Annexin A5 Inhibits Endothelial Inflammation Induced by Lipopolysaccharide-Activated Platelets and Microvesicles via Phosphatidylserine Binding
Abstract
1. Introduction
2. Results
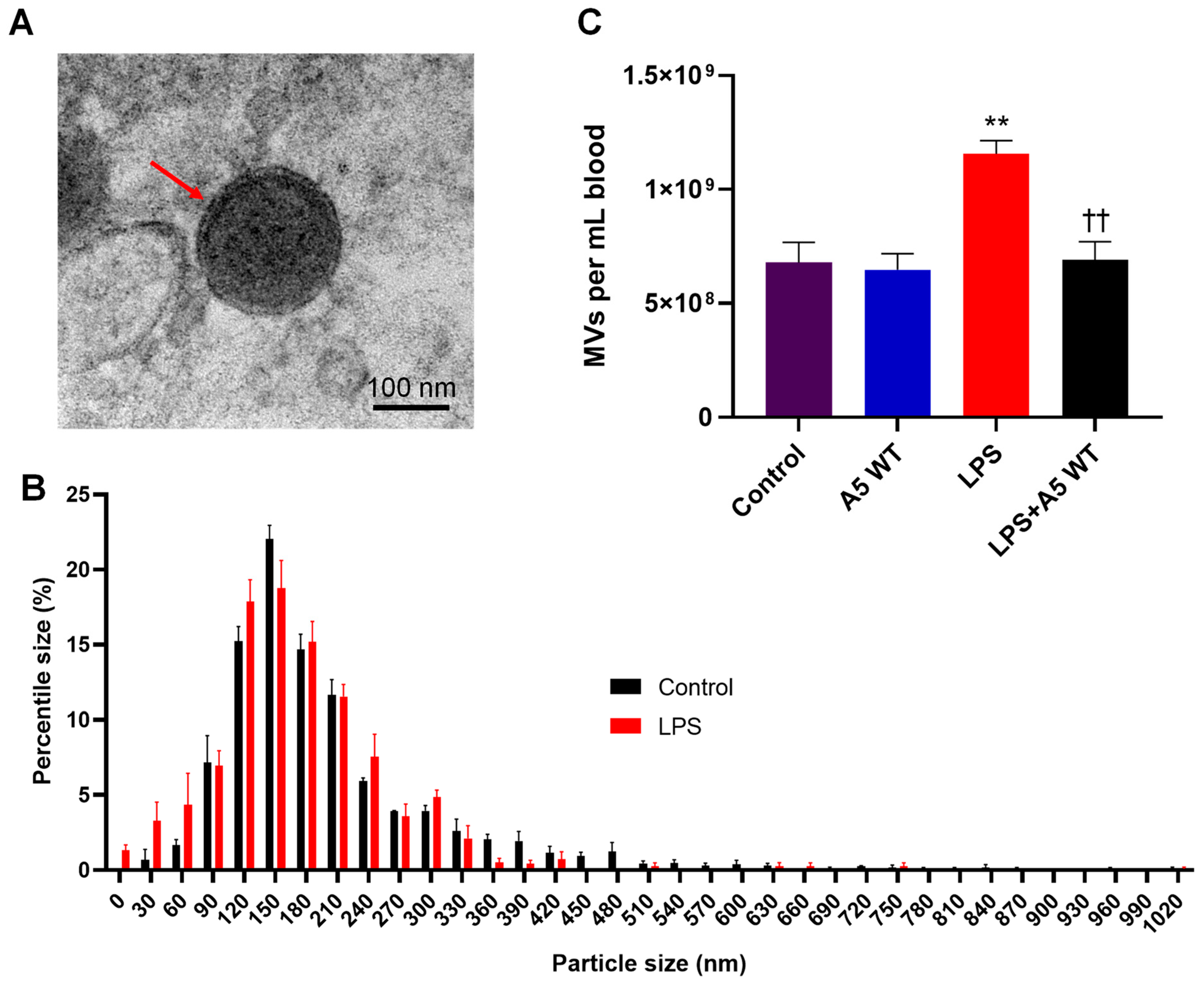
2.1. MV Morphology, Size, and Quantity in Endotoxemic Mice
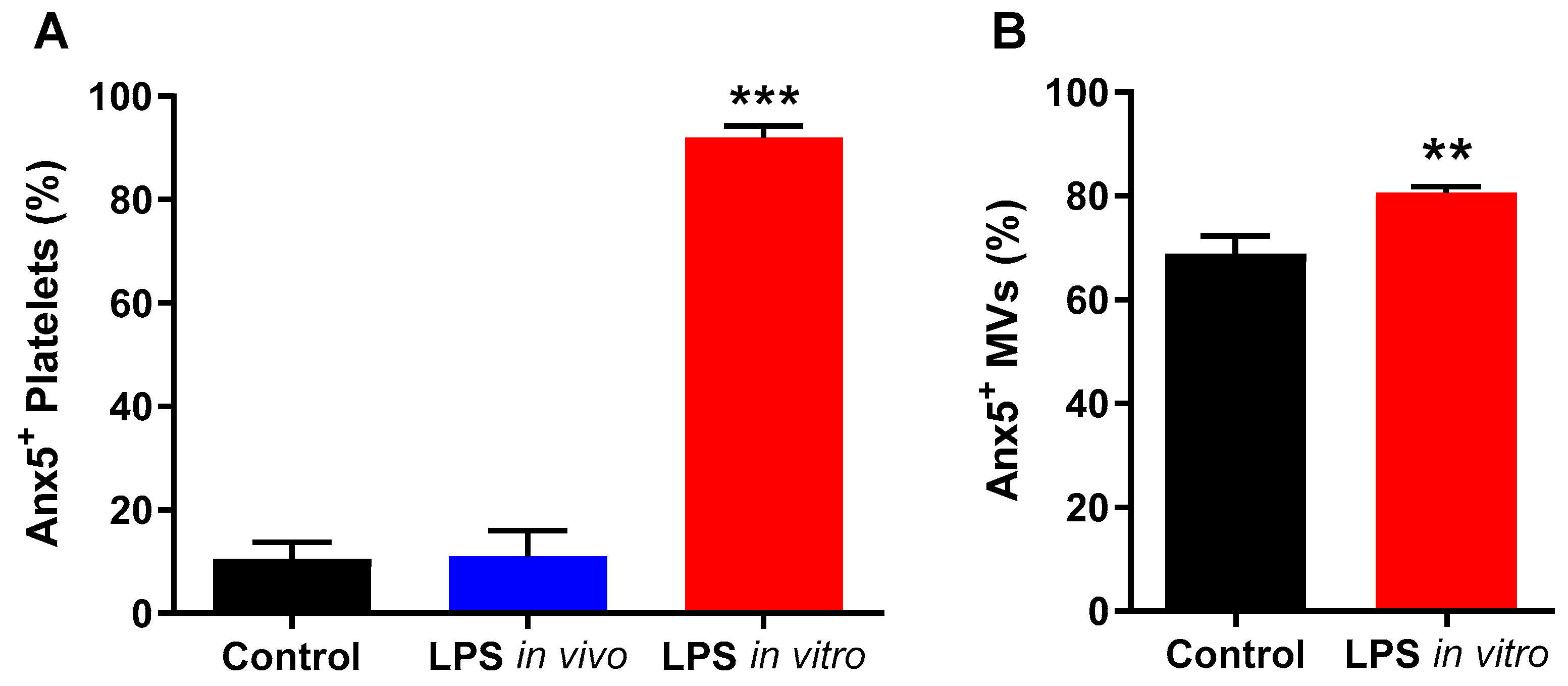
2.2. In Vitro LPS Stimulation Leads to Higher Phosphatidylserine Exposure on Platelets and MVs
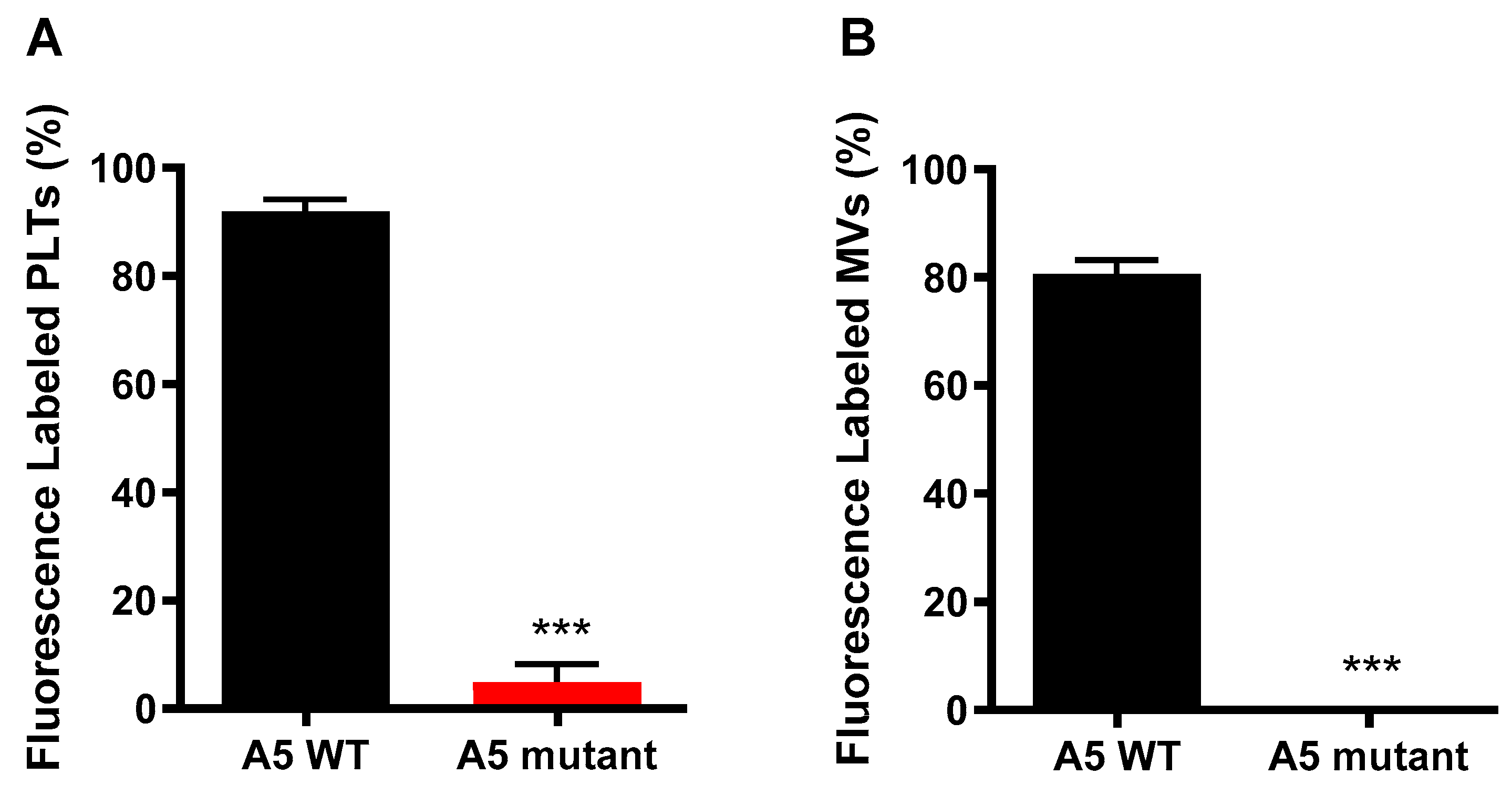
2.3. Anx5 Mutant Is Unable to Bind to Externalized Phosphatidylserine on LPS-Activated Platelets or MVs
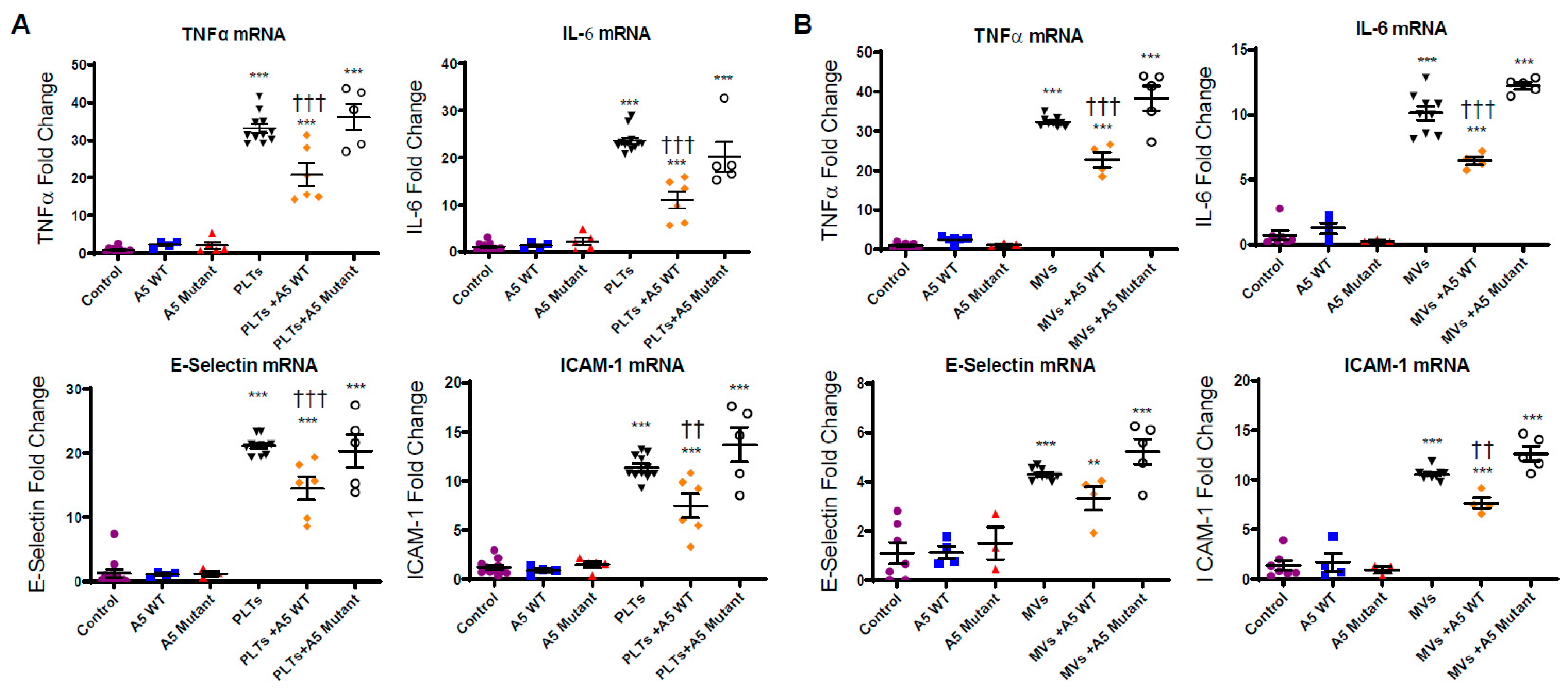
2.4. Anx5 Attenuates Pro-Inflammatory and Pro-Adhesive Responses of LPS-Stimulated Platelets and MVs via Phosphatidylserine Binding
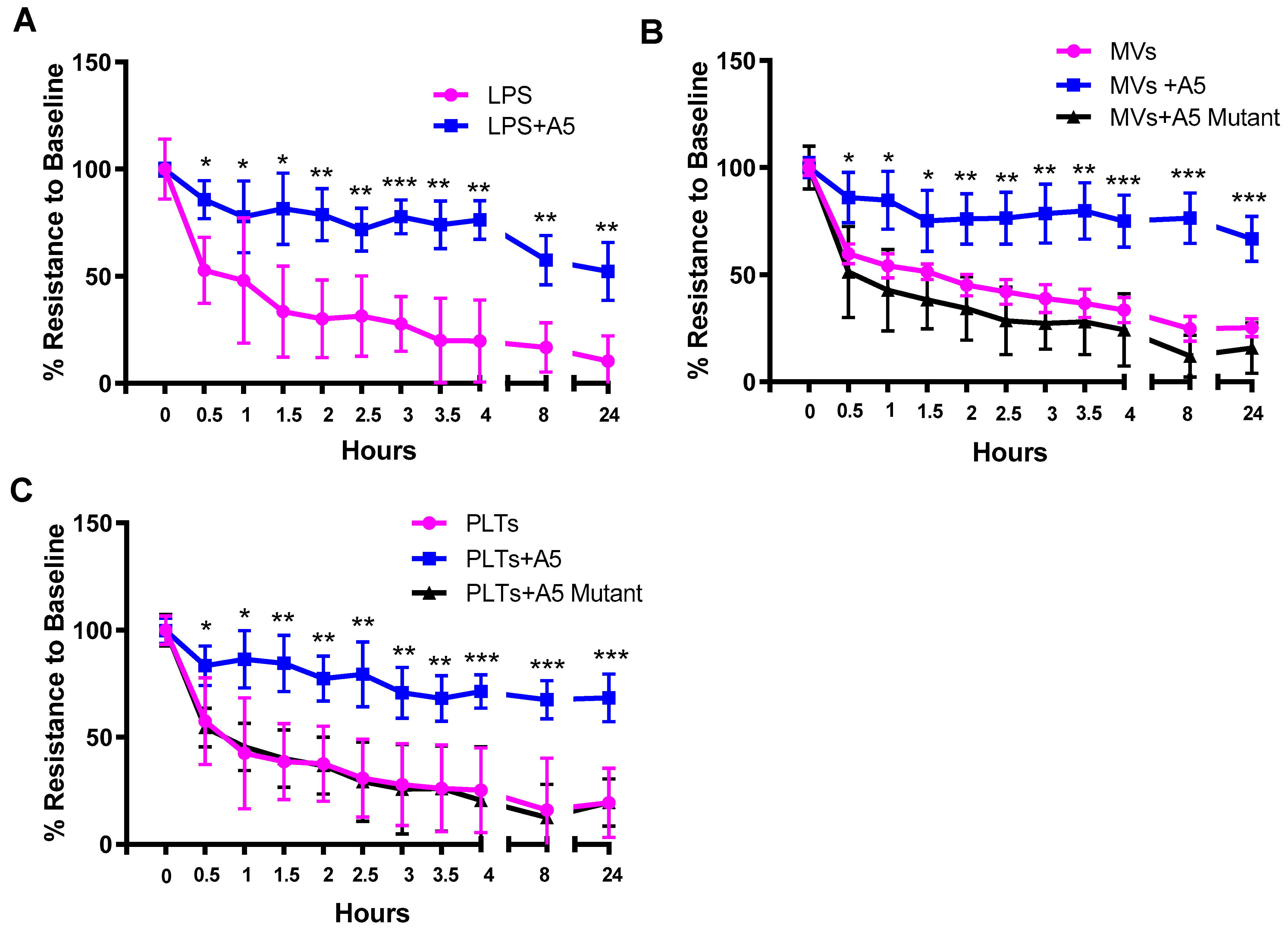
2.5. Trans-Endothelial Electrical Resistance (TEER) in Septic Conditions
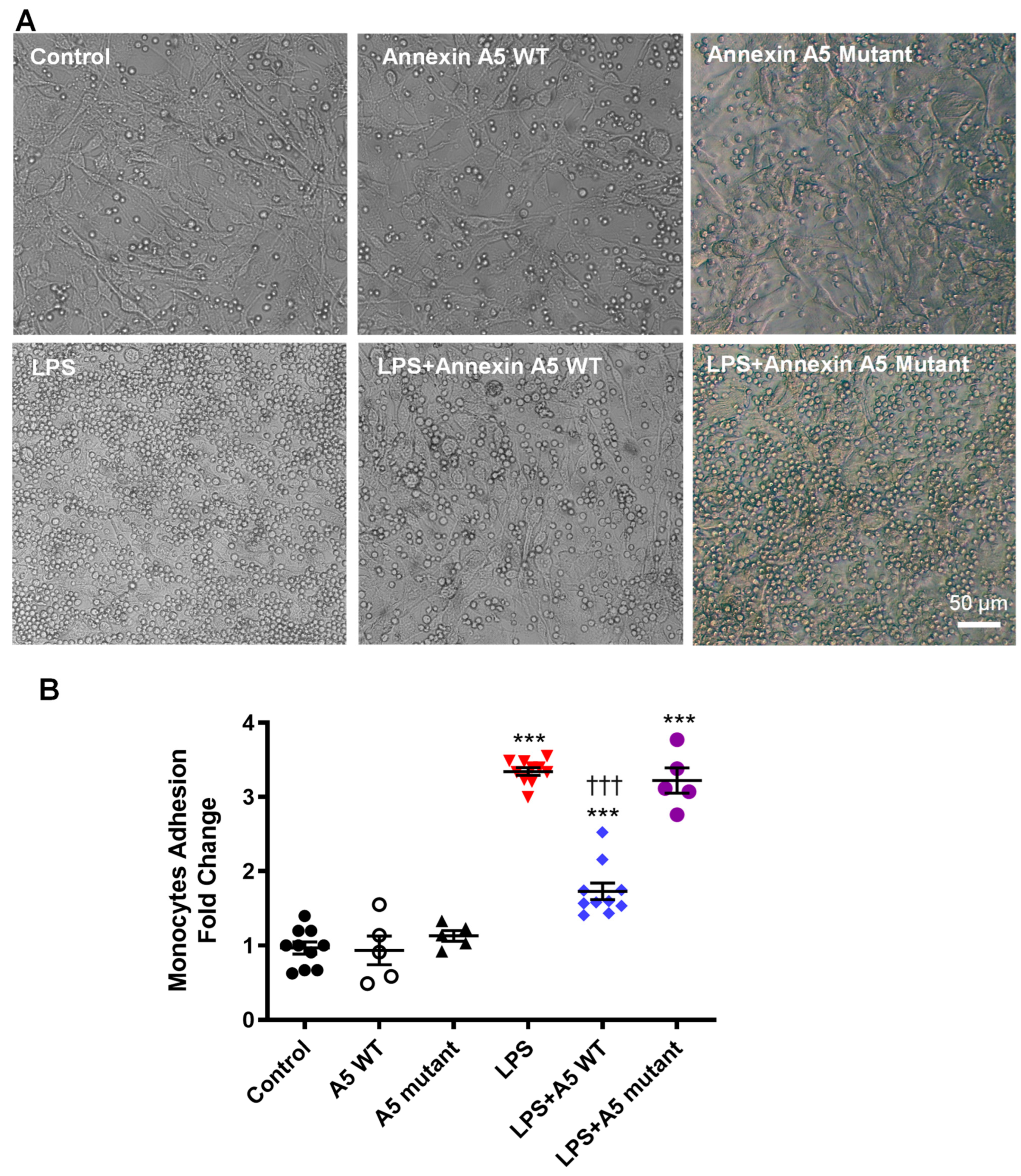
2.6. Anx5 Inhibits LPS-Induced Monocyte Adhesion to Endothelial Cells
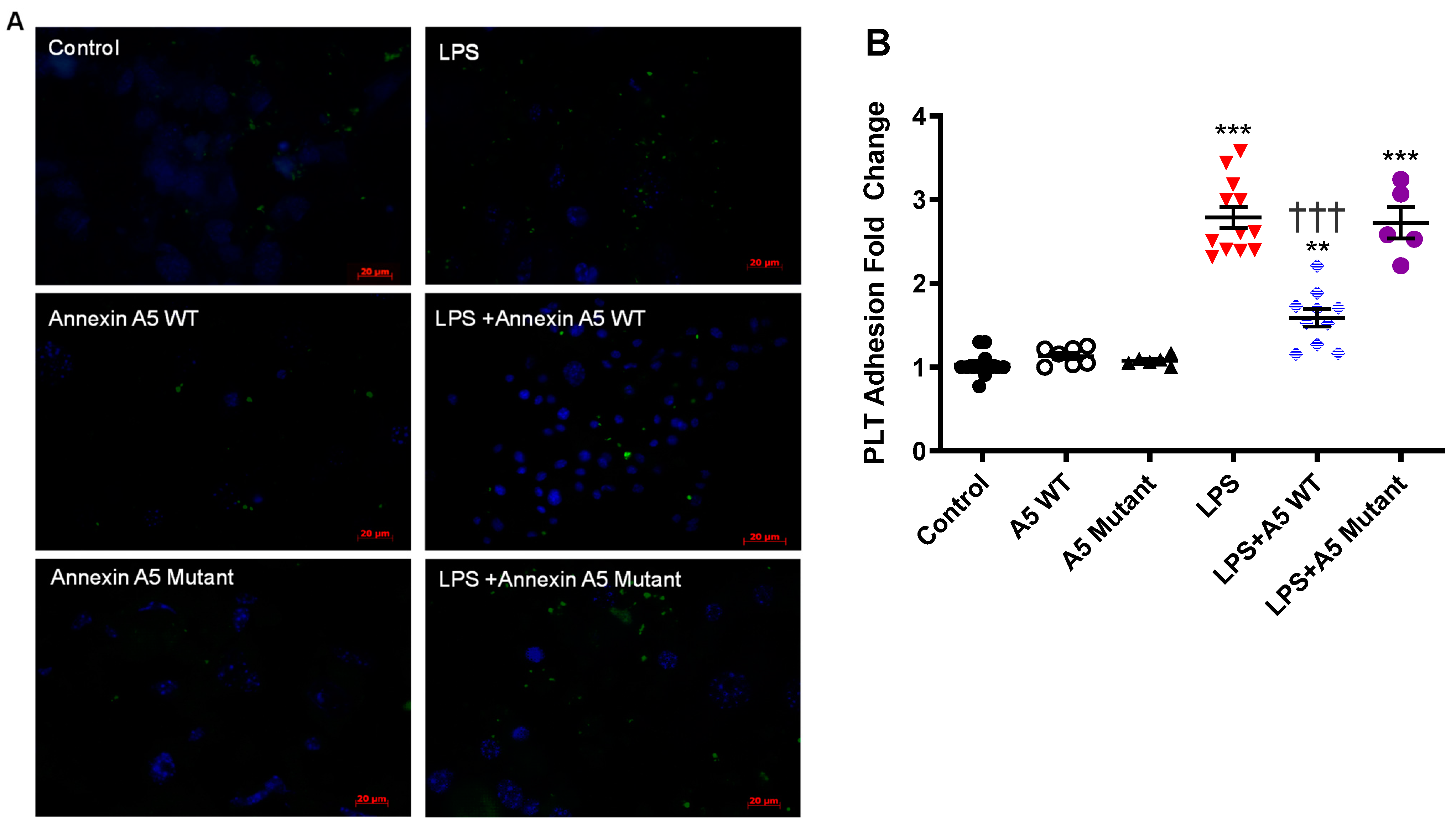
2.7. Anx5 Inhibits LPS-Induced Platelet Adhesion to Endothelial Cells
3. Discussion
4. Methods
4.1. Experimental Animals and Ethics
4.2. In Vivo Endotoxemia
4.3. Mouse Skeletal Muscle Endothelial Cell Culture
4.4. Platelet and Microvesicle Isolation
4.5. Incubation of In Vitro LPS-Stimulated Platelets or MVs with Cultured Endothelial Cells
4.6. Recombinant Human Wildtype and Quadruple Mutant of Anx5
4.7. Nanoparticle Tracking Analysis of Extracellular Vesicles
4.8. Transmission Electron Microscopic Analysis
4.9. Nanoflow Cytometric Analysis
4.10. Platelet Flow Cytometry
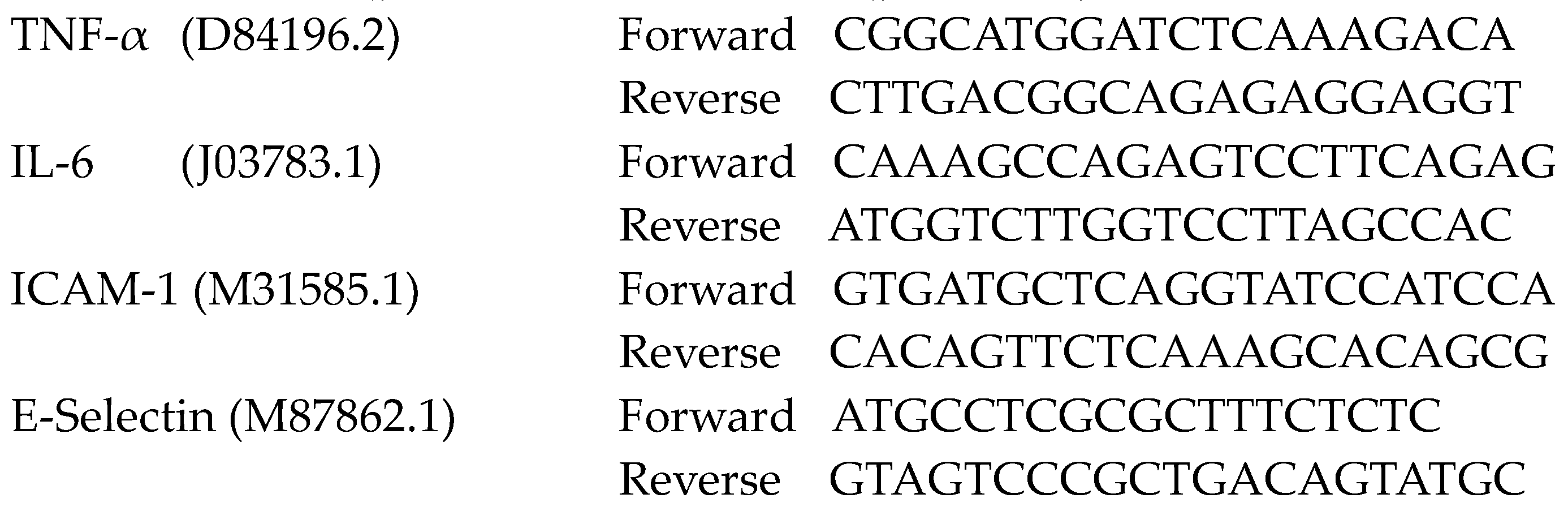
4.11. RT-qPCR Analysis
4.12. Trans-Endothelial Electrical Resistance (TEER)
4.13. Monocyte Isolation and Adhesion Assay
4.14. Platelet Adhesion Assay
4.15. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Hotchkiss, R.S.; Moldawer, L.L.; Opal, S.M.; Reinhart, K.; Turnbull, I.R.; Vincent, J.-L. Sepsis and septic shock. Nat. Rev. Dis. Primers 2016, 2, 16045. [Google Scholar] [CrossRef] [PubMed]
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: Analysis for the global burden of disease study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Fathi, M.; Markazi-Moghaddam, N.; Ramezankhani, A. A systematic review on risk factors associated with sepsis in patients admitted to intensive care units. Aust. Crit. Care 2019, 32, 155–164. [Google Scholar] [CrossRef]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Crit. Care Med. 2021, 49, e1063. [Google Scholar] [CrossRef]
- Chousterman, B.G.; Swirski, F.K.; Weber, G.F. Cytokine storm and sepsis disease pathogenesis. Semin. Immunopathol. 2017, 39, 517–528. [Google Scholar] [CrossRef]
- Matsuda, R.; Kaneko, N.; Kikuchi, M.; Chiwaki, F.; Toda, M.; Ieiri, T.; Horikawa, Y.; Shimizu, M.; Shimamoto, K. Clinical significance of measurement of plasma annexin V concentration of patients in the emergency room. Resuscitation 2003, 57, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Mui, L.; Martin, C.M.; Tschirhart, B.J.; Feng, Q. Therapeutic Potential of Annexins in Sepsis and COVID-19. Front. Pharmacol. 2021, 12, 735472. [Google Scholar] [CrossRef]
- Schutters, K.; Reutelingsperger, C. Phosphatidylserine targeting for diagnosis and treatment of human diseases. Apoptosis 2010, 15, 1072–1082. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Smith, C.; Hsieh, H.-Y.; Gibson, D.F.; Tait, J.F. Essential role of B-helix calcium binding sites in annexin V-membrane binding. J. Biol. Chem. 2004, 279, 40351–40357. [Google Scholar] [CrossRef]
- van Genderen, H.O.; Kenis, H.; Hofstra, L.; Narula, J.; Reutelingsperger, C.P.M. Extracellular annexin A5: Functions of phosphatidylserine-binding and two-dimensional crystallization. Biochim. Biophys. Acta 2008, 1783, 953–963. [Google Scholar] [CrossRef]
- Tontanahal, A.; Arvidsson, I.; Karpman, D. Annexin induces cellular uptake of extracellular vesicles and delays disease in Escherichia coli O157:H7 infection. Microorganisms 2021, 9, 1143. [Google Scholar] [CrossRef] [PubMed]
- Arnold, P.; Lu, X.; Amirahmadi, F.; Brandl, K.; Arnold, J.M.; Feng, Q. Recombinant human annexin A5 inhibits proinflammatory response and improves cardiac function and survival in mice with endotoxemia. Crit. Care Med. 2014, 42, e32–e41. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Jang, J.-H.; Choi, E.J.; Kim, Y.S.; Lee, E.J.; Jung, I.D.; Han, H.D.; Wu, T.-C.; Hung, C.-F.; Kang, T.H.; et al. Annexin A5 increases survival in murine sepsis model by inhibiting HMGB1-mediated pro-inflammation and coagulation. Mol. Med. 2016, 22, 424–436. [Google Scholar] [CrossRef]
- Kawasaki, T.; Kawai, T. Toll-Like Receptor Signaling Pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef] [PubMed]
- Presseizen, K.; Friedman, Z.; Shapiro, H.; Radnay, J.; Ellis, M.H. Phosphatidylserine expression on the platelet membrane of patients with myeloproliferative disorders and its effect on platelet-dependent thrombin formation. Clin. Appl. Thromb. Hemost. 2002, 8, 33–39. [Google Scholar] [CrossRef]
- Ma, R.; Xie, R.; Yu, C.; Si, Y.; Wu, X.; Zhao, L.; Yao, Z.; Fang, S.; Chen, H.; Novakovic, V.; et al. Phosphatidylserine-mediated platelet clearance by endothelium decreases platelet aggregates and procoagulant activity in sepsis. Sci. Rep. 2017, 7, 4978. [Google Scholar] [CrossRef]
- Bach, R.; Gentry, R.; Nemerson, Y. Factor VII binding to tissue factor in reconstituted phospholipid vesicles: Induction of cooperativity by phosphatidylserine. Biochemistry 1986, 25, 4007–4020. [Google Scholar] [CrossRef] [PubMed]
- Argañaraz, G.A.; Palmeira, J.d.F.; Argañaraz, E.R. Phosphatidylserine inside out: A possible underlying mechanism in the inflammation and coagulation abnormalities of COVID-19. Cell Commun. Signal. 2020, 18, 190. [Google Scholar] [CrossRef] [PubMed]
- Burger, D.; Schock, S.; Thompson, C.S.; Montezano, A.C.; Hakim, A.M.; Touyz, R.M. Microparticles: Biomarkers and beyond. Clin. Sci. 2013, 124, 423–441. [Google Scholar] [CrossRef]
- Andres, J.; Smith, L.C.; Murray, A.; Jin, Y.; Businaro, R.; Laskin, J.D.; Laskin, D.L. Role of extracellular vesicles in cell-cell communication and inflammation following exposure to pulmonary toxicants. Cytokine Growth Factor Rev. 2020, 51, 12–18. [Google Scholar] [CrossRef]
- Zarenezhad, E.; Abdulabbas, H.T.; Marzi, M.; Ghazy, E.; Ekrahi, M.; Pezeshki, B.; Ghasemian, A.; Moawad, A.A. Nickel Nanoparticles: Applications and Antimicrobial Role against Methicillin-Resistant Staphylococcus aureus Infections. Antibiotics 2022, 11, 1208. [Google Scholar] [CrossRef] [PubMed]
- Zarenezhad, E.; Kanaan, M.H.G.; Abdollah, S.S.; Vakil, M.K.; Marzi, M.; Mazarzaei, A.; Ghasemian, A. Metallic Nanoparticles: Their Potential Role in Breast Cancer Immunotherapy via Trained Immunity Provocation. Biomedicines 2023, 11, 1245. [Google Scholar] [CrossRef]
- Zhang, Y.; Meng, H.; Ma, R.; He, Z.; Wu, X.; Cao, M.; Yao, Z.; Zhao, L.; Li, T.; Deng, R.; et al. Circulating Microparticles, Blood Cells, and Endothelium Induce Procoagulant Activity in Sepsis Through Phosphatidylserine Exposure. Shock 2016, 45, 299–307. [Google Scholar] [CrossRef]
- Sinauridze, E.I.; Kireev, D.A.; Popenko, N.Y.; Pichugin, A.V.; Panteleev, M.A.; Krymskaya, O.V.; Ataullakhanov, F.I. Platelet microparticle membranes have 50- to 100-fold higher specific procoagulant activity than activated platelets. Thromb. Haemost. 2007, 97, 425–434. [Google Scholar] [PubMed]
- Kay, J.G.; Grinstein, S. Phosphatidylserine-mediated cellular signaling. Adv. Exp. Med. Biol. 2013, 991, 177–193. [Google Scholar] [PubMed]
- Whitlock, J.M.; Chernomordik, L.V. Flagging fusion: Phosphatidylserine signaling in cell–cell fusion. J. Biol. Chem. 2021, 296, 100411. [Google Scholar] [CrossRef] [PubMed]
- Pomorski, T.G.; Menon, A.K. Lipid somersaults: Uncovering the mechanisms of protein-mediated lipid flipping. Prog. Lipid Res. 2016, 64, 69–84. [Google Scholar] [CrossRef]
- Sakuragi, T.; Nagata, S. Regulation of phospholipid distribution in the lipid bilayer by flippases and scramblases. Nat. Rev. Mol. Cell Biol. 2023, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Cheng, X.; Tang, Y.; Qiu, X.; Wang, Y.; Kang, H.; Wu, J.; Wang, Z.; Liu, Y.; Chen, F.; et al. Bacterial Endotoxin Activates the Coagulation Cascade through Gasdermin D-Dependent Phosphatidylserine Exposure. Immunity 2019, 51, 983–996.e6. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yu, C.; Zhuang, J.; Qi, W.; Jiang, J.; Liu, X.; Zhao, W.; Cao, Y.; Wu, H.; Qi, J.; et al. The role of phosphatidylserine on the membrane in immunity and blood coagulation. Biomark. Res. 2022, 10, 4. [Google Scholar] [CrossRef]
- Cauvi, D.M.; Hawisher, D.; Dores-Silva, P.R.; Lizardo, R.E.; De Maio, A. Macrophage reprogramming by negatively charged membrane phospholipids controls infection. FASEB J. 2019, 33, 2995–3009. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, B.; Chen, G.; Zhang, Y.; Wang, Q.; Zhao, L.; Zhou, H. Cryopreserved platelets augment the inflammatory response: Role of phosphatidylserine- and P-selectin–mediated platelet phagocytosis in macrophages. Transfusion 2019, 59, 1799–1808. [Google Scholar] [CrossRef] [PubMed]
- Schrottmaier, W.C.; Kral, J.B.; Zeitlinger, M.; Salzmann, M.; Jilma, B.; Assinger, A. Platelet activation at the onset of human endotoxemia is undetectable in vivo. Platelets 2016, 27, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Han, J.; Welch, E.J.; Ye, R.D.; Voyno-Yasenetskaya, T.A.; Malik, A.B.; Du, X.; Li, Z. LPS stimulates platelet secretion and potentiates platelet aggregation via TLR4/MyD88 and the cGMP-dependent protein kinase pathway. J. Immunol. 2009, 182, 7997–8004. [Google Scholar] [CrossRef] [PubMed]
- Assinger, A.; Schrottmaier, W.C.; Salzmann, M.; Rayes, J. Platelets in Sepsis: An Update on Experimental Models and Clinical Data. Front. Immunol. 2019, 10, 1687. [Google Scholar] [CrossRef] [PubMed]
- Scherlinger, M.; Richez, C.; Tsokos, G.C.; Boilard, E.; Blanco, P. The role of platelets in immune-mediated inflammatory diseases. Nat. Rev. Immunol. 2023, 1–16. [Google Scholar] [CrossRef]
- Aslam, R.; Speck, E.R.; Kim, M.; Crow, A.R.; Bang, K.W.A.; Nestel, F.P.; Ni, H.; Lazarus, A.H.; Freedman, J.; Semple, J.W. Platelet Toll-like receptor expression modulates lipopolysaccharide-induced thrombocytopenia and tumor necrosis factor-α production in vivo. Blood 2006, 107, 637–641. [Google Scholar] [CrossRef]
- Wei, H.; Malcor, J.-D.M.; Harper, M.T. Lipid rafts are essential for release of phosphatidylserine-exposing extracellular vesicles from platelets. Sci. Rep. 2018, 8, 9987. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, A.; Takahashi, Y.; Ogata, K.; Kitamura, S.; Nakagawa, N.; Yamamoto, A.; Ishihama, Y.; Takakura, Y. Phosphatidylserine-deficient small extracellular vesicle is a major somatic cell-derived sEV subpopulation in blood. iScience 2021, 24, 102839. [Google Scholar] [CrossRef] [PubMed]
- Burger, D.; Montezano, A.C.; Nishigaki, N.; He, Y.; Carter, A.; Touyz, R.M. Endothelial Microparticle Formation by Angiotensin II Is Mediated via Ang II Receptor Type I/NADPH Oxidase/ Rho Kinase Pathways Targeted to Lipid Rafts. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1898–1907. [Google Scholar] [CrossRef] [PubMed]
- Šibíková, M.; Živný, J.; Janota, J. Cell Membrane-Derived Microvesicles in Systemic Inflammatory Response. Folia Biol. 2018, 64, 113–124. [Google Scholar]
- Joffre, J.; Hellman, J.; Ince, C.; Ait-Oufella, H. Endothelial Responses in Sepsis. Am. J. Respir. Crit. Care Med. 2020, 202, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Abolbaghaei, A.; Turner, M.; Thibodeau, J.-F.; Holterman, C.E.; Kennedy, C.R.J.; Burger, D. The Proteome of Circulating Large Extracellular Vesicles in Diabetes and Hypertension. Int. J. Mol. Sci. 2023, 24, 4930. [Google Scholar] [CrossRef]
- Gomes, J.; Lucien, F.; Cooper, T.T.; Kim, Y.; Williams, K.C.; Liao, X.; Kaufman, L.; Lagugné-Labarthet, F.; Kenyon, O.; Boysen, J.; et al. Analytical Considerations in Nanoscale Flow Cytometry of Extracellular Vesicles to Achieve Data Linearity. Thromb. Haemost. 2018, 118, 1612–1624. [Google Scholar] [CrossRef] [PubMed]
- Tschirhart, B.J.; Lu, X.; Feng, Q. Abstract 14208: Annexin A5 Inhibits Endothelial Inflammation Induced by Lipopolysaccharide-Activated Platelets and Extracellular Vesicles. Circulation 2021, 144, A14208. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tschirhart, B.J.; Lu, X.; Gomes, J.; Chandrabalan, A.; Bell, G.; Hess, D.A.; Xing, G.; Ling, H.; Burger, D.; Feng, Q. Annexin A5 Inhibits Endothelial Inflammation Induced by Lipopolysaccharide-Activated Platelets and Microvesicles via Phosphatidylserine Binding. Pharmaceuticals 2023, 16, 837. https://doi.org/10.3390/ph16060837
Tschirhart BJ, Lu X, Gomes J, Chandrabalan A, Bell G, Hess DA, Xing G, Ling H, Burger D, Feng Q. Annexin A5 Inhibits Endothelial Inflammation Induced by Lipopolysaccharide-Activated Platelets and Microvesicles via Phosphatidylserine Binding. Pharmaceuticals. 2023; 16(6):837. https://doi.org/10.3390/ph16060837
Chicago/Turabian StyleTschirhart, Brent J., Xiangru Lu, Janice Gomes, Arundhasa Chandrabalan, Gillian Bell, David A. Hess, Guangxin Xing, Hong Ling, Dylan Burger, and Qingping Feng. 2023. "Annexin A5 Inhibits Endothelial Inflammation Induced by Lipopolysaccharide-Activated Platelets and Microvesicles via Phosphatidylserine Binding" Pharmaceuticals 16, no. 6: 837. https://doi.org/10.3390/ph16060837
APA StyleTschirhart, B. J., Lu, X., Gomes, J., Chandrabalan, A., Bell, G., Hess, D. A., Xing, G., Ling, H., Burger, D., & Feng, Q. (2023). Annexin A5 Inhibits Endothelial Inflammation Induced by Lipopolysaccharide-Activated Platelets and Microvesicles via Phosphatidylserine Binding. Pharmaceuticals, 16(6), 837. https://doi.org/10.3390/ph16060837







