Mexican Native Black Bean Anthocyanin-Rich Extracts Modulate Biological Markers Associated with Inflammation
Abstract
1. Introduction
2. Results and Discussion
2.1. Study Description
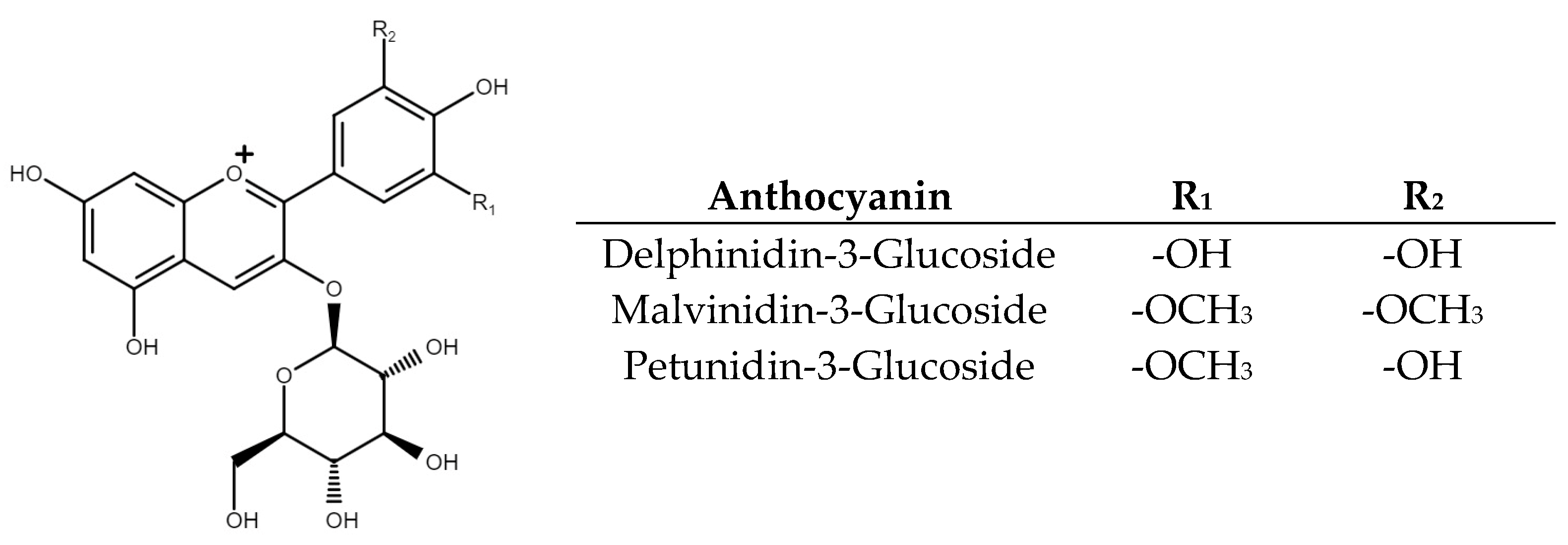
2.2. Phenolic and Anthocyanin Quantification
2.3. Tentative Identification and Quantification of Phenolic Compounds in Treatments
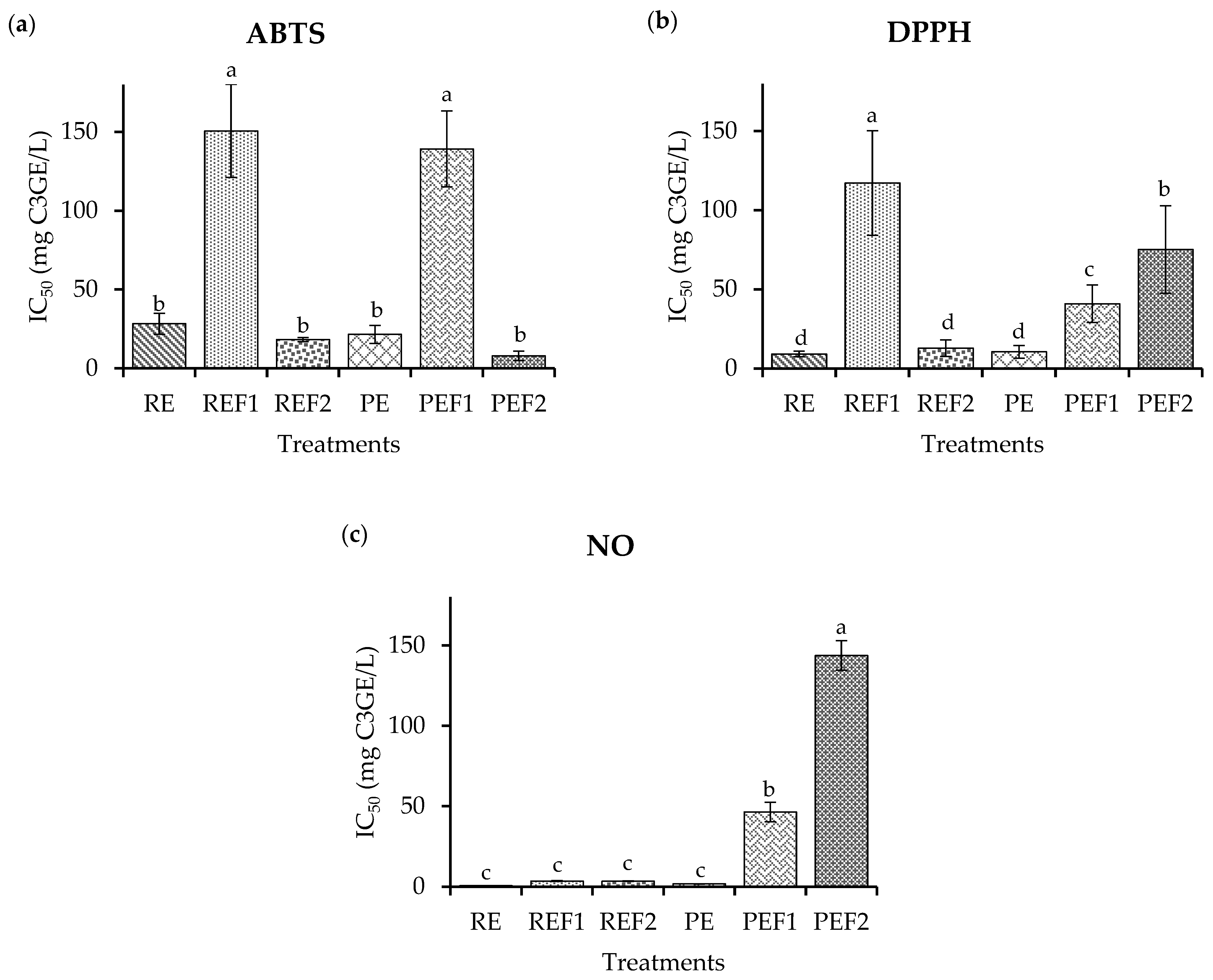
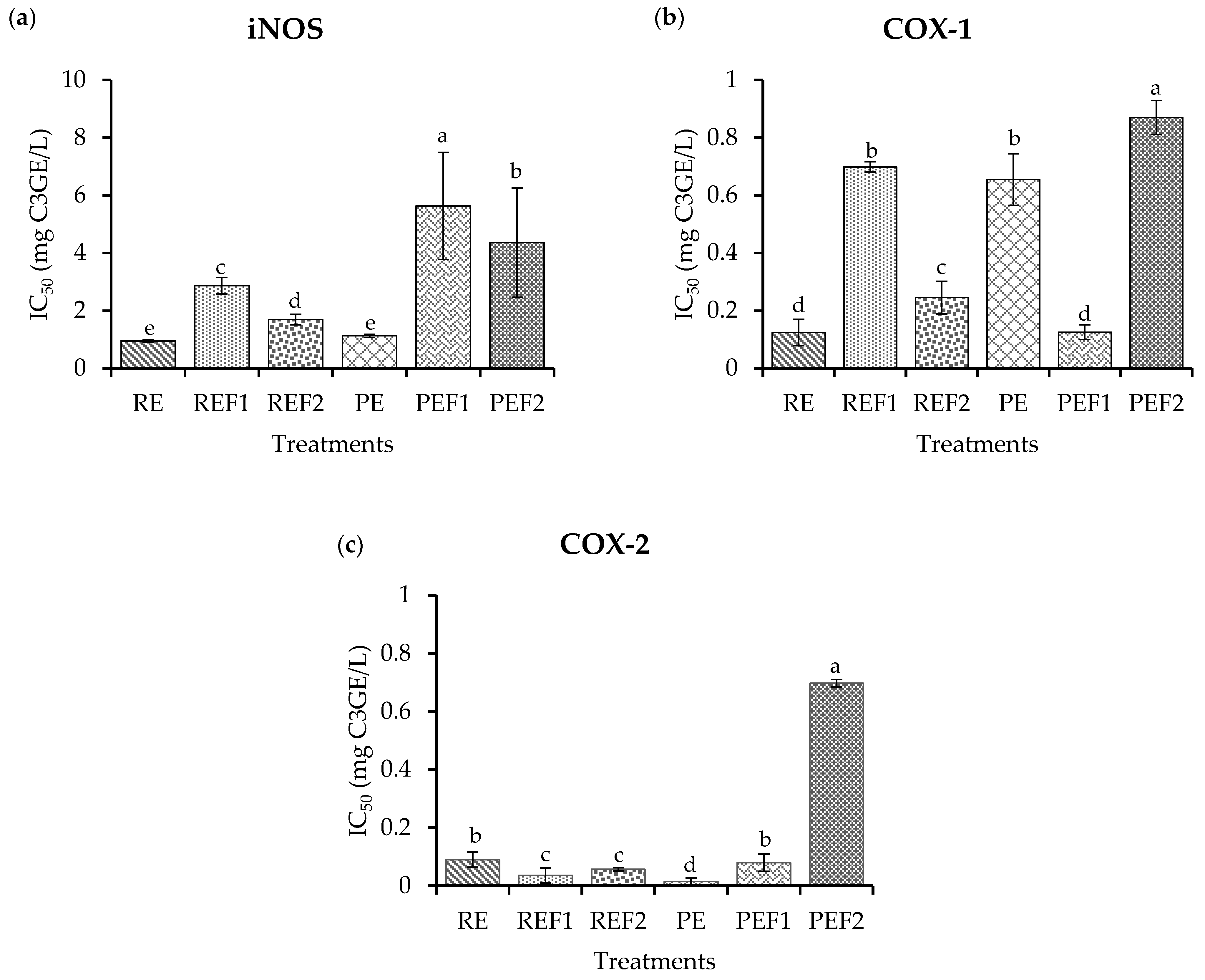
2.4. Biological Potential
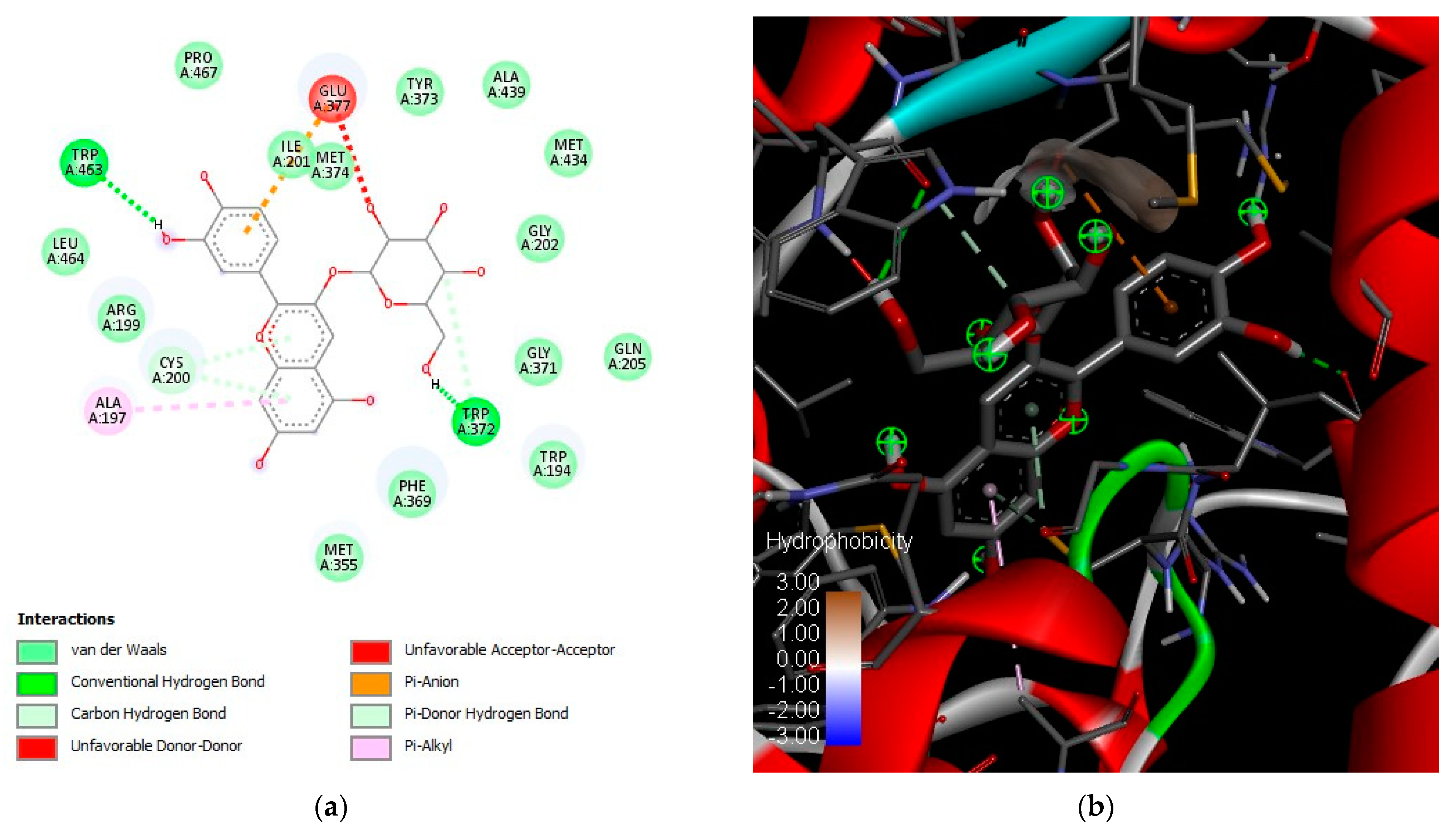
2.5. In Silico Analysis
3. Materials and Methods
3.1. Materials
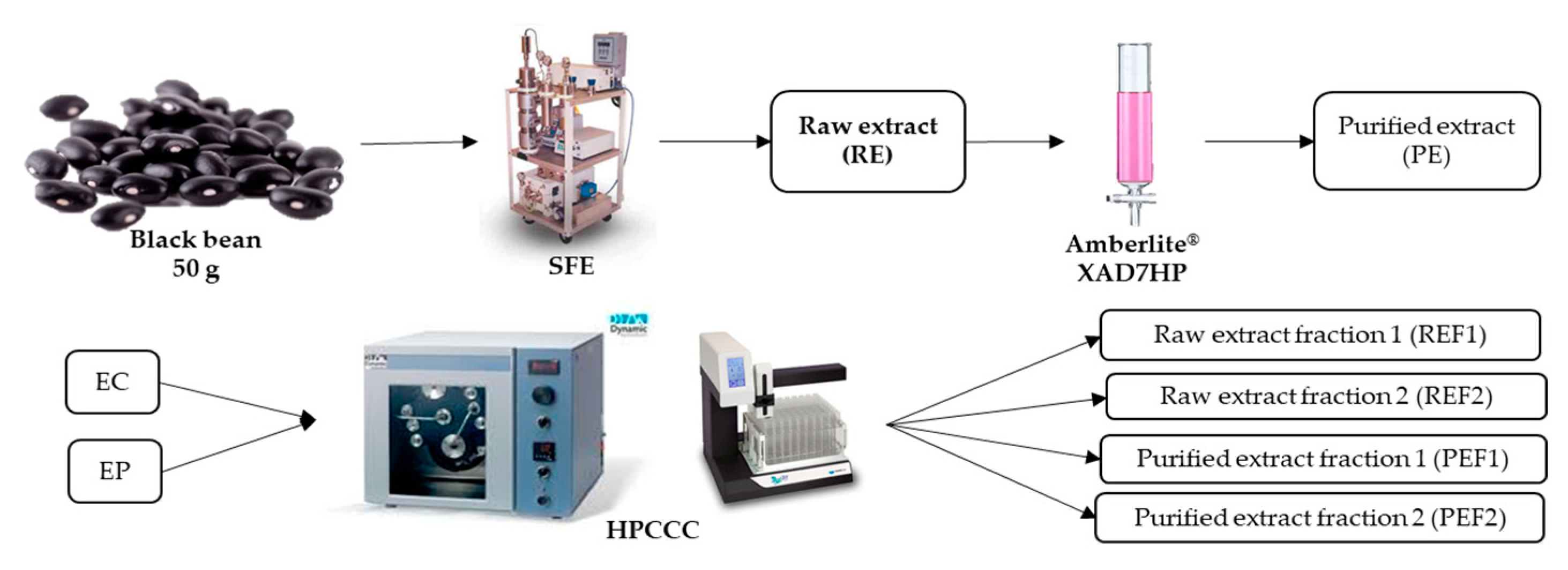
3.2. Supercritical Extraction
3.3. Purification by Column Chromatography
3.4. Fractionation by High-Pressure Countercurrent Chromatography
3.5. Determination of Total Phenolic Content and Total Anthocyanins Content
3.6. Tentative Identification of Phenolic Compounds on Direct Analysis in Real-Time Mass Spectrometry (DART-MS)
3.7. Quantification of Anthocyanins and Phenolic Compounds by High-Pressure Liquid Chromatography
3.8. Antioxidant Potential
3.8.1. DPPH Radical Scavenging
3.8.2. ABTS Radical Scavenging
3.8.3. Nitric Oxide Species Inhibition
3.9. Anti-Inflammatory Potential
3.9.1. Inducible Nitric Oxide Synthase Inhibition Assay
3.9.2. Cyclooxygenases 1 and 2 Isoforms Inhibition Assays
3.10. Molecular Docking
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chávez-Mendoza, C.; Sánchez, E. Bioactive compounds from Mexican varieties of the common bean (Phaseolus vulgaris): Implications for health. Molecules 2017, 22, 1360. [Google Scholar] [CrossRef] [PubMed]
- Servicio de Información Agroalimentaria y Pesquera (SIAP). Panorama Agroalimentario 2022, 2022nd ed.; SIAP: Ciudad de México, México, 2022; pp. 77–78. Available online: https://nube.siap.gob.mx/gobmx_publicaciones_siap/pag/2022/Panorama-Agroalimentario-2022 (accessed on 25 March 2023).
- Gomes Basso Loss, F.; Ferreira Zielinski, A.A.; Wojeicchowski, J.P.; Nogueira, A.; Mottin Demiate, I. Beans (Phaseolus vulgaris L.): Whole seeds with complex chemical composition. Curr. Opin. Food Sci. 2018, 19, 63–71. [Google Scholar] [CrossRef]
- Ramírez-Jiménez, A.K.; Gaytán-Martínez, M.; Morales-Sánchez, E.; Loarca-Piña, G. Functional properties and sensory value of snack bars added with common bean flour as a source of bioactive compounds. LWT-Food Sci. Technol. 2018, 89, 674–680. [Google Scholar] [CrossRef]
- Yang, Q.; Gan, R.; Ge, Y.; Zhang, D.; Corke, H. Polyphenols in Common Beans (Phaseolus vulgaris L.): Chemistry, Analysis, and Factors Affecting Composition. Comp. Rev. Food Sci. Food Saf. 2018, 17, 1518–1539. [Google Scholar] [CrossRef] [PubMed]
- De los Santos Ramos, M.; Romero Rosales, T.; Bobadilla Soto, E.E. Dinámica de la producción de maíz y frijol en México de 1980 a 2014. Agron. Mesoam. 2017, 28, 439–453. [Google Scholar] [CrossRef]
- Delgado-Salinas, A.; Gama-López, S. Diversidad y distribución de los frijoles silvestres en México. Rev. Dig. Univ. 2015, 16, 1–11. [Google Scholar]
- Alcázar-Valle, M.; Lugo-Cervantes, E.; Mojica, L.; Morales-Hernández, N.; Reyes-Ramírez, H.; Enríquez-Vara, J.N.; García-Morales, S. Bioactive Compounds, Antioxidant Activity, and Antinutritional Content of Legumes: A Comparison between Four Phaseolus Species. Molecules 2020, 25, 3528. [Google Scholar] [CrossRef]
- Alcázar-Valle, M.; García-Morales, S.; Mojica, L.; Morales-Hernández, N.; Sánchez-Osorio, E.; Flores-López, L.; Enríquez-Vara, J.N.; Lugo-Cervantes, E. Nutritional, Antinutritional Compounds and Nutraceutical Significance of Native Bean Species (Phaseolus spp.) of Mexican Cultivars. Agriculture 2021, 11, 1031. [Google Scholar] [CrossRef]
- Fonseca-Hernández, D.; Lugo-Cervantes, E.D.C.; Escobedo-Reyes, A.; Mojica, L. Black Bean (Phaseolus vulgaris L.) Polyphenolic Extract Exerts Antioxidant and Antiaging Potential. Molecules 2021, 26, 6716. [Google Scholar] [CrossRef]
- Ramírez-Jaspeado, R.; Palacios-Rojas, N.; Nutti, M.; Pérez, S. Estados potenciales en México para la producción y consumo de frijol biofortificado con Hierro y Zinc. Rev. Fitotec. Mex. 2020, 43, 11–23. Available online: https://www.scielo.org.mx/pdf/rfm/v43n1/0187-7380-rfm-43-01-11.pdf (accessed on 7 June 2023). [CrossRef]
- Mattioli, R.; Francioso, A.; Mosca, L.; Silva, P. Anthocyanins: A Comprehensive Review of Their Chemical Properties and Health Effects on Cardiovascular and Neurodegenerative Diseases. Molecules 2020, 25, 3809. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wang, P.; Luo, Y.; Zhao, M.; Chen, F. Health benefits of anthocyanins and molecular mechanisms: Update from recent decade. Crit. Rev. Food Sci. Nutr. 2017, 57, 1729–1741. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Wang, R.; Wang, J.; Li, Y.; Luo, H.; Chen, S.; Zeng, X.; Han, Z. Acylation of Anthocyanins and Their Applications in the Food Industry: Mechanisms and Recent Research Advances. Foods 2022, 11, 2166. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Giusti, M.M. Anthocyanins: Natural colorants with health-promoting properties. Annu. Rev. Food Sci. Techol. 2010, 1, 163–187. [Google Scholar] [CrossRef]
- Salehi, B.; Sharifi-Rad, J.; Cappellini, F.; Reiner, Z.; Zorzan, D.; Imran, M.; Sener, B.; Kilic, M.; El-Shazly, M.; Fahmy, N.M.; et al. The Therapeutic Potential of Anthocyanins: Current Approaches Based on Their Molecular Mechanism of Action. Front. Pharmacol. 2020, 11, 1300. [Google Scholar] [CrossRef]
- Enaru, B.; Drețcanu, G.; Pop, T.D.; Stǎnilǎ, A.; Diaconeasa, Z. Anthocyanins: Factors Affecting Their Stability and Degradation. Antioxidants 2021, 10, 1967. [Google Scholar] [CrossRef]
- Khalifa, I.; Zhu, W.; Li, K.; Li, C. Polyphenols of mulberry fruits as multifaceted compounds: Compositions, metabolism, health benefits, and stability—A structural review. J. Funct. Foods 2018, 40, 28–43. [Google Scholar] [CrossRef]
- Suárez-Martínez, S.E.; Ferriz-Martínez, R.A.; Campos-Vega, R.; Elton-Puente, J.E.; De La Torre Carbot, K.; García-Gasca, T. Bean seeds: Leading nutraceutical source for human health. CYTA–J. Food 2016, 14, 131–137. [Google Scholar] [CrossRef]
- Mojica, L.; Meyer, A.; Berhow, M.; Gonzalez de Mejia, E. Bean cultivars (Phaseolus vulgaris L.) have similar high antioxidant capacity, in vitro inhibition of α-amylase and α-glucosidase while diverse phenolic composition and concentration. Food Res. Int. 2015, 69, 38–48. [Google Scholar] [CrossRef]
- Aguilera, Y.; Mojica, L.; Rebollo-Hernanz, M.; Berhow, M.; Gonzalez de Mejía, E.; Martín-Cabrejas, M.A. Black bean coats: New source of anthocyanins stabilized by β-cyclodextrin copigmentation in a sport beverage. Food Chem. 2016, 212, 561–570. [Google Scholar] [CrossRef]
- Fonseca-Hernández, D.; Mojica, L.; Berhow, M.; Brownstein, K.; Lugo-Cervantes, E.; Gonzalez de Mejia, E. Black and pinto beans (Phaseolus vulgaris L.) unique Mexican varieties exhibit antioxidant and anti-inflammatory potential. Food Res. Int. 2023, 169, 112816. [Google Scholar] [CrossRef]
- Antonopoulou, I.; Varriale, S.; Topakas, E.; Rova, U.; Christakopoulos, P.; Faraco, V. Enzymatic synthesis of bioactive compounds with high potential for cosmeceutical application. Appl. Microbiol. Biotechnol. 2016, 100, 6519–6543. [Google Scholar] [CrossRef]
- García-Lafuente, A.; Moro, C.; Manchón, N.; Gonzalo-Ruiz, A.; Villares, A.; Guillamón, E.; Rostagno, M.; Mateo-Vivaracho, L. In vitro antiinflammatory activity of phenolic rich extracts from white and red common beans. Food Chem. 2016, 161, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves Bortolini, D.; Maciel, G.M.; Arruda Fernandes, I.A.; Rossetto, R.; Brugnari, T.; Rampazzo Ribeiro, V.; Isidoro Haminiuk, C.W. Biological potential and technological applications of red fruits: An overview. Food Chem. Adv. 2022, 1, 100014. [Google Scholar] [CrossRef]
- Solverson, P. Anthocyanin Bioactivity in Obesity and Diabetes: The Essential Role of Glucose Transporters in the Gut and Periphery. Cells 2020, 9, 2515. [Google Scholar] [CrossRef]
- Mojica, L.; Berhow, M.; Gonzalez de Mejia, E. Black bean anthocyanin-rich extracts as food colorants: Physicochemical stability and antidiabetes potential. Food Chem. 2017, 229, 628–639. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Costa, E.M.; Calhau, C.; Morais, R.M.; Pintado, M.E. Anthocyanin extraction from plant tissues: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 3072–3083. [Google Scholar] [CrossRef] [PubMed]
- Farr, J.E.; Sigurdson, G.T.; Giusti, M.M. Stereochemistry and glycosidic linkages of C3-glycosylations affected the reactivity of cyanidin derivatives. Food Chem. 2019, 278, 443–451. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef]
- Tan, J.; Han, Y.; Han, B.; Qi, X.; Cai, X.; Ge, S.; Xue, H. Extraction and purification of anthocyanins: A review. J. Agric. Food Res. 2022, 8, 100306. [Google Scholar] [CrossRef]
- Maran, J.P.; Priya, B.; Manikandan, S. Modeling and optimization of supercritical fluid extraction of anthocyanin and phenolic compounds from Syzygium cumini fruit pulp. J. Food Sci. Technol. 2014, 51, 1938–1946. [Google Scholar] [CrossRef]
- Nunes, A.N.; Borges, A.; Matias, A.A.; Bronze, M.R.; Oliveira, J. Alternative Extraction and Downstream Purification Processes for Anthocyanins. Molecules 2022, 27, 368. [Google Scholar] [CrossRef]
- Khan, B.M.; Liu, Y. High speed counter current chromatography: Overview of solvent-system and elution-mode. J. Liq. Chromatogr. Relat. Technol. 2018, 41, 629–636. [Google Scholar] [CrossRef]
- Wang, T.; Wang, Q.; Li, P.; Yang, H. High-Speed Countercurrent Chromatography-Based Method for Simultaneous Recovery and Separation of Natural Products from Deep Eutectic Solvent Extracts. ACS Sustain. Chem. Eng. 2020, 8, 2073–2080. [Google Scholar] [CrossRef]
- Kicel, A.; Owczarek, A.; Michel, P.; Skalicka-Woźniak, K.; Kiss, A.K.; Olszewska, M.A. Application of HPCCC, UHPLC-PDA-ESI-MS3 and HPLC-PDA methods for rapid, one-step preparative separation and quantification of rutin in Forsythia flowers. Ind. Crops Prod. 2015, 76, 86–94. [Google Scholar] [CrossRef]
- Li, L.; Zhao, J.; Yang, T.; Sun, B. High-speed countercurrent chromatography as an efficient technique for large separation of plant polyphenols: A review. Food Res. Int. 2022, 153, 110956. [Google Scholar] [CrossRef] [PubMed]
- Ostka, T.; Ostberg-Potthoff, J.J.; Stärke, J.; Guigas, C.; Matsugo, S.; Mirčeski, V.; Stojanov, L.; Veličkovska, S.K.; Winterhalter, P.; Esatbeyoglu, T. Bioactive Phenolic Compounds from Lingonberry (Vaccinium vitis-idaea L.): Extraction, Chemical Characterization, Fractionation and Cellular Antioxidant Activity. Antioxidants 2022, 11, 467. [Google Scholar] [CrossRef]
- Kostka, T.; Ostberg-Potthoff, J.J.; Briviba, K.; Matsugo, S.; Winterhalter, P.; Esatbeyoglu, T. Pomegranate (Punica granatum L.) Extract and Its Anthocyanin and Copigment Fractions—Free Radical Scavenging Activity and Influence on Cellular Oxidative Stress. Foods 2020, 9, 1617. [Google Scholar] [CrossRef] [PubMed]
- Vieira, M.N.; Winterhalter, P.; Jerz, G. Flavonoids from the flowers of Impatiens glandulifera Royle isolated by high performance countercurrent chromatography. Phytochem. Anal. 2016, 27, 116–125. [Google Scholar] [CrossRef]
- Hsieh-Lo, M.; Castillo-Herrera, G.; Mojica, L. Black Bean Anthocyanin-Rich Extract from Supercritical and Pressurized Extraction Increased In Vitro Antidiabetic Potential, While Having Similar Storage Stability. Foods 2020, 9, 655. [Google Scholar] [CrossRef] [PubMed]
- Gençdağ, E.; Özdemir, E.E.; Demirci, K.; Görgüç, A.; Yılmaz, F.M. Copigmentation and stabilization of anthocyanins using organic molecules and encapsulation techniques. Curr. Plant Bio. 2022, 29, 100238. [Google Scholar] [CrossRef]
- Silva, M.; Castellanos, L.; Ottens, M. Capture and Purification of Polyphenols Using Functionalized Hydrophobic Resins. Ind. Eng. Chem. Res. 2018, 57, 5359–5369. [Google Scholar] [CrossRef] [PubMed]
- Green, B.V.; Ford, T.W.; Goldsborrough, H.; Abdelmotalab, M.; Ristvey, A.G.; Sauder, D.G.; Volkis, V.V. Extraction of Antioxidants from Aronia mitschurinii Juice Using Macroporous Resins. ACS Omega 2022, 7, 29877–29885. [Google Scholar] [CrossRef] [PubMed]
- López, A.; El-Naggar, T.; Duenas, M.; Ortega, T.; Estrella, I.; Hernández, T.; Gómez-Serranillos, M.P.; Palomino, O.M.; Emilia Carretero, M. Effect of cooking and germination on phenolic composition and biological properties of dark beans (Phaseolus vulgaris L.). Food Chem. 2013, 138, 547–555. [Google Scholar] [CrossRef]
- Moreno-Jiménez, M.R.; Cervantes-Cardoza, V.; Gallegos-Infante, J.A.; González-Laredo, R.F.; Estrella, I.; García-Gasca, T.; Herrera-Carrera, E.; Díaz-Rivas, J.O.; Rocha-Guzmán, N.E. Phenolic composition changes of processed common beans: Their antioxidant and anti-inflammatory effects in intestinal cancer cells. Food Res. Int. 2015, 76, 79–85. [Google Scholar] [CrossRef]
- Duenas, M.; Sarmento, T.; Aguilera, Y.; Benítez, V.; Molla, E.; Esteban, R.M.; Martín-Cabrejas, M.A. Impact of cooking and germination on phenolic composition and dietary fiber fractions in dark beans (Phaseolus vulgaris L.) and lentils (Lens culinaris L.). LWT-Food Sci. Techol. 2016, 66, 72–78. [Google Scholar] [CrossRef]
- Lin, L.Z.; Harnly, J.M.; Pastor-Corrales, M.S.; Luthria, D.L. The polyphenolic profiles of common bean (Phaseolus vulgaris L.). Food Chem. 2008, 107, 399–410. [Google Scholar] [CrossRef]
- Xu, B.; Chang, S.K.C. Total phenolic, phenolic acid, anthocyanin, flavan-3-ol, and flavonol profiles and antioxidant properties of pinto and black beans (Phaseolus vulgaris L.) as affected by thermal processing. J. Agric. Food Chem. 2009, 57, 4754–4764. [Google Scholar] [CrossRef]
- Meenu, M.; Chen, P.; Mradula, M.; Chang, S.K.C.; Xu, B. New insights into chemical compositions and health-promoting effects of black beans (Phaseolus vulgaris L.). Food Front. 2023, 00, 1–20. [Google Scholar] [CrossRef]
- Chen, L.; Xin, X.; Feng, H.; Li, S.; Cao, Q.; Wang, X.; Vriesekoop, F. Isolation and Identification of Anthocyanin Component in the Fruits of Acanthopanax Sessiliflorus (Rupr. & Maxim.) Seem. by Means of High Speed Counter Current Chromatography and Evaluation of Its Antioxidant Activity. Molecules 2020, 25, 1781. [Google Scholar] [CrossRef] [PubMed]
- Abdin, M.; Hamed, Y.S.; Akhtar, H.M.S.; Chen, D.; Chen, G.; Wan, P.; Zeng, X. Antioxidant and anti-inflammatory activities of target anthocyanins di-glucosides isolated from Syzygium cumini pulp by high speed counter-current chromatography. J. Food Biochem. 2020, 44, 1050–1062. [Google Scholar] [CrossRef]
- Du, Q.; Jerz, G.; Winterhalter, P. Isolation of two anthocyanin sambubiosides from bilberry (Vaccinium myrtillus) by high-speed counter-current chromatography. J. Chromatogr. A 2004, 1045, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Möller, M.N.; Rios, N.; Trujillo, M.; Radi, R.; Denicola, A.; Alvarez, B. Detection and quantification of nitric oxide–derived oxidants in biological systems. J. Biol. Chem. 2019, 294, 14776–14802. [Google Scholar] [CrossRef] [PubMed]
- Goshi, E.; Zhou, G.; He, Q. Nitric oxide detection methods in vitro and in vivo. Med. Gas Res. 2019, 9, 192–207. [Google Scholar] [CrossRef]
- Jayarathne, S.; Koboziev, I.; Park, O.H.; Oldewage-Theron, W.; Shen, C.L.; Moustaid-Moussa, N. Anti-Inflammatory and Anti-Obesity Properties of Food Bioactive Components: Effects on Adipose Tissue. Prev. Nutr. Food Sci. 2017, 4, 251–262. [Google Scholar] [CrossRef]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 20, e00370. [Google Scholar] [CrossRef] [PubMed]
- Ombra, M.N.; d’Acierno, A.; Nazzaro, F.; Riccardi, R.; Spigno, P.; Zaccardelli, M.; Pane, C.; Maione, M.; Fratianni, F. Phenolic composition and antioxidant and antiproliferative activities of the extracts of twelve common bean (Phaseolus vulgaris L.) endemic ecotypes of Southern Italy before and after cooking. Oxidative Med. Cell. Longev. 2016, 2016, 1398298. [Google Scholar] [CrossRef]
- Romani, A.; Vignolini, P.; Falvino, M.; Heimler, D. Polyphenol content and antiradical activity of “sarconi” beans (Phaseolus vulgaris L.) ecotype. Ital. J. Food Sci. 2013, 25, 322–328. Available online: https://www.researchgate.net/publication/286579172_Polyphenol_content_and_antiradical_activity_of_sarconi_beans_Phaseolus_vulgaris_L_ecotype (accessed on 14 January 2023).
- Ongkowijoyo, P.; Luna-Vital, D.A.; Gonzalez de Mejia, E. Extraction techniques and analysis of anthocyanins from food sources by mass spectrometry: An update. Food Chem. 2018, 250, 113–126. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, H.; Song, L.; Yang, Z.; Qiu, M.; Wang, J.; Shi, S. Anthocyanins: Promising Natural Products with Diverse Pharmacological Activities. Molecules 2021, 26, 3807. [Google Scholar] [CrossRef]
- Tena, N.; Martín, J.; Asuero, A.G. State of the Art of Anthocyanins: Antioxidant Activity, Sources, Bioavailability, and Therapeutic Effect in Human Health. Antioxidants 2020, 9, 451. [Google Scholar] [CrossRef]
- Boccellino, M.; D’Angelo, S. Anti-Obesity Effects of Polyphenol Intake: Current Status and Future Possibilities. Int. J. Mol. Sci. 2020, 21, 5642. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2017, 9, 7204–7218. [Google Scholar] [CrossRef]
- Contreras, J.; Herrera-González, A.; Arrizon, J.; Lugo-Cervantes, E.; Mojica, L. Mexican Endemic Black Bean Phenolic Extract Antioxidant and Anti-Inflammatory Potential. Curr. Dev. Nutr. 2020, 4 (Suppl. S2), 382. [Google Scholar] [CrossRef]
- Maldonado-Rojas, W.; Olivero-Verbel, J. Food-Related Compounds That Modulate Expression of Inducible Nitric Oxide Synthase May Act as Its Inhibitors. Molecules 2012, 17, 8118–8135. [Google Scholar] [CrossRef] [PubMed]
- Cinelli, M.A.; Do, H.T.; Miley, G.P.; Silverman, R.B. Inducible nitric oxide synthase: Regulation, structure, and inhibition. Med. Res. Rev. 2020, 40, 158–189. [Google Scholar] [CrossRef] [PubMed]
- Abdelgawad, M.A.; Musa, A.; Almalki, A.H.; Alzarea, S.I.; Mostafa, E.M.; Hegazy, M.M.; Mostafa-Hedeab, G.; Ghoneim, M.M.; Parambi, D.G.T.; Bakr, R.B.; et al. Novel Phenolic Compounds as Potential Dual EGFR and COX-2 Inhibitors: Design, Semisynthesis, in vitro Biological Evaluation and in silico Insights. Drug Des. Dev. Ther. 2021, 15, 2325–2337. [Google Scholar] [CrossRef]
- Hwang, Y.P.; Choi, J.H.; Yun, H.J.; Han, E.H.; Kim, H.G.; Kim, J.Y.; Park, B.H.; Khanal, T.; Choi, J.M.; Chung, Y.C.; et al. Anthocyanins from purple sweet potato attenuate dimethylnitrosamine-induced liver injury in rats by inducing Nrf2-mediated antioxidant enzymes and reducing COX-2 and iNOS expression. Food Chem. Toxicol. 2011, 49, 93–99. [Google Scholar] [CrossRef]
- Dia, V.P.; Berhow, M.; Gonzalez-De Mejia, E. Bowman-Birk inhibitor and genistein among soy compounds that synergistically inhibit nitric oxide and prostaglandin E2 pathways in lipopolysaccharide-induced macrophages. J. Agric. Food Chem. 2008, 56, 11707–11717. [Google Scholar] [CrossRef]
- Chung, W.G.; Miranda, C.L.; Stevens, J.F.; Maier, C.S. Hop proanthocyanidins induce apoptosis, protein carbonylation, and cytoskeleton disorganization in human colorectal adenocarcinoma cells via reactive oxygen species. Food Chem. Toxicol. 2009, 47, 827–836. [Google Scholar] [CrossRef]
- Kowalski, R.; Gonzalez de Mejia, E. Phenolic composition, antioxidant capacity and physical characterization of ten blackcurrant (Ribes nigrum) cultivars, their juices, and the inhibition of type 2 diabetes and inflammation biochemical markers. Food Chem. 2021, 359, 129889. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Somavat, P.; Singh, V.; Gonzalez de Mejia, E. Chemical characterization of proanthocyanidins in purple, blue, and red maize coproducts from different milling processes and their anti-inflammatory properties. Ind. Crops Prod. 2017, 109, 464–475. [Google Scholar] [CrossRef]
- Ganesan, K.; Xu, B. Polyphenol-Rich Dry Common Beans (Phaseolus vulgaris L.) and Their Health Benefits. Int. J. Mol. Sci. 2017, 18, 2331. [Google Scholar] [CrossRef]
- Khan, A.; Pervaiz, A.; Ansari, B.; Ullah, R.; Shah, S.M.M.; Khan, H.; Saeed Jan, M.; Hussain, F.; Ijaz Khan, M.; Albadrani, G.M.; et al. Phytochemical Profiling, Anti-Inflammatory, Anti-Oxidant and In-Silico Approach of Cornus macrophylla Bioss (Bark). Molecules 2022, 27, 4081. [Google Scholar] [CrossRef]
- Jara-Gutiérrez, Á.; Baladrón, V. The Role of Prostaglandins in Different Types of Cancer. Cells 2021, 10, 1487. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Zou, H.; Yuan, C.}.; Hong, Y.H.; Kuklev, D.V.; Smith, W.L. Different Fatty Acids Compete with Arachidonic Acid for Binding to the Allosteric or Catalytic Subunits of Cyclooxygenases to Regulate Prostanoid Synthesis. J. Biol. Chem. 2016, 291, 4069–4078. [Google Scholar] [CrossRef]
- Serreli, G.; Deiana, M. Role of Dietary Polyphenols in the Activity and Expression of Nitric Oxide Synthases: A Review. Antioxidants 2023, 12, 147. [Google Scholar] [CrossRef]
- Jokioja, J.; Yang, B.; Linderborg, K.M. Acylated anthocyanins: A review on their bioavailability and effects on postprandial carbohydrate metabolism and inflammation. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5570–5615. [Google Scholar] [CrossRef] [PubMed]
- Iwakiri, Y. Nitric oxide in liver fibrosis: The role of inducible nitric oxide synthase. Clin. Mol. Hepatol. 2015, 21, 319–325. [Google Scholar] [CrossRef]
- Xu, Z.P.; Liu, Y.; Wang, S.Y.; Li, Z.W.; Lu, D.X.; Jiang, P.; Pan, J.; Guan, W.; Kuang, H.X.; Yang, B.I. Phenolic compounds of Solanum xanthocarpum play an important role in anti-inflammatory effects. Arab. J. Chem. 2022, 15, 103877. [Google Scholar] [CrossRef]
- Lescano, C.H.; Freitas de Lima, F.; Mendes-Silvério, C.B.; Justo, A.F.O.; da Silva-Baldivia, D.; Vieira, C.P.; Sanjinez-Argandoña, E.J.; Cardoso, C.A.L.; Mónica, F.Z.; Pires de Oliveira, I. Effect of Polyphenols From Campomanesia adamantium on Platelet Aggregation and Inhibition of Cyclooxygenases: Molecular Docking and in Vitro Analysis. Front. Pharmacol. 2018, 9, 617. [Google Scholar] [CrossRef] [PubMed]
- Ilari, S.; Dagostino, C.; Malafoglia, V.; Lauro, F.; Giancotti, L.A.; Spila, A.; Proietti, S.; Ventrice, D.; Rizzo, M.; Gliozzi, M.; et al. Protective Effect of Antioxidants in Nitric Oxide/COX-2 Interaction during Inflammatory Pain: The Role of Nitration. Antioxidants 2020, 9, 1284. [Google Scholar] [CrossRef]
- Mittraphab, Y.; Amen, Y.; Nagata, M.; Matsumoto, M.; Wang, D.; Shimizu, K. Anti-Phototoxicity Effect of Phenolic Compounds from Acetone Extract of Entada phaseoloides Leaves via Activation of COX-2 and iNOS in Human Epidermal Keratinocytes. Molecules 2022, 27, 440. [Google Scholar] [CrossRef]
- Zou, D.; Du, Y.; Kuang, J.; Sun, S.; Ma, J.; Jiang, R. PH-zone-refining counter-current chromatography with a hydrophilic organic/salt-containing two-phase solvent system for preparative separation of polar alkaloids from natural products. J. Chromatogr. A 2018, 1553, 1–6. [Google Scholar] [CrossRef]
- Klisurova, D.; Petrova, I.; Ognyanov, M.; Georgiev, Y.; Kratchanova, M.; Denev, P. Co-pigmentation of black chokeberry (Aronia melanocarpa) anthocyanins with phenolic co-pigments and herbal extracts. Food Chem. 2019, 279, 162–170. [Google Scholar] [CrossRef]
- Virgen-Carrillo, C.A.; Valdés Miramontes, E.H.; Fonseca Hernández, D.; Luna-Vital, D.A.; Mojica, L. West Mexico Berries Modulate α-Amylase, α-Glucosidase and Pancreatic Lipase Using In Vitro and In Silico Approaches. Pharmaceuticals 2022, 15, 1081. [Google Scholar] [CrossRef] [PubMed]
- Floegel, A.; Kim, D.O.; Chung, S.J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Comp. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Tsai, M.L.; Lin, C.C.; Lin, W.C.; Yang, C.H. Antimicrobial, antioxidant, and anti-inflammatory activities of essential oils from five selected herbs. Biosci. Biotechnol. Biochem. 2011, 75, 1977–1983. [Google Scholar] [CrossRef]
- Grancieri, M.; Martino, H.S.D.; Gonzalez de Mejia, E. Digested total protein and protein fractions from chia seed (Salvia hispanica L.) had high scavenging capacity and inhibited 5-LOX, COX-1-2, and iNOS enzymes. Food Chem. 2019, 289, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Grande, F.; Giordano, F.; Occhiuzzi, M.A.; Rocca, C.; Ioele, G.; De Luca, M.; Garofalo, A. Toward multitasking pharmacological COX-targeting agents: Non-steroidal anti-inflammatory prodrugs with antiproliferative effects. Molecules 2021, 26, 3940. [Google Scholar] [CrossRef]
- Arias, F.; Franco-Montalban, F.; Romero, M.; Carri´on, M.D.; Camacho, M.E. Synthesis, bioevaluation and docking studies of new imidamide derivatives as nitric oxide synthase inhibitors. Bioorg. Med. Chem. 2021, 44, 116294. [Google Scholar] [CrossRef] [PubMed]
| Treatment | Total Phenolic Content (mg GAE/L) | Total Anthocyanin Content (mg C3GE/L) |
|---|---|---|
| RE | 358.4 ± 24.3 a | 52.2 ± 4.4 b |
| REF1 | 109.1 ± 8.2 c | 27.8 ± 0.1 c |
| REF2 | 315.0 ± 15.8 a | 66.3 ± 1.1 a |
| PE | 185.4 ± 34.0 b | 28.1 ± 1.0 c |
| PEF1 | 40.3 ± 10.8 d | 10.7 ± 0.5 d |
| PEF2 | 48.0 ± 20.0 d | 13.1 ± 0.5 d |
| RE | REF1 | REF2 | PE | PEF1 | PEF2 | |
|---|---|---|---|---|---|---|
| Tentative Identification | m/z Experimental | |||||
| Quercetin-3-galactoside | 463.1255 | 463.0462 | 463.0638 | 463.1255 | 463.055 | 463.0374 |
| Malvidin-3-glucoside | 331.0715 | 331.0752 | 331.0603 | 331.0715 | 331.0603 | 331.0603 |
| Delphinidin 3-glucoside | 303.0402 | 303.0794 | - | 303.0402 | - | 303.0794 |
| Cyanidin 3-glucoside | 447.1372 | 447.0766 | 447.0679 | 447.1372 | 447.0679 | 447.0766 |
| Petunidin-3-glucoside | 447.1285 | 447.0679 | 447.0766 | 447.1372 | 447.0679 | 447.0766 |
| Gallic acid | 169.0633 | 169.026 | 169.026 | 169.0686 | 169.0313 | 169.042 |
| Genistein | 269.189 | 269.2226 | - | 269.1957 | 269.2226 | 269.2293 |
| Protocatechuic acid | 153.07 | 153.0244 | 153.0244 | 153.07 | 153.0497 | 153.0497 |
| Rutin | 609.1727 | - | - | 609.1525 | 609.0716 | - |
| Naringenin | 271.0636 | 271.2356 | - | 271.1951 | 271.2423 | 271.2423 |
| Catechin | 289.0836 | - | - | 289.0975 | 289.0488 | - |
| Glycitin | 285.0322 | 285.1669 | 285.1635 | 285.0322 | 285.1635 | 285.1704 |
| Myricetin | 317.1151 | - | - | 317.1115 | 317.0677 | 317.075 |
| Ferulic acid | 193.0749 | 193.0493 | 193.0493 | 193.0749 | 193.0493 | 193.0493 |
| p-coumaric acid | 165.014 | - | 165.0508 | 165.0114 | 165.0486 | - |
| Caffeic acid | 179.0856 | 179.0829 | 179.0856 | 179.0911 | 179.0856 | 179.0637 |
| Rosmarinic acid | 359.1975 | 359.1432 | - | 359.1199 | 359.1509 | 359.1509 |
| Phenolic Compounds Concentration (mg/L) | Anthocyanins Concentration (mg/L) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | GA | G | M | A7G | EGCG | K3G | Q3G | Q3Ga | D3G | M3G | P3G |
| RE | 180.1 | - | - | - | 9.7 | 30.4 | 10.3 | - | 31.26 | 29.00 | 35.84 |
| REF1 | - | - | - | - | - | - | 19.8 | 3.9 | 2.71 | 7.34 | 3.65 |
| REF2 | 170.4 | 35.2 | - | 15.2 | 5.3 | 25.3 | 15.1 | - | 40.33 | 17.61 | 23.18 |
| PE | - | - | 10.4 | - | 14.8 | 39.5 | 70.4 | 15.2 | 4.51 | 5.91 | 3.17 |
| PEF1 | - | - | - | - | - | - | 10.2 | 4.1 | 0.71 | - | 1.65 |
| PEF2 | - | - | - | - | - | 30.2 | 14.6 | - | 3.40 | 16.81 | 5.90 |
| Compound | iNOS (kcal/mol) | COX-1 (kcal/mol) | COX-2 (kcal/mol) |
|---|---|---|---|
| Quercetin-3-galactoside | −1.8 | −3.2 | −3.3 |
| Malvidin-3-glucoside | −5.3 | −8.45 | −6.3 |
| Delphinidin 3-glucoside | −8.5 | −4.3 | −4.5 |
| Cyanidin 3-glucoside | −3.4 | −5.4 | −7.5 |
| Petunidin-3-glucoside | −4.2 | −6.3 | −6.3 |
| Gallic acid | −2.5 | −7.2 | −2.8 |
| Genistein | −1.6 | −6.2 | −1.8 |
| Protocatechuic acid | −4.8 | −1.4 | −6.3 |
| Rutin | −4.4 | −5.6 | −1.5 |
| Naringenin | −7.2 | −8.3 | −4.3 |
| Catechin | −6.4 | −2.4 | −8.3 |
| Glycitin | −4.2 | −3.5 | −3.2 |
| Myricetin | −3.4 | −4.15 | −4.2 |
| Ferulic acid | −6.7 | −2.36 | −8.2 |
| p-coumaric acid | −2.5 | −6.2 | −3.4 |
| Caffeic acid | −3.8 | −4.7 | −4.4 |
| Rosmarinic acid | −2.7 | −5.2 | −6.2 |
| Mofezolac® | - | −8.4 | - |
| Celecoxib® | - | - | −7.2 |
| L-arginine | −8.3 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Contreras, J.; Alcázar-Valle, M.; Lugo-Cervantes, E.; Luna-Vital, D.A.; Mojica, L. Mexican Native Black Bean Anthocyanin-Rich Extracts Modulate Biological Markers Associated with Inflammation. Pharmaceuticals 2023, 16, 874. https://doi.org/10.3390/ph16060874
Contreras J, Alcázar-Valle M, Lugo-Cervantes E, Luna-Vital DA, Mojica L. Mexican Native Black Bean Anthocyanin-Rich Extracts Modulate Biological Markers Associated with Inflammation. Pharmaceuticals. 2023; 16(6):874. https://doi.org/10.3390/ph16060874
Chicago/Turabian StyleContreras, Jonhatan, Montserrat Alcázar-Valle, Eugenia Lugo-Cervantes, Diego A. Luna-Vital, and Luis Mojica. 2023. "Mexican Native Black Bean Anthocyanin-Rich Extracts Modulate Biological Markers Associated with Inflammation" Pharmaceuticals 16, no. 6: 874. https://doi.org/10.3390/ph16060874
APA StyleContreras, J., Alcázar-Valle, M., Lugo-Cervantes, E., Luna-Vital, D. A., & Mojica, L. (2023). Mexican Native Black Bean Anthocyanin-Rich Extracts Modulate Biological Markers Associated with Inflammation. Pharmaceuticals, 16(6), 874. https://doi.org/10.3390/ph16060874








