Abstract
Multiple myeloma is a malignancy of immunoglobulin-secreting plasma cells that is now often treated in the newly diagnosed and relapsed and/or refractory settings with monoclonal antibodies targeting lineage-specific markers used either alone or in rationally designed combination regimens. Among these are the anti-CD38 antibodies daratumumab and isatuximab, and the anti-Signaling lymphocytic activation molecule family member 7 antibody elotuzumab, all of which are used in their unconjugated formats. Single-chain variable fragments from antibodies also form a key element of the chimeric antigen receptors (CARs) in the B-cell maturation antigen (BCMA)-targeted CAR T-cell products idecabtagene vicleucel and ciltacabtagene autoleucel, which are approved in the advanced setting. Most recently, the bispecific anti-BCMA and T-cell-engaging antibody teclistamab has become available, again for patients with relapsed/refractory disease. Another format into which antibodies can be converted to exert anti-tumor efficacy is as antibody–drug conjugates (ADCs), and belantamab mafodotin, which also targets BCMA, represented the first such agent that gained a foothold in myeloma. Negative results from a recent Phase III study have prompted the initiation of a process for withdrawal of its marketing authorization. However, belantamab remains a drug with some promise, and many other ADCs targeting either BCMA or other plasma cell surface markers are in development and showing potential. This contribution will provide an overview of some of the current data supporting the possibility that ADCs will remain a part of our chemotherapeutic armamentarium against myeloma moving forward, and also highlight areas for future development.
1. Introduction
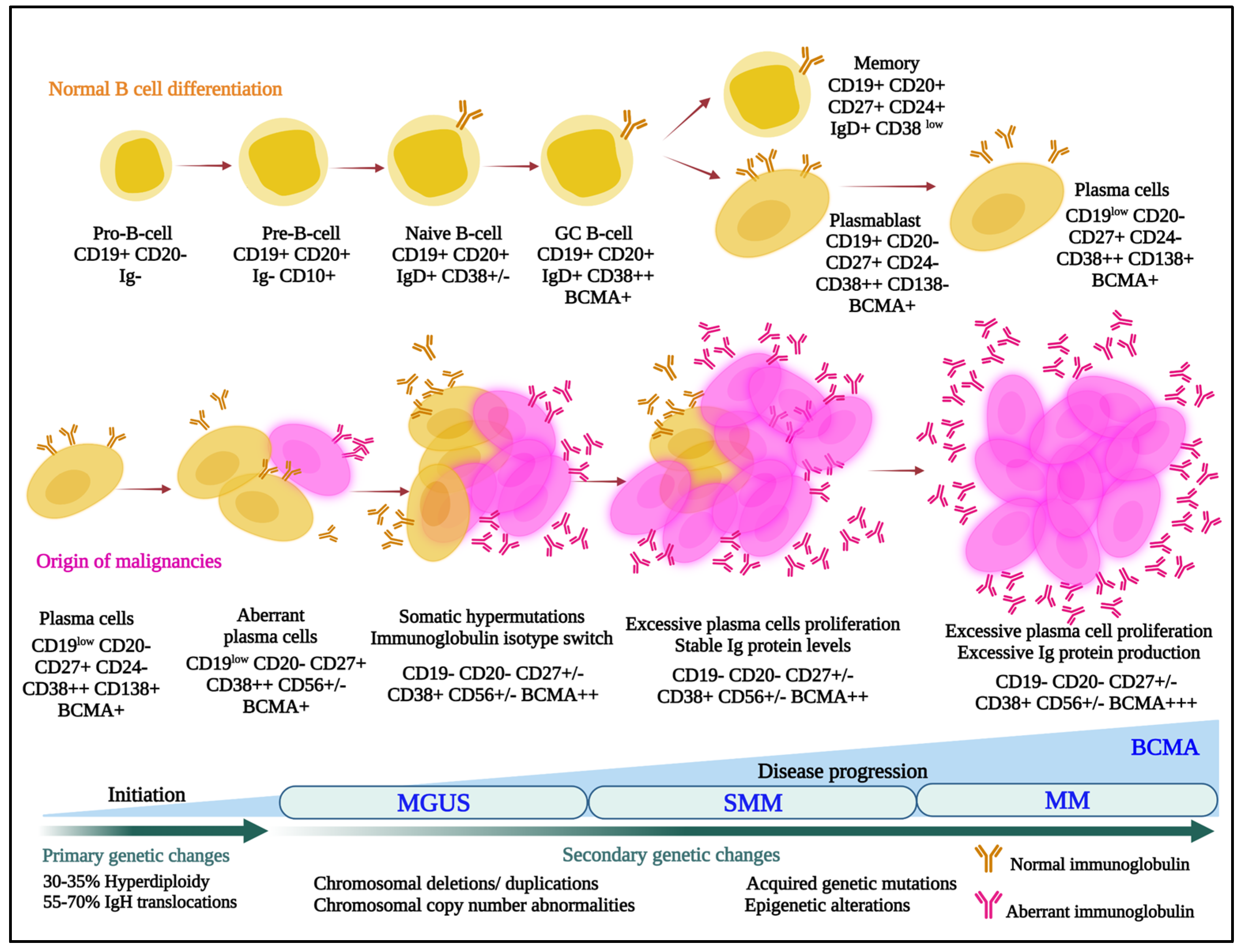
Multiple myeloma is the second most commonly diagnosed hematologic malignancy in most developed countries. It is characterized by infiltration and poorly controlled proliferation of malignant plasma cells in the bone marrow, and sometimes in extramedullary sites as well. These cells represent the terminally differentiated versions of pro-B-cells that originate in the bone marrow and develop to transitional cells. Next, they traffic to lymph nodes, where they are exposed to antigens and become plasmablasts, and, finally, return to the marrow as plasma cells (Figure 1) [1].
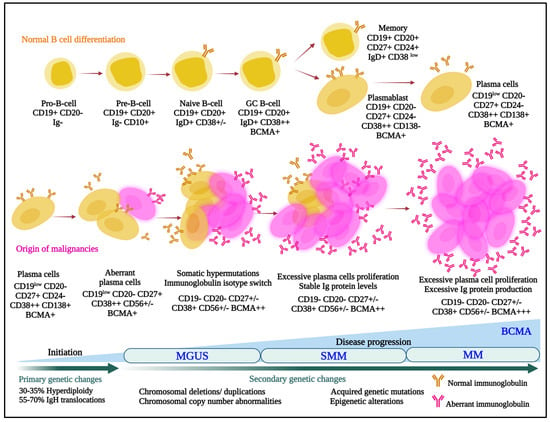
Figure 1.
Normal (upper panel) and aberrant (lower panel) B-cell differentiation processes are outlined, and cells are annotated with some of the lineage-specific surface markers that may be targets for antibody–drug conjugates. MGUS, monoclonal gammopathy of undetermined significance; SMM, smoldering multiple myeloma; MM, multiple myeloma; BCMA, B-cell maturation antigen; IgH, immunoglobulin heavy locus.
In that myeloma cells retain many characteristics of plasma cells, they typically continue to secrete intact immunoglobulins (Igs) such as IgG or IgA, and rarely IgD or IgE, or immunoglobulin fragments, including either free light or heavy chains. Notably, this characteristic was leveraged by Georges Köhler and César Milstein when they fused murine myeloma cells with mouse spleen cells from an immunized donor to form hybridomas [2], which allowed mass production of monoclonal antibodies. This earned them the 1984 Nobel Prize in Physiology or Medicine, and ushered in a new era, especially starting in 1997, when the anti-CD20 monoclonal rituximab became the first approved antibody for cancer therapy. Uncontrolled secretion of monoclonal Igs in myeloma also serves as a quantifiable biomarker for disease burden. Unfortunately, these Igs can also be pathogenic and contribute to organ dysfunction, such as of the heart and kidneys, especially in the setting of light chain (AL) amyloidosis [3]. Notably, outcomes for patients with myeloma have been improving dramatically [4,5,6,7,8] with the advent of novel therapies [9], and especially of immunomodulatory agents (IMiDs), proteasome inhibitors (PIs), anti-CD38 antibodies, and B-cell maturation antigen (BCMA)-targeted drugs. However, even patients with newly diagnosed myeloma do not uniformly enter complete remissions. Also, those that do still develop relapsed and relapsed/refractory disease that is characterized by shorter remission durations with each subsequent therapy, and myeloma remains incurable in the vast majority [10,11,12]. This supports the presence of a persistent unmet medical need in several areas spanning the spectrum of myeloma, and the possibility that new drug categories with new mechanisms of action could still play a significant role in the treatment of patients. Moreover, the stepwise process through which plasma cells develop leads to enhanced expression and exposure of lineage-specific cell surface proteins that can serve as targets for antibody-based therapeutics (Figure 1). This fact, along with the flexibility of the antibody–drug conjugate (ADC) platform, suggests that ADCs could play a substantial role in myeloma therapy. Our task will be to provide an overview of what has been accomplished to date, and highlight possible future promising directions, with a focus on those ADCs that either have been or are in clinical trials or are about to enter the clinic.
2. Rationale for ADCs in Myeloma
2.1. Mechanisms of Action of ADCs against Cancer
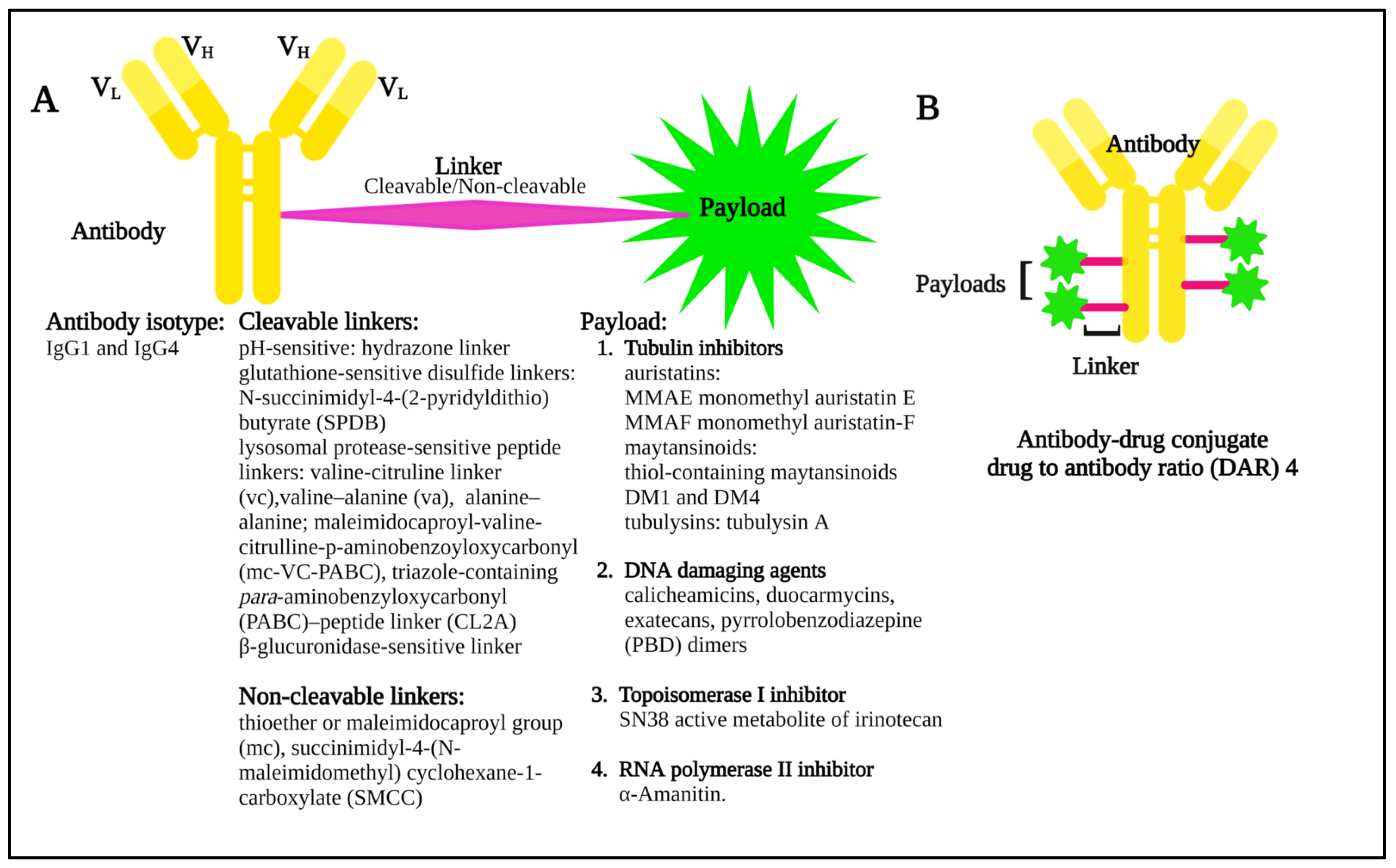
Many cell surface molecules are subject to internalization through phagocytosis, pinocytosis, or receptor-mediated endocytosis, the last of which can be mediated by mechanisms dependent on clathrin or caveolae, or that are independent of both [13]. These pathways provide portals of entry into the cell interior that can be leveraged by ADCs, which are broadly structured to include an antibody to target the cell of interest, a linker, and a payload, or warhead of interest (Figure 2) that induces cytotoxicity.
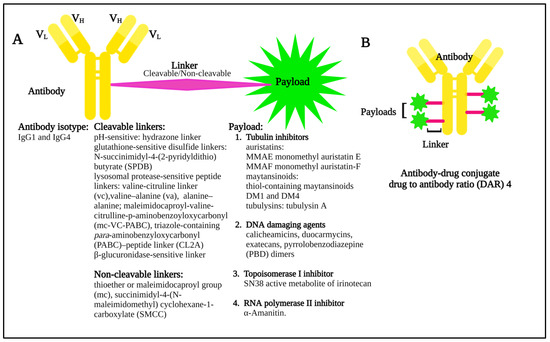
Figure 2.
(A) Diagram showing the basic components of an antibody–drug conjugate, including some of the possible linker and payload combinations. (B) Model structure of an antibody–drug conjugate with a drug to antibody ratio (DAR) 4.
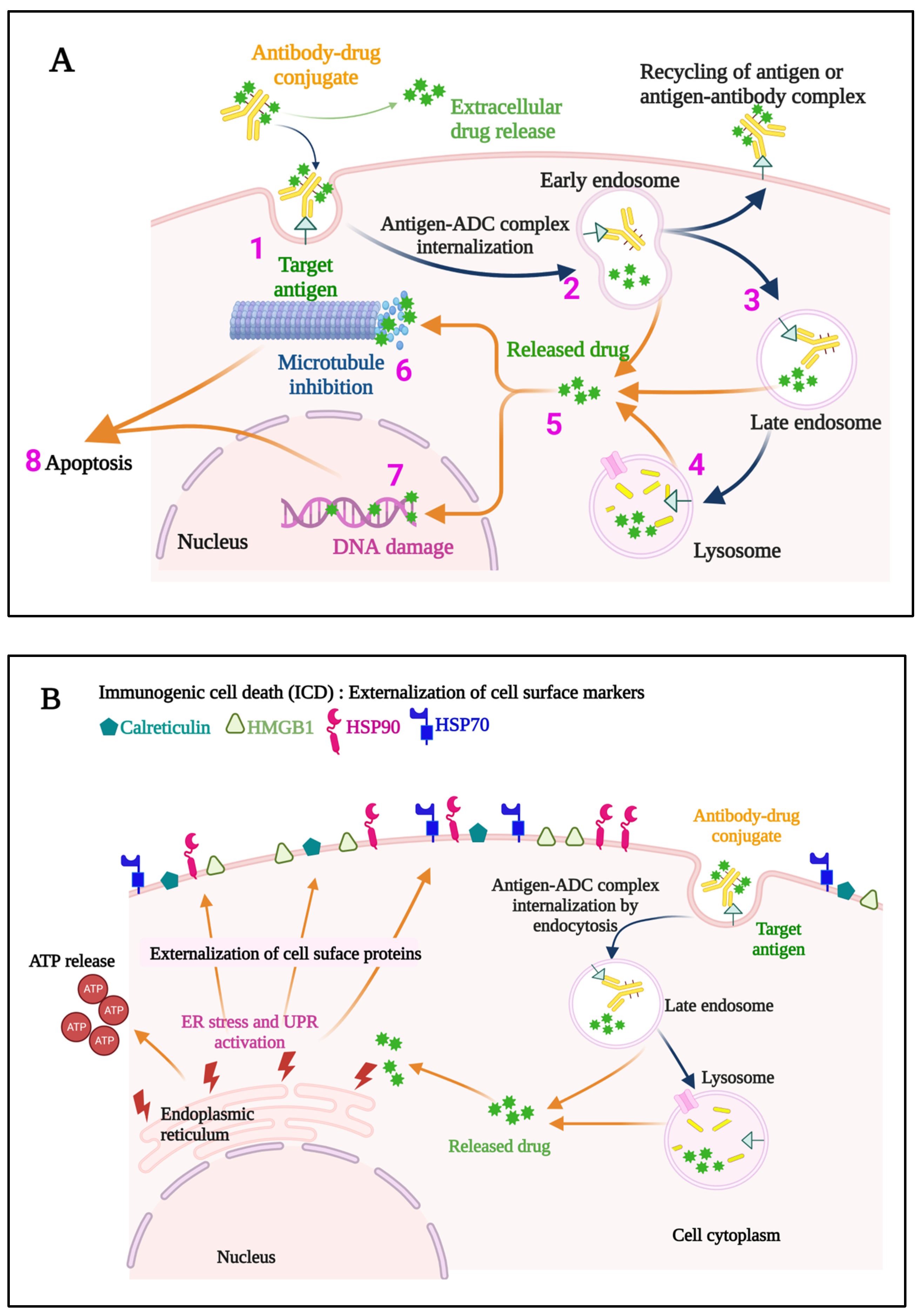
Once internalized, ADCs release their payloads and, in the case of anti-microtubule agents, for example, these can induce cell cycle arrest, resulting ultimately in the triggering of apoptosis (Figure 3A). Indeed, the story of the Greek Trojan Horse is often used as an analogy to describe the means by which ADCs enter cells and exert this portion of their mechanism of action. Additional anti-tumor mechanisms leveraged by ADCs include induction of immunogenic cell death (ICD) (Figure 3B), and Fc-mediated effector functions, including antibody-dependent cellular cytotoxicity (ADCC), phagocytosis (ADCP), and complement-dependent cytotoxicity (CDC). The modular design of ADCs (Figure 2) and the multiple options available for each component confer a high level of flexibility to this class of drugs. A full discussion of these options is beyond the scope of our work, and the interested reader is thus referred to several recent excellent reviews in these areas [14,15]. Briefly, however, these include using antibody isotypes or modifications to enhance or decrease/delete Fc-mediated effector functions, linkers that are cleavable or non-cleavable, and even acid-labile for release into the tumor microenvironment (TME), and a variety of drug classes targeting different mechanisms [14,15].
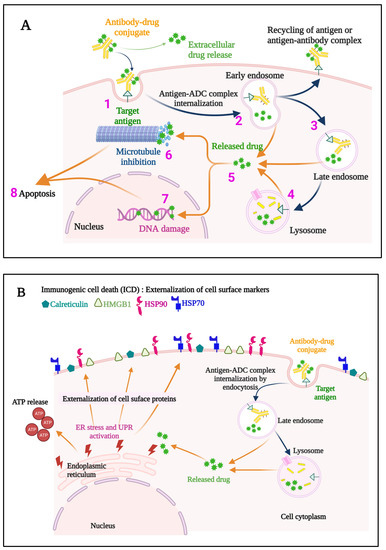
Figure 3.
A representation of the events occurring both at the cell membrane and intracellularly that contribute to the direct cytotoxic mechanisms of action of an ADC (A) is provided. The ADC binds to the specific target antigen on the cell surface (1), the ADC-antigen complex is internalized (2), and endosome/lysosome mediated processing (3,4) results in the release of the cytotoxic payload into the cytosol (5). If an anti-microtubule agent is the payload, aberrant spindle formation may occur causing cell cycle arrest and apoptosis (6), while DNA intercalators cause DNA damage (7), ultimately leading to apoptosis of the cell (8). Some ADCs also induce immunogenic cell death (ICD) (B), which elicits a host immune anti-tumor response. Hallmarks of ICD include externalization of Calreticulin, and Heat shock protein 90 (HSP90) and HSP70, externalization and later release of High mobility group box 1 (HMGB1), and release of ATP. ER, endoplasmic reticulum; UPR, unfolded protein response; ADC, antibody–drug conjugate.
2.2. Why ADCs Should Be Good Therapeutics against Myeloma
Other than the strong case for ADCs as anti-cancer therapies in general, the rationale in myeloma is based in part on the availability of lineage-specific markers that can be used to identify plasma cells (Figure 1). Techniques have been established and validated that allow for identification of neoplastic plasma cells, such as the so-called EuroFlow next generation flow cytometry [16]. When this is used for minimal residual disease testing, it involves multi-parameter flow to detect CD19, CD27, CD38, CD45, CD56, CD81, CD117, CD138, and cytoplasmic Igλ and Igκ. Notably, the ability to differentiate between normal and neoplastic plasma cells could theoretically enhance the therapeutic index of an ADC-based approach. In addition, the Fc gamma receptor 2B (FcɣRIIB) is expressed on plasma cells, and may provide another portal of ADC entry into myeloma cells [17], thereby potentially enhancing cytotoxicity and highlighting why ADCs could be especially effective against myeloma. However, internalization through FcɣRs has been described as a mechanism for off-target toxicity of ADCs, especially when these are aggregated, in cells that express these receptors [18]. Moreover, soluble FcɣRs may be increased in myeloma with the stage of the disease [19] and could theoretically protect myeloma cells by binding to ADCs and thwarting this route for internalization. Finally, disease progression in myeloma is often associated with clonal evolution and the development of genetically complex and heterogeneous subclones that follow different pathways through time and space [20,21,22,23,24], but these often retain stable surface proteomes [25] that can be targeted multiple times. The latter is in part borne out by the remarkably high and deep response rates achieved by BCMA-directed CAR T-cell therapies, providing a roadmap for the optimization of other cell surface-targeted therapies such as ADCs.
3. Clinical Progress in Leveraging ADCs against Myeloma
The first clinical trial of an ADC against myeloma, which is now of more historical interest, utilized the CD19-targeted anti-B4 antibody linked to blocked ricin and was based on the rationale that CD19 may be present on myeloma clonogenic cells [26,27]. Out of five patients treated, no responses were seen, and toxicities included transaminase elevations, myalgias, thrombocytopenia, nausea, vomiting, neurologic toxicity, decreased performance status, and capillary leak syndrome. Modern studies have targeted some of the now more widely accepted myeloma cell surface markers (Figure 1), and the relevant data are reviewed below for these antigens and the various agents either previously or currently in development.
3.1. Targeting BCMA
BCMA, also known as CD269 or Tumor necrosis factor receptor superfamily member 17, has been one of the most popular targets for ADCs, and for CAR T-cells and bispecific T-cell engagers. This is in part due to its high expression on neoplastic plasma cells and late mature B-cells [28], and the prominent role of B-cell activating factor and A proliferation-inducing ligand-mediated activation of BCMA in promoting myeloma cell survival and therefore BCMA-dependence in the TME [29].
3.1.1. AMG 224
This Amgen-developed ADC is an IgG1 anti-human BCMA ADC conjugated with mertansine (DM1), an anti-tubulin maytansinoid through a non-cleavable linker (Table 1), which therefore relies on lysosomal degradation of the antibody for drug release. A completed Phase I study targeted relapsed/refractory myeloma patients who had received a median of 7 prior lines of therapy (LOT). AMG 224 was administered every three weeks intravenously at doses ranging from 30 to 300 mg [30]. Common Grade 3 or higher adverse events (AEs) included thrombocytopenia and anemia, and ocular treatment-emergent adverse events (TEAEs) were seen. These included dry eyes (in 7%), and increased lacrimation, conjunctival hemorrhage, diplopia, eye irritation or pruritus, blurred vision, reduced visual acuity, visual impairment, and vitreous hemorrhage in 3% each. Other AEs of note included infusion-related reaction, fatigue, transaminitis, musculoskeletal pain, and myalgias. Among 40 patients who received at least one drug dose, the objective response rate was 23%, and while the median duration of response (DOR) in the dose escalation was 14.7 months, these data led Amgen to discontinue development of AMG 224. Notably, of patients with available serum samples for soluble BCMA (sBCMA) testing, responders tended to have lower sBCMA levels.

Table 1.
Antibody–Drug Conjugates in Clinical Trials in Multiple Myeloma.
3.1.2. Belantamab Mafodotin (Previously GSK2857916)
Belantamab mafodotin (BelaMaf), a humanized IgG1 anti-BCMA monoclonal antibody conjugated to the microtubule inhibitory drug monomethyl auristatin F (MMAF) (Table 1), was the first and, so far, only ADC to receive regulatory approval for myeloma from the Food and Drug Administration. In addition to inducing direct cytotoxic cell death after the release of MMAF, which inhibits microtubule polymerization and induces G2/M arrest and apoptosis, BelaMaf activated ADCC and ADCP [25,31], as well as ICD [32]. A Phase I trial targeted patients whose myeloma had progressed after prior stem cell transplantation, alkylators, PIs, and IMiDs, and administered this ADC as an intravenous infusion once every three weeks. This identified a dose of 3.40 mg/kg as the recommended Phase II dose (RP2D) based on safety, with Grade 3 and 4 toxicities including anemia and thrombocytopenia, and corneal toxicity being the other main adverse event [33,34], which was predominantly of Grade 1 or 2 severity. Preferred AE terms for the corneal toxicities seen in more than one patient included blurred vision, dry eyes, photophobia, and visual impairment. Detailed ophthalmologic examination described that these were associated with corneal microcyst-like epithelial changes following BelaMaf internalization leading to epithelial cell apoptosis [35]. These changes were reversible once the apoptotic cells were replaced with new corneal epithelial cells. In a second part of Phase I that included patients treated at the RP2D, 21/35 (60%) had a response, defined as an at least 50% reduction in biochemical parameters of disease burden, which translated into a median progression-free survival (PFS) of 12 months and a median DOR of 14.3 months. Baseline sBCMA levels were also compared with myeloma responses as measured by reduction in serum and/or urine monoclonal proteins and/or serum free light chain concentrations, but no clear relationship was reported.
These encouraging data prompted a Phase II, registration-enabling trial known as DREAMM-2, which enrolled almost 200 patients who were randomized to a lower dose of 2.5 mg/kg and the previously identified 3.4 mg/kg [36]. Notably, a more advanced patient population was enrolled in this trial since patients were required to have suffered disease progression after three or more LOT, and their myeloma had to be refractory to both IMiDs and PIs, and either refractory or intolerant (or both) to an anti-CD38 antibody. Indeed, the median number of prior LOT for the 2.5 mg/kg cohort was 7, and this was 6 for the 3.4 mg/kg cohort. Perhaps not unexpectedly, therefore, a lower ORR was seen, which was 31% and 34% in the two cohorts, while the median PFS was 2.9 and 4.9 months, respectively. The most common Grade 3–4 AEs were keratopathy in 21% and 27% of patients at the lower and higher doses, respectively, and anemia and thrombocytopenia were also described. Thrombocytopenia occurred at a median time to onset of 22 days in 35% of patients who received the 2.5 mg/kg dose, and in 58% of patients at the 3.4 mg/kg dose level. Anemia was also commonly observed and seen in 24% and 37% of patients at the 2.5 mg/kg and 3.4 mg/kg doses, respectively. Finally, Grade 1–2 infusion-related reactions were observed with the first infusion in 18% (2.5 mg/kg dosing) and 15% (3.4 mg/kg dosing) of patients, respectively. Due in part to the paucity of other available agents for so-called triple-class refractory (TCR) disease, defined as having progressed through prior PIs, IMiDs, and anti-CD38 monoclonals, BelaMaf was approved on 5 August 2020 [37].
Subsequent reports of so-called real-world experiences with BelaMaf have suggested similar to perhaps improved patient outcomes compared to DREAMM-2. For example, Vaxman et al. noted a response rate of 33% but a median PFS and overall survival (OS) of only 2 and 6.5 months, respectively, even though some patients received BelaMaf-based combinations [38]. However, this cohort was generally more heavily pre-treated, with a median of 8 prior LOT, and included patients who had received CAR T-cell-based treatment. In contrast, Atieh et al. [39] noted an ORR of 43% and a median PFS and OS of 4.9 and 10.7 months, respectively, in 35 TCR patients with a median of 5 prior LOT. Similarly, Shragai et al. [40] reported an ORR of 45.5% and a median PFS and OS of 4.7 and 14.5 months, respectively, in 106 patients with a median of 6 prior LOT.
Current research into BelaMaf has focused on two central aspects, the first being to reduce the incidence of ocular toxicities, which is an AE that is seen with other ADCs as well [41]. Consensus guidelines recommend eye examinations before and during each cycle, and also upon any worsening of symptoms, close collaboration between hematologist/oncologists and eye care professionals to grade toxicities, liberal use of preservative-free eye drops, and dose and/or schedule modifications [42]. Equally of note, a subgroup analysis of DREAMM-2 [43] suggested that 88% of patients (14/16) who had a clinical response and a prolonged treatment delay (>63 day) nonetheless maintained or even deepened their response. This suggested that lower and/or less frequent BelaMaf dosing may maintain clinical efficacy, and it has prompted studies of such approaches. However, a smaller, observational study suggested that BelaMaf dose reduction or discontinuation was associated with progression within 3 months [44], which may undermine this hypothesis.
A second area of focus for ongoing studies (Table 2) has been on combining BelaMaf with other anti-myeloma agents to enhance its efficacy and, potentially, to allow less frequent dosing and mitigate toxicities. For example, one single institution experience of BelaMaf with dexamethasone in 35 TCR patients [39] reported an ORR of 43%, and a median PFS and OS of 4.9 and 10.7 months, respectively. Formal clinical trials are underway with many partner agents, such as in the DREAMM-3 study [45], which included a cohort with the gamma-secretase inhibitor nirogacestat, which should enhance cell surface BCMA expression [46] and could act synergistically with BelaMaf. While final results have not appeared in the peer-reviewed literature, a preliminary report suggested the possibility of enhanced efficacy based on an increase in the number of patients with a stringent complete response (sCR) or CR with nirogacestat and BelaMaf [47]. Another interesting combination from DREAMM-3 could be BelaMaf with bortezomib and dexamethasone, which showed a 78% ORR in relapsed and/or refractory myeloma patients with a median of 3 prior LOT [48]. In addition, BelaMaf on four different doses and schedules is being combined with lenalidomide and dexamethasone as part of the DREAMM-6 trial, an interim report of which focused on 45 patients with a median of 3 prior LOT and the majority (58%) having had prior lenalidomide [49]. The ORR ranged from 42% when BelaMaf was given at 1.9 mg/kg every 8 weeks to 75% when this same dose was given on an every four-week schedule, though a robustly powered and randomized study would be needed to confirm the possibility that the latter was superior. BelaMaf’s role in activating ICD informed a study combining it with the checkpoint inhibitor pembrolizumab, and a recent report of the primary analysis focused on 34 patients with a median of 5 prior LOT [50]. This regimen demonstrated an ORR of 47%, with most (10/16) responders having an at least VGPR, while the DOR was 8.0 months and the median PFS was 3.4 months. Finally, BelaMaf with pomalidomide and dexamethasone (Pd) could be especially attractive as well, with an ORR of 86.0% in 50 response-evaluable triple-class exposed patients with a median of 3 prior LOT, of whom 72% were TCR, while the median PFS was 15.6 months [51].

Table 2.
Currently Active Clinical Trials of Antibody–Drug Conjugates in Multiple Myeloma.
Beyond the relapsed and/or refractory setting, BelaMaf is also being evaluated as part of induction therapy for newly diagnosed symptomatic myeloma. For example, one Phase I/II study is examining transplant-ineligible patients receiving BelaMaf at 1.4 to 2.5 mg/kg given every 8 weeks with lenalidomide and dexamethasone [52]. Among 36 patients in the analysis, a preliminary ORR of 97.2% is being reported, including 52.8% of patients achieving a very good partial remission (VGPR), defined as an at least 90% disease burden reduction, and no episodes of progressive disease were noted. A second study is evaluating transplant-eligible patients receiving BelaMaf at 2.5 mg/kg given every 8 weeks with bortezomib, lenalidomide and dexamethasone (VRd) using an every four-week VRd schedule [53]. Among 40 patients with a median follow-up of 6 months, the PFS has been 89.3% and the ORR was 82.1% after four cycles, with VGPR or better seen in 69.2% and all CR patients had achieved minimal residual disease (MRD) negativity. BelaMaf is also being studied in multiple other myeloma settings, including as a maintenance therapy and in smoldering disease (Table 2).
Finally, BelaMaf may be promising in patients with relapsed/refractory AL amyloidosis, a plasma cell dyscrasia that can be detected in the absence or presence of myeloma, based on two early reports. Zhang et al. reported on six patients with a median of 6 prior LOT who received standard of care BelaMaf [54]. Five (83%) achieved a hematologic response, including three with a CR, and cardiac responses were seen in all but one patient (80%) with baseline cardiac involvement. Encouraging also was a report from Khwaja et al. detailing outcomes in eleven AL patients with a median of 3 prior LOT and a median of two organs involved [55]. An ORR of 64% was noted, and 55% of patients experienced a VGPR or CR, while the PFS at a median follow-up of 7.1 months was 83%. These findings prompted an ongoing Phase II study of BelaMaf within the European Myeloma Network for this population, and a preliminary report is indicating a best hematologic response rate of 52.9% among seventeen patients with a median of 3 prior LOT [56]. Organ responses are being seen as well, with 29.4% of patients seeing any organ response at 3 months, and the median follow-up of 8.4 months suggests that this figure could improve further. One other study of BelaMaf in AL amyloidosis is currently planned, as is a study for plasmablastic lymphoma, which also is often BCMA-positive.
3.1.3. HDP-101
Amanitin is an agent that is being investigated as a warhead for ADCs by Heidelberg Pharma, and functions through the potent and specific inhibition of RNA polymerase II [57] (Table 1). In pre-clinical models, HDP-101 showed efficacy against both proliferating and resting myeloma cells [58], potentially providing a mechanism to attack quiescent myeloma stem-like or myeloma-initiating cells. Intriguingly, in vivo experiments documented cures in some animals after just a single dose of drug, reflecting potentially the strong potency of the amanitin-based warhead. Moreover, other studies suggested that it may show greater potency than BelaMaf [59], and preferential activity against high-risk deletion 17p models, in part due to co-deletion producing haploinsufficiency of RNA polymerase IIA [60]. Clinically, this agent is in a first-in-human (FIH) trial (Table 2) using an adaptive Bayesian logistic regression model with overdose control principle to guide dose escalation. A preliminary report on the first four patients treated with a median of 11 prior LOT indicated that the initial two cohorts were well tolerated without DLTs, and specifically with no keratopathy [61].
3.1.4. MEDI2228
MEDI2228 is a BCMA-targeted ADC that leverages pyrrolobenzodiazepine dimers in its warhead (Table 1), which are released after proteolytic linker cleavage in lysosomes and produce highly cytotoxic DNA crosslinks [62]. Consistent with this mechanism of action, pre-clinical studies of MEDI2228 have shown potent activation of DNA damage responses (DDR) in myeloma models, and synergy with DDR inhibitors targeting ATM/ATR/WEE1 checkpoint kinases [63] as well as bortezomib. Interestingly, MEDI2228 also has shown synergy with daratumumab through interferon-driven immune responses and enhanced CD38 expression [64]. In a Phase I dose-escalation study, MEDI2228 was administered intravenously every 3 weeks to 82 relapsed/refractory myeloma patients who had received 2–11 prior LOT, and 0.14 mg/kg was identified as the maximum tolerated dose (MTD) [65]. Notable TEAEs at this level included photophobia (53.7%), thrombocytopenia (31.7%), rash (29.3%), increased gamma-glutamyltransferase (24.4%), dry eyes (19.5%), and pleural effusion (19.5%). The ORR in this cohort was 61.0% and included 10 patients (24.4%) who achieved a VGPR, and a median DOR was not reached. Interestingly, BCMA expression was examined by immunohistochemistry, and there was no difference in the bone marrows of responders compared with non-responders. Despite these interesting findings, a press release indicated that AstraZeneca decided to halt further development of this ADC.
3.2. Targeting CD38
CD38, or Cluster of differentiation 38, is a type II membrane glycoprotein highly expressed on plasma cells that encodes a bifunctional enzyme having both ADP-ribosyl cyclase and cyclic ADP-ribose hydrolase activities [66]. As CD38 is not expressed on pluripotent hematopoietic progenitor cells and normal resting lymphocytes, this therefore provides potential for a therapeutic index.
3.2.1. STI-6129
STI-6129, also known as LNDS1001, is a fully human anti-CD38 antibody conjugated to Duostatin 5.2, an MMAF-derived microtubule inhibitor [67] (Table 1). Pre-clinical studies showed that it induced CD38-dependent cytotoxic activity in vitro without homotypic aggregation of tumor cells, as is seen with daratumumab, and broad efficacy in multiple xenograft models. Several clinical trials of STI-6129 are recruiting patients, including one for relapsed/refractory myeloma and another for AL amyloidosis (Table 2).
3.2.2. MT-0169
MT-0169, previously known as TAK-169, is a second generation engineered toxin body composed of a single-chain variable fragment with an affinity for CD38 that is fused to the enzymatically active de-immunized Shiga-like toxin-A subunit (Table 1). In pre-clinical studies, this agent induced irreversible ribosome inactivation and potent cytotoxicity against CD38-positive human myeloma models [68]. Among tested primary samples, TAK-169 was equally effective against plasma cells from patients with newly diagnosed and relapsed/refractory myeloma, though a fraction of cells from daratumumab-refractory patients were less sensitive [69]. A very preliminary report of the Phase I study in four patients with relapsed/refractory myeloma and at least 5 prior LOT noted one TEAE of asymptomatic Grade 2 reversible myocarditis and Grade 3 troponin elevation [70], raising concern about its further development.
3.2.3. TAK-573
While not a classical ADC in that it does not have a directly cytotoxic warhead, TAK-573, also known as Modakafusp alfa, is a first-in-class, humanized, anti-CD38, IgG4 antibody genetically fused to two attenuated interferon alpha-2b molecules. This agent has shown direct anti-proliferative activity against myeloma cell lines pre-clinically, and also combined well with standard of care agents in vivo, potentially in part through modulation of immune responses [71]. In an ongoing Phase I/II study, TAK-573 is being administered as a 1–4-h intravenous infusion at 10 different dose levels and on 4 different schedules, and a dose expansion was opened at 1.5 mg/kg every 4 weeks when anti-myeloma activity was seen [72]. Among 30 patients on this dose and schedule, frequent Grade 3–4 TEAEs included neutropenia (63%), thrombocytopenia (43%), leukopenia (40%), anemia (30%), and lymphopenia (30%), while other notable Grade 3/4 events included 1 bleeding event and 3 infections. An ORR of 40% has been reported despite the fact that this cohort had a median of 7 prior LOT, and 37% of patients who were previously anti-CD38 refractory responded, while the median PFS was 6 months, and the median DOR has not been reached. This prompted initiation of a broader development effort in myeloma, with several studies either planned or underway.
3.3. Targeting CD46
CD46, a type I membrane protein that is a negative regulator of the complement system, was felt to be an especially attractive target for 1q amplified patients since this gene is located at cytogenetic band 1q32.2 [73], whose amplification is associated with an adverse prognosis [74]. Notably, CD46-targeted ADCs did indeed show anti-proliferative activity pre-clinically against myeloma cell lines and primary samples, and in an orthometastatic xenograft model [73]. In part with this rationale, Fortis Therapeutics advanced FOR46, which was an MMAF-based ADC, into the clinic for relapsed or refractory myeloma. Unfortunately, while 31 participants were enrolled into the Phase I study, no safety or efficacy data have been reported, and the study is now listed as having been terminated on clinicaltrials.gov.
3.4. Targeting CD48
CD48, or Signaling lymphocyte activation molecule family member 2 (SLAMF2), is a glycosylphosphatidylinositol-anchored membrane protein expressed on myeloma cells that participates in activation and differentiation pathways. SGN-CD48A, an ADC linked to MMAE, was examined in fourteen myeloma patients in a Phase I trial, but no safety or efficacy data have been reported, and the study has been terminated according to clinicaltrials.gov.
3.5. Targeting CD54
CD54, also known as Intracellular adhesion molecule 1 (ICAM1), or BB2, is a transmembrane glycoprotein of the immunoglobulin superfamily that is aberrantly overexpressed in several cancers, including myeloma [75]. A CD54 ADC has been developed by Sherbenou et al., who conjugated the anti-ICAM1 M10A12 IgG1 to MMAF and showed it was effective in vitro and in vivo [76]. However, clinical translation of CD54-targeted ADCs may be challenging considering the prior experience with unconjugated CD54 antibodies such as BI-505. This agent initially showed good safety in relapsed/refractory myeloma patients from a Phase I study that did not identify an MTD [77], but a second study in smoldering myeloma showed no activity [78], and its development was later stopped due to an adverse cardiovascular event.
3.6. Targeting CD56
CD56, also known as Neural cell adhesion molecule 1, is aberrantly expressed on transformed plasma cells in up to 80% or more of patients and used in the assessment of MRD negativity by multi-parameter flow [79], suggesting it is a valid therapeutic target. Lorvotuzumab mertansine (IMGN901) is an ADC based on the anti-CD56 antibody N901 to which the maytansinoid DM1 was added with a disulfide linker (Table 1). Pre-clinical studies demonstrated that IMGN901-depleted CD56-positive myeloma cells from mixed cultures reduced serum paraprotein secretion, inhibited tumor growth, and increased survival in a murine model [80]. While a number of single-agent and combination clinical trials were completed, final data have been published only from the Phase I single-agent study, which enrolled 37 relapsed patients into the dose-escalation portion [81]. The toxicity profile was felt to be favorable, with the only Grade 3 or 4 AE reported in more than one patient being fatigue. Unfortunately, among the 35 response-evaluable patients, a PR was noted in two (5.7%), while an additional four (11.4%) had minor responses, defined as between a 25% and 50% reduction in disease burden, and further development has therefore halted.
3.7. Targeting CD74
CD74 is a non-polymorphic type II transmembrane glycoprotein that functions as a major histocompatibility complex (MHC) class II chaperone, and participates in other non-MHC protein trafficking, such as of angiotensin II type I receptor [82]. Moreover, it serves as the high-affinity receptor for macrophage migration inhibitory factor, which may play a role in myeloma pathobiology [83]. Since it is highly expressed on B-cells and plasma cells, CD74 has been seen as a valid myeloma target [84].
3.7.1. Milatuzumab Doxorubicin
Milatuzumab doxorubicin, also known as hLL1-DOX and IMMU-110, was developed as an ADC for myeloma in part because it showed efficacy in pre-clinical models [85], and since a prior Phase I trial of unconjugated milatuzumab did not show efficacy [86]. A Phase I study of the ADC was performed, and 17 patients were enrolled, but neither safety nor efficacy results have been reported, and its clinicaltrials.gov record indicates that this trial was terminated.
3.7.2. STRO-001
STRO-001 from Sutro Biopharma is a fully human aglycosylated anti-CD74 antibody with a site-specific non-cleavable linker-maytansinoid warhead generated through a novel cell-free protein synthesis technology (Table 1). Pre-clinical studies confirmed its potent efficacy against myeloma cell lines and primary cells, and in in vitro and in vivo models [87]. A Phase I study targeting patients with non-Hodgkin lymphoma or myeloma is underway, and preliminary results of the lymphoma cohort have been presented [88]. Treatment emergent adverse events (TEAEs) occurring in ≥20% of patients included chills, fatigue, nausea, anemia, headache, pyrexia, infusion reaction, decreased appetite, and abdominal pain. One dose limiting toxicity (DLT) included a Grade 3 thromboembolic event, while no ocular toxicities were noted, and the clinical benefit/disease control rate was 25% (4/16). Notably, Sutro Biopharma is also producing CC-99712, a BCMA-targeted ADC conjugated through a non-cleavable linker to a maytansinoid with a drug:antibody ratio (DAR) of 4, which is in Phase I testing through Celgene and Bristol-Myers Squibb.
3.8. Targeting CD319
SLAMF7, also known as CD319 and previously as CD2 subset 1, is a validated myeloma target bound by elotuzumab. This agent is approved in combination with both lenalidomide and dexamethasone [89], as well as Pd [90], in the relapsed and/or refractory setting, providing a rationale for ADCs to target CD319. ABBV-838 has just such an ADC, made from a humanized recombinant IgG1κ antibody to SLAMF7 attached through a valine-citrulline linker to monomethyl auristatin E (MMAE, Table 1). The FIH Phase I trial included 75 patients who received at least one dose, with the most common TEAEs being neutropenia and anemia, fatigue, and nausea, while 16% of patients developed corneal deposits [91]. An ORR of 10.7% was reported, including VGPR in 2 (2.7%) and PR in 6 (8.0%) of patients, which did not support its further development.
3.9. Targeting CD138
CD138, also known as Syndecan-1, is a type I transmembrane proteoglycan cell adhesion molecule overexpressed on transformed plasma cells that promotes angiogenesis, tumor growth and metastases, and adhesion to the TME [92,93], making it a viable target.
Indatuximab Ravtansine
nBT06, a murine/human chimeric CD138-specific antibody conjugated with highly cytotoxic maytansinoid (DM4) derivatives (Table 1), initially showed anti-myeloma efficacy in pre-clinical models [94]. Prominent features of its mechanism of action included induction of G2/M cell cycle arrest, followed by apoptosis with cleavage of caspases, and blockade of adhesion to bone marrow stromal cells. On translation to the clinic for relapsed and/or refractory myeloma patients previously treated with an IMiD and a PI, BT062 was administered on two different schedules that showed MTDs ranging from 140 to 160 mg/m2 [95]. Most AEs were Grade 1 or 2, with the most common being diarrhea and fatigue, and over 75% of pretreated patients achieved stable disease or better on a once every 3-week schedule, while minor and partial responses occurred in 14.7% with a median PFS of 3 months and OS of 26.7 months on the more intensive, weekly schedule. More recently, combination study results were reported in which indatuximab ravtansine was given with lenalidomide/dexamethasone (IrRd) or pomalidomide/dexamethasone (IrPd) [96]. Common Grade 3–4 AEs for both were neutropenia, anemia, and thrombocytopenia, while TEAEs leading to discontinuation occurred in 35/64 (55%), including five patients (8%) with a fatal TEAE, though these were not attributed to the drug. Objective responses for IrRd were noted in 33/46 patients (71.7%), and in 12/17 (70.6%) who received IrPd.
3.10. Targeting CD307
Fc-receptor-like 5 (FCRL5), also known as FCRH5 and CD307, is a member of the immunoglobulin receptor superfamily and the Fc-receptor-like family that may be involved in B-cell development and differentiation and is expressed on malignant plasma cells (Table 1). DFRF4539A, an anti-FcRH5 ADC linked to MMAE, was evaluated in a Phase I study in relapsed/refractory myeloma, and while Grade 3 anemia was the most common TEAE, there were two episodes of Grade 3 renal failure [97]. In part as a result of these, the MTD was not reached, and DFRF4539A demonstrated limited activity with only 2 PRs (5%) as the best responses.
4. Future Directions for ADCs in Myeloma
Despite an abundance of targets available on the myeloma cell surface, the history of ADC-based drug development in multiple myeloma has not been encouraging so far, with many failures and, indeed, only one drug, BelaMaf, reaching a regulatory approval. Of further concern is the competition provided by two commercially available CAR T-cells targeting BCMA, including idecabtagene vicleucel [98] and ciltacabtagene autoleucel [99], and the BCMA bispecific teclistamab [100]. All three of these have substantially higher overall response rates and response durations, and while randomized trials are formally needed to make such comparisons, the results of such efforts would not seem to be in doubt. This could be further borne out by a comparison of cevostamab, a CD305-targeted bispecific which is showing robust efficacy and durability, to the history of the CD305-targeted ADC, which showed marginal efficacy. Finally, even BelaMaf is now in question since GlaxoSmithKline initiated the process for withdrawal of its US marketing approval based upon the outcome of the DREAMM-3 Phase III trial. In this open-label randomized head-to-head superiority study, 325 patients were randomized in a 2:1 ratio to receive BelaMaf at 2.5 mg/kg every 3 weeks or Pd. The observed median PFS was longer for BelaMaf compared to Pd at 11.2 versus 7 months, respectively, and the VGPR or better rate was better with BelaMaf than Pd, at 25% versus 8%, respectively. However, this PFS represented a hazard ratio of only 1.03 (95% confidence interval of 0.72–1.47), indicating a negative trial. Do these and other arguments indicate that this is the beginning of the end for the utility of ADCs in myeloma?
Fortunately, all is not lost for ADCs for myeloma in general, and this is true for BelaMaf as well. With regard to the latter, considering the excellent ORR of 86.0% with BelaMaf in combination with Pd and the median PFS of 15.6 months noted earlier [51], it seems likely that a Phase III trial comparing Pd to BelaMaf with Pd would be positive. In addition, the ongoing DREAMM-8 Phase III study comparing BelaMaf/Pd to bortezomib/Pd has an estimated completion date of 31 March 2023, and since the latter regimen previously conferred a PFS of 11.2 months [101], it is possible that positive data from this trial could lead to full approval of BelaMaf.
Moreover, looking beyond lineage-specific targets expressed on plasma cells of all myeloma patients to those that may be expressed in molecularly defined subsets could be a productive line of research. For example, Fibroblast growth factor receptor 3 (FGRF3) is frequently overexpressed and sometimes activated in patients with the t(4;14) translocation [102]. However, the Phase I study of the FGFR3-targeted ADC LY3076226, which showed an acceptable safety profile, did not enroll any myeloma patients [103]. Thus, it could be of interest to revisit this agent which, if active, would be an especially exciting development considering that t(4;14) myeloma is considered a high-risk subtype with an inferior prognosis [104].
Beyond better clinical trial design, reducing the toxicities of ADCs could allow patients to receive more consistent therapy with a better quality of life and potentially improved long-term outcomes. Ocular toxicities are clearly a challenge for several ADCs and, interestingly, pre-clinical studies have suggested that the mechanism responsible may be their uptake into corneal epithelial cells through macropinocytosis, resulting in inhibition of cellular proliferation [105]. Importantly, since uptake was facilitated by positive charges or hydrophobic residues on the surface of macromolecules, the authors found that decreasing ADC hydrophobicity by attaching polyethylene glycol moieties reduced cytotoxicity, suggesting possible future approaches. Hematologic toxicities in general, and thrombocytopenia in particular, can similarly be a challenge in some patients. In this regard, some studies have described a role for FcɣR-mediated uptake of microtubule-targeted ADCs into megakaryocytes, producing a disrupted cytoskeletal structure [106] and inhibition of megakaryocyte maturation [107] as possible contributors. Thus, ADC re-engineering to avoid this impact could be of benefit in some cases, such as by using IgG4 isotypes, or IgG1 isotypes with decreased/deleted Fc-mediated effector functions as backbones that would not engage FcɣRs. However, this risks a loss of ADCC, ADCP, and CDC mechanisms that IgG1 antibodies can engage, and should be considered only with caution if these mechanisms substantially add to the anti-tumor activity of a specific ADC.
Improved antibody design will likely also play a critical role in improving the standing of ADCs in myeloma moving forward. For example, optimizing the binding affinity of an antibody to a myeloma cell surface target may enhance anti-tumor efficacy [108]. Moreover, optimization of the drug conjugate for the biology of myeloma may be needed as many ADCs have so far employed anti-microtubule agents, and yet myeloma is not classically considered to be a highly proliferative malignancy [109,110]. Thus, agents that do not rely on brisk cell cycle progression, such as amanitins, or that are more potent, such as pyrrolobenzodiazepine dimers, may prove to be superior. Finally, studies aimed at obtaining a better understanding of the determinants of ADC internalization into myeloma cells specifically, as well as factors that determine ADC sensitivity and resistance [111], will be critical. Some patients have tolerated ADCs well and derived substantial long-term benefits, and our ability to ideally identify patients who are most appropriate for CAR T-cell-based therapies is only beginning to emerge [112]. Moreover, initial data in the post-BelaMaf space suggest that patients previously treated with this ADC who then progress suffer from neither a significant loss of BCMA expression nor from significant immune impairment [113], while BCMA loss has been described in at least some patients who have received CAR T-cell-based therapies [114,115]. This suggests the possibility that BelaMaf or other such ADCs could be rationally used earlier in the disease course without compromising the later use of bispecifics or CAR T-cells. Ultimately, if we can identify those patients who are less likely to benefit from CAR T-cells and bispecifics and are likely to show sensitivity with good tolerability to optimized ADCs, then this class of drugs will truly make a significant contribution to our therapeutic armamentarium against myeloma. Therefore, we could indeed be just at the beginning of understanding how best to leverage the exciting power of ADCs against myeloma.
Author Contributions
Conceptualization, U.R. and R.Z.O.; writing—original draft preparation, U.R.; writing—review and editing, R.Z.O.; funding acquisition, R.Z.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Cancer Institute, grant number R01 CA266612.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
RZO: the Florence Maude Thomas Cancer Research Professor, would like to acknowledge support from the Leukemia & Lymphoma Society (SCOR-12206-17), the National Cancer Institute (R01 CA266612), the Miriam and Sheldon G. Adelson Medical Research Foundation, and the Riney Family Multiple Myeloma Research Fund at MD Anderson from the Paula and Rodger Riney Foundation. Additional support came from the Jake and Nina Kamin Endowment for Multiple Myeloma Research, and the James B. and Marie R. Baker, and David and Sara Anne Baker Hopkins Research Endowment. Supported by the generous philanthropic contributions to The University of Texas MD Anderson Cancer Center Moon Shots Program™. The figures were created with BioRender.com, and two of them were adapted from the BioRender templates with modification.
Conflicts of Interest
UR has no conflict of interest. RZO declares: laboratory research funding from Asylia Therapeutics, Inc. (Houston, TX, USA), BioTheryX (Chappaqua, NY, USA), and Heidelberg Pharma (Munich, Germany); clinical research funding from CARsgen Therapeutics (Shanghai, China), Celgene (Summit, NJ), Exelixis (Alameda, CA), Heidelberg Pharma (Ladenburg, Germany), Janssen Biotech (Horsham Township, PA), Sanofi-Aventis (Paris, France), and Takeda Pharmaceuticals North America Inc.( Lexington, MA); service on scientific advisory boards for Amgen, Inc., Bristol-Myers Squibb, Celgene, GSK Biologicals, Janssen Biotech, Karyopharm Therapeutics, Neoleukin Corporation, Oncopeptides AB, Regeneron Pharmaceuticals, Sanofi-Aventis, Servier, and Takeda; and a role as Founder with equity interest in Asylia Therapeutics, Inc.
Abbreviations
ADCC, antibody-dependent cellular cytotoxicity; ADCs, antibody–drug conjugates; ADCP, antibody-dependent cellular phagocytosis; AEs, adverse events; AL, light chain amyloidosis; BCMA, B-cell maturation antigen; BelaMaf, Belantamab mafodotin; CARs, chimeric antigen receptors; CDC, complement-dependent cytotoxicity; CR, complete response; DAR, drug:antibody ratio; DDR, DNA damage response; DM1, mertansine; DOR, duration of response; FCRL5, Fc-receptor-like 5; FcγR, Fcgamma receptor; FGFR3, Fibroblast growth factor receptor 3; FIH, first-in-human; ICAM, Intracellular adhesion molecule; ICD, immunogenic cell death; Igs, immunoglobulins; IMiD, immunomodulatory drug; IrRd, indatuximab ravtansine with lenalidomide and dexamethasone; IrPd, indatuximab ravtansine with pomalidomide and dexamethasone; LOT, lines of therapy; MHC, major histocompatibility complex; MMAE, monomethyl auristatin E; MMAF, monomethyl auristatin F; MRD, minimal residual disease; MTD, maximum tolerated dose; ORR, overall response rate; OS, overall survival; Pd, pomalidomide and dexamethasone; PFS, progression-free survival; PIs, proteasome inhibitors; RP2D, recommended Phase II dose; sBCMA, soluble BCMA; sCR, stringent complete response; SLAMF, Signaling lymphocyte activation molecule family member; TCR, triple-class refractory; TEAEs, treatment-emergent adverse events; TME, tumor microenvironment; VGPR, very good partial remission.
References
- Kumar, S.K.; Rajkumar, V.; Kyle, R.A.; van Duin, M.; Sonneveld, P.; Mateos, M.V.; Gay, F.; Anderson, K.C. Multiple myeloma. Nat. Rev. Dis. Prim. 2017, 3, 17046. [Google Scholar] [CrossRef]
- Kohler, G.; Milstein, C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 1975, 256, 495–497. [Google Scholar] [CrossRef]
- Merlini, G.; Dispenzieri, A.; Sanchorawala, V.; Schonland, S.O.; Palladini, G.; Hawkins, P.N.; Gertz, M.A. Systemic immunoglobulin light chain amyloidosis. Nat. Rev. Dis. Prim. 2018, 4, 38. [Google Scholar] [CrossRef]
- Corre, J.; Perrot, A.; Hulin, C.; Caillot, D.; Stoppa, A.M.; Facon, T.; Leleu, X.; Dib, M.; Karlin, L.; Moreau, P.; et al. Improved survival in multiple myeloma during the 2005–2009 and 2010–2014 periods. Leukemia 2021, 35, 3600–3603. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Shimazu, Y.; Mizuno, S.; Fuchida, S.I.; Suzuki, K.; Tsukada, N.; Hanagaishi, A.; Itagaki, M.; Kataoka, K.; Kako, S.; Sakaida, E.; et al. Improved survival of multiple myeloma patients treated with autologous transplantation in the modern era of new medicine. Cancer Sci. 2021, 112, 5034–5045. [Google Scholar] [CrossRef]
- Tang, C.H.; Hou, H.A.; Huang, K.C.; Qiu, H.; Liu, Y. Treatment evolution and improved survival in multiple myeloma in Taiwan. Ann. Hematol. 2020, 99, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Thorsteinsdottir, S.; Dickman, P.W.; Landgren, O.; Blimark, C.; Hultcrantz, M.; Turesson, I.; Bjorkholm, M.; Kristinsson, S.Y. Dramatically improved survival in multiple myeloma patients in the recent decade: Results from a Swedish population-based study. Haematologica 2018, 103, e412–e415. [Google Scholar] [CrossRef]
- Kunacheewa, C.; Orlowski, R.Z. New Drugs in Multiple Myeloma. Annu. Rev. Med. 2019, 70, 521–547. [Google Scholar] [CrossRef]
- Branagan, A.; Lei, M.; Lou, U.; Raje, N. Current Treatment Strategies for Multiple Myeloma. JCO Oncol. Pract. 2020, 16, 5–14. [Google Scholar] [CrossRef]
- Rajkumar, S.V. Sequencing of myeloma therapy: Finding the right path among many standards. Hematol. Oncol. 2021, 39 (Suppl. S1), 68–72. [Google Scholar] [CrossRef] [PubMed]
- Ravi, P.; Kumar, S.K.; Cerhan, J.R.; Maurer, M.J.; Dingli, D.; Ansell, S.M.; Rajkumar, S.V. Defining cure in multiple myeloma: A comparative study of outcomes of young individuals with myeloma and curable hematologic malignancies. Blood Cancer J. 2018, 8, 26. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Steeg, P.S. Endocytosis: A pivotal pathway for regulating metastasis. Br. J. Cancer 2021, 124, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Khongorzul, P.; Ling, C.J.; Khan, F.U.; Ihsan, A.U.; Zhang, J. Antibody-Drug Conjugates: A Comprehensive Review. Mol. Cancer Res. 2020, 18, 3–19. [Google Scholar] [CrossRef]
- Joubert, N.; Beck, A.; Dumontet, C.; Denevault-Sabourin, C. Antibody-Drug Conjugates: The Last Decade. Pharmaceuticals 2020, 13, 245. [Google Scholar] [CrossRef]
- Flores-Montero, J.; Sanoja-Flores, L.; Paiva, B.; Puig, N.; Garcia-Sanchez, O.; Bottcher, S.; van der Velden, V.H.J.; Perez-Moran, J.J.; Vidriales, M.B.; Garcia-Sanz, R.; et al. Next Generation Flow for highly sensitive and standardized detection of minimal residual disease in multiple myeloma. Leukemia 2017, 31, 2094–2103. [Google Scholar] [CrossRef]
- Anania, J.C.; Chenoweth, A.M.; Wines, B.D.; Hogarth, P.M. The Human FcgammaRII (CD32) Family of Leukocyte FcR in Health and Disease. Front. Immunol. 2019, 10, 464. [Google Scholar] [CrossRef]
- Aoyama, M.; Tada, M.; Yokoo, H.; Demizu, Y.; Ishii-Watabe, A. Fcgamma Receptor-Dependent Internalization and Off-Target Cytotoxicity of Antibody-Drug Conjugate Aggregates. Pharm. Res. 2022, 39, 89–103. [Google Scholar] [CrossRef]
- Fridman, W.H.; Teillaud, J.L.; Bouchard, C.; Teillaud, C.; Astier, A.; Tartour, E.; Galon, J.; Mathiot, C.; Sautes, C. Soluble Fc gamma receptors. J. Leukoc. Biol. 1993, 54, 504–512. [Google Scholar] [CrossRef]
- Bolli, N.; Avet-Loiseau, H.; Wedge, D.C.; Van Loo, P.; Alexandrov, L.B.; Martincorena, I.; Dawson, K.J.; Iorio, F.; Nik-Zainal, S.; Bignell, G.R.; et al. Heterogeneity of genomic evolution and mutational profiles in multiple myeloma. Nat. Commun. 2014, 5, 2997. [Google Scholar] [CrossRef]
- Melchor, L.; Brioli, A.; Wardell, C.P.; Murison, A.; Potter, N.E.; Kaiser, M.F.; Fryer, R.A.; Johnson, D.C.; Begum, D.B.; Hulkki Wilson, S.; et al. Single-cell genetic analysis reveals the composition of initiating clones and phylogenetic patterns of branching and parallel evolution in myeloma. Leukemia 2014, 28, 1705–1715. [Google Scholar] [CrossRef] [PubMed]
- Weinhold, N.; Ashby, C.; Rasche, L.; Chavan, S.S.; Stein, C.; Stephens, O.W.; Tytarenko, R.; Bauer, M.A.; Meissner, T.; Deshpande, S.; et al. Clonal selection and double-hit events involving tumor suppressor genes underlie relapse in myeloma. Blood 2016, 128, 1735–1744. [Google Scholar] [CrossRef] [PubMed]
- Maura, F.; Bolli, N.; Angelopoulos, N.; Dawson, K.J.; Leongamornlert, D.; Martincorena, I.; Mitchell, T.J.; Fullam, A.; Gonzalez, S.; Szalat, R.; et al. Genomic landscape and chronological reconstruction of driver events in multiple myeloma. Nat. Commun. 2019, 10, 3835. [Google Scholar] [CrossRef]
- Rasche, L.; Chavan, S.S.; Stephens, O.W.; Patel, P.H.; Tytarenko, R.; Ashby, C.; Bauer, M.; Stein, C.; Deshpande, S.; Wardell, C.; et al. Spatial genomic heterogeneity in multiple myeloma revealed by multi-region sequencing. Nat. Commun. 2017, 8, 268. [Google Scholar] [CrossRef]
- Lee, L.; Bounds, D.; Paterson, J.; Herledan, G.; Sully, K.; Seestaller-Wehr, L.M.; Fieles, W.E.; Tunstead, J.; McCahon, L.; Germaschewski, F.M.; et al. Evaluation of B cell maturation antigen as a target for antibody drug conjugate mediated cytotoxicity in multiple myeloma. Br. J. Haematol. 2016, 174, 911–922. [Google Scholar] [CrossRef]
- O’Toole, J.E.; Esseltine, D.; Lynch, T.J.; Lambert, J.M.; Grossbard, M.L. Clinical trials with blocked ricin immunotoxins. Curr. Top. Microbiol. Immunol. 1998, 234, 35–56. [Google Scholar] [CrossRef]
- Grossbard, M.L.; Fidias, P.; Kinsella, J.; O’Toole, J.; Lambert, J.M.; Blattler, W.A.; Esseltine, D.; Braman, G.; Nadler, L.M.; Anderson, K.C. Anti-B4-blocked ricin: A phase II trial of 7 day continuous infusion in patients with multiple myeloma. Br. J. Haematol. 1998, 102, 509–515. [Google Scholar] [CrossRef]
- Yu, B.; Jiang, T.; Liu, D. BCMA-targeted immunotherapy for multiple myeloma. J. Hematol. Oncol. 2020, 13, 125. [Google Scholar] [CrossRef]
- Tai, Y.T.; Acharya, C.; An, G.; Moschetta, M.; Zhong, M.Y.; Feng, X.; Cea, M.; Cagnetta, A.; Wen, K.; van Eenennaam, H.; et al. APRIL and BCMA promote human multiple myeloma growth and immunosuppression in the bone marrow microenvironment. Blood 2016, 127, 3225–3236. [Google Scholar] [CrossRef]
- Lee, H.C.; Raje, N.S.; Landgren, O.; Upreti, V.V.; Wang, J.; Avilion, A.A.; Hu, X.; Rasmussen, E.; Ngarmchamnanrith, G.; Fujii, H.; et al. Phase 1 study of the anti-BCMA antibody-drug conjugate AMG 224 in patients with relapsed/refractory multiple myeloma. Leukemia 2021, 35, 255–258. [Google Scholar] [CrossRef]
- Tai, Y.T.; Mayes, P.A.; Acharya, C.; Zhong, M.Y.; Cea, M.; Cagnetta, A.; Craigen, J.; Yates, J.; Gliddon, L.; Fieles, W.; et al. Novel anti-B-cell maturation antigen antibody-drug conjugate (GSK2857916) selectively induces killing of multiple myeloma. Blood 2014, 123, 3128–3138. [Google Scholar] [CrossRef] [PubMed]
- Montes de Oca, R.; Alavi, A.S.; Vitali, N.; Bhattacharya, S.; Blackwell, C.; Patel, K.; Seestaller-Wehr, L.; Kaczynski, H.; Shi, H.; Dobrzynski, E.; et al. Belantamab Mafodotin (GSK2857916) Drives Immunogenic Cell Death and Immune-mediated Antitumor Responses In Vivo. Mol. Cancer Ther. 2021, 20, 1941–1955. [Google Scholar] [CrossRef] [PubMed]
- Trudel, S.; Lendvai, N.; Popat, R.; Voorhees, P.M.; Reeves, B.; Libby, E.N.; Richardson, P.G.; Anderson, L.D., Jr.; Sutherland, H.J.; Yong, K.; et al. Targeting B-cell maturation antigen with GSK2857916 antibody-drug conjugate in relapsed or refractory multiple myeloma (BMA117159): A dose escalation and expansion phase 1 trial. Lancet Oncol. 2018, 19, 1641–1653. [Google Scholar] [CrossRef] [PubMed]
- Trudel, S.; Lendvai, N.; Popat, R.; Voorhees, P.M.; Reeves, B.; Libby, E.N.; Richardson, P.G.; Hoos, A.; Gupta, I.; Bragulat, V.; et al. Antibody-drug conjugate, GSK2857916, in relapsed/refractory multiple myeloma: An update on safety and efficacy from dose expansion phase I study. Blood Cancer J. 2019, 9, 37. [Google Scholar] [CrossRef]
- Farooq, A.V.; Degli Esposti, S.; Popat, R.; Thulasi, P.; Lonial, S.; Nooka, A.K.; Jakubowiak, A.; Sborov, D.; Zaugg, B.E.; Badros, A.Z.; et al. Corneal Epithelial Findings in Patients with Multiple Myeloma Treated with Antibody-Drug Conjugate Belantamab Mafodotin in the Pivotal, Randomized, DREAMM-2 Study. Ophthalmol. Ther. 2020, 9, 889–911. [Google Scholar] [CrossRef]
- Lonial, S.; Lee, H.C.; Badros, A.; Trudel, S.; Nooka, A.K.; Chari, A.; Abdallah, A.O.; Callander, N.; Lendvai, N.; Sborov, D.; et al. Belantamab mafodotin for relapsed or refractory multiple myeloma (DREAMM-2): A two-arm, randomised, open-label, phase 2 study. Lancet Oncol. 2020, 21, 207–221. [Google Scholar] [CrossRef]
- Baines, A.C.; Ershler, R.; Kanapuru, B.; Xu, Q.; Shen, G.; Li, L.; Ma, L.; Okusanya, O.O.; Simpson, N.E.; Nguyen, W.; et al. FDA Approval Summary: Belantamab Mafodotin for Patients with Relapsed or Refractory Multiple Myeloma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2022, 28, 4629–4633. [Google Scholar] [CrossRef]
- Vaxman, I.; Abeykoon, J.; Dispenzieri, A.; Kumar, S.K.; Buadi, F.; Lacy, M.Q.; Dingli, D.; Hwa, Y.; Fonder, A.; Hobbs, M.; et al. “Real-life” data of the efficacy and safety of belantamab mafodotin in relapsed multiple myeloma-the Mayo Clinic experience. Blood Cancer J. 2021, 11, 196. [Google Scholar] [CrossRef]
- Atieh, T.; Atrash, S.; Ahmed, N.; Mohan, M.; Cui, W.; Shune, L.; Hajjar, S.; Mahmoudjafari, Z.; Quick, J.; Wishna, A.; et al. Belantamab in Combination with Dexamethasone in Patients with Triple-class Relapsed/Refractory Multiple Myeloma. Clin. Lymphoma Myeloma Leuk. 2022, 22, 912–919. [Google Scholar] [CrossRef]
- Shragai, T.; Magen, H.; Lavi, N.; Gatt, M.; Trestman, S.; Zektser, M.; Ganzel, C.; Jarchowsky, O.; Berger, T.; Tadmor, T.; et al. Real-world experience with belantamab mafodotin therapy for relapsed/refractory multiple myeloma: A multicentre retrospective study. Br. J. Haematol. 2022, 200, 45–53. [Google Scholar] [CrossRef]
- Eaton, J.S.; Miller, P.E.; Mannis, M.J.; Murphy, C.J. Ocular Adverse Events Associated with Antibody-Drug Conjugates in Human Clinical Trials. J. Ocul. Pharmacol. Ther. 2015, 31, 589–604. [Google Scholar] [CrossRef] [PubMed]
- Lonial, S.; Nooka, A.K.; Thulasi, P.; Badros, A.Z.; Jeng, B.H.; Callander, N.S.; Potter, H.A.; Sborov, D.; Zaugg, B.E.; Popat, R.; et al. Management of belantamab mafodotin-associated corneal events in patients with relapsed or refractory multiple myeloma (RRMM). Blood Cancer J. 2021, 11, 103. [Google Scholar] [CrossRef] [PubMed]
- Lonial, S.; Lee, H.C.; Badros, A.; Trudel, S.; Nooka, A.K.; Chari, A.; Abdallah, A.O.; Callander, N.; Sborov, D.; Suvannasankha, A.; et al. Longer term outcomes with single-agent belantamab mafodotin in patients with relapsed or refractory multiple myeloma: 13-month follow-up from the pivotal DREAMM-2 study. Cancer 2021, 127, 4198–4212. [Google Scholar] [CrossRef]
- Abeykoon, J.P.; Vaxman, J.; Patel, S.V.; Kumar, S.; Malave, G.C.; Young, K.S.; Ailawadhi, S.; Larsen, J.T.; Dispenzieri, A.; Muchtar, E.; et al. Impact of belantamab mafodotin-induced ocular toxicity on outcomes of patients with advanced multiple myeloma. Br. J. Haematol. 2022, 199, 95–99. [Google Scholar] [CrossRef]
- Nooka, A.K.; Weisel, K.; van de Donk, N.W.; Routledge, D.; Otero, P.R.; Song, K.; Quach, H.; Callander, N.; Minnema, M.C.; Trudel, S.; et al. Belantamab mafodotin in combination with novel agents in relapsed/refractory multiple myeloma: DREAMM-5 study design. Future Oncol. 2021, 17, 1987–2003. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.A.; Hoffmann, F.S.; Kuhn, P.H.; Cheng, Q.; Chu, Y.; Schmidt-Supprian, M.; Hauck, S.M.; Schuh, E.; Krumbholz, M.; Rubsamen, H.; et al. gamma-Secretase directly sheds the survival receptor BCMA from plasma cells. Nat. Commun. 2015, 6, 7333. [Google Scholar] [CrossRef]
- Lonial, S.; Grosicki, S.; Hus, M.; Song, K.W.; Facon, T.; Callander, N.S.; Ribrag, V.; Uttervall, K.; Quach, H.; Vorobyev, V.I.; et al. Synergistic effects of low-dose belantamab mafodotin in combination with a gamma-secretase inhibitor (nirogacestat) in patients with relapsed/refractory multiple myeloma (RRMM): DREAMM-5 study. J. Clin. Oncol. 2022, 40, 8019. [Google Scholar] [CrossRef]
- Popat, R.; Nooka, A.; Stockerl-Goldstein, K.; Abonour, R.; Ramaekers, R.; Khot, A.; Forbes, A.; Cindy Lee, C.; Augustson, B.; Spencer, A.; et al. DREAMM-6: Safety, Tolerability and Clinical Activity of Belantamab Mafodotin (Belamaf) in Combination with Bortezomib/Dexamethasone (BorDex) in Relapsed/Refractory Multiple Myeloma (RRMM). In Proceedings of the 62nd ASH Annual Meeting and Exposition, San Diego, CA, USA, 5–8 December 2020. Abstract 1419. [Google Scholar]
- Quach, H.; Gironella, M.; Lee, C.; Popat, R.; Cannell, P.; Kasinathan, R.; Chopra, B.; Rogers, R.; Ferron-Brady, G.; Shafi-Harji, S.; et al. Safety and clinical activity of belantamab mafodotin with lenalidomide plus dexamethasone in patients with relapsed/refractory multiple myeloma (RRMM): DREAMM-6 arm-A interim analysis. J. Clin. Oncol. 2022, 40, 8017. [Google Scholar] [CrossRef]
- Suvannasankha, A.; Bahlis, N.J.; Trudel, S.; Weisel, K.; Koenecke, C.; Rocafiguera, A.O.; Voorhees, P.M.; Alonso, A.A.; Callander, N.S.; Mateos, M.-V.; et al. Safety and clinical activity of belantamab mafodotin with pembrolizumab in patients with relapsed/refractory multiple myeloma (RRMM): DREAMM-4 Study. J. Clin. Oncol. 2022, 40, 8018. [Google Scholar] [CrossRef]
- Trudel, S.; McCurdy, A.; Fu, M.; Sutherland, H.J.; Louzada, M.L.; Chu, M.P.; White, D.J.; Mian, H.S.; Kotb, R.; Othman, I.; et al. Belantamab Mafodotin in Combination with Pomalidomide and Dexamethasone Demonstrates Durable Responses in Triple Class Exposed/Refractory Multiple Myeloma. In Proceedings of the 64th ASH Annual Meeting and Exposition, New Orleans, LA, USA, 10–13 December 2022. Abstract 3248. [Google Scholar]
- Terpos, E.; Gavriatopoulou, M.; Ntanasis-Stathopoulos, I.; Malandrakis, P.; Fotiou, D.; Kanellias, N.; Migkou, M.; Theodorakakou, F.; Spiliopoulou, V.; Syrigou, R.; et al. A Phase 1/2, Dose and Schedule Evaluation Study to Investigate the Safety and Clinical Activity of Belantamab Mafodotin Administered in Combination with Lenalidomide and Dexamethasone in Transplant-Ineligible Patients with Newly Diagnosed Multiple Myeloma. In Proceedings of the 64th ASH Annual Meeting and Exposition, New Orleans, LA, USA, 10–13 December 2022. Abstract 1920. [Google Scholar]
- Gonzalez-Calle, V.; Otero, P.R.; Rey-Bua, B.; De La Rubia, J.; De Arriba, F.; Cabañas, V.; Garcia, E.G.; Ocio, E.M.; Encinas, C.; Cabrera, A.S.; et al. Belantamab Mafodotin in Combination with Vrd for the Treatment of Newly Diagnosed Transplant Eligible Multiple Myeloma Patients: Results from the Phase II, Open Label, Multicenter, GEM-BELA-Vrd Trial. In Proceedings of the 64th ASH Annual Meeting and Exposition, New Orleans, LA, USA, 10–13 December 2022. Abstract 3241. [Google Scholar]
- Zhang, Y.; Godara, A.; Pan, S.; Toskic, D.; Mann, H.; Sborov, D.; Comenzo, R.; Kansagra, A. Belantamab mafodotin in patients with relapsed/refractory AL amyloidosis with myeloma. Ann. Hematol. 2022, 101, 2119–2121. [Google Scholar] [CrossRef]
- Khwaja, J.; Bomsztyk, J.; Mahmood, S.; Wisniowski, B.; Shah, R.; Tailor, A.; Yong, K.; Popat, R.; Rabin, N.; Kyriakou, C.; et al. High response rates with single-agent belantamab mafodotin in relapsed systemic AL amyloidosis. Blood Cancer J. 2022, 12, 128. [Google Scholar] [CrossRef] [PubMed]
- Kastritis, E.; Palladini, G.; Dimopoulos, M.-A.; Jaccard, A.; Merlini, G.; Theodorakakou, F.; Fotiou, D.; Minnema, M.C.; Wechalekar, A.D.; Varghese, S.; et al. Efficacy and Safety of Belantamab Mafodotin Monotherapy in Patients with Relapsed or Refractory Light Chain Amyloidosis: A Phase 2 Study By the European Myeloma Network. In Proceedings of the 64th ASH Annual Meeting and Exposition, New Orleans, LA, USA, 10–13 December 2022. Abstract 3244. [Google Scholar]
- Pahl, A.; Lutz, C.; Hechler, T. Amanitins and their development as a payload for antibody-drug conjugates. Drug Discov. Today Technol 2018, 30, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Figueroa-Vazquez, V.; Ko, J.; Breunig, C.; Baumann, A.; Giesen, N.; Palfi, A.; Muller, C.; Lutz, C.; Hechler, T.; Kulke, M.; et al. HDP-101, an Anti-BCMA Antibody-Drug Conjugate, Safely Delivers Amanitin to Induce Cell Death in Proliferating and Resting Multiple Myeloma Cells. Mol. Cancer Ther. 2021, 20, 367–378. [Google Scholar] [CrossRef]
- Singh, R.K.; Jones, R.J.; Hong, S.; Shirazi, F.; Wang, H.; Kuiatse, I.; Pahl, A.; Orlowski, R.Z. HDP101, a Novel B-Cell Maturation Antigen (BCMA)-Targeted Antibody Conjugated to α-Amanitin, Is Active against Myeloma with Preferential Efficacy Against Pre-Clinical Models of Deletion 17p. Blood 2018, 132, 593. [Google Scholar] [CrossRef]
- Singh, R.K.; Jones, R.J.; Shirazi, F.M.; Zou, J.M.; Wang, H.; Lee, H.C.; Manasanch, E.E.; Kuiatse, I.; Pahl, A.; Orlowski, R.Z. The anti-B-cell maturation antigen (BCMA) antibody-α-amanitin conjugate HDP-101 induces immunogenic cell death and immunologic memory in models of multiple myeloma. Blood 2020, 136, 9–10, ASH Abstract 668. [Google Scholar] [CrossRef]
- Kaufman, J.L.; Orlowski, R.Z.; Strassz, A.; Pahl, A.; Michaels, T.; Last, A.; Szaboki, H.; Jentsch, G.; Schoenborn-Kellenberger, O.; Raab, M.-S. Hdp-101, an Anti-BCMA Antibody-Drug Conjugate with a Novel Payload Amanitin in Patients with Relapsed Multiple Myeloma, Initial Findings of the First in Human Study. In Proceedings of the 64th ASH Annual Meeting and Exposition, New Orleans, LA, USA, 10–13 December 2022. Abstract 3219. [Google Scholar]
- Hartley, J.A. Antibody-drug conjugates (ADCs) delivering pyrrolobenzodiazepine (PBD) dimers for cancer therapy. Expert Opin. Biol. Ther. 2021, 21, 931–943. [Google Scholar] [CrossRef]
- Xing, L.; Lin, L.; Yu, T.; Li, Y.; Cho, S.F.; Liu, J.; Wen, K.; Hsieh, P.A.; Kinneer, K.; Munshi, N.; et al. A novel BCMA PBD-ADC with ATM/ATR/WEE1 inhibitors or bortezomib induce synergistic lethality in multiple myeloma. Leukemia 2020, 34, 2150–2162. [Google Scholar] [CrossRef]
- Xing, L.; Wang, S.; Liu, J.; Yu, T.; Chen, H.; Wen, K.; Li, Y.; Lin, L.; Hsieh, P.A.; Cho, S.F.; et al. BCMA-Specific ADC MEDI2228 and Daratumumab Induce Synergistic Myeloma Cytotoxicity via IFN-Driven Immune Responses and Enhanced CD38 Expression. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2021, 27, 5376–5388. [Google Scholar] [CrossRef]
- Kumar, S.K.; Migkou, M.; Bhutani, M.; Spencer, A.; Ailawadhi, S.; Kalff, A.; Walcott, F.; Pore, N.; Gibson, D.; Wang, F.; et al. Phase 1, First-in-Human Study of MEDI2228, a BCMA-Targeted ADC in Patients with Relapsed/Refractory Multiple Myeloma. In Proceedings of the 62nd ASH Annual Meeting and Exposition, San Diego, CA, USA, 5–8 December 2020. Abstract 179. [Google Scholar]
- Morandi, F.; Airoldi, I.; Marimpietri, D.; Bracci, C.; Faini, A.C.; Gramignoli, R. CD38, a Receptor with Multifunctional Activities: From Modulatory Functions on Regulatory Cell Subsets and Extracellular Vesicles, to a Target for Therapeutic Strategies. Cells 2019, 8, 1527. [Google Scholar] [CrossRef]
- Li, L.; Hau, A.; Tong, W.; Lau, M.; Zhu, T.; Fells, K.; Wei, A.; Li, X.; Deng, D.; Sun, Y.; et al. Abstract LB-227: Preclinical development and characterization of STI-6129, an anti-CD38 antibody-drug conjugate, as a new therapeutic agent for multiple myeloma. Cancer Res. 2020, 80, LB-227. [Google Scholar] [CrossRef]
- Willert, E.K.; Robinson, G.L.; Higgins, J.P.; Liu, J.; Lee, J.; Syed, S.; Zhang, Y.; Tavares, D.; Lublinsky, A.; Chattopadhyay, N.; et al. Abstract 2384: TAK-169, an exceptionally potent CD38 targeted engineered toxin body, as a novel direct cell kill approach for the treatment of multiple myeloma. Cancer Res. 2019, 79, 2384. [Google Scholar] [CrossRef]
- Bruins, W.S.C.; Zheng, W.; Higgins, J.P.; Willert, E.K.; Newcomb, J.; Dash, A.B.; Van De Donk, N.W.C.J.; Zweegman, S.; Mutis, T. TAK-169, a Novel Recombinant Immunotoxin Specific for CD38, Induces Powerful Preclinical Activity Against Patient-Derived Multiple Myeloma Cells. Blood 2020, 136, 11–12. [Google Scholar] [CrossRef]
- Kumar, S.; Mamuye, A.; Dabovic, K.; Wang, J.; Anand, B.; Yuet, A.; Dholaria, B.; Roy, V. 447 Interim results of a phase 1 study of the novel engineered toxin body TAK-169 in patients with relapsed or refractory multiple myeloma. J. ImmunoTherapy Cancer 2021, 9, A475. [Google Scholar] [CrossRef]
- Fatholahi, M.; Valencia, M.; Mark, A.; Bi, M.; Syed, S.; Zhang, Y.; Taura, T.; Yun, Y.; Wilson, D.; Chattopadhyay, N.; et al. TAK-573, an anti-CD38-targeted attenuated interferon alpha (IFNα) fusion protein, showed anti-myeloma tumor responses in combination with standard of care (SOC) agents in multiple myeloma (MM) xenograft tumor models in vivo. Clin. Lymphoma Myeloma Leuk. 2019, 19, e116. [Google Scholar] [CrossRef]
- Kaufman, J.L.; Atrash, S.; Holstein, S.A.; Nadeem, O.; Benson, D.; Suryanarayan, K.; Liu, Y.; Li, C.; Yang, L.; Parot, X.; et al. S181: Modakafusp Alfa (TAK-573): Updated Clinical, Pharmacokinetic (PK), and Immunogenicity Results from a Phase 1/2 Study in Patients (PTS) with Relapsed/Refractory Multiple Myeloma (RRMM). HemaSphere 2022, 6, 82–83. [Google Scholar] [CrossRef]
- Sherbenou, D.W.; Aftab, B.T.; Su, Y.; Behrens, C.R.; Wiita, A.; Logan, A.C.; Acosta-Alvear, D.; Hann, B.C.; Walter, P.; Shuman, M.A.; et al. Antibody-drug conjugate targeting CD46 eliminates multiple myeloma cells. J. Clin. Investig. 2016, 126, 4640–4653. [Google Scholar] [CrossRef]
- Davies, F.E.; Pawlyn, C.; Usmani, S.Z.; San-Miguel, J.F.; Einsele, H.; Boyle, E.M.; Corre, J.; Auclair, D.; Cho, H.J.; Lonial, S.; et al. Perspectives on the Risk-Stratified Treatment of Multiple Myeloma. Blood Cancer Discov. 2022, 3, 273–284. [Google Scholar] [CrossRef]
- Reina, M.; Espel, E. Role of LFA-1 and ICAM-1 in Cancer. Cancers 2017, 9, 153. [Google Scholar] [CrossRef]
- Sherbenou, D.W.; Su, Y.; Behrens, C.R.; Aftab, B.T.; Perez de Acha, O.; Murnane, M.; Bearrows, S.C.; Hann, B.C.; Wolf, J.L.; Martin, T.G.; et al. Potent Activity of an Anti-ICAM1 Antibody-Drug Conjugate against Multiple Myeloma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2020, 26, 6028–6038. [Google Scholar] [CrossRef]
- Hansson, M.; Gimsing, P.; Badros, A.; Niskanen, T.M.; Nahi, H.; Offner, F.; Salomo, M.; Sonesson, E.; Mau-Sorensen, M.; Stenberg, Y.; et al. A Phase I Dose-Escalation Study of Antibody BI-505 in Relapsed/Refractory Multiple Myeloma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2015, 21, 2730–2736. [Google Scholar] [CrossRef]
- Wichert, S.; Juliusson, G.; Johansson, A.; Sonesson, E.; Teige, I.; Wickenberg, A.T.; Frendeus, B.; Korsgren, M.; Hansson, M. A single-arm, open-label, phase 2 clinical trial evaluating disease response following treatment with BI-505, a human anti-intercellular adhesion molecule-1 monoclonal antibody, in patients with smoldering multiple myeloma. PLoS ONE 2017, 12, e0171205. [Google Scholar] [CrossRef] [PubMed]
- Flores-Montero, J.; de Tute, R.; Paiva, B.; Perez, J.J.; Bottcher, S.; Wind, H.; Sanoja, L.; Puig, N.; Lecrevisse, Q.; Vidriales, M.B.; et al. Immunophenotype of normal vs. myeloma plasma cells: Toward antibody panel specifications for MRD detection in multiple myeloma. Cytometry. Part B Clin. Cytom. 2016, 90, 61–72. [Google Scholar] [CrossRef]
- Tassone, P.; Gozzini, A.; Goldmacher, V.; Shammas, M.A.; Whiteman, K.R.; Carrasco, D.R.; Li, C.; Allam, C.K.; Venuta, S.; Anderson, K.C.; et al. In vitro and in vivo activity of the maytansinoid immunoconjugate huN901-N2′-deacetyl-N2′-(3-mercapto-1-oxopropyl)-maytansine against CD56+ multiple myeloma cells. Cancer Res. 2004, 64, 4629–4636. [Google Scholar] [CrossRef] [PubMed]
- Ailawadhi, S.; Kelly, K.R.; Vescio, R.A.; Jagannath, S.; Wolf, J.; Gharibo, M.; Sher, T.; Bojanini, L.; Kirby, M.; Chanan-Khan, A. A Phase I Study to Assess the Safety and Pharmacokinetics of Single-agent Lorvotuzumab Mertansine (IMGN901) in Patients with Relapsed and/or Refractory CD-56-positive Multiple Myeloma. Clin. Lymphoma Myeloma Leuk. 2019, 19, 29–34. [Google Scholar] [CrossRef]
- Su, H.; Na, N.; Zhang, X.; Zhao, Y. The biological function and significance of CD74 in immune diseases. Inflamm. Res. 2017, 66, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wang, Q.; Li, T.; Qian, J.; Lu, Y.; Li, Y.; Bi, E.; Reu, F.; Qin, Y.; Drazba, J.; et al. Role of Myeloma-Derived MIF in Myeloma Cell Adhesion to Bone Marrow and Chemotherapy Response. J. Natl. Cancer Inst. 2016, 108, djw131. [Google Scholar] [CrossRef]
- Burton, J.D.; Ely, S.; Reddy, P.K.; Stein, R.; Gold, D.V.; Cardillo, T.M.; Goldenberg, D.M. CD74 is expressed by multiple myeloma and is a promising target for therapy. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2004, 10, 6606–6611. [Google Scholar] [CrossRef] [PubMed]
- Sapra, P.; Stein, R.; Pickett, J.; Qu, Z.; Govindan, S.V.; Cardillo, T.M.; Hansen, H.J.; Horak, I.D.; Griffiths, G.L.; Goldenberg, D.M. Anti-CD74 antibody-doxorubicin conjugate, IMMU-110, in a human multiple myeloma xenograft and in monkeys. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2005, 11, 5257–5264. [Google Scholar] [CrossRef]
- Kaufman, J.L.; Niesvizky, R.; Stadtmauer, E.A.; Chanan-Khan, A.; Siegel, D.; Horne, H.; Wegener, W.A.; Goldenberg, D.M. Phase I, multicentre, dose-escalation trial of monotherapy with milatuzumab (humanized anti-CD74 monoclonal antibody) in relapsed or refractory multiple myeloma. Br. J. Haematol. 2013, 163, 478–486. [Google Scholar] [CrossRef]
- Abrahams, C.L.; Li, X.; Embry, M.; Yu, A.; Krimm, S.; Krueger, S.; Greenland, N.Y.; Wen, K.W.; Jones, C.; DeAlmeida, V.; et al. Targeting CD74 in multiple myeloma with the novel, site-specific antibody-drug conjugate STRO-001. Oncotarget 2018, 9, 37700–37714. [Google Scholar] [CrossRef]
- Shah, N.N.; Mattour, A.H.; Popplewell, L.L.; Andreadis, C.; Melear, J.M.; Spira, A.I.; Shulman, J.; Manda, S.; Burke, J.M.; Chhabra, S.; et al. Preliminary Results of an Ongoing Phase 1 Dose Escalation Study of the Novel Anti-CD74 Antibody Drug Conjugate (ADC), STRO-001, in Patients with B-Cell Non-Hodgkin Lymphoma. Blood 2020, 136, 29–30. [Google Scholar] [CrossRef]
- Lonial, S.; Dimopoulos, M.; Palumbo, A.; White, D.; Grosicki, S.; Spicka, I.; Walter-Croneck, A.; Moreau, P.; Mateos, M.V.; Magen, H.; et al. Elotuzumab Therapy for Relapsed or Refractory Multiple Myeloma. N. Engl. J. Med. 2015, 373, 621–631. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Dytfeld, D.; Grosicki, S.; Moreau, P.; Takezako, N.; Hori, M.; Leleu, X.; LeBlanc, R.; Suzuki, K.; Raab, M.S.; et al. Elotuzumab plus Pomalidomide and Dexamethasone for Multiple Myeloma. N. Engl. J. Med. 2018, 379, 1811–1822. [Google Scholar] [CrossRef]
- Vij, R.; Nath, R.; Afar, D.E.H.; Mateos, M.V.; Berdeja, J.G.; Raab, M.S.; Guenther, A.; Martinez-Lopez, J.; Jakubowiak, A.J.; Leleu, X.; et al. First-in-Human Phase I Study of ABBV-838, an Antibody-Drug Conjugate Targeting SLAMF7/CS1 in Patients with Relapsed and Refractory Multiple Myeloma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2020, 26, 2308–2317. [Google Scholar] [CrossRef]
- Lamorte, S.; Ferrero, S.; Aschero, S.; Monitillo, L.; Bussolati, B.; Omede, P.; Ladetto, M.; Camussi, G. Syndecan-1 promotes the angiogenic phenotype of multiple myeloma endothelial cells. Leukemia 2012, 26, 1081–1090. [Google Scholar] [CrossRef]
- Beauvais, D.M.; Jung, O.; Yang, Y.; Sanderson, R.D.; Rapraeger, A.C. Syndecan-1 (CD138) Suppresses Apoptosis in Multiple Myeloma by Activating IGF1 Receptor: Prevention by SynstatinIGF1R Inhibits Tumor Growth. Cancer Res. 2016, 76, 4981–4993. [Google Scholar] [CrossRef]
- Ikeda, H.; Hideshima, T.; Fulciniti, M.; Lutz, R.J.; Yasui, H.; Okawa, Y.; Kiziltepe, T.; Vallet, S.; Pozzi, S.; Santo, L.; et al. The monoclonal antibody nBT062 conjugated to cytotoxic Maytansinoids has selective cytotoxicity against CD138-positive multiple myeloma cells in vitro and in vivo. Clin. Cancer Res. 2009, 15, 4028–4037. [Google Scholar] [CrossRef]
- Jagannath, S.; Heffner, L.T., Jr.; Ailawadhi, S.; Munshi, N.C.; Zimmerman, T.M.; Rosenblatt, J.; Lonial, S.; Chanan-Khan, A.; Ruehle, M.; Rharbaoui, F.; et al. Indatuximab Ravtansine (BT062) Monotherapy in Patients With Relapsed and/or Refractory Multiple Myeloma. Clin. Lymphoma Myeloma Leuk. 2019, 19, 372–380. [Google Scholar] [CrossRef]
- Kelly, K.R.; Ailawadhi, S.; Siegel, D.S.; Heffner, L.T.; Somlo, G.; Jagannath, S.; Zimmerman, T.M.; Munshi, N.C.; Madan, S.; Chanan-Khan, A.; et al. Indatuximab ravtansine plus dexamethasone with lenalidomide or pomalidomide in relapsed or refractory multiple myeloma: A multicentre, phase 1/2a study. Lancet Haematol. 2021, 8, e794–e807. [Google Scholar] [CrossRef]
- Stewart, A.K.; Krishnan, A.Y.; Singhal, S.; Boccia, R.V.; Patel, M.R.; Niesvizky, R.; Chanan-Khan, A.A.; Ailawadhi, S.; Brumm, J.; Mundt, K.E.; et al. Phase I study of the anti-FcRH5 antibody-drug conjugate DFRF4539A in relapsed or refractory multiple myeloma. Blood Cancer J. 2019, 9, 17. [Google Scholar] [CrossRef]
- Munshi, N.C.; Anderson, L.D., Jr.; Shah, N.; Madduri, D.; Berdeja, J.; Lonial, S.; Raje, N.; Lin, Y.; Siegel, D.; Oriol, A.; et al. Idecabtagene Vicleucel in Relapsed and Refractory Multiple Myeloma. N. Engl. J. Med. 2021, 384, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Berdeja, J.G.; Madduri, D.; Usmani, S.Z.; Jakubowiak, A.; Agha, M.; Cohen, A.D.; Stewart, A.K.; Hari, P.; Htut, M.; Lesokhin, A.; et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): A phase 1b/2 open-label study. Lancet 2021, 398, 314–324. [Google Scholar] [CrossRef]
- Moreau, P.; Garfall, A.L.; van de Donk, N.; Nahi, H.; San-Miguel, J.F.; Oriol, A.; Nooka, A.K.; Martin, T.; Rosinol, L.; Chari, A.; et al. Teclistamab in Relapsed or Refractory Multiple Myeloma. N. Engl. J. Med. 2022, 387, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Richardson, P.G.; Oriol, A.; Beksac, M.; Liberati, A.M.; Galli, M.; Schjesvold, F.; Lindsay, J.; Weisel, K.; White, D.; Facon, T.; et al. Pomalidomide, bortezomib, and dexamethasone for patients with relapsed or refractory multiple myeloma previously treated with lenalidomide (OPTIMISMM): A randomised, open-label, phase 3 trial. Lancet Oncol. 2019, 20, 781–794. [Google Scholar] [CrossRef] [PubMed]
- Chesi, M.; Nardini, E.; Brents, L.A.; Schrock, E.; Ried, T.; Kuehl, W.M.; Bergsagel, P.L. Frequent translocation t(4;14)(p16.3;q32.3) in multiple myeloma is associated with increased expression and activating mutations of fibroblast growth factor receptor 3. Nat. Genet. 1997, 16, 260–264. [Google Scholar] [CrossRef]
- Kollmannsberger, C.; Britten, C.D.; Olszanski, A.J.; Walker, J.A.; Zang, W.; Willard, M.D.; Radtke, D.B.; Farrington, D.L.; Bell-McGuinn, K.M.; Patnaik, A. A phase 1 study of LY3076226, a fibroblast growth factor receptor 3 (FGFR3) antibody-drug conjugate, in patients with advanced or metastatic cancer. Investig. New Drugs 2021, 39, 1613–1623. [Google Scholar] [CrossRef]
- Sonneveld, P.; Avet-Loiseau, H.; Lonial, S.; Usmani, S.; Siegel, D.; Anderson, K.C.; Chng, W.J.; Moreau, P.; Attal, M.; Kyle, R.A.; et al. Treatment of multiple myeloma with high-risk cytogenetics: A consensus of the International Myeloma Working Group. Blood 2016, 127, 2955–2962. [Google Scholar] [CrossRef]
- Zhao, H.; Atkinson, J.; Gulesserian, S.; Zeng, Z.; Nater, J.; Ou, J.; Yang, P.; Morrison, K.; Coleman, J.; Malik, F.; et al. Modulation of Macropinocytosis-Mediated Internalization Decreases Ocular Toxicity of Antibody-Drug Conjugates. Cancer Res. 2018, 78, 2115–2126. [Google Scholar] [CrossRef]
- Uppal, H.; Doudement, E.; Mahapatra, K.; Darbonne, W.C.; Bumbaca, D.; Shen, B.Q.; Du, X.; Saad, O.; Bowles, K.; Olsen, S.; et al. Potential mechanisms for thrombocytopenia development with trastuzumab emtansine (T-DM1). Clin. Cancer Res. 2015, 21, 123–133. [Google Scholar] [CrossRef]
- Zhao, H.; Gulesserian, S.; Ganesan, S.K.; Ou, J.; Morrison, K.; Zeng, Z.; Robles, V.; Snyder, J.; Do, L.; Avina, H.; et al. Inhibition of Megakaryocyte Differentiation by Antibody-Drug Conjugates (ADCs) is Mediated by Macropinocytosis: Implications for ADC-induced Thrombocytopenia. Mol. Cancer Ther. 2017, 16, 1877–1886. [Google Scholar] [CrossRef]
- Yu, T.; Chaganty, B.; Lin, L.; Xing, L.; Ramakrishnan, B.; Wen, K.; Hsieh, P.A.; Wollacott, A.; Viswanathan, K.; Adari, H.; et al. VIS832, a novel CD138-targeting monoclonal antibody, potently induces killing of human multiple myeloma and further synergizes with IMiDs or bortezomib in vitro and in vivo. Blood Cancer J. 2020, 10, 110. [Google Scholar] [CrossRef] [PubMed]
- Drewinko, B.; Alexanian, R. Growth kinetics of plasma cell myeloma. J. Natl. Cancer Inst. 1977, 58, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Durie, B.G.; Salmon, S.E.; Moon, T.E. Pretreatment tumor mass, cell kinetics, and prognosis in multiple myeloma. Blood 1980, 55, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Ramkumar, P.; Abarientos, A.B.; Tian, R.; Seyler, M.; Leong, J.T.; Chen, M.; Choudhry, P.; Hechler, T.; Shah, N.; Wong, S.W.; et al. CRISPR-based screens uncover determinants of immunotherapy response in multiple myeloma. Blood Adv. 2020, 4, 2899–2911. [Google Scholar] [CrossRef] [PubMed]
- Amini, L.; Silbert, S.K.; Maude, S.L.; Nastoupil, L.J.; Ramos, C.A.; Brentjens, R.J.; Sauter, C.S.; Shah, N.N.; Abou-El-Enein, M. Preparing for CAR T cell therapy: Patient selection, bridging therapies and lymphodepletion. Nat. Rev. Clin. Oncol. 2022, 19, 342–355. [Google Scholar] [CrossRef] [PubMed]
- Lowther, D.E.; Houseman, E.A.; Han, G.; Kleanthous, E.; Knoblock, D.; Zhou, X.; Banerjee, S.; Patel, S.; Figueroa, D. No Evidence of BCMA Expression Loss or Systemic Immune Impairment after Treatment with the BCMA-Targeted Antibody-Drug Conjugate (ADC) Belantamab Mafodotin (Belamaf) in the DREAMM-1 and DREAMM-2 Trials of Patients with Relapsed/Refractory Multiple Myeloma (RRMM). In Proceedings of the 64th ASH Annual Meeting and Exposition, New Orleans, LA, USA, 10–13 December 2022. Abstract 248. [Google Scholar]
- Samur, M.K.; Fulciniti, M.; Aktas Samur, A.; Bazarbachi, A.H.; Tai, Y.T.; Prabhala, R.; Alonso, A.; Sperling, A.S.; Campbell, T.; Petrocca, F.; et al. Biallelic loss of BCMA as a resistance mechanism to CAR T cell therapy in a patient with multiple myeloma. Nat. Commun. 2021, 12, 868. [Google Scholar] [CrossRef]
- Da Via, M.C.; Dietrich, O.; Truger, M.; Arampatzi, P.; Duell, J.; Heidemeier, A.; Zhou, X.; Danhof, S.; Kraus, S.; Chatterjee, M.; et al. Homozygous BCMA gene deletion in response to anti-BCMA CAR T cells in a patient with multiple myeloma. Nat. Med. 2021, 27, 616–619. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).