate Enterococcus faecalis populations. eLlife 2017, 6, e26664. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, Y.; Dong, N.; Shen, L.; Zhou, H.; Hu, Y.; Gu, D.; Chen, S.; Zhang, R.; Ji, Q. Application of CRISPR/Cas9-based genome editing in studying the mechanism of pandrug resistance in Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2019, 63, e00113-19. [Google Scholar] [CrossRef]
- Song, X.; Huang, H.; Xiong, Z.; Ai, L.; Yang, S. CRISPR-Cas9D10A nickase-assisted genome editing in Lactobacillus casei. Appl. Environ. Microbiol. 2017, 83, e01259-17. [Google Scholar] [CrossRef]
- Sanozky-Dawes, R.; Selle, K.; O’Flaherty, S.; Klaenhammer, T.; Barrangou, R. Occurrence and activity of a type II CRISPR-Cas system in Lactobacillus gasseri. Microbiology 2015, 161, 1752–1761. [Google Scholar] [CrossRef]
- Oh, J.-H.; van Pijkeren, J.-P. CRISPR–Cas9-assisted recombineering in Lactobacillus reuteri. Nucleic Acids Res. 2014, 42, e131. [Google Scholar] [CrossRef]
- Singh, A.K.; Carette, X.; Potluri, L.-P.; Sharp, J.D.; Xu, R.; Prisic, S.; Husson, R.N. Investigating essential gene function in Mycobacterium tuberculosis using an efficient CRISPR interference system. Nucleic Acids Res. 2016, 44, e143. [Google Scholar] [CrossRef]
- Choudhary, E.; Thakur, P.; Pareek, M.; Agarwal, N. Gene silencing by CRISPR interference in mycobacteria. Nat. Commun. 2015, 6, 6267. [Google Scholar] [CrossRef]
- Rock, J.M.; Hopkins, F.F.; Chavez, A.; Diallo, M.; Chase, M.R.; Gerrick, E.R.; Pritchard, J.R.; Church, G.M.; Rubin, E.J.; Sassetti, C.M. Programmable transcriptional repression in mycobacteria using an orthogonal CRISPR interference platform. Nat. Microbiol. 2017, 2, 16274. [Google Scholar] [CrossRef]
- Xiang, L.; Qi, F.; Jiang, L.; Tan, J.; Deng, C.; Wei, Z.; Jin, S.; Huang, G. CRISPR-dCas9-mediated knockdown of prtR, an essential gene in Pseudomonas aeruginosa. Lett. Appl. Microbiol. 2020, 71, 386–393. [Google Scholar] [CrossRef]
- Tan, S.Z.; Reisch, C.R.; Prather, K.L. A robust CRISPR interference gene repression system in Pseudomonas. J. Bacteriol. 2018, 200, e00575-17. [Google Scholar] [CrossRef]
- Gu, T.; Zhao, S.; Pi, Y.; Chen, W.; Chen, C.; Liu, Q.; Li, M.; Han, D.; Ji, Q. Highly efficient base editing in Staphylococcus aureus using an engineered CRISPR RNA-guided cytidine deaminase. Chem. Sci. 2018, 9, 3248–3253. [Google Scholar] [CrossRef]
- Liu, Q.; Jiang, Y.; Shao, L.; Yang, P.; Sun, B.; Yang, S.; Chen, D. CRISPR/Cas9-based efficient genome editing in Staphylococcus aureus. Acta Biochim. Biophys. Sin. 2017, 49, 764–770. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhang, Y.; Yeo, W.-S.; Bae, T.; Ji, Q. Rapid and efficient genome editing in Staphylococcus aureus by using an engineered CRISPR/Cas9 system. J. Am. Chem. Soc. 2017, 139, 3790–3795. [Google Scholar] [CrossRef] [PubMed]
- Cobb, L.H.; Park, J.; Swanson, E.A.; Beard, M.C.; McCabe, E.M.; Rourke, A.S.; Seo, K.S.; Olivier, A.K.; Priddy, L.B. CRISPR-Cas9 modified bacteriophage for treatment of Staphylococcus aureus induced osteomyelitis and soft tissue infection. PLoS ONE 2019, 14, e0220421. [Google Scholar] [CrossRef]
- Guan, J.; Wang, W.; Sun, B. Chromosomal targeting by the type III-A CRISPR-Cas system can reshape genomes in Staphylococcus aureus. mSphere 2017, 2, e00403-17. [Google Scholar] [CrossRef] [PubMed]
- Penewit, K.; Holmes, E.A.; McLean, K.; Ren, M.; Waalkes, A.; Salipante, S.J. Efficient and scalable precision genome editing in Staphylococcus aureus through conditional recombineering and CRISPR/Cas9-mediated counterselection. mBio 2018, 9, e00067-18. [Google Scholar] [CrossRef]
- Park, J.Y.; Moon, B.Y.; Park, J.W.; Thornton, J.A.; Park, Y.H.; Seo, K.S. Genetic engineering of a temperate phage-based delivery system for CRISPR/Cas9 antimicrobials against Staphylococcus aureus. Sci. Rep. 2017, 7, 44929. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cobb, R.; Zhao, H. High-efficiency genome editing of Streptomyces species by an engineered CRISPR/Cas system. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2016; Volume 575, pp. 271–284. [Google Scholar]
- Li, L.; Wei, K.; Zheng, G.; Liu, X.; Chen, S.; Jiang, W.; Lu, Y. CRISPR-Cpf1-assisted multiplex genome editing and transcriptional repression in Streptomyces. Appl. Environ. Microbiol. 2018, 84, e00827-18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.M.; Wong, F.T.; Wang, Y.; Luo, S.; Lim, Y.H.; Heng, E.; Yeo, W.L.; Cobb, R.E.; Enghiad, B.; Ang, E.L. CRISPR–Cas9 strategy for activation of silent Streptomyces biosynthetic gene clusters. Nat. Chem. Biol. 2017, 13, 607–609. [Google Scholar] [CrossRef]
- Zeng, H.; Wen, S.; Xu, W.; He, Z.; Zhai, G.; Liu, Y.; Deng, Z.; Sun, Y. Highly efficient editing of the actinorhodin polyketide chain length factor gene in Streptomyces coelicolor M145 using CRISPR/Cas9-CodA (sm) combined system. Appl. Microbiol. Biotechnol. 2015, 99, 10575–10585. [Google Scholar] [CrossRef]
- Qiu, Y.; Wang, S.; Chen, Z.; Guo, Y.; Song, Y. An active type IE CRISPR-Cas system identified in Streptomyces avermitilis. PLoS ONE 2016, 11, e0149533. [Google Scholar] [CrossRef]
- Jia, H.; Zhang, L.; Wang, T.; Han, J.; Tang, H.; Zhang, L. Development of a CRISPR/Cas9-mediated gene-editing tool in Streptomyces rimosus. Microbiology 2017, 163, 1148–1155. [Google Scholar] [CrossRef]
- Cobb, R.E.; Wang, Y.; Zhao, H. High-efficiency multiplex genome editing of Streptomyces species using an engineered CRISPR/Cas system. ACS Synth. Biol. 2015, 4, 723–728. [Google Scholar] [CrossRef]
- Huang, H.; Zheng, G.; Jiang, W.; Hu, H.; Lu, Y. One-step high-efficiency CRISPR/Cas9-mediated genome editing in Streptomyces. Acta Biochim. Biophys. Sin. 2015, 47, 231–243. [Google Scholar] [CrossRef]
- Wendt, K.E.; Ungerer, J.; Cobb, R.E.; Zhao, H.; Pakrasi, H.B. CRISPR/Cas9 mediated targeted mutagenesis of the fast growing cyanobacterium Synechococcus elongatus UTEX 2973. Microb. Cell Factories 2016, 15, 115. [Google Scholar] [CrossRef]
- González de Aledo, M.; González-Bardanca, M.; Blasco, L.; Pacios, O.; Bleriot, I.; Fernández-García, L.; Fernández-Quejo, M.; López, M.; Bou, G.; Tomás, M. CRISPR-cas, a revolution in the treatment and study of ESKAPE infections: Pre-clinical studies. Antibiotics 2021, 10, 756. [Google Scholar] [CrossRef]
- Liu, Z.; Dong, H.; Cui, Y.; Cong, L.; Zhang, D. Application of different types of CRISPR/Cas-based systems in bacteria. Microb. Cell Factories 2020, 19, 172. [Google Scholar] [CrossRef]
- Karah, N.; Samuelsen, Ø.; Zarrilli, R.; Sahl, J.W.; Wai, S.N.; Uhlin, B.E. CRISPR-cas subtype I-Fb in Acinetobacter baumannii: Evolution and utilization for strain subtyping. PLoS ONE 2015, 10, e0118205. [Google Scholar] [CrossRef]
- Karah, N.; Wai, S.N.; Uhlin, B.E. CRISPR-based subtyping to track the evolutionary history of a global clone of Acinetobacter baumannii. Infect. Genet. Evol. 2021, 90, 104774. [Google Scholar] [CrossRef]
- Pourcel, C.; Touchon, M.; Villeriot, N.; Vernadet, J.P.; Couvin, D.; Toffano-Nioche, C.; Vergnaud, G. CRISPRCasdb a successor of CRISPRdb containing CRISPR arrays and cas genes from complete genome sequences, and tools to download and query lists of repeats and spacers. Nucleic Acids Res. 2020, 48, D535–D544. [Google Scholar] [CrossRef]
- Mangas, E.L.; Rubio, A.; Álvarez-Marín, R.; Labrador-Herrera, G.; Pachón, J.; Pachón-Ibáñez, M.E.; Divina, F.; Pérez-Pulido, A.J. Pangenome of Acinetobacter baumannii uncovers two groups of genomes, one of them with genes involved in CRISPR/Cas defence systems associated with the absence of plasmids and exclusive genes for biofilm formation. Microb. Genom. 2019, 5, e000309. [Google Scholar] [CrossRef]
- Di Nocera, P.P.; Rocco, F.; Giannouli, M.; Triassi, M.; Zarrilli, R. Genome organization of epidemic Acinetobacter baumannii strains. BMC Microbiol. 2011, 11, 224. [Google Scholar] [CrossRef] [PubMed]
- Grissa, I.; Vergnaud, G.; Pourcel, C. The CRISPRdb database and tools to display CRISPRs and to generate dictionaries of spacers and repeats. BMC Bioinform. 2007, 8, 172. [Google Scholar] [CrossRef] [PubMed]
- Hauck, Y.; Soler, C.; Jault, P.; Mérens, A.; Gérome, P.; Nab, C.M.; Trueba, F.; Bargues, L.; Thien, H.V.; Vergnaud, G. Diversity of Acinetobacter baumannii in four French military hospitals, as assessed by multiple locus variable number of tandem repeats analysis. PLoS ONE 2012, 7, e44597. [Google Scholar] [CrossRef] [PubMed]
- Tyumentseva, M.; Mikhaylova, Y.; Prelovskaya, A.; Tyumentsev, A.; Petrova, L.; Fomina, V.; Zamyatin, M.; Shelenkov, A.; Akimkin, V. Genomic and phenotypic analysis of multidrug-resistant Acinetobacter baumannii clinical isolates carrying different types of CRISPR/Cas systems. Pathogens 2021, 10, 205. [Google Scholar] [CrossRef] [PubMed]
- Horvath, P.; Romero, D.A.; Coûté-Monvoisin, A.-C.; Richards, M.; Deveau, H.; Moineau, S.; Boyaval, P.; Fremaux, C.; Barrangou, R. Diversity, activity, and evolution of CRISPR loci in Streptococcus thermophilus. J. Bacteriol. 2008, 190, 1401–1412. [Google Scholar] [CrossRef]
- Mohanraju, P.; Saha, C.; van Baarlen, P.; Louwen, R.; Staals, R.H.; van der Oost, J. Alternative functions of CRISPR–Cas systems in the evolutionary arms race. Nat. Rev. Microbiol. 2022, 20, 351–364. [Google Scholar] [CrossRef]
- Pinilla-Redondo, R.; Mayo-Muñoz, D.; Russel, J.; Garrett, R.A.; Randau, L.; Sørensen, S.J.; Shah, S.A. Type IV CRISPR–Cas systems are highly diverse and involved in competition between plasmids. Nucleic Acids Res. 2020, 48, 2000–2012. [Google Scholar] [CrossRef]
- Dong, J.F.; Feng, C.J.; Wang, P.; Li, R.Q.; Zou, Q.H. Comparative genomics analysis of Acinetobacter baumannii multi-drug resistant and drug sensitive strains in China. Microb. Pathog. 2022, 165, 105492. [Google Scholar] [CrossRef]
- Jalal, D.; Elzayat, M.G.; Diab, A.A.; El-Shqanqery, H.E.; Samir, O.; Bakry, U.; Hassan, R.; Elanany, M.; Shalaby, L.; Sayed, A.A. Deciphering multidrug-resistant Acinetobacter baumannii from a pediatric cancer hospital in Egypt. mSphere 2021, 6, e00725-21. [Google Scholar] [CrossRef]
- Montaña, S.; Vilacoba, E.; Fernandez, J.S.; Traglia, G.M.; Sucari, A.; Pennini, M.; Iriarte, A.; Centron, D.; Melano, R.G.; Ramírez, M.S. Genomic analysis of two Acinetobacter baumannii strains belonging to two different sequence types (ST172 and ST25). J. Glob. Antimicrob. Resist. 2020, 23, 154–161. [Google Scholar] [CrossRef]
- Mortensen, K.; Lam, T.J.; Ye, Y. Comparison of CRISPR–Cas immune systems in healthcare-related pathogens. Front. Microbiol. 2021, 12, 758782. [Google Scholar] [CrossRef]
- Shelenkov, A.; Petrova, L.; Zamyatin, M.; Mikhaylova, Y.; Akimkin, V. Diversity of international high-risk clones of Acinetobacter baumannii revealed in a Russian multidisciplinary medical center during 2017–2019. Antibiotics 2021, 10, 1009. [Google Scholar] [CrossRef]
- Pursey, E.; Dimitriu, T.; Paganelli, F.L.; Westra, E.R.; van Houte, S. CRISPR-Cas is associated with fewer antibiotic resistance genes in bacterial pathogens. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2022, 377, 20200464. [Google Scholar] [CrossRef]
- Shehreen, S.; Chyou, T.Y.; Fineran, P.C.; Brown, C.M. Genome-wide correlation analysis suggests different roles of CRISPR-Cas systems in the acquisition of antibiotic resistance genes in diverse species. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2019, 374, 20180384. [Google Scholar] [CrossRef]
- Yadav, G.; Singh, R. In silico analysis reveals the co-existence of CRISPR-Cas type I-F1 and type I-F2 systems and its association with restricted phage invasion in Acinetobacter baumannii. Front. Microbiol. 2022, 13, 909886. [Google Scholar] [CrossRef]
- Carte, J.; Christopher, R.T.; Smith, J.T.; Olson, S.; Barrangou, R.; Moineau, S.; Glover III, C.V.; Graveley, B.R.; Terns, R.M.; Terns, M.P. The three major types of CRISPR-Cas systems function independently in CRISPR RNA biogenesis in Streptococcus thermophilus. Mol. Microbiol. 2014, 93, 98–112. [Google Scholar] [CrossRef]
- Majumdar, S.; Zhao, P.; Pfister, N.T.; Compton, M.; Olson, S.; Glover, C.V.; Wells, L.; Graveley, B.R.; Terns, R.M.; Terns, M.P. Three CRISPR-Cas immune effector complexes coexist in Pyrococcus furiosus. RNA 2015, 21, 1147–1158. [Google Scholar] [CrossRef]
- Silas, S.; Lucas-Elio, P.; Jackson, S.A.; Aroca-Crevillén, A.; Hansen, L.L.; Fineran, P.C.; Fire, A.Z.; Sánchez-Amat, A. Correction: Type III CRISPR-Cas systems can provide redundancy to counteract viral escape from type I systems. eLife 2018, 7, e36853. [Google Scholar] [CrossRef]
- Nussenzweig, P.M.; Marraffini, L.A. Molecular mechanisms of CRISPR-Cas immunity in bacteria. Annu. Rev. Genet. 2020, 54, 93–120. [Google Scholar] [CrossRef]
- Sykes, E.M.; Deo, S.; Kumar, A. Recent advances in genetic tools for Acinetobacter baumannii. Front. Genet. 2020, 11, 601380. [Google Scholar] [CrossRef]
- Aydin, S.; Personne, Y.; Newire, E.; Laverick, R.; Russell, O.; Roberts, A.P.; Enne, V.I. Presence of Type IF CRISPR/Cas systems is associated with antimicrobial susceptibility in Escherichia coli. J. Antimicrob. Chemother. 2017, 72, 2213–2218. [Google Scholar] [CrossRef] [PubMed]
- Leungtongkam, U.; Thummeepak, R.; Kitti, T.; Tasanapak, K.; Wongwigkarn, J.; Styles, K.M.; Wellington, E.M.; Millard, A.D.; Sagona, A.P.; Sitthisak, S. Genomic analysis reveals high virulence and antibiotic resistance amongst phage susceptible Acinetobacter baumannii. Sci. Rep. 2020, 10, 16154. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.D.; Scott, D.A.; Weinstein, J.A.; Ran, F.A.; Konermann, S.; Agarwala, V.; Li, Y.; Fine, E.J.; Wu, X.; Shalem, O. DNA targeting specificity of RNA-guided Cas9 nucleases. Nat. Biotechnol. 2013, 31, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Yang, J.; Sun, X.; Wang, Y.; Yang, L.; Kong, G.; Jiao, H.; Bao, G.; Li, G. Whole-Genome Analysis of Acinetobacter baumannii Strain AB43 Containing a Type I-Fb CRISPR-Cas System: Insights into the Relationship with Drug Resistance. Molecules 2022, 27, 5665. [Google Scholar] [CrossRef]
- Ten, K.E.; Md Zoqratt, M.Z.H.; Ayub, Q.; Tan, H.S. Characterization of multidrug-resistant Acinetobacter baumannii strain ATCC BAA1605 using whole-genome sequencing. BMC Res. Notes 2021, 14, 83. [Google Scholar] [CrossRef]
- Mayer, C.; Muras, A.; Romero, M.; López, M.; Tomás, M.; Otero, A. Multiple quorum quenching enzymes are active in the nosocomial pathogen Acinetobacter baumannii ATCC17978. Front. Cell. Infect. Microbiol. 2018, 8, 310. [Google Scholar] [CrossRef]
- Karlapudi, A.P.; Venkateswarulu, T.; Tammineedi, J.; Srirama, K.; Kanumuri, L.; Kodali, V.P. In silico sgRNA tool design for CRISPR control of quorum sensing in Acinetobacter species. Genes Dis. 2018, 5, 123–129. [Google Scholar] [CrossRef]
- Tucker, A.T.; Nowicki, E.M.; Boll, J.M.; Knauf, G.A.; Burdis, N.C.; Trent, M.S.; Davies, B.W. Defining gene-phenotype relationships in Acinetobacter baumannii through one-step chromosomal gene inactivation. mBio 2014, 5, e01313-14. [Google Scholar] [CrossRef]
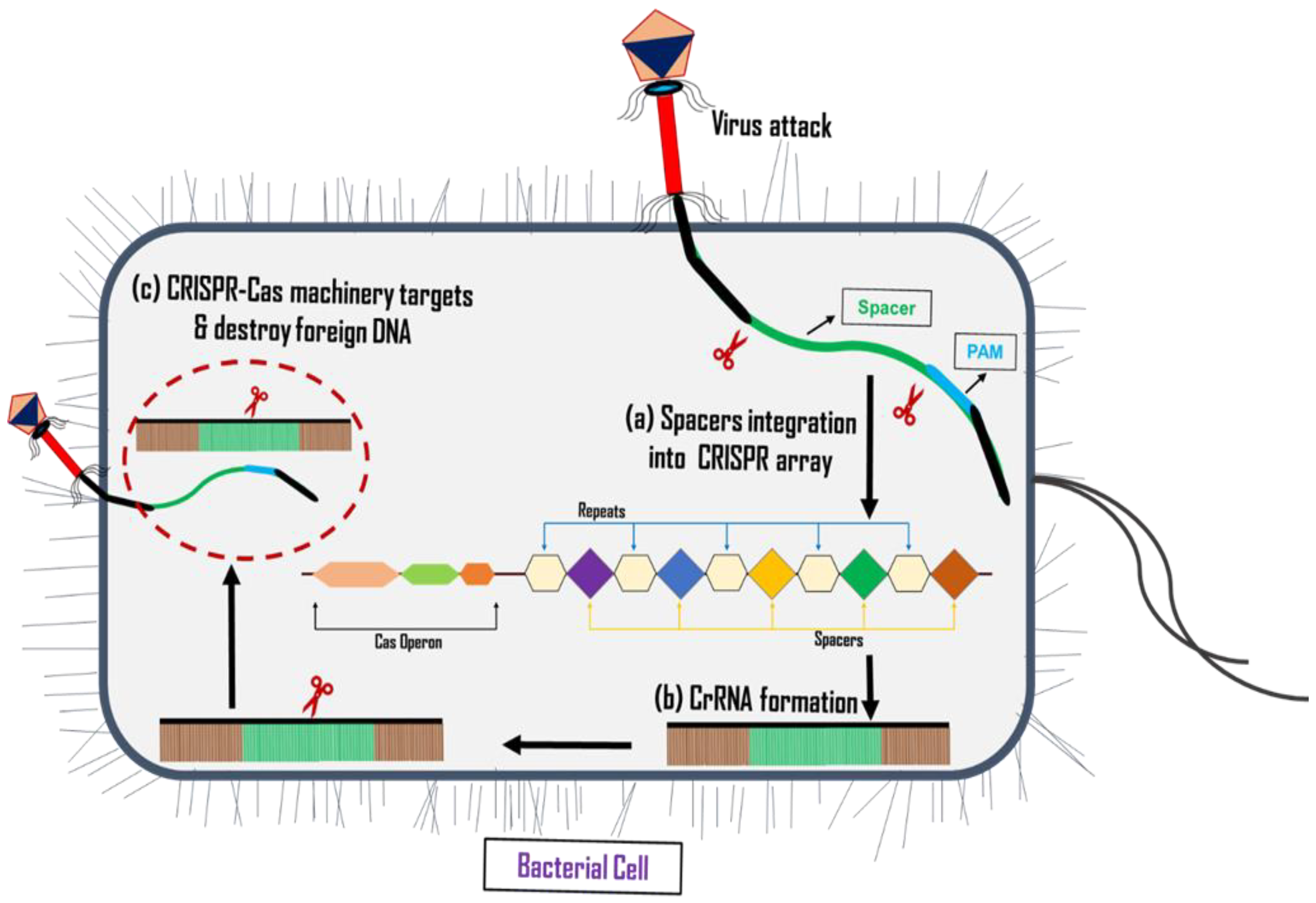
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Junaid, M.; Thirapanmethee, K.; Khuntayaporn, P.; Chomnawang, M.T. CRISPR-Based Gene Editing in Acinetobacter baumannii to Combat Antimicrobial Resistance. Pharmaceuticals 2023, 16, 920. https://doi.org/10.3390/ph16070920
Junaid M, Thirapanmethee K, Khuntayaporn P, Chomnawang MT. CRISPR-Based Gene Editing in Acinetobacter baumannii to Combat Antimicrobial Resistance. Pharmaceuticals. 2023; 16(7):920. https://doi.org/10.3390/ph16070920
Chicago/Turabian StyleJunaid, Muhammad, Krit Thirapanmethee, Piyatip Khuntayaporn, and Mullika Traidej Chomnawang. 2023. "CRISPR-Based Gene Editing in Acinetobacter baumannii to Combat Antimicrobial Resistance" Pharmaceuticals 16, no. 7: 920. https://doi.org/10.3390/ph16070920
APA StyleJunaid, M., Thirapanmethee, K., Khuntayaporn, P., & Chomnawang, M. T. (2023). CRISPR-Based Gene Editing in Acinetobacter baumannii to Combat Antimicrobial Resistance. Pharmaceuticals, 16(7), 920. https://doi.org/10.3390/ph16070920





