Super Carbonate Apatite-miR-497a-5p Complex Is a Promising Therapeutic Option against Inflammatory Bowel Disease
Abstract
1. Introduction
2. Results and Discussion
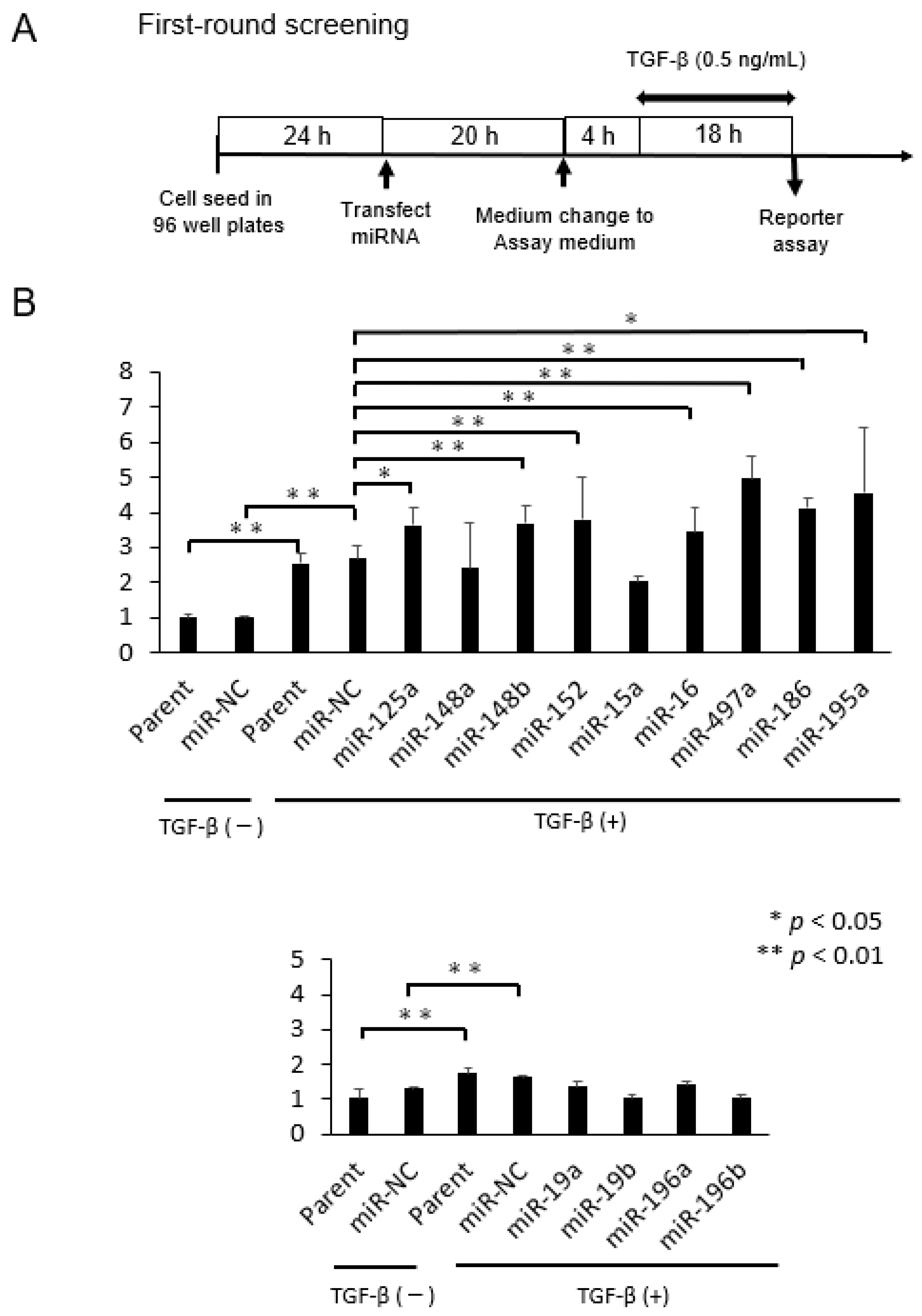
2.1. Selection of microRNAs That Up-Regulate TGF-β/Smad Signal Pathway
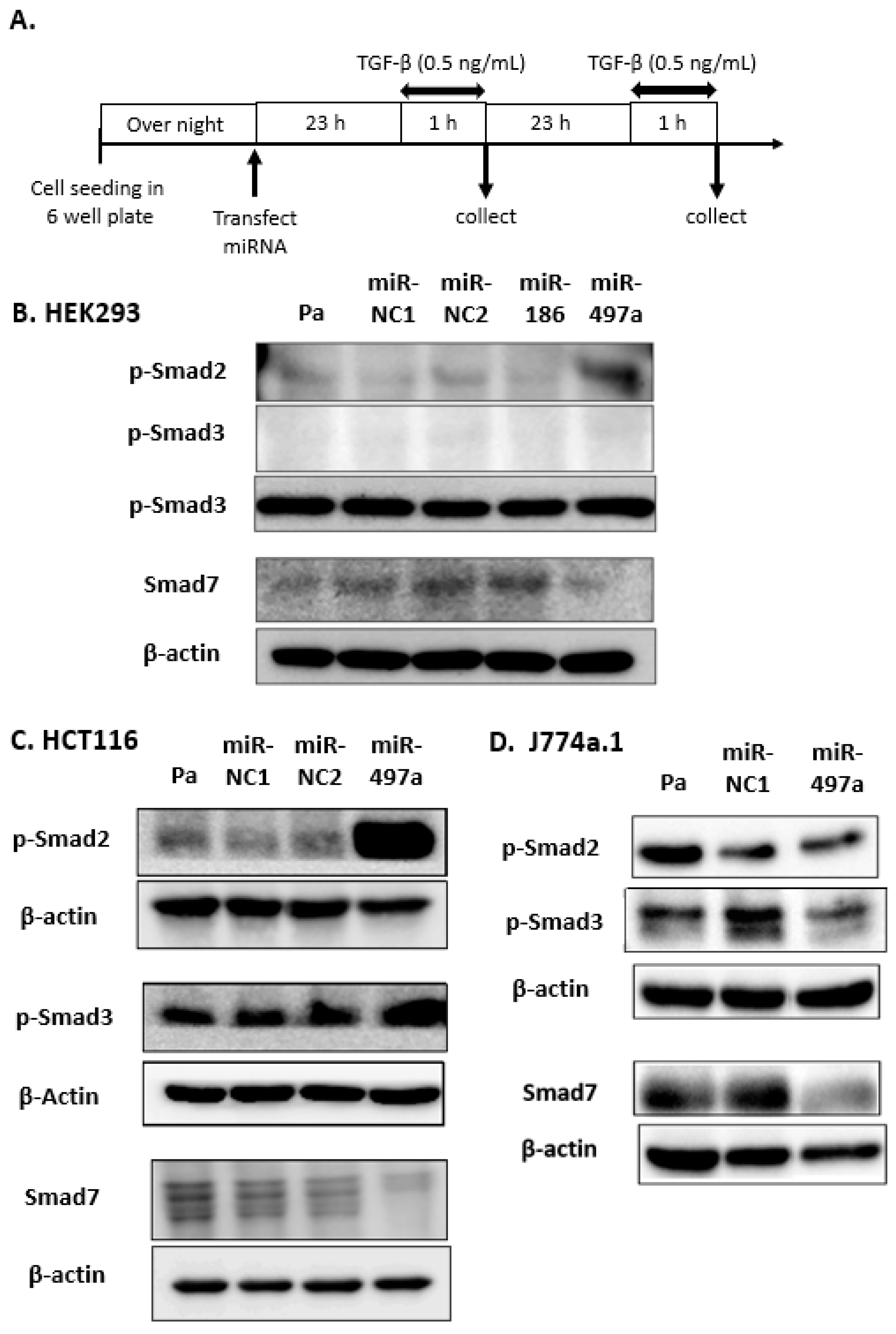
2.2. Effect of miRNA Treatment on Smad Expression
2.3. Smad 7 Is a Direct Target of miR-497a-5p
2.4. MiR-497a-5p Suppressed Expression of Inflammatory Cytokines in Mouse Macrophage J774a.1
2.5. sCA Delivered miRNA to Macrophages in Colonic Mucosa
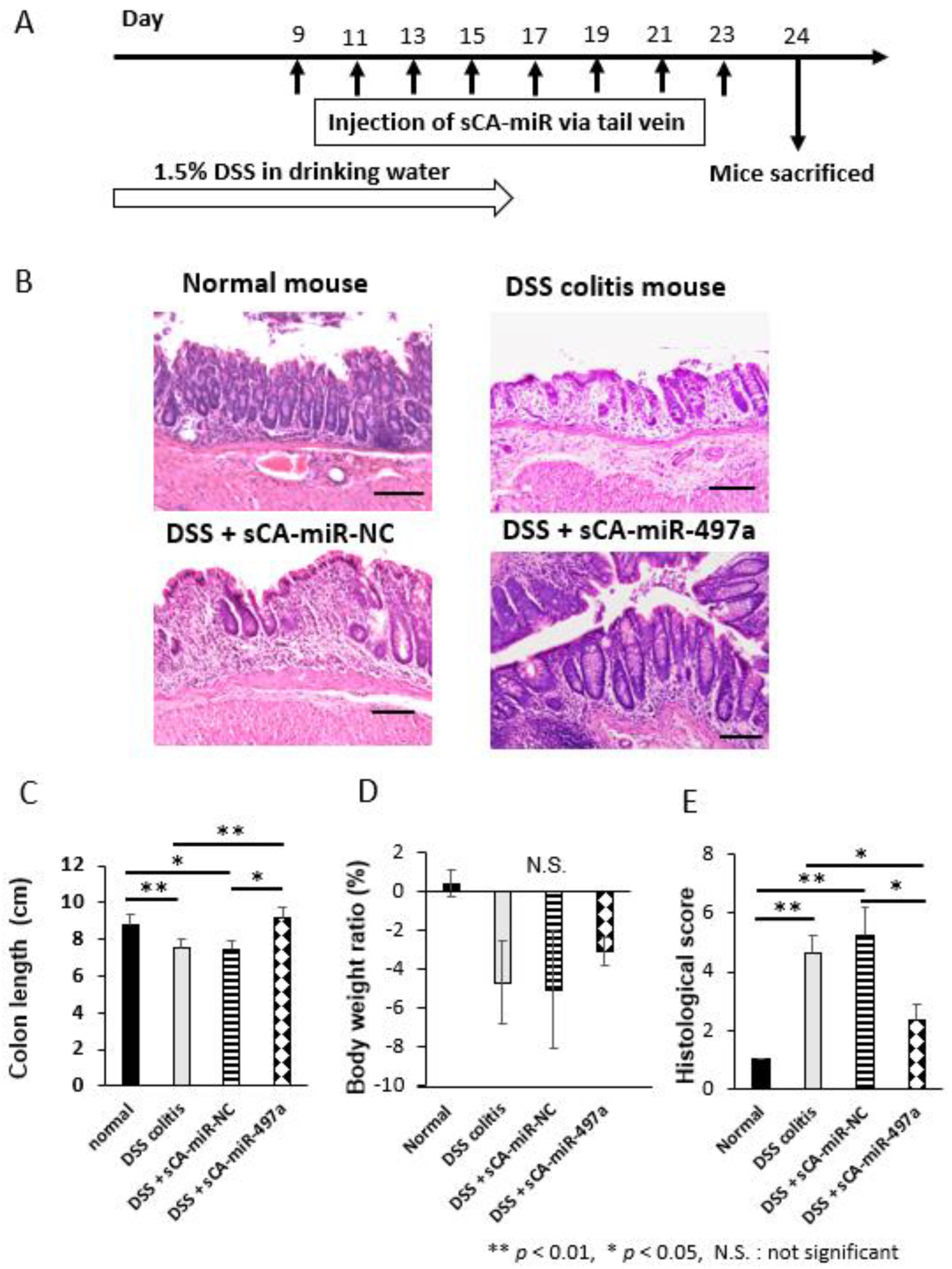
2.6. Therapeutic Efficacy of Systemic Administration of sCA-miR-497a-5p on Mouse DSS-Induced Colitis
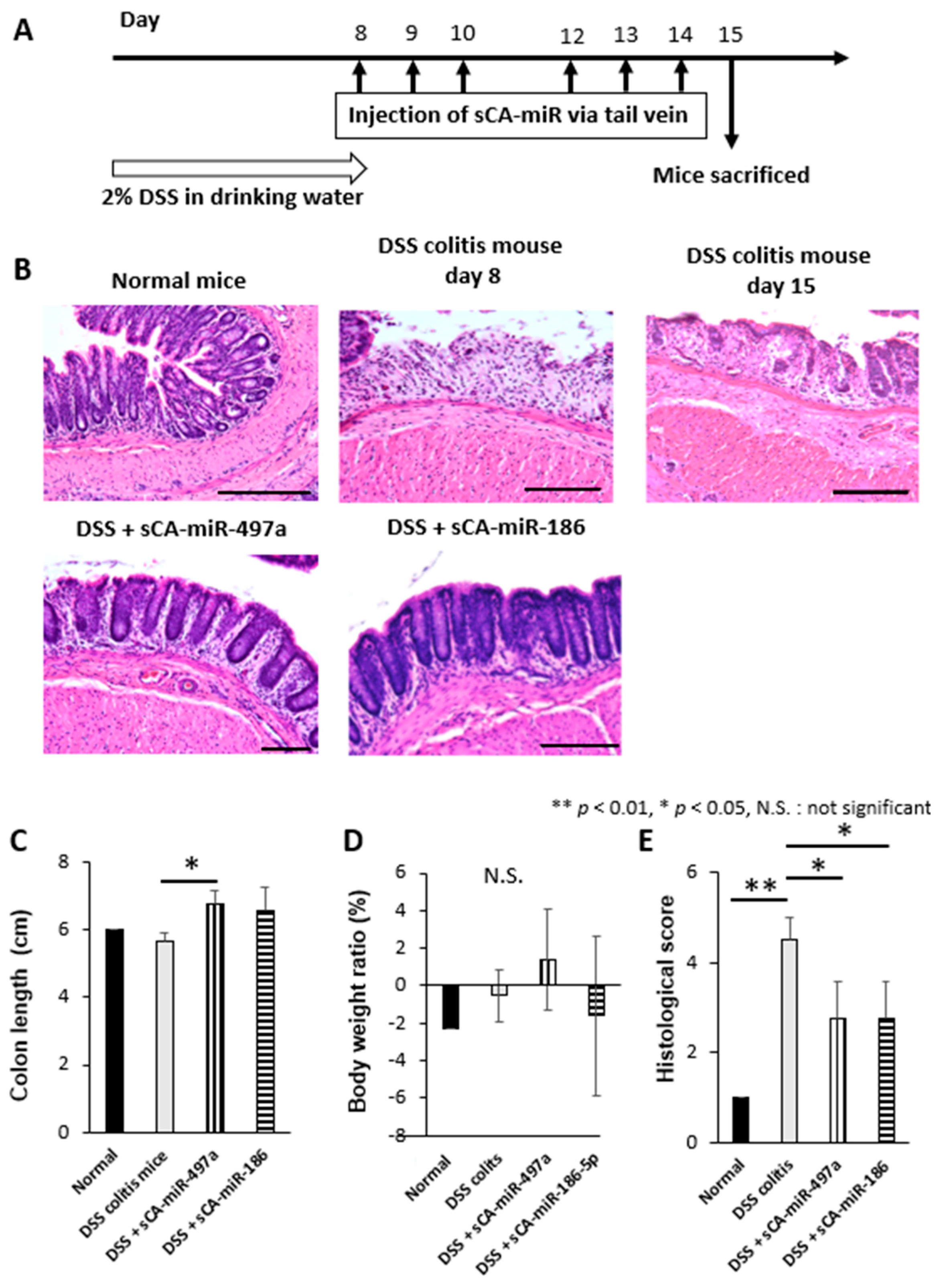
2.7. Therapeutic Efficacy of Systemic Administration of sCA-miR-186-5p on Mouse DSS-Induced Colitis
2.8. Limitation and Future Perspective
3. Materials and Methods
3.1. Cell Lines and Cell Culture
3.2. miRNAs
3.3. TGF-β Pathway-Responsive Reporter Assays
3.4. Transfection
3.5. Western Blotting
3.6. Binding Assay Using pmirGLO Plasmid Vector
3.7. qRT-PCR
- TNF-α: 5′-CGTCAGCCGATTTGCTATCT-3′ (forward) and 5′-CGGACTCCGCAAAGTCTAAG-3′ (reverse).
- IL-6: 5′-AGTTGCCTTCTTGGGACTGA-3′ (forward) and 5′-CAGAATTGCCATTGCACAAC-3′ (reverse).
- IL-12p40: 5′-AGGTGCGTTCCTCGTAGAGA-3′ (forward) and 5′-AAAGCCAACCAAGCAGAAGA-3′ (reverse).
- GAPDH: 5′-AGGTCGGTGTGAACGGATTTG-3′ (forward) and 5′-TGTAGACCATGTAGTTGAGGTCA-3′ (reverse).
3.8. Therapeutic Model for DSS-Induced Mouse Colitis
3.9. Histological Inflammation Scoring of DSS Colitis Mice
3.10. Production of sCA
3.11. Fluorescent Immunostaining of Macrophages at Propria Muscularis of Colon Mucosa
3.12. Statistics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaplan, G.G. The global burden of IBD: From 2015 to 2025. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Windsor, J.W.; Kaplan, G.G. Evolving Epidemiology of IBD. Curr. Gastroenterol. Rep. 2019, 21, 40. [Google Scholar] [CrossRef] [PubMed]
- Ungaro, R.; Mehandru, S.; Allen, P.; Peyrun-Biroulet, L.; Colombel, J.F. Ulcerative colitis. Lancet 2017, 389, 1756–1770. [Google Scholar] [CrossRef] [PubMed]
- Bouguen, G.; Levesque, B.G.; Feagan, B.G.; Kavanaugh, A.; Peyrin-Biroulet, L.; Jean-Frederic, C.; Hanauer, S.B.; Sandborn, W.J. Treat to target: A proposed new paradigm for the management of Crohnés disease. Clin. Gastroenterol. Hepatol. 2015, 13, 1042–1050. [Google Scholar] [CrossRef] [PubMed]
- Peyrin-Biroulet, L.; Sandborn, W.; Sands, B.E.; Reinisch, W.; Bemelman, W.; Bryant, R.V.; D’Haens, G.; Dotan, I.; Dubinsky, M.; Feagan, B.; et al. Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE): Determining Therapeutic Goals for Treat-to-Target. Am. J. Gastroenterol. 2015, 110, 1324–1338. [Google Scholar] [CrossRef] [PubMed]
- Sandborn, W.J.; Baert, F.; Danese, S.; Krznaric, Z.; Kobayashi, T.; Yao, X.; Chen, J.; Rosario, M.; Bhatia, S.; Kisfalvi, K.; et al. Efficacy and Safety of Vedolizumab Subcutaneous Formulation in a Randomized Trial of Patients With Ulcerative Colitis. Gastroenterology 2020, 158, 562–572. [Google Scholar] [CrossRef] [PubMed]
- Sands, B.E.; Snadborn, W.J.; Panaccione, R.; O’Brien, C.D.; Zhang, H.; Johanns, J.; Adedokun, O.J.; Li, K.; Biroulet, L.; Assche, G.V.; et al. Ustekinumab as Induction and Maintenance Therapy for Ulcerative Colitis. N. Engl. J. Med. 2019, 381, 1201–1214. [Google Scholar] [CrossRef] [PubMed]
- Sandborn, W.J.; Rebuck, R.; Wang, Y.; Zou, B.; Adedokun, O.J.; Gasink, C.; Sands, B.E.; Hanauer, S.B.; Targan, S.; Ghosh, S.; et al. Five-Year Efficacy and Safety of Ustekinumab Treatment in Crohn’s Disease: The IM-UNITI Trial. Clin. Gastroenterol. Hepatol. 2022, 20, 578–590. [Google Scholar] [CrossRef]
- Salas, A.; Rocha, C.; Duijvestein, M.; Faubion, W.; McGovern, D.; Vermeire, S.; Vetrano, S.; Casteele, N.V. JAK-STAT pathway targeting for the treatment of inflammatory bowel disease. Nav. Rev. Gastroenterol. Hepatol. 2020, 17, 323–337. [Google Scholar] [CrossRef]
- Mentella, M.C.; Scaldaferii, F.; Pizzoferrato, M.; Gasbarrini, A.; Miggiano, G.A.D. Nutrition, IBD and Gut Microbiota A review. Nutrients 2020, 12, 944. [Google Scholar] [CrossRef]
- Guan, Q. A Comprehensive Review and Update on the Pathogenesis of Inflammatory Bowel Disease. J. Immunol. Res. 2019, 2019, 7247238. [Google Scholar] [CrossRef]
- Glassner, K.L.; Abraham, B.P.; Quigley, E.M.M. The microbiome and inflammatory bowel disease. J. Allergy Clin. Immunol. 2020, 145, 16–27. [Google Scholar] [CrossRef]
- Littman, D.R.; Rudensky, A.Y. Th17 and regulatory T cells in mediating and restraining inflammation. Cell 2010, 140, 845–858. [Google Scholar] [CrossRef]
- Omenetti, S.; Pizarro, T.T. The Treg/Th17 Axis: A Dynamic Balance Regulated by the Gut Microbiome. Front. Immunol. 2015, 6, 639. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Luo, M.; Chen, Z.; He, B. The Function and Role of the Th17/Treg Cell Balance in Inflammatory Bowel Disease. J. Immunol. Res. 2020, 2020, 8813558. [Google Scholar] [CrossRef] [PubMed]
- Bai, B.; Li, H.; Han, L.; Mei, Y.; Hu, C.; Mei, Q.; Xu, J.; Liu, X. Molecular mechanism of the TGF-β/Smad7 signaling pathway in ulcerative colitis. Mol. Med. Rep. 2022, 25, 116. [Google Scholar] [CrossRef] [PubMed]
- Monteleone, G.; Kumberova, A.; Croft, N.M.; McKenzie, C.; Steer, H.W.; MacDonald, T.T. Blocking Smad7 restores TGF-βeta1 signaling in chronic inflammatory bowel disease. J. Clin. Investig. 2001, 108, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Sedda, S.; Marafini, I.; Dinallo, V.; Fusco, D.D.; Monteleone, G. The TGF-β/Smad System in IBD Pathogenesis. Inflamm. Bowel Dis. 2015, 21, 2921–2925. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.S. Transcriptional Control by the SMADs. Cold Spring Harb. Perspect. Biol. 2016, 8, a022079. [Google Scholar] [CrossRef]
- Hata, A.; Chen, Y.G. TGF-β Signaling from Receptors to Smads. Cold Spring Harb. Perspect. Biol. 2016, 8, a022061. [Google Scholar] [CrossRef] [PubMed]
- Monteleone, G.; Neurath, M.F.; Di Ardizzone, S.; Sabatino, A.; Fantini, M.C.; Castiglione, F.; Scribano, M.L.; Armuzzi, A.; Caprioli, F.; Sturniolo, G.C.; et al. Mongersen, an oral SMAD7 antisense oligonucleotide, and Crohn’s disease. N. Engl. J. Med. 2015, 372, 1104–1113. [Google Scholar] [CrossRef]
- Ogino, T.; Takeda, K. Immunoregulation by antigen-presenting cells in human intestinal lamina propria. Front. Immunol. 2023, 14, 1138971. [Google Scholar] [CrossRef] [PubMed]
- Koelink, P.J.; Bloemendaal, F.M.; Li, B.; Westera, L.; Vogels, E.W.M.; Roest, M.; Gloudemans, A.K.; Wout, A.; Korf, H.; Vermerire, S.; et al. Anti-TNF therapy in IBD exerts its therapeutic effect through macrophage IL-10 signaling. Gut 2020, 69, 1053–1063. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Li, E.; Wang, S.; Zhang, P.; Wang, Q.; Xiao, J.; Zhang, C.; Zheng, X.; Xu, X.; Xue, S.; et al. YAP Aggravates Inflammatory Bowel Disease by Regulating M1/M2 Macrophage Polarization and Gut Microbial Homeostasis. Cell Rep. 2019, 27, 1176–1189. [Google Scholar] [CrossRef] [PubMed]
- Ogino, T.; Nishimura, J.; Barman, S.; Kayama, H.; Uematsu, S.; Okuzaki, D.; Osawa, H.; Haraguchi, N.; Uemura, M.; Hata, T.; et al. Increased Th17-inducing activity of CD14+ CD163 low myeloid cells in intestinal lamina propria of patients with Crohn’s disease. Gastroenterology 2013, 145, 1380–1391. [Google Scholar] [CrossRef] [PubMed]
- Diag, A.; Schilling, M.; Klironomos, F.; Ayoub, S.; Rajewsky, N. Spatiotemporal m(i)RNA Architecture and 3’ UTR Regulation in the C. elegans Germline. Dev. Cell 2018, 47, 785–800. [Google Scholar] [CrossRef]
- Kabekkodu, S.; Shakla, V.; Varghese, V.K.; Souza, J.D.; Chakrabarty, S.; Satyamoorthy, K. Clustered miRNAs and their role in biological functions and diseases. Biol. Rev. Camb. Philos. Soc. 2018, 93, 1955–1986. [Google Scholar] [CrossRef]
- Barresi, V.; Musmeci, C.; Rinaldi, A.; Condorelli, D.F. Transcript-Targeted Therapy Based on RNA Interference and Antisense Oligonucleotides: Current Applications and Novel Molecular Targets. Int. J. Mol. Sci. 2022, 23, 8875. [Google Scholar] [CrossRef]
- Fogli, S.; Re, M.D.; Rofi, E.; Posarelli, C.; Figus, M.; Danesi, R. Clinical pharmacology of intravitreal anti-VEGF drugs. Eye 2018, 32, 1010–1020. [Google Scholar] [CrossRef]
- Patel, N.; Hegele, R.A. Mipomersen as a potential adjunctive therapy for hypercholesterolemia. Expert Opin. Pharmacother. 2010, 11, 2569–2572. [Google Scholar] [CrossRef]
- Lim, K.; Maruyama, R.; Yokota, T. Eteplirsen in the treatment of Duchenne muscular dystrophy. Drug Des. Dev. Ther. 2017, 11, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Chiriboga, C.A. Nusinersen for the treatment of spinal muscular atrophy. Expert Rev. Neurother. 2017, 17, 955–962. [Google Scholar] [CrossRef]
- Cooper, C.; Mackie, D. Hepatitis B surface antigen-1018 ISS adjuvant-containing vaccine: A review of HEPLISAV™ safety and efficacy. Expert Rev. Vaccines 2011, 10, 417–427. [Google Scholar] [CrossRef]
- Benson, M.; Dasgupta, N.R.; Monia, B. Inotersen (transthyretin-specific antisense oligonucleotide) for treatment of transthyretin amyloidosis. Neurodegener. Dis. Manag. 2019, 9, 25–30. [Google Scholar] [CrossRef]
- Yang, J. Patisiran for the treatment of hereditary transthyretin-mediated amyloidosis. Expert Rev. Clin. Pharmacol. 2019, 12, 95–99. [Google Scholar] [CrossRef]
- Cai, X.; Zhang, Z.Y.; Yuan, J.T.; Ocansey, D.K.W.; Tu, Q.; Zhang, X.; Qian, H.; Xu, W.R.; Qiu, W.; Mao, F. hucMSC-derived exosomes attenuate colitis by regulating macrophage pyroptosis via the miR-378a-5p/NLRP3 axis. Stem Cell Res. Ther. 2021, 12, 416. [Google Scholar] [CrossRef]
- Feng, Q.; Li, Y.; Zhang, H.; Wang, Z.; Nie, X.; Yao, D.; Han, L.; Chen, W.; Wang, Y. Deficiency of miRNA-149-3p shaped gut microbiota and enhanced dextran sulfate sodium-induced colitis. Mol. Ther. Nucleic Acids 2022, 30, 208–225. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, S. miR-330 alleviates dextran sodium sulfate-induced ulcerative colitis through targeting IRAK1 in rats. Kaohsiung J. Med. Sci. 2021, 37, 497–504. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, C.; Guo, Z.; Zhu, W.; Li, Q. miR-223 improves intestinal inflammation through inhibiting the IL-6/STAT3 signal pathway in dextran sodium sulfate-induced experimental colitis. Immune Inflamm. Dis. 2021, 9, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Jiao, Y.; Zhou, Y.; Meng, C.; Zhou, X.; Song, L.; Jiao, X.; Pan, Z. MicroRNA-5112 Targets IKKγ to Dampen the Inflammatory Response and Improve Clinical Symptoms in Both Bacterial Infection and DSS-Induced Colitis. Front. Immunol. 2022, 13, 779770. [Google Scholar] [CrossRef]
- Scalavino, V.; Piccinno, E.; Bianco, G.; Schena, N.; Armentano, R.; Giannelli, G.; Serino, G. The Increase of miR-195-5p Reduces Intestinal Permeability in Ulcerative Colitis, Modulating Tight Junctions’ Expression. Int. J. Mol. Sci. 2022, 23, 5840. [Google Scholar] [CrossRef]
- Wang, M.; Guo, J.; Zhao, Y.; Wang, J. IL-21 mediates microRNA-423-5p /claudin-5 signal pathway and intestinal barrier function in inflammatory bowel disease. Aging 2020, 12, 16099–16110. [Google Scholar] [CrossRef]
- Jin, X.; Chen, D.; Zheng, R.; Zhang, H.; Chen, Y.; Xiang, Z. miRNA-133a-UCP2 pathway regulates inflammatory bowel disease progress by influencing inflammation, oxidative stress and energy metabolism. World J. Gastroenterol. 2017, 23, 76–86. [Google Scholar] [CrossRef]
- Wu, X.; Yamamoto, H.; Nakanishi, H.; Yamamoto, Y.; Inoue, A.; Tei, M.; Hirose, H.; Uemura, M.; Nishimura, J.; Hata, T.; et al. Innovative Delivery of siRNA to Solid Tumors by Super Carbonate Apatite. PLoS ONE 2015, 10, e0116022. [Google Scholar] [CrossRef]
- Wang, J.; Yokoyama, Y.; Hirose, H.; Shimomura, Y.; Bonkobara, S.; Itakura, H.; Kouda, S.; Morimoto, Y.; Minami, K.; Takahashi, H.; et al. Functional assessment of miR-1291 in colon cancer cells. Int. J. Oncol. 2022, 60, 13. [Google Scholar] [CrossRef]
- Fukata, T.; Mizushima, T.; Nishimura, J.; Okuzaki, D.; Wu, X.; Hirose, H.; Yokoyama, Y.; Kubota, Y.; Nagata, K.; Tsujimura, N.; et al. The Supercarbonate Apatite-MicroRNA Complex Inhibits Dextran Sodium Sulfate-Induced Colitis. Mol. Nucleic Acids 2018, 12, 658–671. [Google Scholar] [CrossRef] [PubMed]
- Takeyama, H.; Yamamoto, H.; Yamashita, S.; Wu, X.; Takahashi, H.; Nishimura, J.; Haraguchi, N.; Miyake, Y.; Suzuki, R.; Murata, K.; et al. Decreased miR-340 expression in bone marrow is associated with liver metastasis of colorectal cancer. Mol. Cancer Ther. 2014, 13, 976–985. [Google Scholar] [CrossRef] [PubMed]
- Hiraki, M.; Nishimura, J.; Takahashi, H.; Wu, X.; Takahashi, Y.; Miyo, M.; Nishida, N.; Uemura, M.; Hata, T.; Takemasa, I.; et al. Concurrent Targeting of KRAS and AKT by MiR-4689 Is a Novel Treatment Against Mutant KRAS Colorectal Cancer. Mol. Nucleic Acids 2015, 4, e231. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, H.; Wu, X.; Kawamoto, K.; Nishida, N.; Konno, M.; Koseki, J.; Matsui, H.; Noguchi, K.; Gotoh, N.; Yamamoto, T.; et al. MicroRNAs Induce Epigenetic Reprogramming and Suppress Malignant Phenotypes of Human Colon Cancer Cells. PLoS ONE 2015, 10, e0127119. [Google Scholar] [CrossRef]
- Takahashi, H.; Nishimura, J.; Kagawa, Y.; Kano, Y.; Takahashi, Y.; Wu, X.; Hiraki, M.; Hamabe, A.; Konno, M.; Haraguchi, N.; et al. Significance of Polypyrimidine Tract-Binding Protein 1 Expression in Colorectal Cancer. Mol. Cancer Ther. 2015, 14, 1705–1716. [Google Scholar] [CrossRef]
- Inoue, A.; Mizushima, T.; Wu, X.; Okuzaki, D.; Kambara, N.; Ishikawa, S.; Wang, J.; Qian, Y.; Hirose, H.; Yokoyama, Y.; et al. A miR-29b Byproduct Sequence Exhibits Potent Tumor-Suppressive Activities via Inhibition of NF-κB Signaling in KRAS-Mutant Colon Cancer Cells. Mol. Cancer Ther. 2018, 17, 977–987. [Google Scholar] [CrossRef]
- Takahashi, H.; Misato, K.; Aoshi, T.; Yamamoto, Y.; Kubota, Y.; Wu, X.; Kuroda, E.; Ishii, K.J.; Yamamoto, H.; Yoshioka, Y. Carbonate Apatite Nanoparticles Act as Potent Vaccine Adjuvant Delivery Vehicles by Enhancing Cytokine Production Induced by Encapsulated Cytosine-Phosphate-Guanine Oligodeoxynucleotides. Front. Immunol. 2018, 9, 783. [Google Scholar] [CrossRef] [PubMed]
- Tamai, K.; Mizushima, T.; Wu, X.; Inoue, A.; Ota, M.; Yokoyama, Y.; Miyoshi, N.; Haraguchi, N.; Takahashi, H.; Nishimura, J.; et al. Photodynamic Therapy Using Indocyanine Green Loaded on Super Carbonate Apatite as Minimally Invasive Cancer Treatment. Mol. Cancer Ther. 2018, 17, 1613–1622. [Google Scholar] [CrossRef]
- Morimoto, Y.; Mizushima, T.; Wu, X.; Okuzaki, D.; Yokoyama, Y.; Inoue, A.; Hata, T.; Hirose, H.; Qian, Y.; Wang, J.; et al. miR-4711-5p regulates cancer stemness and cell cycle progression via KLF5, MDM2 and TFDP1 in colon cancer cells. Br. J. Cancer 2020, 122, 1037–1049. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Yokoyama, Y.; Takahashi, H.; Kouda, S.; Yamamoto, H.; Wang, J.; Morimoto, Y.; Minami, K.; Hata, T.; Shamma, A.; et al. Improved In Vivo Delivery of Small RNA Based on the Calcium Phosphate Method. J. Pers. Med. 2021, 11, 1160. [Google Scholar] [CrossRef]
- Monteleone, G.; Stolfi, C.; Marafini, I.; Atreya, R.; Neurath, M.F. Smad7 Antisense Oligonucleotide-Based Therapy in Crohn’s Disease: Is it Time to Re-Evaluate? Mol. Diagn. Ther. 2022, 26, 477–481. [Google Scholar] [CrossRef]
- Sands, B.; Feagan, B.; Sandborn, W.; Schreiber, S.; Laurent, P.B.; Colombel, J.; Rossiter, G.; Usiskin, K.; Ather, S.; Zhan, X.; et al. Mongersen (GED-0301) for Active Crohn’s Disease: Results of a Phase 3 Study. Am. J. Gastroenterol. 2020, 115, 738–745. [Google Scholar] [CrossRef]
- Marafini, I.; Stolfi, C.; Troncone, E.; Lolli, E.; Onali, S.; Paoluzi, O.; Fantini, M.; Biancone, L.; Calabrese, E.; Grazia, A.; et al. A Pharmacological Batch of Mongersen that Downregulates Smad7 is Effective as Induction Therapy in Active Crohn’s Disease: A Phase II, Open-Label Study. BioDrugs 2021, 35, 325–336. [Google Scholar] [CrossRef]
- Bewtra, M.; Lichtenstein, G. Mongersen and SMAD-7 Inhibition, Not a Lucky 7 for Patients With IBD: When Trial Design Is as Important as Disease Therapy. Am. J. Gastroenterol. 2020, 115, 687–688. [Google Scholar] [CrossRef] [PubMed]
- TargetScan. Available online: https://www.targetscan.org/vert_80/ (accessed on 1 July 2017).
- miRbase. Available online: https://www.mirbase.org (accessed on 1 July 2017).
- Viviana, S.; Emanuele, P.; Antonio, L.; Angela, T.; Raffaele, A.; Gianluigi, G.; Grazia, S. miR-195-5p Regulates Tight Junctions Expression via Claudin-2 Downregulation in Ulcerative Colitis. Biomedicines 2022, 10, 919. [Google Scholar]
- Chapel, A.; Caligaris, C.; Fenouil, T.; Savary, C.; Aires, S.; Martel, S.; Huchede, P.; Chassot, C.; Chauvet, V.; Ruffino, V.; et al. SMAD2/3 mediate oncogenic effects of TGF-β in the absence of SMAD4. Commun. Biol. 2022, 5, 1068. [Google Scholar] [CrossRef] [PubMed]
- Buwaneka, P.; Ralko, A.; Gorai, S.; Pham, H.; Cho, W. Phosphoinositide-binding activity of Smad2 is essential for its function in TGF-β signaling. J. Biol. Chem. 2021, 297, 101303. [Google Scholar] [CrossRef]
- Mohankumar, K.; Namachivayam, K.; Chapalamadugu, K.C.; Garzon, S.A.; Premkumar, M.H.; Tipparaju, S.M.; Maheshwari, A. Smad7 interrupts TGF-β signaling in intestinal macrophages and promotes inflammatory activation of these cells during necrotizing enterocolitis. Pediatr. Res. 2016, 79, 951–961. [Google Scholar] [CrossRef]
- Chen, X.; Shi, C.; Wang, C.; Liu, W.; Chu, Y.; Xiang, Z.; Hu, K.; Dong, P.; Han, X. The role of miR-497-5p in myofibroblast differentiation of LR-MSCs and pulmonary fibrogenesis. Sci. Rep. 2017, 7, 40958. [Google Scholar] [CrossRef]
- Satsu, H.; Ishimotoa, Y.; Nakanoa, T.; Mochizukia, T.; Iwanaga, T.; Shimizu, M. Induction by activated macrophage-like THP-1 cells of apoptotic and necrotic cell death in intestinal epithelial Caco-2 monolayers via tumor necrosis factor-alpha. Exp. Cell Res. 2006, 312, 3909–3919. [Google Scholar] [CrossRef] [PubMed]
- Petric, Z.; Goncalves, J.; Paixao, P. Under the Umbrella of Clinical Pharmacology: Inflammatory Bowel Disease, Infliximab and Adalimumab, and a Bridge to an Era of Biosimilars. Pharmaceutics 2022, 14, 1766. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, K.; Makino, H.; Aoki, M.; Miyake, T.; Yasumasa, N.; Osako, M.; Nakagami, H.; Rakugi, H.; Morishita, R. Therapeutic effect of ribbon-type nuclear factor-κB decoy oligonucleotides in a rat model of inflammatory bowel disease. Curr. Gene Ther. 2012, 12, 484–492. [Google Scholar] [CrossRef]
- Tahara, K.; Samura, S.; Tsuji, K.; Yamamoto, H.; Tsukada, Y.; Bando, Y.; Tsujimoto, H.; Morishita, R.; Kawashima, K. Oral nuclear factor-κB decoy oligonucleotides delivery system with chitosan modified poly(D,L-lactide-co-glycolide) nanospheres for inflammatory bowel disease. Biomaterials 2011, 32, 870–878. [Google Scholar] [CrossRef]
- Kim, J.J.; Shajib, M.S.; Manocha, M.M.; Khan, W.I. Investigating intestinal inflammation in DSS-induced model of IBD. J. Vis. Exp. 2012, 60, 3678. [Google Scholar]
- Hoffmann, M.; Schwertassek, U.; Seydel, A.; Weber, K.; Falk, W.; Hauschildt, S.; Lehmann, J. A refined and translationally relevant model of chronic DSS colitis in BALB/c mice. Lab. Anim. 2018, 52, 240–252. [Google Scholar] [CrossRef]
- Chassaing, B.; Aitken, J.D.; Malleshappa, M.; Kumar, M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr. Protoc. Immunol. 2014, 104, 15.25.1–15.25.14. [Google Scholar] [CrossRef]
- Lei, J.; Liu, L.; Zhang, M.; Zhang, Z. METTL3/LINC00662/miR-186-5p feedback loop regulates docetaxel resistance in triple negative breast cancer. Sci. Rep. 2022, 12, 16715. [Google Scholar]
- Ting, H.; Lina, G.; Na, G.; Chaochao, W.; Wuli, G.; Xiaojie, Z.; Qi, L. miR-221-5p and miR-186-5p Are the Critical Bladder Cancer Derived Exosomal miRNAs in Natural Killer Cell Dysfunction. Int. J. Mol. Sci. 2022, 23, 15177. [Google Scholar]
- Rui, W.; Hongbo, B.; Shihua, Z.; Ruiyan, L.; Lijie, C.; Yulan, Z. miR-186-5p Promotes Apoptosis by Targeting IGF-1 in SH-SY5Y OGD/R Model. Cancer Cell Int. 2021, 21, 114. [Google Scholar]
- Zhang, Z.; Wen, Z.; Junsheng, M.; Zheng, X.; Mingyu, F. miR-186-5p Functions as a Tumor Suppressor in Human Osteosarcoma by Targeting FOXK1. Cell Physiol. Biochem. 2019, 52, 553–564. [Google Scholar] [PubMed]
- Ang, L.; Lei, F.; Xiaoya, N.; Qihui, Z.; Bei, L.; Zhen, Y. Downregulation of OIP5-AS1 affects proNGF-induced pancreatic cancer metastasis by inhibiting p75NTR levels. Aging 2021, 13, 10688–10702. [Google Scholar]
- Xian, Z.; Yanli, W.; Rong, D.; Hailong, Z.; Jinzhuo, D.; Haihua, Y.; Guofang, H.; Yuzhang, D.; Qin, C.; Jianxiu, Y. miR186 suppresses prostate cancer progression by targeting Twist1. Oncotarget 2016, 7, 33136–33151. [Google Scholar]
- Zhang, M.; Yang, D.; Yu, H.; Li, Q. MicroRNA-497 inhibits inflammation in DSS-induced IBD model mice and lipopolysaccharide-induced RAW264.7 cells via Wnt/β-catenin pathway. Int. Immunopharmacol. 2021, 101 Pt B, 108318. [Google Scholar] [CrossRef]
- Abd-Aziz, N.; Kamaruzman, N.I.; Poh, C.L. Development of MicroRNAs as Potential Therapeutics against Cancer. J. Oncol. 2020, 2020, 8029721. [Google Scholar] [CrossRef] [PubMed]
- Forterre, A.; Komuro, H.; Aminova, S.; Harada, M. A Comprehensive Review of Cancer MicroRNA Therapeutic Delivery Strategies. Cancers 2020, 12, 1852. [Google Scholar] [CrossRef]
- Merhautova, J.; Demlova, R.; Slaby, O. MicroRNA-Based Therapy in Animal Models of Selected Gastrointestinal Cancers. Front. Pharmacol. 2016, 7, 329. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, R.U.; Prieto-Vila, M.; Kohama, I.; Ochiya, T. Development of miRNA-based therapeutic approaches for cancer patients. Cancer Sci. 2019, 110, 1140–1147. [Google Scholar] [CrossRef] [PubMed]




| Gene | mmu miRNA | Position in the UTR | Seed Match Count |
|---|---|---|---|
| SMURF1 | 125a-5p | 2315–2322 | 8mer |
| 125b-5p | 2315–2322 | 8mer | |
| 15a-5p | 2628–2634 | 7mer-m8 | |
| 15b-5p | 2628–2634 | 7mer-m8 | |
| 16-5p | 2628–2634 | 7mer-m8 | |
| 19a-3p | 642–649 | 8mer | |
| 19b-3p | 642–649 | 8mer | |
| SMURF2 | 497a-5p | 205–211 | 7mer-1A |
| 322-5p | 205–211 | 7mer-1A | |
| 15a-5p | 205–211 | 7mer-1A | |
| 15b-5p | 205–211 | 7mer-1A | |
| 16-5p | 205–211 | 7mer-1A | |
| 195a-5p | 205–211 | 7mer-1A | |
| 19b-3p | 2572–2578 | 7mer-m8 | |
| 19a-3p | 2572–2578 | 7mer-m8 | |
| 148a-3p | 2574–2580 | 7mer-m8 | |
| 152-3p | 2574–2580 | 7mer-m8 | |
| 186-5p | 2441–2447 | 7mer-m8 | |
| LTBP1 | 152-3p | 37–43 | 7mer-m8 |
| 148a-3p | 37–43 | 7mer-m8 | |
| 148b-3p | 37–43 | 7mer-m8 | |
| SMAD6 | 196b-5p | 102–108 | 7mer-1A |
| 196a-5p | 102–108 | 7mer-1A | |
| 186-5p | 248–254 | 7mer-m8 | |
| SMAD7 | 15a-5p | 69–76 | 8mer |
| 497a-5p | 69–76 | 8mer | |
| 195a-5p | 69–76 | 8mer | |
| 15b-5p | 69–76 | 8mer | |
| 16-5p | 69–76 | 8mer | |
| 322-5p | 69–76 | 8mer | |
| TGIF | 19a-3p | 625–632 | 8mer |
| 19b-5p | 543–549 | 7mer-1A | |
| 6965-5p | 192–198 | 7mer-m8 | |
| 7075-5p | 195–202 | 8mer | |
| 148b-3p | 126–132 | 7mer-m8 | |
| 148a-3p | 126–132 | 7mer-m8 | |
| 15a-5p | 1709–1715 | 7mer-m8 | |
| 16-5p | 1709–1715 | 7mer-m8 | |
| 152-3p | 1678–1685 | 8mer | |
| 195a-5p | 1709–1715 | 7mer-m8 | |
| 322-5p | 1709–1715 | 7mer-m8 | |
| 497a-5p | 1709–1715 | 7mer-m8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsujimura, N.; Ogino, T.; Hiraki, M.; Kai, T.; Yamamoto, H.; Hirose, H.; Yokoyama, Y.; Sekido, Y.; Hata, T.; Miyoshi, N.; et al. Super Carbonate Apatite-miR-497a-5p Complex Is a Promising Therapeutic Option against Inflammatory Bowel Disease. Pharmaceuticals 2023, 16, 618. https://doi.org/10.3390/ph16040618
Tsujimura N, Ogino T, Hiraki M, Kai T, Yamamoto H, Hirose H, Yokoyama Y, Sekido Y, Hata T, Miyoshi N, et al. Super Carbonate Apatite-miR-497a-5p Complex Is a Promising Therapeutic Option against Inflammatory Bowel Disease. Pharmaceuticals. 2023; 16(4):618. https://doi.org/10.3390/ph16040618
Chicago/Turabian StyleTsujimura, Naoto, Takayuki Ogino, Masayuki Hiraki, Taisei Kai, Hiroyuki Yamamoto, Haruka Hirose, Yuhki Yokoyama, Yuki Sekido, Tsuyoshi Hata, Norikatsu Miyoshi, and et al. 2023. "Super Carbonate Apatite-miR-497a-5p Complex Is a Promising Therapeutic Option against Inflammatory Bowel Disease" Pharmaceuticals 16, no. 4: 618. https://doi.org/10.3390/ph16040618
APA StyleTsujimura, N., Ogino, T., Hiraki, M., Kai, T., Yamamoto, H., Hirose, H., Yokoyama, Y., Sekido, Y., Hata, T., Miyoshi, N., Takahashi, H., Uemura, M., Mizushima, T., Doki, Y., Eguchi, H., & Yamamoto, H. (2023). Super Carbonate Apatite-miR-497a-5p Complex Is a Promising Therapeutic Option against Inflammatory Bowel Disease. Pharmaceuticals, 16(4), 618. https://doi.org/10.3390/ph16040618







