EYE-503: A Novel Retinoic Acid Drug for Treating Retinal Neurodegeneration
Abstract
1. Introduction
2. Results
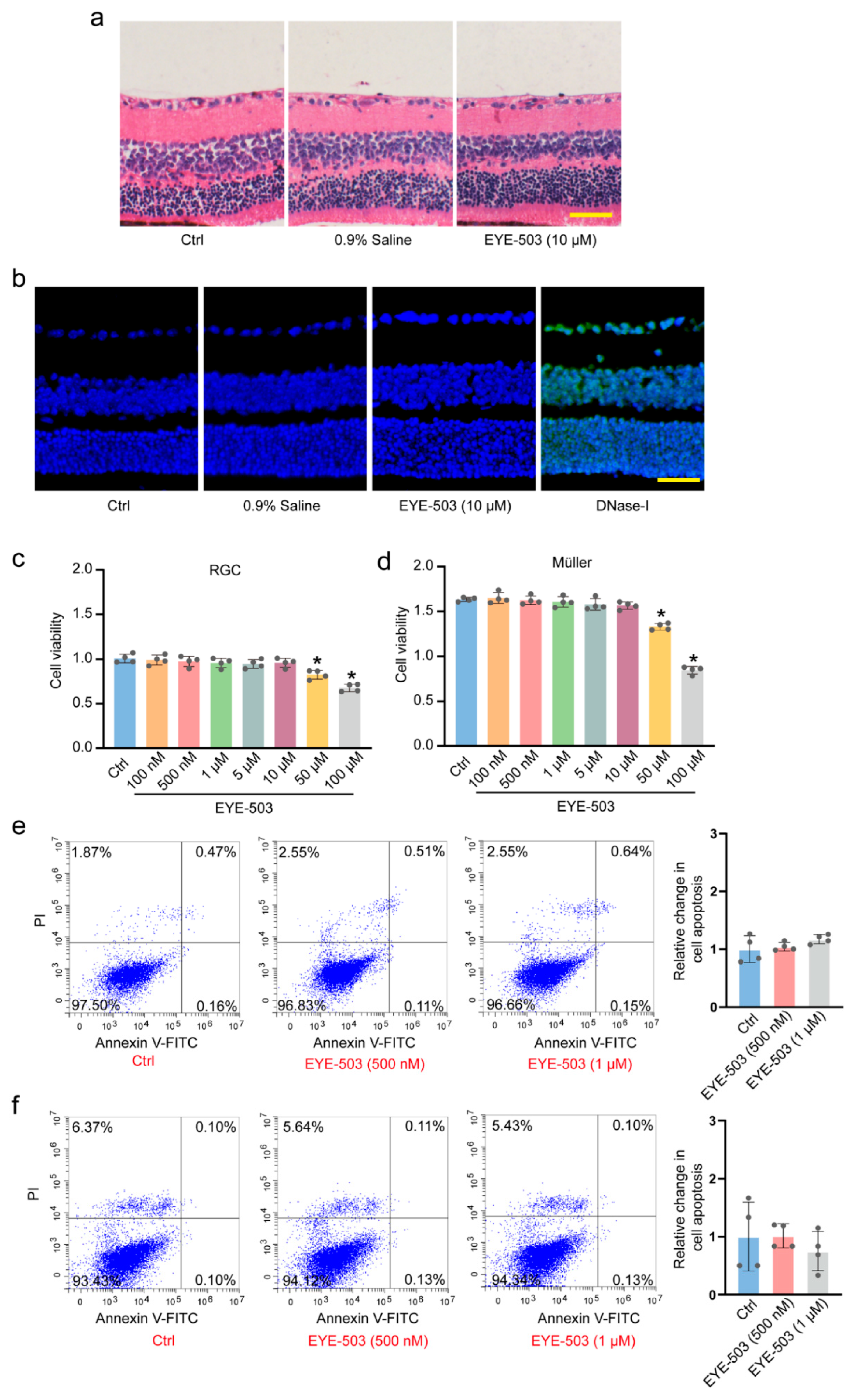
2.1. EYE-503 Has No Detectable Cytotoxicity and Tissue Toxicity
2.2. EYE-503 Administration Inhibits Retinal Reactive Gliosis and Contributes to RGC Survival
2.3. EYE-503 Administration Alleviates ONC-Induced Retinal Injury and Optic Nerve Injury In Vivo
2.4. EYE-503 Administration Regulates Müller Cell and RGC Function In Vitro
2.5. EYE-503 Administration Induces Bcl-2 Downregulation and Bax Translocation
2.6. The Targets of EYE-503 Were Predicted by Network Pharmacology
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Optic Nerve Crush (ONC) Model
4.3. Intravitreal Injection
4.4. Flash Visual Evoked Potentials (F-VEP) Recording
4.5. Hematoxylin and Eosin (H&E) Staining
4.6. Terminal Deoxynucleotidyl Transferase dUTP Nick-End Labeling (TUNEL) Assay
4.7. Immunohistochemistry
4.8. Retinal Flat-Mounts and TUBB3 Staining
4.9. Cell Culture and Treatment
4.10. Cell Counting Kit-8 Assay
4.11. Calcein-AM and Propidium Iodide (PI) Double Staining
4.12. EdU Incorporation Assay
4.13. Rhodamine123 Staining
4.14. Flow Cytometry Analysis
4.15. Western Blot
4.16. Screening for the Targets of EYE-503
4.17. PPI Network Analysis
4.18. GO Enrichment and KEGG Pathway Analysis
4.19. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khalilpour, S.; Latifi, S.; Behnammanesh, G.; Majid, A.; Majid, A.S.A.; Tamayol, A. Ischemic optic neuropathy as a model of neurodegenerative disorder: A review of pathogenic mechanism of axonal degeneration and the role of neuroprotection. J. Neurol. Sci. 2017, 375, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Marchesi, N.; Fahmideh, F.; Boschi, F.; Pascale, A.; Barbieri, A. Ocular Neurodegenerative Diseases: Interconnection between Retina and Cortical Areas. Cells 2021, 10, 2394. [Google Scholar] [CrossRef] [PubMed]
- Simo, R.; Stitt, A.W.; Gardner, T.W. Neurodegeneration in diabetic retinopathy: Does it really matter? Diabetologia 2018, 61, 1902–1912. [Google Scholar] [CrossRef] [PubMed]
- Kojima, K.; Kitaoka, Y.; Munemasa, Y.; Hirano, A.; Sase, K.; Takagi, H. Axonal protection by modulation of p62 expression in TNF-induced optic nerve degeneration. Neurosci. Lett. 2014, 581, 37–41. [Google Scholar] [CrossRef]
- Eastlake, K.; Luis, J.; Limb, G.A. Potential of Muller Glia for Retina Neuroprotection. Curr. Eye Res. 2020, 45, 339–348. [Google Scholar] [CrossRef]
- Fernandez-Albarral, J.A.; de Hoz, R.; Ramirez, A.I.; Lopez-Cuenca, I.; Salobrar-Garcia, E.; Pinazo-Duran, M.D.; Ramirez, J.M.; Salazar, J.J. Beneficial effects of saffron (Crocus sativus L.) in ocular pathologies, particularly neurodegenerative retinal diseases. Neural Regen. Res. 2020, 15, 1408–1416. [Google Scholar] [CrossRef]
- Perron, N.R.; Nasarre, C.; Bandyopadhyay, M.; Beeson, C.C.; Rohrer, B. SAHA is neuroprotective in in vitro and in situ models of retinitis pigmentosa. Mol. Vis. 2021, 27, 151–160. [Google Scholar]
- Li, G.Y.; Fan, B.; Zheng, Y.C. Calcium overload is a critical step in programmed necrosis of ARPE-19 cells induced by high-concentration H2O2. Biomed. Environ. Sci. 2010, 23, 371–377. [Google Scholar] [CrossRef]
- Galindo-Romero, C.; Harun-Or-Rashid, M.; Jimenez-Lopez, M.; Vidal-Sanz, M.; Agudo-Barriuso, M.; Hallbook, F. Neuroprotection by alpha2-Adrenergic Receptor Stimulation after Excitotoxic Retinal Injury: A Study of the Total Population of Retinal Ganglion Cells and Their Distribution in the Chicken Retina. PLoS ONE 2016, 11, e0161862. [Google Scholar] [CrossRef]
- Opere, C.A.; Heruye, S.; Njie-Mbye, Y.F.; Ohia, S.E.; Sharif, N.A. Regulation of Excitatory Amino Acid Transmission in the Retina: Studies on Neuroprotection. J. Ocul. Pharmacol. Ther. 2018, 34, 107–118. [Google Scholar] [CrossRef]
- Martinez-Solis, I.; Acero, N.; Bosch-Morell, F.; Castillo, E.; Gonzalez-Rosende, M.E.; Munoz-Mingarro, D.; Ortega, T.; Sanahuja, M.A.; Villagrasa, V. Neuroprotective Potential of Ginkgo biloba in Retinal Diseases. Planta Med. 2019, 85, 1292–1303. [Google Scholar] [CrossRef] [PubMed]
- Fudalej, E.; Justyniarska, M.; Kasarello, K.; Dziedziak, J.; Szaflik, J.P.; Cudnoch-Jedrzejewska, A. Neuroprotective Factors of the Retina and Their Role in Promoting Survival of Retinal Ganglion Cells: A Review. Ophthalmic Res. 2021, 64, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Pethe, P. Differential expression of retinoic acid alpha and beta receptors in neuronal progenitors generated from human embryonic stem cells in response to TTNPB (a retinoic acid mimetic). Differentiation 2021, 121, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Dewett, D.; Lam-Kamath, K.; Poupault, C.; Khurana, H.; Rister, J. Mechanisms of vitamin A metabolism and deficiency in the mammalian and fly visual system. Dev. Biol. 2021, 476, 68–78. [Google Scholar] [CrossRef]
- Kang, J.B.; Park, D.J.; Shah, M.A.; Koh, P.O. Retinoic acid exerts neuroprotective effects against focal cerebral ischemia by preventing apoptotic cell death. Neurosci. Lett. 2021, 757, 135979. [Google Scholar] [CrossRef]
- Regen, F.; Hellmann-Regen, J.; Costantini, E.; Reale, M. Neuroinflammation and Alzheimer’s Disease: Implications for Microglial Activation. Curr. Alzheimer Res. 2017, 14, 1140–1148. [Google Scholar] [CrossRef]
- Chang, K.H.; Chen, C.M. The Role of Oxidative Stress in Parkinson’s Disease. Antioxidants 2020, 9, 597. [Google Scholar] [CrossRef]
- Mohsin Alvi, A.; Tariq Al Kury, L.; Umar Ijaz, M.; Ali Shah, F.; Tariq Khan, M.; Sadiq Sheikh, A.; Nadeem, H.; Khan, A.U.; Zeb, A.; Li, S. Post-Treatment of Synthetic Polyphenolic 1,3,4 Oxadiazole Compound A3, Attenuated Ischemic Stroke-Induced Neuroinflammation and Neurodegeneration. Biomolecules 2020, 10, 816. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, Y.; Yang, F.; Chen, W.; Jiang, J.; He, P.; Jiang, S.; Li, M.; Xu, R. All-Trans Retinoic Acid Exerts Neuroprotective Effects in Amyotrophic Lateral Sclerosis-Like Tg (SOD1*G93A)1Gur Mice. Mol. Neurobiol. 2020, 57, 3603–3615. [Google Scholar] [CrossRef]
- Cai, W.; Wang, J.; Hu, M.; Chen, X.; Lu, Z.; Bellanti, J.A.; Zheng, S.G. All trans-retinoic acid protects against acute ischemic stroke by modulating neutrophil functions through STAT1 signaling. J. Neuroinflamm. 2019, 16, 175. [Google Scholar] [CrossRef]
- Sabbaghziarani, F.; Mortezaee, K.; Akbari, M.; Kashani, I.R.; Soleimani, M.; Moini, A.; Ataeinejad, N.; Zendedel, A.; Hassanzadeh, G. Retinoic acid-pretreated Wharton’s jelly mesenchymal stem cells in combination with triiodothyronine improve expression of neurotrophic factors in the subventricular zone of the rat ischemic brain injury. Metab. Brain Dis. 2017, 32, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Takamura, R.; Watamura, N.; Nikkuni, M.; Ohshima, T. All-trans retinoic acid improved impaired proliferation of neural stem cells and suppressed microglial activation in the hippocampus in an Alzheimer’s mouse model. J. Neurosci. Res. 2017, 95, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Isla-Magrane, H.; Zufiaurre-Seijo, M.; Garcia-Arumi, J.; Duarri, A. All-trans retinoic acid modulates pigmentation, neuroretinal maturation, and corneal transparency in human multiocular organoids. Stem Cell Res. Ther. 2022, 13, 376. [Google Scholar] [CrossRef] [PubMed]
- Telias, M.; Denlinger, B.; Helft, Z.; Thornton, C.; Beckwith-Cohen, B.; Kramer, R.H. Retinoic Acid Induces Hyperactivity, and Blocking Its Receptor Unmasks Light Responses and Augments Vision in Retinal Degeneration. Neuron 2019, 102, 574–586.e575. [Google Scholar] [CrossRef]
- Lin, H.S.; Chean, C.S.; Ng, Y.Y.; Chan, S.Y.; Ho, P.C. 2-hydroxypropyl-beta-cyclodextrin increases aqueous solubility and photostability of all-trans-retinoic acid. J. Clin. Pharm. Ther. 2000, 25, 265–269. [Google Scholar] [CrossRef]
- Heynen, G.J.; Nevedomskaya, E.; Palit, S.; Jagalur Basheer, N.; Lieftink, C.; Schlicker, A.; Zwart, W.; Bernards, R.; Bajpe, P.K. Mastermind-Like 3 Controls Proliferation and Differentiation in Neuroblastoma. Mol. Cancer Res. 2016, 14, 411–422. [Google Scholar] [CrossRef]
- Toops, K.A.; Hagemann, T.L.; Messing, A.; Nickells, R.W. The effect of glial fibrillary acidic protein expression on neurite outgrowth from retinal explants in a permissive environment. BMC Res. Notes 2012, 5, 693. [Google Scholar] [CrossRef]
- Bringmann, A.; Grosche, A.; Pannicke, T.; Reichenbach, A. GABA and Glutamate Uptake and Metabolism in Retinal Glial (Müller) Cells. Front. Endocrinol. 2013, 4, 48. [Google Scholar] [CrossRef]
- Reinehr, S.; Koch, D.; Weiss, M.; Froemel, F.; Voss, C.; Dick, H.B.; Fuchshofer, R.; Joachim, S.C. Loss of retinal ganglion cells in a new genetic mouse model for primary open-angle glaucoma. J. Cell. Mol. Med. 2019, 23, 5497–5507. [Google Scholar] [CrossRef]
- Navneet, S.; Zhao, J.; Wang, J.; Mysona, B.; Barwick, S.; Ammal Kaidery, N.; Saul, A.; Kaddour-Djebbar, I.; Bollag, W.B.; Thomas, B.; et al. Hyperhomocysteinemia-induced death of retinal ganglion cells: The role of Muller glial cells and NRF2. Redox Biol. 2019, 24, 101199. [Google Scholar] [CrossRef]
- Rosignol, I.; Villarejo-Zori, B.; Teresak, P.; Sierra-Filardi, E.; Pereiro, X.; Rodriguez-Muela, N.; Vecino, E.; Vieira, H.L.A.; Bell, K.; Boya, P. The mito-QC Reporter for Quantitative Mitophagy Assessment in Primary Retinal Ganglion Cells and Experimental Glaucoma Models. Int. J. Mol. Sci. 2020, 21, 1882. [Google Scholar] [CrossRef] [PubMed]
- Luna-Vargas, M.P.A.; Chipuk, J.E. Physiological and Pharmacological Control of BAK, BAX, and Beyond. Trends Cell Biol. 2016, 26, 906–917. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S.; Davis, R.J. Cell Signaling and Stress Responses. Cold Spring Harb. Perspect. Biol. 2016, 8, 6072. [Google Scholar] [CrossRef] [PubMed]
- Coulthard, L.R.; White, D.E.; Jones, D.L.; McDermott, M.F.; Burchill, S.A. p38(MAPK): Stress responses from molecular mechanisms to therapeutics. Trends Mol. Med. 2009, 15, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Mester, L.; Szabo, A.; Atlasz, T.; Szabadfi, K.; Reglodi, D.; Kiss, P.; Racz, B.; Tamas, A.; Gallyas, F., Jr.; Sumegi, B.; et al. Protection against chronic hypoperfusion-induced retinal neurodegeneration by PARP inhibition via activation of PI-3-kinase Akt pathway and suppression of JNK and p38 MAP kinases. Neurotox. Res. 2009, 16, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Al-Gayyar, M.M.; Mysona, B.A.; Matragoon, S.; Abdelsaid, M.A.; El-Azab, M.F.; Shanab, A.Y.; Ha, Y.; Smith, S.B.; Bollinger, K.E.; El-Remessy, A.B. Diabetes and overexpression of proNGF cause retinal neurodegeneration via activation of RhoA pathway. PLoS ONE 2013, 8, e54692. [Google Scholar] [CrossRef]
- Lane, M.A.; Bailey, S.J. Role of retinoid signalling in the adult brain. Prog. Neurobiol. 2005, 75, 275–293. [Google Scholar] [CrossRef]
- Lenz, M.; Kruse, P.; Eichler, A.; Straehle, J.; Beck, J.; Deller, T.; Vlachos, A. All-trans retinoic acid induces synaptic plasticity in human cortical neurons. eLife 2021, 10, 63026. [Google Scholar] [CrossRef]
- Maden, M. Retinoic acid in the development, regeneration and maintenance of the nervous system. Nat. Rev. Neurosci. 2007, 8, 755–765. [Google Scholar] [CrossRef]
- Duprey-Diaz, M.V.; Blagburn, J.M.; Blanco, R.E. Optic nerve injury upregulates retinoic acid signaling in the adult frog visual system. J. Chem. Neuroanat. 2016, 77, 80–92. [Google Scholar] [CrossRef]
- Pang, I.H.; Johnson, E.C.; Jia, L.; Cepurna, W.O.; Shepard, A.R.; Hellberg, M.R.; Clark, A.F.; Morrison, J.C. Evaluation of inducible nitric oxide synthase in glaucomatous optic neuropathy and pressure-induced optic nerve damage. Investig. Ophthalmol. Vis. Sci. 2005, 46, 1313–1321. [Google Scholar] [CrossRef]
- Duprey-Diaz, M.V.; Blagburn, J.M.; Blanco, R.E. Exogenous Modulation of Retinoic Acid Signaling Affects Adult RGC Survival in the Frog Visual System after Optic Nerve Injury. PLoS ONE 2016, 11, e0162626. [Google Scholar] [CrossRef]
- Tomlinson, C.W.E.; Cornish, K.A.S.; Whiting, A.; Pohl, E. Structure-functional relationship of cellular retinoic acid-binding proteins I and II interacting with natural and synthetic ligands. Acta Crystallogr. D Struct. Biol. 2021, 77, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Mrugacz, M.; Bryl, A.; Zorena, K. Retinal Vascular Endothelial Cell Dysfunction and Neuroretinal Degeneration in Diabetic Patients. J. Clin. Med. 2021, 10, 458. [Google Scholar] [CrossRef] [PubMed]
- Zafar, S.; Sachdeva, M.; Frankfort, B.J.; Channa, R. Retinal Neurodegeneration as an Early Manifestation of Diabetic Eye Disease and Potential Neuroprotective Therapies. Curr. Diab Rep. 2019, 19, 17. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, M.R. The optic nerve head in glaucoma: Role of astrocytes in tissue remodeling. Prog. Retin. Eye Res. 2000, 19, 297–321. [Google Scholar] [CrossRef]
- Ramírez, J.M.; Ramírez, A.I.; Salazar, J.J.; de Hoz, R.; Triviño, A. Changes of astrocytes in retinal ageing and age-related macular degeneration. Exp. Eye Res. 2001, 73, 601–615. [Google Scholar] [CrossRef]
- Sakamoto, K.; Hiraiwa, M.; Saito, M.; Nakahara, T.; Sato, Y.; Nagao, T.; Ishii, K. Protective effect of all-trans retinoic acid on NMDA-induced neuronal cell death in rat retina. Eur. J. Pharmacol. 2010, 635, 56–61. [Google Scholar] [CrossRef]
- Vecino, E.; Rodriguez, F.D.; Ruzafa, N.; Pereiro, X.; Sharma, S.C. Glia-neuron interactions in the mammalian retina. Prog. Retin. Eye Res. 2016, 51, 1–40. [Google Scholar] [CrossRef]
- Cuenca, N.; Fernández-Sánchez, L.; Campello, L.; Maneu, V.; De la Villa, P.; Lax, P.; Pinilla, I. Cellular responses following retinal injuries and therapeutic approaches for neurodegenerative diseases. Prog. Retin. Eye Res. 2014, 43, 17–75. [Google Scholar] [CrossRef]
- Pillar, S.; Moisseiev, E.; Sokolovska, J.; Grzybowski, A. Recent Developments in Diabetic Retinal Neurodegeneration: A Literature Review. J. Diabetes Res. 2020, 2020, 5728674. [Google Scholar] [CrossRef] [PubMed]
- Dun, Y.; Mysona, B.; Van Ells, T.; Amarnath, L.; Ola, M.S.; Ganapathy, V.; Smith, S.B. Expression of the cystine-glutamate exchanger (xc-) in retinal ganglion cells and regulation by nitric oxide and oxidative stress. Cell Tissue Res. 2006, 324, 189–202. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Ji, Y.; Li, H.; Ren, L.; Zhu, J.; Yang, T.; Li, X.; Yao, J.; Cao, X.; Yan, B. EYE-503: A Novel Retinoic Acid Drug for Treating Retinal Neurodegeneration. Pharmaceuticals 2023, 16, 1033. https://doi.org/10.3390/ph16071033
Liu S, Ji Y, Li H, Ren L, Zhu J, Yang T, Li X, Yao J, Cao X, Yan B. EYE-503: A Novel Retinoic Acid Drug for Treating Retinal Neurodegeneration. Pharmaceuticals. 2023; 16(7):1033. https://doi.org/10.3390/ph16071033
Chicago/Turabian StyleLiu, Sha, Yuke Ji, Huan Li, Ling Ren, Junya Zhu, Tianjing Yang, Xiumiao Li, Jin Yao, Xin Cao, and Biao Yan. 2023. "EYE-503: A Novel Retinoic Acid Drug for Treating Retinal Neurodegeneration" Pharmaceuticals 16, no. 7: 1033. https://doi.org/10.3390/ph16071033
APA StyleLiu, S., Ji, Y., Li, H., Ren, L., Zhu, J., Yang, T., Li, X., Yao, J., Cao, X., & Yan, B. (2023). EYE-503: A Novel Retinoic Acid Drug for Treating Retinal Neurodegeneration. Pharmaceuticals, 16(7), 1033. https://doi.org/10.3390/ph16071033






