Factor XIa Inhibitors as a Novel Anticoagulation Target: Recent Clinical Research Advances
Abstract
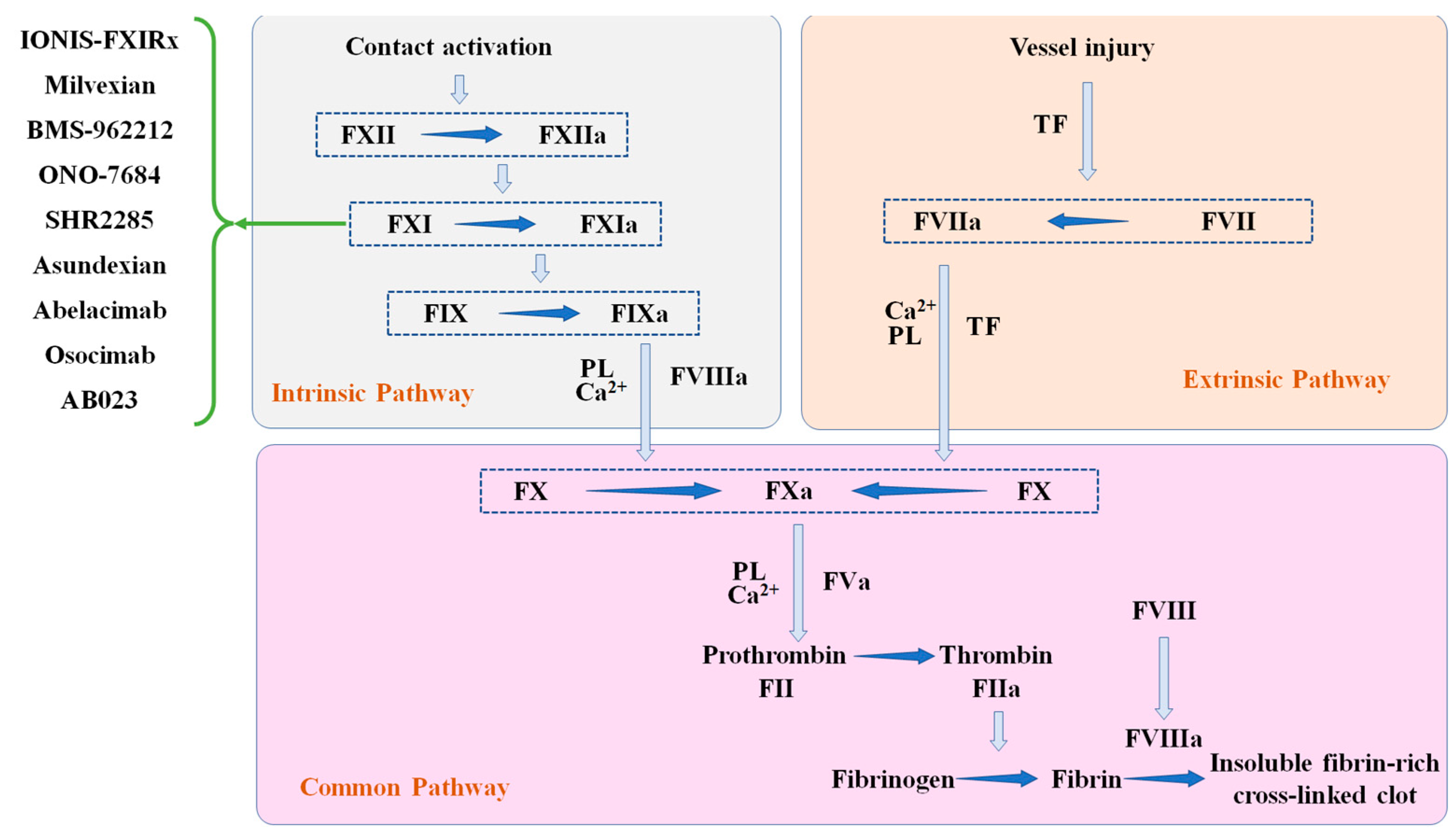
1. Background
2. Results
2.1. ASO
IONIS-FXIRX (BAY2306001)
2.2. Small Molecule Drugs
2.2.1. BMS-962212
2.2.2. Milvexian (JNJ70033093, BMS-986177)
2.2.3. SHR2285
2.2.4. Asundexian (BAY 2433334)
2.3. Antibody
2.3.1. Abelacimab (MAA868)
2.3.2. Osocimab (BAY1213790)
2.3.3. AB023 (Xisomab)
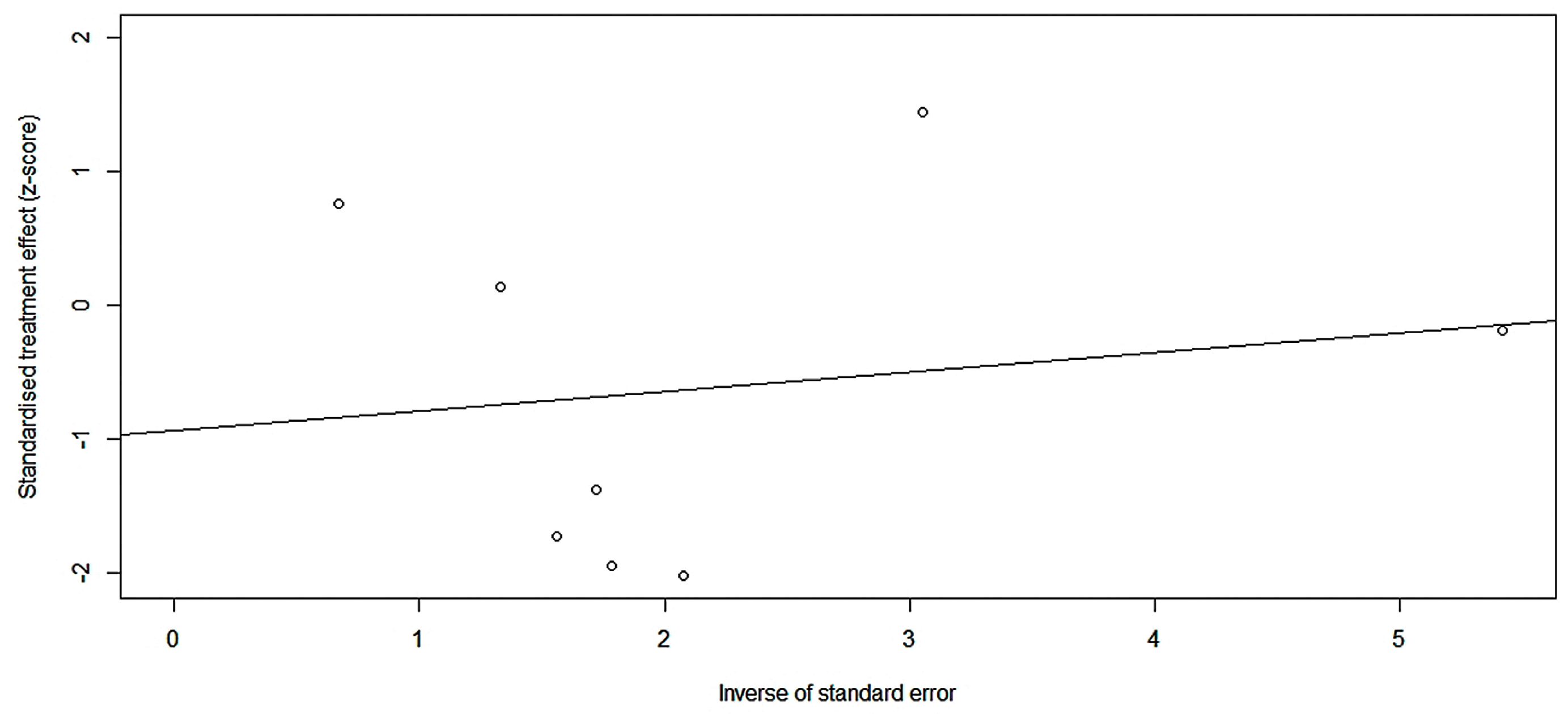
2.4. Safety Meta-Analysis of XIa Inhibitors
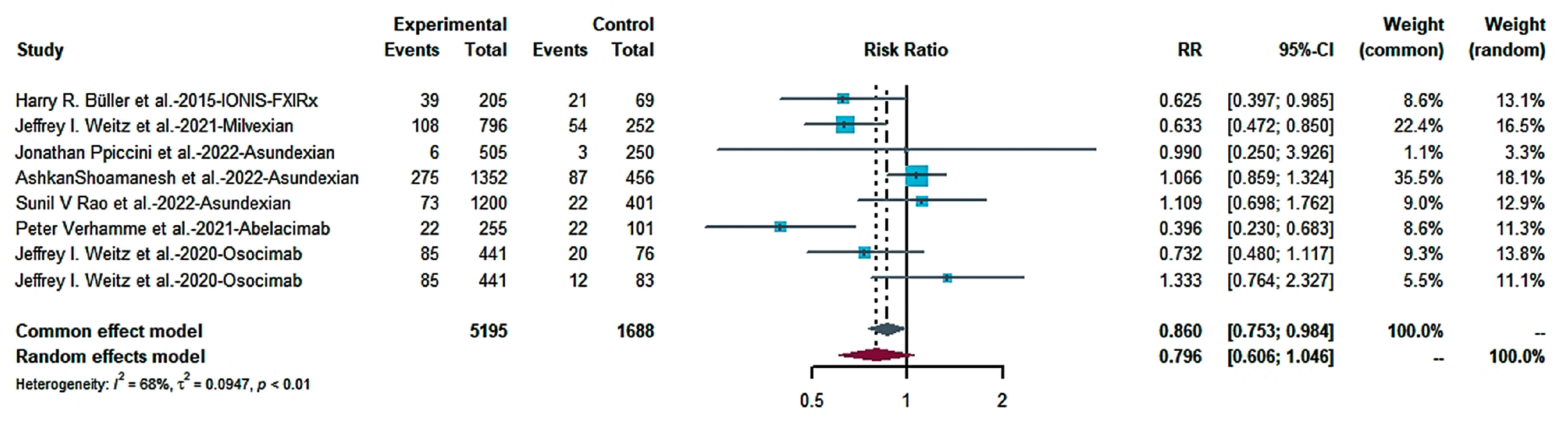
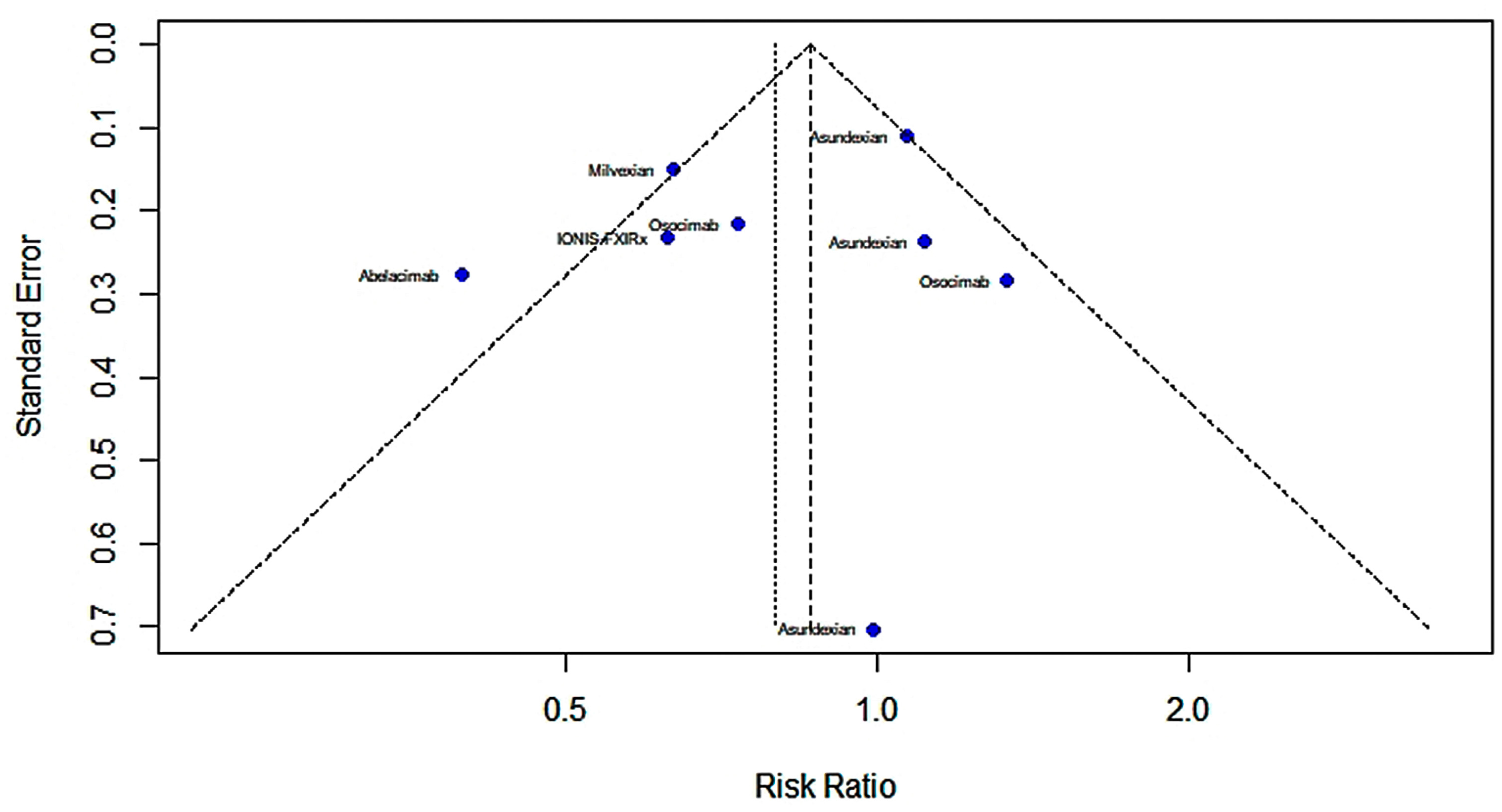
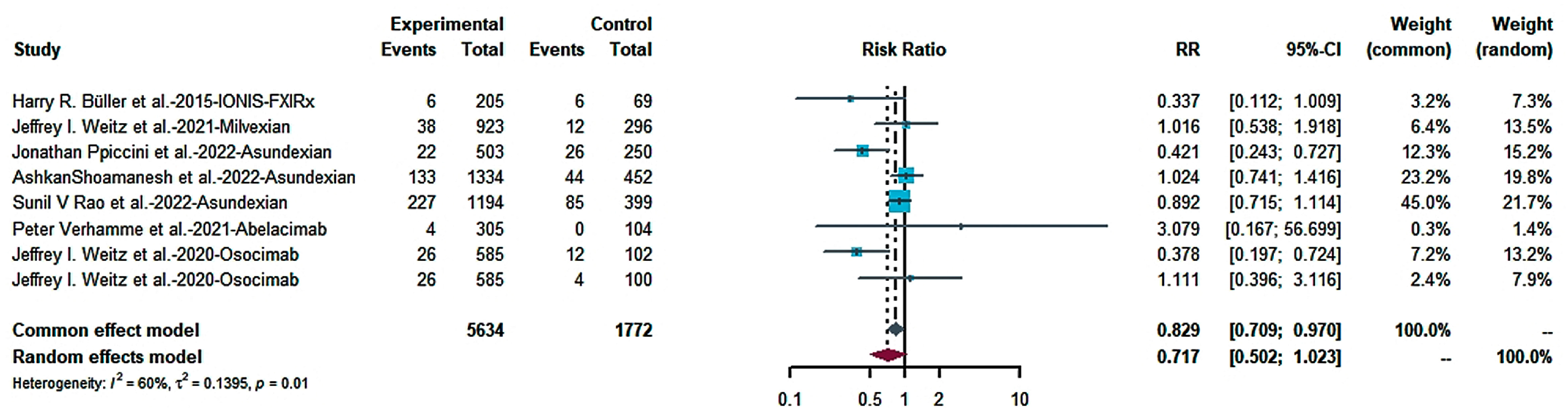
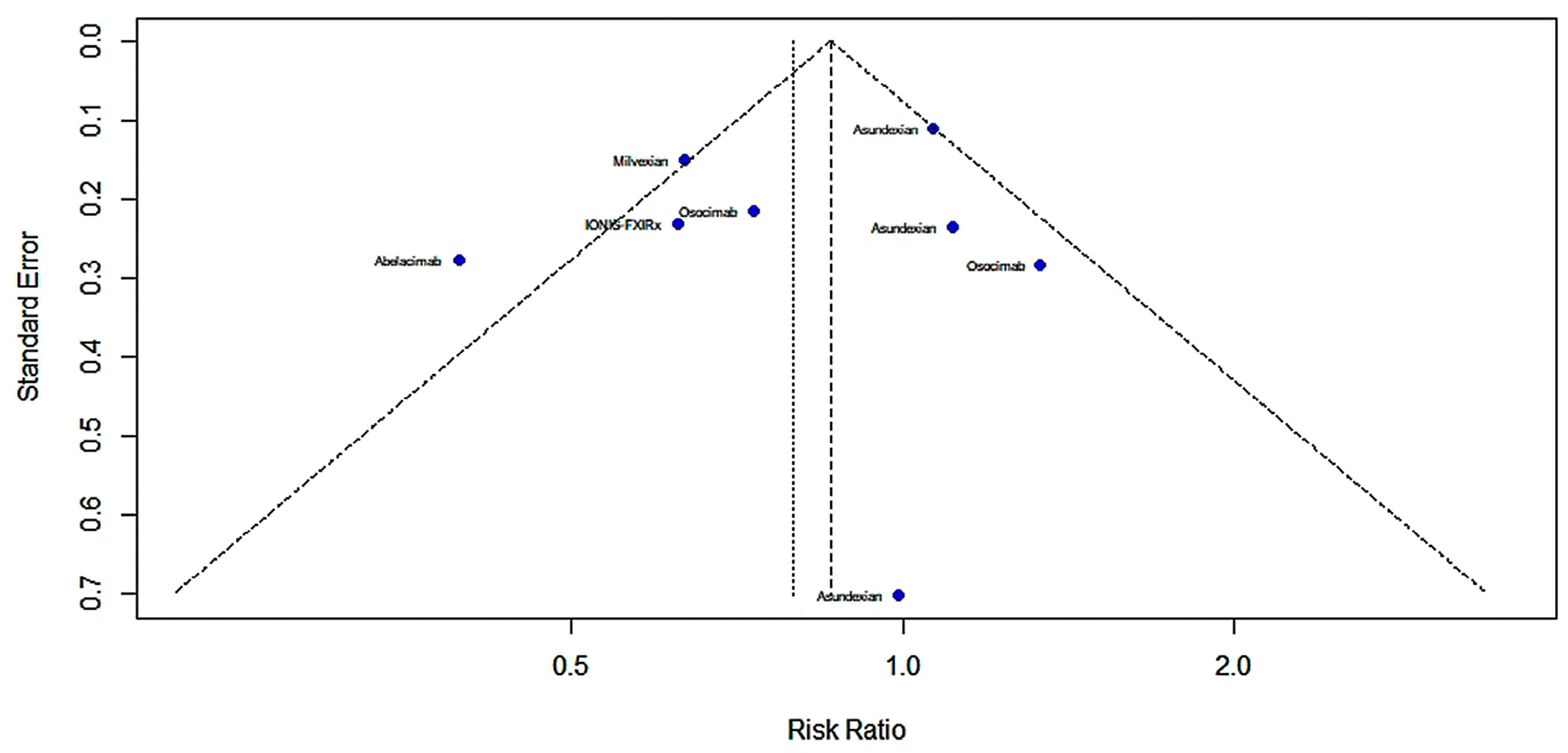
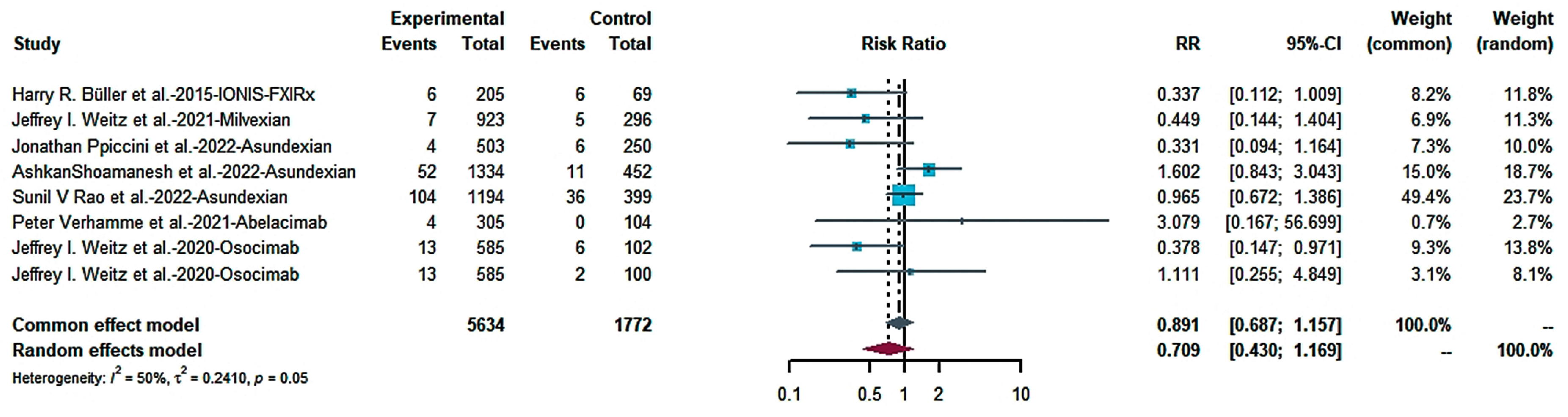
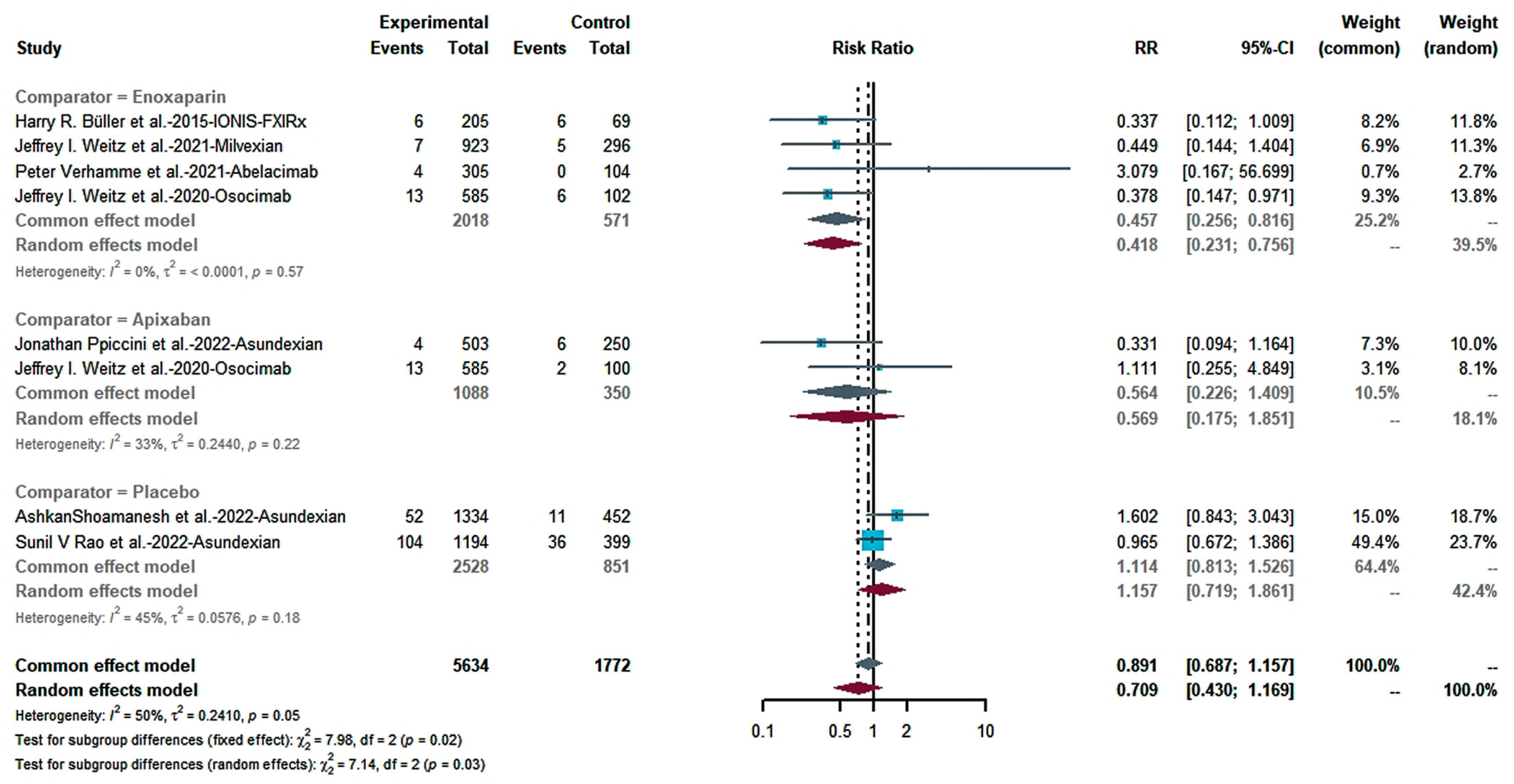
2.4.1. Efficacy
2.4.2. Risk of Bleeding
3. Methods
3.1. Search Strategy
3.2. Literature Screening
3.3. Statistical Analysis
4. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Raskob, G.; Angchaisuksiri, P.; Blanco, A.; Buller, H.; Gallus, A.; Hunt, B.; Hylek, E.; Kakkar, A.; Konstantinides, S.; McCumber, M.; et al. Thrombosis: A major contributor to global disease burden. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2363–2371. [Google Scholar] [CrossRef]
- Hunter, D.; Fineberg, H. Convergence to common purpose in global health. N. Engl. J. Med. 2014, 370, 1753–1755. [Google Scholar] [CrossRef] [PubMed]
- Jamison, D.T.; Summers, L.H.; Alleyne, G.; Arrow, K.J.; Berkley, S.; Binagwaho, A.; Bustreo, F.; Evans, D.; Feachem, R.G.; Frenk, J.; et al. Global health 2035: A world converging within a generation. Lancet 2013, 382, 1898–1955. [Google Scholar] [CrossRef] [PubMed]
- Hirsh, J.; Eikelboom, J.W.; Chan, N.C. Fifty years of research on antithrombotic therapy: Achievements and disappointments. Eur. J. Intern. Med. 2019, 70, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Schulman, S.; Ageno, W.; Konstantinides, S. Venous thromboembolism: Past, present and future. Thromb. Haemost. 2017, 117, 1219–1229. [Google Scholar] [CrossRef] [PubMed]
- Al-Horani, R.A.; Desai, U.R. Factor XIa inhibitors: A review of the patent literature. Expert Opin. Ther. Pat. 2016, 26, 323–345. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Zhao, L.; Gao, N.; Yin, R.; Li, S.; Sun, H.; Zhou, L.; Zhao, G.; Purcell, S.W.; Zhao, J. From multi-target anticoagulants to DOACs, and intrinsic coagulation factor inhibitors. Blood Rev. 2020, 39, 100615. [Google Scholar] [CrossRef]
- Gailani, D. Making anticoagulation safer. Lancet 2022, 399, 1360–1361. [Google Scholar] [CrossRef]
- Szapáry, L.; Tornyos, D.; Kupó, P.; Lukács, R.; Abdallaoui, O.E.A.E.; Komócsi, A. Combination of antiplatelet and anticoagulant therapy, component network meta-analysis of randomized controlled trials. Front. Cardiovasc. Med. 2022, 9, 1036609. [Google Scholar] [CrossRef] [PubMed]
- Kupó, P.; Szakács, Z.; Solymár, M.; Habon, T.; Czopf, L.; Hategan, L.; Csányi, B.; Borbás, J.; Tringer, A.; Varga, G.; et al. Direct Anticoagulants and Risk of Myocardial Infarction, a Multiple Treatment Network Meta-Analysis. Angiology 2020, 71, 27–37. [Google Scholar] [CrossRef]
- Ruff, C.T.; Giugliano, R.P.; Braunwald, E.; Hoffman, E.B.; Deenadayalu, N.; Ezekowitz, M.D.; Camm, A.J.; Weitz, J.I.; Lewis, B.S.; Parkhomenko, A.; et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: A meta-analysis of randomised trials. Lancet 2014, 383, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Alamneh, E.; Chalmers, L.; Bereznicki, L. Suboptimal Use of Oral Anticoagulants in Atrial Fibrillation: Has the Introduction of Direct Oral Anticoagulants Improved Prescribing Practices? Am. J. Cardiovasc. Drugs Drugs Devices Other Interv. 2016, 16, 183–200. [Google Scholar] [CrossRef] [PubMed]
- Büller, H.R.; Bethune, C.; Bhanot, S.; Gailani, D.; Monia, B.P.; Raskob, G.E.; Segers, A.; Verhamme, P.; Weitz, J.I. Factor XI Antisense Oligonucleotide for Prevention of Venous Thrombosis. N. Engl. J. Med. 2015, 372, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Seligsohn, U. Factor XI deficiency in humans. J. Thromb. Haemost. 2009, 7 (Suppl. S1), 84–87. [Google Scholar] [CrossRef]
- Rosenthal, R.L.; Dreskin, O.H.; Rosenthal, N. Plasma thromboplastin antecedent (PTA) deficiency; clinical, coagulation, therapeutic and hereditary aspects of a new hemophilia-like disease. Blood 1955, 10, 120–131. [Google Scholar] [CrossRef]
- Franchini, M.; Veneri, D.; Lippi, G. Inherited factor XI deficiency: A concise review. Hematology 2006, 11, 307–309. [Google Scholar] [CrossRef]
- Lee, S.E.; Choi, Y.J.; Chi, S.-I.; Kim, H.-J.; Seo, K.-S. Factor XI deficiency and orthognathic surgery: A case report on anesthesia management. J. Dent. Anesth. Pain Med. 2015, 15, 25–29. [Google Scholar] [CrossRef]
- Meijers, J.C.; Tekelenburg, W.L.; Bouma, B.N.; Bertina, R.M.; Rosendaal, F.R. High Levels of Coagulation Factor XI as a Risk Factor for Venous Thrombosis. N. Engl. J. Med. 2000, 342, 696–701. [Google Scholar] [CrossRef]
- Merlo, C.; Wuillemin, W.; Redondo, M.; Furlan, M.; Sulzer, I.; Kremer-Hovinga, J.; Binder, B.R.; Lämmle, B. Elevated levels of plasma prekallikrein, high molecular weight kininogen and factor XI in coronary heart disease. Atherosclerosis 2002, 161, 261–267. [Google Scholar] [CrossRef]
- Berliner, I.J.; Rybicki, A.C.; Kaplan, R.C.; Monrad, E.; Freeman, R.; Billett, H.H. Elevated levels of Factor XI are associated with cardiovascular disease in women. Thromb. Res. 2002, 107, 55–60. [Google Scholar] [CrossRef]
- Zhang, H.; Löwenberg, E.C.; Crosby, J.R.; MacLeod, A.R.; Zhao, C.; Gao, D.; Black, C.; Revenko, A.S.; Meijers, J.C.M.; Stroes, E.S.; et al. Inhibition of the intrinsic coagulation pathway factor XI by antisense oligonucleotides: A novel antithrombotic strategy with lowered bleeding risk. Blood 2010, 116, 4684–4692. [Google Scholar] [CrossRef] [PubMed]
- Weitz, J. Factor XI and factor XII as targets for new anticoagulants. Thromb. Res. 2016, 2 (Suppl. S141), S40–S45. [Google Scholar] [CrossRef] [PubMed]
- Whelihan, M.; Orfeo, T.; Gissel, M.; Mann, K. Coagulation procofactor activation by factor XIa. J. Thromb. Haemost. 2010, 8, 1532–1539. [Google Scholar] [CrossRef] [PubMed]
- Matafonov, A.; Cheng, Q.; Geng, Y.; Verhamme, I.M.; Umunakwe, O.; Tucker, E.I.; Sun, M.; Serebrov, V.; Gruber, A.; Gailani, D. Evidence for factor IX-independent roles for factor XIa in blood coagulation. J. Thromb. Haemost. 2013, 11, 2118–2127. [Google Scholar] [CrossRef] [PubMed]
- Puy, C.; Tucker, E.I.; Matafonov, A.; Cheng, Q.; Zientek, K.D.; Gailani, D.; Gruber, A.; McCarty, O.J.T. Activated factor XI increases the procoagulant activity of the extrinsic pathway by inactivating tissue factor pathway inhibitor. Blood 2015, 125, 1488–1496. [Google Scholar] [CrossRef]
- Mohammed, B.M.; Matafonov, A.; Ivanov, I.; Sun, M.-F.; Cheng, Q.; Dickeson, S.K.; Li, C.; Sun, D.; Verhamme, I.M.; Emsley, J.; et al. An update on factor XI structure and function. Thromb. Res. 2018, 161, 94–105. [Google Scholar] [CrossRef]
- Al-Horani, R.A.; Afosah, D.K. Recent advances in the discovery and development of factor XI/XIa inhibitors. Med. Res. Rev. 2018, 38, 1974–2023. [Google Scholar] [CrossRef]
- Weitz, J.; Strony, J.; Ageno, W.; Gailani, D.; Hylek, E.; Lassen, M.; Mahaffey, K.; Notani, R.; Roberts, R.; Segers, A.; et al. Milvexian for the Prevention of Venous Thromboembolism. N. Engl. J. Med. 2021, 385, 2161–2172. [Google Scholar] [CrossRef]
- Piccini, J.P.; Caso, V.; Connolly, S.J.; Fox, K.; Oldgren, J.; Jones, W.S.; Gorog, D.; Durdil, V.; Viethen, T.; Neumann, C.; et al. Safety of the oral factor XIa inhibitor asundexian compared with apixaban in patients with atrial fibrillation (PACIFIC-AF): A multicentre, randomised, double-blind, double-dummy, dose-finding phase 2 study. Lancet 2022, 399, 1383–1390. [Google Scholar] [CrossRef]
- Shoamanesh, A.; Mundl, H.; Smith, E.E.; Masjuan, J.; Milanov, I.; Hirano, T.; Agafina, A.; Campbell, B.; Caso, V.; Mas, J.-L.; et al. Factor XIa inhibition with asundexian after acute non-cardioembolic ischaemic stroke (PACIFIC-Stroke): An international, randomised, double-blind, placebo-controlled, phase 2b trial. Lancet 2022, 400, 997–1007. [Google Scholar] [CrossRef]
- Rao, S.V.; Kirsch, B.; Bhatt, D.L.; Budaj, A.; Coppolecchia, R.; Eikelboom, J.; James, S.K.; Jones, W.S.; Merkely, B.; Keller, L.; et al. A Multicenter, Phase 2, Randomized, Placebo-Controlled, Double-Blind, Parallel-Group, Dose-Finding Trial of the Oral Factor XIa Inhibitor Asundexian to Prevent Adverse Cardiovascular Outcomes After Acute Myocardial Infarction. Circulation 2022, 146, 1196–1206. [Google Scholar] [CrossRef] [PubMed]
- Verhamme, P.; Yi, B.; Segers, A.; Salter, J.; Bloomfield, D.; Büller, H.; Raskob, G.; Weitz, J. Abelacimab for Prevention of Venous Thromboembolism. N. Engl. J. Med. 2021, 385, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Weitz, J.; Bauersachs, R.; Becker, B.; Berkowitz, S.; Freitas, M.; Lassen, M.; Metzig, C.; Raskob, G. Effect of Osocimab in Preventing Venous Thromboembolism Among Patients Undergoing Knee Arthroplasty: The FOXTROT Randomized Clinical Trial. Jama 2020, 323, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Walsh, M.; Bethune, C.; Smyth, A.; Tyrwhitt, J.; Jung, S.; Yu, R.; Wang, Y.; Geary, R.; Weitz, J.; Bhanot, S. Phase 2 Study of the Factor XI Antisense Inhibitor IONIS-FXI(Rx) in Patients With ESRD. Kidney Int. Rep. 2022, 7, 200–209. [Google Scholar] [CrossRef]
- Perera, V.; Luettgen, J.M.; Wang, Z.; Frost, C.E.; Yones, C.; Russo, C.; Lee, J.; Zhao, Y.; LaCreta, F.P.; Ma, X.; et al. First-in-human study to assess the safety, pharmacokinetics and pharmacodynamics of BMS-962212, a direct, reversible, small molecule factor XIa inhibitor in non-Japanese and Japanese healthy subjects. Br. J. Clin. Pharmacol. 2018, 84, 876–887. [Google Scholar] [CrossRef]
- Perera, V.; Abelian, G.; Li, D.; Wang, Z.; Zhang, L.; Lubin, S.; Chen, W.; Bello, A.; Murthy, B. Single-Dose Pharmacokinetics of Milvexian in Participants with Mild or Moderate Hepatic Impairment Compared with Healthy Participants. Clin. Pharmacokinet. 2022, 61, 857–867. [Google Scholar] [CrossRef]
- Perera, V.; Abelian, G.; Li, D.; Wang, Z.; Zhang, L.; Lubin, S.; Bello, A.; Murthy, B. Single-Dose Pharmacokinetics of Milvexian in Participants with Normal Renal Function and Participants with Moderate or Severe Renal Impairment. Clin. Pharmacokinet. 2022, 61, 1405–1416. [Google Scholar] [CrossRef]
- Perera, V.; Wang, Z.; Luettgen, J.; Li, D.; DeSouza, M.; Cerra, M.; Seiffert, D. First-in-human study of milvexian, an oral, direct, small molecule factor XIa inhibitor. Clin. Transl. Sci. 2022, 15, 330–342. [Google Scholar] [CrossRef]
- Perera, V.; Wang, Z.; Lubin, S.; Ueno, T.; Shiozaki, T.; Chen, W.; Xu, X.; Seiffert, D.; DeSouza, M.; Murthy, B. Safety, pharmacokinetics, and pharmacodynamics of milvexian in healthy Japanese participants. Sci. Rep. 2022, 12, 5165. [Google Scholar] [CrossRef]
- Perera, V.; Wang, Z.; Lubin, S.; Christopher, L.J.; Chen, W.; Xu, S.; Seiffert, D.; DeSouza, M.; Murthy, B. Effects of Itraconazole and Diltiazem on the Pharmacokinetics and Pharmacodynamics of Milvexian, A Factor XIa Inhibitor. Cardiol. Ther. 2022, 11, 407–419. [Google Scholar] [CrossRef]
- Perera, V.; Wang, Z.; Lubin, S.; Christopher, L.J.; Chen, W.; Xu, S.; Seiffert, D.; DeSouza, M.; Murthy, B. Effects of rifampin on the pharmacokinetics and pharmacodynamics of milvexian, a potent, selective, oral small molecule factor XIa inhibitor. Sci. Rep. 2022, 12, 22239. [Google Scholar] [CrossRef] [PubMed]
- Beale, D.; Dennison, J.; Boyce, M.; Mazzo, F.; Honda, N.; Smith, P.; Bruce, M. ONO-7684 a novel oral FXIa inhibitor: Safety, tolerability, pharmacokinetics and pharmacodynamics in a first-in-human study. Br. J. Clin. Pharmacol. 2021, 87, 3177–3189. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Guan, X.; Hu, P.; Dong, Y.; Zhu, Y.; Zhang, T.; Zou, J.; Zhang, S. First-In-Human Study to Assess the Safety, Pharmacokinetics, and Pharmacodynamics of SHR2285, a Small-Molecule Factor XIa Inhibitor in Healthy Subjects. Front. Pharmacol. 2022, 13, 197. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Dong, Y.; Huang, L.; Yang, Y.; Geng, Y.; Fei, F.; Xie, P.; Zhao, Y.; Lin, H.; Yang, Z.; et al. SHR2285, the first selectively oral FXIa inhibitor in China: Safety, tolerability, pharmacokinetics and pharmacodynamics combined with aspirin, clopidogrel or ticagrelor. Front. Pharmacol. 2022, 13, 1027627. [Google Scholar] [CrossRef]
- Yi, B.; Freedholm, D.; Widener, N.; Wang, X.; Simard, E.; Cullen, C.; Al-Saady, N.; Lepor, N.; Coulter, S.; Lovern, M.; et al. Pharmacokinetics and pharmacodynamics of Abelacimab (MAA868), a novel dual inhibitor of Factor XI and Factor XIa. J. Thromb. Haemost. 2022, 20, 307–315. [Google Scholar] [CrossRef]
- Thomas, D.; Thelen, K.; Kraff, S.; Schwers, S.; Schiffer, S.; Unger, S.; Yassen, A.; Boxnick, S. BAY 1213790, a fully human IgG1 antibody targeting coagulation factor XIa: First evaluation of safety, pharmacodynamics, and pharmacokinetics. Res. Pract. Thromb. Haemost. 2019, 3, 242–253. [Google Scholar] [CrossRef]
- Lorentz, C.; Verbout, N.; Wallisch, M.; Hagen, M.; Shatzel, J.; Olson, S.; Puy, C.; Hinds, M.; McCarty, O.; Gailani, D.; et al. Contact Activation Inhibitor and Factor XI Antibody, AB023, Produces Safe, Dose-Dependent Anticoagulation in a Phase 1 First-In-Human Trial. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 799–809. [Google Scholar] [CrossRef]
- Lorentz, C.U.; Tucker, E.I.; Verbout, N.G.; Shatzel, J.J.; Olson, S.R.; Markway, B.D.; Wallisch, M.; Ralle, M.; Hinds, M.T.; McCarty, O.J.T.; et al. The contact activation inhibitor AB023 in heparin-free hemodialysis: Results of a randomized phase 2 clinical trial. Blood 2021, 138, 2173–2184. [Google Scholar] [CrossRef]
- Harenberg, J.; Mattiuzzi, C.; Favaloro, E.J.; Lippi, G. Next Generation Antithrombotic Therapy: Focus on Antisense Therapy against Coagulation Factor XI. Semin. Thromb. Hemost. 2015, 41, 255–262. [Google Scholar] [CrossRef]
- Heitmeier, S.; Visser, M.; Tersteegen, A.; Dietze-Torres, J.; Glunz, J.; Gerdes, C.; Laux, V.; Stampfuss, J.; Roehrig, S. Pharmacological profile of asundexian, a novel, orally bioavailable inhibitor of factor XIa. J. Thromb. Haemost. 2022, 20, 1400–1411. [Google Scholar] [CrossRef]
- Beavers, C.J.P.; Wayne, N.B.P. Osocimab: A Novel Agent in Preventing Venous Thromboembolism. J. Cardiovasc. Pharmacol. 2020, 76, 645–649. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Smith, S.A.; Morrissey, J.H. Polyphosphate is a cofactor for the activation of factor XI by thrombin. Blood 2011, 118, 6963–6970. [Google Scholar] [CrossRef] [PubMed]
- Gailani, D.; Broze, G.J. Factor XII-independent activation of factor XI in plasma: Effects of sulfatides on tissue factor-induced coagulation. Blood 1993, 82, 813–819. [Google Scholar] [CrossRef] [PubMed]
- Naito, K.; Fujikawa, K. Activation of human blood coagulation factor XI independent of factor XII. Factor XI is activated by thrombin and factor XIa in the presence of negatively charged surfaces. J. Biol. Chem. 1991, 266, 7353–7358. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Leeflang, M.M.; Deeks, J.J.; Rutjes, A.W.; Reitsma, J.B.; Bossuyt, P.M. Bivariate meta-analysis of predictive values of diagnostic tests can be an alternative to bivariate meta-analysis of sensitivity and specificity. J. Clin. Epidemiol. 2012, 65, 1088–1097. [Google Scholar] [CrossRef]
- Cate, H.T.; Guzik, T.J.; Eikelboom, J.; Spronk, H.M.H. Pleiotropic actions of factor Xa inhibition in cardiovascular prevention: Mechanistic insights and implications for anti-thrombotic treatment. Cardiovasc. Res. 2021, 117, 2030–2044. [Google Scholar] [CrossRef]
- Visser, M.; Heitmeier, S.; Cate, H.T.; Spronk, H.M.H. Role of Factor XIa and Plasma Kallikrein in Arterial and Venous Thrombosis. Thromb. Haemost. 2020, 120, 883–993. [Google Scholar] [CrossRef]
- Nagy, M.; Cate, H.T. What to expect from drug targeting factor XI? Cardiovasc. Res. 2022, 118, e72–e74. [Google Scholar] [CrossRef]
| Number | Author | Year | Compound | Type | Comparator | Subject | Age | Total Number | Study Design | Trial Registration | Status |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 [34] | Michael Walsh et al. | 2022 | IONIS-FXIRx | ASO | placebo | end-stage renal disease | 29–80 | 49 | Part1: open label, phase 2 Part2: randomized, double-blin, phase 2 | NCT02553889 | published |
| 2 [13] | Harry R. Büller et al. | 2015 | IONIS-FXIRx | ASO | Enoxaparin | undergoing total knee arthroplasty | 18–80 | 293 | randomized, open-label, parallel group, phase 2 | NCT01713361 | published |
| 3 [35] | Vidya Perera et al. | 2018 | BMS-962212 | small molecular | placebo | Japanese and non-Japanese healthy subjects | 18–45 | 74 | randomized, double-blind, sequential, ascending dose, placebo-controlled, phase 1 | NCT03197779 | published |
| 4 [28] | Jeffrey I. Weitz et al. | 2021 | Milvexian | small molecular | Enoxaparin | undergoing total knee arthroplasty | 50–90 | 1242 | randomized, parallel-group, open label, phase 2 | NCT03891524 | published |
| 5 [36] | Vidya Perera et al. | 2022 | Milvexian | small molecular | none | patients with liver injury healthy subjects | 18–70 | 26 | nonrandomized, single oral dose, parallel-group, open-label, phase 1 | NCT02982707 | published |
| 6 [37] | Vidya Perera et al. | 2022 | Milvexian | small molecular | none | moderate or severe renal impairment | 18–70 | 43 | nonrandomized, open-label, parallel-group, single dose, phase 1 | NCT03196206 | published |
| 7 [38] | Vidya Perera et al. | 2022 | Milvexian | small molecular | placebo | healthy subjects | 18–55 | 104 | randomized, double-blind, placebo-controlled, sequential, SAD and MAD, phase 1 | NCT02608970 | published |
| 8 [39] | Vidya Perera et al. | 2022 | Milvexian | small molecular | placebo | healthy subjects in Japanese | 18–55 | 33 | randomized, double-blind, placebo-controlled, multiple ascending-dose, phase 1 | NCT03224260 | published |
| 9 [40] | Vidya Perera et al. | 2022 | Milvexian | small molecular | none | healthy subjects | 18–55 | 28 | nonrandomized, open label, two-period, crossover, phase 1 | NCT02807909 | published |
| 10 [41] | Vidya Perera et al. | 2022 | Milvexian | small molecular | none | healthy subjects | 18–55 | 16 | nonrandomized, open-label, single-sequence, phase 1 | NCT02959060 | published |
| 11 [42] | Dominic Beale et al. | 2021 | ONO-7684 | small molecule | placebo | healthy subjects | 18–55 | 72 | randomized, double-blind, single and multiple dose, phase 1 | NCT03919890 | published |
| 12 [43] | Rui Chen et al. | 2022 | SHR2285 | small molecular | placebo | healthy subjects | 15–45 | 28 | randomized, double-blind, dose-ascending, single-dosing, phase 1 | NCT03769831 | published |
| 13 [44] | Tingting Ma et al. | 2022 | SHR2285 | small molecular | placebo | healthy subjects | 18–55 | 52 | randomized, double-blind, single-center, placebo-controlled, phase 1 | NCT04945616 | published |
| 14 [29] | Jonathan Ppiccini et al. | 2022 | Asundexian | small molecular | Apixaban | atrial fibrillation | mean: 75 | 753 | randomized, double-blind, multicenter, phase 2 | NCT04218266 | published |
| 15 [30] | AshkanShoamanesh et al. | 2022 | Asundexian | small molecular | placebo | non-cardioembolic ischaemic stroke | mean: 67 | 1808 | randomized, double-blind, parallel-group, placebo-controlled, phase 2b | NCT04304508 | published |
| 16 [31] | Sunil V Rao et al. | 2022 | Asundexian | small molecular | placebo | acute myocardial infarction | mean: 65 | 1601 | randomized, double-blind, parallel-group, phase 2 | NCT04304534 | published |
| 17 [32] | Peter Verhamme et al. | 2021 | Abelacimab | antibody | Enoxaparin | undergoing total knee arthroplasty | 18–80 | 412 | randomized, parallel-group, prospective, phase 2 | EudraCT 2019-003756-37 | published |
| 18 [45] | B. Alexander Yi et al. | 2022 | Abelacimab | antibody | placebo | healthy subjects and atrial fibrillation | ANT-003: 18–60 ANT-004: 18–85 | ANT-003: 32 ANT-004: 18 | randomized, subject/patient-and investigator-blinded, placebo-controlled, multiple ascending dose | none | published |
| 19 [46] | Dirk Thomas et al. | 2019 | Osocimab | antibody | placebo | healthy subjects | 18–55 | 83 | randomized, single-blind, parallel-group, placebo-controlled, dose-escalation, phase 1 | EudraCT 2014-003816-35 | published |
| 20 [33] | Jeffrey I. Weitz et al. | 2020 | Osocimab | antibody | Enoxaparin, Apixaban | undergoing total knee arthroplasty | mean: 66.5 | 813 | randomized, open-label, parallel-group, phase 2 | NCT03276143 | published |
| 21 [47] | Christina U Lorentz et al. | 2019 | AB023 | antibody | placebo | healthy subjects | 18–48 | 21 | randomized, double-blind, placebo-controlled, single ascending bolus dose, phase 1 | NCT03097341 | published |
| 22 [48] | Christina U Lorentz et al. | 2021 | AB023 | antibody | placebo | end-stage renal disease (ESRD) | 18–80 | 24 | randomized, double-blind, placebo-controlled, single-dose, phase 2 | NCT03612856 | published |
| Number | Compound | Type | Comparator | Subject | Age | Total Number | Study Design | Trial Registration | Status |
|---|---|---|---|---|---|---|---|---|---|
| 1 | IONIS-FXIRX | ASO | placebo | end-stage renal disease (ESRD) | 18–85 | 213 | randomized, double-blind, placebo-controlled, phase 2 | NCT03358030 | Completed unpublished |
| 2 | FXI-LICA | ASO | placebo | end-stage renal disease (ESRD) | ≥18 | 307 | randomized, double-blind, placebo-controlled, phase 2 | NCT04534114 | Completed unpublished |
| 3 | Milvexian | small molecular | Placebo, Clopidogrel, Aspirin | Acute Ischemic Stroke, Transient Ischemic Attack (TIA) | ≥40 | 2366 | randomized, double-blind, placebo-controlled, dose-ranging, phase 2 | NCT03766581 | Completed unpublished |
| 4 | SHR2285 | small molecular | Enoxaparin | undergoing elective unilateral total knee arthroplasty | 40–75 | 500 | randomized, double-blind, open-label, multicenter, positive-controlled, phase 2 | NCT05203705 | Recruiting |
| 5 | BMS-986209 | small molecular | Placebo, Itraconazole, Diltiazem | healthy subjects | 18–55 | 114 | randomized, single and multiple doses, phase 1 | NCT04154800 | Completed unpublished |
| 6 | Abelacimab | antibody | Dalteparin | Gastrointestinal/Genitourinary Cancer and Associated VTE | ≥18 | 1020 | randomized, blinded endpoint evaluation, multicenter, phase 3 | NCT05171075 | Recruiting |
| 7 | Abelacimab | antibody | Apixaban | Cancer associated thrombosis | ≥18 | 1655 | randomized, blinded endpoint evaluation, multicenter, phase 3 | NCT05171049 | Recruiting |
| 8 | Osocimab | antibody | placebo | end-stage renal disease (ESRD) | ≥20 | 686 | randomized, double-blind, placebo-controlled, multicenter, parallel-group, phase 2 | NCT04523220 | Completed unpublished |
| 9 | Osocimab | antibody | placebo | end-stage renal disease (ESRD) | 18–80 | 55 | randomized, observer-blind, multicenter, placebo-controlled, parallel-group, phase 1 | NCT03787368 | Completed unpublished |
| 10 | AB023 | antibody | none | patients with cancer receiving chemotherapy | ≥18 | 50 | single-group, open-label, phase 2 | NCT04465760 | Recruiting |
| 11 | MK-2060 | antibody | placebo | end-stage renal disease (ESRD) | ≥18 | 489 | randomized, double-blind, placebo-controlled, multicenter, parallel-group, phase 2 | NCT05027074 | Recruiting |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, Y.; Hu, Y.; Tang, L. Factor XIa Inhibitors as a Novel Anticoagulation Target: Recent Clinical Research Advances. Pharmaceuticals 2023, 16, 866. https://doi.org/10.3390/ph16060866
Xia Y, Hu Y, Tang L. Factor XIa Inhibitors as a Novel Anticoagulation Target: Recent Clinical Research Advances. Pharmaceuticals. 2023; 16(6):866. https://doi.org/10.3390/ph16060866
Chicago/Turabian StyleXia, Yunqing, Yu Hu, and Liang Tang. 2023. "Factor XIa Inhibitors as a Novel Anticoagulation Target: Recent Clinical Research Advances" Pharmaceuticals 16, no. 6: 866. https://doi.org/10.3390/ph16060866
APA StyleXia, Y., Hu, Y., & Tang, L. (2023). Factor XIa Inhibitors as a Novel Anticoagulation Target: Recent Clinical Research Advances. Pharmaceuticals, 16(6), 866. https://doi.org/10.3390/ph16060866