Isolated Fraction of Gastric-Digested Camel Milk Yogurt with Carao (Cassia grandis) Pulp Fortification Enhances the Anti-Inflammatory Properties of HT-29 Human Intestinal Epithelial Cells
Abstract
1. Introduction
2. Results and Discussion
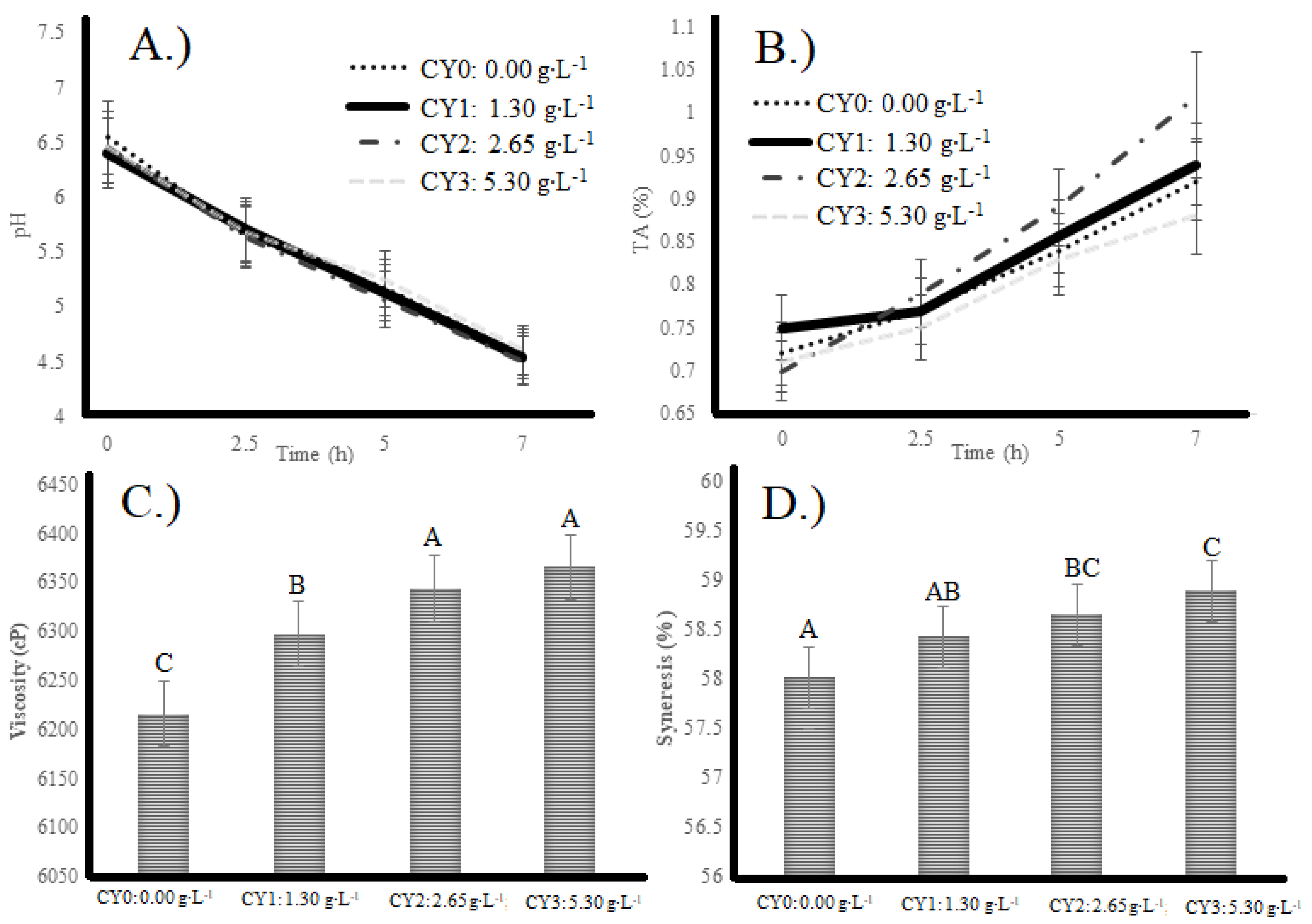
2.1. Physico-Chemical Evaluations
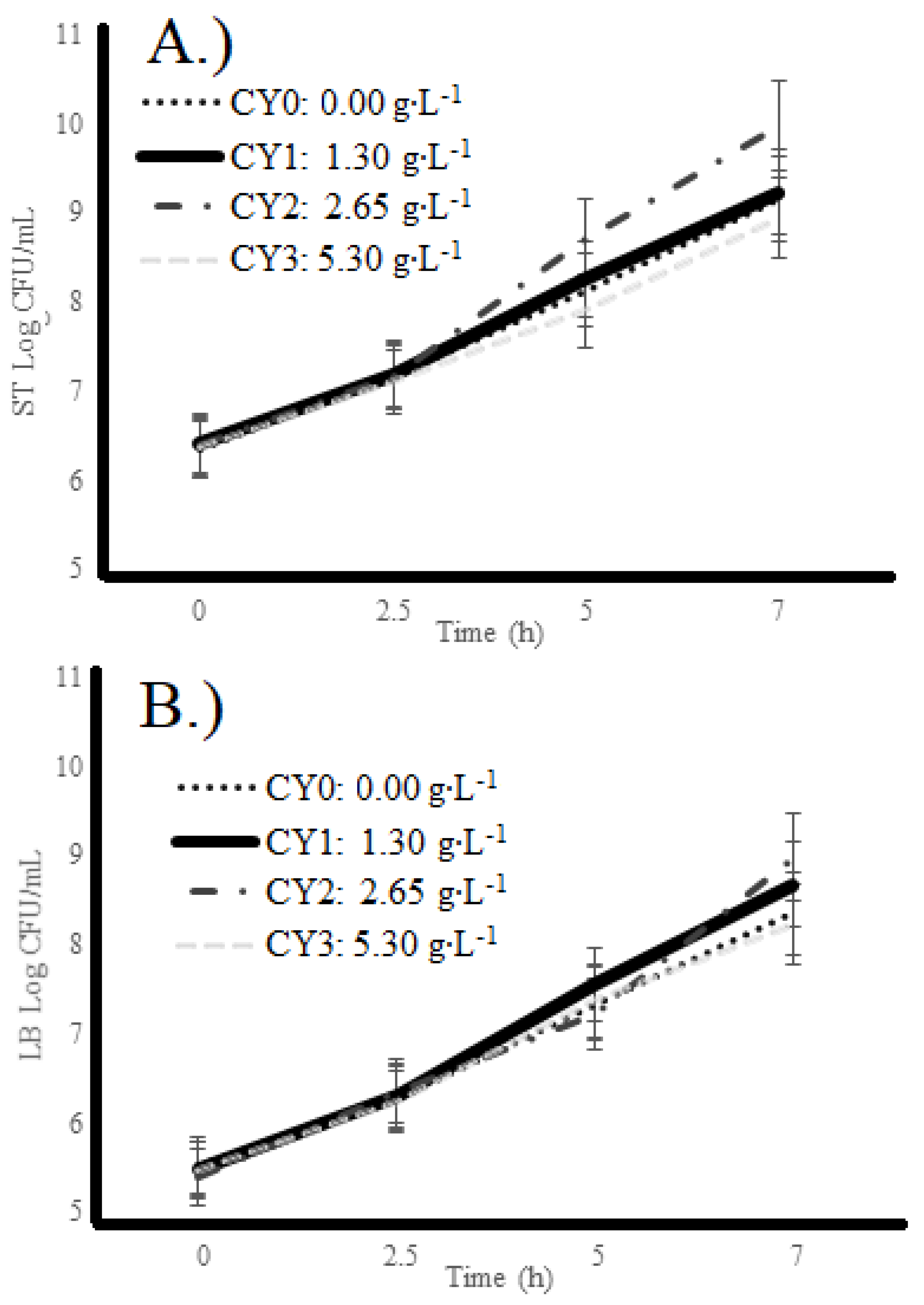
2.2. Microbial Counts
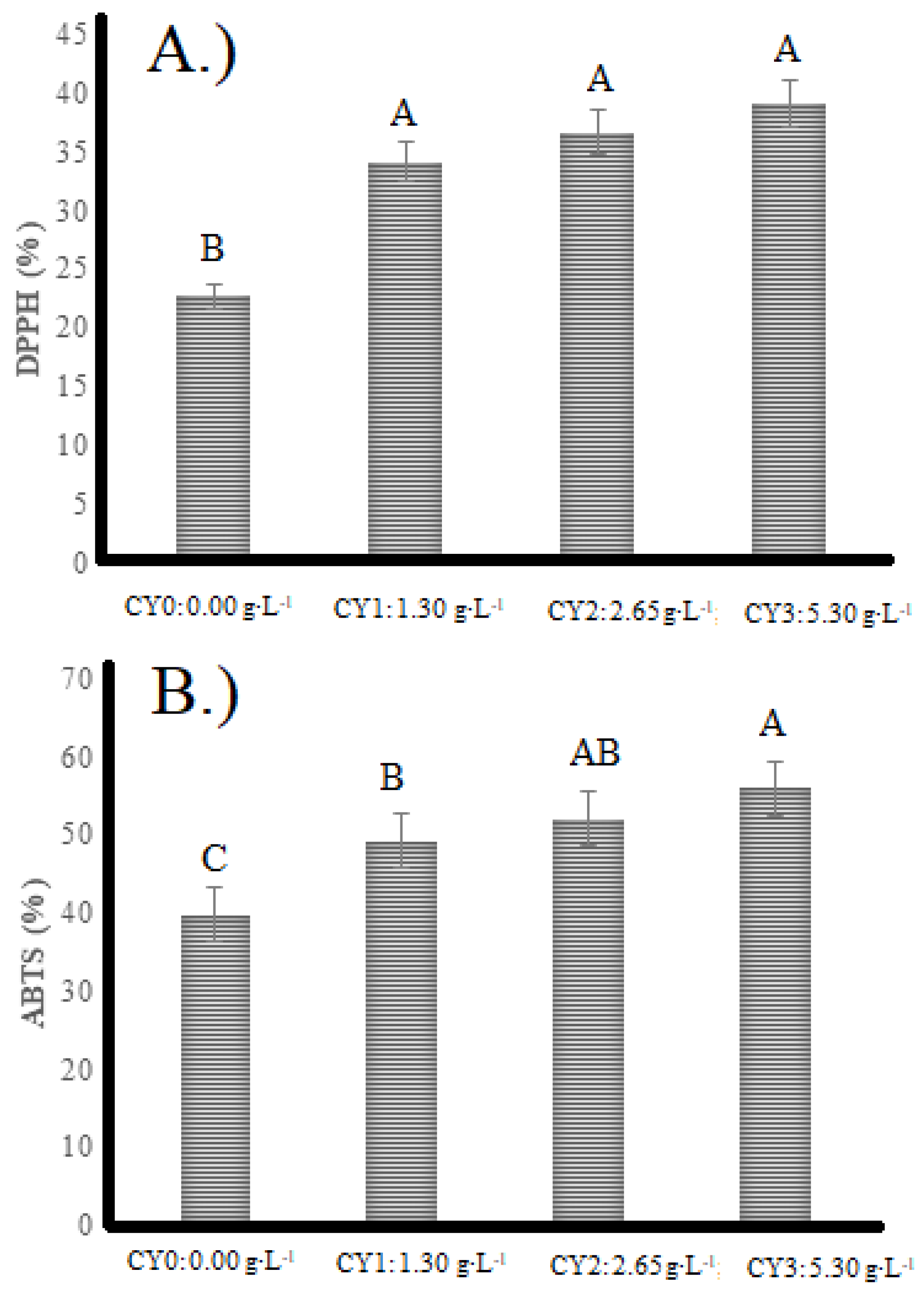
2.3. Radical Scavenging Ability Evaluations
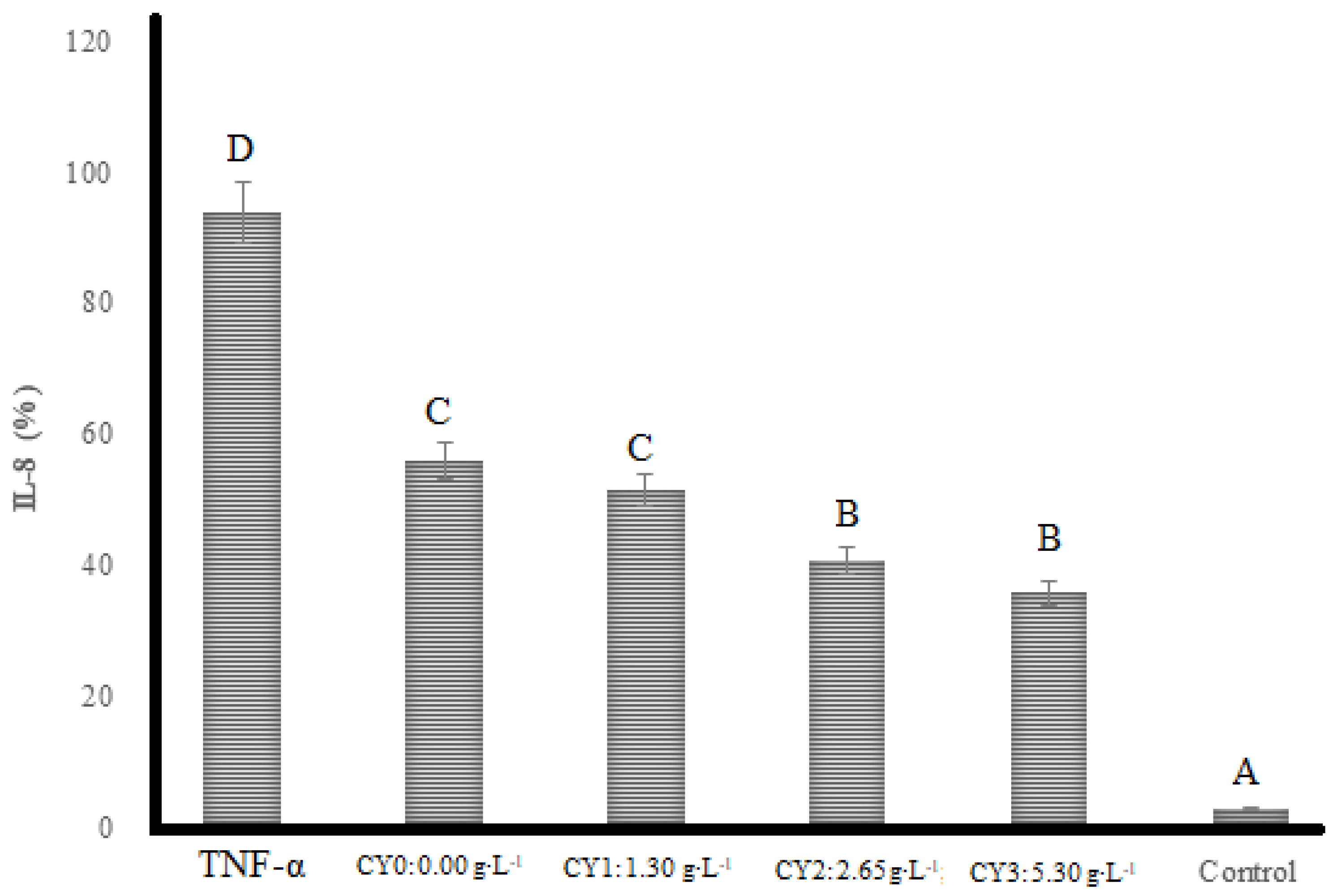
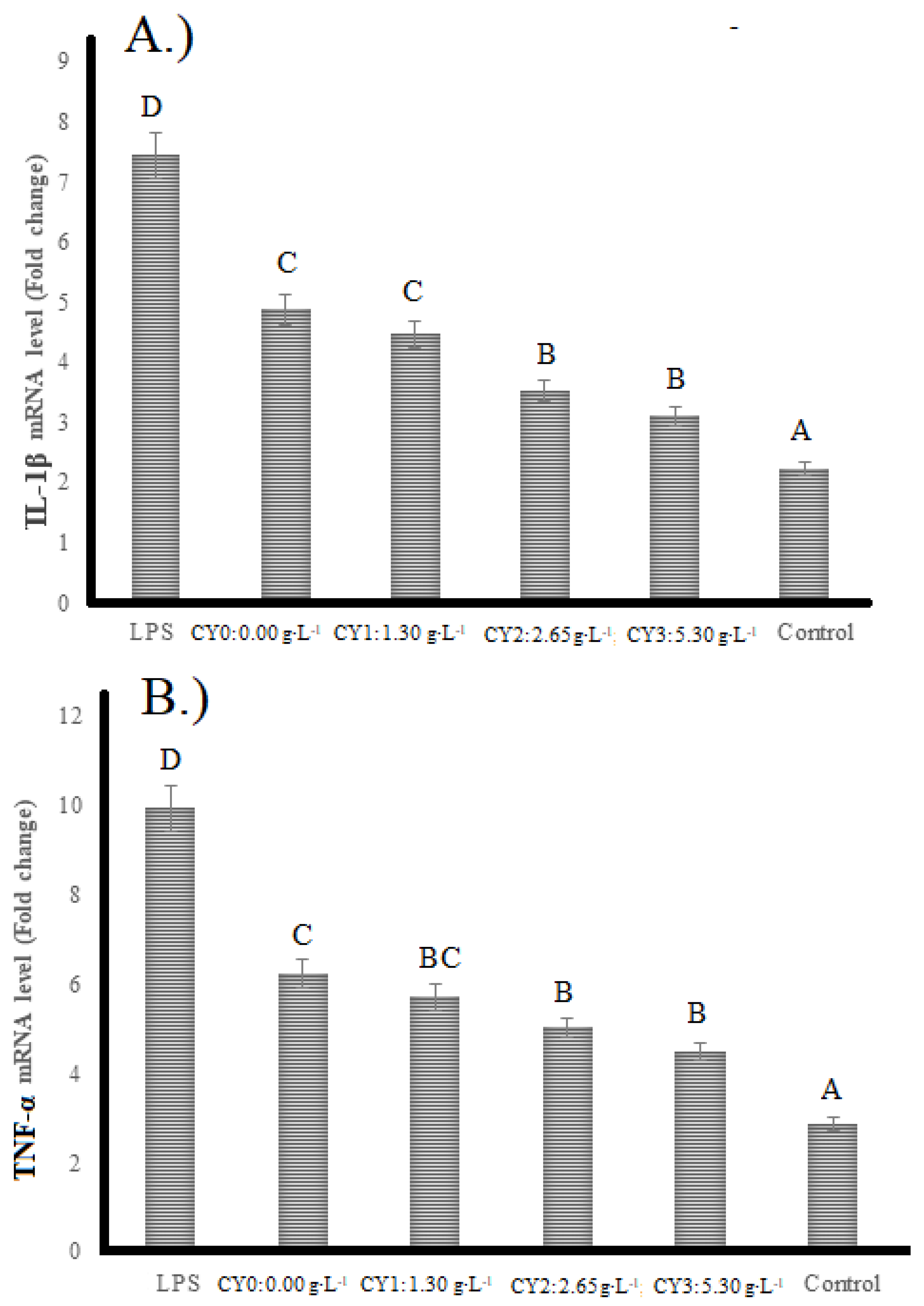
2.4. Anti-Inflammatory Activity
2.5. mRNA Levels of Interleukin-1β (IL-1β) and Tumor Necrosis Factor-α (TNF-α)
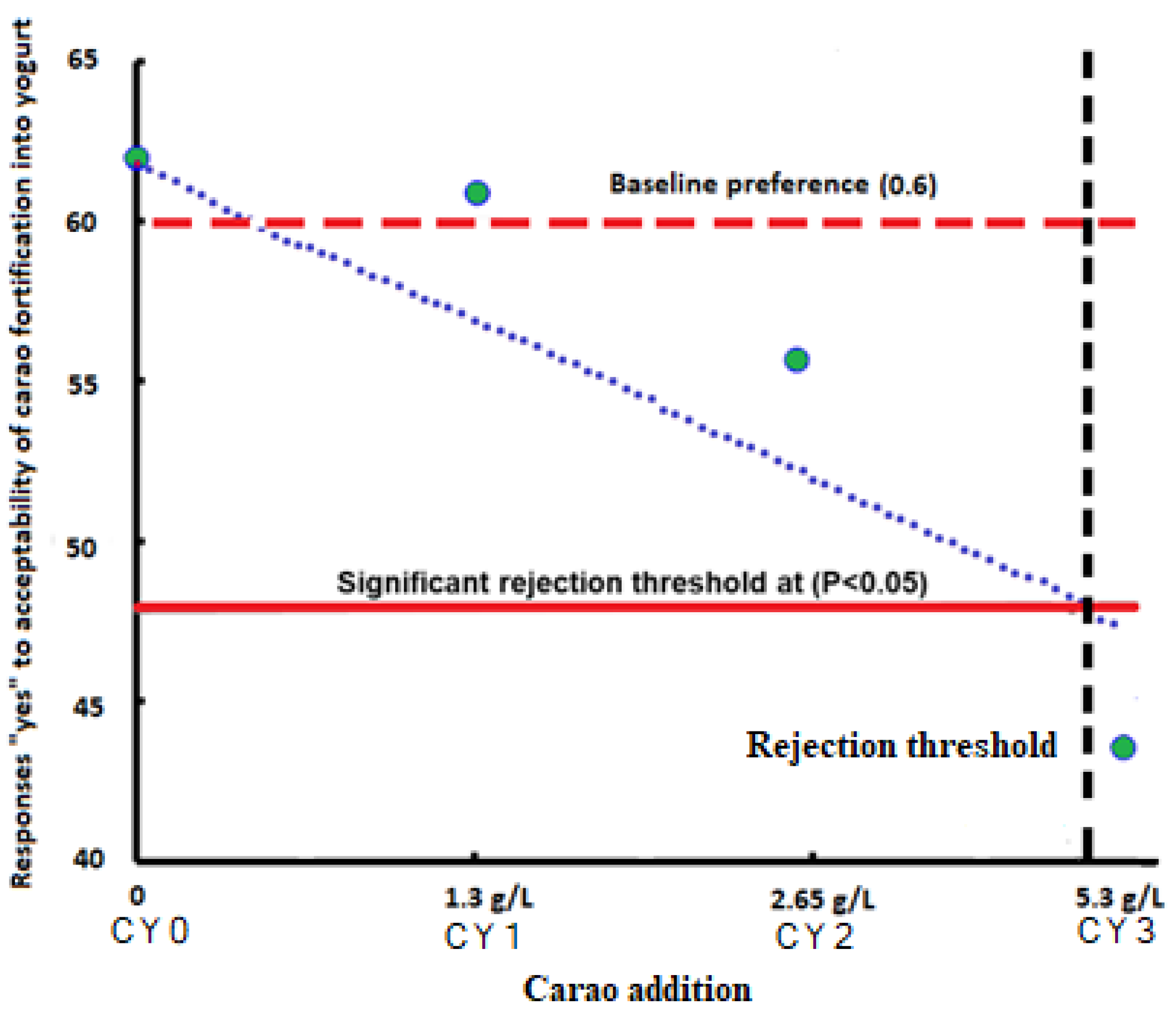
2.6. Sensory Evaluation of Camel Milk Yogurt
3. Materials and Methods
3.1. Milk and Plant Material
3.2. Yogurt Preparation
3.3. Analysis
3.3.1. Physico-Chemical Evaluations
3.3.2. Microbial Analysis
3.3.3. Simulated Gastric Phase Digestion of Camel Milk Yogurt
3.3.4. Radical Scavenging Assay of the Digested Camel Milk Yogurt
3.3.5. HT-29 Cell Lines Culture
3.3.6. Anti-Inflammatory Activity of Digested Camel Milk Yogurt
3.3.7. Real-Time Reverse-Transcriptase Polymerase Chain Reaction Analysis
3.3.8. Sensory Evaluation
3.3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sánchez, R.; Martín-Tornero, E.; Lozano, J.; Fernández, A.; Arroyo, P.; Meléndez, F.; Martín-Vertedor, D. Electronic nose application for the discrimination of sterilization treatments applied to Californian-style black olive varieties. J. Sci. Food Agric. 2022, 102, 2232–2241. [Google Scholar] [CrossRef] [PubMed]
- Shahbandeh, M.U.S. Yogurt Market—STATISTICS & Facts. Available online: https://www.statista.com/topics/1739/yogurt/#topicOverview (accessed on 14 July 2023).
- Ferreira, S.R.; Santos, L. Incorporation of phenolic extracts fromm different by-products in yoghurts to create fotified and sustainable foods. Food Sci. 2022, 51, 102293. [Google Scholar]
- Kamal-Eldin, A.; Alhammadi, A.; Gharsallaoui, A.; Hamed, F.; Ghnimi, S. Physicochemical, rheological, and micro-structural properties of yogurts produced from mixtures of camel and bovine milks. NFS J. 2020, 19, 26–33. [Google Scholar] [CrossRef]
- Khan, B.; Iqbal, A. Production and Composition of Camel Milk, Review. Pak. J. Agric. Sci. 2001, 38, 64–69. [Google Scholar]
- Swelum, A.A.; El-Saadony, M.T.; Abdo, M.; Ombarak, R.A.; Hussein, E.O.; Suliman, G. Nutritional, antimicrobial and medicinal properties of Camel’s milk: A review. Saudi J. Biol. Sci. 2021, 28, 3126–3136. [Google Scholar] [CrossRef] [PubMed]
- Al-Zoreky, N.S.; Al-Otaibi, M.M. Suitability of camel milk for making yogurt. Food Sci. Biotechnol. 2015, 24, 601–606. [Google Scholar] [CrossRef]
- Laleye, L.C.; Jobe, B.; Wasesa, A.A.H. Comparative Study on Heat Stability and Functionality of Camel and Bovine Milk Whey Proteins. J. Dairy Sci. 2008, 91, 4527–4534. [Google Scholar] [CrossRef]
- Mudgil, P.; Jumah, B.; Ahmad, M.; Hamed, F.; Maqsood, S. Rheological, micro-structural and sensorial properties of camel milk yogurt as influenced by gelatin. LWT 2018, 98, 646–653. [Google Scholar] [CrossRef]
- Abou-Soliman, N.H.I.; Sakr, S.S.; Awad, S. Physico-chemical, microstructural and rheological properties of camel-milk yogurt as enhanced by microbial transglutaminase. J. Food Sci. Technol. 2017, 54, 1616–1627. [Google Scholar] [CrossRef]
- Oselu, S.; Ebere, R.; Huka, G.; Musalia, L.; Marete, E.; Mathara, J.M. Production and characterisation of camel milk yoghurt containing different types of stabilising agents. Heliyon 2022, 8, e11816. [Google Scholar] [CrossRef]
- Salmerón-Manzano, E.; Garrido-Cardenas, J.A.; Manzano-Agugliaro, F. Worldwide research trends on medicinal plants. Int. J. Environ. Res. Public Health 2020, 17, 3376. [Google Scholar] [CrossRef] [PubMed]
- Godic, A.; Poljšak, B.; Adamic, M.; Dahmane, R. The role of antioxidants in skin cancer prevention and treatment. Oxidative Med. Cell Longev. 2014, 2014, 860479. [Google Scholar] [CrossRef]
- Aleman, R.S.; Marcia, J.; Page, R.; Kazemzadeh, P.S.; Martín-Vertedor, D.; Manrique-Fernández, V.; Montero-Fernández, I. Effects of Yogurt with Carao (Cassia grandis) on Intestinal Barrier Dysfunction, α-glycosidase Activity, Lipase Activity, Hypoglycemic Effect, and Antioxidant Activity. Fermentation 2023, 9, 566. [Google Scholar] [CrossRef]
- Alemán, R.S.; Moncada, M.; Aryana, K.L. Leaky Gut and the Ingredients that Hepl Treat it: A Review. Molecules 2023, 28, 619. [Google Scholar] [CrossRef]
- Fuentes, J.A.M.; Fernández, I.M.; Fernández, H.Z.; Sánchez, J.L.; Alemán, R.S.; Navarro-Alarcon, M. Quantification of bioactive molecules, minerals and bromatological analysis in Carao (Cassia grandis). J. Agric. Sci. 2020, 12, 88. [Google Scholar] [CrossRef]
- Fuentes, J.A.M.; Fernández, I.M.; Maldonado, S.A.S.; Murillo, I.M.V.; Altamirano, C.M.S.; Bonilla, F.J.H.; Tejada, E.G.C.; Dereck, B.F.C.; Fernández, H.Z.; de Jesús Alvarez Gil, M. Physical-chemical evaluation of the Cassia grandis L. as fortifying egg powder. J. Agric. Sci. 2020, 12, 277. [Google Scholar] [CrossRef]
- Fuentes, J.A.M.; López-Salas, L.; Borrás-Linares, I.; Navarro-Alarcón, M.; Segura-Carretero, A.; Lozano-Sánchez, J. Development of an innovative pressurized liquid extraction procedure by response surface methodology to recover bioactive compounds from carao Tree Seeds. Foods 2021, 10, 398. [Google Scholar] [CrossRef]
- Marcía-Fuentes, J.; Santos-Aleman, R.; Borrás-Linares, I.; Sánchez, J.L. The Carao (Cassia grandis L.): Its Potential Usage in Pharmacological, Nutritional, and Medicinal Applications. In Innovations in Biotechnology for a Sustainable Future; Springer: Berlin/Heidelberg, Germany, 2021; pp. 403–427. [Google Scholar]
- Marcia, J.; Aleman, R.S.; Montero-Fernández, I.; Martín-Vertedor, D.; Manrique-Fernández, V.; Moncada, M. Attributes of Lactobacillus acidophilus as Effected by Carao (Cassia grandis) Pulp Powder. Fermentation 2023, 9, 408. [Google Scholar] [CrossRef]
- Joshi, H.; Kapoor, V.P. Cassia grandis Linn. f. seed galactomannan: Structural and crystallographical studies. Carbohydr. Res. 2003, 338, 1907–1912. [Google Scholar] [CrossRef] [PubMed]
- Kotipalli, H.; Battu-Ganga, R.; Devarakonda, R. Qualitative physicochemical, phytochemical analysis and quantitative estimation of total phenols, flavonoids and alkaloids of Cassia grandis. J. Glob. Trends Pharm. Sci. 2017, 8, 3860–3867. [Google Scholar]
- Aleman, R.S.; Paz, D.; Cedillos, R.; Tabora, M.; Olson, D.W.; Aryana, K. Attributes of Culture Bacteria as Influenced by Ingredients That Help Treat Leaky Gut. Microorganisms 2023, 11, 893. [Google Scholar] [CrossRef]
- Hegazi, N.M.; Hashim, A.N. Grandisin, 2-methoxy 6,7,2′,6′-tetrahydroxy flavanone 6-O-glucoside, from Cassia grandis leaves—Antioxidant and cytotoxic activities. Pharmacology 2016, 71, 544–547. [Google Scholar]
- Prada, A.L.; Amado, J.R.R.; Keita, H.; Zapata, E.P.; Carvalho, H.; Lima, E.S. Cassia grandis fruit extract reduces the blood glucose level in alloxan-induced diabetic rats. Biomed. Pharmacother. 2018, 103, 421–428. [Google Scholar] [CrossRef]
- Paz, D.; Aleman, R.S.; Cedillos, R.; Olson, D.W.; Aryana, K.; Marcia, J. Probiotic Characteristics of Streptococcus thermophilus and Lactobacillus delbrueckii ssp. bulgaricus as Influenced by Carao (Cassia grandis). Fermentation 2022, 8, 499. [Google Scholar] [CrossRef]
- Medina, L.; Aleman, R.S.; Cedillos, R.; Aryana, K.; Olson, D.W.; Marcia, J. Effects of carao (Cassia grandis L.) on physico-chemical, microbiological and rheological characteristics of yogurt. LWT 2023, 183, 114891. [Google Scholar] [CrossRef]
- Montero-Fernández, I.; Marcía-Fuentes, J.A.; Cascos, G.; Saravia-Maldonado, S.A.; Lozano, J.; Martín-Vertedor, D. Masking effect of Cassia grandis sensory defect with flavoured stuffed olives. Foods 2022, 11, 2305. [Google Scholar] [CrossRef] [PubMed]
- Hashim, I.B.; Khalil, A.H.; Habib, H. Quality and acceptability of a set-type yogurt made from camel milk. J. Dairy Sci. 2009, 92, 857–862. [Google Scholar] [CrossRef]
- Lucey, J.A. Cultured dairy products: An overview of their gelation and texture properties. Int. J. Dairy Technol. 2004, 57, 77–84. [Google Scholar] [CrossRef]
- Bhaturiwala, R.; Bagban, M.A.; Singh, T.A.; Modi, H.A. Partial purification and application of β-mannanase for the preparation of low molecular weight galacto and glucomannan. Biocatal. Agric. Biotechnol. 2021, 36, 102155. [Google Scholar] [CrossRef]
- Buchilina, A.; Aryana, K. Physicochemical and microbiological characteristics of camel milk yogurt as influenced by monk fruit sweetener. J. Dairy Sci. 2021, 104, 1484–1493. [Google Scholar] [CrossRef] [PubMed]
- Safdari, Y.; Vazifedoost, M.; Didar, Z.; Hajirostamloo, B. The effect of banana fiber and banana peel fiber on the chemical and rheological properties of symbiotic yogurt made from camel milk. Int. J. Food Sci. 2021, 2021, 5230882. [Google Scholar] [CrossRef] [PubMed]
- Ranadheera, C.S.; Evans, C.A.; Adams, M.C.; Baines, S.K. In vitro analysis of gastrointestinal tolerance and intestinal cell adhesion of probiotics in goat’s milk ice cream and yogurt. Food Res. Int. 2012, 49, 619–625. [Google Scholar] [CrossRef]
- Rahman, I.E.A.; Dirar, H.A.; Osman, M.A. Microbiological and biochemical changes and sensory evaluation of camel milk fermented by selected bacterial starter cultures. Afr. J. Food Sci. 2009, 3, 398–405. [Google Scholar]
- Fguiri, I.; Ziadi, M.; Rekaya, K.; Arroum, S.; Khorchani, T. Isolation and Characterization of Lactic Acid Bacteria Strains from Raw Camel Milk for Potential Use in the Production of Yogurt. Food Sci. Nutr. 2017, 3, 26. [Google Scholar] [CrossRef]
- El-Deeb, A.; Dyab, A.; Elkot, W. Production of Flavoured Fermented Camel Milk. Ismailia J. Dairy Sci. Technol. 2017, 5, 9–20. [Google Scholar] [CrossRef]
- El-Sayed, M.I.; Awad, S.; Abou-Soliman, N.H.I.; El-Sayed, M.I.; Awad, S.; Abou-Soliman, N.H.I. Improving the Antioxidant Properties of Fermented Camel Milk Using Some Strains of Lactobacillus. Food Nutr. Sci. 2021, 12, 352–371. [Google Scholar]
- Al-Dhaheri, A.S.; Al-Hemeiri, R.; Kizhakkayil, J.; Al-Nabulsi, A.; Abushelaibi, A.; Shah, N.P. Health-promoting benefits of low-fat akawi cheese made by exopolysaccharide-producing probiotic Lactobacillus plantarum isolated from camel milk. J. Dairy Sci. 2017, 100, 7771–7779. [Google Scholar] [CrossRef]
- Dimitrellou, D.; Solomakou, N.; Kokkinomagoulos, E.; Kandylis, P. Yogurts supplemented with juices from grapes and berries. Foods 2020, 9, 1158. [Google Scholar] [CrossRef]
- Baniasadi, M.; Azizkhani, M.; Saris, P.E.J.; Tooryan, F. Comparative antioxidant potential of kefir and yogurt of bovine and non-bovine origins. J. Food Sci. Technol. 2022, 59, 1307–1316. [Google Scholar] [CrossRef] [PubMed]
- Moslehishad, M.; Ehsani, M.R.; Salami, M.; Mirdamadi, S.; Ezzatpanah, H.; Naslaji, A.N. The comparative assessment of ACE-inhibitory and antioxidant activities of peptide fractions obtained from fermented camel and bovine milk by Lactobacillus rhamnosus PTCC 1637. Int. Dairy J. 2013, 29, 82–87. [Google Scholar] [CrossRef]
- Zepeda-Hernández, A.; Garcia-Amezquita, L.E.; Requena, T.; García-Cayuela, T. Probiotics, prebiotics, and synbiotics added to dairy products: Uses and applications to manage type 2 diabetes. Food Res. Int. 2021, 142, 110208. [Google Scholar] [CrossRef]
- Jeong, C.H.; Ryu, H.; Zhang, T.; Lee, C.H.; Seo, H.G.; Han, S.G. Green tea powder supplementation enhances fermentation and antioxidant activity of set-type yogurt. Food Sci. Biotechnol. 2018, 27, 1419–1427. [Google Scholar] [CrossRef]
- Meng, Z.; Yan, C.; Deng, Q.; Gao, D.F.; Niu, X.L. Curcumin inhibits LPS-induced inflammation in rat vascular smooth muscle cells in vitro via ROS-relative TLR4-MAPK/NF-κB pathways. Acta Pharmacol. Sin. 2013, 34, 901–911. [Google Scholar] [CrossRef]
- Li, G.; Le, G.; Shi, Y.; Shrestha, S. Angiotensin I-converting enzyme inhibitory peptides derived from food proteins and therir physiological and pharmacologucal effects. Nutr. Res. 2004, 24, 469–486. [Google Scholar] [CrossRef]
- Dharmisthaben, P.; Basciawmoit, B.; Sakure, A.; Das, S.; Mauyra, R.; Bishnoi, M. Exploring potential of antioxidative, anti-inflamatory activities and production of bioactive peptides in lactic fermented camel milk. Food Biosci. 2021, 44, 101404. [Google Scholar] [CrossRef]
- Shukla, P.; Sakure, A.; Pipaliya, R.; Basaiawmoit, B.; Maurya, R.; Bishnoi, M. Exploring the potential of Lacticaseibacillus paracasei M11 on antidiabetic, anti-inflammatory, and ACE inhibitory effects of fermented dromedary camel milk (Camelus dromedaries) and the release of antidiabetic and anti-hypertensive peptides. J. Food Biochem. 2022, 46, e14449. [Google Scholar] [CrossRef] [PubMed]
- Ardoin, R.; Marx, B.D.; Boeneke, C.; Prinyawiwatkul, W. Effects of cricket powder on selected physical properties and US consumer perceptions of whole-wheat snack crackers. Int. J. Food Sci. Technol. 2021, 56, 4070–4080. [Google Scholar] [CrossRef]
- Ayyash, M.; Al-Dhaheri, A.S.; Al Mahadin, S.; Kizhakkayil, J.; Abushelaibi, A. In vitro investigation of anticancer, antihypertensive, antidiabetic, and antioxidant activities of camel milk fermented with camel milk probiotic: A comparative study with fermented bovine milk. J. Dairy Sci. 2018, 101, 900–911. [Google Scholar] [CrossRef] [PubMed]
- Maqsood, S.; Al-Dowaila, A.; Mudgil, P.; Kamal, H.; Jobe, B.; Hassan, H.M. Comparative characterization of protein and lipid fractions from camel and cow milk, their functionality, antioxidant and antihypertensive properties upon simulated gastro-intestinal digestion. Food Chem. 2019, 279, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, J.; Xia, X.; Zhao, Y.; Shao, W. Probiotic potential of Lactobacillus paracasei FM-LP-4 isolated from Xinjiang camel milk yogurt. Int. Dairy J. 2016, 62, 28–34. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, H.; Liu, R.; Mats, L.; Zhu, H.; Pauls, K.P. Antioxidant and anti-inflammatory polyphenols and peptides of common bean (Phaseolus vulgaris L.) milk and yogurt in Caco-2 and HT-29 cell models. J. Funct. Foods 2019, 53, 125–135. [Google Scholar] [CrossRef]
| Formulations | Appearance | Color | Consistency | Aroma | Sweetness |
|---|---|---|---|---|---|
| CY0 | 6.43 ± 1.54 a | 6.57 ± 1.602 | 6.51 ± 1.91 a | 6.38 ± 1.80 a | 6.19 ± 1.54 a |
| CY1 | 6.34 ± 1.33 a | 6.21 ± 1.55 a | 6.28 ± 1.35 ab | 6.02 ± 1.26 a | 6.02 ± 1.88 a |
| CY2 | 6.25 ± 1.38 ab | 6.06 ± 2.11 b | 6.06 ± 1.89 b | 5.11 ± 1.58 b | 6.07 ± 1.21 a |
| CY3 | 6.01 ± 1.74 b | 6.04 ± 2.12 b | 6.03 ± 1.79 b | 5.28 ± 1.85 b | 6.41 ± 1.94 a |
| Formulations | Sourness | After-Taste | Thickness | Flavor | Overall Liking |
| CY0 | 6.30 ± 1.25 a | 6.44 ± 1.77 a | 6.15 ± 1.47 a | 6.77 ± 1.30 a | 6.68 ± 1.49 a |
| CY1 | 6.13 ± 1.96 a | 6.45 ± 1.38 a | 6.17 ± 1.24 a | 6.66 ± 1.68 a | 6.49 ± 1.16 a |
| CY2 | 6.11 ± 1.87 a | 6.26 ± 1.37 a | 6.42 ± 1.73 a | 6.05 ± 1.27 b | 5.92 ± 1.79 b |
| CY3 | 6.34 ± 1.79 a | 6.15 ± 1.48 a | 6.17 ± 1.90 a | 6.15 ± 1.94 b | 5.76 ± 1.72 b |
| Formulations | Purchase Intent (PI, %) | Ranking (Rank Sums) | |
|---|---|---|---|
| PI-Before | PI-After | Preference | |
| CY0 | 68.23% a,A | - | 160 a |
| CY1 | 59.54% a,A | 71.45% a,B | 165 a,b |
| CY2 | 61.56% a,A | 68.56% a,B | 183 b |
| CY3 | 55.67% b,A | 67.32% a,B | 180 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcia, J.A.; Aleman, R.S.; Kazemzadeh, S.; Manrique Fernández, V.; Martín Vertedor, D.; Kayanush, A.; Montero Fernández, I. Isolated Fraction of Gastric-Digested Camel Milk Yogurt with Carao (Cassia grandis) Pulp Fortification Enhances the Anti-Inflammatory Properties of HT-29 Human Intestinal Epithelial Cells. Pharmaceuticals 2023, 16, 1032. https://doi.org/10.3390/ph16071032
Marcia JA, Aleman RS, Kazemzadeh S, Manrique Fernández V, Martín Vertedor D, Kayanush A, Montero Fernández I. Isolated Fraction of Gastric-Digested Camel Milk Yogurt with Carao (Cassia grandis) Pulp Fortification Enhances the Anti-Inflammatory Properties of HT-29 Human Intestinal Epithelial Cells. Pharmaceuticals. 2023; 16(7):1032. https://doi.org/10.3390/ph16071032
Chicago/Turabian StyleMarcia, Jhunior Abrahan, Ricardo S. Aleman, Shirin Kazemzadeh, Víctor Manrique Fernández, Daniel Martín Vertedor, Aryana Kayanush, and Ismael Montero Fernández. 2023. "Isolated Fraction of Gastric-Digested Camel Milk Yogurt with Carao (Cassia grandis) Pulp Fortification Enhances the Anti-Inflammatory Properties of HT-29 Human Intestinal Epithelial Cells" Pharmaceuticals 16, no. 7: 1032. https://doi.org/10.3390/ph16071032
APA StyleMarcia, J. A., Aleman, R. S., Kazemzadeh, S., Manrique Fernández, V., Martín Vertedor, D., Kayanush, A., & Montero Fernández, I. (2023). Isolated Fraction of Gastric-Digested Camel Milk Yogurt with Carao (Cassia grandis) Pulp Fortification Enhances the Anti-Inflammatory Properties of HT-29 Human Intestinal Epithelial Cells. Pharmaceuticals, 16(7), 1032. https://doi.org/10.3390/ph16071032








