Chaperone Activity and Protective Effect against Aβ-Induced Cytotoxicity of Artocarpus camansi Blanco and Amaranthus dubius Mart. ex Thell Seed Protein Extracts
Abstract
1. Introduction
2. Results and Discussion
2.1. Preparation of Protein Extracts and Protein Concentration
2.2. Protein Profiling with SDS-PAGE
2.3. CD Spectra and Estimation of Protein Secondary Structure Content
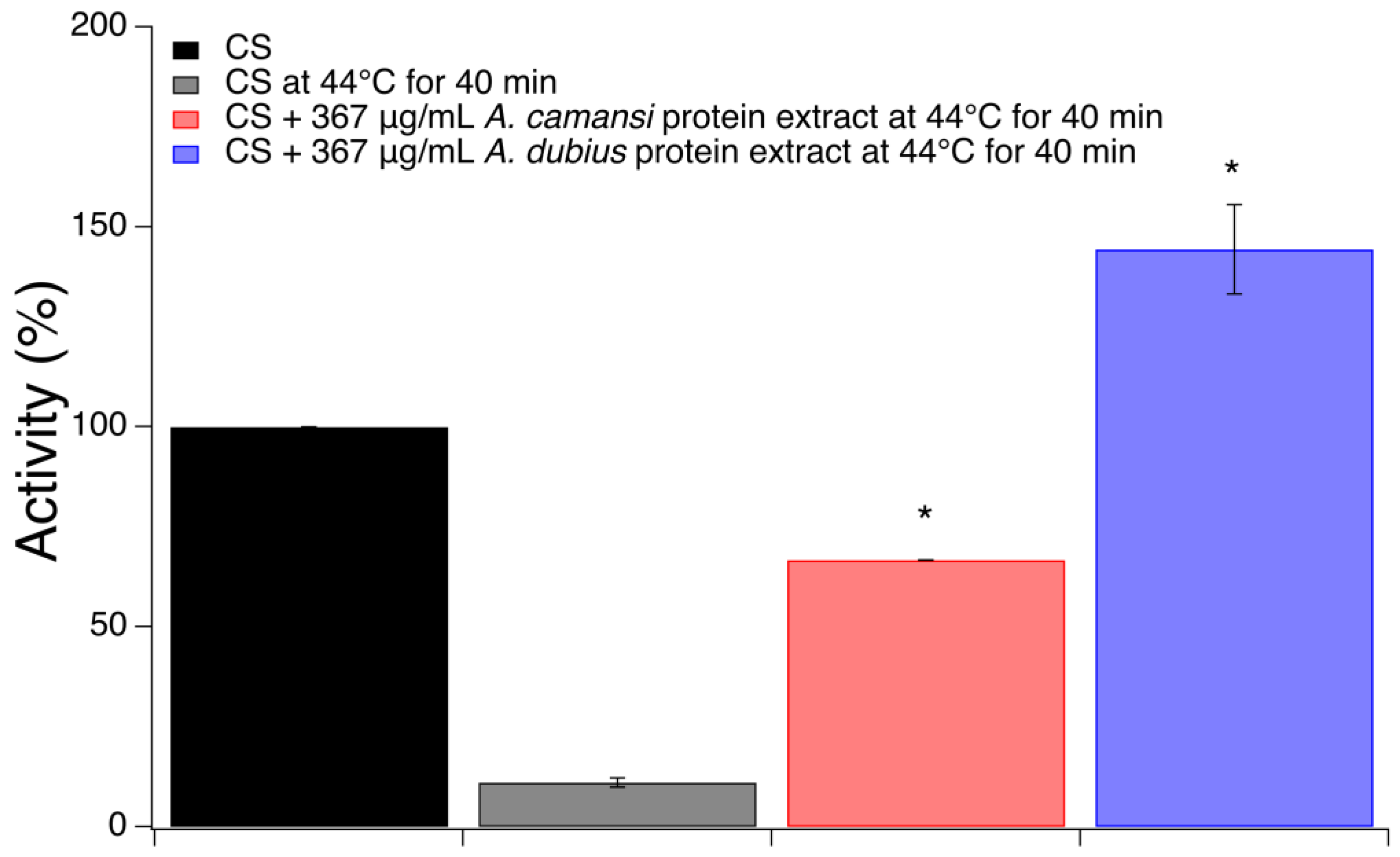
2.4. Chaperone Activity of Seed Protein Extracts of A. camansi and A. dubius
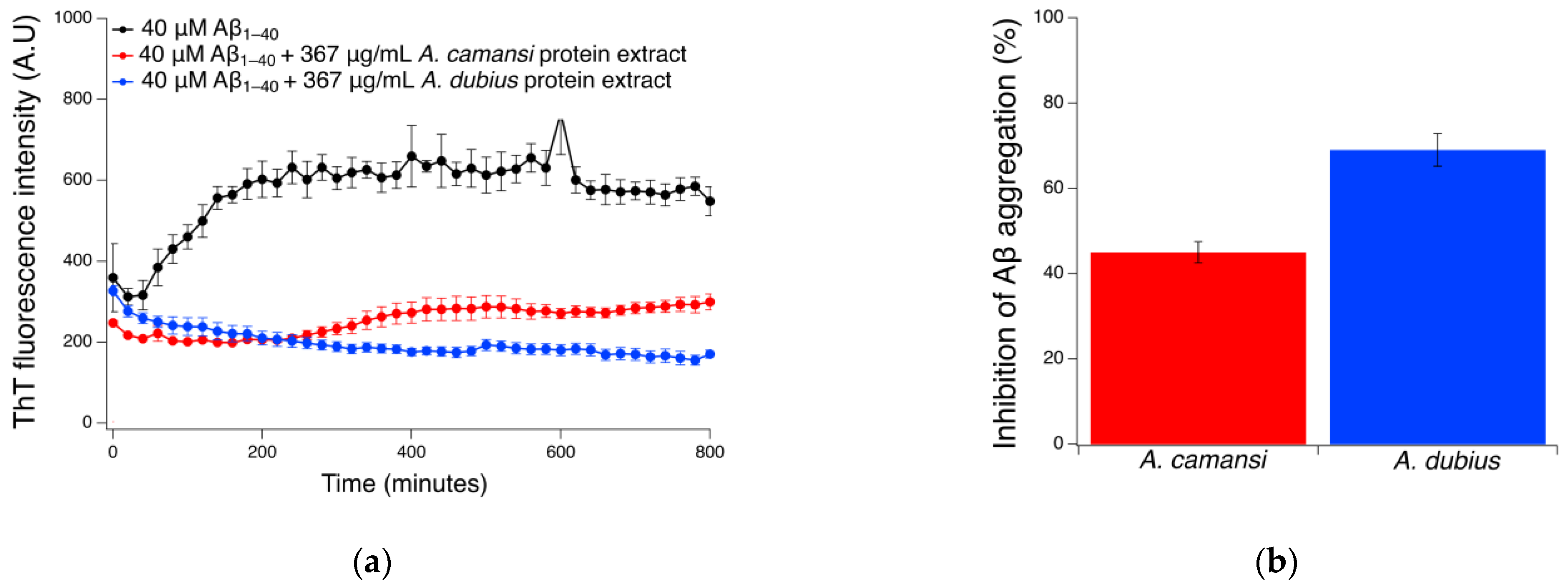
2.5. Inhibition of Aβ1–40 Fibrillation Using ThT Assay
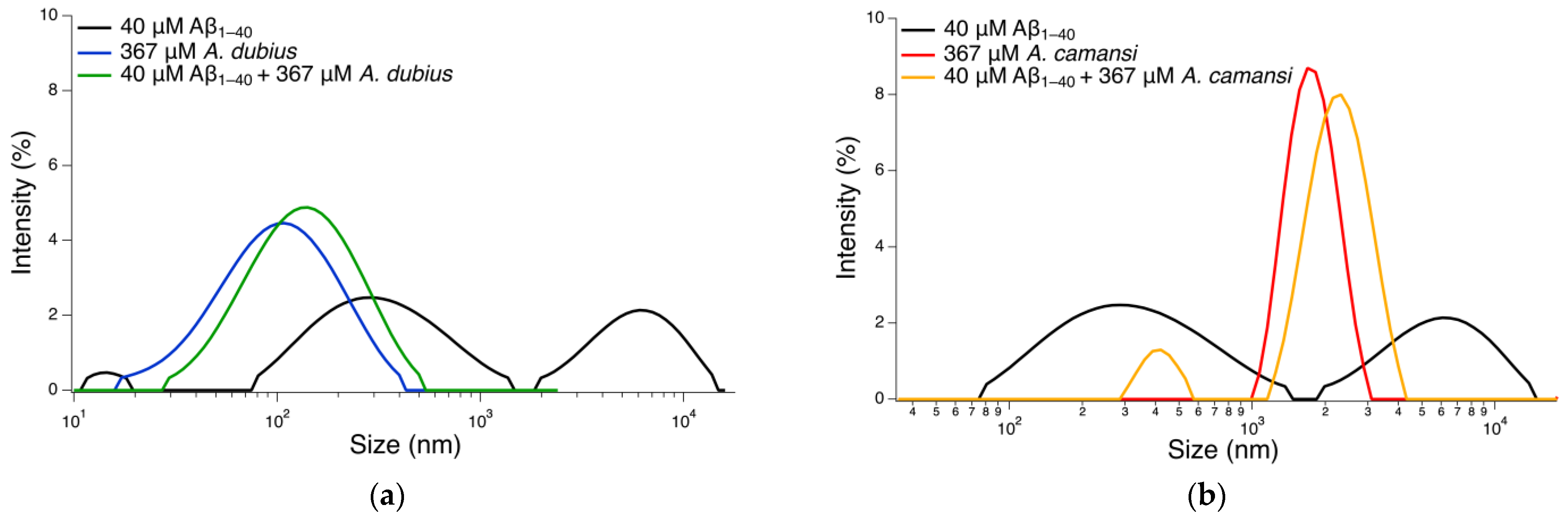
2.6. Size Distribution Analysis with DLS
2.7. Hydrolysis of Seed Protein Extracts Using the Alcalase Enzyme
2.8. Cytotoxic Effect of Protein Extracts on SH-SY5Y Cells
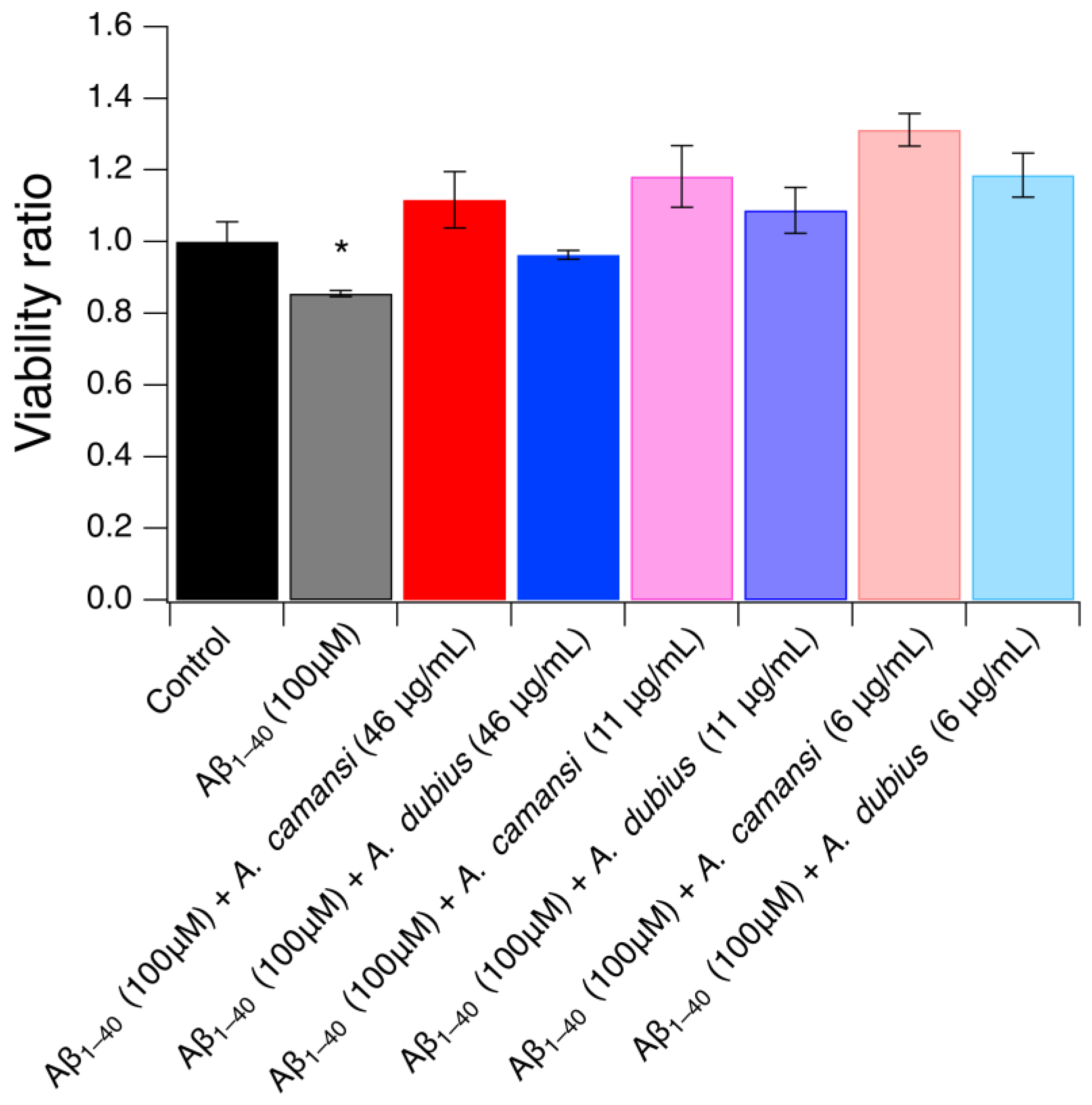
2.9. Neuroprotective Effect of A. camansi and A. dubius Protein Extracts in SH-SY5Y Cells
3. Materials and Methods
3.1. Materials
3.2. Plant Material
3.3. Protein Extraction and Purification
3.4. SDS-PAGE Pattern of Seed Protein Extracts
3.5. Circular Dichroism Spectroscopy
3.6. Chaperone Activity Determination
3.7. Thioflavin T Fluorescence Measurements
3.8. DLS Measurements of Aβ1–40 Fibrils in the Presence or Absence of Seed Protein Extracts
3.9. Alcalase Hydrolysis of Seed Protein Extracts
3.10. Cell Culture
3.11. Cytotoxicity Studies of Seed Protein Extracts
3.12. Neuroprotective Evaluation of Protein Extracts
3.13. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- 2022 Alzheimer’s disease facts and figures. Alzheimers Dement. 2022, 18, 700–789. [CrossRef] [PubMed]
- Rajan, K.B.; Weuve, J.; Barnes, L.L.; McAninch, E.A.; Wilson, R.S.; Evans, D.A. Population estimate of people with clinical Alzheimer’s disease and mild cognitive impairment in the United States (2020–2060). Alzheimers Dement. 2021, 17, 1966–1975. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Li, J.; Li, X.; Ma, L.; Hou, M.; Zhou, H.; Zhou, R. Based on molecular structures: Amyloid-beta generation, clearance, toxicity and therapeutic strategies. Front. Mol. Neurosci. 2022, 15, 927530. [Google Scholar] [CrossRef] [PubMed]
- Ricciarelli, R.; Fedele, E. The Amyloid Cascade Hypothesis in Alzheimer’s Disease: It’s Time to Change Our Mind. Curr. Neuropharmacol. 2017, 15, 926–935. [Google Scholar] [CrossRef]
- Sevigny, J.; Chiao, P.; Bussiere, T.; Weinreb, P.H.; Williams, L.; Maier, M.; Dunstan, R.; Salloway, S.; Chen, T.; Ling, Y.; et al. Addendum: The antibody aducanumab reduces Abeta plaques in Alzheimer’s disease. Nature 2017, 546, 564. [Google Scholar] [CrossRef]
- Gu, L.; Guo, Z. Alzheimer’s Abeta42 and Abeta40 peptides form interlaced amyloid fibrils. J. Neurochem. 2013, 126, 305–311. [Google Scholar] [CrossRef]
- Aleksis, R.; Oleskovs, F.; Jaudzems, K.; Pahnke, J.; Biverstål, H. Structural studies of amyloid-β peptides: Unlocking the mechanism of aggregation and the associated toxicity. Biochimie 2017, 140, 176–192. [Google Scholar] [CrossRef]
- Ruifang, E.; Shi, Y.; Wang, W.; Qi, M. Callistephin inhibits amyloid-β protein aggregation and determined cytotoxicity against cerebrovascular smooth muscle cells as an in vitro model of cerebral amyloid angiopathy. Arab. J. Chem. 2022, 15, 103605. [Google Scholar] [CrossRef]
- Reitz, C. Alzheimer’s disease and the amyloid cascade hypothesis: A critical review. Int. J. Alzheimers Dis. 2012, 2012, 369808. [Google Scholar] [CrossRef]
- Lannfelt, L.; Blennow, K.; Zetterberg, H.; Batsman, S.; Ames, D.; Harrison, J.; Masters, C.L.; Targum, S.; Bush, A.I.; Murdoch, R.; et al. Safety, efficacy, and biomarker findings of PBT2 in targeting Abeta as a modifying therapy for Alzheimer’s disease: A phase IIa, double-blind, randomised, placebo-controlled trial. Lancet Neurol. 2008, 7, 779–786. [Google Scholar] [CrossRef]
- Wilkinson, D.G.; Francis, P.T.; Schwam, E.; Payne-Parrish, J. Cholinesterase inhibitors used in the treatment of Alzheimer’s disease: The relationship between pharmacological effects and clinical efficacy. Drugs Aging 2004, 21, 453–478. [Google Scholar] [CrossRef] [PubMed]
- Adlard, P.A.; Cherny, R.A.; Finkelstein, D.I.; Gautier, E.; Robb, E.; Cortes, M.; Volitakis, I.; Liu, X.; Smith, J.P.; Perez, K.; et al. Rapid restoration of cognition in Alzheimer’s transgenic mice with 8-hydroxy quinoline analogs is associated with decreased interstitial Abeta. Neuron 2008, 59, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Aisen, P.S.; Saumier, D.; Briand, R.; Laurin, J.; Gervais, F.; Tremblay, P.; Garceau, D. A Phase II study targeting amyloid-beta with 3APS in mild-to-moderate Alzheimer disease. Neurology 2006, 67, 1757–1763. [Google Scholar] [CrossRef] [PubMed]
- McLaurin, J.; Kierstead, M.E.; Brown, M.E.; Hawkes, C.A.; Lambermon, M.H.; Phinney, A.L.; Darabie, A.A.; Cousins, J.E.; French, J.E.; Lan, M.F.; et al. Cyclohexanehexol inhibitors of Abeta aggregation prevent and reverse Alzheimer phenotype in a mouse model. Nat. Med. 2006, 12, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Opazo, C.; Luza, S.; Villemagne, V.L.; Volitakis, I.; Rowe, C.; Barnham, K.J.; Strozyk, D.; Masters, C.L.; Cherny, R.A.; Bush, A.I. Radioiodinated clioquinol as a biomarker for beta-amyloid: Zn complexes in Alzheimer’s disease. Aging Cell. 2006, 5, 69–79. [Google Scholar] [CrossRef]
- Vaz, M.; Silva, V.; Monteiro, C.; Silvestre, S. Role of Aducanumab in the Treatment of Alzheimer’s Disease: Challenges and Opportunities. Clin. Interv. Aging 2022, 17, 797–810. [Google Scholar] [CrossRef]
- Bhat, B.A.; Almilaibary, A.; Mir, R.A.; Aljarallah, B.M.; Mir, W.R.; Ahmad, F.; Mir, M.A. Natural Therapeutics in Aid of Treating Alzheimer’s Disease: A Green Gateway Toward Ending Quest for Treating Neurological Disorders. Front. Neurosci. 2022, 16, 884345. [Google Scholar] [CrossRef]
- Chen, X.; Drew, J.; Berney, W.; Lei, W. Neuroprotective Natural Products for Alzheimer’s Disease. Cells 2021, 10, 1309. [Google Scholar] [CrossRef]
- Hoi, C.P.; Ho, Y.P.; Baum, L.; Chow, A.H. Neuroprotective effect of honokiol and magnolol, compounds from Magnolia officinalis, on beta-amyloid-induced toxicity in PC12 cells. Phytother. Res. 2010, 24, 1538–1542. [Google Scholar] [CrossRef]
- Tuzimski, T.; Petruczynik, A. Determination of Anti-Alzheimer’s Disease Activity of Selected Plant Ingredients. Molecules 2022, 27, 3222. [Google Scholar] [CrossRef]
- Tan, M.A.; An, S.S.A. Neuroprotective potential of the oxindole alkaloids isomitraphylline and mitraphylline in human neuroblastoma SH-SY5Y cells. 3 Biotech 2020, 10, 517. [Google Scholar] [CrossRef] [PubMed]
- Vrabec, R.; Blunden, G.; Cahlikova, L. Natural Alkaloids as Multi-Target Compounds towards Factors Implicated in Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 4399. [Google Scholar] [CrossRef]
- Yoo, K.Y.; Park, S.Y. Terpenoids as potential anti-Alzheimer’s disease therapeutics. Molecules 2012, 17, 3524–3538. [Google Scholar] [CrossRef]
- Hamaguchi, T.; Ono, K.; Murase, A.; Yamada, M. Phenolic compounds prevent Alzheimer’s pathology through different effects on the amyloid-beta aggregation pathway. Am. J. Pathol. 2009, 175, 2557–2565. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Li, L.; Takamura, Y.; Yoshiike, Y.; Zhu, L.; Han, F.; Mao, X.; Ikeda, T.; Takasaki, J.; Nishijo, H.; et al. Phenolic compounds prevent amyloid beta-protein oligomerization and synaptic dysfunction by site-specific binding. J. Biol. Chem. 2012, 287, 14631–14643. [Google Scholar] [CrossRef]
- Bastianetto, S.; Quirion, R. Natural extracts as possible protective agents of brain aging. Neurobiol. Aging 2002, 23, 891–897. [Google Scholar] [CrossRef]
- Boubakri, A.; Leri, M.; Bucciantini, M.; Najjaa, H.; Ben Arfa, A.; Stefani, M.; Neffati, M. Allium roseum L. extract inhibits amyloid beta aggregation and toxicity involved in Alzheimer’s disease. PLoS ONE 2020, 15, e0223815. [Google Scholar] [CrossRef] [PubMed]
- Dhouafli, Z.; Rigacci, S.; Leri, M.; Bucciantini, M.; Mahjoub, B.; Tounsi, M.S.; Wannes, W.A.; Stefani, M.; Hayouni, E.A. Screening for amyloid-β aggregation inhibitor and neuronal toxicity of eight Tunisian medicinal plants. Ind. Crops Prod. 2018, 111, 823–833. [Google Scholar] [CrossRef]
- Chen, G.; Andrade-Talavera, Y.; Tambaro, S.; Leppert, A.; Nilsson, H.E.; Zhong, X.; Landreh, M.; Nilsson, P.; Hebert, H.; Biverstal, H.; et al. Augmentation of Bri2 molecular chaperone activity against amyloid-beta reduces neurotoxicity in mouse hippocampus in vitro. Commun. Biol. 2020, 3, 32. [Google Scholar] [CrossRef] [PubMed]
- Kastenholz, B.; Garfin, D.E. Medicinal plants: A natural chaperones source for treating neurological disorders. Protein Pept. Lett. 2009, 16, 116–120. [Google Scholar] [CrossRef]
- Kastenholz, B.; Horst, B.; Horst, J. Can Plant-Made Copper Chaperones Heal Early Alzheimer’s Disease? Nat. Preced. 2011. [Google Scholar] [CrossRef]
- Lazarev, V.F.; Mikhaylova, E.R.; Guzhova, I.V.; Margulis, B.A. Possible Function of Molecular Chaperones in Diseases Caused by Propagating Amyloid Aggregates. Front. Neurosci. 2017, 11, 277. [Google Scholar] [CrossRef] [PubMed]
- Mansson, C.; Kakkar, V.; Monsellier, E.; Sourigues, Y.; Harmark, J.; Kampinga, H.H.; Melki, R.; Emanuelsson, C. DNAJB6 is a peptide-binding chaperone which can suppress amyloid fibrillation of polyglutamine peptides at substoichiometric molar ratios. Cell. Stress Chaperones 2014, 19, 227–239. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, L.Q.; Perrett, S. Studying the effects of chaperones on amyloid fibril formation. Methods 2011, 53, 285–294. [Google Scholar] [CrossRef]
- Saibil, H. Chaperone machines for protein folding, unfolding and disaggregation. Nat. Rev. Mol. Cell. Biol. 2013, 14, 630–642. [Google Scholar] [CrossRef]
- Hochberg, G.K.; Ecroyd, H.; Liu, C.; Cox, D.; Cascio, D.; Sawaya, M.R.; Collier, M.P.; Stroud, J.; Carver, J.A.; Baldwin, A.J.; et al. The structured core domain of alphaB-crystallin can prevent amyloid fibrillation and associated toxicity. Proc. Natl. Acad. Sci. USA 2014, 111, E1562–E1570. [Google Scholar] [CrossRef]
- Arosio, P.; Michaels, T.C.; Linse, S.; Mansson, C.; Emanuelsson, C.; Presto, J.; Johansson, J.; Vendruscolo, M.; Dobson, C.M.; Knowles, T.P. Kinetic analysis reveals the diversity of microscopic mechanisms through which molecular chaperones suppress amyloid formation. Nat. Commun. 2016, 7, 10948. [Google Scholar] [CrossRef] [PubMed]
- Bernd, K. Phytochemical approach and bioanalytical strategy to develop chaperone-based medications. Open. Biochem. J. 2008, 2, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Jagtap, U.B.; Bapat, V.A. Artocarpus: A review of its traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. 2010, 129, 142–166. [Google Scholar] [CrossRef]
- Baraniak, J.; Kania-Dobrowolska, M. The Dual Nature of Amaranth-Functional Food and Potential Medicine. Foods 2022, 11, 618. [Google Scholar] [CrossRef]
- House, N.C.; Puthenparampil, D.; Malayil, D.; Narayanankutty, A. Variation in the polyphenol composition, antioxidant, and anticancer activity among different Amaranthus species. S. Afr. J. Bot. 2020, 135, 408–412. [Google Scholar] [CrossRef]
- Silalahi, M. Keluwih (Artocarpus camansi Blanco): Potential utilization as foodstuff and its bioactivity. Biol. Pharm. Sci. 2022, 19, 310–315. [Google Scholar] [CrossRef]
- Bojorquez-Velazquez, E.; Barrera-Pacheco, A.; Espitia-Rangel, E.; Herrera-Estrella, A.; Barba de la Rosa, A.P. Protein analysis reveals differential accumulation of late embryogenesis abundant and storage proteins in seeds of wild and cultivated amaranth species. BMC Plant. Biol. 2019, 19, 59. [Google Scholar] [CrossRef]
- Kaur, H.; Petla, B.; Kamble, N.; Singh, A.; Rao, V.; Salvi, P.; Ghosh, S.; Majee, M. Differentially expressed seed aging responsive heat shock protein OsHSP18.2 implicates in seed vigor, longevity and improves germination and seedling establishment under abiotic stress. Front. Plant Sci. 2015, 6, 713. [Google Scholar] [CrossRef]
- Mukesh, S.; Sikarwar, B.J.H.; Subramaniam, K.; Valeisamy, B.D.; Yean, L.K.; Balaji, K. A Review on Artocarpus altilis (Parkinson) Fosberg (breadfruit). J. Appl. Pharm. Sci. 2014, 4, 091–097. [Google Scholar]
- Rodríguez, P.; Pérez, E.; Romel, G.; Dufour, D. Characterization of the protein’s fractions extracted from leaves of Amaranthus dubius (Amaranthus spp.). Afr. J. Food Sci. 2011, 5, 417–424. [Google Scholar]
- Fenner, M. Relationships Between Seed Weight, Ash Content, and Seedling Growth in Twenty-Four Species of Compositae. New Phytol. 1983, 95, 697–706. [Google Scholar] [CrossRef]
- Adeleke, R.A.; Abiodun, O.A. Nutritional composition of breadnut seeds (Artocarpus camansi). Afr. J. Agric. Res. 2010, 5, 1273–1276. [Google Scholar]
- Rehana Asghar, R.S.; Afzal, M.; Akhtar, S. Inter and Intra-Specific Variation in SDS-PAGE of Total Seed Protein in Rice (Oryza sativa L.) Germplasm. Pak. J. Biol. Sci. 2004, 7, 139–143. [Google Scholar] [CrossRef]
- Micsonai, A.; Wien, F.; Bulyaki, E.; Kun, J.; Moussong, E.; Lee, Y.H.; Goto, Y.; Refregiers, M.; Kardos, J. BeStSel: A web server for accurate protein secondary structure prediction and fold recognition from the circular dichroism spectra. Nucleic Acids Res. 2018, 46, W315–W322. [Google Scholar] [CrossRef]
- Xiao, B.; Liu, Y.; Luo, M.; Yang, T.; Guo, X.; Yi, H. Evaluation of the secondary structures of protein in the extracellular polymeric substances extracted from activated sludge by different methods. J. Environ. Sci. (China) 2019, 80, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-H.; Wang, Z.; Xu, S.-Y. Preparation and characterization of sericin powder extracted from silk industry wastewater. Food Chem. 2007, 103, 1255–1262. [Google Scholar] [CrossRef]
- Hristozova, N.; Tompa, P.; Kovacs, D. A Novel Method for Assessing the Chaperone Activity of Proteins. PLoS ONE 2016, 11, e0161970. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, D.; Agoston, B.; Tompa, P. Disordered plant LEA proteins as molecular chaperones. Plant. Signal. Behav. 2008, 3, 710–713. [Google Scholar] [CrossRef]
- Sun, X.; Chen, W.D.; Wang, Y.D. beta-Amyloid: The key peptide in the pathogenesis of Alzheimer’s disease. Front. Pharm. 2015, 6, 221. [Google Scholar] [CrossRef]
- Khurana, R.; Coleman, C.; Ionescu-Zanetti, C.; Carter, S.A.; Krishna, V.; Grover, R.K.; Roy, R.; Singh, S. Mechanism of thioflavin T binding to amyloid fibrils. J. Struct. Biol. 2005, 151, 229–238. [Google Scholar] [CrossRef]
- Sudhakar, S.; Kalipillai, P.; Santhosh, P.B.; Mani, E. Role of Surface Charge of Inhibitors on Amyloid Beta Fibrillation. J. Phys. Chem. C 2017, 121, 6339–6348. [Google Scholar] [CrossRef]
- Witter, S.; Witter, R.; Vilu, R.; Samoson, A. Medical Plants and Nutraceuticals for Amyloid-beta Fibrillation Inhibition. J. Alzheimers Dis. Rep. 2018, 2, 239–252. [Google Scholar] [CrossRef]
- Kannaian, B.; Sharma, B.; Phillips, M.; Chowdhury, A.; Manimekalai, M.S.S.; Adav, S.S.; Ng, J.T.Y.; Kumar, A.; Lim, S.; Mu, Y.; et al. Abundant neuroprotective chaperone Lipocalin-type prostaglandin D synthase (L-PGDS) disassembles the Amyloid-beta fibrils. Sci. Rep. 2019, 9, 12579. [Google Scholar] [CrossRef]
- Pryor, N.E.; Moss, M.A.; Hestekin, C.N. Unraveling the early events of amyloid-beta protein (Abeta) aggregation: Techniques for the determination of Abeta aggregate size. Int. J. Mol. Sci. 2012, 13, 3038–3072. [Google Scholar] [CrossRef]
- Mansson, C.; Arosio, P.; Hussein, R.; Kampinga, H.H.; Hashem, R.M.; Boelens, W.C.; Dobson, C.M.; Knowles, T.P.; Linse, S.; Emanuelsson, C. Interaction of the molecular chaperone DNAJB6 with growing amyloid-beta 42 (Abeta42) aggregates leads to sub-stoichiometric inhibition of amyloid formation. J. Biol. Chem. 2014, 289, 31066–31076. [Google Scholar] [CrossRef]
- Arimon, M.; Grimminger, V.; Sanz, F.; Lashuel, H.A. Hsp104 targets multiple intermediates on the amyloid pathway and suppresses the seeding capacity of Abeta fibrils and protofibrils. J. Mol. Biol. 2008, 384, 1157–1173. [Google Scholar] [CrossRef]
- Mannini, B.; Chiti, F. Chaperones as Suppressors of Protein Misfolded Oligomer Toxicity. Front. Mol. Neurosci. 2017, 10, 98. [Google Scholar] [CrossRef]
- Evans, C.G.; Wisén, S.; Gestwicki, J.E. Heat Shock Proteins 70 and 90 Inhibit Early Stages of Amyloid β-(1–42) Aggregation in Vitro*. J. Biol. Chem. 2006, 281, 33182–33191. [Google Scholar] [CrossRef]
- Parekh, M.I.K. Antimicrobial and Hemolytic Activity of Seed Protein Extracts from Selected Medicinal Plants against Tooth Decaying Microorganisms. Int. J. Res. Stud. Microbiol. Biotechnol. 2020, 6, 38–48. [Google Scholar]
- Walker, J.M. The bicinchoninic acid (BCA) assay for protein quantitation. Methods Mol. Biol. 1994, 32, 5–8. [Google Scholar] [CrossRef]
- Soto-Madrid, D.; Perez, N.; Gutierrez-Cutino, M.; Matiacevich, S.; Zuniga, R.N. Structural and Physicochemical Characterization of Extracted Proteins Fractions from Chickpea (Cicer arietinum L.) as a Potential Food Ingredient to Replace Ovalbumin in Foams and Emulsions. Polymers 2022, 15, 110. [Google Scholar] [CrossRef]
- Kanekiyo, T.; Ban, T.; Aritake, K.; Huang, Z.L.; Qu, W.M.; Okazaki, I.; Mohri, I.; Murayama, S.; Ozono, K.; Taniike, M.; et al. Lipocalin-type prostaglandin D synthase/beta-trace is a major amyloid beta-chaperone in human cerebrospinal fluid. Proc. Natl. Acad. Sci. USA 2007, 104, 6412–6417. [Google Scholar] [CrossRef]
- Serpell, L.C. Alzheimer’s amyloid fibrils: Structure and assembly. Biochim. Biophys. Acta 2000, 1502, 16–30. [Google Scholar] [CrossRef]
- Villanueva, A.; Clemente, A.; Bautista, J.; Millán, F. Production of an extensive sunflower protein hydrolysate by sequential hydrolysis with endo- and exo-proteases. Grasas Y Aceites 1999, 50, 472–476. [Google Scholar] [CrossRef]




| Conformation | A. dubius (%) | A. camansi (%) |
|---|---|---|
| α-Helix | 5.9 | 0.0 |
| β-Sheet | 25.6 | 36.8 |
| β-Turn | 13.5 | 12.4 |
| Others | 54.9 | 50.7 |
| Scientific Names | Local Names | Parts Used |
|---|---|---|
| Amaranthus dubius Mart. ex Thell | Red Spinach, Pig’s weed, Bledo | Seeds |
| Artocarpus camansi Blanco | Breadnut, Pana de Pepita | Seeds |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanchez-Rodriguez, D.; Gonzalez-Figueroa, I.; Alvarez-Berríos, M.P. Chaperone Activity and Protective Effect against Aβ-Induced Cytotoxicity of Artocarpus camansi Blanco and Amaranthus dubius Mart. ex Thell Seed Protein Extracts. Pharmaceuticals 2023, 16, 820. https://doi.org/10.3390/ph16060820
Sanchez-Rodriguez D, Gonzalez-Figueroa I, Alvarez-Berríos MP. Chaperone Activity and Protective Effect against Aβ-Induced Cytotoxicity of Artocarpus camansi Blanco and Amaranthus dubius Mart. ex Thell Seed Protein Extracts. Pharmaceuticals. 2023; 16(6):820. https://doi.org/10.3390/ph16060820
Chicago/Turabian StyleSanchez-Rodriguez, David, Idsa Gonzalez-Figueroa, and Merlis P. Alvarez-Berríos. 2023. "Chaperone Activity and Protective Effect against Aβ-Induced Cytotoxicity of Artocarpus camansi Blanco and Amaranthus dubius Mart. ex Thell Seed Protein Extracts" Pharmaceuticals 16, no. 6: 820. https://doi.org/10.3390/ph16060820
APA StyleSanchez-Rodriguez, D., Gonzalez-Figueroa, I., & Alvarez-Berríos, M. P. (2023). Chaperone Activity and Protective Effect against Aβ-Induced Cytotoxicity of Artocarpus camansi Blanco and Amaranthus dubius Mart. ex Thell Seed Protein Extracts. Pharmaceuticals, 16(6), 820. https://doi.org/10.3390/ph16060820






