Elevated Intraocular Pressure and Glaucomatous Optic Neuropathy: Genes to Disease Mechanisms, Therapeutic Drugs, and Gene Therapies
Abstract
1. Introduction
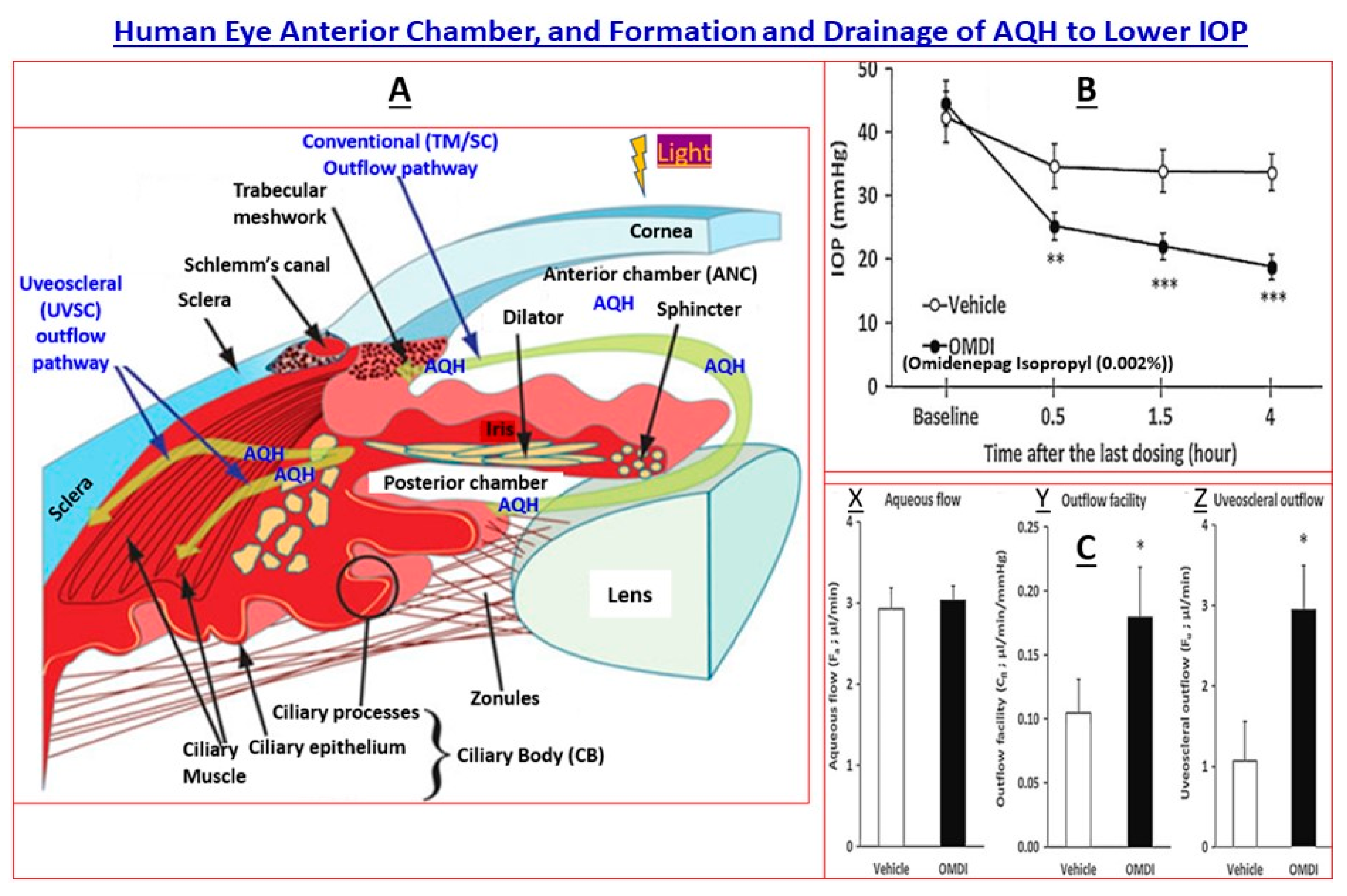
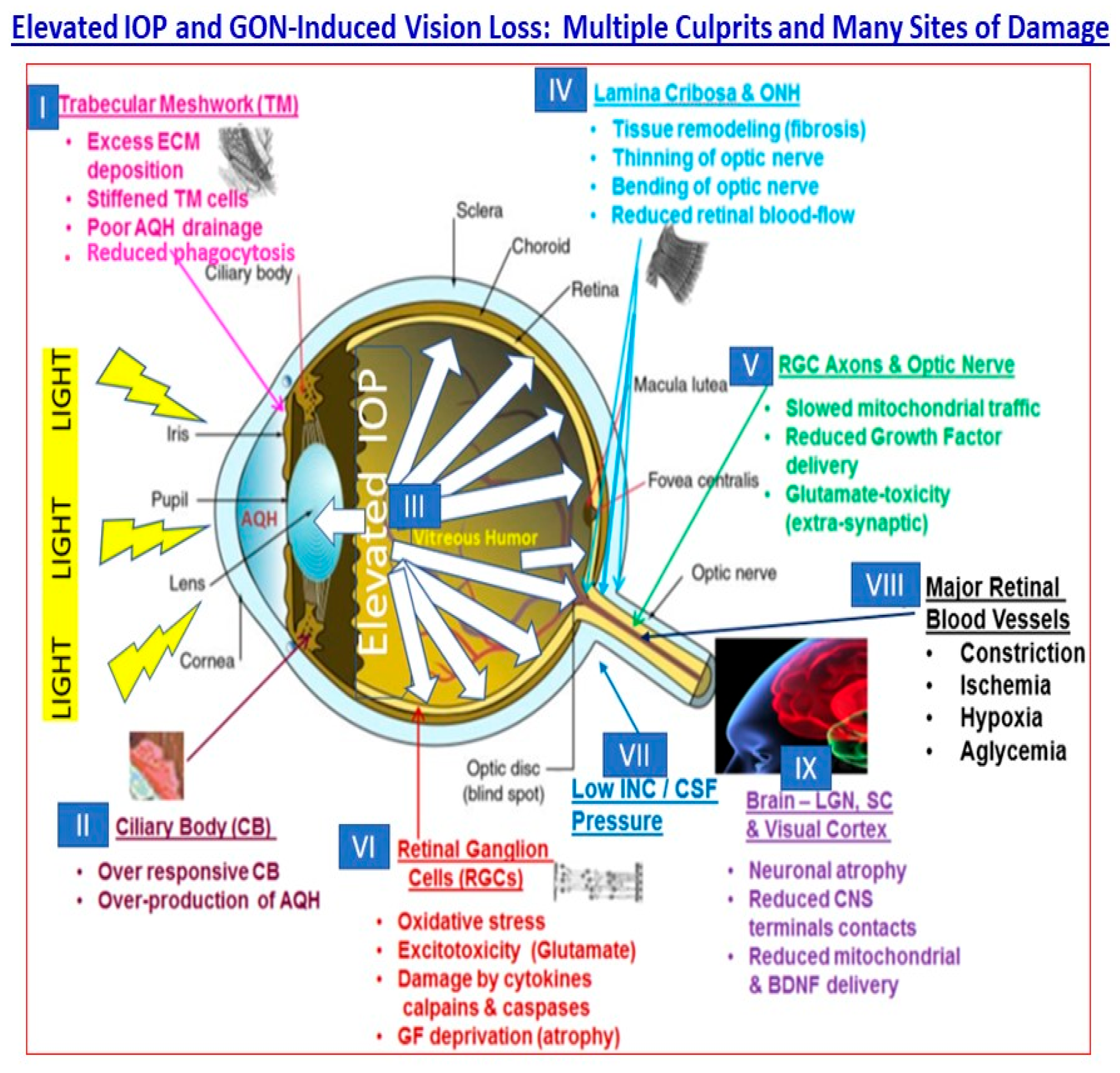
2. Pathogenesis of cOHT and PAOG
3. Some Examples of GWAS for Different Forms of Glaucoma—Translational Research
4. Basics of Gene Therapy for Eye Diseases
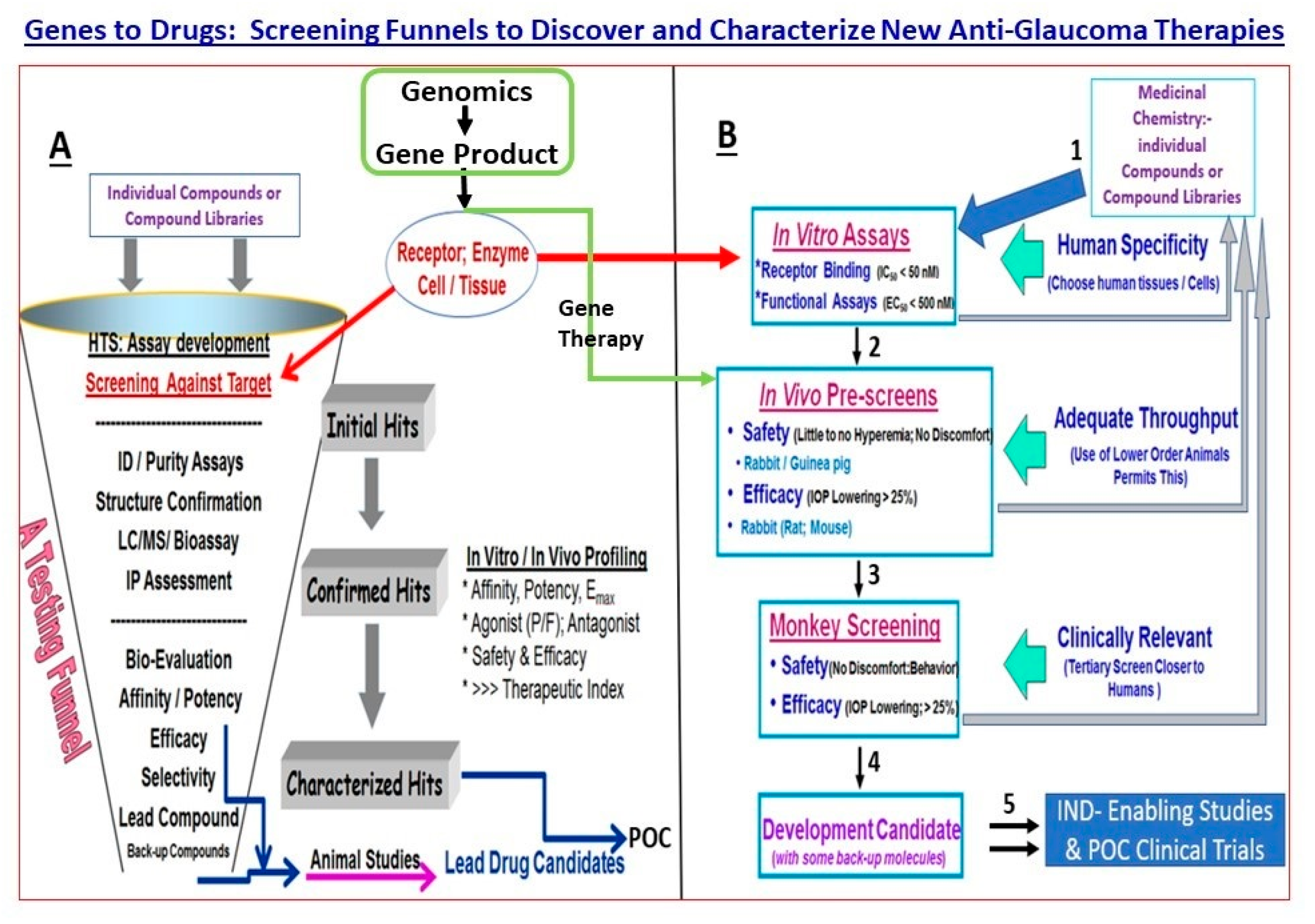
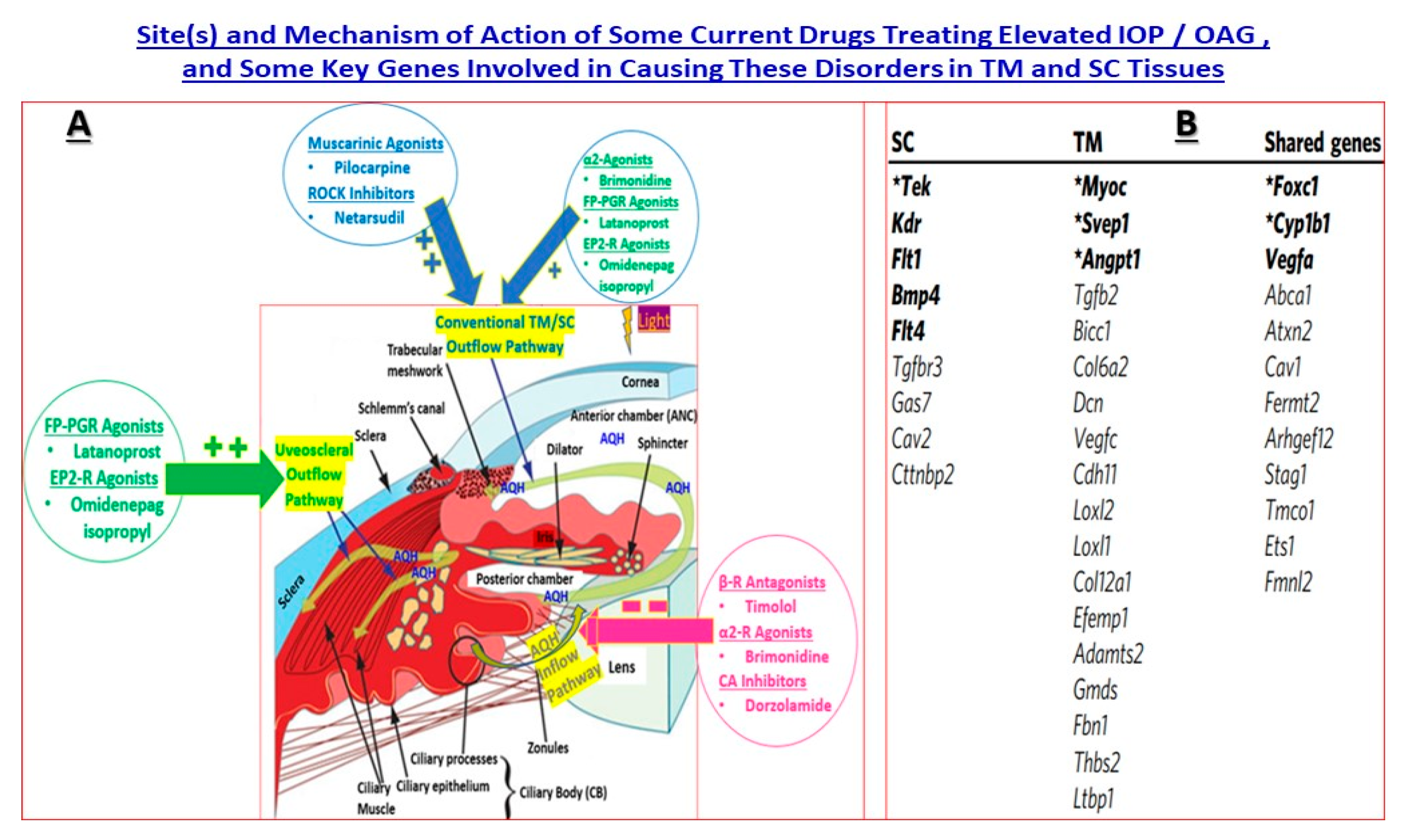
5. Drug Discovery and Development for cOHT/POAG—Genes and Pathway Analysis
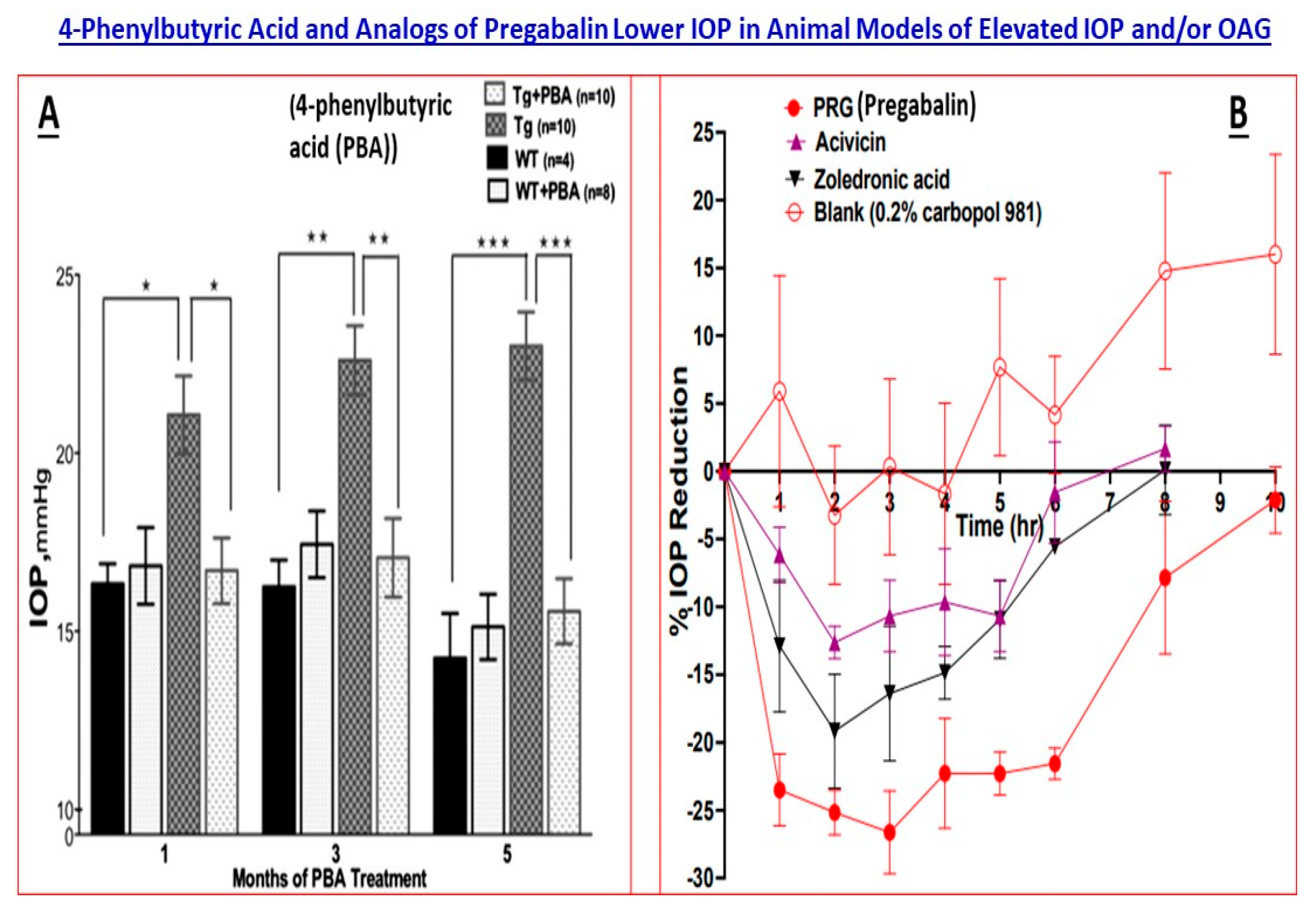
5.1. MYOC Gene and Mutant Myocilin Raises IOP
5.2. Cacna2d1 Gene and Linkage to a Ca2+-Channel
5.3. CAV1/CAV2 Genes and Caveolin Proteins
5.4. TMCO1 Gene and Its Protein
5.5. GAS7 Gene and Its Protein
5.6. ABCA1 Gene and Its Transporter Protein
5.7. ANGPT1 Gene and Its Angiopoitin-1 Protein
5.8. CDKN2BAS Gene and Its Cyclin Proteins
5.9. SIX1/SIX6 Gene and Its Protein
5.10. NTF4 Gene and Its Protein
5.11. OPTN Gene and Its Adapter Protein
5.12. TBK1 Gene and Its Kinase Protein
6. Genes That Affect Patient Responsiveness to Drug Treatments
7. POAG and Gene Therapy
8. Concluding Remarks
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saldanha, I.J.; Lindsley, K.; Do, D.V.; Chuck, R.S.; Meyerle, C.; Jones, L.S.; Coleman, A.L.; Jampel, H.D.; Dickersin, K.; Virgili, G. Comparison of clinical trial and systematic review outcomes for the 4 most prevalent eye diseases. JAMA Ophthalmol. 2017, 135, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Assi, L.; Chamseddine, F.; Ibrahim, P.; Sabbagh, H.; Rosman, L.; Congdon, N.; Evans, J.; Ramke, J.; Kuper, H.; Burton, M.J.; et al. A global assessment of eye health and quality of life: A systematic review of systematic reviews. JAMA Ophthalmol. 2021, 139, 526–541. [Google Scholar] [CrossRef] [PubMed]
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The pathophysiology and treatment of glaucoma: A review. J. Am. Med. Assoc. 2014, 311, 1901–1911. [Google Scholar] [CrossRef] [PubMed]
- Sharif, N.A. Ocular hypertension and glaucoma: A review and current perspectives. Int. J. Ophthalmol. Vis. Sci. 2017, 2, 22–36. [Google Scholar]
- Tham, Y.C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef]
- Sharif, N.A. iDrugs and iDevices discovery and development- preclinical assays, techniques and animal model studies for ocular hypotensives and neuroprotectants. J. Ocul. Pharmacol. Ther. 2018, 34, 7–39. [Google Scholar] [CrossRef]
- Sharif, N.A. Therapeutic drugs and devices for tackling ocular hypertension and glaucoma, and need for neuroprotection and cytoprotective therapies. Front. Pharmacol. 2021, 12, 729249. [Google Scholar] [CrossRef]
- Botto, C.; Rucli, M.; Tekinsoy, M.D.; Pulman, J.; Sahel, J.A.; Dalkara, D. Early and late-stage gene therapy interventions for inherited retinal degenerations. Prog. Retin. Eye Res. 2022, 86, 100975. [Google Scholar] [CrossRef]
- Moraru, A.D.; Costin, D.; Iorga, R.E.; Munteanu, M.; Moraru, R.L.; Branisteanu, D.C. Current trends in gene therapy for retinal diseases (review). Exp. Ther. Med. 2022, 23, 26. [Google Scholar] [CrossRef]
- Choquet, H.; Thai, K.K.; Yin, J.; Hoffmann, T.J.; Kvale, M.N.; Banda, Y.; Schaefer, C.; Risch, N.; Nair, K.S.; Melles, R.; et al. A large multi-ethnic genome-wide association study identifies novel genetic loci for intraocular pressure. Nat. Commun. 2017, 8, 2108. [Google Scholar] [CrossRef]
- Canut, M.I.; Villa, O.; Kudsieh, B.; Mattlin, H.; Banchs, I.; González, J.R.; Armengol, L.; Casaroli-Marano, R.P. MLIP genotype as a predictor of pharmacological response in primary open-angle glaucoma and ocular hypertension. Sci. Rep. 2021, 11, 1583. [Google Scholar] [CrossRef] [PubMed]
- Ussa, F.; Fernandez, I.; Brion, M.; Carracedo, A.; Blazquez, F.; Garcia, M.T.; Sanchez-Jara, A.; De Juan-Marcos, L.; Jimenez-Carmona, S.; Juberias, J.R.; et al. Association between SNPs of metalloproteinases and prostaglandin F2α receptor genes and latanoprost response in open-angle glaucoma. Ophthalmology 2015, 122, 1040–1048.e4. [Google Scholar] [CrossRef] [PubMed]
- Doucette, L.P.; Footz, T.; Walter, M.A. FOXC1 Regulates Expression of prostaglandin receptors leading to an attenuated response to latanoprost. Investig. Ophthalmol. Vis. Sci. 2018, 59, 2548–2554. [Google Scholar] [CrossRef]
- Xu, K.; Yu, L.; Wang, Z.; Lin, P.; Zhang, N.; Xing, Y.; Yang, N. Use of gene therapy for optic nerve protection: Current concepts. Front. Neurosci. 2023, 17, 1158030. [Google Scholar] [CrossRef] [PubMed]
- Sharif, N.A. Pharmacodynamic Evaluation: Ocular Pharmacology. In Drug Discovery and Evaluation: Methods in Clinical Pharmacology; Hock, F.J., Gralinski, M.R., Eds.; Springer Publishing Company: Berlin/Heidelberg, Germany, 2020; Chapter 54; pp. 1–46. [Google Scholar] [CrossRef]
- Ohia, S.E.; Sharif, N.A. (Eds.) Handbook of Basic and Clinical Ocular Pharmacology and Therapeutics; Academic Press: Oxford, UK, 2022; 47p. [Google Scholar]
- Guo, L.; Moss, S.E.; Alexander, R.A.; Ali, R.R.; Fitzke, F.W.; Cordeiro, M.F. Retinal ganglion cell apoptosis in glaucoma is related to intraocular pressure and IOP-induced effects on extracellular matrix. Investig. Ophthalmol. Vis. Sci. 2005, 46, 175–182. [Google Scholar] [CrossRef]
- Leske, M.C.; Heijl, A.; Hussein, M.; Bengtsson, B.; Hyman, L.; Komaroff, E. Factors for glaucoma progression and the effect of treatment: The early manifest glaucoma trial. Arch. Ophthalmol. 2003, 121, 48–56. [Google Scholar] [CrossRef]
- de Voogd, S.; Ikram, M.K.; Wolfs, R.C.; Jansonius, N.M.; Hofman, A.; de Jong, P.T. Incidence of open-angle glaucoma in a general elderly population: The Rotterdam Study. Ophthalmology 2005, 112, 1487–1493. [Google Scholar] [CrossRef]
- Khawaja, A.P.; Cooke Bailey, J.N.; Wareham, N.J.; Scott, R.A.; Simcoe, M.; Igo, R.P., Jr.; Song, Y.E.; Wojciechowski, R.; Cheng, C.Y.; Khaw, P.T.; et al. Genome-wide analyses identify 68 new loci associated with intraocular pressure and improve risk prediction for primary open-angle glaucoma. Nat. Genet. 2018, 50, 778–782. [Google Scholar] [CrossRef]
- Acott, T.S.; Vranka, J.A.; Keller, K.E.; Raghunathan, V.; Kelley, M.J. Normal and glaucomatous outflow regulation. Prog. Retin. Eye Res. 2020, 11, 100897. [Google Scholar] [CrossRef]
- Calkins, D.J.; Horner, P.J. The cell and molecular biology of glaucoma: Axonopathy and the brain. Investig. Ophthalmol. Vis. Sci. 2012, 53, 2482–2484. [Google Scholar] [CrossRef]
- Gupta, N.; Ly, T.; Zhang, Q.; Kaufman, P.L.; Weinreb, R.N.; Yücel, Y.H. Chronic ocular hypertension induces dendrite pathology in the lateral geniculate nucleus of the brain. Exp. Eye Res. 2007, 84, 176–184. [Google Scholar] [CrossRef]
- Sharif, N.A. Pathogenesis of elevated intraocular pressure and glaucoma-related retinal and optic nerve degeneration: Diverse mitigation strategies and treatment modalities. EC Ophthalmol. 2022, 13, 43–67. [Google Scholar]
- Thomson, B.R.; Liu, P.; Onay, T.; Du, J.; Tompson, S.W.; Misener, S.; Purohit, R.R.; Young, T.L.; Jin, J.; Quaggin, S.E. Cellular crosstalk regulates the aqueous humor outflow pathway and provides new targets for glaucoma therapies. Nat. Commun. 2021, 12, 6072. [Google Scholar] [CrossRef] [PubMed]
- Alward, W.L.; Fingert, J.H.; Coote, M.A.; Johnson, A.T.; Lerner, S.F.; Junqua, D.; Durcan, F.J.; McCartney, P.J.; Mackey, D.A.; Sheffield, V.C.; et al. Clinical features associated with mutations in the chromosome 1 open-angle glaucoma gene (GLC1A). N. Engl. J. Med. 1998, 38, 1022–1027. [Google Scholar] [CrossRef]
- Stone, E.M.; Fingert, J.H.; Alward, W.L.; Nguyen, T.D.; Polansky, J.R.; Sunden, S.L.; Nishimura, D.; Clark, A.F.; Nystuen, A.; Nichols, B.E.; et al. Identification of a gene that causes primary open angle glaucoma. Science 1997, 275, 668–670. [Google Scholar] [CrossRef] [PubMed]
- Aung, T.; Khor, C.C. Glaucoma genetics: Recent advances and future directions. Asia Pac. J. Ophthalmol. (Phila.) 2016, 5, 256–259. [Google Scholar] [CrossRef] [PubMed]
- Bailey, J.N.; Loomis, S.J.; Kang, J.H.; Allingham, R.R.; Gharahkhani, P.; Khor, C.C.; Burdon, K.P.; Aschard, H.; Chasman, D.I.; Igo, R.P., Jr.; et al. Genome-wide association analysis identifies TXNRD2, ATXN2 and FOXC1 as susceptibility loci for primary open-angle glaucoma. Nat. Genet. 2016, 48, 189–194. [Google Scholar] [CrossRef]
- Springelkamp, H.; Iglesias, A.I.; Mishra, A.; Höhn, R.; Wojciechowski, R.; Khawaja, A.P.; Nag, A.; Wang, Y.X.; Wang, J.J.; Cuellar-Partida, G.; et al. New insights into the genetics of primary open-angle glaucoma based on meta-analyses of intraocular pressure and optic disc characteristics. Hum. Mol. Genet. 2017, 26, 438–453. [Google Scholar] [CrossRef]
- Danford, I.D.; Verkuil, L.D.; Choi, D.J.; Collins, D.W.; Gudiseva, H.V.; Uyhazi, K.E.; Lau, M.K.; Kanu, L.N.; Grant, G.R.; Chavali, V.R.; et al. Characterizing the “POAGome”: A bioinformatics-driven approach to primary open-angle glaucoma. Prog. Retin. Eye Res. 2017, 58, 89–114. [Google Scholar] [CrossRef]
- Borrás, T. The pathway from genes to gene therapy in glaucoma: A review of possibilities for using genes as glaucoma drugs. Asia Pac. J. Ophthalmol. (Phila.) 2017, 6, 80–93. [Google Scholar]
- Shiga, Y.; Akiyama, M.; Nishiguchi, K.M.; Sato, K.; Shimozawa, N.; Takahashi, A.; Momozawa, Y.; Hirata, M.; Matsuda, K.; Yamaji, T.; et al. Genome-wide association study identifies seven novel susceptibility loci for primary open-angle glaucoma. Hum. Mol. Genet. 2018, 27, 1486–1496. [Google Scholar] [CrossRef]
- Youngblood, H.; Hauser, M.A.; Liu, Y. Update on the genetics of primary open-angle glaucoma. Exp. Eye Res. 2019, 188, 107795. [Google Scholar] [CrossRef] [PubMed]
- Qassim, A.; Souzeau, E.; Siggs, O.M.; Hassall, M.M.; Han, X.; Griffiths, H.L.; Frost, N.A.; Vallabh, N.A.; Kirwan, J.F.; Menon, G.; et al. An intraocular pressure polygenic risk score stratifies multiple primary open-angle glaucoma parameters including treatment intensity. Ophthalmology 2020, 127, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Trivli, A.; Zervou, M.I.; Goulielmos, G.N.; Spandidos, D.A.; Detorakis, E.T. Primary open angle glaucoma genetics: The common variants and their clinical associations (Review). Mol. Med. Rep. 2020, 22, 1103–1110. [Google Scholar] [CrossRef]
- Aung, T.; Yong, V.H.; Chew, P.T.; Seah, S.K.; Gazzard, G.; Foster, P.J.; Vithana, E.N. Molecular analysis of the myocilin gene in Chinese subjects with chronic primary-angle closure glaucoma. Investig. Ophthalmol. Vis. Sci. 2005, 46, 1303–1306. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.C.; Shei, W.; Chan, A.S.; Chua, B.T.; Goh, S.R.; Chong, Y.F.; Hilmy, M.H.; Nongpiur, M.E.; Baskaran, M.; Khor, C.C.; et al. Primary angle closure glaucoma (PACG) susceptibility gene PLEKHA7 encodes a novel Rac1/Cdc42 GAP that modulates cell migration and blood-aqueous barrier function. Hum. Mol. Genet. 2017, 26, 4011–4027. [Google Scholar] [CrossRef]
- Khor, C.C.; Do, T.; Jia, H.; Nakano, M.; George, R.; Abu-Amero, K.; Duvesh, R.; Chen, L.J.; Li, Z.; Nongpiur, M.E.; et al. Genome-wide association study identifies five new susceptibility loci for primary angle closure glaucoma. Nat. Genet. 2016, 48, 556–562. [Google Scholar] [CrossRef]
- Sakurada, Y.; Mabuchi, F.; Kashiwagi, K. Genetics of primary open-angle glaucoma and its endophenotypes. Prog. Brain Res. 2020, 256, 31–47. [Google Scholar]
- Aung, T.; Chan, A.S.; Khor, C.C. Genetics of exfoliation syndrome. J. Glaucoma 2018, 27 (Suppl. 1), S12–S14. [Google Scholar] [CrossRef]
- Berner, D.; Hoja, U.; Zenkel, M.; Ross, J.J.; Uebe, S.; Paoli, D.; Frezzotti, P.; Rautenbach, R.M.; Ziskind, A.; Williams, S.E.; et al. The protective variant rs7173049 at LOXL1 locus impacts on retinoic acid signaling pathway in pseudoexfoliation syndrome. Hum. Mol. Genet. 2019, 28, 2531–2548. [Google Scholar] [CrossRef]
- Genetics of Exfoliation Syndrome Partnership; Li, Z.; Wang, Z.; Lee, M.C.; Zenkel, M.; Peh, E.; Ozaki, M.; Topouzis, F.; Nakano, S.; Chan, A.; et al. Association of rare CYP39A1 variants with exfoliation syndrome involving the anterior chamber of the eye. JAMA 2021, 325, 753–764. [Google Scholar]
- Schlötzer-Schrehardt, U.; Khor, C.C. Pseudoexfoliation syndrome and glaucoma: From genes to disease mechanisms. Curr. Opin. Ophthalmol. 2021, 32, 118–128. [Google Scholar] [CrossRef]
- Aung, T.; Ocaka, L.; Ebenezer, N.D.; Morris, A.G.; Krawczak, M.; Thiselton, D.L.; Alexander, C.; Votruba, M.; Brice, G.; Child, A.H.; et al. A major marker for normal tension glaucoma: Association with polymorphisms in the OPA1 gene. Hum. Genet. 2002, 110, 52–56. [Google Scholar] [CrossRef]
- Simcoe, M.J.; Weisschuh, N.; Wissinger, B.; Hysi, P.G.; Hammond, C.J. Genetic heritability of pigmentary glaucoma and associations with other eye phenotypes. JAMA Ophthalmol. 2019, 138, 294–299. [Google Scholar] [CrossRef]
- Rozpędek-Kamińska, W.; Wojtczak, R.; Szaflik, J.P.; Szaflik, J.; Majsterek, I. The genetic and endoplasmic reticulum-mediated molecular mechanisms of primary open-angle glaucoma. Int. J. Mol. Sci. 2020, 21, 4171. [Google Scholar] [CrossRef] [PubMed]
- Rozpędek-Kamińska, W.; Galita, G.; Siwecka, N.; Carroll, S.L.; Diehl, J.A.; Kucharska, E.; Pytel, D.; Majsterek, I. The potential role of small-molecule PERK inhibitor LDN-0060609 in primary open-angle glaucoma treatment. Int. J. Mol. Sci. 2021, 22, 4494. [Google Scholar] [CrossRef] [PubMed]
- Liesenborghs, I.; Eijssen, L.M.; Kutmon, M.; Gorgels, T.G.; Evelo, C.T.; Beckers, H.J.; Webers, C.A.; Schouten, J.S. Comprehensive bioinformatics analysis of trabecular meshwork gene expression data to unravel the molecular pathogenesis of primary open-angle glaucoma. Acta Ophthalmol. 2020, 98, 48–57. [Google Scholar] [CrossRef]
- Chai, X.; Low, K.Y.; Tham, Y.C.; Chee, M.L.; Thakur, S.; Zhang, L.; Tan, N.Y.; Khor, C.C.; Aung, T.; Wong, T.Y.; et al. Association of glaucoma risk genes with retinal nerve fiber layer in a multi-ethnic Asian population: The Singapore epidemiology of eye diseases study. Investig. Ophthalmol. Vis. Sci. 2020, 61, 37. [Google Scholar] [CrossRef] [PubMed]
- Hysi, P.G.; Cheng, C.Y.; Springelkamp, H.; Macgregor, S.; Bailey, J.N.C.; Wojciechowski, R.; Vitart, V.; Nag, A.; Hewitt, A.W.; Höhn, R.; et al. Genome-wide analysis of multi-ancestry cohorts identifies new loci influencing intraocular pressure and susceptibility to glaucoma. Nat. Genet. 2014, 46, 1126–1130. [Google Scholar] [CrossRef]
- Chintalapudi, S.R.; Maria, D.; Di Wang, X.; Bailey, J.N.C.; NEIGHBORHOOD consortium; International Glaucoma Genetics consortium; Hysi, P.G.; Wiggs, J.L.; Williams, R.W. Systems genetics identifies a role for Cacna2d1 regulation in elevated intraocular pressure and glaucoma susceptibility. Nat. Commun. 2017, 8, 1755. [Google Scholar] [CrossRef]
- Iomdina, E.N.; Tikhomirova, N.K.; Bessmertny, A.M.; Serebryakova, M.V.; Baksheeva, V.E.; Zalevsky, A.O.; Kotelin, V.I.; Kiseleva, O.A.; Kosakyan, S.M.; Zamyatnin, A.A., Jr.; et al. Alterations in proteome of human sclera associated with primary open-angle glaucoma involve proteins participating in regulation of the extracellular matrix. Mol. Vis. 2020, 26, 623–640. [Google Scholar] [PubMed]
- Vithana, E.N.; Nongpiur, M.E.; Venkataraman, D.; Chan, S.H.; Mavinahalli, J.; Aung, T. Identification of a novel mutation in the NTF4 gene that causes primary open-angle glaucoma in a Chinese population. Mol. Vis. 2010, 16, 1640–1645. [Google Scholar] [PubMed]
- Asefa, N.G.; Kamali, Z.; Pereira, S.; Vaez, A.; Jansonius, N.; Bergen, A.A.; Snieder, H. Bioinformatic prioritization and functional annotation of GWAS-based candidate genes for primary open-angle glaucoma. Genes 2022, 13, 1055. [Google Scholar] [CrossRef] [PubMed]
- Polansky, J.R.; Fauss, D.J.; Zimmerman, C.C. Regulation of TIGR/MYOC gene expression in human trabecular meshwork cells. Eye 2000, 14, 503–514. [Google Scholar] [CrossRef]
- Zode, G.S.; Bugge, K.E.; Mohan, K.; Grozdanic, S.D.; Peters, J.C.; Koehn, D.R.; Anderson, M.G.; Kardon, R.H.; Stone, E.M.; Sheffield, V.C. Topical ocular sodium 4-phenylbutyrate rescues glaucoma in a myocilin mouse model of primary open-angle glaucoma. Investig. Ophthalmol. Vis. Sci. 2012, 53, 1557–1565. [Google Scholar] [CrossRef]
- Huard, D.J.; Crowley, V.M.; Du, Y.; Cordova, R.A.; Sun, Z.; Tomlin, M.O.; Dickey, C.A.; Koren, J., III; Blair, L.; Fu, H.; et al. Trifunctional high-throughput screen identifies promising scaffold to inhibit Grp94 and treat myocilin-associated glaucoma. ACS Chem. Biol. 2018, 13, 933–941. [Google Scholar] [CrossRef]
- Li, H.; Ibrahim, M.M.; Chen, H.; Li, W.; Jablonski, M.M. In silico screening and in vivo evaluation of potential CACNA2D1 antagonists as intraocular pressure-reducing agents in glaucoma therapy. Pharmaceuticals 2021, 14, 887. [Google Scholar] [CrossRef]
- Kim, J.Y.; Abdi, S.; Huh, B.; Kim, K.H. Mirogabalin: Could it be the next generation gabapentin or pregabalin? Korean J. Pain 2021, 34, 4–18. [Google Scholar] [CrossRef]
- Thorleifsson, G.; Walters, G.B.; Hewitt, A.W.; Masson, G.; Helgason, A.; DeWan, A.; Sigurdsson, A.; Jonasdottir, A.; Gudjonsson, S.A.; Magnusson, K.P.; et al. Common variants near CAV1 and CAV2 are associated with primary open-angle glaucoma. Nat. Genet. 2010, 42, 906–909. [Google Scholar] [CrossRef]
- Aga, M.; Bradley, J.M.; Wanchu, R.; Yang, Y.F.; Acott, T.S.; Keller, K.E. Differential effects of caveolin-1 and -2 knockdown on aqueous outflow and altered extracellular matrix turnover in caveolin-silenced trabecular meshwork cells. Investig. Ophthalmol. Vis. Sci. 2014, 55, 5497–5509. [Google Scholar] [CrossRef]
- Xi, X.; Chen, Q.; Ma, J.; Wang, X.; Xia, Y.; Wen, X.; Cai, B.; Li, Y. Acteoside protects retinal ganglion cells from experimental glaucoma by activating the PI3K/AKT signaling pathway via caveolin 1 upregulation. Ann. Transl. Med. 2022, 10, 312. [Google Scholar] [CrossRef]
- Hu, C.; Niu, L.; Li, L.; Song, M.; Zhang, Y.; Lei, Y.; Chen, Y.; Sun, X. ABCA1 regulates IOP by modulating Cav1/eNOS/NO signaling pathway. Investig. Ophthalmol. Vis. Sci. 2020, 61, 33. [Google Scholar] [CrossRef] [PubMed]
- Burdon, K.P.; Macgregor, S.; Hewitt, A.W.; Sharma, S.; Chidlow, G.; Mills, R.A.; Danoy, P.; Casson, R.; Viswanathan, A.C.; Liu, J.Z.; et al. Genome-wide association study identifies susceptibility loci for open angle glaucoma at TMCO1 and CDKN2B-AS1. Nat. Genet. 2011, 43, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Verkuil, L.; Danford, I.; Pistilli, M.; Collins, D.W.; Gudiseva, H.V.; Trachtman, B.T.; He, J.; Rathi, S.; Haider, N.; Ying, G.S.; et al. SNP located in an AluJb repeat downstream of TMCO1, rs4657473, is protective for POAG in African Americans. Br. J. Ophthalmol. 2019, 103, 1530–1536. [Google Scholar] [CrossRef]
- Xu, J.; Luo, H.; Yu, M.; Yang, C.; Shu, Y.; Gong, B.; Lin, Y.; Wang, J. Association of polymorphism rs11656696 in GAS7 with primary open-angle glaucoma in a Chinese population. Ophthalmic Genet. 2019, 40, 237–241. [Google Scholar] [CrossRef]
- Luo, J.; Wang, S.; Zhou, Z.; Zhao, Y. Ad- and AAV8-mediated ABCA1 gene therapy in a murine model with retinal ischemia/reperfusion injuries. Mol. Ther. Methods Clin. Dev. 2021, 20, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Thackaberry, E.A.; Zhou, Y.; de Zafra, C.L.Z.; Fuh, G.; Lee, C.V.; Sanowar, S.; Ridgway, J.B.; Kusi, A.M.; Farman, C.; Booler, H.; et al. Rapid development of glaucoma via ITV nonselective ANGPT 1/2 antibody: A potential role for ANGPT/TIE2 signaling in primate aqueous humor outflow. Investig. Ophthalmol. Vis. Sci. 2019, 60, 4097–4108. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Nottebaum, A.F.; Brigell, M.; Navarro, I.D.; Ipe, U.; Mishra, S.; Gomez-Caraballo, M.; Schmitt, H.; Soldo, B.; Pakola, S.; et al. A small molecule inhibitor of VE-PTP activates Tie2 in Schlemm’s canal increasing outflow facility and reducing intraocular pressure. Investig. Ophthalmol. Vis. Sci. 2020, 61, 12. [Google Scholar] [CrossRef]
- Philomenadin, F.S.; Asokan, R.; George, R.; Lingam, V.; Sarangapani, S. Genetic association of SNPs near ATOH7, CARD10, CDKN2B, CDC7 and SIX1/SIX6 with the endophenotypes of primary open angle glaucoma in Indian population. PLoS ONE 2015, 10, e0119703. [Google Scholar] [CrossRef]
- Pfeiffer, N.; Voykov, B.; Renieri, G.; Bell, K.; Richter, P.; Weigel, M.; Thieme, H.; Wilhelm, B.; Lorenz, K.; Feindor, M.; et al. First-in-human phase I study of ISTH0036, an antisense oligonucleotide selectively targeting transforming growth factor beta 2 (TGF-β2), in subjects with open-angle glaucoma undergoing glaucoma filtration surgery. PLoS ONE 2017, 12, e0188899. [Google Scholar] [CrossRef]
- Lu, S.Y.; He, Z.Z.; Xu, J.X.; Yang, C.; Chen, L.J.; Gong, B. Association of polymorphisms at the SIX1-SIX6 locus with primary open-angle glaucoma. Investig. Ophthalmol. Vis. Sci. 2019, 60, 2914–2924. [Google Scholar] [CrossRef]
- Osborne, A.; Khatib, T.Z.; Songra, L.; Barber, A.C.; Hall, K.; Kong, G.Y.; Widdowson, P.S.; Martin, K.R. Neuroprotection of retinal ganglion cells by a novel gene therapy construct that achieves sustained enhancement of brain-derived neurotrophic factor/tropomyosin-related kinase receptor-B signaling. Cell Death Dis. 2018, 9, 1007. [Google Scholar] [CrossRef] [PubMed]
- Rezaie, T.; Child, A.; Hitchings, R.; Brice, G.; Miller, L.; Coca-Prados, M.; Héon, E.; Krupin, T.; Ritch, R.; Kreutzer, D.; et al. Adult-onset primary open-angle glaucoma caused by mutations in optineurin. Science 2002, 295, 1077–1079. [Google Scholar] [CrossRef] [PubMed]
- Minegishi, Y.; Iejima, D.; Kobayashi, H.; Chi, Z.L.; Kawase, K.; Yamamoto, T.; Seki, T.; Yuasa, S.; Fukuda, K.; Iwata, T. Enhanced optineurin E50K-TBK1 interaction evokes protein insolubility and initiates familial primary open-angle glaucoma. Hum. Mol. Genet. 2013, 22, 3559–3567. [Google Scholar] [CrossRef] [PubMed]
- Hou, M.; Shao, Z.; Zhang, S.; Liu, X.; Fan, P.; Jiang, M.; Zhao, Y.; Xiao, R.; Yuan, H. Age-related visual impairments and retinal ganglion cells axonal degeneration in a mouse model harboring OPTN (E50K) mutation. Cell Death Dis. 2022, 13, 362. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Wang, Z.; Jie, W.; Fu, X.; Li, B.; Xu, H.; Liu, Y.; Li, M.; Kim, E.; Yang, Y.; et al. The kinase inhibitor BX795 suppresses the inflammatory response via multiple kinases. Biochem. Pharmacol. 2020, 174, 113797. [Google Scholar] [CrossRef] [PubMed]
- Klimko, P.; Sharif, N.A. Discovery, characterization and clinical utility of prostaglandin agonists for treatment of glaucoma. Br. J. Pharmacol. 2019, 176, 1051–1058. [Google Scholar] [CrossRef]
- Sakurai, M.; Higashide, T.; Takahashi, M.; Sugiyama, K. Association between genetic polymorphisms of the prostaglandin F2alpha receptor gene and response to latanoprost. Ophthalmology 2007, 114, 1039–1045. [Google Scholar] [CrossRef]
- Sakurai, M.; Higashide, T.; Ohkubo, S.; Takeda, H.; Sugiyama, K. Association between genetic polymorphisms of the prostaglandin F2α receptor gene, and response to latanoprost in patients with glaucoma and ocular hypertension. Br. J. Ophthalmol. 2014, 98, 469–473. [Google Scholar] [CrossRef]
- Zhang, P.; Jiang, B.; Xie, L.; Huang, W. PTGFR and SLCO2A1 gene polymorphisms determine intraocular pressure response to latanoprost in Han Chinese patients with glaucoma. Curr. Eye Res. 2016, 41, 1561–1565. [Google Scholar] [CrossRef]
- Gerometta, R.; Spiga, M.G.; Borrás, T.; Candia, O.A. Treatment of sheep steroid-induced ocular hypertension with a glucocorticoid-inducible MMP1 gene therapy virus. Investig. Ophthalmol. Vis. Sci. 2010, 51, 3042–3048. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, J.; Crosbie, D.E.; Cassidy, P.S.; Sherwood, J.M.; Fluegel-Koch, C.; Luetjen-Drecoll, E.; Humphries, M.M.; Reina-Torres, E.; Wallace, D.; Kiang, A.S.; et al. Therapeutic potential of AAV-mediated MMP-3 secretion from corneal endothelium in treating glaucoma. Hum. Mol. Genet. 2017, 26, 1230–1246. [Google Scholar] [CrossRef]
- Barraza, R.A.; McLaren, J.W.; Poeschla, E.M. Prostaglandin pathway gene therapy for sustained reduction of intraocular pressure. Mol. Ther. 2010, 18, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Martínez, T.; González, M.V.; Roehl, I.; Wright, N.; Pañeda, C.; Jiménez, A.I. In vitro and in vivo efficacy of SYL040012, a novel siRNA compound for treatment of glaucoma. Mol. Ther. 2014, 22, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Montañés, J.; Sádaba, B.; Ruz, V.; Gómez-Guiu, A.; Zarranz, J.; González, M.V.; Pañeda, C.; Jimenez, A.I. Phase I clinical trial of SYL040012, a small interfering RNA targeting β-adrenergic receptor 2, for lowering intraocular pressure. Mol. Ther. 2014, 22, 226–232. [Google Scholar] [CrossRef]
- Sun, D.; Zhan, Z.; Zeng, R.; Liu, X.; Wang, B.; Yang, F.; Huang, S.; Li, Y.; Yang, Z.; Su, Y.; et al. Long-term and potent IOP-lowering effect of IκBα-siRNA in a nonhuman primate model of chronic ocular hypertension. iScience 2022, 25, 104149. [Google Scholar] [CrossRef]
- Tan, J.; Liu, G.; Lan, C.; Pang, I.H.; Luo, X.; Wu, S.; Fan, N.; Zhang, J.; Wang, N.; Liu, X. Lentiviral vector-mediated expression of C3 transferase attenuates retinal ischemia and reperfusion injury in rats. Life Sci. 2021, 272, 119269. [Google Scholar] [CrossRef]
- Visuvanathan, S.; Baker, A.N.; Lagali, P.S.; Coupland, S.G.; Miller, G.; Hauswirth, W.W.; Tsilfidis, C. XIAP gene therapy effects on retinal ganglion cell structure and function in a mouse model of glaucoma. Gene Ther. 2022, 29, 147–156. [Google Scholar] [CrossRef]
- Donahue, R.J.; Fehrman, R.L.; Gustafson, J.R.; Nickells, R.W. BCLXL gene therapy moderates neuropathology in the DBA/2J mouse model of inherited glaucoma. Cell Death Dis. 2021, 12, 781. [Google Scholar] [CrossRef]
- Lani-Louzada, R.; Marra, C.; Dias, M.S.; de Araújo, V.G.; Abreu, C.A.; Ribas, V.T.; Adesse, D.; Allodi, S.; Chiodo, V.; Hauswirth, W.; et al. Neuroprotective gene therapy by overexpression of the transcription factor MAX in rat models of glaucomatous neurodegeneration. Investig. Ophthalmol. Vis. Sci. 2022, 63, 5. [Google Scholar] [CrossRef]
- Wu, J.; Bell, O.H.; Copland, D.A.; Young, A.; Pooley, J.R.; Maswood, R.; Evans, R.S.; Khaw, P.T.; Ali, R.R.; Dick, A.D.; et al. Gene therapy for glaucoma by ciliary body aquaporin 1 disruption using CRISPR-Cas9. Mol. Ther. 2020, 28, 820–829. [Google Scholar] [CrossRef]
- Amador, C.; Shah, R.; Ghiam, S.; Kramerov, A.A.; Ljubimov, A.V. Gene therapy in the anterior eye segment. Curr. Gene Ther. 2022, 22, 104–1131. [Google Scholar] [CrossRef]
- Levin, L.A.; Patrick, C.; Choudry, N.B.; Sharif, N.A.; Goldberg, J.L. Neuroprotection in neurodegenerations of the brain and eye: Lessons from the past and directions for the future. Front. Neurol. 2022, 13, 964197. [Google Scholar] [CrossRef]
- Sharif, N.A. Electrical, electromagnetic, ultrasound wave therapies and electronic implants for neuronal rejuvenation, neuroprotection, axonal regeneration and IOP reduction. J. Ocul. Pharmacol. Ther. 2023, in press. [Google Scholar] [CrossRef]
- Springelkamp, H.; Iglesias, A.I.; Cuellar-Partida, G.; Amin, N.; Burdon, K.P.; van Leeuwen, E.M.; Gharahkhani, P.; Mishra, A.; van der Lee, S.J.; Hewitt, A.W.; et al. ARHGEF12 influences the risk of glaucoma by increasing intraocular pressure. Hum. Mol. Genet. 2015, 24, 2689–2699. [Google Scholar] [CrossRef] [PubMed]
- Gharahkhani, P.; Jorgenson, E.; Hysi, P.; Khawaja, A.P.; Pendergrass, S.; Han, X.; Ong, J.S.; Hewitt, A.W.; Segrè, A.V.; Rouhana, J.M.; et al. Genome-wide meta-analysis identifies 127 open-angle glaucoma loci with consistent effect across ancestries. Nat. Commun. 2021, 12, 1258. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, U.; Xie, B.; Xie, E.F.; D’Souza, M.; Dao, D.; Sulakhe, D.; Skondra, D. Using advanced bioinformatics tools to identify novel therapeutic candidates for age-related macular degeneration. Transl. Vis. Sci. Technol. 2022, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Sahel, J.A.; Marazova, K.; Audo, I. Clinical characteristics and current therapies for inherited retinal degenerations. Cold Spring Harb. Perspect. Med. 2015, 5, a017111. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharif, N.A. Elevated Intraocular Pressure and Glaucomatous Optic Neuropathy: Genes to Disease Mechanisms, Therapeutic Drugs, and Gene Therapies. Pharmaceuticals 2023, 16, 870. https://doi.org/10.3390/ph16060870
Sharif NA. Elevated Intraocular Pressure and Glaucomatous Optic Neuropathy: Genes to Disease Mechanisms, Therapeutic Drugs, and Gene Therapies. Pharmaceuticals. 2023; 16(6):870. https://doi.org/10.3390/ph16060870
Chicago/Turabian StyleSharif, Najam A. 2023. "Elevated Intraocular Pressure and Glaucomatous Optic Neuropathy: Genes to Disease Mechanisms, Therapeutic Drugs, and Gene Therapies" Pharmaceuticals 16, no. 6: 870. https://doi.org/10.3390/ph16060870
APA StyleSharif, N. A. (2023). Elevated Intraocular Pressure and Glaucomatous Optic Neuropathy: Genes to Disease Mechanisms, Therapeutic Drugs, and Gene Therapies. Pharmaceuticals, 16(6), 870. https://doi.org/10.3390/ph16060870




