Live Cell Imaging by Förster Resonance Energy Transfer Fluorescence to Study Trafficking of PLGA Nanoparticles and the Release of a Loaded Peptide in Dendritic Cells
Abstract
1. Introduction
2. Results and Discussion
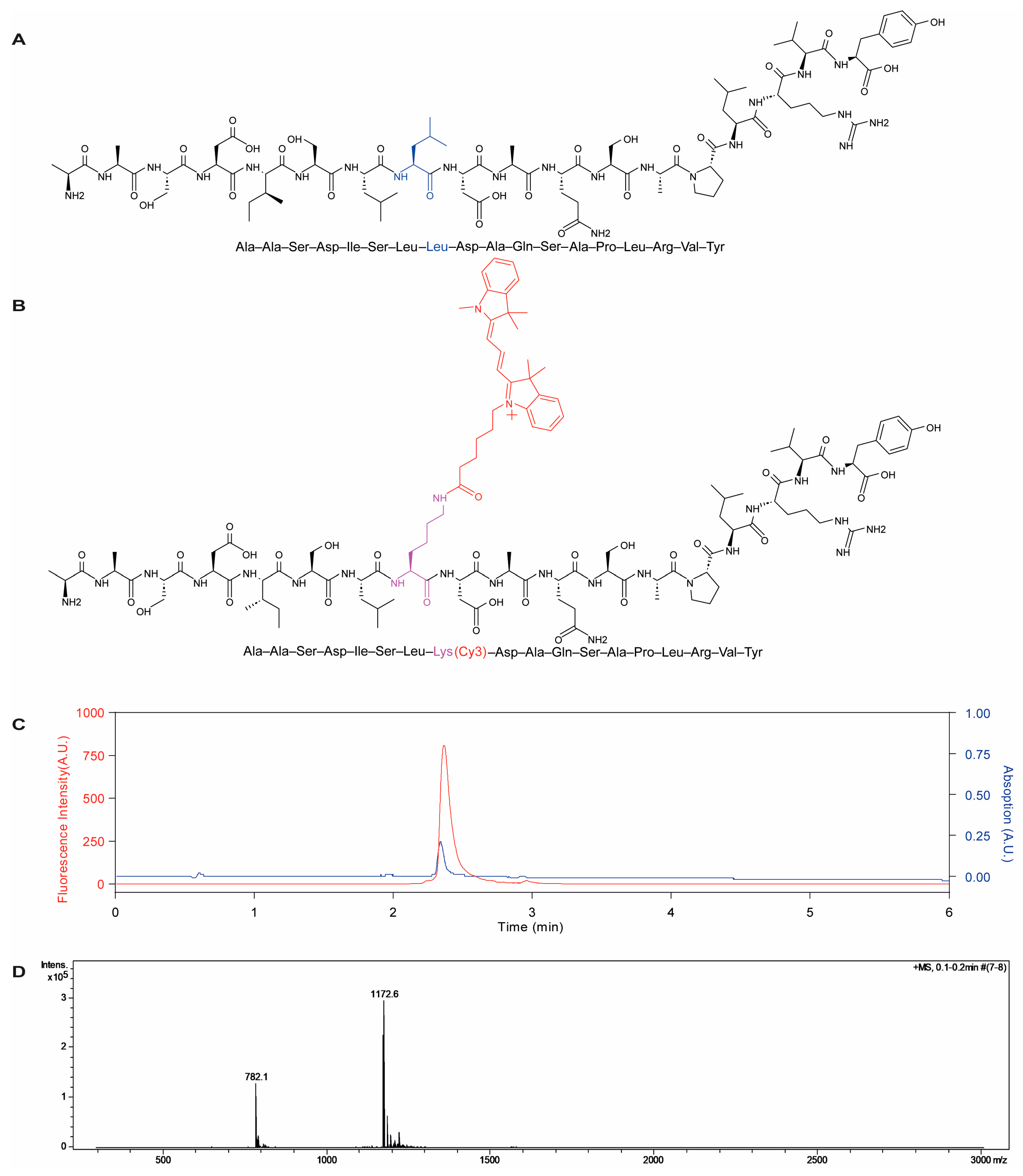
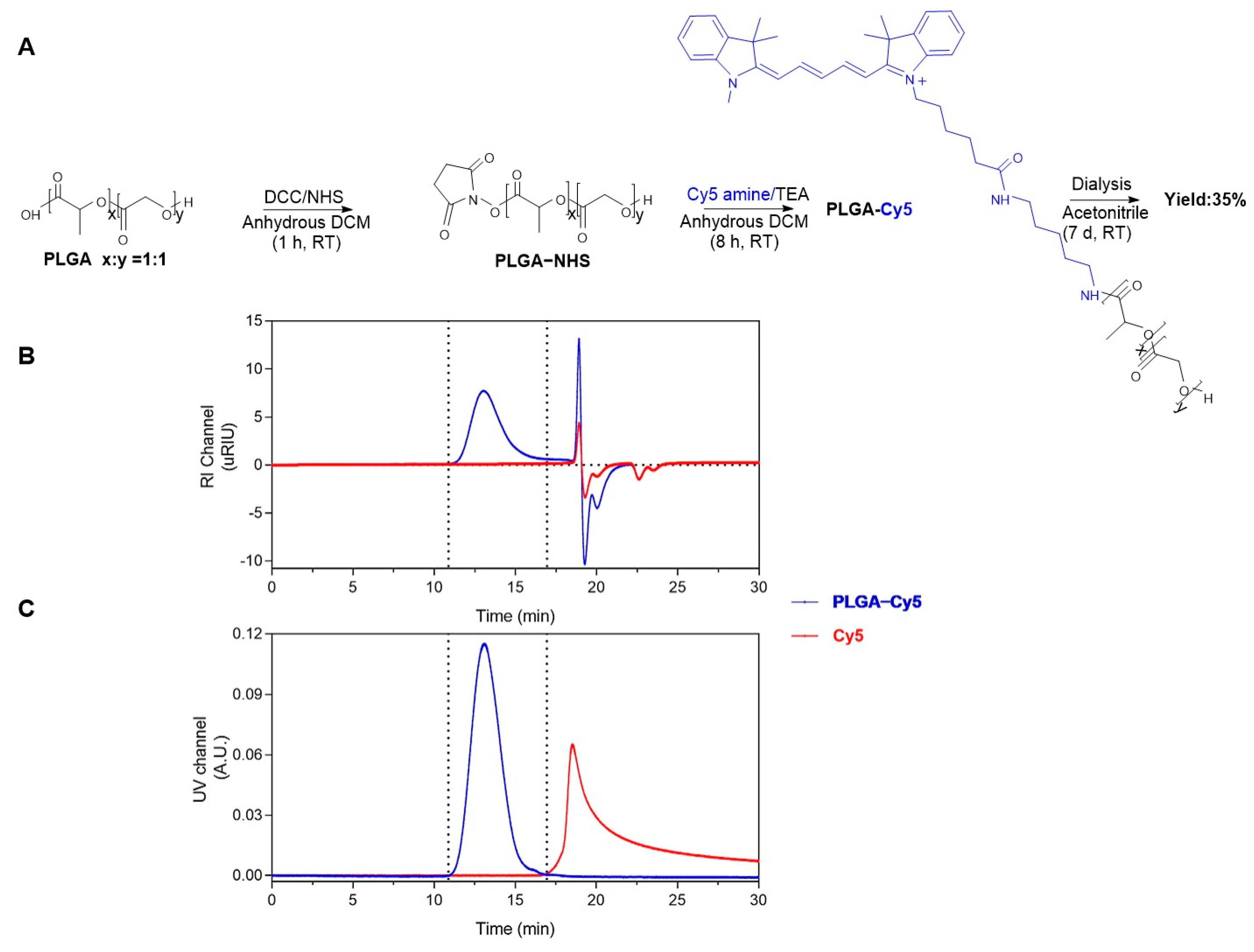
2.1. Preparation and Characterization of the Cy3-Labeled β-Lactoglobulin-Derived Peptide and Cy5-Labeled PLGA
2.2. Characteristics of Single- and Dual-Labeled PLGA Nanoparticles
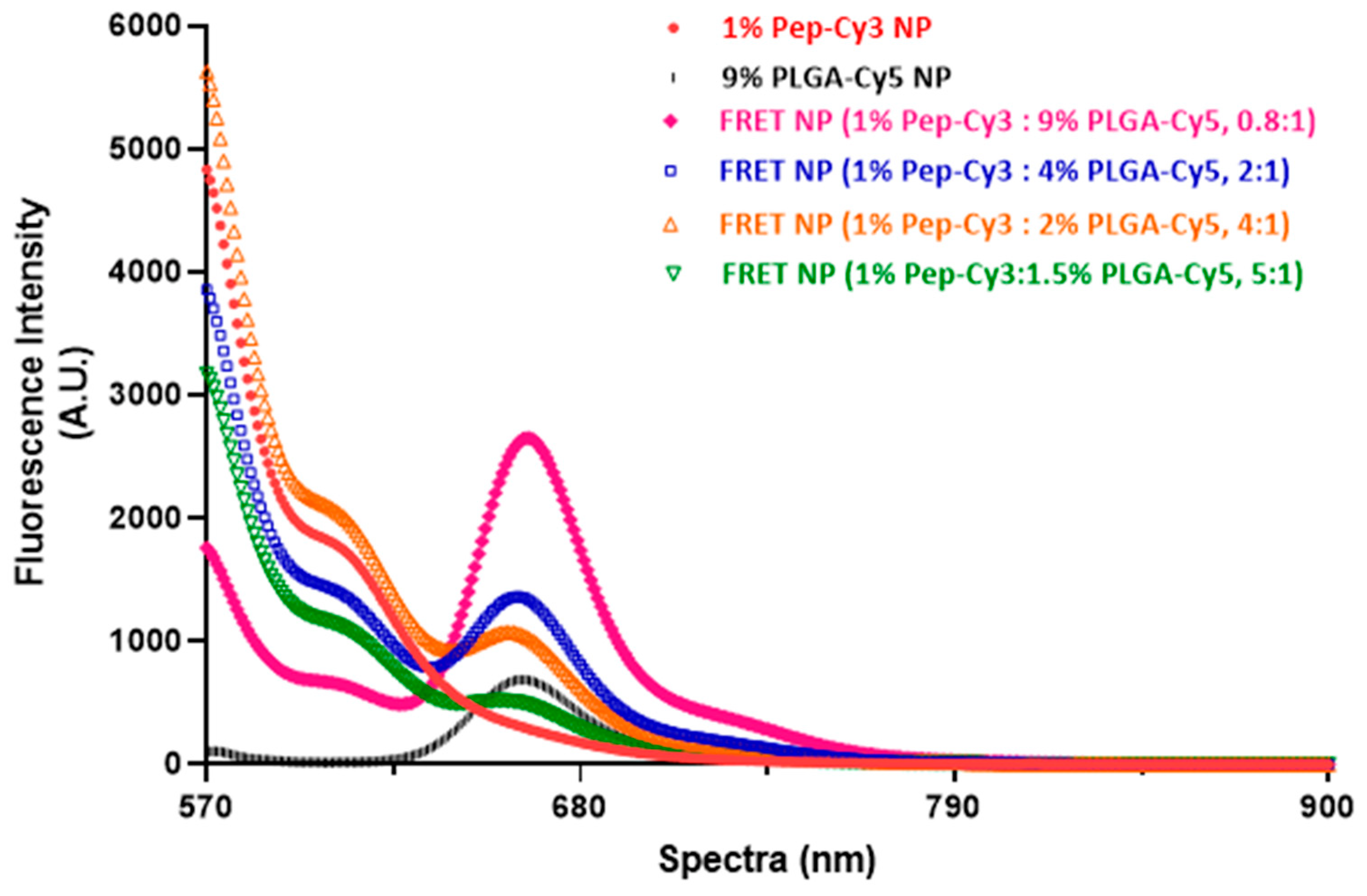
2.3. Optimization of FRET Efficiency of the NPs Labeled with the Cy3 and Cy5
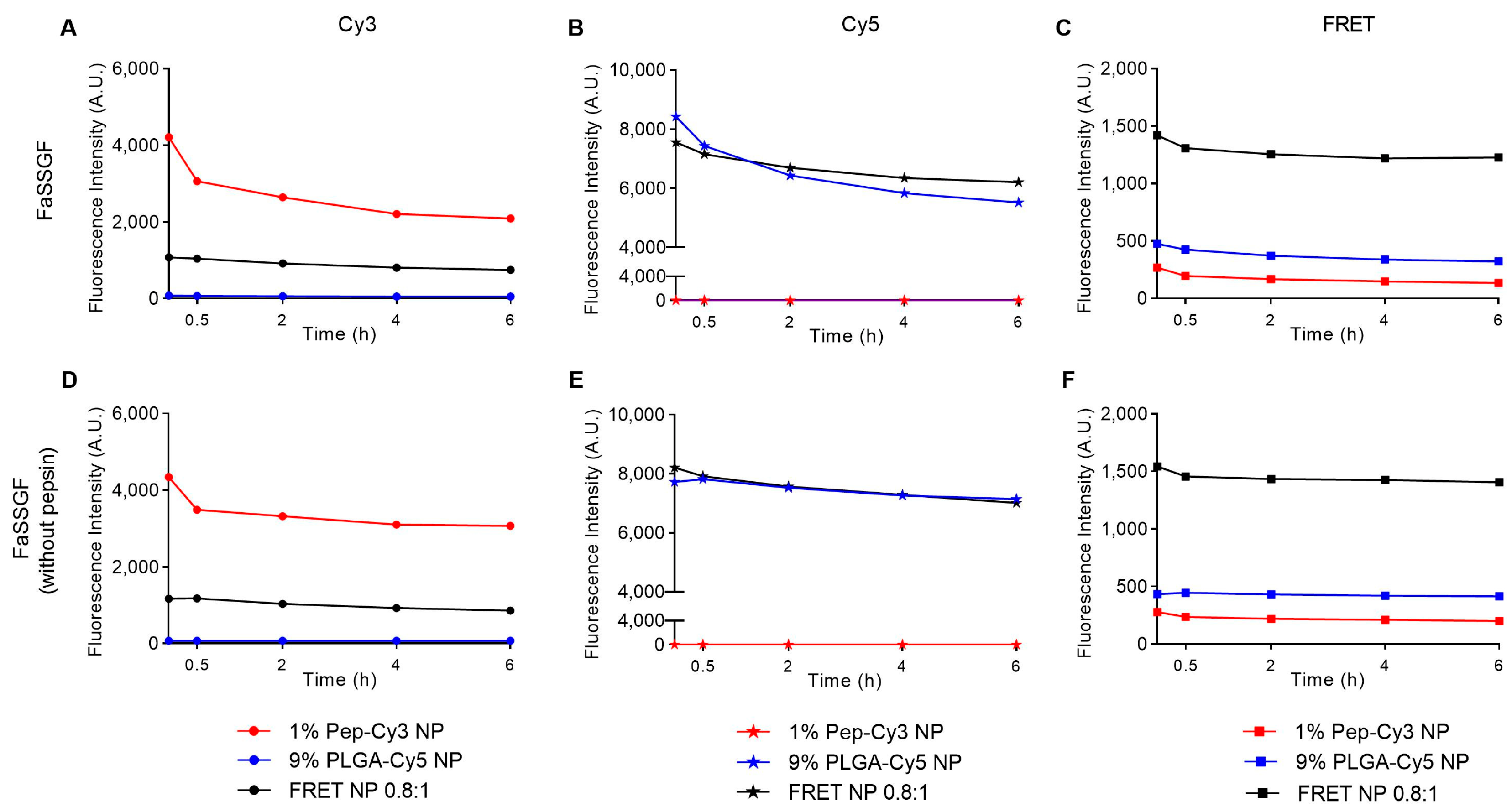
2.4. Colloidal Stability and Time-Dependent Fluorescence of Labeled PLGA NP in PBS and Biorelevant Fasted-State-Simulated Gastric Fluid
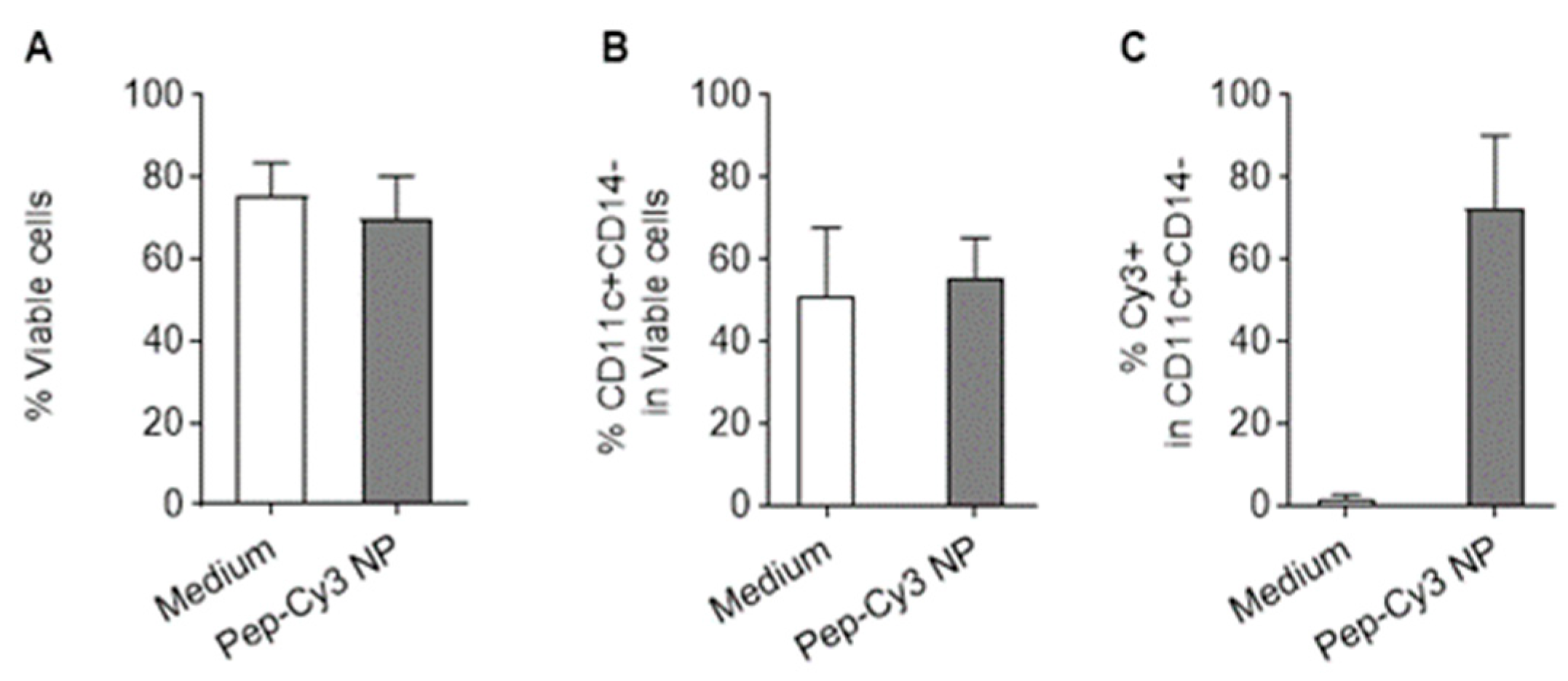
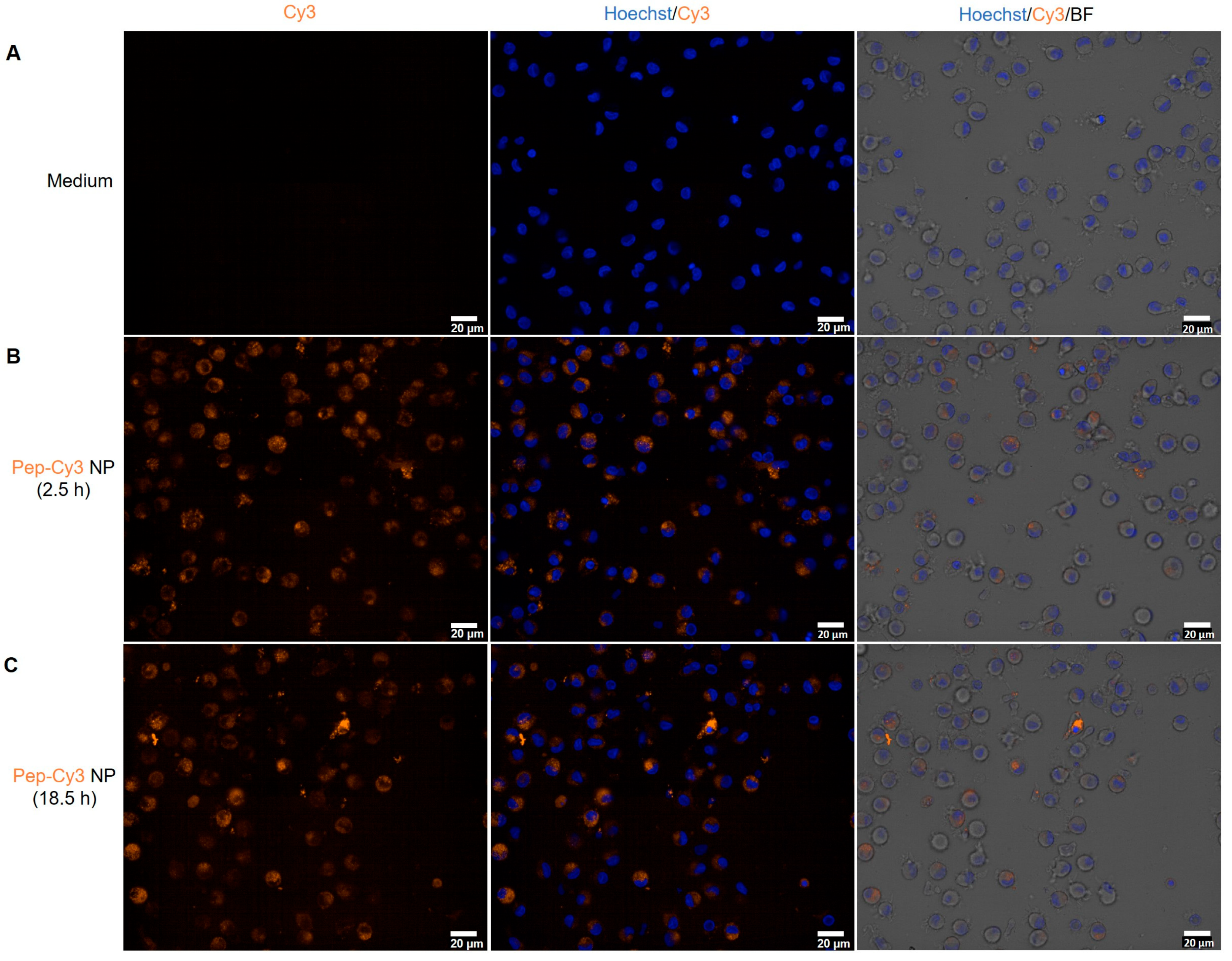
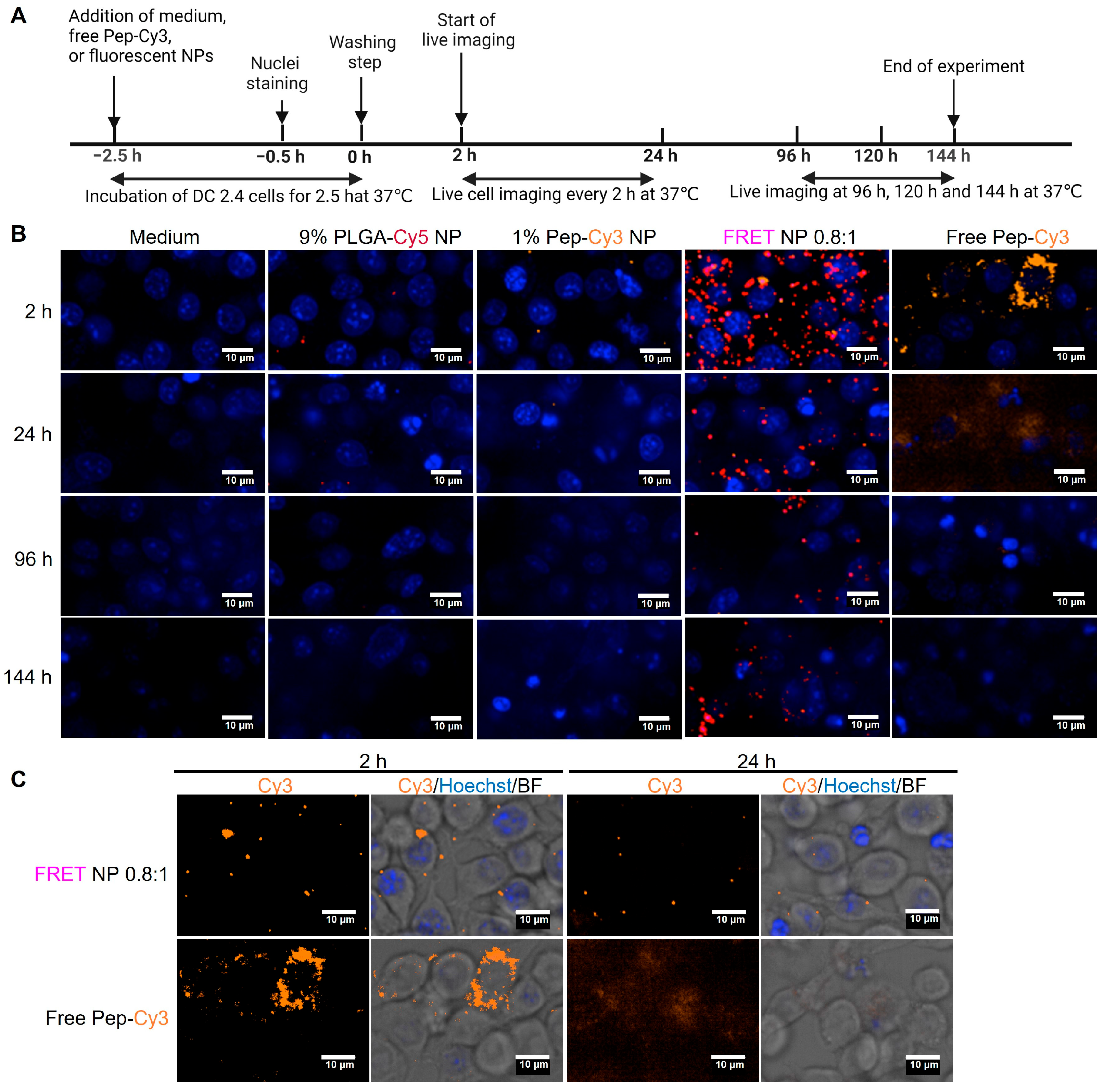
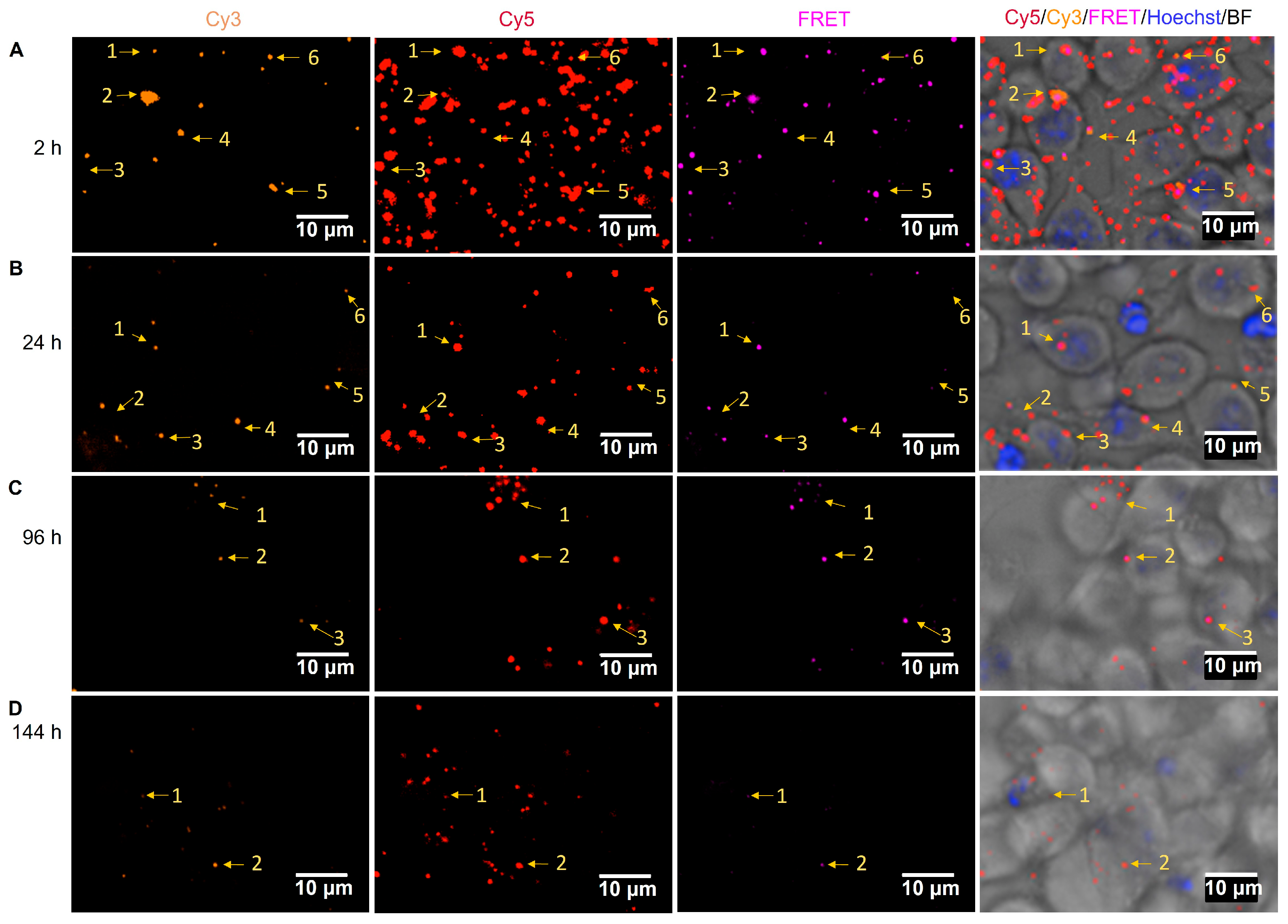
2.5. Cellular Uptake of FRET NPs by Human Immature moDC and Their Intracellular Trafficking in Murine Dendritic Cells DC 2.4
3. Materials and Methods
3.1. Materials
3.2. Solid-Phase Peptide Synthesis and Characterization
3.3. Conjugation of Cy5 to PLGA-COOH
3.4. Preparation of Nanoparticles
3.5. Characterization of the Nanoparticles
3.6. Peptide Encapsulation Efficiency
3.7. Fluorescence Characteristics of Labeled Nanoparticles
3.8. Fluorescence Intensity of Labeled PLGA NP in PBS or Fasted-State-Simulated Gastric Fluid
3.9. Culture of Human-Monocytes-Derived Dendritic Cells (moDC)
3.10. Culture of Murine Bone-Marrow-Derived Dendritic Cell Line DC 2.4
3.11. Internalization of Pep-Cy3/NP by Human moDC Determined by Fluorescence Microscopy and Flow Cytometry
3.12. Cellular Uptake and Intracellular Trafficking of Nanoparticles by Murine DC 2.4
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xiong, Y.; Xu, G.; Chen, M.; Ma, H. Intestinal Uptake and Tolerance to Food Antigens. Front. Immunol. 2022, 13, 906122. [Google Scholar] [CrossRef]
- Domogalla, M.P.; Rostan, P.V.; Raker, V.K.; Steinbrink, K. Tolerance through Education: How Tolerogenic Dendritic Cells Shape Immunity. Front. Immunol. 2017, 8, 1764. [Google Scholar] [CrossRef]
- van Esch, B.C.; Schouten, B.; de Kivit, S.; Hofman, G.A.; Knippels, L.M.; Willemsen, L.E.; Garssen, J. Oral tolerance induction by partially hydrolyzed whey protein in mice is associated with enhanced numbers of Foxp3+ regulatory T-cells in the mesenteric lymph nodes. Pediatr. Allergy Immunol. 2011, 22, 820–826. [Google Scholar] [CrossRef]
- Meulenbroek, L.A.; van Esch, B.C.; Hofman, G.A.; den Hartog Jager, C.F.; Nauta, A.J.; Willemsen, L.E.; Bruijnzeel-Koomen, C.A.; Garssen, J.; van Hoffen, E.; Knippels, L.M. Oral treatment with beta-lactoglobulin peptides prevents clinical symptoms in a mouse model for cow’s milk allergy. Pediatr. Allergy Immunol. 2013, 24, 656–664. [Google Scholar] [CrossRef]
- Liu, M.; Thijssen, S.; van Nostrum, C.F.; Hennink, W.E.; Garssen, J.; Willemsen, L.E.M. Inhibition of cow’s milk allergy development in mice by oral delivery of beta-lactoglobulin-derived peptides loaded PLGA nanoparticles is associated with systemic whey-specific immune silencing. Clin. Exp. Allergy 2022, 52, 137–148. [Google Scholar] [CrossRef]
- Miquel-Clopes, A.; Bentley, E.G.; Stewart, J.P.; Carding, S.R. Mucosal vaccines and technology. Clin. Exp. Immunol. 2019, 196, 205–214. [Google Scholar] [CrossRef]
- Gu, P.; Wusiman, A.; Zhang, Y.; Liu, Z.; Bo, R.; Hu, Y.; Liu, J.; Wang, D. Rational Design of PLGA Nanoparticle Vaccine Delivery Systems To Improve Immune Responses. Mol. Pharm. 2019, 16, 5000–5012. [Google Scholar] [CrossRef]
- Silva, A.L.; Rosalia, R.A.; Varypataki, E.; Sibuea, S.; Ossendorp, F.; Jiskoot, W. Poly-(lactic-co-glycolic-acid)-based particulate vaccines: Particle uptake by dendritic cells is a key parameter for immune activation. Vaccine 2015, 33, 847–854. [Google Scholar] [CrossRef]
- Cappellano, G.; Comi, C.; Chiocchetti, A.; Dianzani, U. Exploiting PLGA-Based Biocompatible Nanoparticles for Next-Generation Tolerogenic Vaccines against Autoimmune Disease. Int. J. Mol. Sci. 2019, 20, 204. [Google Scholar] [CrossRef]
- Chen, T.; Liu, W.; Xiong, S.; Li, D.; Fang, S.; Wu, Z.; Wang, Q.; Chen, X. Nanoparticles Mediating the Sustained Puerarin Release Facilitate Improved Brain Delivery to Treat Parkinson’s Disease. ACS Appl. Mater. Interfaces 2019, 11, 45276–45289. [Google Scholar] [CrossRef]
- Rahimian, S.; Fransen, M.F.; Kleinovink, J.W.; Amidi, M.; Ossendorp, F.; Hennink, W.E. Particulate Systems Based on Poly(Lactic-co-Glycolic)Acid (pLGA) for Immunotherapy of Cancer. Curr. Pharm. Des. 2015, 21, 4201–4216. [Google Scholar] [CrossRef]
- Gouw, J.W.; Jo, J.; Meulenbroek, L.; Heijjer, T.S.; Kremer, E.; Sandalova, E.; Knulst, A.C.; Jeurink, P.V.; Garssen, J.; Rijnierse, A.; et al. Identification of peptides with tolerogenic potential in a hydrolysed whey-based infant formula. Clin. Exp. Allergy 2018, 48, 1345–1353. [Google Scholar] [CrossRef]
- Sturniolo, T.; Bono, E.; Ding, J.; Raddrizzani, L.; Tuereci, O.; Sahin, U.; Braxenthaler, M.; Gallazzi, F.; Protti, M.P.; Sinigaglia, F. Generation of tissue-specific and promiscuous HLA ligand databases using DNA microarrays and virtual HLA class II matrices. Nat. Biotechnol. 1999, 17, 555–561. [Google Scholar]
- Holland, C.J.; Cole, D.K.; Godkin, A. Re-Directing CD4(+) T Cell Responses with the Flanking Residues of MHC Class II-Bound Peptides: The Core is Not Enough. Front. Immunol. 2013, 4, 172. [Google Scholar] [CrossRef]
- Rudolph, M.G.; Stanfield, R.L.; Wilson, I.A. How TCRs bind MHCs, peptides, and coreceptors. Annu. Rev. Immunol. 2006, 24, 419–466. [Google Scholar] [CrossRef]
- Landsverk, O.J.; Bakke, O.; Gregers, T.F. MHC II and the endocytic pathway: Regulation by invariant chain. Scand. J. Immunol. 2009, 70, 184–193. [Google Scholar] [CrossRef]
- Schulze, M.S.; Wucherpfennig, K.W. The mechanism of HLA-DM induced peptide exchange in the MHC class II antigen presentation pathway. Curr. Opin. Immunol. 2012, 24, 105–111. [Google Scholar] [CrossRef]
- Weber, D.A.; Evavold, B.D.; Jensen, P.E. Enhanced Dissociation of HLA-DR-Bound Peptides in the Presence of HLA-DM. Science 1996, 274, 618–620. [Google Scholar]
- Lovitch, S.B.; Pu, Z.; Unanue, E.R. Amino-terminal flanking residues determine the conformation of a peptide-class II MHC complex. J. Immunol. 2006, 176, 2958–2968. [Google Scholar] [CrossRef]
- Godkin, A.J.; Smith, K.J.; Willis, A.; Tejada-Simon, M.V.; Zhang, J.; Elliott, T.; Hill, A.V. Naturally processed HLA class II peptides reveal highly conserved immunogenic flanking region sequence preferences that reflect antigen processing rather than peptide-MHC interactions. J. Immunol. 2001, 166, 6720–6727. [Google Scholar] [CrossRef]
- Ishikawa-Ankerhold, H.C.; Ankerhold, R.; Drummen, G.P. Advanced fluorescence microscopy techniques—FRAP, FLIP, FLAP, FRET and FLIM. Molecules 2012, 17, 4047–4132. [Google Scholar] [CrossRef]
- Algar, W.R.; Hildebrandt, N.; Vogel, S.S.; Medintz, I.L. FRET as a biomolecular research tool—understanding its potential while avoiding pitfalls. Nat. Methods 2019, 16, 815–829. [Google Scholar] [CrossRef]
- Kaeokhamloed, N.; Legeay, S.; Roger, E. FRET as the tool for in vivo nanomedicine tracking. J. Control. Release 2022, 349, 156–173. [Google Scholar] [CrossRef]
- Chen, T.; He, B.; Tao, J.; He, Y.; Deng, H.; Wang, X.; Zheng, Y. Application of Forster Resonance Energy Transfer (FRET) technique to elucidate intracellular and In Vivo biofate of nanomedicines. Adv. Drug Deliv. Rev. 2019, 143, 177–205. [Google Scholar] [CrossRef]
- Swider, E.; Maharjan, S.; Houkes, K.; van Riessen, N.K.; Figdor, C.; Srinivas, M.; Tagit, O. Forster Resonance Energy Transfer-Based Stability Assessment of PLGA Nanoparticles in Vitro and in Vivo. ACS Appl. Bio Mater. 2019, 2, 1131–1140. [Google Scholar] [CrossRef]
- Zhang, H.; Li, H.; Cao, Z.; Du, J.; Yan, L.; Wang, J. Investigation of the in vivo integrity of polymeric micelles via large Stokes shift fluorophore-based FRET. J. Control. Release 2020, 324, 47–54. [Google Scholar] [CrossRef]
- Lin, Z.; Xi, L.; Chen, S.; Tao, J.; Wang, Y.; Chen, X.; Li, P.; Wang, Z.; Zheng, Y. Uptake and trafficking of different sized PLGA nanoparticles by dendritic cells in imiquimod-induced psoriasis-like mice model. Acta Pharm. Sin. B 2021, 11, 1047–1055. [Google Scholar] [CrossRef]
- Merrifield, B. Solid phase synthesis. Science 1986, 232, 341–347. [Google Scholar] [CrossRef]
- Swami, A.; Reagan, M.R.; Basto, P.; Mishima, Y.; Kamaly, N.; Glavey, S.; Zhang, S.; Moschetta, M.; Seevaratnam, D.; Zhang, Y.; et al. Engineered nanomedicine for myeloma and bone microenvironment targeting. Proc. Natl. Acad. Sci. USA 2014, 111, 10287–10292. [Google Scholar] [CrossRef]
- Yang, G.; Liu, Y.; Hui, Y.; Tengjisi; Chen, D.; Weitz, D.A.; Zhao, C.X. Implications of Quenching-to-Dequenching Switch in Quantitative Cell Uptake and Biodistribution of Dye-Labeled Nanoparticles. Angew. Chem. Int. Ed. 2021, 60, 15426–15435. [Google Scholar] [CrossRef]
- Filipe, V.; Hawe, A.; Jiskoot, W. Critical evaluation of Nanoparticle Tracking Analysis (NTA) by NanoSight for the measurement of nanoparticles and protein aggregates. Pharm. Res. 2010, 27, 796–810. [Google Scholar] [CrossRef]
- Preus, S.; Wilhelmsson, L.M. Advances in quantitative FRET-based methods for studying nucleic acids. ChemBioChem 2012, 13, 1990–2001. [Google Scholar] [CrossRef]
- Chen, H.; Puhl, H.L., 3rd; Koushik, S.V.; Vogel, S.S.; Ikeda, S.R. Measurement of FRET efficiency and ratio of donor to acceptor concentration in living cells. Biophys. J. 2006, 91, L39–L41. [Google Scholar] [CrossRef]
- Rescignano, N.; Tarpani, L.; Romani, A.; Bicchi, I.; Mattioli, S.; Emiliani, C.; Torre, L.; Kenny, J.M.; Martino, S.; Latterini, L.; et al. In-vitro degradation of PLGA nanoparticles in aqueous medium and in stem cell cultures by monitoring the cargo fluorescence spectrum. Polym. Degrad. Stab. 2016, 134, 296–304. [Google Scholar] [CrossRef]
- Liu, M.; Thijssen, S.; Hennink, W.E.; Garssen, J.; van Nostrum, C.F.; Willemsen, L.E.M. Oral pretreatment with β-lactoglobulin derived peptide and CpG co-encapsulated in PLGA nanoparticles prior to sensitizations attenuates cow’s milk allergy development in mice. Front. Immunol. 2023, 13, 3107. [Google Scholar] [CrossRef]
- Koerner, J.; Horvath, D.; Groettrup, M. Harnessing Dendritic Cells for Poly (D,L-lactide-co-glycolide) Microspheres (PLGA MS)-Mediated Anti-tumor Therapy. Front. Immunol. 2019, 10, 707. [Google Scholar] [CrossRef]
- Kostadinova, A.I.; Middelburg, J.; Ciulla, M.; Garssen, J.; Hennink, W.E.; Knippels, L.M.J.; van Nostrum, C.F.; Willemsen, L.E.M. PLGA nanoparticles loaded with beta-lactoglobulin-derived peptides modulate mucosal immunity and may facilitate cow’s milk allergy prevention. Eur. J. Pharmacol. 2018, 818, 211–220. [Google Scholar] [CrossRef]
- Waeckerle-Men, Y.; Scandella, E.; Uetz-Von Allmen, E.; Ludewig, B.; Gillessen, S.; Merkle, H.P.; Gander, B.; Groettrup, M. Phenotype and functional analysis of human monocyte-derived dendritic cells loaded with biodegradable poly(lactide-co-glycolide) microspheres for immunotherapy. J. Immunol. Methods 2004, 287, 109–124. [Google Scholar] [CrossRef]
- Lim, J.P.; Gleeson, P.A. Macropinocytosis: An endocytic pathway for internalising large gulps. Immunol. Cell Biol. 2011, 89, 836–843. [Google Scholar] [CrossRef]
- Diwan, M.; Elamanchili, P.; Lane, H.; Gainer, A.; Samuel, J. Biodegradable Nanoparticle Mediated Antigen Delivery to Human Cord Blood Derived Dendritic Cells for Induction of Primary T Cell Responses. J. Drug Target. 2008, 11, 495–507. [Google Scholar] [CrossRef]
- Panyam, J.; Labhasetwar, V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv. Drug Deliv. Rev. 2003, 55, 329–347. [Google Scholar] [CrossRef]
- Cartiera, M.S.; Johnson, K.M.; Rajendran, V.; Caplan, M.J.; Saltzman, W.M. The uptake and intracellular fate of PLGA nanoparticles in epithelial cells. Biomaterials 2009, 30, 2790–2798. [Google Scholar] [CrossRef]
- Schuurhuis, D.H.; Ioan-Facsinay, A.; Nagelkerken, B.; van Schip, J.J.; Sedlik, C.; Melief, C.J.; Verbeek, J.S.; Ossendorp, F. Antigen-antibody immune complexes empower dendritic cells to efficiently prime specific CD8+ CTL responses in vivo. J. Immunol. 2002, 168, 2240–2246. [Google Scholar] [CrossRef]
- van Montfoort, N.N.; Camps, M.G.; Khan, S.; Filippov, D.V.; Weterings, J.J.; Griffith, J.M.; Geuze, H.J.; van Hall, T.; Verbeek, J.S.; Melief, C.J.; et al. Antigen storage compartments in mature dendritic cells facilitate prolonged cytotoxic T lymphocyte cross-priming capacity. Proc. Natl. Acad. Sci. USA 2009, 106, 6730–6735. [Google Scholar]
- Frohlich, E. Cellular elimination of nanoparticles. Environ. Toxicol. Pharmacol. 2016, 46, 90–94. [Google Scholar] [CrossRef]
- Labhasetwar, J.P.a.V. Dynamics of Endocytosis and Exocytosis of Poly(D,L-Lactide-co-Glycolide) Nanoparticles in Vascular Smooth Muscle Cells. Pharm. Res. 2003, 20, 212–220. [Google Scholar]
- Liu, Z.; Roche, P.A. Macropinocytosis in phagocytes: Regulation of MHC class-II-restricted antigen presentation in dendritic cells. Front. Physiol. 2015, 6, 1. [Google Scholar] [CrossRef]
- Hamdy, S.; Haddadi, A.; Hung, R.W.; Lavasanifar, A. Targeting dendritic cells with nano-particulate PLGA cancer vaccine formulations. Adv. Drug Deliv. Rev. 2011, 63, 943–955. [Google Scholar] [CrossRef]
- Donahue, N.D.; Acar, H.; Wilhelm, S. Concepts of nanoparticle cellular uptake, intracellular trafficking, and kinetics in nanomedicine. Adv. Drug Deliv. Rev. 2019, 143, 68–96. [Google Scholar] [CrossRef]
- Kamath, A.T.; Henri, S.; Battye, F.; Tough, D.F.; Shortman, K. Developmental kinetics and lifespan ofdendritic cells in mouse lymphoid organs. Blood 2002, 100, 1734–1741. [Google Scholar]
- Vertzoni, M.; Dressman, J.; Butler, J.; Hempenstall, J.; Reppas, C. Simulation of fasting gastric conditions and its importance for the in vivo dissolution of lipophilic compounds. Eur. J. Pharm. Biopharm. 2005, 60, 413–417. [Google Scholar] [CrossRef]
- Vanier, G.S. Microwave-Assisted Solid-Phase Peptide Synthesis Based on the Fmoc Protecting Group Strategy (CEM). In Peptide Synthesis and Applications; Jensen, K., Tofteng Shelton, P., Pedersen, S., Eds.; Humana Press: Totowa, NJ, USA, 2013; Volume 1047. [Google Scholar]
- Bhattacharjee, S. DLS and zeta potential—What they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef]
- Ali, H.; Weigmann, B.; Collnot, E.M.; Khan, S.A.; Windbergs, M.; Lehr, C.M. Budesonide Loaded PLGA Nanoparticles for Targeting the Inflamed Intestinal Mucosa--Pharmaceutical Characterization and Fluorescence Imaging. Pharm. Res. 2016, 33, 1085–1092. [Google Scholar] [CrossRef]
- Monkare, J.; Pontier, M.; van Kampen, E.E.M.; Du, G.; Leone, M.; Romeijn, S.; Nejadnik, M.R.; O’Mahony, C.; Slutter, B.; Jiskoot, W.; et al. Development of PLGA nanoparticle loaded dissolving microneedles and comparison with hollow microneedles in intradermal vaccine delivery. Eur. J. Pharm. Biopharm. 2018, 129, 111–121. [Google Scholar] [CrossRef]
- Macri, C.; Morgan, H.; Villadangos, J.A.; Mintern, J.D. Regulation of dendritic cell function by Fc-gamma-receptors and the neonatal Fc receptor. Mol. Immunol. 2021, 139, 193–201. [Google Scholar] [CrossRef]

| Formulation (n = 1) | Nanosight Analysis 1 | Zetasizer Nano S 2 | Zeta-Potential (mV) | EE 3 (%) | LC 4 (%) | Actual Molar Ratio Pep-Cy3: PLGA-Cy5 | Yield (%) | EPR 5 (%) | |
|---|---|---|---|---|---|---|---|---|---|
| Dh (nm) | Dh (nm) | PDI | |||||||
| 0.1% Pep-Cy3 NP * | - | 616 ± 24 | 0.15 ± 0.06 | −2.5 ± 0.1 | - | - | 63 | - | |
| 0.5% Pep-Cy3 NP * | - | 463 ± 19 | 0.22 ± 0.01 | −2.1 ± 0.2 | 31 | 0.16 | 82 | - | |
| 1% Pep-Cy3 NP * | - | 338 ± 10 | 0.15 ± 0.01 | −1.9 ± 0.4 | 31 | 0.31 | 73 | - | |
| 1.5% Pep-Cy3 NP * | - | 526 ± 10 | 0.20 ± 0.04 | −1.8 ± 0.1 | 44 | 0.66 | 62 | - | |
| 2% Pep-Cy3 NP * | - | 574 ± 22 | 0.15 ± 0.02 | −2.1 ± 0.2 | 51 | 1.02 | 77 | - | |
| Empty PLGA NP | 291 ± 5 | 358 ± 5 | 0.13 ± 0.01 | −2.5 ± 0.4 | - | - | 73 | - | |
| 1.5% PLGA-Cy5 NP | 330 ± 7 | 345 ± 5 | 0.09 ± 0.05 | −2.6 ± 0.3 | - | - | 73 | - | |
| 2% PLGA-Cy5 NP | 304 ± 14 | 372 ± 9 | 0.12 ± 0.02 | −2.5 ± 0.3 | - | - | 85 | - | |
| 4% PLGA-Cy5 NP | 309 ± 3 | 249 ± 6 | 0.08 ± 0.01 | −3.2 ± 0.5 | - | - | 66 | - | |
| 7% PLGA-Cy5 NP | 361 ± 10 | 600 ± 6 | 0.29 ± 0.01 | −2.5 ± 1.6 | - | - | 66 | - | |
| 9% PLGA-Cy5 NP | 170 ± 21 | 631 ± 5 | 0.21 ± 0.01 | −2.1 ± 0.3 | - | - | 68 | - | |
| 1% Pep-Cy3 NP | 260 ± 12 | 253 ± 2 | 0.08 ± 0.02 | −2.7 ± 0.3 | 30 | 0.30 | 56 | - | |
| FRET NP 0.8:1 (1% Pep-Cy3: 9% PLGA-Cy5) | 227 ± 26 | 236 ± 3 | 0.05 ± 0.01 | −2.1 ± 0.3 | 70 | 0.70 | 0.56:1 | 38 | 60 |
| FRET NP 2:1 (1% Pep-Cy3: 4% PLGA-Cy5) | 276 ± 5 | 240 ± 4 | 0.10 ± 0.03 | −2.5 ± 0.5 | 24 | 0.24 | 0.43:1 | 48 | 26 |
| FRET NP 4:1 (1% Pep-Cy3: 2% PLGA-Cy5) | 295 ± 8 | 259 ± 2 | 0.09 ± 0.02 | −2.6 ± 0.4 | 30 | 0.30 | 1.11:1 | 60 | 16 |
| FRET NP 5:1 (1% Pep-Cy3: 1.5% PLGA-Cy5) | 265 ± 6 | 217 ± 6 | 0.07 ± 0.04 | −3.7 ± 1.0 | 16 | 0.16 | 0.77:1 | 58 | 14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; Lau, C.Y.J.; Cabello, I.T.; Garssen, J.; Willemsen, L.E.M.; Hennink, W.E.; van Nostrum, C.F. Live Cell Imaging by Förster Resonance Energy Transfer Fluorescence to Study Trafficking of PLGA Nanoparticles and the Release of a Loaded Peptide in Dendritic Cells. Pharmaceuticals 2023, 16, 818. https://doi.org/10.3390/ph16060818
Liu M, Lau CYJ, Cabello IT, Garssen J, Willemsen LEM, Hennink WE, van Nostrum CF. Live Cell Imaging by Förster Resonance Energy Transfer Fluorescence to Study Trafficking of PLGA Nanoparticles and the Release of a Loaded Peptide in Dendritic Cells. Pharmaceuticals. 2023; 16(6):818. https://doi.org/10.3390/ph16060818
Chicago/Turabian StyleLiu, Mengshan, Chun Yin Jerry Lau, Irene Trillo Cabello, Johan Garssen, Linette E. M. Willemsen, Wim E. Hennink, and Cornelus F. van Nostrum. 2023. "Live Cell Imaging by Förster Resonance Energy Transfer Fluorescence to Study Trafficking of PLGA Nanoparticles and the Release of a Loaded Peptide in Dendritic Cells" Pharmaceuticals 16, no. 6: 818. https://doi.org/10.3390/ph16060818
APA StyleLiu, M., Lau, C. Y. J., Cabello, I. T., Garssen, J., Willemsen, L. E. M., Hennink, W. E., & van Nostrum, C. F. (2023). Live Cell Imaging by Förster Resonance Energy Transfer Fluorescence to Study Trafficking of PLGA Nanoparticles and the Release of a Loaded Peptide in Dendritic Cells. Pharmaceuticals, 16(6), 818. https://doi.org/10.3390/ph16060818






