Acute Toxicity and Pharmacokinetic Profile of an EU-GMP-Certified Cannabis sativa L. in Rodents
Abstract
1. Introduction
2. Results
2.1. Acute Oral Toxicity Assessment in Rats
2.1.1. Primary Monitoring and General Health Status
- Writhing for the 1–24 h interval from administration for the dose group 5 mg/kg and the dose group 300 mg/kg;
- Straub phenomena in the 1–8 h interval, for the dose group 5 mg/kg and the dose group 300 mg/kg;
- Moderate sedation, maintained for 24 h, for the dose group 50 mg/kg, 300 mg kg and 2000 mg/kg;
- Mild motor incoordination for the 50 and 300 mg/kg dose groups. This effect occurred in the first 8 h from administration for the 50 mg/kg dose group and later, after 12–15 h for the 300 mg/kg dose group;
- Stereotypes (head movements, chewing and sniffing) occurred exclusively for the 50 mg/kg group in the first 8 h;
- Short-duration facial tremors and abortive seizures during the initial 8 h, limited to 16% of the subjects, for the 300 mg/kg group;
- Loss of reactivity to touch during the first 8 h for 16% of the subjects, for the 2000 mg/kg group.

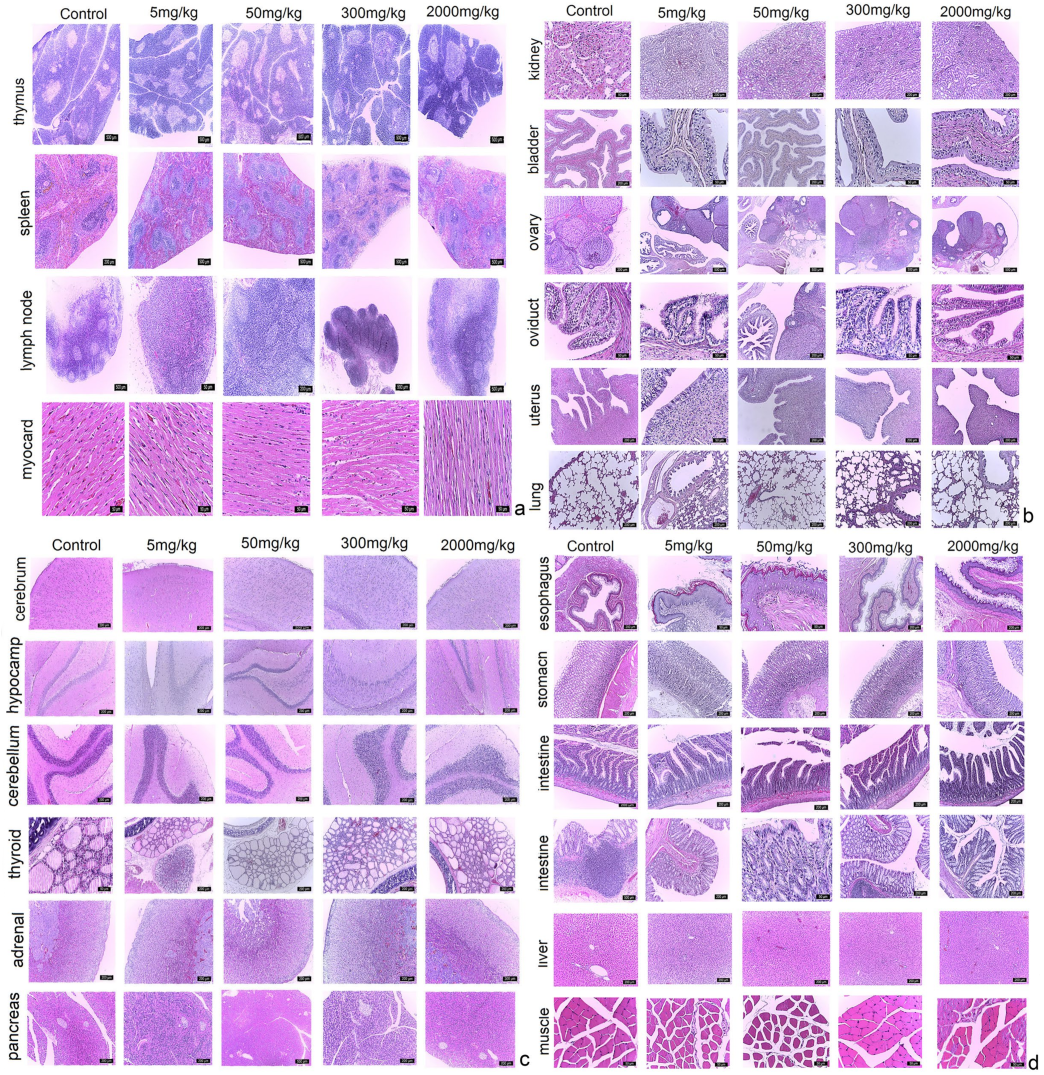
2.1.2. Paraclinical Evaluation: Gross Necropsy, Biochemistry and Histopathology
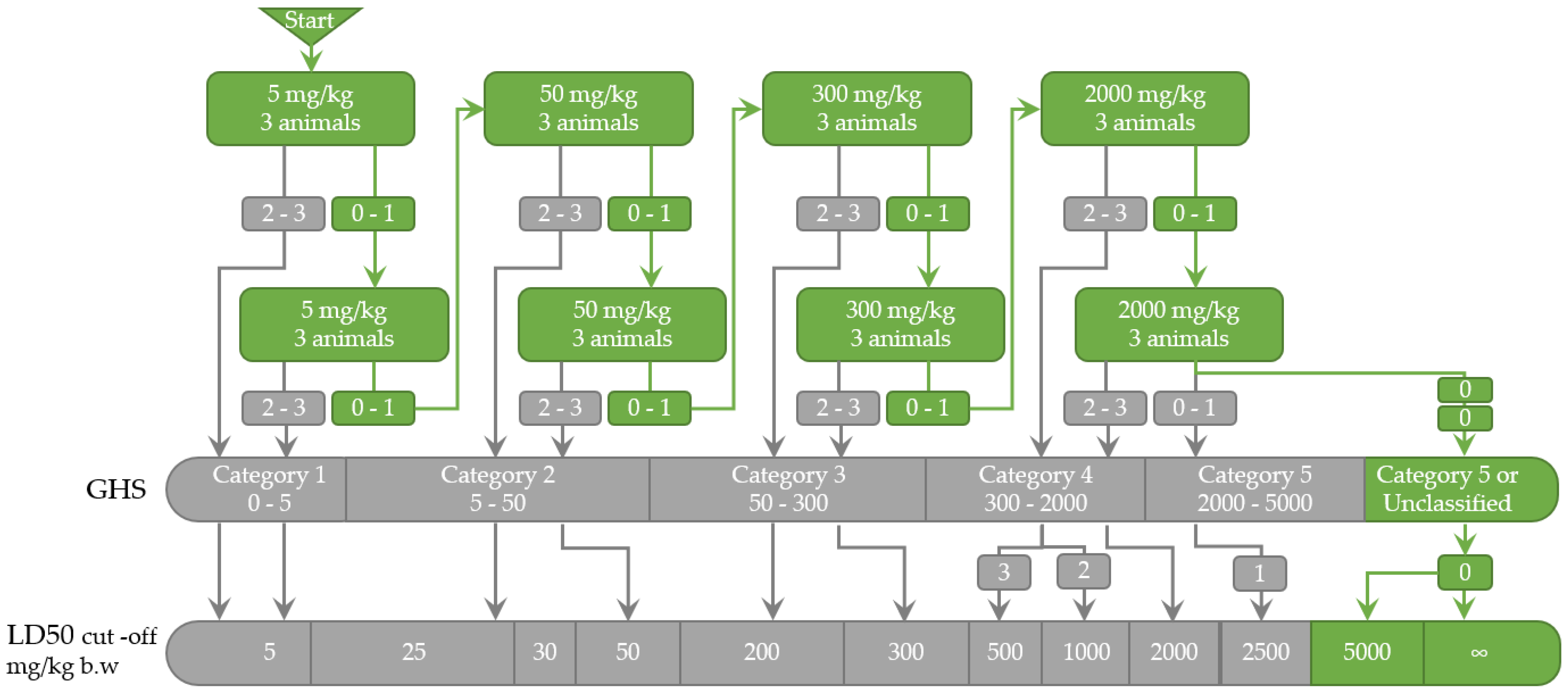
2.1.3. LD50 Estimation
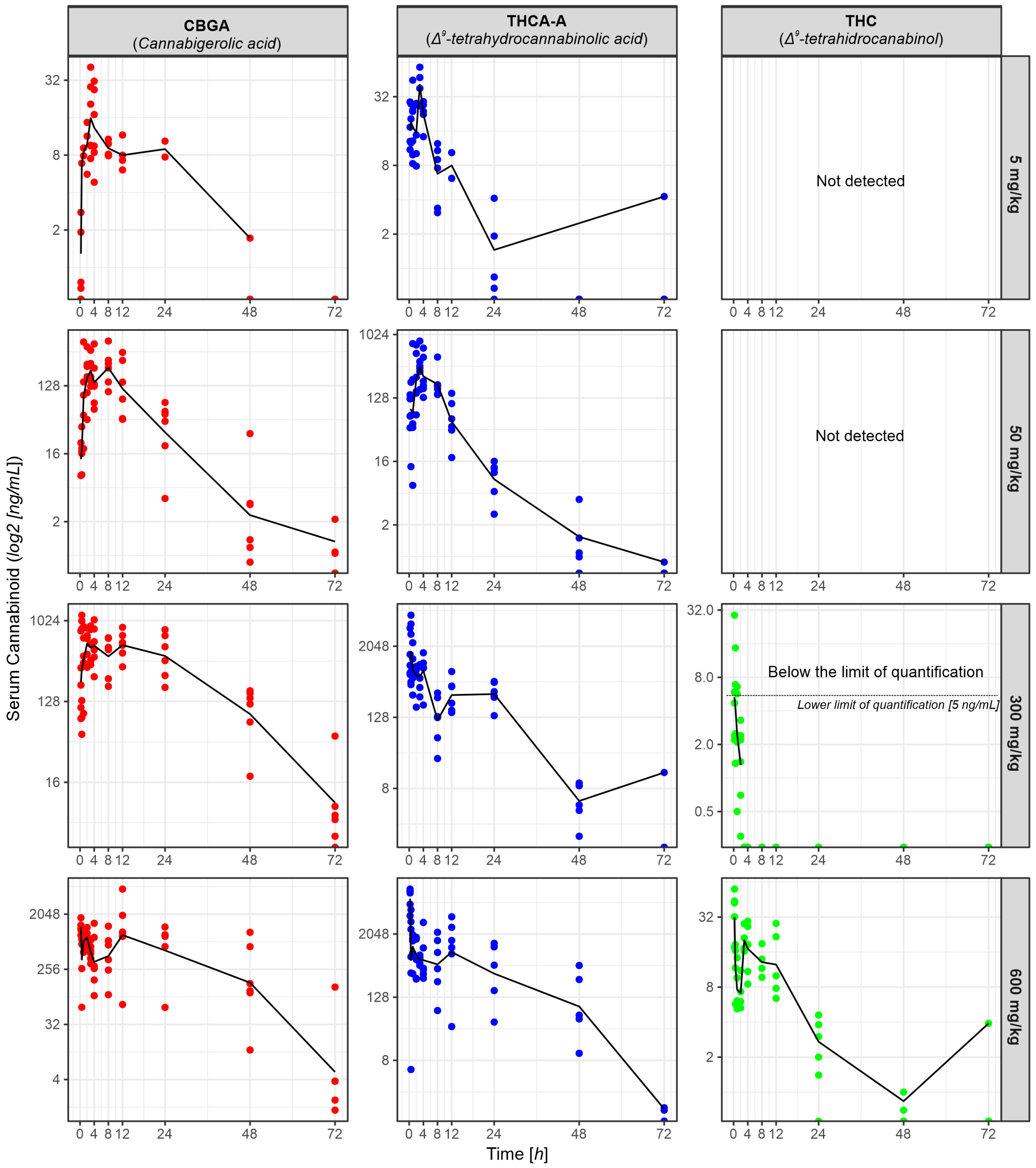
2.2. Pharmacokinetic Profile
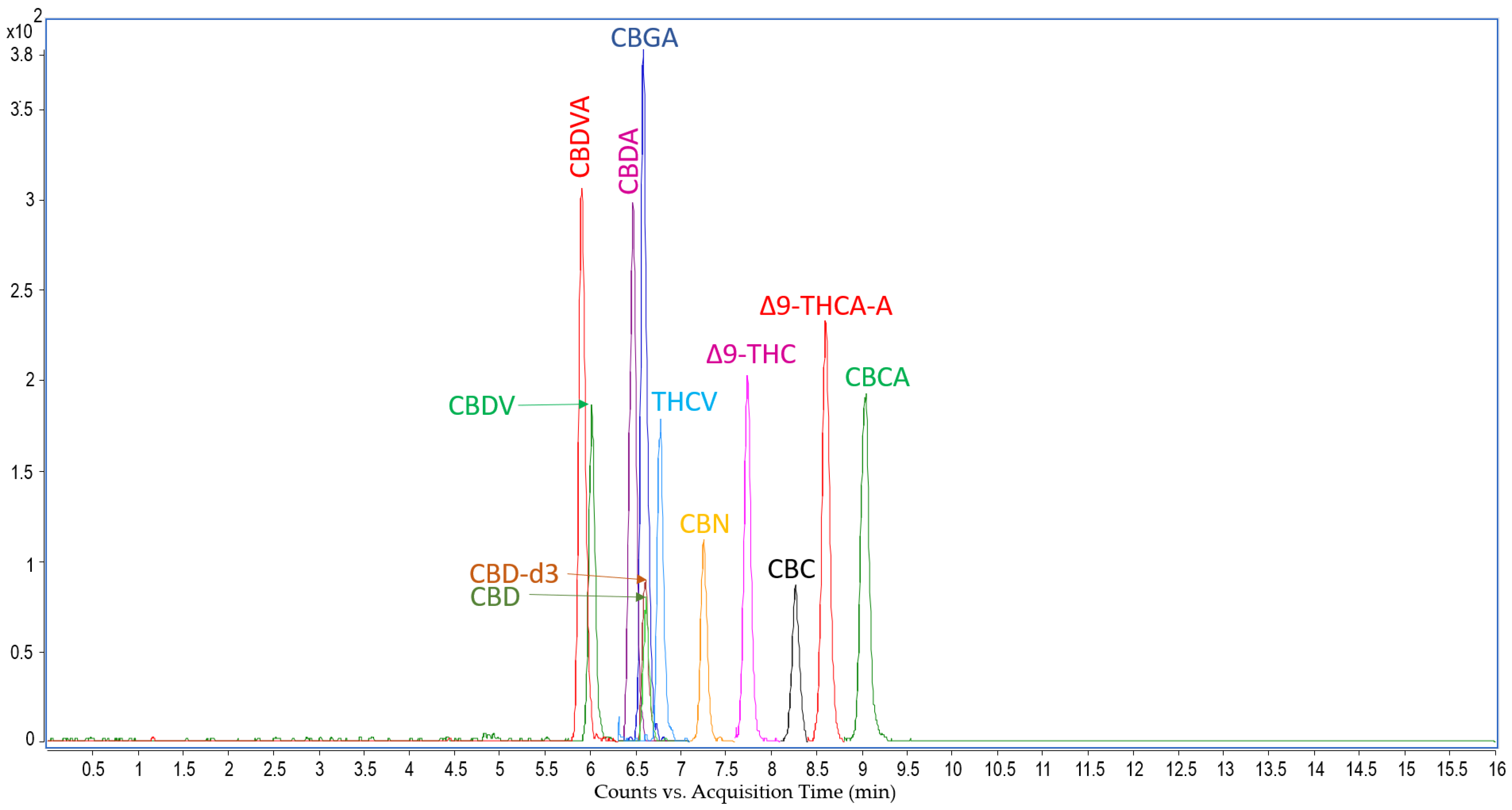
LC-MS Analysis: Cannabinoids Concentration in Rat Serum
3. Discussion
3.1. Acute Oral Toxicity
3.2. Pharmacokinetics
4. Materials and Methods
4.1. Plant Material
4.2. Reagents
4.3. Animals
4.4. Acute Oral Toxicity Evaluation
4.4.1. LD50 Estimation
4.4.2. Monitoring and Measurement of Vital Signs
4.4.3. Paraclinical Evaluation: Gross Necropsy, Biochemistry, and Histopathology
4.5. Pharmacokinetic Profile Evaluation
4.5.1. Cannabinoids Analysis in Rat Serum
4.5.2. Instrumentation for Cannabinoids Extraction and LC-MS/MS Analysis
4.5.3. Pharmacokinetic Analysis
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bungau, S.G.; Popa, V.C. Between Religion and Science: Some Aspects: Concerning Illness and Healing in Antiquity. Transylv. Rev. 2015, 24, 3–19. [Google Scholar]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef]
- Skoglund, G.; Nockert, M.; Holst, B. Viking and Early Middle Ages Northern Scandinavian Textiles Proven to be made with Hemp. Sci. Rep. 2013, 3, 2686. [Google Scholar] [CrossRef]
- European Monitoring Centre for Drugs and Drug Addiction (EMCDDA). Cannabis Legislation in Europe; Publications Office of the European Union: Luxembourg, 2018; pp. 3–30. ISBN 978-92-9497-328-3. [Google Scholar] [CrossRef]
- Elser, H.; Humphreys, K.; Kiang, M.V.; Mehta, S.; Yoon, J.H.; Faustman, W.O.; Matthay, E.C. State Cannabis Legalization and Psychosis-Related Health Care Utilization. JAMA Netw Open 2023, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Moran, L.V.; Tsang, E.S.; Ongur, D.; Hsu, J.; Choi, M.Y. Geographical variation in hospitalization for psychosis associated with cannabis use and cannabis legalization in the United States: Submit to: Psychiatry Research. Psychiatry Res. 2022, 308, 114387. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves-Pinho, M.; Bragança, M.; Freitas, A. Psychotic disorders hospitalizations associated with cannabis abuse or dependence: A nationwide big data analysis. Int. J. Methods Psychiatr. Res. 2020, 29, e1813. [Google Scholar] [CrossRef] [PubMed]
- State Medical Cannabis Laws. Available online: https://www.ncsl.org/health/state-medical-cannabis-laws (accessed on 25 February 2023).
- Vasincu, A.; Rusu, R.-N.; Ababei, D.-C.; Larion, M.; Bild, W.; Stanciu, G.D.; Solcan, C.; Bild, V. Endocannabinoid Modulation in Neurodegenerative Diseases: In Pursuit of Certainty. Biology 2022, 11, 440. [Google Scholar] [CrossRef] [PubMed]
- Tamba, B.I.; Stanciu, G.D.; Urîtu, C.M.; Rezus, E.; Stefanescu, R.; Mihai, C.T.; Luca, A.; Rusu-Zota, G.; Leon-Constantin, M.M.; Cojocaru, E.; et al. Challenges and opportunities in preclinical research of synthetic cannabinoids for pain therapy. Medicina 2020, 56, 24. [Google Scholar] [CrossRef]
- Filipiuc, L.E.; Ababei, D.C.; Alexa-Stratulat, T.; Pricope, C.V.; Bild, V.; Stefanescu, R.; Stanciu, G.D.; Tamba, B.I. Major Phytocannabinoids and Their Related Compounds: Should We Only Search for Drugs That Act on Cannabinoid Receptors? Pharmaceutics 2021, 13, 1823. [Google Scholar] [CrossRef]
- Temple, L.M.; Leikin, J.B. Tetrahydrocannabinol—Friend or foe?—Debate. Clin. Toxicol. (Phila) 2020, 58, 75–81. [Google Scholar] [CrossRef]
- Subramaniam, V.N.; Menezes, A.R.; DeSchutter, A.; Lavie, C.J. The Cardiovascular Effects of Marijuana: Are the Potential Adverse Effects Worth the High? Mo. Med. 2019, 116, 146–153. [Google Scholar]
- Bodine, M.; Kemp, A.K. Medical Cannabis Use In Oncology. StatPearls 2022, 2, 1–11. [Google Scholar]
- Gupta, S.; Fellows, K.; Weinstock-Guttman, B.; Hagemeier, J.; Zivadinov, R.; Ramanathan, M. Marijuana Use by Patients with Multiple Sclerosis. Int. J. MS Care 2019, 21, 57. [Google Scholar] [CrossRef]
- Jhanji, R.; Behl, T.; Sehgal, A.; Bungau, S. Mitochondrial dysfunction and traffic jams in amyotrophic lateral sclerosis. Mitochondrion 2021, 58, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Cassano, T.; Villani, R.; Pace, L.; Carbone, A.; Bukke, V.N.; Orkisz, S.; Avolio, C.; Serviddio, G. From Cannabis sativa to Cannabidiol: Promising Therapeutic Candidate for the Treatment of Neurodegenerative Diseases. Front. Pharmacol. 2020, 11, 124. [Google Scholar] [CrossRef]
- Behl, T.; Makkar, R.; Sehgal, A.; Sharma, N.; Singh, S.; Albratty, M.; Najmi, A.; Meraya, A.M.; Bungau, S.G. Insights into the Explicit Protective Activity of Herbals in Management of Neurodegenerative and Cerebrovascular Disorders. Molecules 2022, 27, 4970. [Google Scholar] [CrossRef]
- Woolridge, E.; Barton, S.; Samuel, J.; Osorio, J.; Dougherty, A.; Holdcroft, A. Cannabis use in HIV for pain and other medical symptoms. J. Pain Symptom Manag. 2005, 29, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.P. Cannabinoids in pain management: CB1, CB2 and non-classic receptor ligands. Expert Opin. Investig. Drugs 2014, 23, 1123–1140. [Google Scholar] [CrossRef]
- Zou, S.; Kumar, U. Cannabinoid Receptors and the Endocannabinoid System: Signaling and Function in the Central Nervous System. Int. J. Mol. Sci. 2018, 19, 833. [Google Scholar] [CrossRef]
- De Petrocellis, L.; Ligresti, A.; Schiano Moriello, A.; Allarà, M.; Bisogno, T.; Petrosino, S.; Stott, C.G.; Marzo, V.D. Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br. J. Pharmacol. 2011, 163, 1479–1494. [Google Scholar] [CrossRef]
- Soderstrom, K.; Soliman, E.; Dross, R. Van Cannabinoids modulate neuronal activity and cancer by CB1 and CB2 receptor-independent mechanisms. Front. Pharmacol. 2017, 8, 720. [Google Scholar] [CrossRef] [PubMed]
- Page 349 of 349 Weedmaps Strains Library. Available online: https://weedmaps.com/strains?page=349 (accessed on 20 February 2023).
- Thompson, G.R.; Rosenkrantz, H.; Schaeppi, U.H.; Braude, M.C. Comparison of acute oral toxicity of cannabinoids in rats, dogs and monkeys. Toxicol. Appl. Pharmacol. 1973, 25, 363–372. [Google Scholar] [CrossRef]
- Phillips, R.N.; Turk, R.F.; Forney, R.B. Acute toxicity of delta-9-tetrahydrocannabinol in rats and mice. Proc. Soc. Exp. Biol. Med. 1971, 136, 260–263. [Google Scholar] [CrossRef]
- Luthra, Y.K.; Rosenkrantz, H. Cannabinoids: Neurochemical aspects after oral chronic administration to rats. Toxicol. Appl. Pharmacol. 1974, 27, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Mahabir, V.K.; Merchant, J.J.; Smith, C.; Garibaldi, A. Medical cannabis use in the United States: A retrospective database study. J. Cannabis Res. 2020, 2, 32. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, W.; Bhatia, R.; Zebardast, N. Retrospective cross-sectional analysis of the changes in marijuana use in the USA, 2005–2018. BMJ Open 2020, 10, 37905. [Google Scholar] [CrossRef]
- Poyatos, L.; Pérez-Acevedo, A.P.; Papaseit, E.; Pérez-Mañá, C.; Martin, S.; Hladun, O.; Siles, A.; Torrens, M.; Busardo, F.P.; Farré, M. Oral Administration of Cannabis and Δ-9-tetrahydrocannabinol (THC) Preparations: A Systematic Review. Medicina 2020, 56, 309. [Google Scholar] [CrossRef] [PubMed]
- Shelef, A.; Barak, Y.; Berger, U.; Paleacu, D.; Tadger, S.; Plopsky, I.; Baruch, Y. Safety and Efficacy of Medical Cannabis Oil for Behavioral and Psychological Symptoms of Dementia: An-Open Label, Add-On, Pilot Study. J. Alzheimer’s Dis. 2016, 51, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Assaf, R.D.; Gorbach, P.M.; Cooper, Z.D. Changes in medical and non-medical cannabis use among United States adults before and during the COVID-19 pandemic. Am. J. Drug Alcohol. Abus. 2022, 48, 321–327. [Google Scholar] [CrossRef]
- Leung, J.; Quinn, C.; Carlyle, M.; Ellem, R.; Tisdale, C.; Davidson, L.; White, M.J.; Kavanagh, D.J.; Hides, L. Retrospective Self-Reports of How Adolescent Substance Use Changed with the COVID-19 Pandemic. Int. J. Environ. Res. Public Health 2022, 19, 13680. [Google Scholar] [CrossRef]
- Ramakrishnan, D.; Sureshanand, S.; Pittman, B.; Radhakrishnan, R. Impact of Cannabis Use, Substance Use Disorders, and Psychiatric Diagnoses on COVID-19 Outcomes: A Retrospective Cohort Study. J. Clin. Psychiatry 2022, 83, 42517. [Google Scholar] [CrossRef]
- Young-Wolff, K.C.; Ray, G.T.; Alexeeff, S.E.; Benowitz, N.; Adams, S.R.; Does, M.B.; Goler, N.; Ansley, D.; Conway, A.; Avalos, L.A. Association of cannabis use during pregnancy with severe acute respiratory syndrome coronavirus 2 infection: A retrospective cohort study. Addiction 2023, 118, 317–326. [Google Scholar] [CrossRef]
- Goel, A.; McGuinness, B.; Jivraj, N.K.; Wijeysundera, D.N.; Mittleman, M.A.; Bateman, B.T.; Clarke, H.; Kotra, L.P.; Ladha, K.S. Cannabis Use Disorder and Perioperative Outcomes in Major Elective SurgeriesA Retrospective Cohort Analysis. Anesthesiology 2020, 132, 625–635. [Google Scholar] [CrossRef]
- Vozoris, N.T.; Zhu, J.; Ryan, C.M.; Chow, C.W.; To, T. Cannabis use and risks of respiratory and all-cause morbidity and mortality: A population-based, data-linkage, cohort study. BMJ Open Respir. Res. 2022, 9, e001216. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, U.; Specka, M.; Roser, P.; Scherbaum, N. Cannabis use, abuse and dependence during the COVID-19 pandemic: A scoping review. J. Neural Transm. 2023, 130, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Services, H. Guidance for industry: Characterization and qualification of cell substrates and other biological starting materials used in the production of viral vaccines for the prevention and treatment of infectious diseases. Biotechnol. Law Rep. 2006, 25, 697–723. [Google Scholar] [CrossRef]
- OECD. Test No. 423: Acute Oral Toxicity—Acute Toxic Class Method. In OECD Guideline for the Testing of Chemicals; OECD: Paris, France, 2002; Section 4; ISBN 9789264071001. [Google Scholar]
- Brunetti, P.; Pichini, S.; Pacifici, R.; Busardò, F.P.; del Rio, A. Herbal Preparations of Medical Cannabis: A Vademecum for Prescribing Doctors. Medicina 2020, 56, 237. [Google Scholar] [CrossRef] [PubMed]
- Horvath, C.; Dalley, C.B.; Grass, N.; Tola, D.H. Marijuana Use in the Anesthetized Patient: History, Pharmacology, and Anesthetic Considerations. AANA J. 2019, 87, 451–458. [Google Scholar]
- Phillips, R.N.; Brown, D.J.; Martz, R.; Hubbard, J.D.; Forney, R.B. Subacute toxicity of aqueous-suspended Δ9-tetrahydrocannabinol in rats. Toxicol. Appl. Pharmacol. 1972, 22, 45–49. [Google Scholar] [CrossRef]
- Sofia, R.D.; Barry, H. Acute and chronic effects of δ9-tetrahydrocannabinol on food intake by rats. Psychopharmacologia 1974, 39, 213–222. [Google Scholar] [CrossRef]
- Thompson, G.R.; Mason, M.M.; Rosenkrantz, H.; Braude, M.C. Chronic oral toxicity of cannabinoids in rats. Toxicol. Appl. Pharmacol. 1973, 25, 373–390. [Google Scholar] [CrossRef]
- Reynoso-Moreno, I.; Tietz, S.; Vallini, E.; Engelhardt, B.; Gertsch, J.; Chicca, A. Selective Endocannabinoid Reuptake Inhibitor WOBE437 Reduces Disease Progression in a Mouse Model of Multiple Sclerosis. ACS Pharmacol. Transl. Sci. 2021, 4, 765–779. [Google Scholar] [CrossRef]
- Anderson, L.L.; Heblinski, M.; Absalom, N.L.; Hawkins, N.A.; Bowen, M.T.; Benson, M.J.; Zhang, F.; Bahceci, D.; Doohan, P.T.; Chebib, M.; et al. Cannabigerolic acid, a major biosynthetic precursor molecule in cannabis, exhibits divergent effects on seizures in mouse models of epilepsy. Br. J. Pharmacol. 2021, 178, 4826–4841. [Google Scholar] [CrossRef]
- Anderson, L.L.; Low, I.K.; Banister, S.D.; McGregor, I.S.; Arnold, J.C. Pharmacokinetics of Phytocannabinoid Acids and Anticonvulsant Effect of Cannabidiolic Acid in a Mouse Model of Dravet Syndrome. J. Nat. Prod. 2019, 82, 3047–3055. [Google Scholar] [CrossRef] [PubMed]
- Feigenbaum, J.J.; Bergmann, F.; Richmond, S.A.; Mechoulam, R.; Nadler, V.; Kloog, Y.; Sokolovsky, M. Nonpsychotropic cannabinoid acts as a functional N-methyl-D-aspartate receptor blocker. Proc. Natl. Acad. Sci. USA 1989, 86, 9584. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.R.; Canseco-Alba, A.; Zhang, H.Y.; Tagliaferro, P.; Chung, M.; Dennis, E.; Sanabria, B.; Schanz, N.; Escosteguy-Neto, J.C.; Ishiguro, H.; et al. Cannabinoid type 2 receptors in dopamine neurons inhibits psychomotor behaviors, alters anxiety, depression and alcohol preference. Sci. Rep. 2017, 7, 17410. [Google Scholar] [CrossRef]
- Berkenkopf, J.W.; Weichman, B.M. Production of prostacyclin in mice following intraperitoneal injection of acetic acid, phenylbenzoquinone and zymosan: Its role in the writhing response. Prostaglandins 1988, 36, 693–709. [Google Scholar] [CrossRef] [PubMed]
- Steubl, D.; Block, M.; Herbst, V.; Nockher, W.A.; Schlumberger, W.; Satanovskij, R.; Angermann, S.; Hasenau, A.L.; Stecher, L.; Heemann, U.; et al. Plasma Uromodulin Correlates With Kidney Function and Identifies Early Stages in Chronic Kidney Disease Patients. Medicine 2016, 95, e3011. [Google Scholar] [CrossRef] [PubMed]
- Delgado, G.E.; Kleber, M.E.; Scharnagl, H.; Krämer, B.K.; März, W.; Scherberich, J.E. Serum Uromodulin and Mortality Risk in Patients Undergoing Coronary Angiography. J. Am. Soc. Nephrol. 2017, 28, 2201–2210. [Google Scholar] [CrossRef]
- Leiherer, A.; Muendlein, A.; Saely, C.H.; Brandtner, E.M.; Geiger, K.; Fraunberger, P.; Drexel, H. The value of uromodulin as a new serum marker to predict decline in renal function. J. Hypertens. 2018, 36, 110–118. [Google Scholar] [CrossRef]
- Scherberich, J.E.; Gruber, R.; Nockher, W.A.; Christensen, E.I.; Schmitt, H.; Herbst, V.; Block, M.; Kaden, J.; Schlumberger, W. Serum uromodulin-a marker of kidney function and renal parenchymal integrity. Nephrol. Dial. Transpl. 2018, 33, 284–295. [Google Scholar] [CrossRef]
- Jian, L.; Fa, X.; Zhou, Z.; Liu, S. Functional analysis of UMOD gene and its effect on inflammatory cytokines in serum of essential hypertension patients. Int. J. Clin. Exp. Pathol. 2015, 8, 11356–11363. [Google Scholar] [PubMed]
- Radhakrishnan, A.; Price, A.M.; Pickup, L.C.; Law, J.P.; Mcgee, K.C.; Fabritz, L.; Senior, R.; Steeds, R.P.; Ferro, C.J.; Townend, J.N. Coronary flow velocity reserve and inflammatory markers in living kidney donors. Int. J. Cardiol. 2020, 320, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Purohit, V.; Rapaka, R.; Shurtleff, D. Role of Cannabinoids in the Development of Fatty Liver (Steatosis). AAPS J. 2010, 12, 233–237. [Google Scholar] [CrossRef]
- Bazwinsky-Wutschke, I.; Zipprich, A.; Dehghani, F. Endocannabinoid System in Hepatic Glucose Metabolism, Fatty Liver Disease, and Cirrhosis. Int. J. Mol. Sci. 2019, 20, 2516. [Google Scholar] [CrossRef]
- Parfieniuk, A.; Flisiak, R. Role of cannabinoids in chronic liver diseases. World J. Gastroenterol. 2008, 14, 6109–6114. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Gao, S.; Niu, J.; Li, P.; Deng, J.; Xu, S.; Wang, Z.; Wang, W.; Kong, D.; Li, C. Cannabinoid 2 Receptor Agonist Improves Systemic Sensitivity to Insulin in High-Fat Diet/Streptozotocin-Induced Diabetic Mice. Cell. Physiol. Biochem. 2016, 40, 1175–1185. [Google Scholar] [CrossRef] [PubMed]
- Bellocchio, L.; Cervino, C.; Pasquali, R.; Pagotto, U. The endocannabinoid system and energy metabolism. J. Neuroendocrinol. 2008, 20, 850–857. [Google Scholar] [CrossRef]
- van Thuijl, H.; Kola, B.; Korbonits, M. Appetite and metabolic effects of ghrelin and cannabinoids: Involvement of AMP-activated protein kinase. Vitam. Horm. 2008, 77, 121–148. [Google Scholar] [CrossRef]
- Moreno-Sanz, G. Can You Pass the Acid Test? Critical Review and Novel Therapeutic Perspectives of Δ 9-Tetrahydrocannabinolic Acid A. Cannabis Cannabinoid Res. 2016, 1, 124–130. [Google Scholar] [CrossRef]
- Hložek, T.; Uttl, L.; Kadeřábek, L.; Balíková, M.; Lhotková, E.; Horsley, R.R.; Nováková, P.; Šíchová, K.; Štefková, K.; Tylš, F.; et al. Pharmacokinetic and behavioural profile of THC, CBD, and THC+CBD combination after pulmonary, oral, and subcutaneous administration in rats and confirmation of conversion in vivo of CBD to THC. Eur. Neuropsychopharmacol. 2017, 27, 1223–1237. [Google Scholar] [CrossRef]
- De Caro, C.; Cristiano, C.; Avagliano, C.; Bertamino, A.; Ostacolo, C.; Campiglia, P.; Gomez-Monterrey, I.; La Rana, G.; Gualillo, O.; Calignano, A.; et al. Characterization of New TRPM8 Modulators in Pain Perception. Int. J. Mol. Sci. 2019, 20, 5544. [Google Scholar] [CrossRef]
- Russo, E. Handbook of Psychotropic Herbs: A Scientific Analysis of Herbal Remedies for Psychiatric Conditions; Routledge: New York, NY, USA, 2001. [Google Scholar]
- Mcpartland, J.M.; Macdonald, C.; Young, M.; Grant, P.S.; Furkert, D.P.; Glass, M. Affinity and Efficacy Studies of Tetrahydrocannabinolic Acid A at Cannabinoid Receptor Types One and Two. Cannabis Cannabinoid Res. 2017, 2, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Jastrząb, A.; Jarocka-Karpowicz, I.; Skrzydlewska, E. The Origin and Biomedical Relevance of Cannabigerol. Int. J. Mol. Sci. 2022, 23, 7929. [Google Scholar] [CrossRef] [PubMed]
- De Petrocellis, L.; Orlando, P.; Schiano Moriello, A.; Aviello, G.; Stott, C.; Izzo, A.A.; Di Marzo, V. Cannabinoid actions at TRPV channels: Effects on TRPV3 and TRPV4 and their potential relevance to gastrointestinal inflammation. Acta Physiol. 2012, 204, 255–266. [Google Scholar] [CrossRef]
- Jara-Oseguera, A.; Simon, S.A.; Rosenbaum, T. TRPV1: On the road to pain relief. Curr. Mol. Pharmacol. 2008, 1, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.M.; Chung, M.-K. Targeting TRPV3 for the Development of Novel Analgesics. Open Pain J. 2013, 6, 119. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Wang, C.; Han, J.; Ding, X.; Tang, S.; Ning, L. Role of TRPV4-P2X7 Pathway in Neuropathic Pain in Rats with Chronic Compression of the Dorsal Root Ganglion. Neurochem. Res. 2021, 46, 2143–2153. [Google Scholar] [CrossRef]
- Naziroǧlu, M. Molecular role of catalase on oxidative stress-induced Ca2+ signaling and TRP cation channel activation in nervous system. J. Recept. Signal Transduct. Res. 2012, 32, 134–141. [Google Scholar] [CrossRef]
- Takeda, S.; Misawa, K.; Yamamoto, I.; Watanabe, K. Cannabidiolic Acid as a Selective Cyclooxygenase-2 Inhibitory Component in Cannabis. Drug Metab. Dispos. 2008, 36, 1917–1921. [Google Scholar] [CrossRef]
- Roux, S.; Sablé, E.; Porsolt, R.D. Primary Observation (Irwin) Test in Rodents for Assessing Acute Toxicity of a Test Agent and its Effects on Behavior and Physiological Function. Curr. Protoc. Pharmacol. 2004, 27, 1–23. [Google Scholar] [CrossRef]
- Ruehl-Fehlert, C.; Kittel, B.; Morawietz, G.; Deslex, P.; Keenan, C.; Mahrt, C.R.; Nolte, T.; Robinson, M.; Stuart, B.P.; Deschl, U. Revised guides for organ sampling and trimming in rats and mice—Part 1. Exp. Toxicol. Pathol. 2003, 55, 91–106. [Google Scholar] [CrossRef] [PubMed]
- Kittel, B.; Ruehl-Fehlert, C.; Morawietz, G.; Klapwijk, J.; Elwell, M.R.; Lenz, B.; O’Sullivan, M.G.; Roth, D.R.; Wadsworth, P.F. Revised guides for organ sampling and trimming in rats and mice—Part 2. Exp. Toxicol. Pathol. 2004, 55, 413–431. [Google Scholar] [CrossRef]
- Morawietz, G.; Ruehl-Fehlert, C.; Kittel, B.; Bube, A.; Keane, K.; Halm, S.; Heuser, A.; Hellmann, J. Revised guides for organ sampling and trimming in rats and mice—Part 3. Exp. Toxicol. Pathol. 2004, 55, 433–449. [Google Scholar] [CrossRef]
- Test No. 417: Toxicokinetics; OECD: Paris, France, 2010; ISBN 9789264070882.
- Wakshlag, J.J.; Schwark, W.S.; Deabold, K.A.; Talsma, B.N.; Cital, S.; Lyubimov, A.; Iqbal, A.; Zakharov, A. Pharmacokinetics of Cannabidiol, Cannabidiolic Acid, Δ9-Tetrahydrocannabinol, Tetrahydrocannabinolic Acid and Related Metabolites in Canine Serum After Dosing With Three Oral Forms of Hemp Extract. Front. Vet. Sci. 2020, 7, 505. [Google Scholar] [CrossRef] [PubMed]
- Ambroziak, K.; Adamowicz, P. Simple screening procedure for 72 synthetic cannabinoids in whole blood by liquid chromatography–tandem mass spectrometry. Forensic Toxicol. 2018, 36, 280–290. [Google Scholar] [CrossRef] [PubMed]
| Dose/Parameter | 0 | 5 | 50 | 300 | 2000 |
|---|---|---|---|---|---|
| mg/kg | |||||
| CRE [mg/dL] | 0.5 ± 0.02 | 0.5 ± 0.04 | 0.6 ± 0.02 | 0.5 ± 0.01 | 0.6 ± 0.03 |
| AST [U/L] | 111 ± 9.15 | 138.1 ± 23.18 | 137.1 ± 0.07 | 111.8 ± 12.19 | 85.9 ± 10.86 |
| ALT [U/L] | 38.7 ± 3.13 | 41.6 ± 4.11 | 42.7 ± 2.65 | 37.5 ± 1.91 | 41.8 ± 4.17 |
| TC [mg/dL] | 98.8 ± 32.01 | 66.8 ± 17.48 | 87.7 ± 17.08 | 91.7 ± 21.51 | 113.8 ± 13.99 |
| GLU [mg/dL] | 98.2 ± 35.65 | 75.5 ± 27.07 | 68.3 ± 11.55 | 83.5 ± 7.50 | 93.3 ± 10.73 |
| ALB [g/L] | 44.2 ± 3.13 | 37.6 ± 6.91 | 44.6 ± 3.81 | 43.2 ± 2.99 | 44.7 ± 2.09 |
| ALP [U/L] | 86.8 ± 14.83 | 110.8 ± 7.89 | 138.7 ± 4.58 | 96.2 ± 7.07 | 125 ± 14.72 |
| TP [g/L] | 72.5 ± 4.49 | 59.7 ± 5.08 | 75.4 ± 3.69 | 69.4 ± 2.48 | 81.4 ± 1.04 |
| UREA [mg/dL] | 35.5 ± 5.14 | 25.1 ± 1.72 | 27.3 ± 5.79 | 26.3 ± 1.00 | 29.4 ± 3.24 |
| TBA [µmol/L] | 61 ± 26.56 | 46.7 ± 14.21 | 73.0 ± 11.51 | 27.8 ± 6.09 | 79.7 ± 32.86 |
| N | 3 | 6 | 6 | 6 | 6 |
| Compound | Cmax (ng/mL) | Tmax (h) | T1/2 (h) | AUC Last (h*ng/mL) | AUC Inf (h*ng/mL) | Vd (L/kg) | Cl (L/h/kg) |
|---|---|---|---|---|---|---|---|
| 5 mg/kg Cannabixir® Medium Flos (0.78 mg Δ9-THCA-A + Δ9-THC) | |||||||
| Δ9-THCA-A | 42.425 | 3 | 5.3374 | 197.236 | 260.802 | 23.0298 | 2.099 |
| Δ9-THC | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 50 mg/kg Cannabixir® Medium Flos (7.8 mg Δ9-THCA-A + Δ9-THC) | |||||||
| Δ9-THCA-A | 404.16 | 3 | 5.4189 | 3163.1937 | 3172.8488 | 19.2193 | 2.4583 |
| Δ9-THC | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 300 mg/kg Cannabixir® Medium Flos (46.8 mg Δ9-THCA-A + Δ9-THC) | |||||||
| Δ9-THCA-A | 2787.617 | 0.5 | 10.291 | 13,323.57 | 13,544.792 | 51.298 | 3.4552 |
| Δ9-THC | BQL | BQL | BQL | BQL | BQL | BQL | BQL |
| 600 mg/kg Cannabixir® Medium Flos (93.6 mg Δ9-THCA-A + Δ9-THC) | |||||||
| Δ9-THCA-A | 10,563.4 | 0.25 | 10.988 | 40,371.48 | 43,261.689 | 34.298 | 2.1635 |
| Δ9-THC | 34.916 | 0.25 | 7.080 | 284.342 | 287.2334 | 3328.818 | 325.867 |
| Time Segment | Compound Name | Precursor Ion | Product Ion | Dwell Time (ms) | Fragmentor Voltage (V) | Collision Energy (V) | Retention Time (min) |
|---|---|---|---|---|---|---|---|
| 1 | Cannabidivarinic acid | 331.2 | 191.1 | 100 | 135 | 30 | 5.93 |
| 1 | Cannabidivarin | 287.2 | 165.1 | 100 | 135 | 22 | 6.02 |
| 2 | Cannabidiolic acid | 359.2 | 219.1 | 40 | 135 | 30 | 6.48 |
| 2 | Cannabidiol | 315.2 | 193.2 | 40 | 135 | 20 | 6.63 |
| 2 | Cannabidiol-d3 | 318.2 | 196.1 | 40 | 135 | 22 | 6.64 |
| 2 | Canabigerolic acid | 361.2 | 219.2 | 40 | 135 | 25 | 6.61 |
| 2 | Tetrahydrocannabivarin | 287.2 | 165.1 | 40 | 135 | 20 | 6.79 |
| 3 | Cannabinol | 311.2 | 223.1 | 200 | 135 | 20 | 7.28 |
| 4 | Δ9-THC | 315.2 | 193.2 | 200 | 135 | 20 | 7.76 |
| 5 | Cannabichromene | 315.2 | 193.2 | 200 | 135 | 17 | 8.3 |
| 6 | Δ9-THCA-A | 359.2 | 219.1 | 200 | 135 | 30 | 8.6 |
| 7 | Cannabicromenic acid | 359.2 | 219.1 | 200 | 135 | 25 | 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filipiuc, L.-E.; Ştefănescu, R.; Solcan, C.; Ciorpac, M.; Szilagyi, A.; Cojocaru, D.; Stanciu, G.D.; Creangă, I.; Caratașu, C.-C.; Ababei, D.-C.; et al. Acute Toxicity and Pharmacokinetic Profile of an EU-GMP-Certified Cannabis sativa L. in Rodents. Pharmaceuticals 2023, 16, 694. https://doi.org/10.3390/ph16050694
Filipiuc L-E, Ştefănescu R, Solcan C, Ciorpac M, Szilagyi A, Cojocaru D, Stanciu GD, Creangă I, Caratașu C-C, Ababei D-C, et al. Acute Toxicity and Pharmacokinetic Profile of an EU-GMP-Certified Cannabis sativa L. in Rodents. Pharmaceuticals. 2023; 16(5):694. https://doi.org/10.3390/ph16050694
Chicago/Turabian StyleFilipiuc, Leontina-Elena, Raluca Ştefănescu, Carmen Solcan, Mitică Ciorpac, Andrei Szilagyi, Dana Cojocaru, Gabriela Dumitrita Stanciu, Ioana Creangă, Cătălin-Cezar Caratașu, Daniela-Carmen Ababei, and et al. 2023. "Acute Toxicity and Pharmacokinetic Profile of an EU-GMP-Certified Cannabis sativa L. in Rodents" Pharmaceuticals 16, no. 5: 694. https://doi.org/10.3390/ph16050694
APA StyleFilipiuc, L.-E., Ştefănescu, R., Solcan, C., Ciorpac, M., Szilagyi, A., Cojocaru, D., Stanciu, G. D., Creangă, I., Caratașu, C.-C., Ababei, D.-C., Gavrila, R.-E., Timofte, A.-D., Filipiuc, S.-I., & Bild, V. (2023). Acute Toxicity and Pharmacokinetic Profile of an EU-GMP-Certified Cannabis sativa L. in Rodents. Pharmaceuticals, 16(5), 694. https://doi.org/10.3390/ph16050694










