Antiplasmodial, Trypanocidal, and Genotoxicity In Vitro Assessment of New Hybrid α,α-Difluorophenylacetamide-statin Derivatives
Abstract
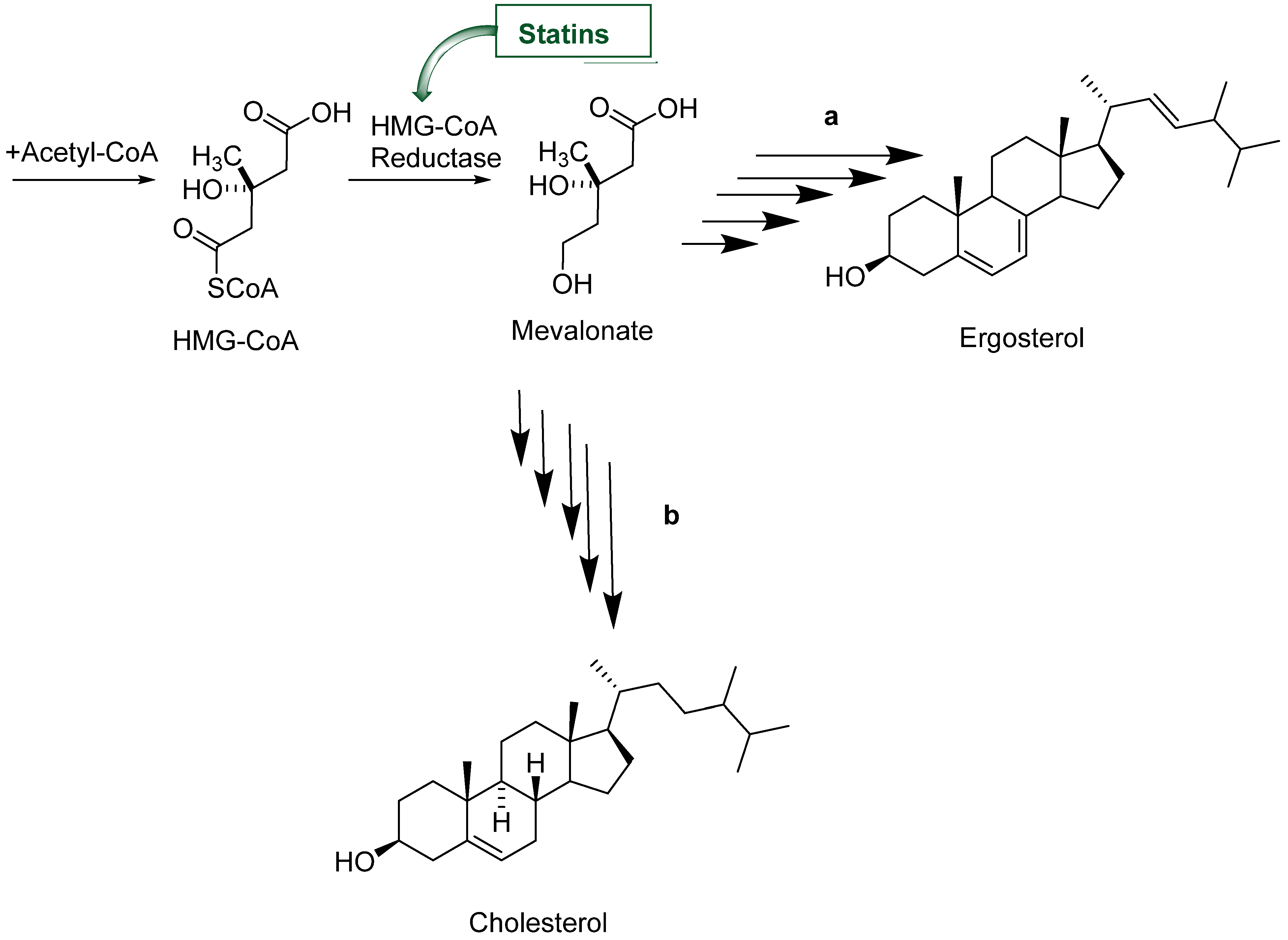
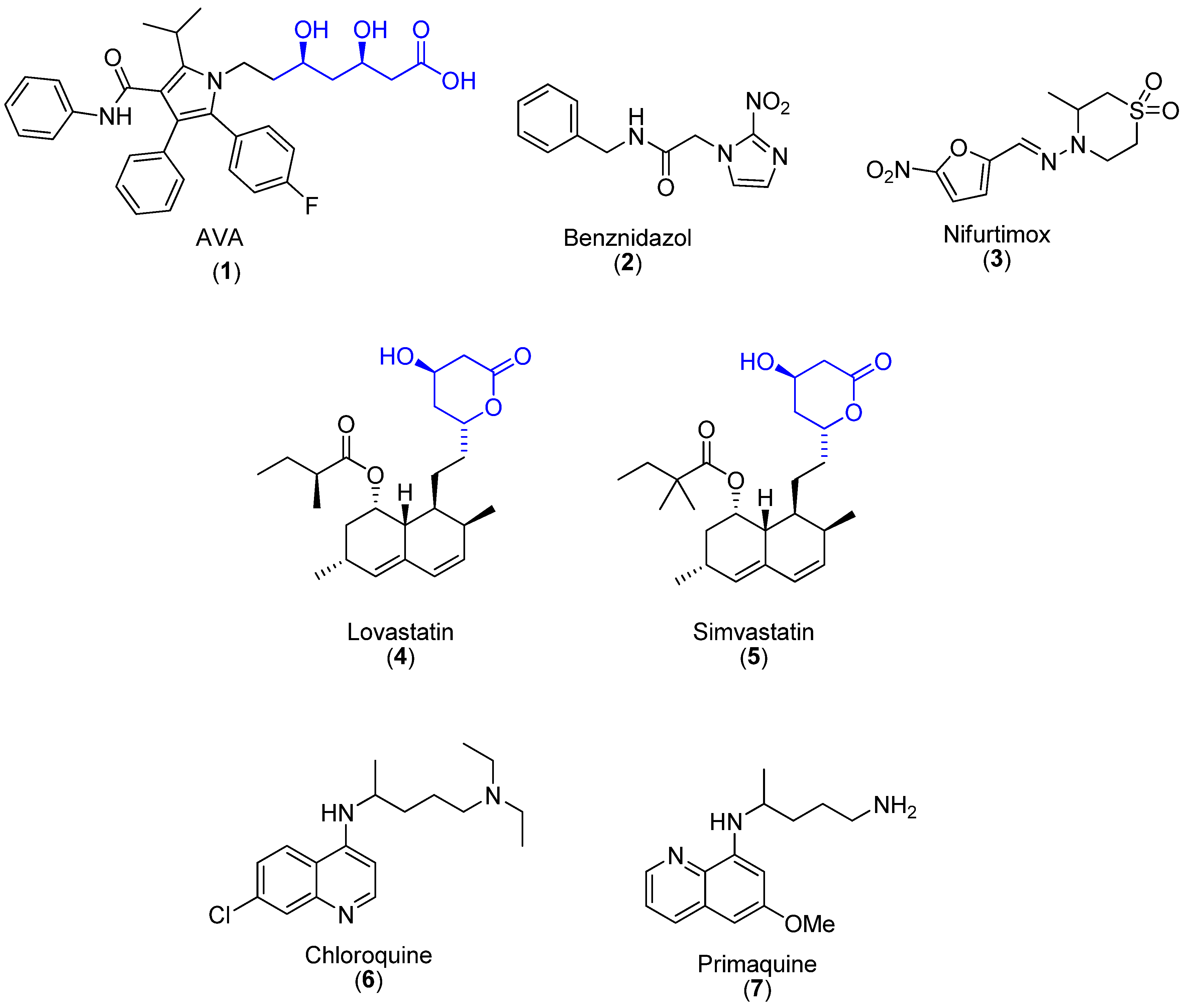
1. Introduction
2. Results and Discussion
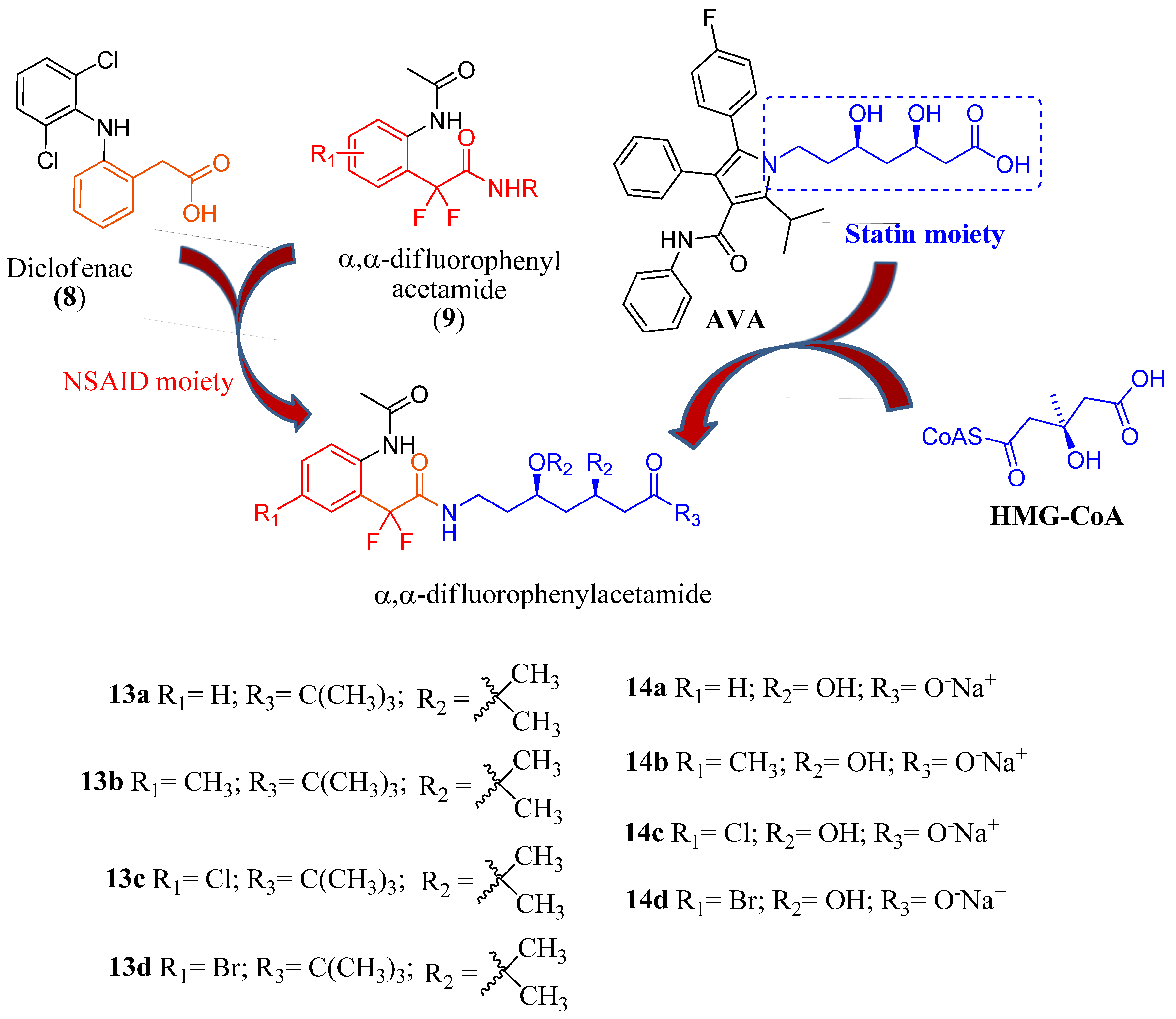
2.1. Chemistry
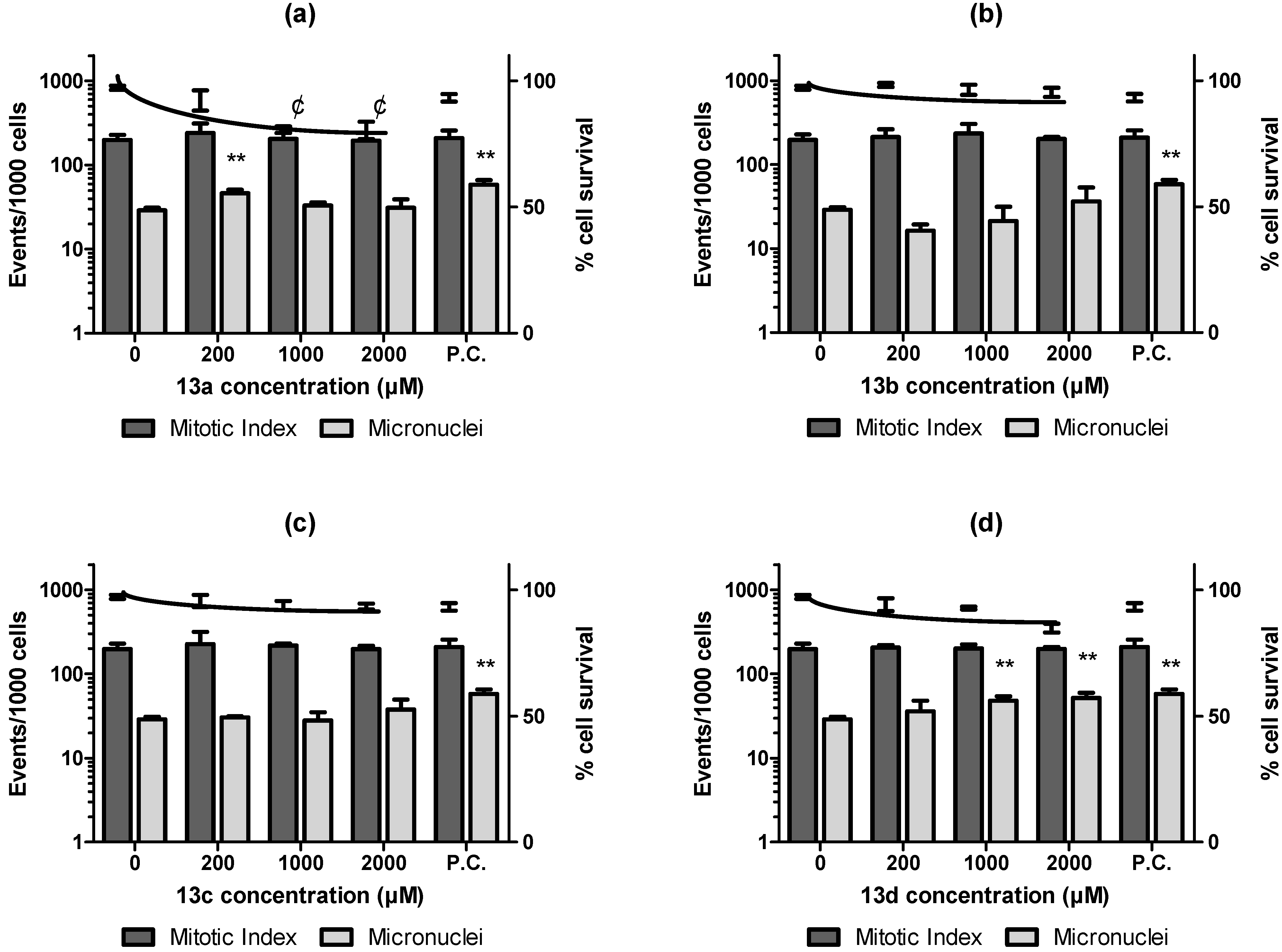
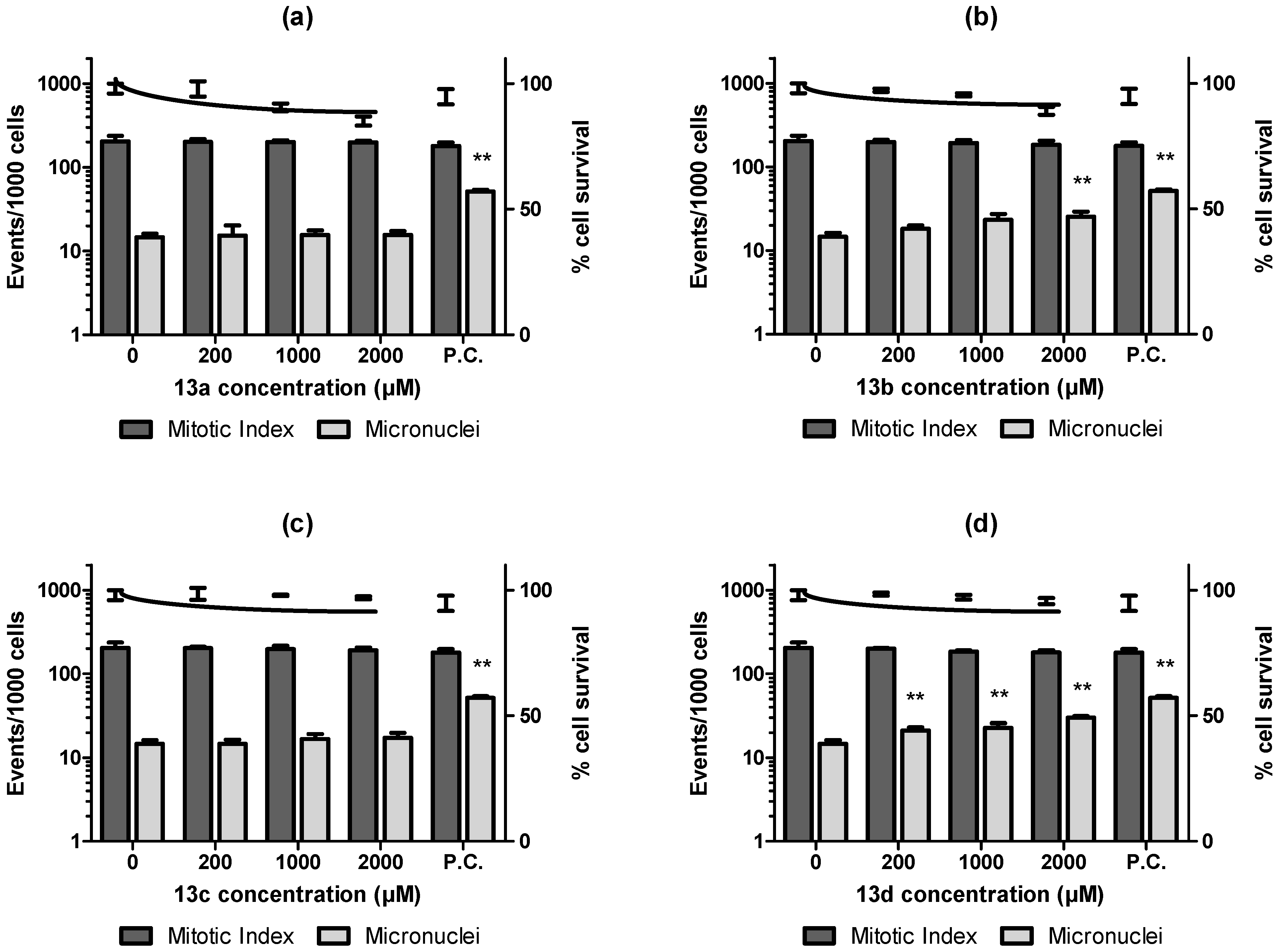
2.2. Biological Evaluation
3. Conclusions
4. Materials and Methods
4.1. Chemistry
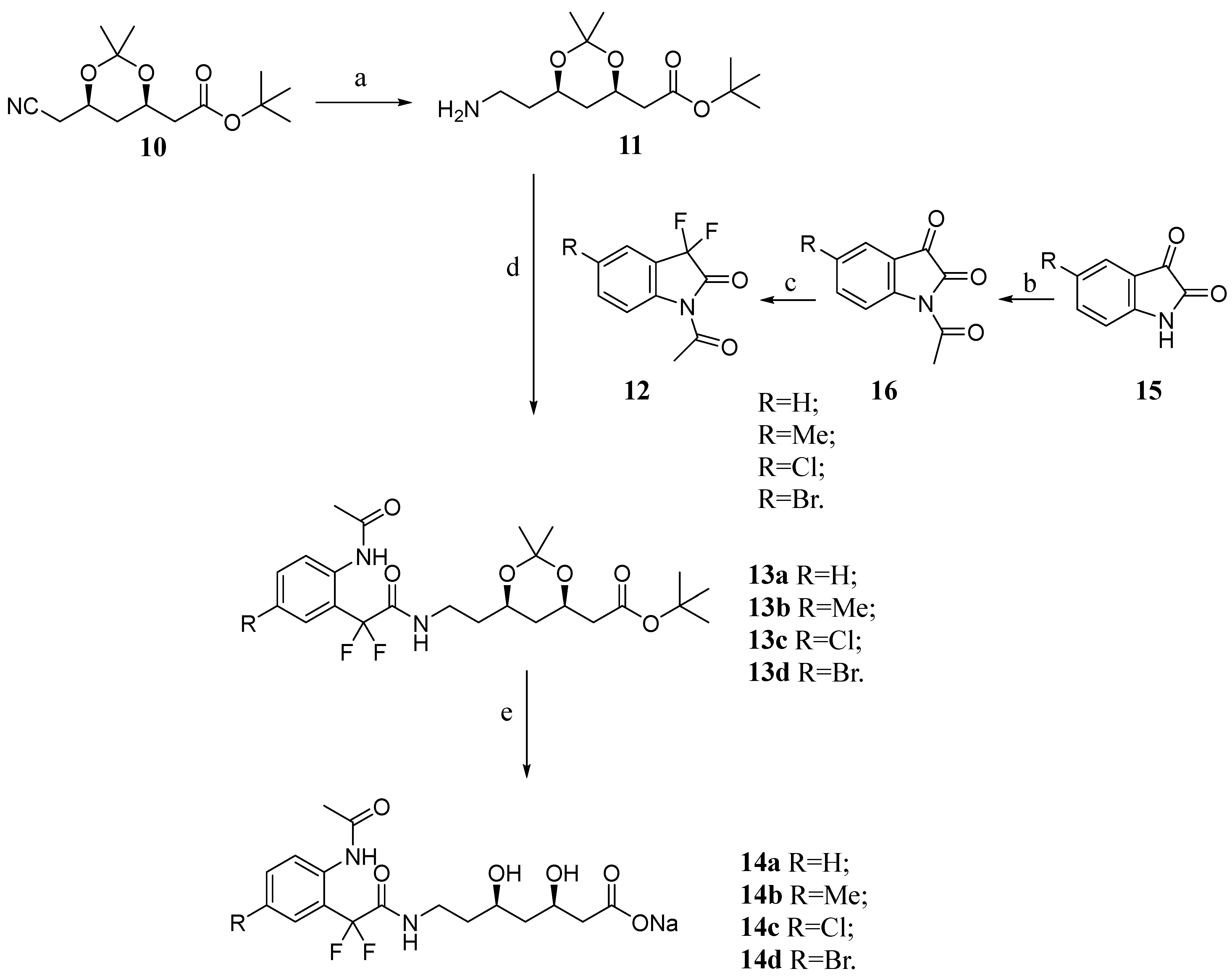
4.2. Synthesis
4.2.1. Preparation of tert-Butyl 2-((4R,6R)-6-(2-Aminoethyl)-2,2-dimethyl-1,3-dioxan-4-yl)acetate (11)
4.2.2. General Preparation of tert-Butyl 2-((4R,6R)-6-(2-(2-(2-Acetamido-5-subtitutedphenyl)-2,2-difluoroacetamido)ethyl)-2,2-dimethyl-1,3-dioxan-4-yl)acetate 13 (a–d)
4.2.3. General Preparation of Sodium (3R,5R)-7-(2-(2-Acetamido-5-substituted-phenyl)-2,2-difluoroacetamide)-3,5-dihydroxyheptanoate salts 14 (a–d)
4.3. Biological Evaluation
4.3.1. Bacteria
4.3.2. Cell Cultures
4.3.3. Cellular Viability in Cell Cultures
4.3.4. Cultures of P. falciparum-Infected Erythrocytes and In Vitro Assays
4.3.5. Trypanocidal In Vitro Phenotypic Screening
4.3.6. Salmonella/Microsome Assay
4.3.7. In Vitro Micronuclei Assay in the Cell Culture (MNvit)
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tavakkoli, A.; Johnston, T.P.; Sahebkar, A. Antifungal aspects of statins. Pharmacol. Ther. 2020, 208, 107483. [Google Scholar] [CrossRef] [PubMed]
- Sirtori, C.R. The pharmacology of statins. Pharmacol. Res. 2014, 88, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Dong, J.; Yu, Z. Pleiotropic use of Statins as non-lipid-lowering drugs. Int. J. Biol. Sci. 2020, 16, 2704–2711. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-H.; Ramakrishnan, S.; Striepen, B.; Moreno, S.N.J. Toxoplasma gondii Relies on Both Host and Parasite Isoprenoids and Can Be Rendered Sensitive to Atorvastatin. PLoS Pathog. 2014, 9, e1003665. [Google Scholar] [CrossRef]
- Araujo-Lima, C.F.; Peres, R.B.; Silva, P.B.; Batista, M.M.; Aiub, C.A.F.; Felzenszwalb, I.; Soeiro, M.N.C. Repurposing Strategy of Atorvastatin against Trypanosoma cruzi: In vitro Monotherapy and Combined Therapy with Benznidazole Exhibit Synergistic Trypanocidal Activity. Antimicrob. Agents Chemother. 2018, 62, e00979-18. [Google Scholar] [CrossRef]
- Mota, S.; Bensalel, J.; Park, D.H.; Gonzalez, S.; Rodriguez, A.; Gallego-Delgado, J. Treatment Reducing Endothelial Activation Protects against Experimental Cerebral Malaria. Pathogens 2022, 11, 643. [Google Scholar] [CrossRef]
- Hirota, T.; Fujita, Y.; Ieiri, I. An updated review of pharmacokinetic drug interactions and pharmacogenetics of statins. Expert Opin. Drug Metab. Toxicol. 2020, 16, 809–822. [Google Scholar] [CrossRef]
- WHO. Chagas Disease (Also Known as American Trypanosomiasis). 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/chagas-disease-(american-trypanosomiasis) (accessed on 14 April 2023).
- PAHO. Chagas Disease. 2023. Available online: https://www.paho.org/en/topics/chagas-disease (accessed on 14 April 2023).
- Drugs for Neglected Diseases Intent (DNDi). Chagas Disease. 2023. Available online: https://dndi.org/diseases/chagas/facts/ (accessed on 14 April 2023).
- Soeiro, M.D.N.C. Perspectives for a new drug candidate for Chagas disease therapy. Mem. Inst. Oswaldo Cruz 2022, 117, e220004. [Google Scholar] [CrossRef]
- Salomão, K.; Menna-Barreto, R.F.S.; De Castro, S.L. Stairway to heaven or hell? Perspectives and limitations of Chagas disease chemotherapy. Curr. Top. Med. Chem. 2016, 16, 2266–2289. [Google Scholar] [CrossRef]
- Concepcion, J.L.; Gonzalez-Pacanowska, D.; Urbina, J.A. 3-Hydroxy-3-methyl-glutaryl-CoA Reductase in Trypanosoma (Schizotrypanum) cruzi: Subcellular Localization and Kinetic Properties. Arch. Biochem. Biophys. 1998, 352, 114–120. [Google Scholar] [CrossRef]
- Peña-Diaz, J.; Montalvetti, A.; Flores, C.-L.; Constán, A.; Hurtado-Guerrero, R.; De Souza, W.; Gancedo, C.; Ruiz-Perez, L.M.; Gonzalez-Pacanowska, D. Mitochondrial Localization of the Mevalonate Pathway Enzyme 3-Hydroxy-3-methyl-glutaryl-CoA Reductase in the Trypanosomatidae. Mol. Biol. Cell 2004, 15, 1356–1363. [Google Scholar] [CrossRef] [PubMed]
- Urbina, J.A.; Lazardi, K.; Marchan, E.; Visbal, G.; Aguirre, T.; Piras, M.M.; Piras, R.; Maldonado, R.A.; Payares, G.; de Souza, W. Mevinolin (lovastatin) potentiates the antiproliferative effects of ketoconazole and terbinafine against Trypanosoma (Schizotrypanum) cruzi: In vitro and in vivo studies. Antimicrob. Agents Chemother. 1993, 37, 580–591. [Google Scholar] [CrossRef]
- Melo, L.; Figueiredo, V.P.; Azevedo, M.A.; Bahia, M.T.; Diniz, L.D.F.; Gonçalves, K.R.; de Lima, W.G.; Talvani, A.; Caldas, I.S.; Nascimento, A.F.D.S.D.; et al. Low Doses of Simvastatin Therapy Ameliorate Cardiac Inflammatory Remodeling in Trypanosoma cruzi-Infected Dogs. Am. J. Trop. Med. Hyg. 2011, 84, 325–331. [Google Scholar] [CrossRef]
- Silva, R.R.; Shrestha-Bajracharya, D.; Almeida-Leite, C.M.; Leite, R.; Bahia, M.T.; Talvani, A. Short-term therapy with simvastatin reduces inflammatory mediators and heart inflammation during the acute phase of experimental Chagas disease. Mem. Inst. Oswaldo Cruz 2012, 107, 513–521. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). World Malaria Report. 2022. Available online: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2022 (accessed on 14 April 2023).
- Savini, H.; Souraud, J.B.; Briolant, S.; Baret, E.; Amalvict, R.; Rogier, C.; Pradines, B. Atorvastatin as a Potential Antimalarial Drug: In vitro Synergy in Combinational Therapy with Dihydroartemisinin. Antimicrob. Agents Chemother. 2010, 54, 966–967. [Google Scholar] [CrossRef] [PubMed]
- Taoufiq, Z.; Pino, P.; N’Dilimabaka, N.; Arrouss, I.; Assi, S.; Soubrier, F.; Rebollo, A.; Mazier, D. Atorvastatin prevents Plasmodium falciparum cytoadherence and endothelial damage. Malar. J. 2011, 10, 52. [Google Scholar] [CrossRef] [PubMed]
- Reis, P.A.; Estato, V.; Da Silva, T.I.; D’Avila, J.; Siqueira, L.D.; Assis, E.F.; Bozza, P.; Bozza, F.A.; Tibiriça, E.V.; Zimmerman, G.A.; et al. Statins Decrease Neuroinflammation and Prevent Cognitive Impairment after Cerebral Malaria. PLoS Pathog. 2012, 8, e1003099. [Google Scholar] [CrossRef] [PubMed]
- Bienvenu, A.-L.; Picot, S. Statins Alone Are Ineffective in Cerebral Malaria but Potentiate Artesunate. Antimicrob. Agents Chemother. 2008, 52, 4203–4204. [Google Scholar] [CrossRef]
- Wong, R.P.M.; Davis, T.M.E. Statins as Potential Antimalarial Drugs: Low Relative Potency and Lack of Synergy with Conventional Antimalarial Drugs. Antimicrob. Agents Chemother. 2009, 53, 2212–2214. [Google Scholar] [CrossRef]
- Parquet, V.; Briolant, S.; Torrentino-Madamet, M.; Henry, M.; Almeras, L.; Amalvict, R.; Baret, E.; Fusai, T.; Rogier, C.; Pradines, B. Atorvastatin Is a Promising Partner for Antimalarial Drugs in Treatment of Plasmodium falciparum Malaria. Antimicrob. Agents Chemother. 2009, 53, 2248–2252. [Google Scholar] [CrossRef]
- Dormoi, J.; Briolant, S.; Pascual, A.; Desgrouas, C.; Travaillé, C.; Pradines, B. Improvement of the efficacy of dihydroartemisinin with atorvastatin in an experimental cerebral malaria murine model. Malar. J. 2013, 12, 302. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, R.C.; Martins, W.A.; Silva, T.P.; Kaiser, C.R.; Bastos, M.M.; Pinheiro, L.C.; Krettli, A.U.; Boechat, N. New pentasubstituted pyrrole hybrid atorvastatin–quinoline derivatives with antiplasmodial activity. Bioorganic Med. Chem. Lett. 2016, 26, 1881–1884. [Google Scholar] [CrossRef]
- Burgess, V.; Maya, J.D. Statin and aspirin use in parasitic infections as a potential therapeutic strategy: A narrative review. Rev. Argent. Microbiol. 2023; in press. [Google Scholar] [CrossRef]
- Boechat, N.; Pinto, A.C. Gem-difluoro derivative of phenylacetamide and phenylacetic acid and their pharmaceutical uses. US Patent 6034266 (CA 132:194195), 7 March 2000. [Google Scholar]
- Boechat, N.; Kover, W.B.; Bastos, M.M.; Pinto, A.C.; Maciel, L.C.; Mayer, L.M.U.; Da Silva, F.S.Q.; Sá, P.M.; Mendonça, J.S.; Wardell, S.M.S.V.; et al. N-Acyl-3,3-difluoro-2-oxoindoles as versatile intermediates for the preparation of different 2,2-difluorophenylacetic derivatives. J. Braz. Chem. Soc. 2008, 19, 445–457. [Google Scholar] [CrossRef]
- Cheah, W.C.; Black, D.S.; Goh, W.K.; Kumar, N. Synthesis of anti-bacterial peptidomimetics derived from N-acylisatins. Tetrahedron Lett. 2008, 49, 2965–2968. [Google Scholar] [CrossRef]
- Obafemi, C.A.; Taiwo, F.O.; Iwalewai, E.O.; Akinpelu, D.A. Synthesis, antibacterial and anti-inflammatory activities of some 2-phenylglyoxylic acid derivatives. Int. J. Life Sci. Pharma. Res. 2012, 2, 22–36. [Google Scholar]
- OECD. Test No. 471: Bacterial Reverse Mutation Test, OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2020; Available online: https://www.oecd-ilibrary.org/environment/test-no-471-bacterial-reverse-mutation-test_9789264071247-en (accessed on 1 January 2023).
- FDA. Guidance on Genotoxicity Testing and Data Interpretation for Pharmaceuticals Intended for Human Use. 2012. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/s2r1-genotoxicity-testing-and-data-interpretation-pharmaceuticals-intended-human-use (accessed on 11 April 2023).
- Jain, S.C.; Sinha, J.; Bhagat, S.; Errington, W.; Olsen, C.E. A Facile Synthesis pf Novel Spiro-[Indole-pyrazolinyl-thiazolidine]-2,4′-dione. Synth. Commun. 2003, 33, 563–577. [Google Scholar] [CrossRef]
- Guidipati, S.; Katkam, S.; Komati, S.; Kudvalli, S.J. Amorphous Atorvastatin Calcium. WO Patent 2006/039441A2, 13 April 2006. [Google Scholar]
- Agarwal, V.K.; Vakil, M.H.; Pandita, K.; Ramakrishna, N.V.S.; Patel, P.R.; Manakiwala, S.C. Process for the Production of Atorvastatin Calcium in Amorphous Form. WO Patent 02/083638A1, 24 October 2002. [Google Scholar]
- De Araújo, J.S.; Da Silva, C.F.; Batista, D.G.J.; Da Silva, P.B.; Meuser, M.B.; Aiub, C.A.F.; da Silva, M.F.V.; Araújo-Lima, C.F.; Banerjee, M.; Farahat, A.A.; et al. In vitro and In vivo Studies of the Biological Activity of Novel Arylimidamides against Trypanosoma cruzi. Antimicrob. Agents Chemother. 2014, 58, 4191–4195. [Google Scholar] [CrossRef]
- Florin-Christensen, M.; Garin, C.; Isola, E.; Brenner, R.; Rasmussen, L. Inhibition of Trypanosoma cruzi growth and sterol biosynthesis by lovastatin. Biochem. Biophys. Res. Commun. 1990, 166, 1441–1445. [Google Scholar] [CrossRef]
- Westerink, W.M.; Schirris, T.J.; Horbach, G.J.; Schoonen, W.G. Development and validation of a high-content screening in vitro micronucleus assay in CHO-k1 and HepG2 cells. Mutat. Res. Toxicol. Environ. Mutagen. 2011, 724, 7–21. [Google Scholar] [CrossRef]
- Ciaravino, V.; Kropko, M.L.; Rothwell, C.E.; Hovey, C.A.; Theiss, J.C. The genotoxicity profile of atorvastatin, a new drug in the treatment of hypercholesterolemia. Mutat. Res. Toxicol. 1995, 343, 95–107. [Google Scholar] [CrossRef]
- Morrow, P.E. Louis James Casarett (1927-1972). Toxicol. Sci. 2001, 63, 151–152. [Google Scholar] [CrossRef] [PubMed]
- Maron, D.M.; Ames, B.N. Revised methods for the Salmonella mutagenicity test. Mutat. Res. Environ. Mutagenes. Relat. Subj. 1983, 113, 173–215. [Google Scholar] [CrossRef]
- Pérez-Garrido, A.; Girón, F.; Helguera, A.M.; Borges, F.; Combes, R. Topological structural alerts modulations of mammalian cell mutagenicity for halogenated derivatives. SAR QSAR Environ. Res. 2014, 25, 17–33. [Google Scholar] [CrossRef] [PubMed]
- Tsuboy, M.; Angeli, J.; Mantovani, M.; Knasmüller, S.; Umbuzeiro, G.; Ribeiro, L. Genotoxic, mutagenic and cytotoxic effects of the commercial dye CI Disperse Blue 291 in the human hepatic cell line HepG2. Toxicol. Vitr. 2007, 21, 1650–1655. [Google Scholar] [CrossRef] [PubMed]
- Holla, B.S.; Sarojini, B.K.; Rao, B.S.; Akberali, P.M.; Kumari, N.S.; Shetty, V. Synthesis of some halogen-containing 1,2,4-triazolo-1,3,4-thiadiazines and their antibacterial and anticancer screening studies—Part I. Il Farm. 2001, 56, 565–570. [Google Scholar] [CrossRef]
- Don, R.; Ioset, J.-R. Screening strategies to identify new chemical diversity for drug development to treat kinetoplastid infections. Parasitology 2014, 141, 140–146. [Google Scholar] [CrossRef]
- Araujo-Lima, C.F.; Carvalho, R.D.C.C.; Peres, R.B.; Fiuza, L.F.D.A.; Galvão, B.V.D.; Castelo-Branco, F.S.; Bastos, M.M.; Boechat, N.; Felzenszwalb, I.; Soeiro, M.D.N.C. In silico and in vitro assessment of anti-Trypanosoma cruzi efficacy, genotoxicity and pharmacokinetics of pentasubstituted pyrrolic Atorvastatin-aminoquinoline hybrid compounds. Acta Trop. 2023, 242, 106924. [Google Scholar] [CrossRef]
- Galvão, B.V.D.; Araujo-Lima, C.F.; dos Santos, M.C.P.; Seljan, M.P.; Carrão-Dantas, E.K.; Aiub, C.A.F.; Cameron, L.C.; Ferreira, M.S.L.; Gonçalves, C.B.D.A.; Felzenszwalb, I. Plinia cauliflora (Mart.) Kausel (Jaboticaba) leaf extract: In vitro anti-Trypanosoma cruzi activity, toxicity assessment and phenolic-targeted UPLC-MS metabolomic analysis. J. Ethnopharmacol. 2021, 277, 114217. [Google Scholar] [CrossRef]
- Timm, B.L.; da Silva, P.B.; Batista, M.M.; da Silva, F.H.G.; da Silva, C.F.; Tidwell, R.R.; Patrick, D.A.; Jones, S.K.; Bakunov, S.A.; Bakunova, S.M.; et al. In vitro and In vivo Biological Effects of Novel Arylimidamide Derivatives against Trypanosoma cruzi. Antimicrob. Agents Chemother. 2014, 58, 3720–3726. [Google Scholar] [CrossRef] [PubMed]
- Meirelles, M.N.; De Araujo-Jorge, T.C.; Miranda, C.F.; De Souza, W.; Barbosa, H. Interaction of Trypanosoma cruzi with heart muscle cells: Ultrastructural and cytochemical analysis of endocytic vacuole formation and effect upon myogenesis in vitro. Eur. J. Cell Biol. 1986, 41, 198–206. [Google Scholar] [PubMed]
- Araujo-Lima, C.F.; Christoni, L.S.A.; Justo, G.; Soeiro, M.N.C.; Aiub, C.A.F.; Felzenszwalb, I. Atorvastatin Downregulates In vitro Methyl Methanesulfonate and Cyclophosphamide Alkylation-Mediated Cellular and DNA Injuries. Oxidative Med. Cell. Longev. 2018, 2018, 7820890. [Google Scholar] [CrossRef] [PubMed]
- Soeiro, M.D.N.C.; de Souza, E.M.; da Silva, C.F.; Batista, D.D.G.J.; Batista, M.M.; Pavão, B.P.; Araújo, J.S.; Aiub, C.A.F.; da Silva, P.B.; Lionel, J.; et al. In vitro and In vivo Studies of the Antiparasitic Activity of Sterol 14α-Demethylase (CYP51) Inhibitor VNI against Drug-Resistant Strains of Trypanosoma cruzi. Antimicrob. Agents Chemother. 2013, 57, 4151–4163. [Google Scholar] [CrossRef]
- Oduola, A.M.; Weatherly, N.F.; Bowdre, J.H.; Desjardins, R.E. Plasmodium falciparum: Cloning by single-erythrocyte micromanipulation and heterogeneity in vitro. Exp. Parasitol. 1988, 66, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Trager, W.; Jensen, J.B. Human Malaria Parasites in Continuous Culture. Science 1976, 193, 673–675. [Google Scholar] [CrossRef] [PubMed]
- Lambros, C.; Vanderberg, J.P. Synchronization of Plasmodium falciparum Erythrocytic Stages in Culture. J. Parasitol. 1979, 65, 418. [Google Scholar] [CrossRef]
- de Andrade-Neto, V.F.; Goulart, M.O.; Filho, J.F.D.S.; da Silva, M.J.; Pinto, M.D.C.F.; Pinto, A.V.; Zalis, M.G.; Carvalho, L.H.; Krettli, A.U. Antimalarial activity of phenazines from lapachol, β-lapachone and its derivatives against Plasmodium falciparum in vitro and Plasmodium berghei in vivo. Bioorganic Med. Chem. Lett. 2004, 14, 1145–1149. [Google Scholar] [CrossRef]
- Romanha, A.J.; De Castro, S.L.; Soeiro, M.D.N.C.; Lannes-Vieira, J.; Ribeiro, I.; Talvani, A.; Bourdin, B.; Blum, B.; Olivieri, B.; Zani, C.; et al. In vitro and in vivo experimental models for drug screening and development for Chagas disease. Mem. Inst. Oswaldo Cruz 2010, 105, 233–238. [Google Scholar] [CrossRef]
- Boechat, N.; Carvalho, A.S.; Salomão, K.; De Castro, S.L.; Araujo-Lima, C.; Mello, F.D.V.C.; Felzenszwalb, I.; Aiub, C.A.F.; Conde, T.R.; Zamith, H.P.S.; et al. Studies of genotoxicity and mutagenicity of nitroimidazoles: Demystifying this critical relationship with the nitro group. Mem. Inst. Oswaldo Cruz 2015, 110, 492–499. [Google Scholar] [CrossRef]
| Compounds | EC50 (µM) | LC50 (µM) | SI |
|---|---|---|---|
| AVA | 10.30 ± 1.2 | >1000 | >98 |
| 13a | >62 | >1000 | Inactive |
| 13b | 14.26 ± 0.2 | >1000 | >70 |
| 13c | 11.78 ± 2.0 | >1000 | >85 |
| 13d | >89 | >1000 | Inactive |
| 14a | >122 | >488 | Inactive |
| 14b | >118 | >472 | Inactive |
| 14c | >112 | >450 | Inactive |
| 14d | >102 | >409 | Inactive |
| Chloroquine | 0.59 ± 0.03 | 1219 | 2066 |
| Compounds | EC50 (µM) | LC50 (µM) | SI |
|---|---|---|---|
| AVA | 7.07 ± 1.78 | 360.7 ± 18.2 | 51 |
| 13a | >500 | >500 | ND |
| 13b | >500 | >500 | ND |
| 13c | 28.20 ± 0.75 | >500 | >17.7 |
| 13d | 23.18 ± 3.13 | >500 | >21.6 |
| 14a | >500 | >500 | ND |
| 14b | >500 | >500 | ND |
| 14c | >500 | >500 | ND |
| 14d | >500 | >500 | ND |
| Benznidazole | 13.00 ± 2.0 | >1000 | >77 |
| Compounds | 10µM (% of Parasite Lysis) | EC50 (µM) | LC50 (µM) | SI |
|---|---|---|---|---|
| AVA | 31.73 ± 5.80 | 45.33 ± 3.06 | 76.13 ± 2.16 | 1.7 |
| 13a | 17.92 ± 11.02 | >50 | 112.33 ± 28.29 | ND |
| 13b | 25.41 ± 10.90 | >50 | 113.67 ± 35.44 | ND |
| 13c | 57.83 ± 2.84 | 9.24 ± 1.06 | 153.33 ± 25.17 | 16.7 |
| 13d | 54.92 ± 16.94 | 11.42 ± 3.29 | 114.00 ± 5.29 | 10 |
| Benznidazole | 87.84 ± 7.08 | 1.83 ± 0.73 | 169.12 ± 27.2 | 92.4 |
| µM | TA97 | TA98 | TA100 | TA102 | TA104 | ||
|---|---|---|---|---|---|---|---|
| 13a | −S9 | 0 | 76 ± 6 | 25 ± 4 | 117 ± 5 | 386 ± 32 | 213 ± 3 |
| −S9 | 3 | 78 ± 6 | 24 ± 3 | 117 ± 43 | 458 ± 19 | 264 ± 27 | |
| −S9 | 15 | 82 ± 8 | 29 ± 6 | 136 ± 13 | 435 ± 22 | 307 ± 18 | |
| −S9 | 30 | 79 ± 8 | 31 ± 8 | 139 ± 13 | 433 ± 7 | 314 ± 26 | |
| −S9 | 150 | 69 ± 10 | 32 ± 3 | 142 ± 5 | 380 ± 12 | 315 ± 36 | |
| −S9 | 300 | 80 ± 11 | 52 ± 4 * | 162 ± 37 | 345 ± 48 | 316 ± 33 | |
| −S9 | 1500 | 90 ± 9 | 61 ± 5 * | 179 ± 34 | Cytotoxic | 350 ± 21 | |
| +S9 | 0 | 225 ± 9 | 29 ± 8 | 213 ± 20 | 344 ± 38 | 369 ± 44 | |
| +S9 | 3 | 214 ± 21 | 25 ± 8 | 211 ± 37 | 384 ± 32 | 390 ± 49 | |
| +S9 | 15 | 217 ± 9 | 25 ± 4 | 223 ± 15 | 399 ± 32 | 417 ± 15 | |
| +S9 | 30 | 235 ± 23 | 26 ± 5 | 241 ± 36 | 407 ± 21 | 467 ± 19 | |
| +S9 | 150 | 237 ± 26 | 27 ± 1 | 244 ± 14 | 411 ± 46 | 330 ± 9 | |
| +S9 | 300 | 237 ± 4 | 33 ± 1 | 270 ± 45 | 448 ± 52 | 268 ± 37 | |
| +S9 | 1500 | 290 ± 28 | 36 ± 3 | 290 ± 31 | 501 ± 20 | Cytotoxic | |
| 13b | −S9 | 0 | 76 ± 6 | 15 ± 3 | 111 ± 13 | 370 ± 20 | 210 ± 3 |
| −S9 | 3 | 69 ± 12 | 18 ± 2 | 145 ± 11 | 388 ± 20 | 210 ± 11 | |
| −S9 | 15 | 76 ± 7 | 19 ± 3 | 146 ± 1 | 393 ± 18 | 267 ± 15 | |
| −S9 | 30 | 75 ± 12 | 21 ± 5 | 145 ± 17 | 423 ± 11 | 27 ± 13 | |
| −S9 | 150 | 74 ± 12 | 22 ± 2 | 143 ± 40 | 503 ± 23 | 326 ± 13 | |
| −S9 | 300 | 84 ± 7 | 22 ± 2 | 144 ± 8 | 543 ± 39 | 350 ± 22 | |
| −S9 | 1500 | 89 ± 27 | 26 ± 4 | 178 ± 10 | 560 ± 41 | 400 ± 11 | |
| +S9 | 0 | 185 ± 8 | 27 ± 8 | 183 ± 20 | 354 ± 38 | 314 ± 5,7 | |
| +S9 | 3 | 189 ± 43 | 27 ± 5 | 179 ± 38 | 365 ± 37 | 355 ± 12 | |
| +S9 | 15 | 183 ± 43 | 27 ± 1 | 188 ± 23 | 386 ± 42 | 356 ± 14 | |
| +S9 | 30 | 216 ± 47 | 27 ± 5 | 228 ± 46 | 403 ± 19 | 421 ± 67 | |
| +S9 | 150 | 231 ± 5 | 27 ± 5 | 260 ± 32 | 396 ± 31 | 447 ± 45 | |
| +S9 | 300 | 244 ± 23 | 39 ± 1 | 306 ± 60 | 372 ± 40 | 449 ± 18 | |
| +S9 | 1500 | 288 ± 33 | 45 ± 4 | 333 ± 45 | 399 ± 18 | 510 ± 13 | |
| 13c | −S9 | 0 | 68 ± 5 | 21 ± 4 | 127 ± 5 | 385 ± 31 | 180 ± 9 |
| −S9 | 3 | 81 ± 6 | 21 ± 2 | 135 ± 38 | 373 ± 50 | 195 ± 9 | |
| −S9 | 15 | 84 ± 8 | 21 ± 5 | 135 ± 21 | 386 ± 35 | 199 ± 7 | |
| −S9 | 30 | 84 ± 6 | 21 ± 3 | 135 ± 30 | 387 ± 33 | 192 ± 3 | |
| −S9 | 150 | 70 ± 6 | 21 ± 2 | 140 ± 31 | 386 ± 5 | 201 ± 17 | |
| −S9 | 300 | 71 ± 11 | 21 ± 1 | 164 ± 17 | 400 ± 24 | 206 ± 8 | |
| −S9 | 1500 | 62 ± 9 | 23 ± 4 | 168 ± 15 | 450 ± 32 | 241 ± 10 | |
| +S9 | 0 | 225 ± 8 | 30 ± 4 | 213 ± 19 | 344 ± 19 | 369 ± 44 | |
| +S9 | 3 | 219 ± 16 | 34 ± 4 | 223 ± 39 | 392 ± 8 | 384 ± 34 | |
| +S9 | 15 | 245 ± 22 | 35 ± 3 | 223 ± 47 | 390 ± 12 | 401 ± 31 | |
| +S9 | 30 | 247 ± 14 | 39 ± 3 | 228 ± 17 | 402 ± 24 | 445 ± 49 | |
| +S9 | 150 | 249 ± 17 | 37 ± 7 | 278 ± 5 | 401 ± 66 | 305 ± 7 | |
| +S9 | 300 | 259 ± 1 | 47 ± 4 | 286 ± 18 | 403 ± 12 | 297 ± 4 | |
| +S9 | 1500 | 261 ± 13 | 49 ± 5 | 300 ± 15 | 430 ± 14 | Cytotoxic | |
| 13d | −S9 | 0 | 71 ± 4 | 20 ± 3 | 103 ± 15 | 325 ± 2 | 403 ± 1 |
| −S9 | 3 | 119 ± 8 | 22 ± 7 | 99 ± 4 | 356 ± 7 | 469 ± 65 | |
| −S9 | 15 | 137 ± 1 | 23 ± 5 | 96 ± 12 | Cytotoxic | 515 ± 61 | |
| −S9 | 30 | 135 ± 4 | 25 ± 1 | 93 ± 1 | - | 570 ± 53 | |
| −S9 | 150 | 134 ± 4 | 25 ± 1 | Cytotoxic | - | 605 ± 61 | |
| −S9 | 300 | 146 ± 6 * | 27 ± 5 | - | - | 615 ± 15 | |
| −S9 | 1500 | Cytotoxic | Cytotoxic | - | - | 620 ± 31 | |
| +S9 | 0 | 119 ± 3 | 24 ± 3 | 139 ± 17 | 124 ± 1 | 382 ± 30 | |
| +S9 | 3 | 134 ± 3 | 28 ± 1 | 156 ± 18 | 153 ± 13 | 490 ± 52 | |
| +S9 | 15 | 135 ± 1 | 28 ± 5 | 166 ± 11 | 155 ± 20 | 513 ± 19 | |
| +S9 | 30 | 201 ± 4 | 31 ± 7 | 173 ± 1 | 160 ± 11 | 530 ± 7 | |
| +S9 | 150 | 203 ± 1 | 34 ± 2 | 174 ± 21 | 162 ± 31 | 541 ± 29 | |
| +S9 | 300 | 288 ± 6 * | 34 ± 4 | 181 ± 15 | 176 ± 11 | 589 ± 5 | |
| +S9 | 1500 | 410 ± 10 * | 35 ± 3 | Cytotoxic | Cytotoxic | 591 ± 11 |
| Antiplasmodial Activity | Trypanocidal Activity | Bacterial Mutagenicity | Mammalian Cell Genotoxicity | |
|---|---|---|---|---|
| 13a | No | No | Yes | Yes |
| 13b | Yes | No | No | Yes |
| 13c | Yes | Yes | No | No |
| 13d | No | Yes | Yes | Yes |
| 14a | No | No | Not Analyzed | Not Analyzed |
| 14b | No | No | Not Analyzed | Not Analyzed |
| 14c | No | No | Not Analyzed | Not Analyzed |
| 14d | No | No | Not Analyzed | Not Analyzed |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araujo-Lima, C.F.; de Cassia Castro Carvalho, R.; Rosario, S.L.; Leite, D.I.; Aguiar, A.C.C.; de Souza Santos, L.V.; de Araujo, J.S.; Salomão, K.; Kaiser, C.R.; Krettli, A.U.; et al. Antiplasmodial, Trypanocidal, and Genotoxicity In Vitro Assessment of New Hybrid α,α-Difluorophenylacetamide-statin Derivatives. Pharmaceuticals 2023, 16, 782. https://doi.org/10.3390/ph16060782
Araujo-Lima CF, de Cassia Castro Carvalho R, Rosario SL, Leite DI, Aguiar ACC, de Souza Santos LV, de Araujo JS, Salomão K, Kaiser CR, Krettli AU, et al. Antiplasmodial, Trypanocidal, and Genotoxicity In Vitro Assessment of New Hybrid α,α-Difluorophenylacetamide-statin Derivatives. Pharmaceuticals. 2023; 16(6):782. https://doi.org/10.3390/ph16060782
Chicago/Turabian StyleAraujo-Lima, Carlos Fernando, Rita de Cassia Castro Carvalho, Sandra Loureiro Rosario, Debora Inacio Leite, Anna Caroline Campos Aguiar, Lizandra Vitoria de Souza Santos, Julianna Siciliano de Araujo, Kelly Salomão, Carlos Roland Kaiser, Antoniana Ursine Krettli, and et al. 2023. "Antiplasmodial, Trypanocidal, and Genotoxicity In Vitro Assessment of New Hybrid α,α-Difluorophenylacetamide-statin Derivatives" Pharmaceuticals 16, no. 6: 782. https://doi.org/10.3390/ph16060782
APA StyleAraujo-Lima, C. F., de Cassia Castro Carvalho, R., Rosario, S. L., Leite, D. I., Aguiar, A. C. C., de Souza Santos, L. V., de Araujo, J. S., Salomão, K., Kaiser, C. R., Krettli, A. U., Bastos, M. M., Aiub, C. A. F., de Nazaré Correia Soeiro, M., Boechat, N., & Felzenszwalb, I. (2023). Antiplasmodial, Trypanocidal, and Genotoxicity In Vitro Assessment of New Hybrid α,α-Difluorophenylacetamide-statin Derivatives. Pharmaceuticals, 16(6), 782. https://doi.org/10.3390/ph16060782






