Aptamers Versus Vascular Endothelial Growth Factor (VEGF): A New Battle against Ovarian Cancer
Abstract
1. Introduction
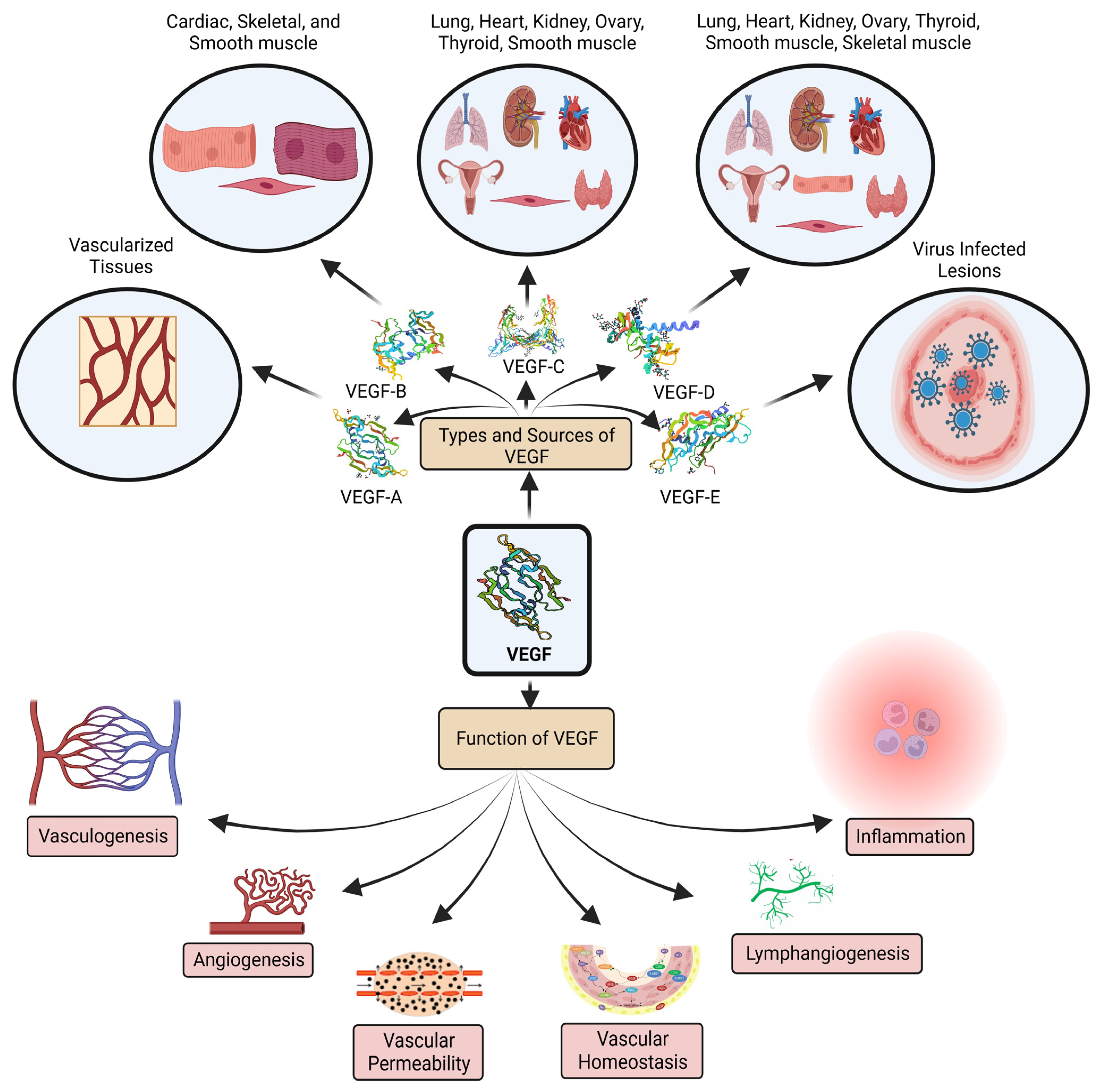
2. Correlation of Ovarian Cancer and VEGF
2.1. VEGF: Biomarker of Ovarian Cancer
2.2. VEGF: Targeted Conventional Treatments and Their Limitations
2.2.1. Anti-VEGF Antibodies
2.2.2. Immune Checkpoint Inhibitors
2.2.3. Tyrosine Kinase Inhibitors
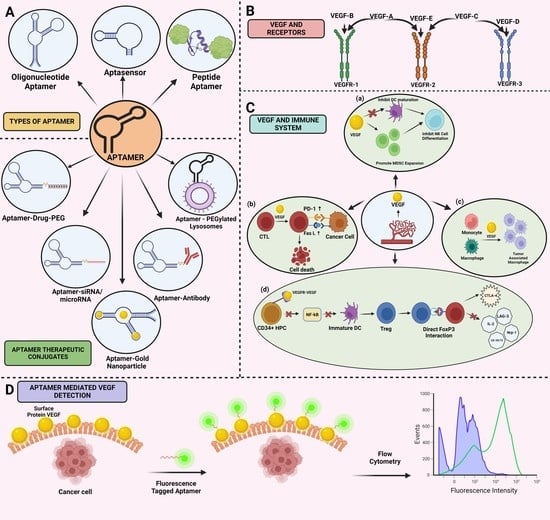
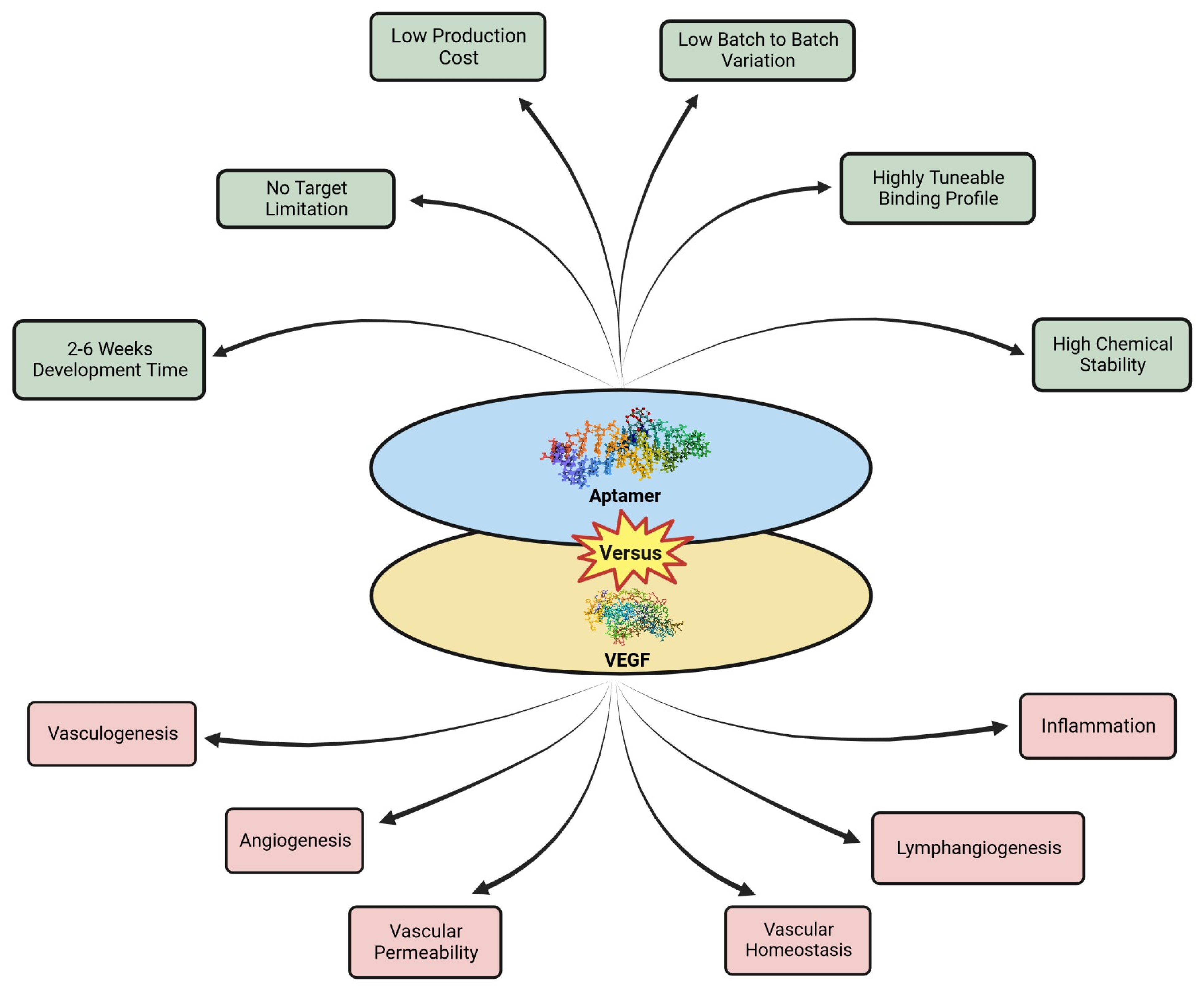
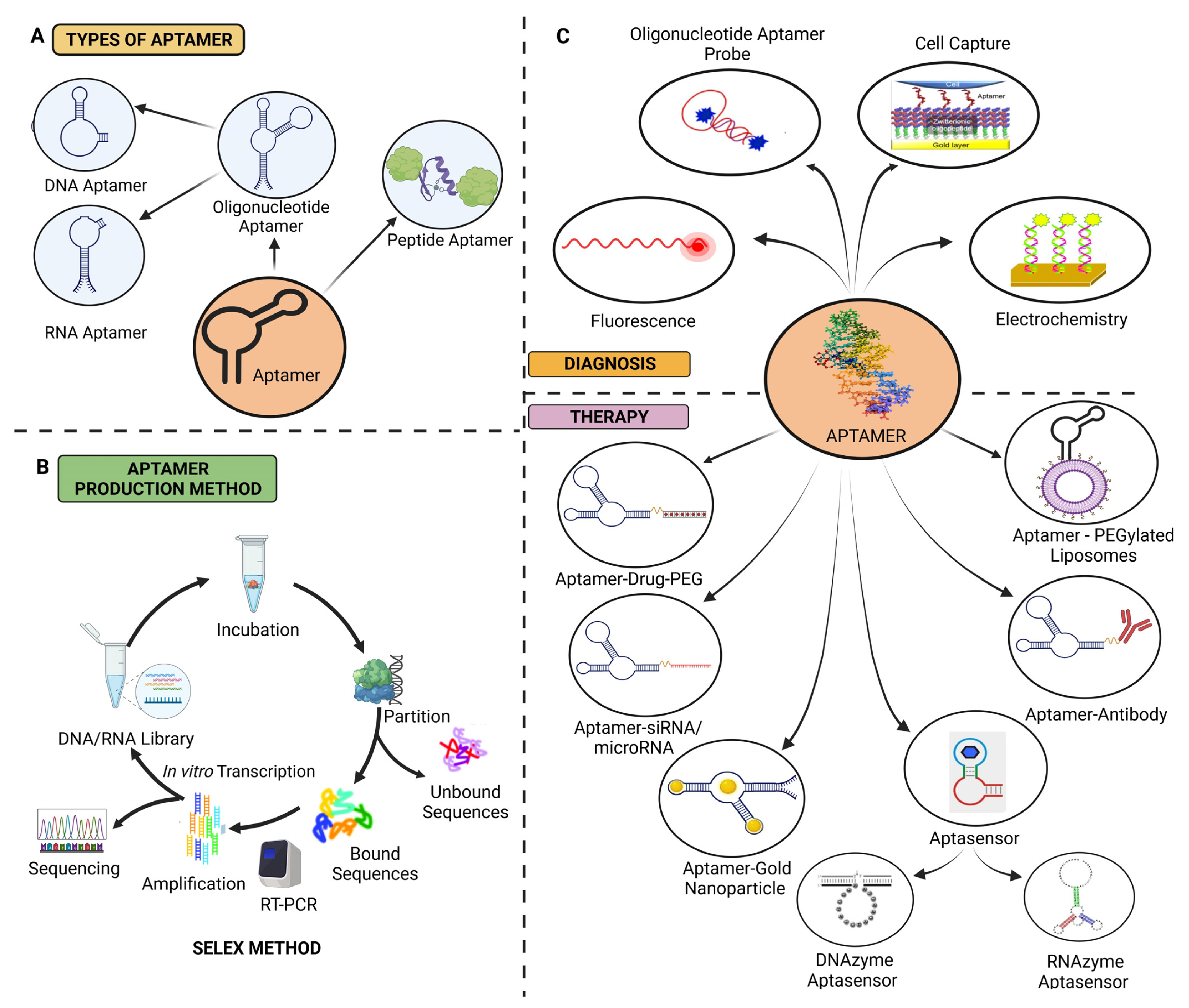
3. Aptamers
3.1. Synthesis of Aptamers
3.1.1. Capillary Electrophoresis SELEX
3.1.2. Capture SELEX
3.1.3. Magnetic-Bead-SELEX
3.1.4. Cell-SELEX Method
3.1.5. Post-SELEX Modifications
3.2. Properties of Aptamers
3.3. Classification of Aptamers
3.3.1. RNA Aptamers
3.3.2. DNA Aptamers
3.3.3. Peptide Aptamers
3.3.4. DNAzyme-Assisted Aptasensors
3.4. Aptamers in Ovarian Cancer Treatment
3.4.1. Detection of Human Epididymis Protein 4
3.4.2. Heat Shock Protein 70 Detection
3.4.3. Molecular Therapy
3.4.4. Aptamer Functionalized Liposome
3.4.5. Aptamer-Magnetic Mesoporous Silica Nanoparticles
3.4.6. Aptasensors
3.5. Toxicological Profile of Aptamers
3.5.1. Aptamer-Based Targeted Chemotherapy
3.5.2. In Vivo and In Vitro Cytotoxicity
4. Aptamer Mediated Targeting of VEGF
4.1. Aptamer-Based Biosensors Detecting VEGF
4.2. RNA and DNA Aptamers Detecting VEGF
5. Aptamers Targeting VEGF in the Ovarian Cancer Therapy
- (i)
- Selection of VEGF-specific aptamers: The process typically involves an in vitro selection method called SELEX, in which a random library of aptamers is generated, and multiple rounds of selection and amplification are performed to enrich the aptamers that bind specifically to VEGF [18].
- (ii)
- Validation of aptamer binding: After several rounds of SELEX, the selected aptamers are tested for their binding affinity and specificity towards VEGF. This can be done using techniques such as surface plasmon resonance (SPR) or fluorescence-based assays.
- (iii)
- Diagnostic applications: Once VEGF-specific aptamers are identified, they can be utilized for diagnostic purposes in OC. For example, aptamers can be conjugated with fluorescent or radioactive labels to develop imaging probes that specifically target VEGF-expressing tumor cells. These probes can help visualize and detect the presence of OC lesions.
- (iv)
- Therapeutic applications: VEGF aptamers can also be explored as therapeutic agents. By binding to VEGF, aptamers can interfere with its activity and inhibit angiogenesis, thus limiting tumor growth and metastasis. Additionally, aptamers can be engineered to deliver therapeutic payloads, such as drugs or siRNAs, specifically to VEGF-expressing cancer cells.
6. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- El-Tanani, M.; Platt-Higgins, A.; Lee, Y.-F.; Al Khatib, A.O.; Haggag, Y.; Sutherland, M.; Zhang, S.-D.; Aljabali, A.A.A.; Mishra, V.; Serrano-Aroca, Á. Matrix metalloproteinase 2 is a target of the Ran-gtp pathway and mediates migration, invasion and metastasis in human breast cancer. Life Sci. 2022, 310, 121046. [Google Scholar] [CrossRef] [PubMed]
- El-Tanani, M.; Al Khatib, A.O.; Al-Najjar, B.O.; Shakya, A.K.; El-Tanani, Y.; Lee, Y.-F.; Serrano-Aroca, Á.; Mishra, V.; Mishra, Y.; Aljabali, A.A.; et al. Cellular and molecular basis of therapeutic approaches to breast cancer. Cell. Signal. 2023, 101, 110492. [Google Scholar] [CrossRef] [PubMed]
- Mishra, Y.; Mishra, V.; Tambuwala, M.M. Tumor adhesion molecule targeting for breast cancer nanomedicine. In Targeted Nanomedicine for Breast Cancer Therapy; Paliwal, S.R., Paliwal, R., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 257–280. ISBN 978-0-12-824476-0. [Google Scholar]
- Khan, R.; Arshad, F.; Hassan, I.U.; Naikoo, G.A.; Pedram, M.Z.; Zedegan, M.S.; Pourfarzad, H.; Aljabali, A.A.A.; Serrano-Aroca, Á.; Haggag, Y.; et al. Advances in nanomaterial-based immunosensors for prostate cancer screening. Biomed. Pharmacother. 2022, 155, 113649. [Google Scholar] [CrossRef]
- Mishra, Y.; Amin, H.I.M.; Mishra, V.; Vyas, M.; Prabhakar, P.K.; Gupta, M.; Kanday, R.; Sudhakar, K.; Saini, S.; Hromić-Jahjefendić, A.; et al. Application of nanotechnology to herbal antioxidants as improved phytomedicine: An expanding horizon. Biomed. Pharmacother. 2022, 153, 113413. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.K.; Tare, M.S.; Mishra, V.; Tripathi, P.K. The development, characterization and in vivo anti-ovarian cancer activity of poly(propylene imine) (PPI)-antibody conjugates containing encapsulated paclitaxel. Nanomed. J. 2015, 11, 207–218. [Google Scholar] [CrossRef]
- Kaur, P.; Mishra, V.; Shunmugaperumal, T.; Goyal, A.K.; Ghosh, G.; Rath, G. Inhalable spray dried lipid nanoparticles for the co-delivery of paclitaxel and doxorubicin in lung cancer. J. Drug Deliv. Sci. Technol. 2020, 56, 101502. [Google Scholar] [CrossRef]
- Mishra, V.; Kesharwani, P.; Amin, M.C.I.M.; Iyer, A. Nanotechnology-Based Approaches for Targeting and Delivery of Drugs and Genes; Academic Press: Cambridge, MA, USA, 2017; ISBN 9780128097182. [Google Scholar]
- El-Tanani, M.; Nsairat, H.; Mishra, V.; Mishra, Y.; Aljabali, A.A.A.; Serrano-Aroca, Á.; Tambuwala, M.M. Ran GTPase and its importance in cellular signaling and malignant phenotype. Int. J. Mol. Sci. 2023, 24, 3065. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, W.; Zuo, L.; Xu, M.; Wu, Y.; Huang, J.; Zhang, X.; Li, Y.; Wang, J.; Chen, J.; et al. The Fibrillin-1/VEGFR2/STAT2 signaling axis promotes chemoresistance via modulating glycolysis and angiogenesis in ovarian cancer organoids and cells. Cancer Commun. 2022, 42, 245–265. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zhao, J.; Guo, Z.; Liu, Y.; Chen, H.; Chen, Z.; He, N. Applications of aptamer-bound nanomaterials in cancer therapy. Biosensors 2021, 11, 344. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, M.; Wang, Z.; Li, S.; Deng, Y.; He, N. Point-of-Care diagnostics for infectious diseases: From methods to devices. Nano Today 2021, 37, 101092. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhang, Y.; Wang, Z.; Zhang, Y.; Zou, J.; Qiu, L. Aptamer-based cancer cell analysis and treatment. ChemistryOpen 2022, 11, e202200141. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Fang, C.; Ouyang, P.; Qing, Y.; Yang, Y.; Li, H.; Wang, Z.; Du, J. Chaperone copolymer assisted G-quadruplex-based signal amplification assay for highly sensitive detection of VEGF. Biosensors 2022, 12, 262. [Google Scholar] [CrossRef] [PubMed]
- Salve, R.; Kumar, P.; Chaudhari, B.P.; Gajbhiye, V. Aptamer tethered bio-responsive mesoporous silica nanoparticles for efficient targeted delivery of paclitaxel to treat ovarian cancer cells. J. Pharm. Sci. 2023, 112, 1450–1459. [Google Scholar] [CrossRef]
- Zhao, J.; Tan, W.; Zheng, J.; Su, Y.; Cui, M. Aptamer nanomaterials for ovarian cancer target theranostics. Front. Bioeng. Biotechnol. 2022, 10, 884405. [Google Scholar] [CrossRef]
- Kohlberger, M.; Gadermaier, G. SELEX: Critical factors and optimization strategies for successful aptamer selection. Biotechnol. Appl. Biochem. 2022, 69, 1771–1792. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Ai, L.; Cui, C.; Fu, T.; Cheng, X.; Qu, F.; Tan, W. Functional aptamer-embedded nanomaterials for diagnostics and therapeutics. ACS Appl. Mater. Interfaces 2021, 13, 9542–9560. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Hinchcliff, E.; Westin, S.N.; Herzog, T.J. State of the science: Contemporary front-line treatment of advanced ovarian cancer. Gynecol. Oncol. 2022, 166, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Bartoletti, M.; Musacchio, L.; Giannone, G.; Tuninetti, V.; Bergamini, A.; Scambia, G.; Lorusso, D.; Valabrega, G.; Mangili, G.; Puglisi, F.; et al. Emerging molecular alterations leading to histology-specific targeted therapies in ovarian cancer beyond PARP inhibitors. Cancer Treat. Rev. 2021, 101, 102298. [Google Scholar] [CrossRef]
- Qiao, L.; Chen, X.; Xi, X.; Chen, X.; Zhang, P.; Dong, H.; Wu, X.; Chen, X. Correlation analysis and clinical significance of CA125, HE4, DDI, and FDP in type II epithelial ovarian cancer. Medicine 2020, 99, e23329. [Google Scholar] [CrossRef]
- Zhang, R.; Siu, M.K.Y.; Ngan, H.Y.S.; Chan, K.K.L. Molecular biomarkers for the early detection of ovarian cancer. Int. J. Mol. Sci. 2022, 23, 12041. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Yuan, M.; Bu, H.; Jin, C. Antiangiogenic strategies in epithelial ovarian cancer: Mechanism, resistance, and combination therapy. J. Oncol. 2022, 2022, e4880355. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Ma, T.; Li, D.; He, M.; Wang, H.; Cui, Y. Circulating vascular endothelial growth factor and cancer risk: A bidirectional mendelian randomization. Front. Genet. 2022, 13, 981032. [Google Scholar] [CrossRef]
- Mabeta, P.; Steenkamp, V. The VEGF/VEGFR axis revisited: Implications for cancer therapy. Int. J. Mol. Sci. 2022, 23, 15585. [Google Scholar] [CrossRef] [PubMed]
- Mamer, S.B.; Wittenkeller, A.; Imoukhuede, P.I. VEGF-A splice variants bind VEGFRs with differential affinities. Sci. Rep. 2020, 10, 14413. [Google Scholar] [CrossRef] [PubMed]
- Vaddepally, R.K.; Kharel, P.; Pandey, R.; Garje, R.; Chandra, A.B. Review of indications of FDA-approved immune checkpoint inhibitors per NCCN guidelines with the level of evidence. Cancers 2020, 12, 738. [Google Scholar] [CrossRef] [PubMed]
- Doo, D.W.; Norian, L.A.; Arend, R.C. Checkpoint inhibitors in ovarian cancer: A review of preclinical data. Gynecol. Oncol. Rep. 2019, 29, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Chandra, A.; Pius, C.; Nabeel, M.; Nair, M.; Vishwanatha, J.K.; Ahmad, S.; Basha, R. Ovarian cancer: Current status and strategies for improving therapeutic outcomes. Cancer Med. 2019, 8, 7018–7031. [Google Scholar] [CrossRef] [PubMed]
- Baci, D.; Bosi, A.; Gallazzi, M.; Rizzi, M.; Noonan, D.M.; Poggi, A.; Bruno, A.; Mortara, L. The ovarian cancer tumor immune microenvironment (TIME) as target for therapy: A focus on innate immunity cells as therapeutic effectors. Int. J. Mol. Sci. 2020, 21, 3125. [Google Scholar] [CrossRef] [PubMed]
- Gasparri, M.L.; Attar, R.; Palaia, I.; Perniola, G.; Marchetti, C.; Di Donato, V.; Farooqi, A.A.; Papadia, A.; Panici, P.B. Tumor infiltrating lymphocytes in ovarian cancer. Asian Pac. J. Cancer Prev. 2015, 16, 3635–3638. [Google Scholar] [CrossRef]
- Kaiser, S.M.; Arepalli, S.; Ehlers, J.P. Current and future anti-VEGF agents for neovascular age-related macular degeneration. J. Exp. Pharmacol. 2021, 29, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Yang, C. Anti-VEGF/VEGFR2 monoclonal antibodies and their combinations with PD-1/PD-L1 inhibitors in clinic. Curr. Cancer Drug Targets 2020, 20, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Shirley, M. Faricimab: First approval. Drugs 2022, 82, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Chen, W.; Han, L.; Bian, Q.; Fan, J.; Cao, Z.; Jin, X.; Ding, T.; Xian, Z.; Guo, Z.; et al. VEGF-B antibody and interleukin-22 fusion protein ameliorates diabetic nephropathy through inhibiting lipid accumulation and inflammatory responses. Acta Pharm. Sin. B 2021, 11, 127–142. [Google Scholar] [CrossRef] [PubMed]
- Doi, T.; Boku, N.; Onozawa, Y.; Takahashi, K.; Kawaguchi, O.; Ohtsu, A. Phase I dose-escalation study of the safety, tolerability, and pharmacokinetics of aflibercept in combination with S-1 in Japanese patients with advanced solid malignancies. Investig. New Drugs 2020, 38, 1390–1399. [Google Scholar] [CrossRef]
- Bumbaca Yadav, D.; Reyes, A.E.; Gupta, P.; Vernes, J.M.; Meng, Y.G.; Schweiger, M.G.; Stainton, S.L.; Fuh, G.; Fielder, P.J.; Kamath, A.V.; et al. Complex formation of anti-VEGF-C with VEGF-C released during blood coagulation resulted in an artifact in its serum pharmacokinetics. Pharmacol. Res. Perspect. 2020, 8, e00573. [Google Scholar] [CrossRef] [PubMed]
- Dumond, A.; Montemagno, C.; Vial, V.; Grépin, R.; Pagès, G. Anti-Vascular endothelial growth factor C antibodies efficiently inhibit the growth of experimental clear cell renal cell carcinomas. Cells 2021, 10, 1222. [Google Scholar] [CrossRef] [PubMed]
- Depetris, R.S.; Lu, D.; Polonskaya, Z.; Zhang, Z.; Luna, X.; Tankard, A.; Kolahi, P.; Drummond, M.; Williams, C.; Ebert, M.C.C.J.C.; et al. Functional antibody characterization via direct structural analysis and information-driven protein-protein docking. Proteins 2022, 90, 919–935. [Google Scholar] [CrossRef] [PubMed]
- Kito, Y.; Satake, H.; Taniguchi, H.; Yamada, T.; Horie, Y.; Esaki, T.; Denda, T.; Yasui, H.; Izawa, N.; Masuishi, T.; et al. Phase Ib study of FOLFOXIRI plus ramucirumab as first-line treatment for patients with metastatic colorectal cancer. Cancer Chemother. Pharmacol. 2020, 86, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Guo, S.; Deng, J.; Shen, J.; Du, F.; Wu, X.; Chen, Y.; Li, M.; Chen, M.; Li, X.; et al. VEGF/VEGFR-targeted therapy and immunotherapy in non-small cell lung cancer: Targeting the tumor microenvironment. Int. J. Biol. Sci. 2022, 18, 3845–3858. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.; Hurwitz, H.I.; Sandler, A.B.; Miles, D.; Coleman, R.L.; Deurloo, R.; Chinot, O.L. Bevacizumab (Avastin®) in cancer treatment: A review of 15 years of clinical experience and future outlook. Cancer Treat. Rev. 2020, 86, 102017. [Google Scholar] [CrossRef]
- Alvarez Secord, A.; Bell Burdett, K.; Owzar, K.; Tritchler, D.; Sibley, A.B.; Liu, Y.; Starr, M.D.; Brady, J.C.; Lankes, H.A.; Hurwitz, H.I.; et al. Predictive blood-based biomarkers in patients with epithelial ovarian cancer treated with carboplatin and paclitaxel with or without bevacizumab: Results from GOG-0218. Clin. Cancer Res. 2020, 26, 1288–1296. [Google Scholar] [CrossRef]
- Nakai, H.; Matsumura, N. The roles and limitations of bevacizumab in the treatment of ovarian cancer. Int. J. Clin. Oncol. 2022, 27, 1120–1126. [Google Scholar] [CrossRef]
- Kajiyama, H.; Suzuki, S.; Yoshihara, M.; Nishino, K.; Yoshikawa, N.; Utsumi, F.; Niimi, K.; Mizuno, M.; Kawai, M.; Oguchi, H.; et al. The possible existence of occult metastasis in patients with ovarian clear-cell carcinoma who underwent complete resection without any residual tumours. Oncotarget 2018, 9, 6298–6307. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Coelho, F.; Martins, F.; Pereira, S.A.; Serpa, J. Anti-angiogenic therapy: Current challenges and future perspectives. Int. J. Mol. Sci. 2021, 22, 3765. [Google Scholar] [CrossRef] [PubMed]
- Tonooka, A.; Ohashi, R. Current trends in anticancer molecular targeted therapies: Renal complications and their histological features. J. Nippon Med. Sch. 2022, 89, 128–138. [Google Scholar] [CrossRef]
- Markóth, C.; File, I.; Szász, R.; Bidiga, L.; Balla, J.; Mátyus, J. Ibrutinib-induced acute kidney injury via interstitial nephritis. Ren. Fail. 2021, 43, 335–339. [Google Scholar] [CrossRef]
- Ștefan, G.; Hosu, O.; De Wael, K.; Lobo-Castañón, M.J.; Cristea, C. Aptamers in biomedicine: Selection strategies and recent advances. Electrochim. Acta 2021, 376, 137994. [Google Scholar] [CrossRef]
- Alhamhoom, Y.; As Sobeai, H.M.; Alsanea, S.; Alhoshani, A. Aptamer-based therapy for targeting key mediators of cancer metastasis. Int. J. Oncol. 2022, 60, 65. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Chen, C.; Larcher, L.M.; Barrero, R.A.; Veedu, R.N. Three decades of nucleic acid aptamer technologies: Lessons learned, progress and opportunities on aptamer development. Biotechnol. Adv. 2019, 37, 28–50. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Yang, G.; Ghulam, M.; Li, L.; Qu, F. Evolution of multi-functional capillary electrophoresis for high-efficiency selection of aptamers. Biotechnol. Adv. 2019, 37, 107432. [Google Scholar] [CrossRef] [PubMed]
- Stoltenburg, R.; Nikolaus, N.; Strehlitz, B. Capture-SELEX: Selection of DNA aptamers for aminoglycoside antibiotics. J. Anal. Methods Chem. 2012, 2012, e415697. [Google Scholar] [CrossRef]
- Pang, Y.; Wan, N.; Shi, L.; Wang, C.; Sun, Z.; Xiao, R.; Wang, S. Dual-recognition surface-enhanced raman scattering(sers)biosensor for pathogenic bacteria detection by using Vancomycin-SERS tags and aptamer-Fe3O4@Au. Anal. Chim. Acta 2019, 1077, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Gregorio, M.R.; González-Barreiro, C.; Rial-Otero, R.; Simal-Gándara, J. Comparison of sanitizing technologies on the quality appearance and antioxidant levels in onion slices. Food Control 2011, 22, 2052–2058. [Google Scholar] [CrossRef]
- Ding, L.; Wu, Y.; Liu, W.; Liu, L.; Yu, F.; Yu, S.; Tian, Y.; Feng, J.; He, L. Magnetic-assisted self-assembled aptamer/protein hybrid probes for efficient capture and rapid detection of cancer cells in whole blood. Talanta 2019, 205, 120129. [Google Scholar] [CrossRef] [PubMed]
- Zumrut, H.E.; Mallikaratchy, P.R. Ligand guided selection (LIGS) of artificial nucleic acid ligands against cell surface targets. ACS Appl. Bio Mater. 2020, 3, 2545–2552. [Google Scholar] [CrossRef] [PubMed]
- Williams, N.B.; Batool, S.; Zumrut, H.E.; Patel, R.; Sosa, G.; Jamal, M.; Mallikaratchy, P. An in vitro selection platform to identify multiple aptamers against multiple cell-surface markers using ligand-guided selection. Biochemistry 2022, 61, 1600–1613. [Google Scholar] [CrossRef] [PubMed]
- Freage, L.; Jamal, D.; Williams, N.B.; Mallikaratchy, P.R. A homodimeric aptamer variant generated from ligand-guided selection activates the t cell receptor cluster of differentiation 3 complex. Mol. Ther. Nucleic Acids 2020, 22, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Moccia, F.; Platella, C.; Musumeci, D.; Batool, S.; Zumrut, H.; Bradshaw, J.; Mallikaratchy, P.; Montesarchio, D. The role of G-quadruplex structures of LIGS-generated aptamers R1.2 and R1.3 in IgM specific recognition. Int. J. Biol. Macromol. 2019, 133, 839–849. [Google Scholar] [CrossRef]
- Abeydeera, N.D.; Egli, M.; Cox, N.; Mercier, K.; Conde, J.N.; Pallan, P.S.; Mizurini, D.M.; Sierant, M.; Hibti, F.-E.; Hassell, T.; et al. Evoking picomolar binding in RNA by a single phosphorodithioate linkage. Nucleic Acids Res. 2016, 44, 8052–8064. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Xiang, J. Aptamers, the nucleic acid antibodies, in cancer therapy. In Advances in Medical Biochemistry, Genomics, Physiology, and Pathology; Jenny Stanford Publishing: Dubai, United Arab Emirates, 2021; ISBN 978-1-00-318044-9. [Google Scholar]
- Narayan, C.; Veeramani, S.; Thiel, W.H. Optimization of RNA aptamer SELEX methods: Improved aptamer transcript 3′-end homogeneity, page purification yield, and target-bound aptamer RNA recovery. Nucleic Acid Ther. 2022, 32, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Devi, S.; Sharma, N.; Ahmed, T.; Huma, Z.I.; Kour, S.; Sahoo, B.; Singh, A.K.; Macesic, N.; Lee, S.J.; Gupta, M.K. Aptamer-based diagnostic and therapeutic approaches in animals: Current potential and challenges. Saudi J. Biol. Sci. 2021, 28, 5081–5093. [Google Scholar] [CrossRef] [PubMed]
- Razlansari, M.; Jafarinejad, S.; Rahdar, A.; Shirvaliloo, M.; Arshad, R.; Fathi-Karkan, S.; Mirinejad, S.; Sargazi, S.; Sheervalilou, R.; Ajalli, N.; et al. Development and classification of RNA aptamers for therapeutic purposes: An updated review with emphasis on cancer. Mol. Cell. Biochem. 2022, 478, 1573–1598. [Google Scholar] [CrossRef]
- Kim, S.-H.; Choi, J.-W.; Kim, A.-R.; Lee, S.-C.; Yoon, M.-Y. Development of SsDNA aptamers for diagnosis and inhibition of the highly pathogenic avian influenza virus subtype H5N1. Biomolecules 2020, 10, 1116. [Google Scholar] [CrossRef] [PubMed]
- Acquah, C.; Agyei, D.; Obeng, E.M.; Pan, S.; Tan, K.X.; Danquah, M.K. Aptamers: An emerging class of bioaffinity ligands in bioactive peptide applications. Crit. Rev. Food Sci. Nutr. 2020, 60, 1195–1206. [Google Scholar] [CrossRef]
- Huang, Z.; Niu, L. RNA aptamers for AMPA receptors. Neuropharmacology 2021, 199, 108761. [Google Scholar] [CrossRef]
- Ma, X.; Ding, W.; Wang, C.; Wu, H.; Tian, X.; Lyu, M.; Wang, S. DNAzyme biosensors for the detection of pathogenic bacteria. Sens. Actuators B Chem. 2021, 331, 129422. [Google Scholar] [CrossRef]
- Kamali, H.; Golmohammadzadeh, S.; Zare, H.; Nosrati, R.; Fereidouni, M.; Safarpour, H. The recent advancements in the early detection of cancer biomarkers by DNAzyme-assisted aptasensors. J. Nanobiotechnol. 2022, 20, 438. [Google Scholar] [CrossRef] [PubMed]
- Heidari, F.; Mohajeri, N.; Zarghami, N. Targeted design of green carbon dot-CA-125 aptamer conjugate for the fluorescence imaging of ovarian cancer cell. Cell Biochem. Biophys. 2022, 80, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Hanžek, A.; Ducongé, F.; Siatka, C.; Duc, A.-C.E. Identification and characterization of aptamers targeting ovarian cancer biomarker human epididymis protein 4 for the application in urine. Cancers 2023, 15, 452. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-N.; Tsai, Y.-C.; Hsu, C.-C.; Liang, Y.-L.; Wu, Y.-Y.; Kang, C.-Y.; Lin, C.-H.; Hsu, P.-H.; Lee, G.-B.; Hsu, K.-F. An aptamer interacting with heat shock protein 70 shows therapeutic effects and prognostic ability in serous ovarian cancer. Mol. Ther. Nucleic Acids 2021, 23, 757–768. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Fang, X.; Zhang, C.; Ji, H.; Pang, Q.; Li, X.; Luo, Z.; Wu, Q.; Zhang, L. Development of aptamer-based molecular tools for rapid intraoperative diagnosis and in vivo imaging of serous ovarian cancer. ACS Appl. Mater. Interfaces 2021, 13, 16118–16126. [Google Scholar] [CrossRef]
- Bates, P.J.; Reyes-Reyes, E.M.; Malik, M.T.; Murphy, E.M.; O’Toole, M.G.; Trent, J.O. G-quadruplex oligonucleotide AS1411 as a cancer-targeting agent: Uses and mechanisms. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 1414–1428. [Google Scholar] [CrossRef] [PubMed]
- Cadinoiu, A.N.; Rata, D.M.; Atanase, L.I.; Mihai, C.T.; Bacaita, S.E.; Popa, M. Formulations based on drug loaded aptamer-conjugated liposomes as a viable strategy for the topical treatment of basal cell carcinoma-in vitro tests. Pharmaceutics 2021, 13, 866. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, Q.; He, M.; Ding, F.; Cai, L.; Zhao, M.; Dong, L.; Wang, Q.; Xu, K. AS1411 aptamer-modified theranostic liposomes co-encapsulating manganese oxide nano-contrast agent and paclitaxel for MRI and therapy of cancer. RSC Adv. 2019, 9, 34837–34846. [Google Scholar] [CrossRef] [PubMed]
- Riccardi, C.; Fàbrega, C.; Grijalvo, S.; Vitiello, G.; D’Errico, G.; Eritja, R.; Montesarchio, D. AS1411-decorated niosomes as effective nanocarriers for Ru(iii)-based drugs in anticancer strategies. J. Mater. Chem. B 2018, 6, 5368–5384. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Wang, H.; Chen, S. Aptamer (AS1411)-conjugated liposome for enhanced therapeutic efficacy of MiRNA-29b in ovarian cancer. J. Nanosci. Nanotechnol. 2020, 20, 2025–2031. [Google Scholar] [CrossRef] [PubMed]
- Torabi, M.; Aghanejad, A.; Savadi, P.; Barzegari, A.; Omidi, Y.; Barar, J. Targeted delivery of sunitinib by MUC-1 aptamer-capped magnetic mesoporous silica nanoparticles. Molecules 2023, 28, 411. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Gopinath, S.C.B.; Lakshmipriya, T.; Chen, Y. Carbon material hybrid construction on an aptasensor for monitoring surgical tumors. J. Anal. Methods Chem. 2022, 2022, e9740784. [Google Scholar] [CrossRef]
- Farzin, L.; Sadjadi, S.; Shamsipur, M.; Sheibani, S.; Mousazadeh, M.H. Employing AgNPs doped amidoxime-modified polyacrylonitrile (PAN-Oxime) nanofibers for target induced strand displacement-based electrochemical aptasensing of CA125 in ovarian cancer patients. Mater. Sci. Eng. C 2019, 97, 679–687. [Google Scholar] [CrossRef]
- Ma, X.; Lakshmipriya, T.; Gopinath, S.C.B. Recent advances in identifying biomarkers and high-affinity aptamers for gyneco logic cancers diagnosis and therapy. J. Anal. Methods Chem. 2019, 2019, e5426974. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Y.; Li, P.-C.; Guo, J.; Huo, F.; Yang, J.; Jia, R.; Wang, J.; Huang, Q.; Theodorescu, D.; et al. Development of novel aptamer-based targeted chemotherapy for bladder cancer. Cancer Res. 2022, 82, 1128–1139. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, Q.; Lu, C. Aptamer-functionalized iron-based metal–organic frameworks (MOFs) for synergistic cascade cancer chemotherapy and chemodynamic therapy. Molecules 2022, 27, 4247. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Cai, X.; Sun, Q.; Guo, X.; Liang, C.; Tang, H.; Huang, H.; Luo, H.; Chen, L.; Chen, J. Design and synthesis of aptamer-cyclometalated iridium(III) complex conjugate targeting cancer cells. Eur. J. Med. Chem. 2022, 236, 114335. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Fu, Y.; Guo, M.; Chen, Y.; Zhang, D.; Wei, Y.; Jin, F.; Zeng, Q.; Wang, Y.; Chai, C.; et al. Dual-aptamer-engineered M1 macrophage with enhanced specific targeting and checkpoint blocking for solid-tumor immunotherapy. Mol. Ther. 2022, 30, 2817–2827. [Google Scholar] [CrossRef] [PubMed]
- Yavari, B.; Athari, S.S.; Omidi, Y.; Jalali, A.; Najafi, R. EpCAM aptamer activated 5-FU-loaded PLGA nanoparticles in CRC treatment; in vitro and in vivo study. J. Drug Target. 2023, 31, 296–309. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhang, W.; Chen, J. Binary Nanodrug-delivery system designed for leukemia therapy: Aptamer- and transferrin-decorated daunorubicin- and luteolin-coloaded nanoparticles. Drug Des. Devel. Ther. 2023, 17, 1–13. [Google Scholar] [CrossRef]
- Riccardi, C.; Napolitano, E.; Platella, C.; Musumeci, D.; Melone, M.A.B.; Montesarchio, D. Anti-VEGF DNA-based aptamers in cancer therapeutics and diagnostics. Med. Res. Rev. 2021, 41, 464–506. [Google Scholar] [CrossRef] [PubMed]
- Marshall, M.L.; Wagstaff, K.M. Internalized functional DNA aptamers as alternative cancer therapies. Front. Pharmacol. 2020, 11, 1115. [Google Scholar] [CrossRef] [PubMed]
- Ahirwar, R.; Khan, N.; Kumar, S. Aptamer-based sensing of breast cancer biomarkers: A comprehensive review of analytical figures of merit. Expert Rev. Mol. Diagn. 2021, 21, 703–721. [Google Scholar] [CrossRef] [PubMed]
- Dakowicz, D.; Zajkowska, M.; Mroczko, B. Relationship between VEGF family members, their receptors and cell death in the neoplastic transformation of colorectal cancer. Int. J. Mol. Sci. 2022, 23, 3375. [Google Scholar] [CrossRef]
- Ceci, C.; Atzori, M.G.; Lacal, P.M.; Graziani, G. Role of VEGFs/VEGFR-1 signaling and its inhibition in modulating tumor invasion: Experimental evidence in different metastatic cancer models. Int. J. Mol. Sci. 2020, 21, 1388. [Google Scholar] [CrossRef]
- Apte, R.S.; Chen, D.S.; Ferrara, N. VEGF in signaling and disease: Beyond discovery and development. Cell 2019, 176, 1248–1264. [Google Scholar] [CrossRef] [PubMed]
- Yoshitomi, T.; Hayashi, M.; Oguro, T.; Kimura, K.; Wayama, F.; Furusho, H.; Yoshimoto, K. Binding and structural properties of DNA aptamers with VEGF-A-mimic activity. Mol. Ther. Nucleic Acids 2020, 19, 1145–1152. [Google Scholar] [CrossRef] [PubMed]
- Kumar Kulabhusan, P.; Hussain, B.; Yüce, M. Current perspectives on aptamers as diagnostic tools and therapeutic agents. Pharmaceutics 2020, 12, 646. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhang, D.; Zeng, Z.; Huang, L.; Lin, X.; Hong, S. Aptamer-based probes for cancer diagnostics and treatment. Life 2022, 12, 1937. [Google Scholar] [CrossRef] [PubMed]
- Roy, T.; James, B.D.; Allen, J.B. Anti-VEGF-R2 aptamer and RGD peptide synergize in a bifunctional hydrogel for enhanced angiogenic potential. Macromol. Biosci. 2021, 21, e2000337. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Hong, M.-S.; Lee, W.-H.; Kim, J.-G.; Kim, K. Highly sensitive electrochemical aptasensor for detecting the VEGF165 tumor marker with PANI/CNT nanocomposites. Biosensors 2021, 11, 114. [Google Scholar] [CrossRef]
- Azuaje-Hualde, E.; de Pancorbo, M.M.; Benito-Lopez, F.; Basabe-Desmonts, L. Paper based microfluidic platform for single-step detection of mesenchymal stromal cells secreted VEGF. Anal. Chim. Acta 2022, 1199, 339588. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Xia, X.; Zhang, J.; Huang, J.; Xie, F.; Li, X.; Chen, D.; Peng, C. A novel “Signal on-off-Super on” sandwich-type aptamer sensor of CRISPR-Cas12a coupled voltage enrichment assay for VEGF detection. Biosens. Bioelectron. 2023, 221, 114424. [Google Scholar] [CrossRef]
- Mei, C.; Zhang, Y.; Pan, L.; Dong, B.; Chen, X.; Gao, Q.; Xu, H.; Xu, W.; Fang, H.; Liu, S.; et al. A one-step electrochemical aptasensor based on signal amplification of metallo nanoenzyme particles for vascular endothelial growth factor. Front. Bioeng. Biotechnol. 2022, 10, 850412. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, T.; Johnson-Buck, A.; Walter, N.G. Highly sensitive protein detection by aptamer-based single-molecule kinetic fingerprinting. Biosens. Bioelectron. 2022, 216, 114639. [Google Scholar] [CrossRef]
- Kalathingal, M.; Rhee, Y.M. Molecular mechanism of binding between a therapeutic RNA aptamer and its protein target VEGF: A molecular dynamics study. J. Comput. Chem. 2023, 44, 1129–1137. [Google Scholar] [CrossRef]
- Nonaka, Y.; Sode, K.; Ikebukuro, K. Screening and improvement of an anti-VEGF DNA aptamer. Molecules 2010, 15, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Nonaka, Y.; Yoshida, W.; Abe, K.; Ferri, S.; Schulze, H.; Bachmann, T.T.; Ikebukuro, K. Affinity improvement of a VEGF aptamer by in silico maturation for a sensitive VEGF-detection system. Anal. Chem. 2013, 85, 1132–1137. [Google Scholar] [CrossRef]
- Moccia, F.; Riccardi, C.; Musumeci, D.; Leone, S.; Oliva, R.; Petraccone, L.; Montesarchio, D. Insights into the G-rich VEGF-binding aptamer V7t1: When two G-quadruplexes are better than one! Nucleic Acids Res. 2019, 47, 8318–8331. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, E.; Riccardi, C.; Gaglione, R.; Arciello, A.; Pirota, V.; Triveri, A.; Doria, F.; Musumeci, D.; Montesarchio, D. Selective light-up of dimeric G-Quadruplex forming aptamers for efficient VEGF165 detection. Int. J. Biol. Macromol. 2023, 224, 344–357. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Xu, Z.; Li, S.; Liang, H.; Zhang, C.; Wang, Z.; Li, J.; Li, J.; Yang, H. Systematic Interrogation of Cellular Signaling in Live Cells Using a Membrane-Anchored DNA Multitasking Processor. Angew. Chem. Int. Ed. 2022, 61, e202113795. [Google Scholar] [CrossRef]
- Chang, C.-C.; Chen, C.-Y.; Chuang, T.-L. Aptamer-based colorimetric detection of proteins using a branched DNA cascade amplification strategy and unmodified gold nanoparticles. Biosens. Bioelectron. 2016, 78, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Davydova, A.; Vorobyeva, M. Aptamer-based biosensors for the colorimetric detection of blood biomarkers: Paving the way to clinical laboratory testing. Biomedicines 2022, 10, 1606. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Xu, X.; Chen, P.; Wu, J.; Jin, Y.; Zhang, L.; Du, S. Base amount-dependent fluorescence enhancement for the assay of vascular endothelial growth factor 165 in human serum using hairpin DNA-Silver nanoclusters and oxidized carbon nanoparticles. Microchim. Acta 2020, 187, 629. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; He, L.; Wang, Y.; Yu, F.; Yu, S.; Liu, L.; Wang, J.; Tian, Y.; Qu, L.; Han, R.; et al. A highly sensitive colorimetric aptasensor for the detection of the vascular endothelial growth factor in human serum. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 226, 117622. [Google Scholar] [CrossRef]
- Petrucci, E.; Pasquini, L.; Bernabei, M.; Saulle, E.; Biffoni, M.; Accarpio, F.; Sibio, S.; Di Giorgio, A.; Di Donato, V.; Casorelli, A.; et al. A small molecule SMAC mimic LBW242 potentiates TRAIL- and anticancer drug-mediated cell death of ovarian cancer cells. PLoS ONE 2012, 7, e35073. [Google Scholar] [CrossRef] [PubMed]
| Aptamer Type | Biomarkers of Ovarian Cancer | Diagnostic Rate | Reference |
|---|---|---|---|
| DNA aptamer | VEGF | 0.185 nM | [85] |
| DNA aptamer | CA125 | 0.05 U/mL | [84] |
| DNA aptamer | HE4 | 13 nM | [74] |
| Tx-01 aptamer | HSP70 | 200 μm | [75] |
| Amine functionalized aptamer | MUC-1 | 0.8 nM | [82] |
| Aptasensor | CEA | 0.5 ng/mL | [83] |
| Aptamer-Based Anti-VEGF Treatments | Effects | Side Effects | Reference |
|---|---|---|---|
| Aptamer-modified magnetic nanocrystal | Accurate identification of angiogenic vessels | No immunogenic responses in vivo | [100] |
| Bifunctional thiolated hyaluronic acid−polyethylene glycol diacrylate hydrogels | Despite exogenous growth agents, stimulating angiogenesis | Boost cell survival, encourage cell movement, and accelerate angiogenesis | [101] |
| Alkalinity-dependent fluorescence enhancement-based treatment | High sensitivity and selectivity | Significant cytotoxicity and improved anti-tumor efficiency | [114] |
| Colorimetric assay | High sensitivity | Lower detection limit | [115] |
| Sandwich-type colorimetric microplate detector | Quick and easy detection of VEGF in blood | Higher anti-tumor efficiency | [116] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mishra, Y.; Chattaraj, A.; Mishra, V.; Ranjan, A.; Tambuwala, M.M. Aptamers Versus Vascular Endothelial Growth Factor (VEGF): A New Battle against Ovarian Cancer. Pharmaceuticals 2023, 16, 849. https://doi.org/10.3390/ph16060849
Mishra Y, Chattaraj A, Mishra V, Ranjan A, Tambuwala MM. Aptamers Versus Vascular Endothelial Growth Factor (VEGF): A New Battle against Ovarian Cancer. Pharmaceuticals. 2023; 16(6):849. https://doi.org/10.3390/ph16060849
Chicago/Turabian StyleMishra, Yachana, Aditi Chattaraj, Vijay Mishra, Abhigyan Ranjan, and Murtaza M. Tambuwala. 2023. "Aptamers Versus Vascular Endothelial Growth Factor (VEGF): A New Battle against Ovarian Cancer" Pharmaceuticals 16, no. 6: 849. https://doi.org/10.3390/ph16060849
APA StyleMishra, Y., Chattaraj, A., Mishra, V., Ranjan, A., & Tambuwala, M. M. (2023). Aptamers Versus Vascular Endothelial Growth Factor (VEGF): A New Battle against Ovarian Cancer. Pharmaceuticals, 16(6), 849. https://doi.org/10.3390/ph16060849