Multifaceted Roles of GLP-1 and Its Analogs: A Review on Molecular Mechanisms with a Cardiotherapeutic Perspective
Abstract
1. Introduction
2. GLP-1 Analogs and Physiological Importance in Humans
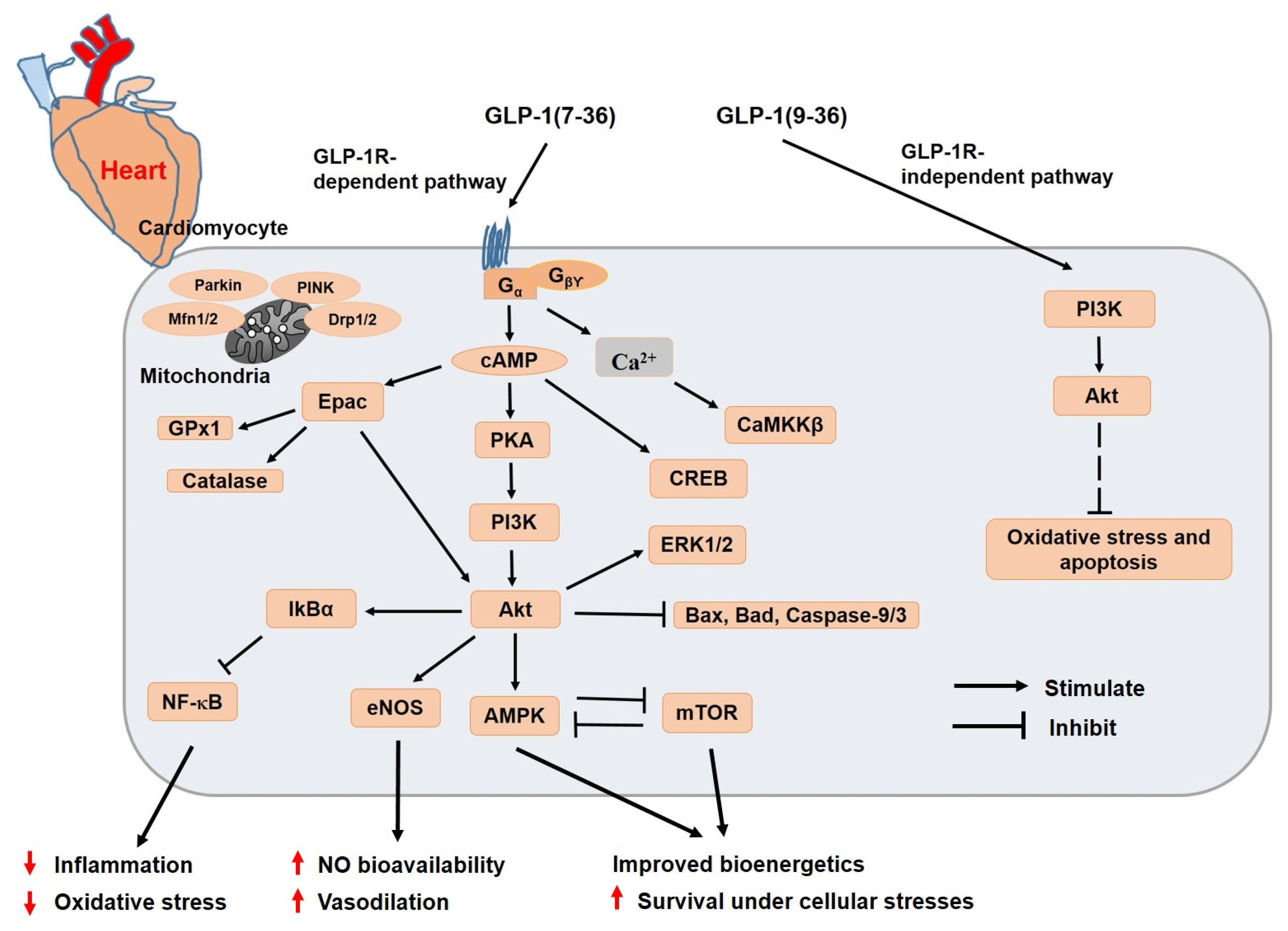
3. Molecular Mechanisms Underlying Cardioprotective Effects of GLP-1 and Its Analogs
3.1. GLP-1 and Its Analogs and Cardiac Functions
3.2. GLP-1 and Its Analogs and Myocardial Glucose Uptake
3.3. GLP-1 Analogs and Cardiac Oxidative Stress as Well as Ischemia/Reperfusion (I/R) Injury
4. Significant Roles of GLP-1 and Its Analogs in Mitochondrial Homeostasis
5. Co-Agonists: A Single Molecule Stimulating Multiple Peptide Receptors
6. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Del Olmo-Garcia, M.I.; Merino-Torres, J.F. GLP-1 receptor agonists and cardiovascular disease in patients with type 2 diabetes. J. Diabetes Res. 2018, 2018, 4020492. [Google Scholar] [CrossRef]
- Jia, G.; Hill, M.A.; Sowers, J.R. Diabetic cardiomyopathy: An update of mechanisms contributing to this clinical entity. Circ. Res. 2018, 122, 624–638. [Google Scholar] [CrossRef]
- Campbell, J.E.; Drucker, D.J. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 2013, 17, 819–837. [Google Scholar] [CrossRef]
- Graaf, C.; Donnelly, D.; Wootten, D.; Lau, J.; Sexton, P.M.; Miller, L.J.; Ahn, J.M.; Liao, J.; Fletcher, M.M.; Yang, D.; et al. Glucagon-like peptide-1 and its class B G protein-coupled receptors: A long march to therapeutic successes. Pharmacol. Rev. 2016, 68, 954–1013. [Google Scholar] [CrossRef]
- Moore, B. On the treatment of diabetus mellitus by acid extract of duodenal mucous membrane. Biochem. J. 1906, 1, 28–38. [Google Scholar] [CrossRef]
- Brown, J.C.; Dryburgh, J.R.; Ross, S.A.; Dupre, J. Identification and actions of gastric inhibitory polypeptide. Recent Prog. Horm. Res. 1975, 31, 487–532. [Google Scholar] [CrossRef]
- Sarson, D.L.; Wood, S.M.; Kansal, P.C.; Bloom, S.R. Glucose-dependent insulinotropic polypeptide augmentation of insulin. Physiology or pharmacology? Diabetes 1984, 33, 389–393. [Google Scholar] [CrossRef]
- Bell, G.I.; Santerre, R.F.; Mullenbach, G.T. Hamster preproglucagon contains the sequence of glucagon and two related peptides. Nature 1983, 302, 716–718. [Google Scholar] [CrossRef]
- Shields, D.; Warren, T.G.; Roth, S.E.; Brenner, M.J. Cell-free synthesis and processing of multiple precursors to glucagon. Nature 1981, 289, 511–514. [Google Scholar] [CrossRef]
- Orskov, C.; Bersani, M.; Johnsen, A.H.; Hojrup, P.; Holst, J.J. Complete sequences of glucagon-like peptide-1 from human and pig small intestine. J. Biol. Chem. 1989, 264, 12826–12829. [Google Scholar] [CrossRef]
- Sandoval, D.A.; D’Alessio, D.A. Physiology of proglucagon peptides: Role of glucagon and GLP-1 in health and disease. Physiol. Rev. 2015, 95, 513–548. [Google Scholar] [CrossRef]
- Doyle, M.E.; Egan, J.M. Mechanisms of action of glucagon-like peptide 1 in the pancreas. Pharmacol. Ther. 2007, 113, 546–593. [Google Scholar] [CrossRef]
- Meloni, A.R.; DeYoung, M.B.; Lowe, C.; Parkes, D.G. GLP-1 receptor activated insulin secretion from pancreatic beta-cells: Mechanism and glucose dependence. Diabetes Obes. Metab. 2013, 15, 15–27. [Google Scholar] [CrossRef]
- Rolin, B.; Deacon, C.F.; Carr, R.D.; Ahren, B. The major glucagon-like peptide-1 metabolite, GLP-1-(9-36)-amide, does not affect glucose or insulin levels in mice. Eur. J. Pharmacol. 2004, 494, 283–288. [Google Scholar] [CrossRef]
- Nuamnaichati, N.; Parichatikanond, W.; Mangmool, S. Cardioprotective effects of glucagon-like peptide-1 (9-36) against oxidative injury in H9c2 cardiomyoblasts: Potential role of the PI3K/Akt/NOS pathway. J. Cardiovasc. Pharmacol. 2022, 79, e50–e63. [Google Scholar] [CrossRef]
- Nikolaidis, L.A.; Elahi, D.; Shen, Y.T.; Shannon, R.P. Active metabolite of GLP-1 mediates myocardial glucose uptake and improves left ventricular performance in conscious dogs with dilated cardiomyopathy. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H2401–H2408. [Google Scholar] [CrossRef]
- Picatoste, B.; Ramirez, E.; Caro-Vadillo, A.; Iborra, C.; Ares-Carrasco, S.; Egido, J.; Tunon, J.; Lorenzo, O. Sitagliptin reduces cardiac apoptosis, hypertrophy and fibrosis primarily by insulin-dependent mechanisms in experimental type-II diabetes. Potential roles of GLP-1 isoforms. PLoS ONE 2013, 8, e78330. [Google Scholar] [CrossRef]
- Poudyal, H. Mechanisms for the cardiovascular effects of glucagon-like peptide-1. Acta Physiol. 2016, 216, 277–313. [Google Scholar] [CrossRef]
- Deacon, C.F.; Johnsen, A.H.; Holst, J.J. Degradation of glucagon-like peptide-1 by human plasma in vitro yields an N-terminally truncated peptide that is a major endogenous metabolite in vivo. J. Clin. Endocrinol. Metab. 1995, 80, 952–957. [Google Scholar] [CrossRef]
- Pabreja, K.; Mohd, M.A.; Koole, C.; Wootten, D.; Furness, S.G. Molecular mechanisms underlying physiological and receptor pleiotropic effects mediated by GLP-1R activation. Br. J. Pharmacol. 2014, 171, 1114–1128. [Google Scholar] [CrossRef]
- Tomas, E.; Habener, J.F. Insulin-like actions of glucagon-like peptide-1: A dual receptor hypothesis. Trends Endocrinol. Metab. 2010, 21, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Stanojevic, V.; Brindamour, L.J.; Habener, J.F. GLP1-derived nonapeptide GLP1(28-36)amide protects pancreatic beta-cells from glucolipotoxicity. J. Endocrinol. 2012, 213, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Tomas, E.; Stanojevic, V.; Habener, J.F. GLP-1-derived nonapeptide GLP-1(28-36)amide targets to mitochondria and suppresses glucose production and oxidative stress in isolated mouse hepatocytes. Regul. Pept. 2011, 167, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.A.; Vardarli, I.; Deacon, C.F.; Holst, J.J.; Meier, J.J. Secretion of glucagon-like peptide-1 (GLP-1) in type 2 diabetes: What is up, what is down? Diabetologia 2011, 54, 10–18. [Google Scholar] [CrossRef]
- Sonne, D.P.; Rehfeld, J.F.; Holst, J.J.; Vilsboll, T.; Knop, F.K. Postprandial gallbladder emptying in patients with type 2 diabetes: Potential implications for bile-induced secretion of glucagon-like peptide 1. Eur. J. Endocrinol. 2014, 171, 407–419. [Google Scholar] [CrossRef]
- Guglielmi, V.; Sbraccia, P. GLP-1 receptor independent pathways: Emerging beneficial effects of GLP-1 breakdown products. Eat. Weight Disord. 2017, 22, 231–240. [Google Scholar] [CrossRef]
- Gupta, V. Glucagon-like peptide-1 analogues: An overview. Indian J. Endocrinol. Metab. 2013, 17, 413–421. [Google Scholar] [CrossRef]
- Kalra, S.; Baruah, M.P.; Sahay, R.K.; Unnikrishnan, A.G.; Uppal, S.; Adetunji, O. Glucagon-like peptide-1 receptor agonists in the treatment of type 2 diabetes: Past, present, and future. Indian J. Endocrinol. Metab. 2016, 20, 254–267. [Google Scholar] [CrossRef]
- Gilbert, M.P.; Pratley, R.E. GLP-1 analogs and DPP-4 inhibitors in type 2 diabetes therapy: Review of head-to-head clinical trials. Front. Endocrinol. 2020, 11, 178. [Google Scholar] [CrossRef]
- Htike, Z.Z.; Zaccardi, F.; Papamargaritis, D.; Webb, D.R.; Khunti, K.; Davies, M.J. Efficacy and safety of glucagon-like peptide-1 receptor agonists in type 2 diabetes: A systematic review and mixed-treatment comparison analysis. Diabetes Obes. Metab. 2017, 19, 524–536. [Google Scholar] [CrossRef]
- MacDonald, P.E.; El-Kholy, W.; Riedel, M.J.; Salapatek, A.M.; Light, P.E.; Wheeler, M.B. The multiple actions of GLP-1 on the process of glucose-stimulated insulin secretion. Diabetes 2002, 51 (Suppl. S3), S434–S442. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Dong, X.; Fisher, T.L.; Dunn, S.; Omer, A.K.; Weir, G.; White, M.F. Exendin-4 uses Irs2 signaling to mediate pancreatic beta cell growth and function. J. Biol. Chem. 2006, 281, 1159–1168. [Google Scholar] [CrossRef] [PubMed]
- Portha, B.; Tourrel-Cuzin, C.; Movassat, J. Activation of the GLP-1 receptor signalling pathway: A relevant strategy to repair a deficient beta-cell mass. Exp. Diabetes Res. 2011, 2011, 376509. [Google Scholar] [CrossRef]
- Meurot, C.; Jacques, C.; Martin, C.; Sudre, L.; Breton, J.; Rattenbach, R.; Bismuth, K.; Berenbaum, F. Targeting the GLP-1/GLP-1R axis to treat osteoarthritis: A new opportunity? J. Orthop. Translat. 2022, 32, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Reed, J.; Bain, S.; Kanamarlapudi, V. Recent advances in understanding the role of glucagon-like peptide 1. F1000Res 2020, 9, 239. [Google Scholar] [CrossRef]
- Peng, W.; Zhou, R.; Sun, Z.F.; Long, J.W.; Gong, Y.Q. Novel insights into the roles and mechanisms of GLP-1 receptor agonists against aging-related diseases. Aging Dis. 2022, 13, 468–490. [Google Scholar] [CrossRef]
- Pratley, R.E.; Jacob, S.; Baek, S.; Trautmann, M.E.; Hompesch, M.; Han, O.; Stewart, J.; Sorli, C.H.; Shaunik, A.; Yoon, K.H. Efficacy and safety of efpeglenatide in key patient subgroups from the BALANCE randomized trial, stratified by pre-diabetes status, BMI, and age at baseline. BMJ Open Diabetes Res. Care 2022, 10, e002207. [Google Scholar] [CrossRef]
- Andersen, A.; Lund, A.; Knop, F.K.; Vilsboll, T. Glucagon-like peptide 1 in health and disease. Nat. Rev. Endocrinol. 2018, 14, 390–403. [Google Scholar] [CrossRef]
- Sposito, A.C.; Berwanger, O.; de Carvalho, L.S.F.; Saraiva, J.F.K. GLP-1RAs in type 2 diabetes: Mechanisms that underlie cardiovascular effects and overview of cardiovascular outcome data. Cardiovasc. Diabetol. 2018, 17, 157. [Google Scholar] [CrossRef]
- Wettergren, A.; Schjoldager, B.; Mortensen, P.E.; Myhre, J.; Christiansen, J.; Holst, J.J. Truncated GLP-1 (proglucagon 78-107-amide) inhibits gastric and pancreatic functions in man. Dig. Dis. Sci. 1993, 38, 665–673. [Google Scholar] [CrossRef]
- Maljaars, P.W.; Peters, H.P.; Mela, D.J.; Masclee, A.A. Ileal brake: A sensible food target for appetite control. A review. Physiol. Behav. 2008, 95, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Schirra, J.; Nicolaus, M.; Roggel, R.; Katschinski, M.; Storr, M.; Woerle, H.J.; Goke, B. Endogenous glucagon-like peptide 1 controls endocrine pancreatic secretion and antro-pyloro-duodenal motility in humans. Gut 2006, 55, 243–251. [Google Scholar] [CrossRef]
- Wettergren, A.; Petersen, H.; Orskov, C.; Christiansen, J.; Sheikh, S.P.; Holst, J.J. Glucagon-like peptide-1 7-36 amide and peptide YY from the L-cell of the ileal mucosa are potent inhibitors of vagally induced gastric acid secretion in man. Scand. J. Gastroenterol. 1994, 29, 501–505. [Google Scholar] [CrossRef]
- Rowlands, J.; Heng, J.; Newsholme, P.; Carlessi, R. Pleiotropic effects of GLP-1 and analogs on cell signaling, metabolism, and function. Front. Endocrinol. 2018, 9, 672. [Google Scholar] [CrossRef]
- Papaetis, G.S.; Kyriacou, A. GLP-1 receptor agonists, polycystic ovary syndrome and reproductive dysfunction: Current research and future horizons. Adv. Clin. Exp. Med. 2022, 31, 1265–1274. [Google Scholar] [CrossRef] [PubMed]
- Daniels, D.; Mietlicki-Baase, E.G. Glucagon-Like Peptide 1 in the Brain: Where Is It Coming From, Where Is It Going? Diabetes 2019, 68, 15–17. [Google Scholar] [CrossRef]
- Holst, J.J. The physiology of glucagon-like peptide 1. Physiol. Rev. 2007, 87, 1409–1439. [Google Scholar] [CrossRef] [PubMed]
- Heppner, K.M.; Perez-Tilve, D. GLP-1 based therapeutics: Simultaneously combating T2DM and obesity. Front. Neurosci. 2015, 9, 92. [Google Scholar] [CrossRef]
- Perry, T.; Lahiri, D.K.; Sambamurti, K.; Chen, D.; Mattson, M.P.; Egan, J.M.; Greig, N.H. Glucagon-like peptide-1 decreases endogenous amyloid-beta peptide (Abeta) levels and protects hippocampal neurons from death induced by Abeta and iron. J. Neurosci. Res. 2003, 72, 603–612. [Google Scholar] [CrossRef]
- Li, Y.; Perry, T.; Kindy, M.S.; Harvey, B.K.; Tweedie, D.; Holloway, H.W.; Powers, K.; Shen, H.; Egan, J.M.; Sambamurti, K.; et al. GLP-1 receptor stimulation preserves primary cortical and dopaminergic neurons in cellular and rodent models of stroke and Parkinsonism. Proc. Natl. Acad. Sci. USA 2009, 106, 1285–1290. [Google Scholar] [CrossRef]
- Coskun, T.; Sloop, K.W.; Loghin, C.; Alsina-Fernandez, J.; Urva, S.; Bokvist, K.B.; Cui, X.; Briere, D.A.; Cabrera, O.; Roell, W.C.; et al. LY3298176, a novel dual GIP and GLP-1 receptor agonist for the treatment of type 2 diabetes mellitus: From discovery to clinical proof of concept. Mol. Metab. 2018, 18, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chigurupati, S.; Holloway, H.W.; Mughal, M.; Tweedie, D.; Bruestle, D.A.; Mattson, M.P.; Wang, Y.; Harvey, B.K.; Ray, B.; et al. Exendin-4 ameliorates motor neuron degeneration in cellular and animal models of amyotrophic lateral sclerosis. PLoS ONE 2012, 7, e32008. [Google Scholar] [CrossRef] [PubMed]
- Baggio, L.L.; Drucker, D.J. Glucagon-like peptide-1 receptors in the brain: Controlling food intake and body weight. J. Clin. Investig. 2014, 124, 4223–4226. [Google Scholar] [CrossRef]
- Burcelin, R.; Da Costa, A.; Drucker, D.; Thorens, B. Glucose competence of the hepatoportal vein sensor requires the presence of an activated glucagon-like peptide-1 receptor. Diabetes 2001, 50, 1720–1728. [Google Scholar] [CrossRef]
- Knauf, C.; Cani, P.D.; Kim, D.H.; Iglesias, M.A.; Chabo, C.; Waget, A.; Colom, A.; Rastrelli, S.; Delzenne, N.M.; Drucker, D.J.; et al. Role of central nervous system glucagon-like peptide-1 receptors in enteric glucose sensing. Diabetes 2008, 57, 2603–2612. [Google Scholar] [CrossRef]
- Almutairi, M.; Al Batran, R.; Ussher, J.R. Glucagon-like peptide-1 receptor action in the vasculature. Peptides 2019, 111, 26–32. [Google Scholar] [CrossRef]
- Forst, T.; Weber, M.M.; Pfutzner, A. Cardiovascular benefits of GLP-1-based herapies in patients with diabetes mellitus type 2: Effects on endothelial and vascular dysfunction beyond glycemic control. Exp. Diabetes Res. 2012, 2012, 635472. [Google Scholar] [CrossRef]
- Reed, J.; Bain, S.; Kanamarlapudi, V. A review of current trends with type 2 diabetes epidemiology, aetiology, pathogenesis, treatments and future perspectives. Diabetes Metab. Syndr. Obes. 2021, 14, 3567–3602. [Google Scholar] [CrossRef]
- Drucker, D.J. The cardiovascular biology of glucagon-like peptide-1. Cell Metab. 2016, 24, 15–30. [Google Scholar] [CrossRef]
- Hadjiyanni, I.; Siminovitch, K.A.; Danska, J.S.; Drucker, D.J. Glucagon-like peptide-1 receptor signalling selectively regulates murine lymphocyte proliferation and maintenance of peripheral regulatory T cells. Diabetologia 2010, 53, 730–740. [Google Scholar] [CrossRef]
- Panjwani, N.; Mulvihill, E.E.; Longuet, C.; Yusta, B.; Campbell, J.E.; Brown, T.J.; Streutker, C.; Holland, D.; Cao, X.; Baggio, L.L.; et al. GLP-1 receptor activation indirectly reduces hepatic lipid accumulation but does not attenuate development of atherosclerosis in diabetic male ApoE(-/-) mice. Endocrinology 2013, 154, 127–139. [Google Scholar] [CrossRef]
- Xu, X.; Lin, L.; Chen, P.; Yu, Y.; Chen, S.; Chen, X.; Shao, Z. Treatment with liraglutide, a glucagon-like peptide-1 analogue, improves effectively the skin lesions of psoriasis patients with type 2 diabetes: A prospective cohort study. Diabetes Res. Clin. Pract. 2019, 150, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Noyan-Ashraf, M.H.; Shikatani, E.A.; Schuiki, I.; Mukovozov, I.; Wu, J.; Li, R.K.; Volchuk, A.; Robinson, L.A.; Billia, F.; Drucker, D.J.; et al. A glucagon-like peptide-1 analog reverses the molecular pathology and cardiac dysfunction of a mouse model of obesity. Circulation 2013, 127, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.; Marso, S.P.; Neeland, I.J. Liraglutide for weight management: A critical review of the evidence. Obes. Sci. Pract. 2017, 3, 3–14. [Google Scholar] [CrossRef]
- Whitten, J.S. Liraglutide (Saxenda) for weight loss. Am. Fam. Physician 2016, 94, 161–166. [Google Scholar]
- Koehler, J.A.; Baggio, L.L.; Lamont, B.J.; Ali, S.; Drucker, D.J. Glucagon-like peptide-1 receptor activation modulates pancreatitis-associated gene expression but does not modify the susceptibility to experimental pancreatitis in mice. Diabetes 2009, 58, 2148–2161. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Pinzon, W.L.; Power, R.F.; Yan, Y.; Wasserfall, C.; Atkinson, M.; Rabinovitch, A. Combination therapy with glucagon-like peptide-1 and gastrin restores normoglycemia in diabetic NOD mice. Diabetes 2008, 57, 3281–3288. [Google Scholar] [CrossRef]
- Muskiet, M.H.A.; Tonneijck, L.; Smits, M.M.; van Baar, M.J.B.; Kramer, M.H.H.; Hoorn, E.J.; Joles, J.A.; van Raalte, D.H. GLP-1 and the kidney: From physiology to pharmacology and outcomes in diabetes. Nat. Rev. Nephrol. 2017, 13, 605–628. [Google Scholar] [CrossRef]
- Skov, J. Effects of GLP-1 in the kidney. Rev. Endocr. Metab. Disord. 2014, 15, 197–207. [Google Scholar] [CrossRef]
- Nuamnaichati, N.; Mangmool, S.; Chattipakorn, N.; Parichatikanond, W. Stimulation of GLP-1 receptor inhibits methylglyoxal-induced mitochondrial dysfunctions in H9c2 cardiomyoblasts: Potential role of Epac/PI3K/Akt pathway. Front. Pharmacol. 2020, 11, 805. [Google Scholar] [CrossRef]
- Mangmool, S.; Hemplueksa, P.; Parichatikanond, W.; Chattipakorn, N. Epac is required for GLP-1R-mediated inhibition of oxidative stress and apoptosis in cardiomyocytes. Mol. Endocrinol. 2015, 29, 583–596. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; He, X.; Chen, Y.; Huang, Y.; Wu, L.; He, J. Exendin-4 attenuates cardiac hypertrophy via AMPK/mTOR signaling pathway activation. Biochem. Biophys. Res. Commun. 2015, 468, 394–399. [Google Scholar] [CrossRef]
- He, W.; Tong, G.; Fan, H.; Zhen, C.; Zeng, L.; Xue, L.; Chen, J.; Sun, Z.; He, P. Exendin-4 alleviates myocardial ischemia reperfusion injury by enhancing autophagy through promoting nuclear translocation of TFEB. Exp. Cell Res. 2023, 423, 113469. [Google Scholar] [CrossRef] [PubMed]
- Lyu, J.; Imachi, H.; Fukunaga, K.; Sato, S.; Kobayashi, T.; Saheki, T.; Japar, S.; Iwama, H.; Matsumura, Y.; Ozaki, M.; et al. Exendin-4 increases scavenger receptor class BI expression via activation of AMPK/FoxO1 in human vascular endothelial cells. Curr. Issues Mol. Biol. 2022, 44, 5474–5484. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.H.; Chen, Y.C.; Lee, T.I.; Kao, Y.H.; Chazo, T.F.; Chen, S.A.; Chen, Y.J. Glucagon-like peptide-1 regulates calcium homeostasis and electrophysiological activities of HL-1 cardiomyocytes. Peptides 2016, 78, 91–98. [Google Scholar] [CrossRef]
- Wang, J.; Fan, S.; Xiong, Q.; Niu, Y.; Zhang, X.; Qin, J.; Shi, Y.; Zhang, L. Glucagon-like peptide-1 attenuates cardiac hypertrophy via the AngII/AT1R/ACE2 and AMPK/mTOR/p70S6K pathways. Acta Biochim. Biophys. Sin. 2021, 53, 1189–1197. [Google Scholar] [CrossRef]
- Li, Q.; Tuo, X.; Li, B.; Deng, Z.; Qiu, Y.; Xie, H. Semaglutide attenuates excessive exercise-induced myocardial injury through inhibiting oxidative stress and inflammation in rats. Life Sci. 2020, 250, 117531. [Google Scholar] [CrossRef]
- Zhu, Q.; Luo, Y.; Wen, Y.; Wang, D.; Li, J.; Fan, Z. Semaglutide inhibits ischemia/reperfusion-induced cardiomyocyte apoptosis through activating PKG/PKCepsilon/ERK1/2 pathway. Biochem. Biophys. Res. Commun. 2023, 647, 1–8. [Google Scholar] [CrossRef]
- Chen, J.; Wang, D.; Wang, F.; Shi, S.; Chen, Y.; Yang, B.; Tang, Y.; Huang, C. Exendin-4 inhibits structural remodeling and improves Ca2+ homeostasis in rats with heart failure via the GLP-1 receptor through the eNOS/cGMP/PKG pathway. Peptides 2017, 90, 69–77. [Google Scholar] [CrossRef]
- Chen, J.; Xu, S.; Wang, L.; Zhou, W.; Li, P.; Deng, N.; Tang, Q.; Li, Y.; Wu, L.; Chen, J.; et al. Exendin-4 inhibits atrial arrhythmogenesis in a model of myocardial infarction-induced heart failure via the GLP-1 receptor signaling pathway. Exp. Ther. Med. 2020, 20, 3669–3678. [Google Scholar] [CrossRef]
- Wei, H.; Bu, R.; Yang, Q.; Jia, J.; Li, T.; Wang, Q.; Chen, Y. Exendin-4 protects against hyperglycemia-induced cardiomyocyte pyroptosis via the AMPK-TXNIP pathway. J. Diabetes Res. 2019, 2019, 8905917. [Google Scholar] [CrossRef] [PubMed]
- Eid, R.A.; Khalil, M.A.; Alkhateeb, M.A.; Eleawa, S.M.; Zaki, M.S.A.; El-Kott, A.F.; Al-Shraim, M.; El-Sayed, F.; Eldeen, M.A.; Bin-Meferij, M.M.; et al. Exendin-4 attenuates remodeling in the remote myocardium of rats after an acute myocardial infarction by activating beta-arrestin-2, protein phosphatase 2A, and glycogen synthase kinase-3 and inhibiting beta-catenin. Cardiovasc. Drugs Ther. 2021, 35, 1095–1110. [Google Scholar] [CrossRef] [PubMed]
- Wassef, M.A.E.; Tork, O.M.; Rashed, L.A.; Ibrahim, W.; Morsi, H.; Rabie, D.M.M. Mitochondrial dysfunction in diabetic cardiomyopathy: Effect of mesenchymal stem cell with PPAR-gamma agonist or exendin-4. Exp. Clin. Endocrinol. Diabetes 2018, 126, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xu, S.; Zhou, W.; Wu, L.; Wang, L.; Li, W. Exendin-4 reduces ventricular arrhythmia activity and calcium sparks-mediated sarcoplasmic reticulum calcium leak in rats with heart failure. Int. Heart J. 2020, 61, 145–152. [Google Scholar] [CrossRef]
- Piao, L.; Zhao, G.; Zhu, E.; Inoue, A.; Shibata, R.; Lei, Y.; Hu, L.; Yu, C.; Yang, G.; Wu, H.; et al. Chronic psychological stress accelerates vascular senescence and impairs ischemia-induced neovascularization: The role of dipeptidyl peptidase-4/glucagon-like peptide-1-adiponectin axis. J. Am. Heart Assoc. 2017, 6, e006421. [Google Scholar] [CrossRef] [PubMed]
- Katsurada, K.; Nakata, M.; Saito, T.; Zhang, B.; Maejima, Y.; Nandi, S.S.; Sharma, N.M.; Patel, K.P.; Kario, K.; Yada, T. Central glucagon-like peptide-1 receptor signaling via brainstem catecholamine neurons counteracts hypertension in spontaneously hypertensive rats. Sci. Rep. 2019, 9, 12986. [Google Scholar] [CrossRef]
- Jojima, T.; Uchida, K.; Akimoto, K.; Tomotsune, T.; Yanagi, K.; Iijima, T.; Suzuki, K.; Kasai, K.; Aso, Y. Liraglutide, a GLP-1 receptor agonist, inhibits vascular smooth muscle cell proliferation by enhancing AMP-activated protein kinase and cell cycle regulation, and delays atherosclerosis in ApoE deficient mice. Atherosclerosis 2017, 261, 44–51. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, X.; Yang, L.; Yang, L.; Ma, H. Liraglutide ameliorates myocardial damage in experimental diabetic rats by inhibiting pyroptosis via Sirt1/AMPK signaling. Iran. J. Basic Med. Sci. 2021, 24, 1358–1365. [Google Scholar] [CrossRef]
- Zheng, R.H.; Zhang, W.W.; Ji, Y.N.; Bai, X.J.; Yan, C.P.; Wang, J.; Bai, F.; Zhao, Z.Q. Exogenous supplement of glucagon like peptide-1 protects the heart against aortic banding induced myocardial fibrosis and dysfunction through inhibiting mTOR/p70S6K signaling and promoting autophagy. Eur. J. Pharmacol. 2020, 883, 173318. [Google Scholar] [CrossRef]
- Scheen, A.J. GLP-1 receptor agonists and heart failure in diabetes. Diabetes Metab. 2017, 43 (Suppl. S1), 2S13–2S19. [Google Scholar] [CrossRef]
- Sokos, G.G.; Nikolaidis, L.A.; Mankad, S.; Elahi, D.; Shannon, R.P. Glucagon-like peptide-1 infusion improves left ventricular ejection fraction and functional status in patients with chronic heart failure. J. Card. Fail. 2006, 12, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Lepore, J.J.; Olson, E.; Demopoulos, L.; Haws, T.; Fang, Z.; Barbour, A.M.; Fossler, M.; Davila-Roman, V.G.; Russell, S.D.; Gropler, R.J. Effects of the novel long-acting GLP-1 agonist, albiglutide, on cardiac function, cardiac metabolism, and exercise capacity in patients with chronic heart failure and reduced ejection fraction. JACC Heart Fail. 2016, 4, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Scalzo, R.L.; Moreau, K.L.; Ozemek, C.; Herlache, L.; McMillin, S.; Gilligan, S.; Huebschmann, A.G.; Bauer, T.A.; Dorosz, J.; Reusch, J.E.; et al. Exenatide improves diastolic function and attenuates arterial stiffness but does not alter exercise capacity in individuals with type 2 diabetes. J. Diabetes Complicat. 2017, 31, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Baggio, L.L.; Ussher, J.R.; McLean, B.A.; Cao, X.; Kabir, M.G.; Mulvihill, E.E.; Mighiu, A.S.; Zhang, H.; Ludwig, A.; Seeley, R.J.; et al. The autonomic nervous system and cardiac GLP-1 receptors control heart rate in mice. Mol. Metab. 2017, 6, 1339–1349. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Lee, C.E.; Marcus, J.N.; Williams, T.D.; Overton, J.M.; Lopez, M.E.; Hollenberg, A.N.; Baggio, L.; Saper, C.B.; Drucker, D.J.; et al. Glucagon-like peptide-1 receptor stimulation increases blood pressure and heart rate and activates autonomic regulatory neurons. J. Clin. Investig. 2002, 110, 43–52. [Google Scholar] [CrossRef]
- Ang, R.; Mastitskaya, S.; Hosford, P.S.; Basalay, M.; Specterman, M.; Aziz, Q.; Li, Y.; Orini, M.; Taggart, P.; Lambiase, P.D.; et al. Modulation of cardiac ventricular excitability by GLP-1 (glucagon-like peptide-1). Circ. Arrhythm. Electrophysiol. 2018, 11, e006740. [Google Scholar] [CrossRef] [PubMed]
- Barragan, J.M.; Eng, J.; Rodriguez, R.; Blazquez, E. Neural contribution to the effect of glucagon-like peptide-1-(7-36) amide on arterial blood pressure in rats. Am. J. Physiol. 1999, 277, E784–E791. [Google Scholar] [CrossRef]
- Barragan, J.M.; Rodriguez, R.E.; Blazquez, E. Changes in arterial blood pressure and heart rate induced by glucagon-like peptide-1-(7-36) amide in rats. Am. J. Physiol. 1994, 266, E459–E466. [Google Scholar] [CrossRef]
- Okerson, T.; Yan, P.; Stonehouse, A.; Brodows, R. Effects of exenatide on systolic blood pressure in subjects with type 2 diabetes. Am. J. Hypertens. 2010, 23, 334–339. [Google Scholar] [CrossRef]
- Blonde, L.; Russell-Jones, D. The safety and efficacy of liraglutide with or without oral antidiabetic drug therapy in type 2 diabetes: An overview of the LEAD 1-5 studies. Diabetes Obes. Metab. 2009, 11 (Suppl. S3), 26–34. [Google Scholar] [CrossRef]
- Klonoff, D.C.; Buse, J.B.; Nielsen, L.L.; Guan, X.; Bowlus, C.L.; Holcombe, J.H.; Wintle, M.E.; Maggs, D.G. Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr. Med. Res. Opin. 2008, 24, 275–286. [Google Scholar] [CrossRef]
- Ravassa, S.; Zudaire, A.; Diez, J. GLP-1 and cardioprotection: From bench to bedside. Cardiovasc. Res. 2012, 94, 316–323. [Google Scholar] [CrossRef]
- Wei, Y.; Mojsov, S. Tissue-specific expression of the human receptor for glucagon-like peptide-I: Brain, heart and pancreatic forms have the same deduced amino acid sequences. FEBS Lett. 1995, 358, 219–224. [Google Scholar] [CrossRef]
- Kim, M.; Platt, M.J.; Shibasaki, T.; Quaggin, S.E.; Backx, P.H.; Seino, S.; Simpson, J.A.; Drucker, D.J. GLP-1 receptor activation and Epac2 link atrial natriuretic peptide secretion to control of blood pressure. Nat. Med. 2013, 19, 567–575. [Google Scholar] [CrossRef]
- Rudovich, N.; Pivovarova, O.; Gogebakan, O.; Sparwasser, A.; Doehner, W.; Anker, S.D.; Arafat, A.M.; Bergmann, A.; Nauck, M.A.; Pfeiffer, A.F. Effect of exogenous intravenous administrations of GLP-1 and/or GIP on circulating pro-atrial natriuretic peptide in subjects with different stages of glucose tolerance. Diabetes Care 2015, 38, e7–e8. [Google Scholar] [CrossRef]
- Zhao, T.; Parikh, P.; Bhashyam, S.; Bolukoglu, H.; Poornima, I.; Shen, Y.T.; Shannon, R.P. Direct effects of glucagon-like peptide-1 on myocardial contractility and glucose uptake in normal and postischemic isolated rat hearts. J. Pharmacol. Exp. Ther. 2006, 317, 1106–1113. [Google Scholar] [CrossRef]
- Lu, K.; Chang, G.; Ye, L.; Zhang, P.; Li, Y.; Zhang, D. Protective effects of extendin-4 on hypoxia/reoxygenation-induced injury in H9c2 cells. Mol. Med. Rep. 2015, 12, 3007–3016. [Google Scholar] [CrossRef]
- Gejl, M.; Søndergaard, H.M.; Stecher, C.; Bibby, B.M.; Møller, N.; Bøtker, H.E.; Hansen, S.B.; Gjedde, A.; Rungby, J.; Brock, B. Exenatide alters myocardial glucose transport and uptake depending on insulin resistance and increases myocardial blood flow in patients with type 2 diabetes. J. Clin. Endocrinol. Metab. 2012, 97, E1165–E1169. [Google Scholar] [CrossRef]
- Gejl, M.; Lerche, S.; Mengel, A.; Møller, N.; Bibby, B.M.; Smidt, K.; Brock, B.; Søndergaard, H.; Bøtker, H.E.; Gjedde, A.; et al. Influence of GLP-1 on myocardial glucose metabolism in healthy men during normo- or hypoglycemia. PLoS ONE 2014, 9, e83758. [Google Scholar] [CrossRef]
- Ying, Y.; Zhu, H.; Liang, Z.; Ma, X.; Li, S. GLP1 protects cardiomyocytes from palmitate-induced apoptosis via Akt/GSK3b/b-catenin pathway. J. Mol. Endocrinol. 2015, 55, 245–262. [Google Scholar] [CrossRef]
- Sonne, D.P.; Engstrom, T.; Treiman, M. Protective effects of GLP-1 analogues exendin-4 and GLP-1(9-36) amide against ischemia-reperfusion injury in rat heart. Regul. Pept. 2008, 146, 243–249. [Google Scholar] [CrossRef]
- Timmers, L.; Henriques, J.P.; de Kleijn, D.P.; Devries, J.H.; Kemperman, H.; Steendijk, P.; Verlaan, C.W.; Kerver, M.; Piek, J.J.; Doevendans, P.A.; et al. Exenatide reduces infarct size and improves cardiac function in a porcine model of ischemia and reperfusion injury. J. Am. Coll. Cardiol. 2009, 53, 501–510. [Google Scholar] [CrossRef]
- Nikolaidis, L.A.; Mankad, S.; Sokos, G.G.; Miske, G.; Shah, A.; Elahi, D.; Shannon, R.P. Effects of glucagon-like peptide-1 in patients with acute myocardial infarction and left ventricular dysfunction after successful reperfusion. Circulation 2004, 109, 962–965. [Google Scholar] [CrossRef]
- Guan, G.; Zhang, J.; Liu, S.; Huang, W.; Gong, Y.; Gu, X. Glucagon-like peptide-1 attenuates endoplasmic reticulum stress-induced apoptosis in H9c2 cardiomyocytes during hypoxia/reoxygenation through the GLP-1R/PI3K/Akt pathways. Naunyn. Schmiedebergs Arch. Pharmacol. 2019, 392, 715–722. [Google Scholar] [CrossRef]
- Ban, K.; Kim, K.H.; Cho, C.K.; Sauve, M.; Diamandis, E.P.; Backx, P.H.; Drucker, D.J.; Husain, M. Glucagon-like peptide (GLP)-1(9-36)amide-mediated cytoprotection is blocked by exendin(9-39) yet does not require the known GLP-1 receptor. Endocrinology 2010, 151, 1520–1531. [Google Scholar] [CrossRef]
- Noyan-Ashraf, M.H.; Momen, M.A.; Ban, K.; Sadi, A.M.; Zhou, Y.Q.; Riazi, A.M.; Baggio, L.L.; Henkelman, R.M.; Husain, M.; Drucker, D.J. GLP-1R agonist liraglutide activates cytoprotective pathways and improves outcomes after experimental myocardial infarction in mice. Diabetes 2009, 58, 975–983. [Google Scholar] [CrossRef]
- Maack, C.; Kartes, T.; Kilter, H.; Schafers, H.J.; Nickenig, G.; Bohm, M.; Laufs, U. Oxygen free radical release in human failing myocardium is associated with increased activity of rac1-GTPase and represents a target for statin treatment. Circulation 2003, 108, 1567–1574. [Google Scholar] [CrossRef]
- Fukui, T.; Yoshiyama, M.; Hanatani, A.; Omura, T.; Yoshikawa, J.; Abe, Y. Expression of p22-phox and gp91-phox, essential components of NADPH oxidase, increases after myocardial infarction. Biochem. Biophys. Res. Commun. 2001, 281, 1200–1206. [Google Scholar] [CrossRef]
- Mangmool, S.; Denkaew, T.; Phosri, S.; Pinthong, D.; Parichatikanond, W.; Shimauchi, T.; Nishida, M. Sustained betaAR stimulation mediates cardiac insulin resistance in a PKA-dependent manner. Mol. Endocrinol. 2016, 30, 118–132. [Google Scholar] [CrossRef]
- Parichatikanond, W.; Nishimura, A.; Nishida, M.; Mangmool, S. Prolonged stimulation of beta(2)-adrenergic receptor with beta(2)-agonists impairs insulin actions in H9c2 cells. J. Pharmacol. Sci. 2018, 138, 184–191. [Google Scholar] [CrossRef]
- Hutchinson, D.S.; Chernogubova, E.; Dallner, O.S.; Cannon, B.; Bengtsson, T. Beta-adrenoceptors, but not alpha-adrenoceptors, stimulate AMP-activated protein kinase in brown adipocytes independently of uncoupling protein-1. Diabetologia 2005, 48, 2386–2395. [Google Scholar] [CrossRef]
- Chiasson, J.L.; Shikama, H.; Chu, D.T.; Exton, J.H. Inhibitory effect of epinephrine on insulin-stimulated glucose uptake by rat skeletal muscle. J. Clin. Investig. 1981, 68, 706–713. [Google Scholar] [CrossRef]
- Ban, K.; Noyan-Ashraf, M.H.; Hoefer, J.; Bolz, S.S.; Drucker, D.J.; Husain, M. Cardioprotective and vasodilatory actions of glucagon-like peptide 1 receptor are mediated through both glucagon-like peptide 1 receptor-dependent and -independent pathways. Circulation 2008, 117, 2340–2350. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Du, X.; Pick, J.E.; Sui, G.; Brownlee, M.; Klann, E. Glucagon-like peptide-1 cleavage product GLP-1(9-36) amide rescues synaptic plasticity and memory deficits in Alzheimer’s disease model mice. J. Neurosci. 2012, 32, 13701–13708. [Google Scholar] [CrossRef] [PubMed]
- Vakifahmetoglu-Norberg, H.; Ouchida, A.T.; Norberg, E. The role of mitochondria in metabolism and cell death. Biochem. Biophys. Res. Commun. 2017, 482, 426–431. [Google Scholar] [CrossRef]
- Volpe, C.M.O.; Villar-Delfino, P.H.; Dos Anjos, P.M.F.; Nogueira-Machado, J.A. Cellular death, reactive oxygen species (ROS) and diabetic complications. Cell Death Dis. 2018, 9, 119. [Google Scholar] [CrossRef]
- Marso, S.P.; Bain, S.C.; Consoli, A.; Eliaschewitz, F.G.; Jodar, E.; Leiter, L.A.; Lingvay, I.; Rosenstock, J.; Seufert, J.; Warren, M.L.; et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 2016, 375, 1834–1844. [Google Scholar] [CrossRef]
- Marso, S.P.; Daniels, G.H.; Brown-Frandsen, K.; Kristensen, P.; Mann, J.F.; Nauck, M.A.; Nissen, S.E.; Pocock, S.; Poulter, N.R.; Ravn, L.S.; et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 2016, 375, 311–322. [Google Scholar] [CrossRef]
- Kyriacou, A.; Ahmed, A.B. Exenatide use in the management of type 2 diabetes mellitus. Pharmaceuticals 2010, 3, 2554–2567. [Google Scholar] [CrossRef]
- Shyangdan, D.; Cummins, E.; Royle, P.; Waugh, N. Liraglutide for the treatment of type 2 diabetes. Health Technol. Assess. 2011, 15 (Suppl. S1), 77–86. [Google Scholar] [CrossRef]
- Nauck, M.A.; Friedrich, N. Do GLP-1-based therapies increase cancer risk? Diabetes Care 2013, 36 (Suppl. S2), S245–S252. [Google Scholar] [CrossRef] [PubMed]
- Vangoitsenhoven, R.; Mathieu, C.; Van der Schueren, B. GLP1 and cancer: Friend or foe? Endocr. Relat. Cancer 2012, 19, F77–F88. [Google Scholar] [CrossRef] [PubMed]
- Chang, G.; Liu, J.; Qin, S.; Jiang, Y.; Zhang, P.; Yu, H.; Lu, K.; Zhang, N.; Cao, L.; Wang, Y.; et al. Cardioprotection by exenatide: A novel mechanism via improving mitochondrial function involving the GLP-1 receptor/cAMP/PKA pathway. Int. J. Mol. Med. 2018, 41, 1693–1703. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Tian, J.; Diao, S.; Zhang, G.; Xiao, M.; Chang, D. GLP-1 receptor agonist liraglutide protects cardiomyocytes from IL-1beta-induced metabolic disturbance and mitochondrial dysfunction. Chem. Biol. Interact. 2020, 332, 109252. [Google Scholar] [CrossRef]
- Yuan, M.; Gong, M.; Zhang, Z.; Meng, L.; Tse, G.; Zhao, Y.; Bao, Q.; Zhang, Y.; Yuan, M.; Liu, X.; et al. Hyperglycemia induces endoplasmic reticulum stress in atrial cardiomyocytes, and mitofusin-2 downregulation prevents mitochondrial dysfunction and subsequent cell death. Oxidative Med. Cell. Longev. 2020, 2020, 6569728. [Google Scholar] [CrossRef]
- Labbadia, J.; Morimoto, R.I. The biology of proteostasis in aging and disease. Annu. Rev. Biochem. 2015, 84, 435–464. [Google Scholar] [CrossRef]
- Sabath, N.; Levy-Adam, F.; Younis, A.; Rozales, K.; Meller, A.; Hadar, S.; Soueid-Baumgarten, S.; Shalgi, R. Cellular proteostasis decline in human senescence. Proc. Natl. Acad. Sci. USA 2020, 117, 31902–31913. [Google Scholar] [CrossRef]
- DeNicola, M.; Du, J.; Wang, Z.; Yano, N.; Zhang, L.; Wang, Y.; Qin, G.; Zhuang, S.; Zhao, T.C. Stimulation of glucagon-like peptide-1 receptor through exendin-4 preserves myocardial performance and prevents cardiac remodeling in infarcted myocardium. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E630–E643. [Google Scholar] [CrossRef]
- Qiao, H.; Ren, H.; Du, H.; Zhang, M.; Xiong, X.; Lv, R. Liraglutide repairs the infarcted heart: The role of the SIRT1/Parkin/mitophagy pathway. Mol. Med. Rep. 2018, 17, 3722–3734. [Google Scholar] [CrossRef]
- Kyhl, K.; Lonborg, J.; Hartmann, B.; Kissow, H.; Poulsen, S.S.; Ali, H.E.; Kjaer, A.; Dela, F.; Engstrom, T.; Treiman, M. Lack of effect of prolonged treatment with liraglutide on cardiac remodeling in rats after acute myocardial infarction. Peptides 2017, 93, 1–12. [Google Scholar] [CrossRef]
- Yu, W.; Zha, W.; Ren, J. Exendin-4 and liraglutide attenuate glucose toxicity-induced cardiac injury through mTOR/ULK1-dependent autophagy. Oxidative Med. Cell. Longev. 2018, 2018, 5396806. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, D.S.; Apovian, C.M.; Aronne, L.J.; Astrup, A.; Cantley, L.C.; Ebbeling, C.B.; Heymsfield, S.B.; Johnson, J.D.; King, J.C.; Krauss, R.M.; et al. Competing paradigms of obesity pathogenesis: Energy balance versus carbohydrate-insulin models. Eur. J. Clin. Nutr. 2022, 76, 1209–1221. [Google Scholar] [CrossRef] [PubMed]
- Finan, B.; Ma, T.; Ottaway, N.; Muller, T.D.; Habegger, K.M.; Heppner, K.M.; Kirchner, H.; Holland, J.; Hembree, J.; Raver, C.; et al. Unimolecular dual incretins maximize metabolic benefits in rodents, monkeys, and humans. Sci. Transl. Med. 2013, 5, 209ra151. [Google Scholar] [CrossRef] [PubMed]
- Adriaenssens, A.E.; Gribble, F.M.; Reimann, F. The glucose-dependent insulinotropic polypeptide signaling axis in the central nervous system. Peptides 2020, 125, 170194. [Google Scholar] [CrossRef]
- NamKoong, C.; Kim, M.S.; Jang, B.T.; Lee, Y.H.; Cho, Y.M.; Choi, H.J. Central administration of GLP-1 and GIP decreases feeding in mice. Biochem. Biophys. Res. Commun. 2017, 490, 247–252. [Google Scholar] [CrossRef]
- Zhang, Q.; Delessa, C.T.; Augustin, R.; Bakhti, M.; Collden, G.; Drucker, D.J.; Feuchtinger, A.; Caceres, C.G.; Grandl, G.; Harger, A.; et al. The glucose-dependent insulinotropic polypeptide (GIP) regulates body weight and food intake via CNS-GIPR signaling. Cell Metab. 2021, 33, 833–844.e5. [Google Scholar] [CrossRef]
- Nauck, M.A.; D’Alessio, D.A. Tirzepatide, a dual GIP/GLP-1 receptor co-agonist for the treatment of type 2 diabetes with unmatched effectiveness regrading glycaemic control and body weight reduction. Cardiovasc. Diabetol. 2022, 21, 169. [Google Scholar] [CrossRef]
- Fisman, E.Z.; Tenenbaum, A. The dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist tirzepatide: A novel cardiometabolic therapeutic prospect. Cardiovasc. Diabetol. 2021, 20, 225. [Google Scholar] [CrossRef]
- Frias, J.P.; Nauck, M.A.; Van, J.; Kutner, M.E.; Cui, X.; Benson, C.; Urva, S.; Gimeno, R.E.; Milicevic, Z.; Robins, D.; et al. Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: A randomised, placebo-controlled and active comparator-controlled phase 2 trial. Lancet 2018, 392, 2180–2193. [Google Scholar] [CrossRef]
- Pirro, V.; Roth, K.D.; Lin, Y.; Willency, J.A.; Milligan, P.L.; Wilson, J.M.; Ruotolo, G.; Haupt, A.; Newgard, C.B.; Duffin, K.L. Effects of tirzepatide, a dual GIP and GLP-1 RA, on lipid and metabolite profiles in subjects with type 2 diabetes. J. Clin. Endocrinol. Metab. 2022, 107, 363–378. [Google Scholar] [CrossRef]
- Wilson, J.M.; Lin, Y.; Luo, M.J.; Considine, G.; Cox, A.L.; Bowsman, L.M.; Robins, D.A.; Haupt, A.; Duffin, K.L.; Ruotolo, G. The dual glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1 receptor agonist tirzepatide improves cardiovascular risk biomarkers in patients with type 2 diabetes: A post hoc analysis. Diabetes Obes. Metab. 2022, 24, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.M.; Nikooienejad, A.; Robins, D.A.; Roell, W.C.; Riesmeyer, J.S.; Haupt, A.; Duffin, K.L.; Taskinen, M.R.; Ruotolo, G. The dual glucose-dependent insulinotropic peptide and glucagon-like peptide-1 receptor agonist, tirzepatide, improves lipoprotein biomarkers associated with insulin resistance and cardiovascular risk in patients with type 2 diabetes. Diabetes Obes. Metab. 2020, 22, 2451–2459. [Google Scholar] [CrossRef] [PubMed]
- Sattar, N.; McGuire, D.K.; Pavo, I.; Weerakkody, G.J.; Nishiyama, H.; Wiese, R.J.; Zoungas, S. Tirzepatide cardiovascular event risk assessment: A pre-specified meta-analysis. Nat. Med. 2022, 28, 591–598. [Google Scholar] [CrossRef] [PubMed]

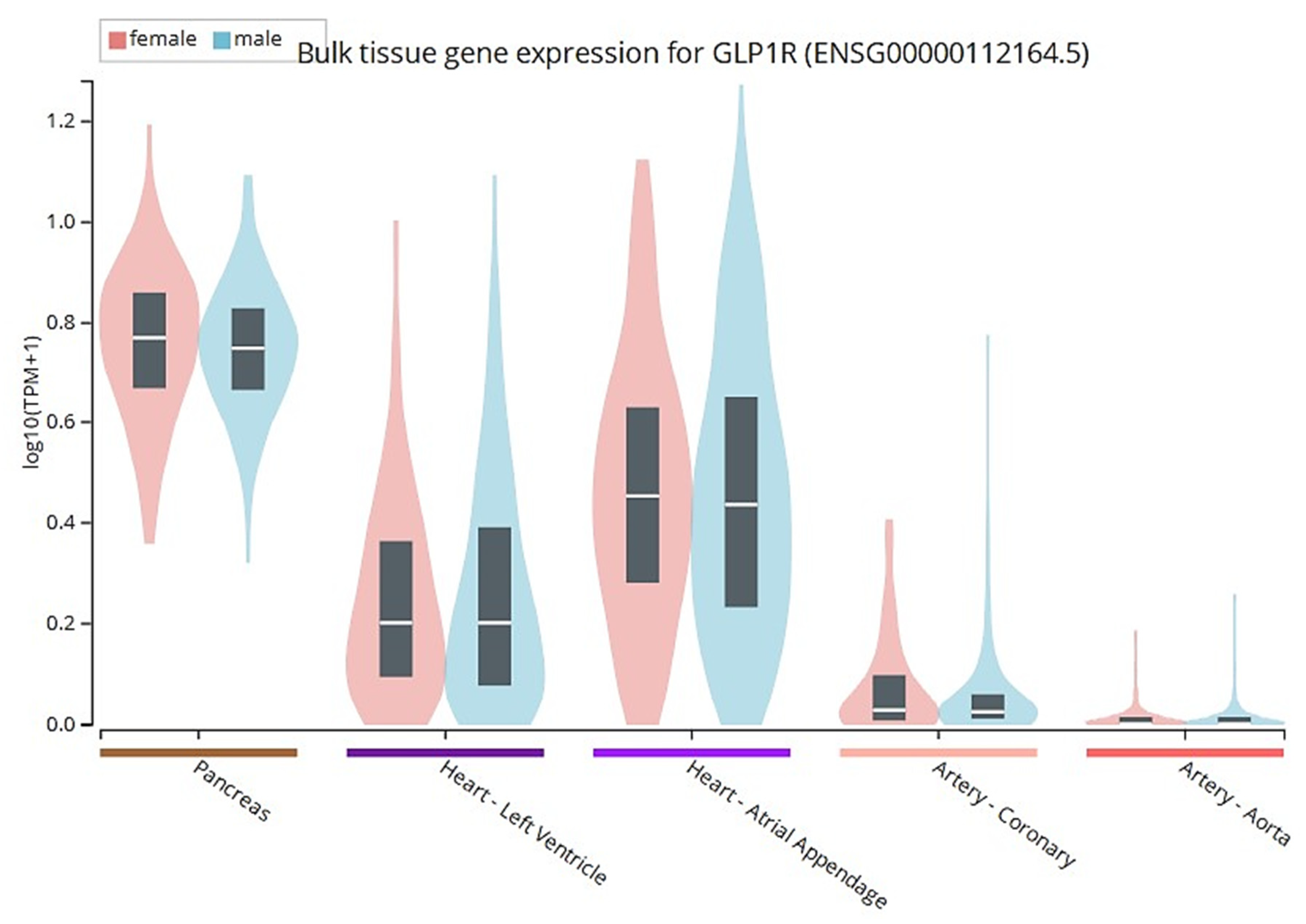
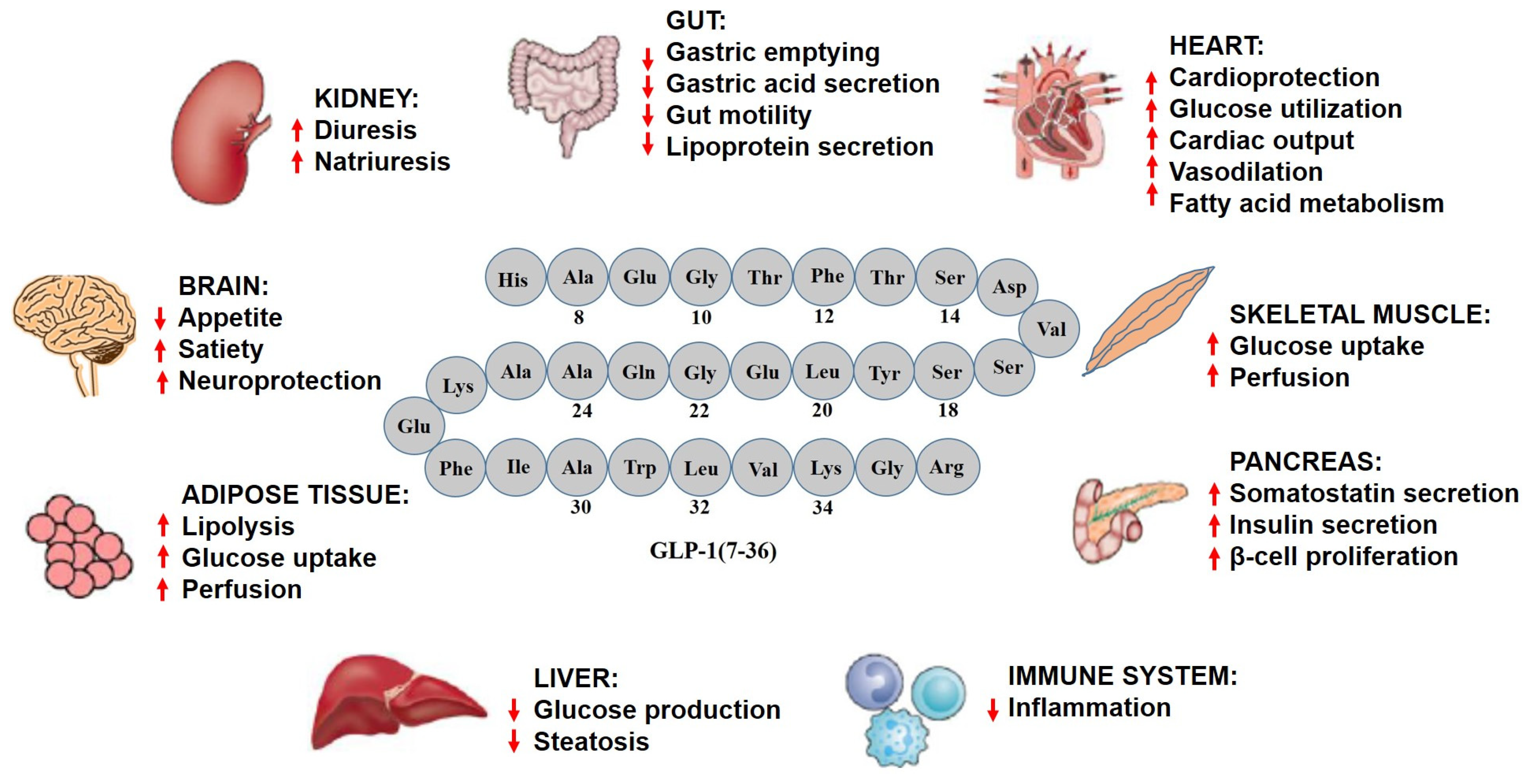
| GLP-1 Analogs | Company | FDA Approval Year for T2DM | Description | Administration | Half-Life |
|---|---|---|---|---|---|
| Human GLP-1 backbone (_glutide) | |||||
| Albiglutide | GlaxoSmithKline | 2014 | Extended release, fused to human albumin | Subcutaneous (SC), once weekly | 5–6 days |
| Dulaglutide | Eli Lilly | 2014 | Extended release, Fc region of human IgG4 | SC, once weekly | 4.7 days |
| Liraglutide | Novo Nordisk | 2010 | Immediate release, linked with free fatty acid | SC, once daily | 12–13 h |
| Semaglutide | Novo Nordisk | 2017 | Extended release, linked with free fatty acid | SC, once weekly | 5–7 days |
| Novo Nordisk | 2020 | Extended release, linked with free fatty acid | Oral, once daily | 5–7 days | |
| Exendin-4 backbone (_natide) | |||||
| Exenatide | Eli Lilly | 2005 | Immediate release | SC, twice daily | 2.4 h |
| AstraZeneca | 2012 | Extended release, encapsulated in microsphere form | SC, once weekly | 3–5 days | |
| Efpeglenatide | Sanofi | Not available | Extended release | SC, once monthly | Not available |
| Lixisenatide | Sanofi | 2016 | Immediate release, linked with poly-lysine tail | SC, once daily | 3–4 h |
| Organs/Tissues | Biological Effects |
|---|---|
| Heart |
|
| Brain |
|
| Liver |
|
| Kidneys |
|
| Pancreas |
|
| Gut |
|
| Adipose tissues |
|
| Immune system |
|
| Skeletal muscle |
|
| Reproductive system |
|
| GLP-1 Analogs | Study Models | Mechanistic Findings | Ref. |
|---|---|---|---|
| Exendin-4 | Methylglyoxal-exposed H9c2 cardiomyoblasts |
| [70] |
| Exendin-4 | Hydrogen peroxide (H2O2) -exposed neonatal rat cardiomyocytes |
| [71] |
| Exendin-4 | Phenylephrine-induced neonatal rat cardiomyocyte |
| [72] |
| Exendin-4 | Oxygen glucose deprivation/reoxygenation (OGD/R) in human ventricular cardiomyocytes |
| [73] |
| Exendin-4 | High-density lipoprotein (HDL)-induced human umbilical vein endothelial cells |
| [74] |
| GLP-1 | HL-1 cells derived from mouse atrial cardiac muscle cells |
| [75] |
| Liraglutide | Angiotensin II-treated H9c2 cells |
| [76] |
| Semaglutide | Lipopolysaccharides-treated H9c2 cells |
| [77] |
| Semaglutide | Hypoxia/reoxygenation (H/R) injury in H9c2 cells |
| [78] |
| GLP-1 Analogs | Study Models | Mechanistic Findings | Ref. |
|---|---|---|---|
| Exendin-4 | Left anterior descending (LAD) coronary artery ligation-induced heart failure rats |
| [79] |
| Exendin-4 | LAD coronary artery ligation-induced myocardial infarction (MI) rats |
| [80] |
| Exendin-4 | High-fat diet (HFD)-induced diabetic rats |
| [81] |
| Exendin-4 | LAD coronary artery ligation-induced MI rats |
| [82] |
| Exendin-4 | Streptozotocin (STZ)-induced diabetic rats |
| [83] |
| Exendin-4 | LAD coronary artery ligation-induced MI rats |
| [84] |
| Exenatide | DPP4-deficient rats; Adiponectin-deficient mice |
| [85] |
| Exendin-4 | LAD coronary artery ligation-induced MI rats |
| [80] |
| Exendin-4 | LAD coronary artery ligation-induced MI rats |
| [79] |
| Liraglutide | Spontaneously hypertensive rats (SHR) |
| [86] |
| Liraglutide | Apolipoprotein E deficient (ApoE−/−) mice |
| [87] |
| Liraglutide | HFD-feeding and STZ-induced diabetic rats |
| [88] |
| Liraglutide | Abdominal aortic constriction-induced cardiac fibrosis rats |
| [89] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pandey, S.; Mangmool, S.; Parichatikanond, W. Multifaceted Roles of GLP-1 and Its Analogs: A Review on Molecular Mechanisms with a Cardiotherapeutic Perspective. Pharmaceuticals 2023, 16, 836. https://doi.org/10.3390/ph16060836
Pandey S, Mangmool S, Parichatikanond W. Multifaceted Roles of GLP-1 and Its Analogs: A Review on Molecular Mechanisms with a Cardiotherapeutic Perspective. Pharmaceuticals. 2023; 16(6):836. https://doi.org/10.3390/ph16060836
Chicago/Turabian StylePandey, Sudhir, Supachoke Mangmool, and Warisara Parichatikanond. 2023. "Multifaceted Roles of GLP-1 and Its Analogs: A Review on Molecular Mechanisms with a Cardiotherapeutic Perspective" Pharmaceuticals 16, no. 6: 836. https://doi.org/10.3390/ph16060836
APA StylePandey, S., Mangmool, S., & Parichatikanond, W. (2023). Multifaceted Roles of GLP-1 and Its Analogs: A Review on Molecular Mechanisms with a Cardiotherapeutic Perspective. Pharmaceuticals, 16(6), 836. https://doi.org/10.3390/ph16060836






