Selenylated Imidazo [1,2-a]pyridine Induces Apoptosis and Oxidative Stress in 2D and 3D Models of Colon Cancer Cells
Abstract
1. Introduction
2. Results
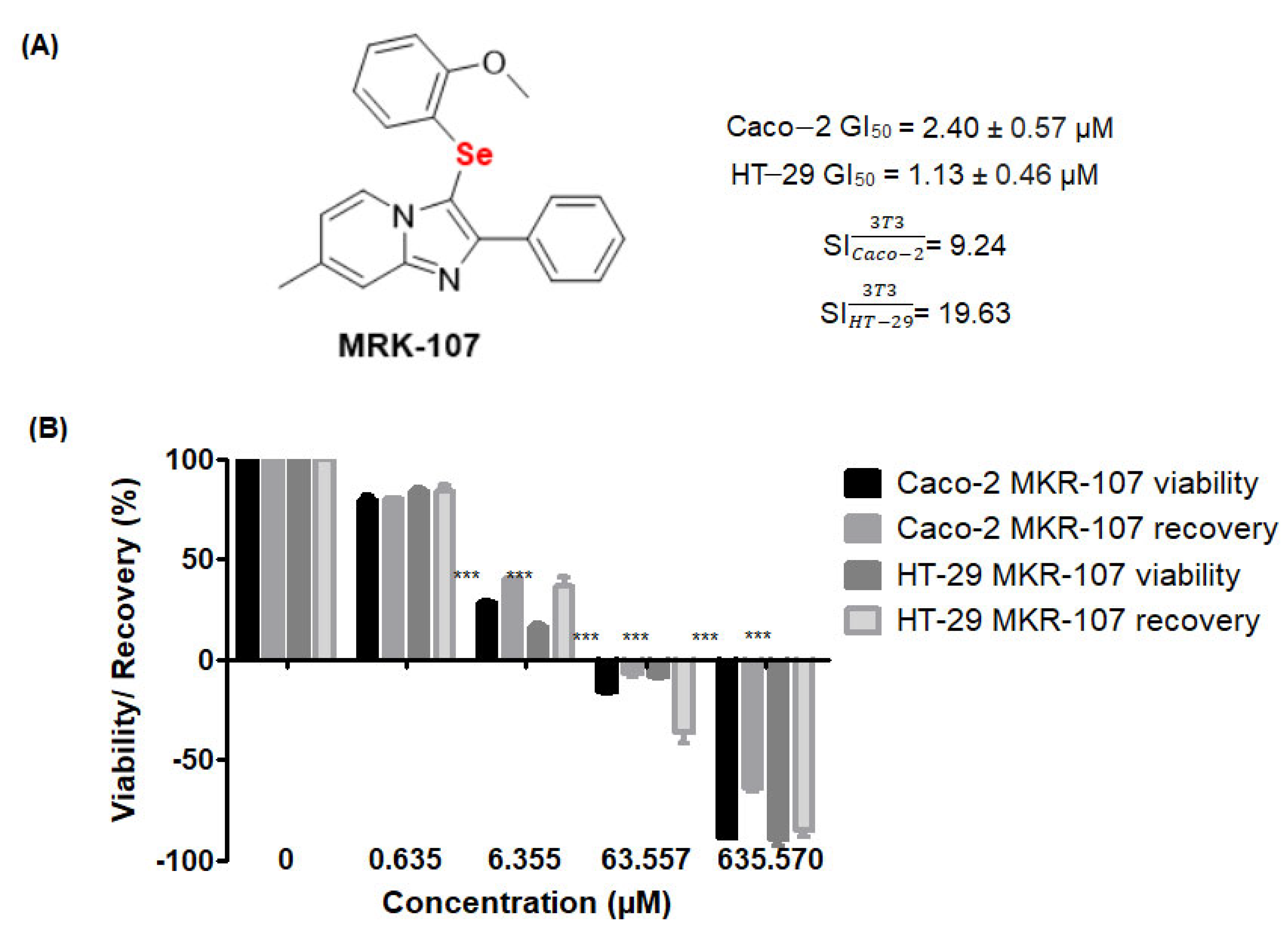
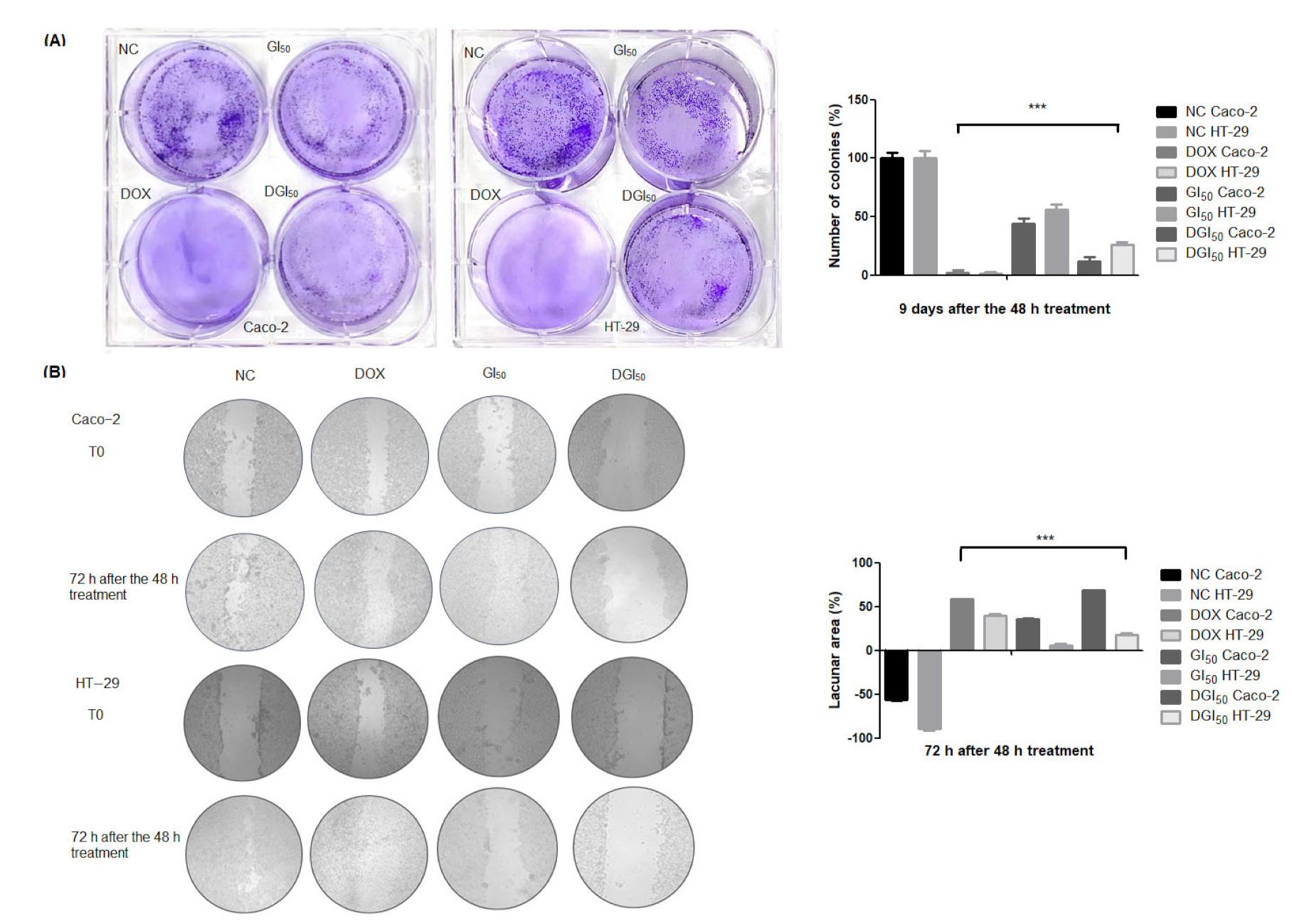
2.1. MRK-107 Selectively Decreased Caco-2 and HT-29 Viability in Monolayers, Affording Colony Reduction and Impaired Healing/Recovery
2.2. MRK-107 Reduces Proliferation
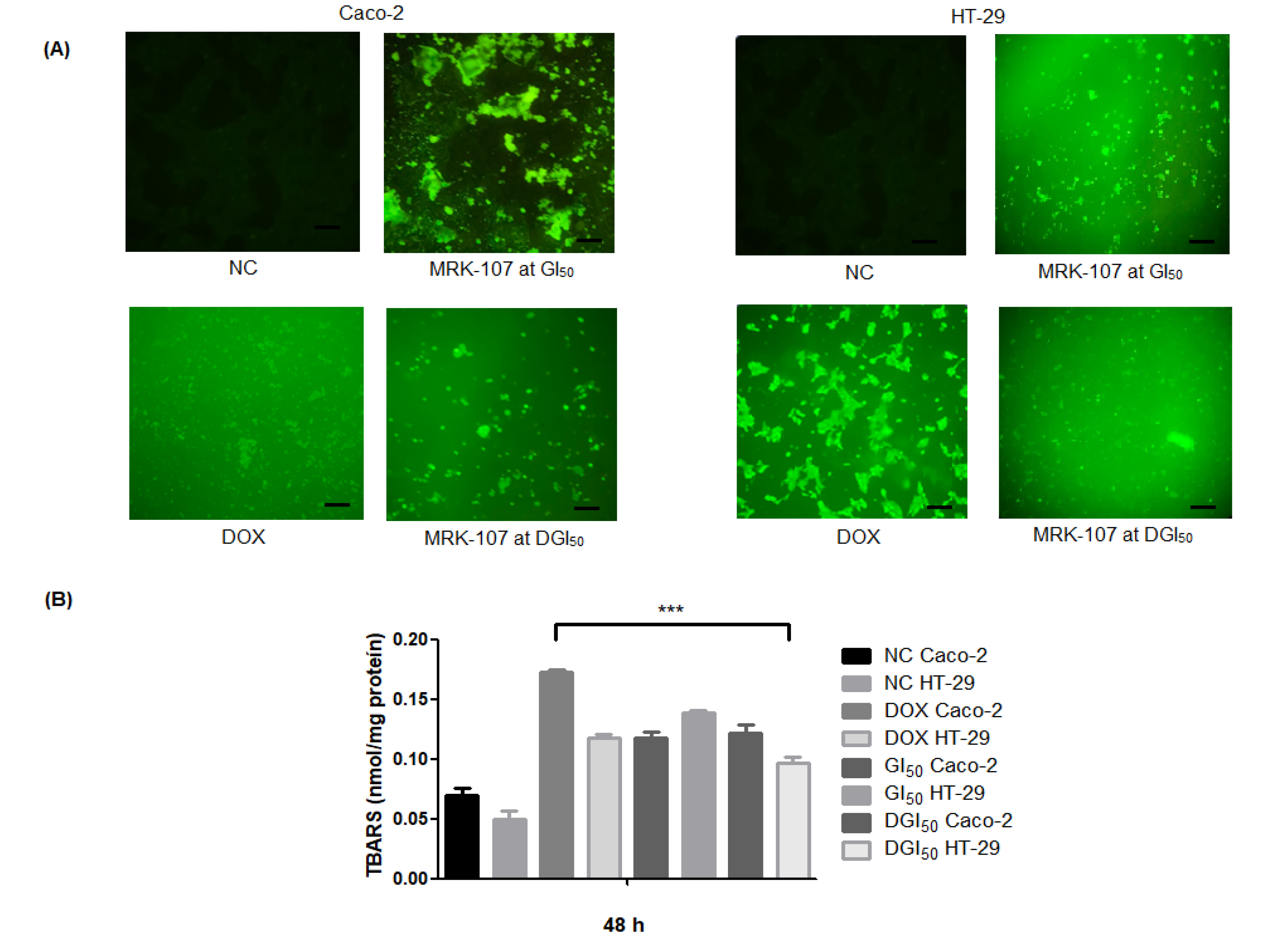
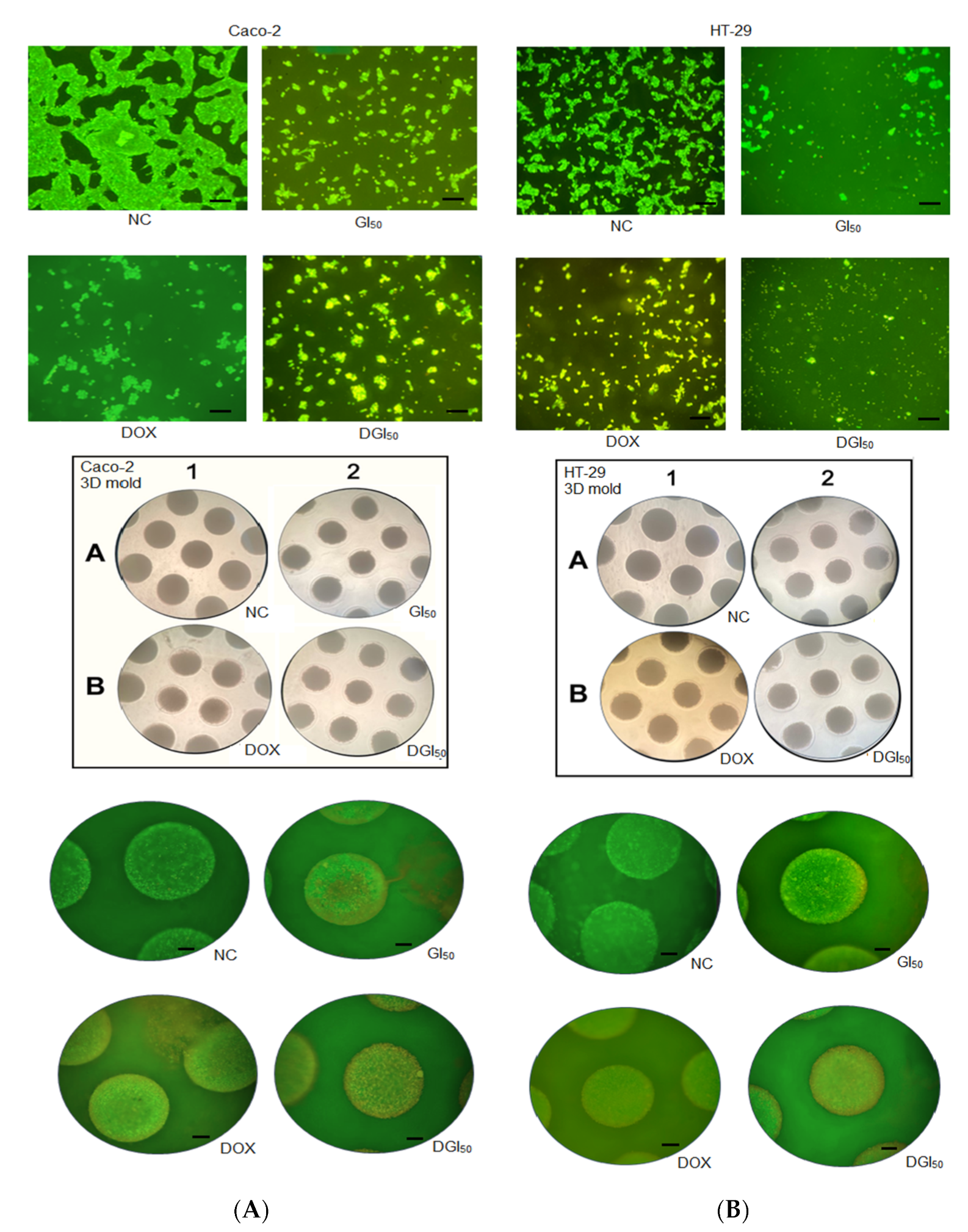
2.3. MRK-107 Causes Oxidative Stress, Generates Protein Damage, and Ruptures DNA in Caco-2 and HT-29 Cells in Monolayer and Spheroid Cultures
2.4. MRK-107 Activates Caspases-3/7 in Caco-2 and HT-29 Cells in Monolayer and Spheroid Cultures
2.5. MRK-107 Induces Apoptosis of Caco-2 and HT-29 Cells
3. Discussion
4. Materials and Methods
4.1. MRK-107 Synthesis
4.2. Cell Lines and Treatments
4.3. Cytotoxicity Assay
4.4. Selectivity Assay
4.5. Assay
4.6. Clonogenic Assay
4.7. Wound-Healing Assay
4.8. Ki-67 Staining
4.9. DCFH-DA
4.10. Spheroid Formation
4.11. TBARS Assay
4.12. Caspases-3/7
4.13. Differential Staining
4.14. Annexin V
4.15. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Wang, D. Inhibition of Colon Cancer Cell Growth by Imidazole Through Activation of Apoptotic Pathway. Med. Sci. Monit. 2019, 25, 7597–7604. [Google Scholar] [CrossRef] [PubMed]
- Kawai, S.; Yamazaki, M.; Shibuya, K.; Fujii, E.; Nakano, K.; Suzuki, M. Three-dimensional culture models mimic colon cancer heterogeneity induced by different microenvironments. Sci. Rep. 2020, 10, 3156. [Google Scholar] [CrossRef] [PubMed]
- Farooqi, A.A.; de la Roche, M.; Djamgoz, M.B.A.; Siddik, Z.H. Overview of the oncogenic signaling pathways in colorectal cancer: Mechanistic insights. Semin. Cancer Biol. 2019, 58, 65–79. [Google Scholar] [CrossRef]
- Radomska, D.; Czarnomysy, R.; Radomski, D.; Bielawski, K. Selenium Compounds as Novel Potential Anticancer Agents. Int. J. Mol. Sci. 2021, 22, 1009. [Google Scholar] [CrossRef]
- Kuršvietienė, L.; Mongirdienė, A.; Bernatonienė, J.; Šulinskienė, J.; Stanevičienė, I. Selenium Anticancer Properties and Impact on Cellular Redox Status. Antioxidants 2020, 9, 80. [Google Scholar] [CrossRef]
- Rodrigues, O.E.D.; de Souza, D.; Soares, L.C.; Dornelles, L.; Burrow, R.A.; Appelt, H.R.; Alves, C.F.; Alves, D.; Braga, A.L. Stereoselective synthesis of selenosteroids. Tetrahedron Lett. 2010, 51, 2237–2240. [Google Scholar] [CrossRef]
- Indira Priyadarsini, K.; Singh, B.G.; Kunwar, A. Current Developments on Synthesis, Redox Reactions and Biochemical Studies of Selenium Antioxidants. Curr. Chem. Biol. 2013, 7, 37–46. [Google Scholar] [CrossRef]
- Frizon, T.E.A.; Cararo, J.H.; Saba, S.; Dal-Pont, G.C.; Michels, M.; Braga, H.C.; Pimentel, T.; Dal-Pizzol, F.; Valvassori, S.S.; Rafique, J. Synthesis of Novel Selenocyanates and Evaluation of Their Effect in Cultured Mouse Neurons Submitted to Oxidative Stress. Oxid. Med. Cell Longev. 2020, 2020, 5417024. [Google Scholar] [CrossRef]
- He, J.; Wu, Z.; Pan, D.; Guo, Y.; Zeng, X. Effect of selenylation modification on antitumor activity of peptidoglycan from Lactobacillus acidophilus. Carbohydr. Polym. 2017, 165, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.K.; Kline, C.L.; Berg, A.; Amin, S.; Irby, R.B. The Akt inhibitor ISC-4 activates prostate apoptosis response protein-4 and reduces colon tumor growth in a nude mouse model. Clin. Cancer Res. 2011, 17, 4474–4483. [Google Scholar] [CrossRef]
- Burkner, G.T.; Dias, D.A.; Souza, K.F.; Araújo, A.J.; Basilio, D.C.; Jacobsen, F.T.; Moraes, A.C.; Silva-Filho, S.E.; Cavalcante, M.F.; Moraes, C.A.; et al. Selenylated Imidazo [1,2-a]pyridine Induces Cell Senescence and Oxidative Stress in Chronic Myeloid Leukemia Cells. Molecules 2023, 28, 893. [Google Scholar] [CrossRef]
- Dos Santos, D.C.; Rafique, J.; Saba, S.; Almeida, G.M.; Siminski, T.; Pádua, C.; Filho, D.W.; Zamoner, A.; Braga, A.L.; Pedrosa, R.C.; et al. Apoptosis oxidative damage-mediated and antiproliferative effect of selenylated imidazo [1,2-a]pyridines on hepatocellular carcinoma HepG2 cells and in vivo. J. Biochem. Mol. Toxicol. 2021, 35, e22663. [Google Scholar] [CrossRef]
- Dos Santos, D.C.; Rafique, J.; Saba, S.; Grinevicius, V.M.A.S.; Filho, D.W.; Zamoner, A.; Braga, A.L.; Pedrosa, R.C.; Ourique, F. IP-Se-06, a Selenylated Imidazo [1,2-a] pyridine, Modulates Intracellular Redox State and Causes Akt/mTOR/HIF-1α and MAPK Signaling Inhibition, Promoting Antiproliferative Effect and Apoptosis in Glioblastoma Cells. Oxid. Med. Cell Longev. 2022, 2022, 3710449. [Google Scholar] [CrossRef] [PubMed]
- Almeida, G.M.; Rafique, J.; Saba, S.; Siminski, T.; Mota, N.S.R.S.; Filho, D.W.; Braga, A.L.; Pedrosa, R.C.; Ourique, F. Novel selenylated imidazo [1,2-a]pyridines for breast cancer chemotherapy: Inhibition of cell proliferation by Akt-mediated regulation, DNA cleavage and apoptosis. Biochem. Biophys. Res. Commun. 2018, 503, 1291–1297. [Google Scholar] [CrossRef]
- Domínguez-Álvarez, E.; Plano, D.; Font, M.; Calvo, A.; Prior, C.; Jacob, C.; Palop, J.A.; Sanmartín, C. Synthesis and antiproliferative activity of novel selenoester derivatives. Eur. J. Med. Chem. 2014, 73, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef]
- Hariharan, S.; Dharmaraj, S. Selenium and selenoproteins: It’s role in regulation of inflammation. Inflammopharmacology 2020, 28, 667–695. [Google Scholar] [CrossRef]
- Boulahjar, R.; Rincon Arias, A.; Bolteau, R.; Renault, N.; Coevoet, M.; Barczyk, A.; Duroux, R.; Yous, S.; Melnyk, P.; Agouridas, L. Design and synthesis of 2,6-disubstituted-8-amino imidazo [1,2a]pyridines, a promising privileged structure. Bioorg. Med. Chem. 2018, 26, 3296–3307. [Google Scholar] [CrossRef]
- Lourenço, D.; Lopes, R.; Pestana, C.; Queirós, A.C.; João, C.; Carneiro, E.A. Patient-Derived Multiple Myeloma 3D Models for Personalized Medicine-Are We There Yet? Int. J. Mol. Sci. 2022, 23, 12888. [Google Scholar] [CrossRef] [PubMed]
- Reidy, E.; Leonard, N.A.; Treacy, O.; Ryan, A.E. A 3D View of Colorectal Cancer Models in Predicting Therapeutic Responses and Resistance. Cancers 2021, 13, 227. [Google Scholar] [CrossRef]
- Fitzgerald, A.A.; Li, E.; Weiner, L.M. 3D Culture Systems for Exploring Cancer Immunology. Cancers 2020, 13, 56. [Google Scholar] [CrossRef] [PubMed]
- Veloso, I.C.; Delanogare, E.; Machado, A.E.; Braga, S.P.; Rosa, G.K.; De Bem, A.F.; Rafique, J.; Saba, S.; da Trindade, R.N.; Galetto, F.Z.; et al. A selanylimidazopyridine (3-SePh-IP) reverses the prodepressant- and anxiogenic-like effects of a high-fat/high-fructose diet in mice. J. Pharm. Pharmacol. 2021, 73, 673–681. [Google Scholar] [CrossRef]
- Franco, M.S.; Saba, S.; Rafique, J.; Braga, A.L. KIO. Angew. Chem. Int. Ed. Engl. 2021, 60, 18454–18460. [Google Scholar] [CrossRef] [PubMed]
- Doerner, C.V.; Neto, J.S.S.; Cabreira, C.R.; Saba, S.; Sandjo, L.P.; Rafique, J.; Braga, A.L.; de Assis, F.F. Synthesis of 3-selanyl-isoflavones from 2-hydroxyphenyl enaminones using trichloroisocyanuric acid (TCCA): A sustainable approach. New J. Chem. 2023, 47, 5598–5602. [Google Scholar] [CrossRef]
- Pedroso, G.J.; Costa, D.M.S.; Felipe Kokuszi, L.T.; da Silva, E.B.V.; Cavalcante, M.F.O.; Junca, E.; Moraes, C.A.O.; Pich, C.T.; de Lima, V.R.; Saba, S.; et al. Selenylated indoles: Synthesis, effects on lipid membrane properties and DNA cleavage. New J. Chem. 2023, 47, 2719–2726. [Google Scholar] [CrossRef]
- Peterle, M.M.; Scheide, M.R.; Silva, L.T.; Saba, S.; Rafique, J.; Braga, A.L. Copper-Catalyzed Three-Component Reaction of Oxadiazoles, Elemental Se/S and Aryl Iodides: Synthesis of Chalcogenyl (Se/S)-Oxadiazoles. ChemistrySelect 2018, 3, 13191–13196. [Google Scholar] [CrossRef]
- Rafique, J.; Farias, G.; Saba, S.; Zapp, E.; Bellettini, I.C.; Momoli Salla, C.A.; Bechtold, I.H.; Scheide, M.R.; Santos Neto, J.S.; Monteiro de Souza Junior, D.; et al. Selenylated-oxadiazoles as promising DNA intercalators: Synthesis, electronic structure, DNA interaction and cleavage. Dyes. Pigm. 2020, 180, 108519. [Google Scholar] [CrossRef]
- Saba, S.; Dos Santos, C.R.; Zavarise, B.R.; Naujorks, A.A.S.; Franco, M.S.; Schneider, A.R.; Scheide, M.R.; Affeldt, R.F.; Rafique, J.; Braga, A.L. Photoinduced, Direct C(sp2)−H Bond Azo Coupling of Imidazoheteroarenes and Imidazoanilines with Aryl Diazonium Salts Catalyzed by Eosin Y. Chemistry 2020, 26, 4461–4466. [Google Scholar] [CrossRef] [PubMed]
- Saba, S.; Preve, N.B.; Granja, I.J.A.; Pedroso, G.J.; Cabreira, C.R.; Dreyer, J.P.; Ribeiro, L.F.B.; Horn, A.P.; Marinho, M.A.G.; Bellettini, I.C.; et al. Synthesis of silver nanoparticles coupled with aromatic diselenides: Greener approach, potential against glioma cells and DNA interaction. New J. Chem. 2023, 47, 2727–2735. [Google Scholar] [CrossRef]
- Morgan, E.; Arnold, M.; Gini, A.; Lorenzoni, V.; Cabasag, C.J.; Laversanne, M.; Vignat, J.; Ferlay, J.; Murphy, N.; Bray, F. Global burden of colorectal cancer in 2020 and 2040: Incidence and mortality estimates from GLOBOCAN. Gut 2023, 72, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.H.; Chen, Y.X.; Fang, J.Y. Comprehensive review of targeted therapy for colorectal cancer. Signal Transduct. Target. Ther. 2020, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.P.; Gandin, V. Selenium compounds as therapeutic agents in cancer. Biochim. Biophys. Acta 2015, 1850, 1642–1660. [Google Scholar] [CrossRef]
- Zakharia, Y.; Bhattacharya, A.; Rustum, Y.M. Selenium targets resistance biomarkers enhancing efficacy while reducing toxicity of anti-cancer drugs: Preclinical and clinical development. Oncotarget 2018, 9, 10765–10783. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Pérez, M.; Ali, W.; Marć, M.A.; Handzlik, J.; Domínguez-Álvarez, E. Selenides and Diselenides: A Review of Their Anticancer and Chemopreventive Activity. Molecules 2018, 23, 628. [Google Scholar] [CrossRef]
- Indrayanto, G.; Putra, G.S.; Suhud, F. Validation of in-vitro bioassay methods: Application in herbal drug research. Profiles Drug Subst. Excip. Relat. Methodol. 2021, 46, 273–307. [Google Scholar] [CrossRef]
- Haddad, M.J.; Sztupecki, W.; Delayre-Orthez, C.; Rhazi, L.; Barbezier, N.; Depeint, F.; Anton, P.M. Complexification of In Vitro Models of Intestinal Barriers, A True Challenge for a More Accurate Alternative Approach. Int. J. Mol. Sci. 2023, 24, 3595. [Google Scholar] [CrossRef] [PubMed]
- Chuai, H.; Zhang, S.Q.; Bai, H.; Li, J.; Wang, Y.; Sun, J.; Wen, E.; Zhang, J.; Xin, M. Small molecule selenium-containing compounds: Recent development and therapeutic applications. Eur. J. Med. Chem. 2021, 223, 113621. [Google Scholar] [CrossRef] [PubMed]
- Begines, P.; Sevilla-Horrillo, L.; Puerta, A.; Puckett, R.; Bayort, S.; Lagunes, I.; Maya, I.; Padrón, J.M.; López, Ó.; Fernández-Bolaños, J.G. Masked Phenolic-Selenium Conjugates: Potent and Selective Antiproliferative Agents Overcoming P-gp Resistance. Pharmaceuticals 2020, 13, 358. [Google Scholar] [CrossRef]
- Gandin, V.; Khalkar, P.; Braude, J.; Fernandes, A.P. Organic selenium compounds as potential chemotherapeutic agents for improved cancer treatment. Free Radic. Biol. Med. 2018, 127, 80–97. [Google Scholar] [CrossRef] [PubMed]
- Stefanello, S.T.; Mizdal, C.R.; Gonçalves, D.F.; Hartmann, D.D.; Dobrachinski, F.; de Carvalho, N.R.; Salman, S.M.; Sauer, A.C.; Dornelles, L.; de Campos, M.M.A.; et al. The insertion of functional groups in organic selenium compounds promote changes in mitochondrial parameters and raise the antibacterial activity. Bioorg. Chem. 2020, 98, 103727. [Google Scholar] [CrossRef]
- Metes-Kosik, N.; Luptak, I.; Dibello, P.M.; Handy, D.E.; Tang, S.S.; Zhi, H.; Qin, F.; Jacobsen, D.W.; Loscalzo, J.; Joseph, J. Both selenium deficiency and modest selenium supplementation lead to myocardial fibrosis in mice via effects on redox-methylation balance. Mol. Nutr. Food Res. 2012, 56, 1812–1824. [Google Scholar] [CrossRef] [PubMed]
- Sebaugh, J.L. Guidelines for accurate EC50/IC50 estimation. Pharm. Stat. 2011, 10, 128–134. [Google Scholar] [CrossRef]
- Brix, N.; Samaga, D.; Belka, C.; Zitzelsberger, H.; Lauber, K. Analysis of clonogenic growth in vitro. Nat. Protoc. 2021, 16, 4963–4991. [Google Scholar] [CrossRef]
- Agena, R.; de Jesús Cortés-Sánchez, A.; Hernández-Sánchez, H.; Jaramillo-Flores, M.E. Pro-Apoptotic Activity of Bioactive Compounds from Seaweeds: Promising Sources for Developing Novel Anticancer Drugs. Mar. Drugs 2023, 21, 182. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Gau, C.C.; Lee, W.F.; Lee, W.I.; Huang, J.L.; Chen, S.H.; Yeh, H.Y.; Liang, C.J.; Fu, S.H. Comparison of [3H]-Thymidine, Carboxyfluorescein Diacetate Succinimidyl Ester and Ki-67 in Lymphocyte Proliferation. Front. Pediatr. 2022, 10, 638549. [Google Scholar] [CrossRef] [PubMed]
- Sobecki, M.; Mrouj, K.; Colinge, J.; Gerbe, F.; Jay, P.; Krasinska, L.; Dulic, V.; Fisher, D. Cell-Cycle Regulation Accounts for Variability in Ki-67 Expression Levels. Cancer Res. 2017, 77, 2722–2734. [Google Scholar] [CrossRef] [PubMed]
- Sanmartín, C.; Plano, D.; Sharma, A.K.; Palop, J.A. Selenium compounds, apoptosis and other types of cell death: An overview for cancer therapy. Int. J. Mol. Sci. 2012, 13, 9649–9672. [Google Scholar] [CrossRef]
- Misra, S.; Boylan, M.; Selvam, A.; Spallholz, J.E.; Björnstedt, M. Redox-active selenium compounds—from toxicity and cell death to cancer treatment. Nutrients 2015, 7, 3536–3556. [Google Scholar] [CrossRef]
- Razaghi, A.; Poorebrahim, M.; Sarhan, D.; Björnstedt, M. Selenium stimulates the antitumour immunity: Insights to future research. Eur. J. Cancer 2021, 155, 256–267. [Google Scholar] [CrossRef]
- Marrocco, I.; Altieri, F.; Peluso, I. Measurement and Clinical Significance of Biomarkers of Oxidative Stress in Humans. Oxid. Med. Cell Longev. 2017, 2017, 6501046. [Google Scholar] [CrossRef] [PubMed]
- Lian, P.; Braber, S.; Varasteh, S.; Wichers, H.J.; Folkerts, G. Hypoxia and heat stress affect epithelial integrity in a Caco-2/HT-29 co-culture. Sci. Rep. 2021, 11, 13186. [Google Scholar] [CrossRef]
- Aboelella, N.S.; Brandle, C.; Kim, T.; Ding, Z.C.; Zhou, G. Oxidative Stress in the Tumor Microenvironment and Its Relevance to Cancer Immunotherapy. Cancers 2021, 13, 986. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, U.; Groscurth, P. Morphological features of cell death. News Physiol. Sci. 2004, 19, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Qiu, Z.; Jin, Q.; Chen, G.; Guo, M. Cell Cycle Arrest and Apoptosis in HT-29 Cells Induced by Dichloromethane Fraction From. Front. Pharmacol. 2018, 9, 629. [Google Scholar] [CrossRef]
- Gupta, R.; Sharma, D. Therapeutic response differences between 2D and 3D tumor models of magnetic hyperthermia. Nanoscale Adv. 2021, 3, 3663–3680. [Google Scholar] [CrossRef]
- Basak, D.; Uddin, M.N.; Hancock, J. The Role of Oxidative Stress and Its Counteractive Utility in Colorectal Cancer (CRC). Cancers 2020, 12, 3336. [Google Scholar] [CrossRef]
- Wang, Y.N.; Zeng, Z.L.; Lu, J.; Wang, Y.; Liu, Z.X.; He, M.M.; Zhao, Q.; Wang, Z.X.; Li, T.; Lu, Y.X.; et al. CPT1A-mediated fatty acid oxidation promotes colorectal cancer cell metastasis by inhibiting anoikis. Oncogene 2018, 37, 6025–6040. [Google Scholar] [CrossRef]
- Darling, N.J.; Mobbs, C.L.; González-Hau, A.L.; Freer, M.; Przyborski, S. Bioengineering Novel. Front. Bioeng. Biotechnol. 2020, 8, 992. [Google Scholar] [CrossRef]
- Koveitypour, Z.; Panahi, F.; Vakilian, M.; Peymani, M.; Seyed Forootan, F.; Nasr Esfahani, M.H.; Ghaedi, K. Signaling pathways involved in colorectal cancer progression. Cell Biosci. 2019, 9, 97. [Google Scholar] [CrossRef]
- Malinowsky, K.; Nitsche, U.; Janssen, K.P.; Bader, F.G.; Späth, C.; Drecoll, E.; Keller, G.; Höfler, H.; Slotta-Huspenina, J.; Becker, K.F. Activation of the PI3K/AKT pathway correlates with prognosis in stage II colon cancer. Br. J. Cancer 2014, 110, 2081–2089. [Google Scholar] [CrossRef] [PubMed]
- Hashem, S.; Ali, T.A.; Akhtar, S.; Nisar, S.; Sageena, G.; Ali, S.; Al-Mannai, S.; Therachiyil, L.; Mir, R.; Elfaki, I.; et al. Targeting cancer signaling pathways by natural products: Exploring promising anti-cancer agents. Biomed. Pharm. 2022, 150, 113054. [Google Scholar] [CrossRef] [PubMed]
- Toubhans, B.; Alkafri, N.; Quintela, M.; James, D.W.; Bissardon, C.; Gazze, S.; Knodel, F.; Proux, O.; Gourlan, A.T.; Rathert, P.; et al. Selenium nanoparticles modulate histone methylation via lysine methyltransferase activity and S-adenosylhomocysteine depletion. Redox Biol. 2023, 61, 102641. [Google Scholar] [CrossRef] [PubMed]
- Paschall, A.V.; Yang, D.; Lu, C.; Choi, J.H.; Li, X.; Liu, F.; Figueroa, M.; Oberlies, N.H.; Pearce, C.; Bollag, W.B.; et al. H3K9 Trimethylation Silences Fas Expression To Confer Colon Carcinoma Immune Escape and 5-Fluorouracil Chemoresistance. J. Immunol. 2015, 195, 1868–1882. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Y.; Zhu, Y.; Li, N.; Li, W.; Shang, C.; Song, G.; Li, S.; Cong, J.; Li, T.; et al. Apoptin induces pyroptosis of colorectal cancer cells via the GSDME-dependent pathway. Int. J. Biol. Sci. 2022, 18, 717–730. [Google Scholar] [CrossRef]
- Protti, S.; Fagnoni, M. Recent Advances in Light-Induced Selenylation. ACS Org. Inorg. Au 2022, 2, 455–463. [Google Scholar] [CrossRef]
- Rusetskaya, N.Y.; Fedotov, I.V.; Koftina, V.A.; Borodulin, V.B. Selenium compounds in redox regulation of inflammation and apoptosis. Biomed. Khim. 2019, 65, 165–179. [Google Scholar] [CrossRef]
- Bettanin, L.; Saba, S.; Doerner, C.V.; Franco, M.S.; Godoi, M.; Rafique, J.; Braga, A.L. NH4I-catalyzed chalcogen(S/Se)-functionalization of 5-membered N-heteroaryls under metal-free conditions. Tetrahedron 2018, 74, 3971–3980. [Google Scholar] [CrossRef]
- Rafique, J.; Saba, S.; Rosário, A.R.; Braga, A.L. Regioselective, Solvent- and Metal-Free Chalcogenation of Imidazo [1,2-a]pyridines by Employing I2/DMSO as the Catalytic Oxidation System. Chemistry 2016, 22, 11854–11862. [Google Scholar] [CrossRef]
- Rafique, J.; Saba, S.; Franco, M.S.; Bettanin, L.; Schneider, A.R.; Silva, L.T.; Braga, A.L. Direct, Metal-free C(sp2)−H Chalcogenation of Indoles and Imidazopyridines with Dichalcogenides Catalysed by KIO3. Chemistry 2018, 24, 4173–4180. [Google Scholar] [CrossRef]
- Saba, S.; Rafique, J.; Franco, M.S.; Schneider, A.R.; Espíndola, L.; Silva, D.O.; Braga, A.L. Rose Bengal catalysed photo-induced selenylation of indoles, imidazoles and arenes: A metal free approach. Org. Biomol. Chem. 2018, 16, 880–885. [Google Scholar] [CrossRef] [PubMed]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Monks, A.; Scudiero, D.; Skehan, P.; Shoemaker, R.; Paull, K.; Vistica, D.; Hose, C.; Langley, J.; Cronise, P.; Vaigro-Wolff, A. Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. J. Natl. Cancer Inst. 1991, 83, 757–766. [Google Scholar] [CrossRef] [PubMed]
- López-Lázaro, M. How many times should we screen a chemical library to discover an anticancer drug? Drug Discov. Today 2015, 20, 167–169. [Google Scholar] [CrossRef]
- Carr, B.I.; Cavallini, A.; Lippolis, C.; D’Alessandro, R.; Messa, C.; Refolo, M.G.; Tafaro, A. Fluoro-Sorafenib (Regorafenib) effects on hepatoma cells: Growth inhibition, quiescence, and recovery. J. Cell Physiol. 2013, 228, 292–297. [Google Scholar] [CrossRef]
- Franken, N.A.; Rodermond, H.M.; Stap, J.; Haveman, J.; van Bree, C. Clonogenic assay of cells in vitro. Nat. Protoc. 2006, 1, 2315–2319. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Rodriguez, L.G.; Wu, X.; Guan, J.L. Wound-healing assay. Methods Mol. Biol. 2005, 294, 23–29. [Google Scholar] [CrossRef]
- Yang, C.W.; Liu, X.J.; Zhao, L.; Che, F.; Yin, Y.; Chen, H.J.; Zhang, B.; Wu, M.; Song, B. Preoperative prediction of gastrointestinal stromal tumors with high Ki-67 proliferation index based on CT features. Ann. Transl. Med. 2021, 9, 1556. [Google Scholar] [CrossRef]
- Possel, H.; Noack, H.; Augustin, W.; Keilhoff, G.; Wolf, G. 2,7-Dihydrodichlorofluorescein diacetate as a fluorescent marker for peroxynitrite formation. FEBS Lett. 1997, 416, 175–178. [Google Scholar] [CrossRef]
- Gomes, G.B.; Zubieta, C.S.; Weber, S.S.; de Lima, D.P.; Reddy, T.N.; Guerrero, A.T.G.; Matos, M.D.F.C.; Parisotto, E.B.; Perdomo, R.T. Thiopyrimidine derivatives induce cytotoxicity, cell cycle arrest and oxidative stress in breast cancer 3D-spheroids. Chem. Pap. 2020, 75, 1211–1220. [Google Scholar] [CrossRef]
- Napolitano, A.P.; Chai, P.; Dean, D.M.; Morgan, J.R. Dynamics of the self-assembly of complex cellular aggregates on micromolded nonadhesive hydrogels. Tissue Eng. 2007, 13, 2087–2094. [Google Scholar] [CrossRef]
- Bird, R.P.; Draper, H.H. Comparative studies on different methods of malonaldehyde determination. Methods Enzymol. 1984, 105, 299–305. [Google Scholar] [CrossRef]
- Wong, W.; Gan, W.L.; Teo, Y.K.; Lew, W.S. Interplay of cell death signaling pathways mediated by alternating magnetic field gradient. Cell Death Discov. 2018, 4, 49. [Google Scholar] [CrossRef]
- McGahon, A.J.; Martin, S.J.; Bissonnette, R.P.; Mahboubi, A.; Shi, Y.; Mogil, R.J.; Nishioka, W.K.; Green, D.R. The end of the (cell) line: Methods for the study of apoptosis in vitro. Methods Cell. Biol. 1995, 46, 153–185. [Google Scholar] [CrossRef]
- Vermes, I.; Haanen, C.; Steffens-Nakken, H.; Reutelingsperger, C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J. Immunol. Methods 1995, 184, 39–51. [Google Scholar] [CrossRef]


| Cell Line | Treatment | Proliferation Index |
|---|---|---|
| Caco-2 | Negative control | 2.67 ± 0.25 |
| Doxorubicin | 1.65 ± 0.27a | |
| GI50 | 1.32 ± 0.15a | |
| HT-29 | DGI50 | 1.25 ± 0.14a |
| Negative control | 2.41 ± 0.22a | |
| Doxorubicin | 1.44 ± 0.19a | |
| GI50 | 1.73 ± 0.27a | |
| DGI50 | 1.33 ± 0.13a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomes, G.B.; Zubieta, C.S.; Guilhermi, J.d.S.; Toffoli-Kadri, M.C.; Beatriz, A.; Rafique, J.; Parisotto, E.B.; Saba, S.; Perdomo, R.T. Selenylated Imidazo [1,2-a]pyridine Induces Apoptosis and Oxidative Stress in 2D and 3D Models of Colon Cancer Cells. Pharmaceuticals 2023, 16, 814. https://doi.org/10.3390/ph16060814
Gomes GB, Zubieta CS, Guilhermi JdS, Toffoli-Kadri MC, Beatriz A, Rafique J, Parisotto EB, Saba S, Perdomo RT. Selenylated Imidazo [1,2-a]pyridine Induces Apoptosis and Oxidative Stress in 2D and 3D Models of Colon Cancer Cells. Pharmaceuticals. 2023; 16(6):814. https://doi.org/10.3390/ph16060814
Chicago/Turabian StyleGomes, Giovana Bicudo, Claudia Stutz Zubieta, Jhefferson dos Santos Guilhermi, Mônica Cristina Toffoli-Kadri, Adilson Beatriz, Jamal Rafique, Eduardo Benedetti Parisotto, Sumbal Saba, and Renata Trentin Perdomo. 2023. "Selenylated Imidazo [1,2-a]pyridine Induces Apoptosis and Oxidative Stress in 2D and 3D Models of Colon Cancer Cells" Pharmaceuticals 16, no. 6: 814. https://doi.org/10.3390/ph16060814
APA StyleGomes, G. B., Zubieta, C. S., Guilhermi, J. d. S., Toffoli-Kadri, M. C., Beatriz, A., Rafique, J., Parisotto, E. B., Saba, S., & Perdomo, R. T. (2023). Selenylated Imidazo [1,2-a]pyridine Induces Apoptosis and Oxidative Stress in 2D and 3D Models of Colon Cancer Cells. Pharmaceuticals, 16(6), 814. https://doi.org/10.3390/ph16060814










