Respiratory Outcomes of Insulin Use in Patients with COPD: A Nationwide Population-Based Cohort Study
Abstract
1. Introduction
2. Results
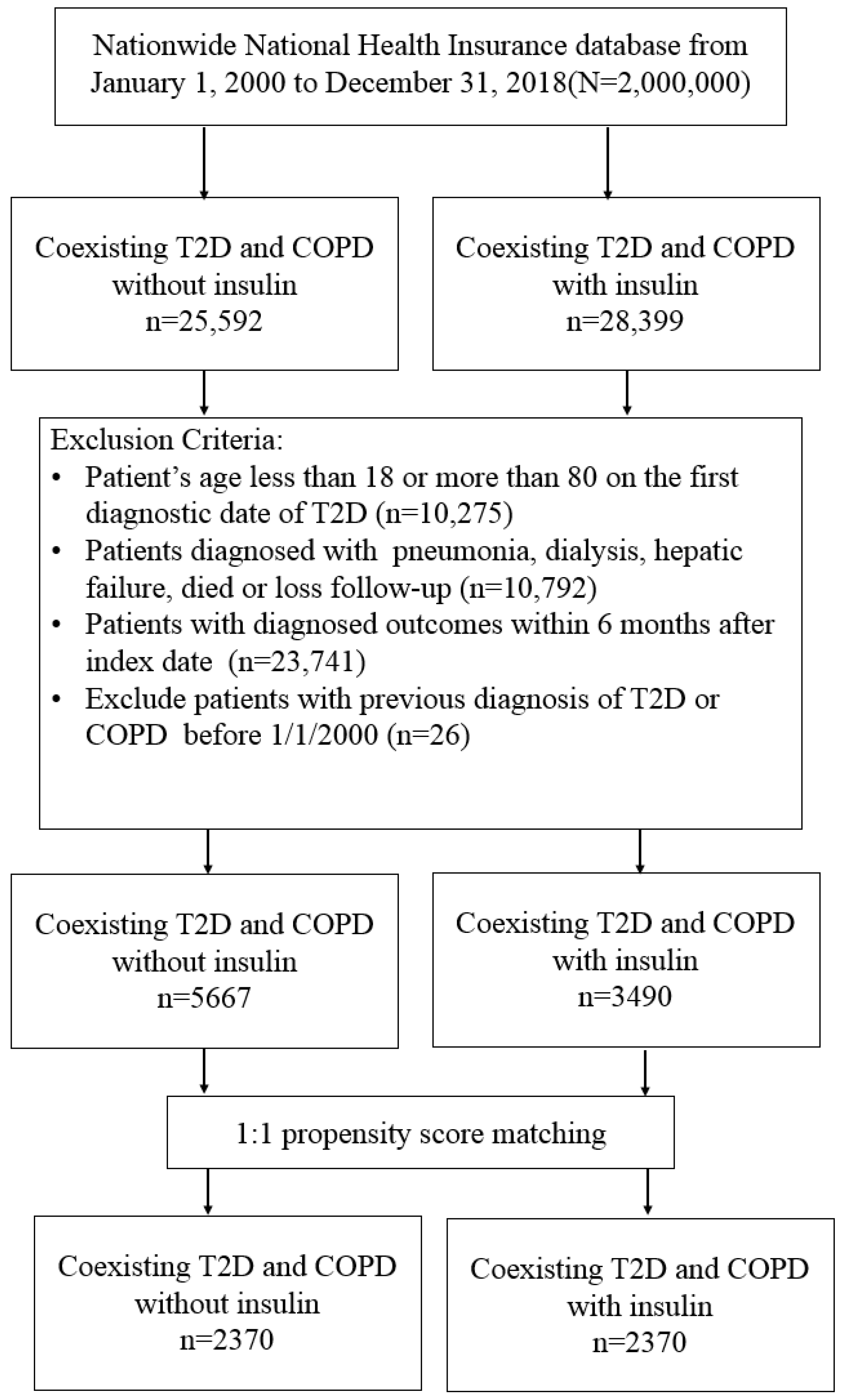
2.1. Participants
2.2. Main Outcomes
2.3. Dose-Response Analysis
3. Discussion
4. Materials and Methods
4.1. Data Source and Population of the Study
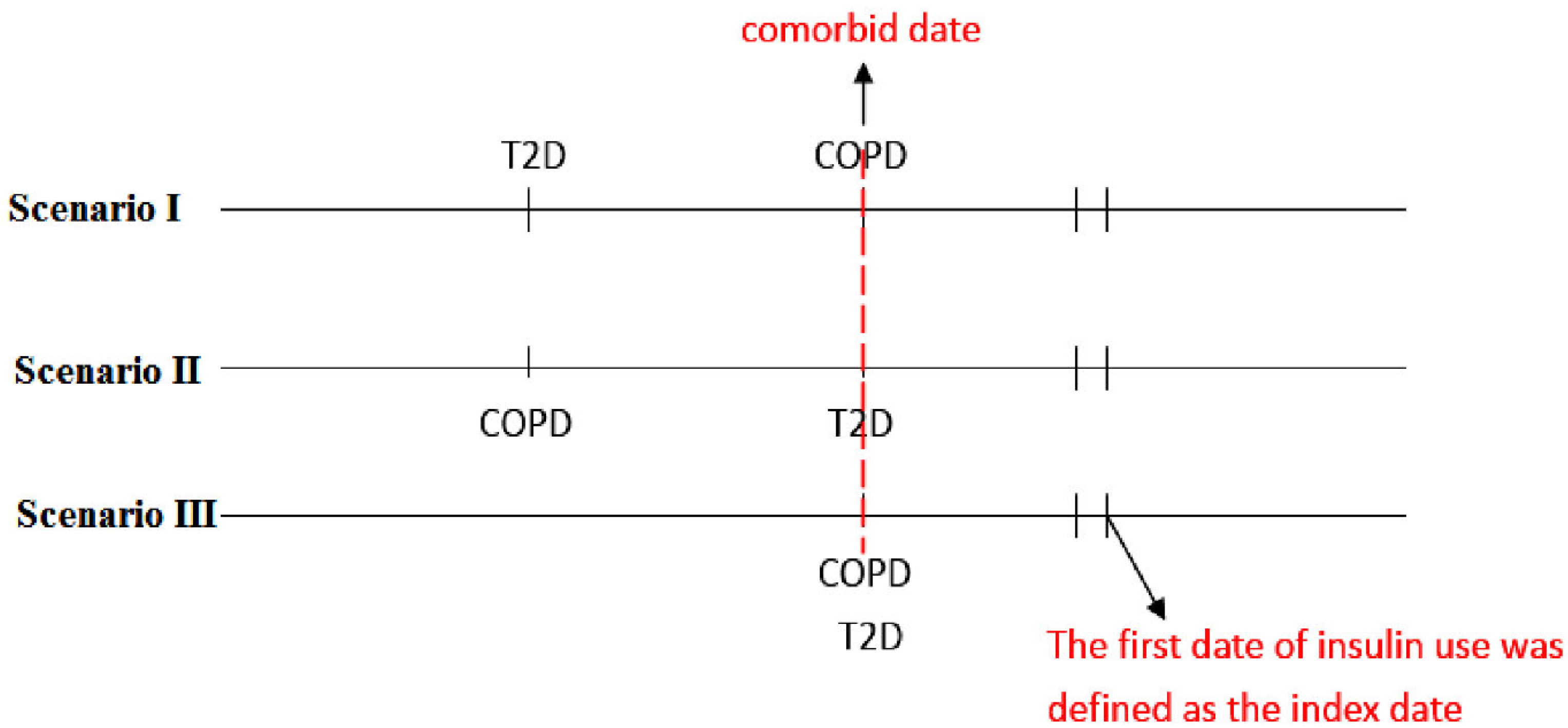
4.2. Study Design
4.3. Procedures
4.4. The Outcomes of Interest
4.5. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Stolz, D.; Mkorombindo, T.; Schumann, D.M.; Agusti, A.; Ash, S.Y.; Bafadhel, M.; Bai, C.; Chalmers, J.D.; Criner, G.J.; Dharmage, S.C.; et al. Towards the elimination of chronic obstructive pulmonary disease: A Lancet commission. Lancet 2022, 400, 921–972. [Google Scholar] [CrossRef] [PubMed]
- Christenson, S.A.; Smith, B.M.; Bafadhel, M.; Putcha, N. Chronic obstructive pulmonary disease. Lancet 2022, 399, 2227–2242. [Google Scholar] [CrossRef] [PubMed]
- Agustí, A.; Hogg, J.C. Update on the pathogenesis of chronic obstructive pulmonary disease. N. Engl. J. Med. 2019, 381, 1248–1256. [Google Scholar] [CrossRef] [PubMed]
- Celli, B.R.; Wedzicha, J.A. Update on clinical aspects of chronic obstructive pulmonary disease. N. Engl. J. Med. 2019, 381, 1257–1266. [Google Scholar] [CrossRef]
- Wedzicha, J.A.; Seemungal, T.A. COPD exacerbations: Defining their cause and prevention. Lancet 2007, 370, 786–796. [Google Scholar] [CrossRef]
- GBD. Institute for Health Metrics and Evaluation, Global Health Data Exchange, Global Burden of Disease Study 2019 (GBD 2019) Data Resources, GBD Results Tool, Terms and Conditions (2019). Available online: https://vizhub.healthdata.org/gbd-results/ (accessed on 9 September 2022).
- Gläser, S.; Krüger, S.; Merkel, M.; Bramlage, P.; Herth, F.J. Chronic obstructive pulmonary disease and diabetes mellitus: A systematic review of the literature. Respiration 2015, 89, 253–264. [Google Scholar] [CrossRef]
- Mirrakhimov, A.E. Chronic obstructive pulmonary disease and glucose metabolism: A bitter sweet symphony. Cardiovasc. Diabetol. 2012, 11, 132. [Google Scholar] [CrossRef]
- Park, S.S.; Perez Perez, J.L.; Perez Gandara, B.; Agudelo, C.W.; Rodriguez Ortega, R.; Ahmed, H.; Garcia-Arcos, I.; McCarthy, C.; Geraghty, P. Mechanisms Linking COPD to Type 1 and 2 Diabetes Mellitus: Is There a Relationship between Diabetes and COPD? Medicina 2022, 58, 1030. [Google Scholar] [CrossRef]
- Gunasekaran, K.; Murthi, S.; Elango, K.; Rahi, M.S.; Thilagar, B.; Ramalingam, S.; Voruganti, D.; Paramasivam, V.K.; Kolandaivel, K.P.; Arora, A.; et al. The Impact of Diabetes Mellitus in Patients with Chronic Obstructive Pulmonary Disease (COPD) Hospitalization. J. Clin. Med. 2021, 10, 235. [Google Scholar] [CrossRef]
- Hegele, R.A.; Maltman, G.M. Insulin’s centenary: The birth of an idea. Lancet Diabetes Endocrinol. 2020, 8, 971–977. [Google Scholar] [CrossRef]
- Cahn, A.; Miccoli, R.; Dardano, A.; Del Prato, S. New forms of insulin and insulin therapies for the treatment of type 2 diabetes. Lancet Diabetes Endocrinol. 2015, 3, 638–652. [Google Scholar] [CrossRef] [PubMed]
- Ohkubo, Y.; Kishikawa, H.; Araki, E.; Miyata, T.; Isami, S.; Motoyoshi, S.; Kojima, Y.; Furuyoshi, N.; Shichiri, M. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: A randomized prospective 6-year study. Diabetes Res. Clin. Pract. 1995, 28, 103–117. [Google Scholar] [CrossRef] [PubMed]
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998, 352, 837–853, Erratum in Lancet 1999, 354, 602. [Google Scholar] [CrossRef]
- Dandona, P.; Chaudhuri, A.; Mohanty, P.; Ghanim, H. Anti-inflammatory effects of insulin. Curr. Opin. Clin. Nutr. Metab. Care 2007, 10, 511–517, Erratum in Curr. Opin. Clin. Nutr. Metab. Care 2007, 10, 660. [Google Scholar] [CrossRef]
- Moran, A.; Brunzell, C.; Cohen, R.C.; Katz, M.; Marshall, B.C.; Onady, G.; Robinson, K.A.; Sabadosa, K.A.; Stecenko, A.; Slovis, B.; et al. Clinical care guidelines for cystic fibrosis-related diabetes: A position statement of the American Diabetes Association and a clinical practice guideline of the Cystic Fibrosis Foundation, endorsed by the Pediatric Endocrine Society. Diabetes Care 2010, 33, 2697–2708. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes-2022. Diabetes Care 2022, 45, S125–S143. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 16. Diabetes Care in the Hospital: Standards of Medical Care in Diabetes-2022. Diabetes Care 2022, 45, S244–S253. [Google Scholar] [CrossRef]
- Rabe, K.F.; Watz, H. Chronic obstructive pulmonary disease. Lancet 2017, 389, 1931–1940. [Google Scholar] [CrossRef]
- Wang, M.T.; Lai, J.H.; Huang, Y.L.; Kuo, F.C.; Wang, Y.H.; Tsai, C.L.; Tu, M.Y. Use of antidiabetic medications and risk of chronic obstructive pulmonary disease exacerbation requiring hospitalization: A disease risk score-matched nested case-control study. Respir. Res. 2020, 21, 319. [Google Scholar] [CrossRef]
- Dandona, P.; Mohanty, P.; Chaudhuri, A.; Garg, R.; Aljada, A. Insulin infusion in acute illness. J. Clin. Investig. 2005, 115, 2069–2072. [Google Scholar] [CrossRef]
- Jiang, S.; Xu, Z.; Shi, Y.; Liang, S.; Jiang, X.; Xiao, M.; Wang, K.; Ding, L. Circulating insulin-like growth factor-1 and risk of lung diseases: A Mendelian randomization analysis. Front. Endocrinol. 2023, 14, 1126397. [Google Scholar] [CrossRef] [PubMed]
- Herman, M.E.; O’Keefe, J.H.; Bell, D.S.H.; Schwartz, S.S. Insulin therapy increases cardiovascular risk in Type 2 diabetes. Prog. Cardiovasc. Dis. 2017, 60, 422–434. [Google Scholar] [CrossRef]
- Kolb, H.; Kempf, K.; Röhling, M.; Martin, S. Insulin: Too much of a good thing is bad. BMC Med. 2020, 18, 224. [Google Scholar] [CrossRef]
- Yen, F.S.; Wei, J.C.; Yang, Y.C.; Hsu, C.C.; Hwu, C.M. Thiazolidinedione use in individuals with Type 2 diabetes and chronic obstructive pulmonary disease. Front. Med. 2021, 8, 729518. [Google Scholar] [CrossRef]
- Hecht, S.S. Tobacco smoke carcinogens and lung cancer. J. Natl. Cancer Inst. 1999, 91, 1194–1210. [Google Scholar] [CrossRef] [PubMed]
- Sasco, A.J.; Secretan, M.B.; Straif, K. Tobacco smoking and cancer: A brief review of recent epidemiological evidence. Lung Cancer 2004, 45, S3–S9. [Google Scholar] [CrossRef]
- Aredo, J.V.; Luo, S.J.; Gardner, R.M.; Sanyal, N.; Choi, E.; Hickey, T.P.; Riley, T.L.; Huang, W.Y.; Kurian, A.W.; Leung, A.N.; et al. Tobacco Smoking and Risk of Second Primary Lung Cancer. J. Thorac. Oncol. 2021, 16, 968–979. [Google Scholar] [CrossRef] [PubMed]
- Philips, B.J.; Meguer, J.X.; Redman, J.; Baker, E.H. Factors determining the appearance of glucose in upper and lower respiratory tract secretions. Intensive Care Med. 2003, 29, 2204–2210. [Google Scholar] [CrossRef]
- Zhu, N.; Zhang, Y.; Gong, Y.I.; He, J.; Chen, X. Metformin and lung cancer risk of patients with type 2 diabetes mellitus: A meta-analysis. Biomed. Rep. 2015, 3, 235–241. [Google Scholar] [CrossRef]
- Bowker, S.L.; Majumdar, S.R.; Veugelers, P.; Johnson, J.A. Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin. Diabetes Care 2006, 29, 254–258. [Google Scholar] [CrossRef]
- ORIGIN Trial Investigators; Gerstein, H.C.; Bosch, J.; Dagenais, G.R.; Díaz, R.; Jung, H.; Maggioni, A.P.; Pogue, J.; Probstfield, J.; Ramachandran, A.; et al. Basal insulin and cardiovascular and other outcomes in dysglycemia. N. Engl. J. Med. 2012, 367, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Kasirye, Y.; Simpson, M.; Mamillapalli, C.K.; Epperla, N.; Liang, H.; Yale, S.H. Association between blood glucose level and outcomes in patients hospitalized for acute exacerbation of chronic obstructive pulmonary disease. WMJ 2013, 112, 244–249, quiz 250. [Google Scholar] [PubMed]
- Joslin, E.P. The Treatment of Diabetes Mellitus, 4th ed.; Lea & Febiger: Philadelphia, PA, USA, 1928. [Google Scholar]
- Yen, F.S.; Chen, W.; Wei, J.C.; Hsu, C.C.; Hwu, C.M. Effects of metformin use on total mortality in patients with type 2 diabetes and chronic obstructive pulmonary disease: A matched-subject design. PLoS ONE 2018, 13, e0204859. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Sander, L.E.; Fitchett, D.H.; Zinman, B.; Pernille, O.A.; Wanner, C.; Vedin, O.; Lauer, S.; Verma, S.; Yaggi, H.K.; et al. Empagliflozin in patients with type 2 diabetes mellitus and chronic obstructive pulmonary disease. Diabetes Res. Clin. Pract. 2022, 186, 109837. [Google Scholar] [CrossRef]
- Lin, C.C.; Lai, M.S.; Syu, C.Y.; Chang, S.C.; Tseng, F.Y. Accuracy of diabetes diagnosis in health insurance claims data in Taiwan. J. Formos. Med. Assoc. 2005, 104, 157–163. [Google Scholar] [PubMed]
- Ho, T.W.; Ruan, S.Y.; Huang, C.T.; Tsai, Y.J.; Lai, F.; Yu, C.J. Validity of ICD9-CM codes to diagnose chronic obstructive pulmonary disease from National Health Insurance claim data in Taiwan. Int. J. Chron. Obstruct. Pulmon. Dis. 2018, 13, 3055–3063. [Google Scholar] [CrossRef]
- Rodriguez-Roisin, R. Towards a consensus definition for COPD exacerbations. Chest 2000, 117, 398S–401S. [Google Scholar] [CrossRef]
- Meduru, P.; Helmer, D.; Rajan, M.; Tseng, C.L.; Pogach, L.; Sambamoorthi, U. Chronic illness with complexity: Implications for performance measurement of optimal glycemic control. J. Gen. Intern. Med. 2007, 22, 408–418. [Google Scholar] [CrossRef]
- Young, B.A.; Lin, E.; Von Korff, M.; Simon, G.; Ciechanowski, P.; Ludman, E.J.; Everson-Stewart, S.; Kinder, L.; Oliver, M.; Boyko, E.J.; et al. Diabetes complications severity index and risk of mortality, hospitalization, and health care utilization. Am. J. Manag. Care 2008, 14, 15–23. [Google Scholar]
- Kelsey, J.L.; Whittemore, A.S.; Evans, A.S.; Thompson, W.D. Methods in Observational Epidemiology, 2nd ed.; Tables 12–15; Oxford University Press: Oxford, UK, 1996. [Google Scholar]
- D’Agostino, R.B., Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat. Med. 1998, 17, 2265–2281. [Google Scholar] [CrossRef]

| Non-Insulin | Insulin | ||||
|---|---|---|---|---|---|
| (N = 2370) | (N = 2370) | ||||
| Variables | n | % | n | % | SMD § |
| Sex | 0.003 | ||||
| female | 894 | 37.72 | 890 | 37.55 | |
| male | 1476 | 62.28 | 1480 | 62.45 | |
| Age | |||||
| 18–40 | 117 | 4.94 | 103 | 4.35 | 0.028 |
| 41–60 | 981 | 41.39 | 988 | 41.69 | 0.006 |
| 61–80 | 1272 | 53.67 | 1279 | 53.97 | 0.006 |
| mean, (SD) † | 60.73 | 11.21 | 61.02 | 11.11 | 0.026 |
| Comorbidities | |||||
| Obesity | 60 | 2.53 | 63 | 2.66 | 0.008 |
| Smoking | 93 | 3.92 | 90 | 3.80 | 0.007 |
| Hypertension | 1541 | 65.02 | 1575 | 66.46 | 0.03 |
| Dyslipidemia | 1572 | 66.33 | 1610 | 67.93 | 0.034 |
| Coronary artery disease | 690 | 29.11 | 713 | 30.08 | 0.021 |
| Atrial fibrillation | 294 | 12.41 | 290 | 12.24 | 0.005 |
| Peripheral arterial disease | 94 | 3.97 | 96 | 4.05 | 0.004 |
| Chronic kidney disease | 323 | 13.63 | 342 | 14.43 | 0.023 |
| Liver cirrhosis | 47 | 1.98 | 47 | 1.98 | <0.001 |
| Moderate exacerbation of COPD | 915 | 38.61 | 953 | 40.21 | 0.033 |
| Severe exacerbation of COPD | 1124 | 47.43 | 1153 | 48.65 | 0.024 |
| Charlson Comorbidity Index | |||||
| 1 | 2134 | 90.04 | 2134 | 90.04 | <0.001 |
| 2–3 | 210 | 8.86 | 207 | 8.73 | 0.004 |
| >3 | 26 | 1.10 | 29 | 1.22 | 0.012 |
| Diabetes Complication Severity Index | |||||
| 0 | 769 | 32.45 | 750 | 31.65 | 0.017 |
| 1 | 554 | 23.38 | 579 | 24.43 | 0.025 |
| ≥2 | 1047 | 44.18 | 1041 | 43.92 | 0.005 |
| Medications | |||||
| Oral systemic corticosteroid | 1723 | 72.70 | 1814 | 76.54 | 0.088 |
| Corticosteroid inhalants | 1954 | 82.45 | 2037 | 85.95 | 0.096 |
| β2bronchodilators inhalants | 29 | 1.22 | 34 | 1.43 | 0.018 |
| Anticholinergic inhalants | 150 | 6.33 | 145 | 6.12 | 0.009 |
| Metformin | 1332 | 56.20 | 1385 | 58.44 | 0.045 |
| Sulfonylureas | 1206 | 50.89 | 1247 | 52.62 | 0.035 |
| Thiazolidinediones | 311 | 13.12 | 315 | 13.29 | 0.005 |
| Dipeptidyl peptidase-4 inhibitors | 317 | 13.38 | 331 | 13.97 | 0.017 |
| Sodium-glucose cotransporter 2 inhibitors | 13 | 0.55 | 19 | 0.80 | 0.031 |
| Alpha-glucosidase inhibitors | 319 | 13.46 | 327 | 13.80 | 0.01 |
| ACEI/ARB | 1256 | 53.00 | 1284 | 54.18 | 0.024 |
| β-blockers | 735 | 31.01 | 722 | 30.46 | 0.012 |
| Calcium-channel blockers | 1353 | 57.09 | 1394 | 58.82 | 0.035 |
| Diuretics | 874 | 36.88 | 899 | 37.93 | 0.022 |
| Statin | 1032 | 43.54 | 1059 | 44.68 | 0.023 |
| Aspirin | 1055 | 44.51 | 1079 | 45.53 | 0.02 |
| Non-Insulin | Insulin | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Outcome | n | PY | IR | n | PY | IR | cHR | (95% CI) | p-Value | aHR a | (95% CI) | p-Value |
| Death | 326 | 15,198 | 21.45 | 357 | 16,026 | 22.28 | 1.04 | (0.89, 1.2) | 0.6482 | 1.08 | (0.93, 1.26) | 0.3076 |
| Hospitalization for COPD | 63 | 14,978 | 4.21 | 112 | 15,638 | 7.16 | 1.69 | (1.24, 2.3) | <0.001 | 1.7 | (1.24, 2.32) | <0.001 |
| NIPPV | 13 | 15,194 | 0.86 | 67 | 15,877 | 4.22 | 4.84 | (2.67, 8.77) | <0.001 | 5.05 | (2.76, 9.22) | <0.001 |
| IMV | 55 | 15,175 | 3.62 | 148 | 15,823 | 9.35 | 2.58 | (1.89, 3.51) | <0.001 | 2.72 | (1.99, 3.72) | <0.001 |
| Bacterial pneumonia | 122 | 14,855 | 8.21 | 286 | 15,154 | 18.87 | 2.29 | (1.85, 2.83) | <0.001 | 2.42 | (1.95, 3) | <0.001 |
| Lung cancer | 10 | 15,181 | 0.66 | 22 | 16,013 | 1.37 | 2.08 | (0.99, 4.4) | 0.0544 | 2.11 | (0.99, 4.49) | 0.0537 |
| Hospitalized hypoglycemia | 12 | 15,155 | 0.79 | 55 | 15,801 | 3.48 | 4.41 | (2.36, 8.23) | <0.001 | 4.71 | (2.5, 8.89) | <0.001 |
| Hospitalization for COPD | |||||||
|---|---|---|---|---|---|---|---|
| Variables | n | PY | IR | cHR | (95% CI) | aHR a | (95% CI) |
| Non-use of Insulin | 63 | 14,978 | 4.21 | 1.00 | (Reference) | 1.00 | (Reference) |
| Cumulative duration of insulin use (days) | |||||||
| <90 | 67 | 10,998 | 6.09 | 1.44 | (1.02, 2.04) * | 1.49 | (1.05, 2.12) * |
| 90–179 | 14 | 1263 | 11.09 | 2.61 | (1.46, 4.66) ** | 2.03 | (1.12, 3.67) * |
| >179 | 31 | 3377 | 9.18 | 2.14 | (1.39, 3.29) *** | 2.15 | (1.38, 3.35) *** |
| Bacterial pneumonia | |||||||
| Variables | n | PY | IR | cHR | (95% CI) | aHR | (95% CI) |
| Non-use of Insulin | 122 | 14,855 | 8.21 | 1.00 | (Reference) | 1.00 | (Reference) |
| Cumulative duration of insulin use (days) | |||||||
| <90 | 155 | 10,778 | 14.38 | 1.75 | (1.38, 2.22) *** | 1.93 | (1.52, 2.46) *** |
| 90–179 | 42 | 1170 | 35.91 | 4.34 | (3.06, 6.17) *** | 3.35 | (2.34, 4.8) *** |
| >179 | 89 | 3206 | 27.76 | 3.35 | (2.55, 4.4) *** | 3.45 | (2.6, 4.58) *** |
| Invasive mechanical ventilation | |||||||
| Variables | n | PY | IR | cHR | (95% CI) | aHR | (95% CI) |
| Non-use of Insulin | 55 | 15,175 | 3.62 | 1.00 | (Reference) | 1.00 | (Reference) |
| Cumulative duration of insulin use (days) | |||||||
| <90 | 76 | 11,134 | 6.83 | 1.88 | (1.33, 2.67) *** | 2.08 | (1.46, 2.96) *** |
| 90–179 | 20 | 1289 | 15.52 | 4.25 | (2.55, 7.09) *** | 3.31 | (1.97, 5.59) *** |
| >179 | 52 | 3401 | 15.29 | 4.2 | (2.87, 6.13) *** | 4.38 | (2.96, 6.47) *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yen, F.-S.; Chang, S.-H.; Wei, J.C.-C.; Shih, Y.-H.; Hwu, C.-M. Respiratory Outcomes of Insulin Use in Patients with COPD: A Nationwide Population-Based Cohort Study. Pharmaceuticals 2023, 16, 643. https://doi.org/10.3390/ph16050643
Yen F-S, Chang S-H, Wei JC-C, Shih Y-H, Hwu C-M. Respiratory Outcomes of Insulin Use in Patients with COPD: A Nationwide Population-Based Cohort Study. Pharmaceuticals. 2023; 16(5):643. https://doi.org/10.3390/ph16050643
Chicago/Turabian StyleYen, Fu-Shun, Shu-Hao Chang, James Cheng-Chung Wei, Ying-Hsiu Shih, and Chii-Min Hwu. 2023. "Respiratory Outcomes of Insulin Use in Patients with COPD: A Nationwide Population-Based Cohort Study" Pharmaceuticals 16, no. 5: 643. https://doi.org/10.3390/ph16050643
APA StyleYen, F.-S., Chang, S.-H., Wei, J. C.-C., Shih, Y.-H., & Hwu, C.-M. (2023). Respiratory Outcomes of Insulin Use in Patients with COPD: A Nationwide Population-Based Cohort Study. Pharmaceuticals, 16(5), 643. https://doi.org/10.3390/ph16050643







