Prescriptive Appropriateness: Inhospital Adherence to Proton Pump Inhibitors Deprescription Flow Chart
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
|
|
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hajjar, E.R.; Cafiero, A.C.; Hanlon, J.T. Polypharmacy in Elderly Patients. Am. J. Geriatr. Pharmacother. 2007, 5, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, S.S.; Shati, M.; Keshtkar, A.; Malakouti, S.K.; Bazargan, M.; Assari, S. Defining Polypharmacy in the Elderly: A Systematic Review Protocol. BMJ Open 2016, 6. [Google Scholar] [CrossRef]
- Masnoon, N.; Shakib, S.; Kalisch-Ellett, L.; Caughey, G.E. What Is Polypharmacy? A Systematic Review of Definitions. BMC Geriatr. 2017, 17, 230. [Google Scholar] [CrossRef] [PubMed]
- Onder, G.; Bonassi, S.; Abbatecola, A.M.; Folino-Gallo, P.; Lapi, F.; Marchionni, N.; Pani, L.; Pecorelli, S.; Sancarlo, D.; Scuteri, A.; et al. High Prevalence of Poor Quality Drug Prescribing in Older Individuals: A Nationwide Report from the Italian Medicines Agency (AIFA). J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Rapporto Nazionale OsMed 2019 Sull’uso Dei Farmaci in Italia. Available online: https://www.aifa.gov.it/en/-/rapporto-osmed-2019 (accessed on 12 November 2022).
- Spinewine, A.; Schmader, K.E.; Barber, N.; Hughes, C.; Lapane, K.L.; Swine, C.; Hanlon, J.T. Appropriate Prescribing in Elderly People: How Well Can It Be Measured and Optimised? Lancet 2007, 370, 173–184. [Google Scholar] [CrossRef]
- Avery, A.J.; Ghaleb, M.; Barber, N.; Dean Franklin, B.; Armstrong, S.J.; Serumaga, B.; Dhillon, S.; Freyer, A.; Howard, R.; Talabi, O.; et al. The Prevalence and Nature of Prescribing and Monitoring Errors in English General Practice: A Retrospective Case Note Review. Br. J. Gen. Pract. 2013, 63, e543–e553. [Google Scholar] [CrossRef]
- Dequito, A.B.; Mol, P.G.M.; Van Doormaal, J.E.; Zaal, R.J.; Van Den Bemt, P.M.L.A.; Haaijer-Ruskamp, F.M.; Kosterink, J.G.W. Preventable and Non-Preventable Adverse Drug Events in Hospitalized Patients: A Prospective Chart Review in the Netherlands. Drug Saf. 2011, 34, 1089–1100. [Google Scholar] [CrossRef]
- Moßhammer, D.; Haumann, H.; Mörike, K.; Joos, S. Polypharmacy-an Upward Trend with Unpredictable Effects. Dtsch. Arztebl. Int. 2016, 113, 627–633. [Google Scholar] [CrossRef]
- OsMed 2020 National Report on Medicines Use in Italy. Available online: https://www.aifa.gov.it/en/-/rapporto-nazionale-osmed-2020-sull-uso-dei-farmaci-in-italia (accessed on 12 November 2022).
- Strand, D.S.; Kim, D.; Peura, D.A. 25 Years of Proton Pump Inhibitors: A Comprehensive Review. Gut Liver 2017, 11, 27–37. [Google Scholar] [CrossRef]
- Lee, L.; Ramos-Alvarez, I.; Ito, T.; Jensen, R.T. Insights into Effects/Risks of Chronic Hypergastrinemia and Lifelong PPI Treatment in Man Based on Studies of Patients with Zollinger-Ellison Syndrome. Int. J. Mol. Sci. 2019, 20, 5128. [Google Scholar] [CrossRef]
- Maret-Ouda, J.; Markar, S.R.; Lagergren, J. Gastroesophageal Reflux Disease: A Review. JAMA 2020, 324, 2536–2547. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.M.; Poly, T.N.; Walther, B.A.; Dubey, N.K.; Anggraini Ningrum, D.N.; Shabbir, S.A.; Li, Y.C. Adverse Outcomes of Long-Term Use of Proton Pump Inhibitors: A Systematic Review and Meta-Analysis. Eur. J. Gastroenterol. Hepatol. 2018, 30, 1395–1405. [Google Scholar] [CrossRef]
- Savarino, V.; Marabotto, E.; Zentilin, P.; Furnari, M.; Bodini, G.; De Maria, C.; Pellegatta, G.; Coppo, C.; Savarino, E. The Appropriate Use of Proton-Pump Inhibitors. Minerva Med. 2018, 109, 386–399. [Google Scholar] [CrossRef]
- Ksiadzyna, D.; Szelag, A.; Paradowski, L. Overuse of Proton Pump Inhibitors. Pol. Arch. Med. Wewn. 2015, 125, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, R.; Echizen, H. Drug-Drug Interaction Profiles of Proton Pump Inhibitors. Clin. Pharmacokinet. 2010, 49, 509–533. [Google Scholar] [CrossRef]
- Cao, F.; Chen, C.X.; Wang, M.; Liao, H.R.; Wang, M.X.; Hua, S.Z.; Huang, B.; Xiong, Y.; Zhang, J.Y.; Xu, Y.L. Updated Meta-Analysis of Controlled Observational Studies: Proton-Pump Inhibitors and Risk of Clostridium Difficile Infection. J. Hosp. Infect. 2018, 98, 4–13. [Google Scholar] [CrossRef]
- Howell, M.D.; Novack, V.; Grgurich, P.; Soulliard, D.; Novack, L.; Pencina, M.; Talmor, D. Iatrogenic Gastric Acid Suppression and the Risk of Nosocomial Clostridium Difficile Infection. Arch. Intern. Med. 2010, 170, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Cea Soriano, L.; Ruigõmez, A.; Johansson, S.; García Rodríguez, L.A. Study of the Association between Hip Fracture and Acid-Suppressive Drug Use in a UK Primary Care Setting. Pharmacotherapy 2014, 34, 570–581. [Google Scholar] [CrossRef]
- Cheungpasitporn, W.; Thongprayoon, C.; Kittanamongkolchai, W.; Srivali, N.; Edmonds, P.J.; Ungprasert, P.; O’Corragain, O.A.; Korpaisarn, S.; Erickson, S.B. Proton Pump Inhibitors Linked to Hypomagnesemia: A Systematic Review and Meta-Analysis of Observational Studies. Ren. Fail. 2015, 37, 1237–1241. [Google Scholar] [CrossRef]
- Lazarus, B.; Chen, Y.; Wilson, F.P.; Sang, Y.; Chang, A.R.; Coresh, J.; Grams, M.E. Proton Pump Inhibitor Use and the Risk of Chronic Kidney Disease. JAMA Intern. Med. 2016, 176, 238–246. [Google Scholar] [CrossRef]
- Gilard, M.; Arnaud, B.; Cornily, J.C.; Le Gal, G.; Lacut, K.; Le Calvez, G.; Mansourati, J.; Mottier, D.; Abgrall, J.F.; Boschat, J. Influence of Omeprazole on the Antiplatelet Action of Clopidogrel Associated with Aspirin: The Randomized, Double-Blind OCLA (Omeprazole CLopidogrel Aspirin) Study. J. Am. Coll. Cardiol. 2008, 51, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Lam, J.R.; Schneider, J.L.; Zhao, W.; Corley, D.A. Proton Pump Inhibitor and Histamine 2 Receptor Antagonist Use and Vitamin B12 Deficiency. JAMA 2013, 310, 2435–2442. [Google Scholar] [CrossRef] [PubMed]
- Gomm, W.; Von Holt, K.; Thomé, F.; Broich, K.; Maier, W.; Fink, A.; Doblhammer, G.; Haenisch, B. Association of Proton Pump Inhibitors With Risk of Dementia: A Pharmacoepidemiological Claims Data Analysis. JAMA Neurol 2016, 73, 410–416. [Google Scholar] [CrossRef]
- Jianu, C.S.; Fossmark, R.; Viset, T.; Qvigstad, G.; Sørdal, O.; Mårvik, R.; Waldum, H.L. Gastric Carcinoids after Long-Term Use of a Proton Pump Inhibitor. Aliment. Pharmacol. Ther. 2012, 36, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Bowe, B.; Li, T.; Xian, H.; Yan, Y.; Al-Aly, Z. Risk of Death among Users of Proton Pump Inhibitors: A Longitudinal Observational Cohort Study of United States Veterans. BMJ Open 2017, 7, e015735. [Google Scholar] [CrossRef]
- Pasina, L.; Nobili, A.; Tettamanti, M.; Salerno, F.; Corrao, S.; Marengoni, A.; Iorio, A.; Marcucci, M.; Mannucci, P.M. Prevalence and Appropriateness of Drug Prescriptions for Peptic Ulcer and Gastro-Esophageal Reflux Disease in a Cohort of Hospitalized Elderly. Eur. J. Intern. Med. 2011, 22, 205–210. [Google Scholar] [CrossRef]
- Farrell, B.; Pottie, K.; Thompson, W.; Boghossian, T.; Pizzola, L.; Rashid, F.J.; Rojas-Fernandez, C.; Walsh, K.; Welch, V.; Moayyedi, P. Deprescribing Proton Pump Inhibitors: Evidence-Based Clinical Practice Guideline. Canadian Family Physician 2017, 63, 354. [Google Scholar]
- Savarino, V.; Marabotto, E.; Zentilin, P.; Furnari, M.; Bodini, G.; De Maria, C.; Pellegatta, G.; Coppo, C.; Savarino, E. Proton Pump Inhibitors: Use and Misuse in the Clinical Setting. Expert Rev. Clin. Pharmacol. 2018, 11, 1123–1134. [Google Scholar] [CrossRef]
- Lanas-Gimeno, A.; Hijos, G.; Lanas, Á. Proton Pump Inhibitors, Adverse Events and Increased Risk of Mortality. Expert Opin Drug Saf. 2019, 18, 1043–1053. [Google Scholar] [CrossRef]
- Reid, M.; Keniston, A.; Heller, J.C.; Miller, M.; Medvedev, S.; Albert, R.K. Inappropriate Prescribing of Proton Pump Inhibitors in Hospitalized Patients. J. Hosp. Med. 2012, 7, 421–425. [Google Scholar] [CrossRef]
- Thomas, L.; Culley, E.J.; Gladowski, P.; Goff, V.; Fong, J.; Marche, S.M. Longitudinal Analysis of the Costs Associated with Inpatient Initiation and Subsequent Outpatient Continuation of Proton Pump Inhibitor Therapy for Stress Ulcer Prophylaxis in a Large Managed Care Organization. J. Manag. Care Pharm. 2010, 16, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Garg, P.; Kottoor, R.; Munoz, J.C.; Jamal, M.M.; Lambiase, L.R.; Vega, K.J. Overuse of Acid Suppression Therapy in Hospitalized Patients. South Med. J. 2010, 103, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Waldum, H.L.; Qvigstad, G.; Fossmark, R.; Kleveland, P.M.; Sandvik, A.K. Rebound Acid Hypersecretion from a Physiological, Pathophysiological and Clinical Viewpoint. Scand. J. Gastroenterol. 2010, 45, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Boghossian, T.A.; Rashid, F.J.; Thompson, W.; Welch, V.; Moayyedi, P.; Rojas-Fernandez, C.; Pottie, K.; Farrell, B. Deprescribing versus Continuation of Chronic Proton Pump Inhibitor Use in Adults. Cochrane Database Syst. Rev. 2017, 2017, CD011969. [Google Scholar] [CrossRef]
- Helgadottir, H.; Bjornsson, E.S. Problems Associated with Deprescribing of Proton Pump Inhibitors. Int. J. Mol. Sci. 2019, 20, 5469. [Google Scholar] [CrossRef]
- Del-Pino, M.; Sanz, E.J. Analysis of Deprescription Strategies of Proton Pump Inhibitors in Primary Care: A Narrative Review. Prim. Health Care Res. Dev. 2023, 24, e14. [Google Scholar] [CrossRef]
- Scarpignato, C.; for the SIF-AIGO-FIMMG Group; Gatta, L.; Zullo, A.; Blandizzi, C. Effective and Safe Proton Pump Inhibitor Therapy in Acid-Related Diseases—A Position Paper Addressing Benefits and Potential Harms of Acid Suppression. BMC Med. 2016, 14, 179. [Google Scholar] [CrossRef]
- Gutiérrez-Valencia, M.; Izquierdo, M.; Cesari, M.; Casas-Herrero, Á.; Inzitari, M.; Martínez-Velilla, N. The Relationship between Frailty and Polypharmacy in Older People: A Systematic Review. Br. J. Clin. Pharmacol. 2018, 84, 1432–1444. [Google Scholar] [CrossRef]
- Nota 01 | Italian Medicines Agency. Available online: https://www.aifa.gov.it/en/nota-01 (accessed on 12 November 2022).
- Nota 48 | Italian Medicines Agency. Available online: https://www.aifa.gov.it/en/nota-48 (accessed on 12 November 2022).
- Pace, F.; Scarlata, P.; Casini, V.; Sarzi-Puttini, P.; Porro, G.B. Validation of the Reflux Disease Questionnaire for an Italian Population of Patients with Gastroesophageal Reflux Disease. Eur. J. Gastroenterol. Hepatol. 2008, 20, 187–190. [Google Scholar] [CrossRef]
- Vakil, N.; Van Zanten, S.V.; Kahrilas, P.; Dent, J.; Jones, R.; Bianchi, L.K.; Cesario, K.B. The Montreal Definition and Classification of Gastroesophageal Reflux Disease: A Global Evidence-Based Consensus. Am. J. Gastroenterol. 2006, 101, 1900–1920. [Google Scholar] [CrossRef]

| Total Population (n = 98) | |||
|---|---|---|---|
| Demographic Characteristics | No. | % | |
| Sex | |||
| Male | 49 | 50 | |
| Female | 49 | 50 | |
| Age, years | |||
| Mean ± SD | 75.6 ± 10.6 | ||
| Polypharmacy (≥5 drugs) | 78 | 79.6 | |
| PPI Prescription Trends | No. | % | Full Dose Prescriptions |
| home-PPI | 54 | 55.1 | 83.3% |
| inhospital-PPI | 44 | 44.9 | 93.2% |
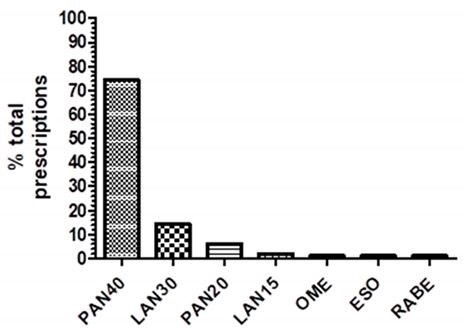 | PAN40: pantoprazole 40 mg LAN30: lansoprazole 30 mg PAN20: pantoprazole 20 mg LAN15: lansoprazole 15 mg OME: omeprazole any strength ESO: esomeprazole any strength RABE: rabeprazole any strength | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baiardi, G.; Calvini, G.; Panarello, S.; Fioravanti, C.; Stella, M.; Martelli, A.; Antonucci, G.; Mattioli, F. Prescriptive Appropriateness: Inhospital Adherence to Proton Pump Inhibitors Deprescription Flow Chart. Pharmaceuticals 2023, 16, 635. https://doi.org/10.3390/ph16050635
Baiardi G, Calvini G, Panarello S, Fioravanti C, Stella M, Martelli A, Antonucci G, Mattioli F. Prescriptive Appropriateness: Inhospital Adherence to Proton Pump Inhibitors Deprescription Flow Chart. Pharmaceuticals. 2023; 16(5):635. https://doi.org/10.3390/ph16050635
Chicago/Turabian StyleBaiardi, Giammarco, Giulia Calvini, Serena Panarello, Chiara Fioravanti, Manuela Stella, Antonietta Martelli, Giancarlo Antonucci, and Francesca Mattioli. 2023. "Prescriptive Appropriateness: Inhospital Adherence to Proton Pump Inhibitors Deprescription Flow Chart" Pharmaceuticals 16, no. 5: 635. https://doi.org/10.3390/ph16050635
APA StyleBaiardi, G., Calvini, G., Panarello, S., Fioravanti, C., Stella, M., Martelli, A., Antonucci, G., & Mattioli, F. (2023). Prescriptive Appropriateness: Inhospital Adherence to Proton Pump Inhibitors Deprescription Flow Chart. Pharmaceuticals, 16(5), 635. https://doi.org/10.3390/ph16050635








