Baicalin—Current Trends in Detection Methods and Health-Promoting Properties
Abstract
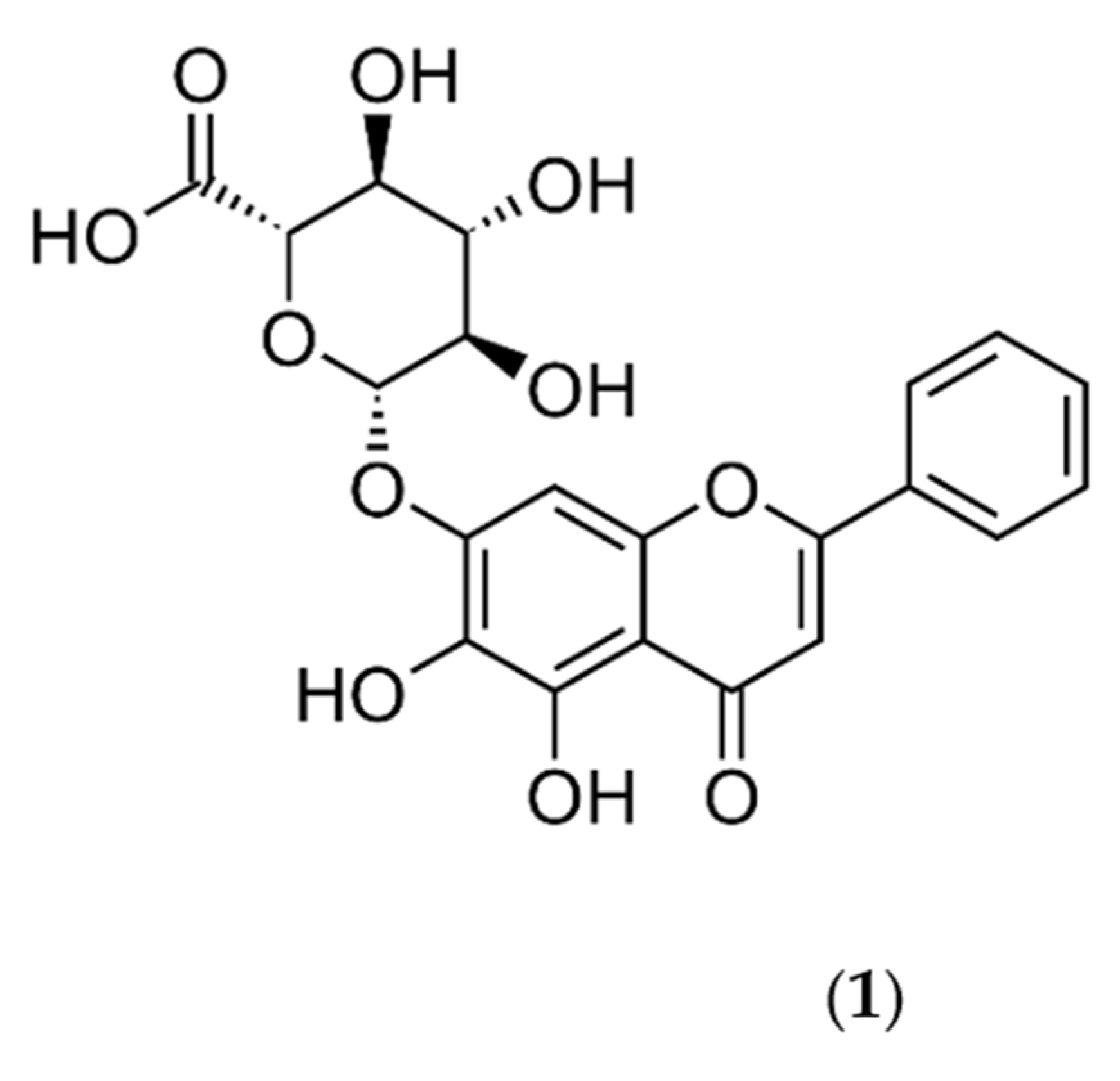
1. Introduction
1.1. Detection Methods
1.1.1. Chromatographic Methods of Detection
| Detection Methods | ||
|---|---|---|
| Ultra-high-performance liquid chromatography (UHPLC) | ||
| Refs. | ||
| Ultra-high-performance liquid chromatography with photodiode-array detectors | UHPLC-PDA | [16] |
| Ultra-performance liquid chromatography/quadrupole time-of-flight mass spectrometry | UPLC-Q-TOF-MS | [13] |
| Ultra-performance liquid chromatography–electrospray ionization with mass spectrometry | UPLC-ESI-MS/MS | [17] |
| Ultra-high-performance liquid chromatography /quadrupole time-of-flight mass spectrometry | UHPLC-Q-TOF-MS | [14] |
| Ultra-performance liquid chromatography with diode array detectors | UPLC-DAD | [5,24] |
| Ultra-performance liquid chromatography with mass spectrometry | UPLC-MS | [27] |
| High-performance liquid chromatography (HPLC) | ||
| High-performance liquid chromatography with mass spectrometry | HPLC | [23] |
| High-performance liquid chromatography with mass spectrometry | HPLC-MS/MS | [15,26] |
| High-performance liquid chromatography photodiode-array with electrospray ionization and mass spectrometry | HPLC–PDA–ESI–MS/MS | [20] |
| High-performance liquid chromatography with electrospray ionization quadrupole time-of-flight and mass spectrometry | HPLC-ESI-Q-TOF-MS | [22] |
| High-performance liquid chromatography with diode array detectors | HPLC-DAD | [4,7] |
| Attenuated-total-reflectance mid-infrared spectroscopy | (ATR-IR) | [28] |
| Near-infrared spectroscopy | (NIR) | [28] |
1.1.2. Electrochemical and Fluorescent Sensors
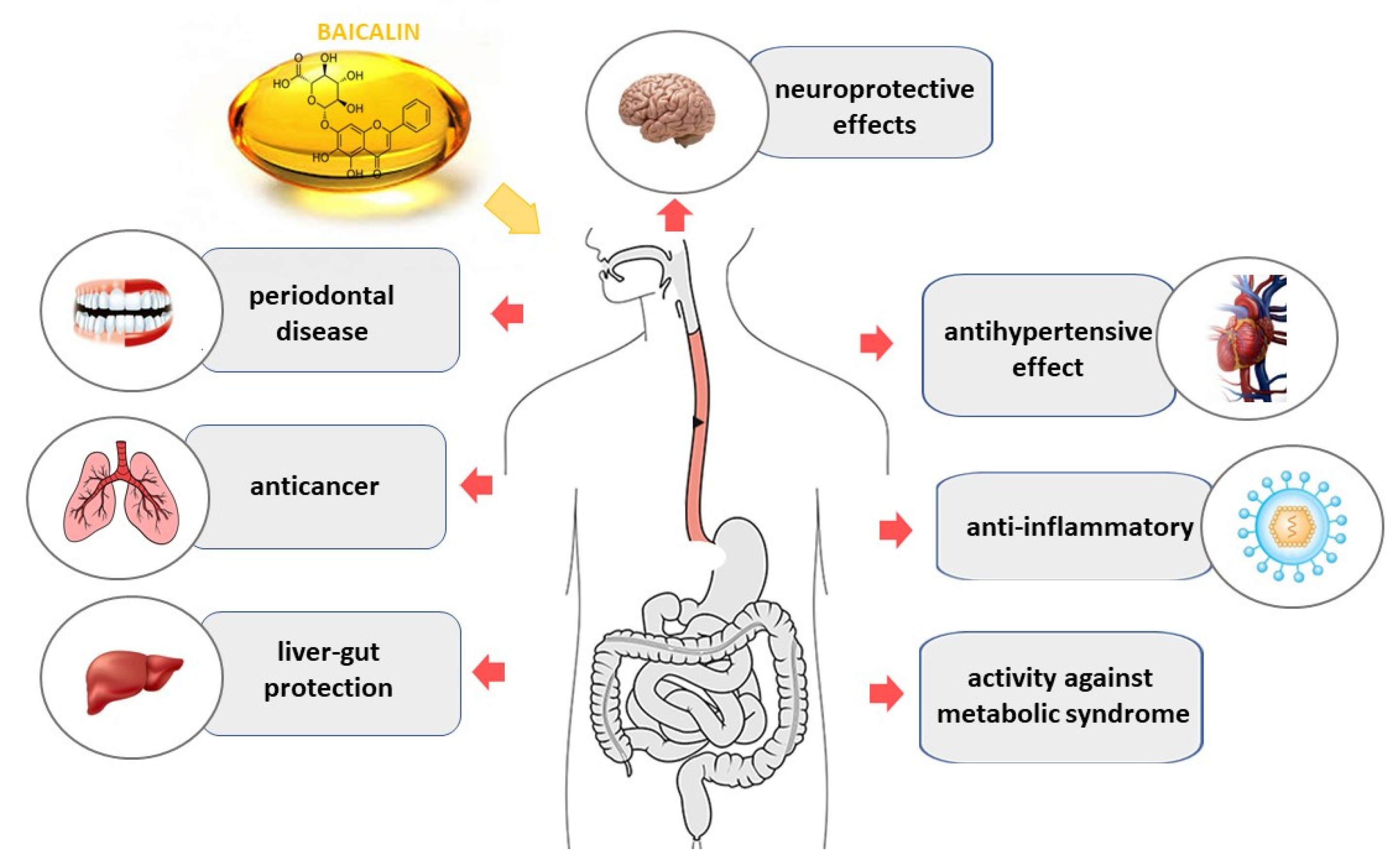
2. Pharmacological Effects of Baicalin
2.1. Anticancer Effect
2.2. Ischemia
2.3. Hypertension
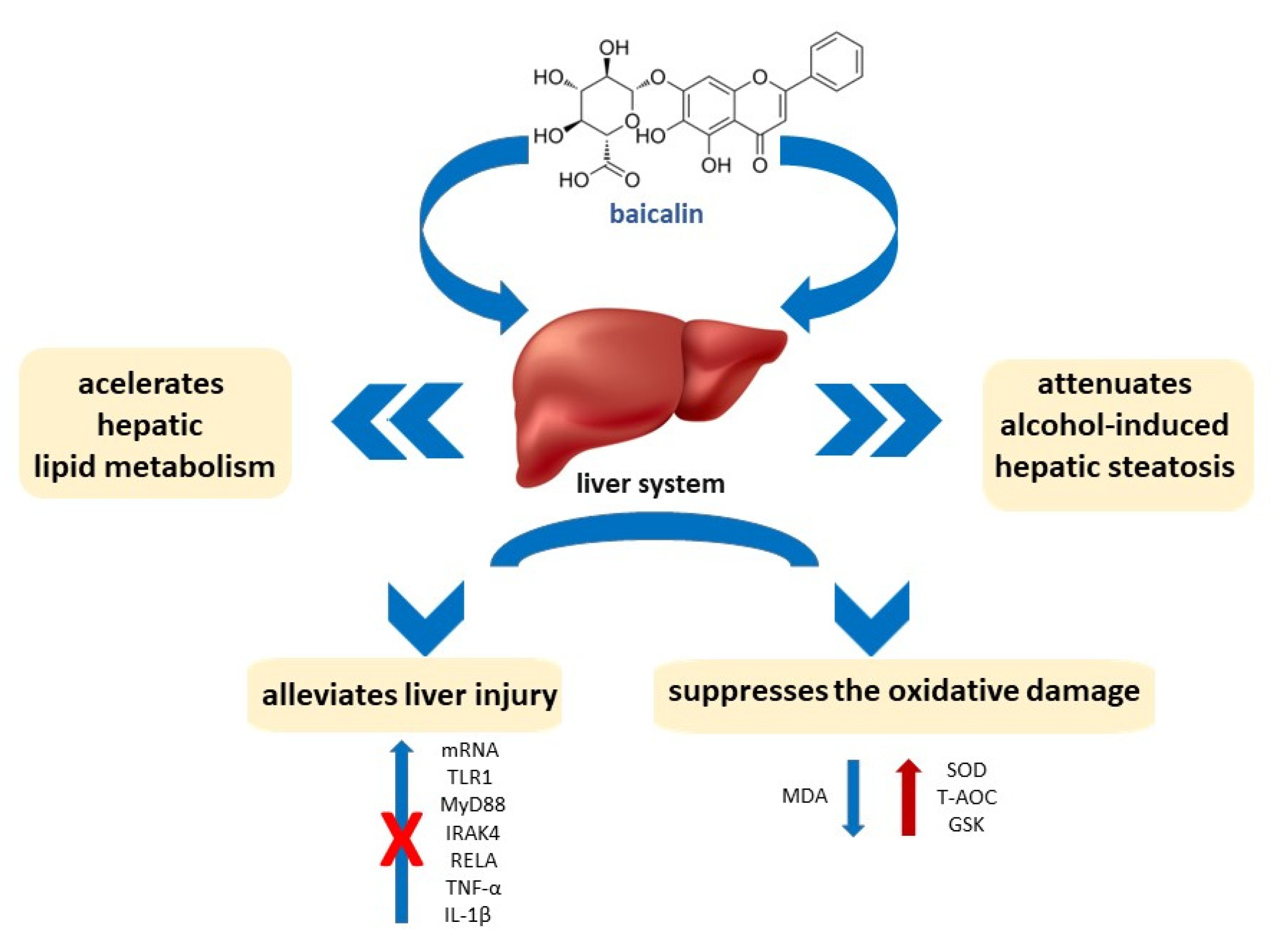
2.4. Liver-Gut System
2.5. Metabolic Syndrome
2.6. Protective Agent
2.7. Periodontal Disease
2.8. Other
2.9. In Vivo Studies
| Pharmacological Effects | Refs. |
|---|---|
| Anticancer effect | [48,59,60,61,62,63,73] |
| Ischemia | [64,65,66,67] |
| Hypertension | [47,68,69,70] |
| Liver-gut system | [71,72,73,74,75] |
| Metabolic syndrome | [76,77] |
| Protective agent | [78,79,80,81] |
| Periodontal disease | [82,84,85] |
| Anti-inflammatory effects | [48,50,54,72] |
| Antiviral properties | [48,55,72] |
| Other | [86,87,88,89,90,91,92,93] |
3. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AgNCs | silver nanoclusters |
| AMPK | 5′AMP-activated protein kinase |
| Ang II | angiotensin II |
| ATGL | adipose triglyceride lipase |
| ATR-IR | attenuated-total-reflectance mid-infrared |
| CMECs | cardiac microvascular endothelial cells |
| DAD | diode array detectors |
| DM-β-CD | 2,6-dimethyl-β-cyclodextrin |
| DON | deoxynivalenol |
| DPV | differential pulse voltammetry |
| GCE | glassy carbon electrode |
| HPLC | high-performance liquid chromatography |
| HSCCC | high-performance countercurrent chromatography |
| LC-MS/MS | liquid chromatography–tandem mass spectrometry |
| IR | ischemia-reperfusion |
| LC-UV | liquid chromatography with ultraviolet detector |
| LOQ | limit of quantification |
| LOT | limit of detection |
| MAPK | mitogen-activated protein kinase |
| MEKC | micellar electrokinetic capillary chromatography |
| MI | myocardial infarction |
| MS | mass spectrometry |
| NAFLD | nonalcoholic fatty liver disease |
| NIR | near-infrared spectroscopy |
| NF-κB | nuclear factor kappa-B |
| NO | nitric oxide |
| OPG | osteoprotegerin |
| UHPLC | ultra-high-performance liquid chromatography |
| UUO | unilateral ureteral obstruction |
| PE | phenylserine |
| PH | pulmonary hypertension |
| PDA | photodiode-array detectors |
| ROS | reactive oxygen species |
| RSD | relative standard deviation |
| SDS | sodium dodecyl sulfate |
| SHRs | spontaneously hypertensive rats |
| SOD | sphincter of oddi dysfunction |
| SREBP1c | sterol regulatory element-binding protein 1c |
| TLC | thin layer chromatography |
| TLRs | toll-like receptors |
| TNF-α | tumor necrosis factor α |
| TYR | tyrosinase |
| VSMCs | vascular smooth muscle cells |
References
- Zhao, Q.; Chen, X.Y.; Martin, C. Scutellaria baicalensis, the Golden Herb from the Garden of Chinese Medicinal Plants. Sci. Bull. 2016, 61, 1391–1398. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-Z.; Yu, C.-H.; Gao, J.; Zhao, G.-R. Effects of Processing and Extracting Methods on Active Components in Radix Scutellariae by HPLC Analysis. Zhongguo Zhongyao Zazhi 2007, 32, 1637–1640. [Google Scholar] [PubMed]
- Seo, C.S.; Shin, H.K. Development of a Simultaneous Analysis Method for Quality Control of a Traditional Herbal Formula, Daeshiho-Tang, Using 10 Marker Components. Appl. Sci. 2021, 11, 242. [Google Scholar] [CrossRef]
- Chen, J.C.; Wu, H.L.; Wang, T.; Dong, M.Y.; Chen, Y.; Yu, R.Q. High-Performance Liquid Chromatography–Diode Array Detection Combined with Chemometrics for Simultaneous Quantitative Analysis of Five Active Constituents in a Chinese Medicine Formula Wen-Qing-Yin. Chemosensors 2022, 10, 238. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, Y.; Zhang, T.; Jiang, X.; Yi, Y.; Zhang, L.; Chen, Y.; Li, T.; Kang, P.; Tian, J. Quantitative Analysis of Twelve Active Components Combined with Chromatographic Fingerprint for Comprehensive Evaluation of Qinma Prescription by Ultra-Performance Liquid Chromatography Coupled with Diode Array Detection. J. Chromatogr. Sci. 2019, 57, 855–865. [Google Scholar] [CrossRef]
- Wu, T.Y.; Chang, F.R.; Liou, J.R.; Lo, I.W.; Chung, T.C.; Lee, L.Y.; Chi, C.C.; Du, Y.C.; Wong, M.H.; Juo, S.H.H.; et al. Rapid HPLC Quantification Approach for Detection of Active Constituents in Modern Combinatorial Formula, San-Huang-Xie-Xin-Tang (SHXXT). Front. Pharmacol. 2016, 7, 374. [Google Scholar] [CrossRef]
- Li, B.Q.; Chen, J.; Li, J.J.; Wang, X.; Zhai, H.L.; Zhang, X.Y. High-Performance Liquid Chromatography with Photodiode Array Detection and Chemometrics Method for the Analysis of Multiple Components in the Traditional Chinese Medicine Shuanghuanglian Oral Liquid. J. Sep. Sci. 2015, 38, 4187–4195. [Google Scholar] [CrossRef]
- Seo, C.S.; Shin, H.K. HPLC-PDA Method for Simultaneous Determination of Nine Marker Components in Banhasasim-Tang. J. Chromatogr. Sci. 2016, 54, 299–304. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Lu, Y.; Xiao, J.; Chen, Y.; Wang, X.H. Concurrent Identification of 11 Major Primary Active Compounds in Huangqin Qingfei Decoction by Liquid Chromatography Tandem Mass Spectrometry via Liquid Chromatography Tandem Mass Spectrometry. Pak. J. Pharm. Sci. 2020, 33, 1005–1013. [Google Scholar] [CrossRef]
- Wang, Z.; An, R.; Du, G.; Liang, K.; Li, G. Validation of an LC–MS/MS Method for Simultaneous Detection of Diverse Components of Qinxing Qingre Zhike Granule in Rat Plasma and Its Application to Pharmacokinetic Study after Oral Administration to Rats. Biomed. Chromatogr. 2019, 33, e4524. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Xiao, J.; Xu, R.; Wang, Q.; Wang, X. Simultaneous Determination of Baicalin, Baicalein, Wogonoside, Wogonin, Scutellarin, Berberine, Coptisine, Ginsenoside Rb1 and Ginsenoside Re of Banxia Xiexin Decoction in Rat Plasma by LC–MS/MS and Its Application to a Pharmacokinetic Study. Biomed. Chromatogr. 2018, 32, e4083. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, J.; Zhang, Y.; Chen, Y.; Cao, J.; An, R.; Wang, X. LC-MS/MS Analysis of Gegen Qinlian Decoction and Its Pharmacokinetics after Oral Administration to Rats. Biomed. Chromatogr. 2015, 29, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Xiong, Y.; Zou, Z.; Yang, Y.; He, J.; Zhong, L.; Wang, Y.; Yang, M. Identifying the Chemical Markers in Raw and Wine-Processed Scutellaria baicalensis by Ultra-Performance Liquid Chromatography/Quadrupole Time-of-Flight Mass Spectrometry Coupled with Multiple Statistical Strategies. Biomed. Chromatogr. 2020, 34, e4849. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Li, Z.; Li, M.; Yuan, Y.; Cui, S.; Chen, J.; Li, R. An Integrated Strategy for Profiling the Chemical Components of Scutellariae Radix and Their Exogenous Substances in Rats by Ultra-High-Performance Liquid Chromatography/Quadrupole Time-of-Flight Mass Spectrometry. Rapid Commun. Mass Spectrom. 2020, 34, e8823. [Google Scholar] [CrossRef] [PubMed]
- Baygildieva, D.I.; Baygildiev, T.M.; Stavrianidi, A.N.; Shpigun, O.A.; Rodin, I.A. Simultaneous Determination of Wogonin, Scutellarin, Baicalin, and Baicalein in Extracts from Scutellariae baicalensis by High-Performance Liquid Chromatography with Tandem Mass Spectrometry. J. Anal. Chem. 2018, 73, 1317–1322. [Google Scholar] [CrossRef]
- Cui, X.; Cai, H.; Li, H.; Tao, Y.; Huang, P.; Qian, X.; Li, J.; Cai, B. Simultaneous Determination of 10 Flavonoids in Crude and Wine-Processed Radix Scutellariae by UHPLC. J. Chromatogr. Sci. 2016, 54, 312–317. [Google Scholar] [CrossRef]
- Cui, X.B.; Qian, X.C.; Huang, P.; Zhang, Y.X.; Li, J.S.; Yang, G.M.; Cai, B.C. Simultaneous Determination of Ten Flavonoids of Crude and Wine-Processed Radix Scutellariae Aqueous Extracts in Rat Plasma by UPLC-ESI-MS/MS and Its Application to a Comparative Pharmacokinetic Study. Biomed. Chromatogr. 2015, 29, 1112–1123. [Google Scholar] [CrossRef]
- Li, H.; Jiang, Y.; Chen, F. Separation Methods Used for Scutellaria Baicalensis Active Components. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2004, 812, 277–290. [Google Scholar] [CrossRef]
- Luo, J.L.; Lu, F.L.; Liu, Y.C.; Lo, C.F. Identification of Scutellaria Baicalensis in Traditional Chinese Medicine Preparations by LC/MS/MS Fingerprinting Method. J. Food Drug Anal. 2012, 20, 29. [Google Scholar] [CrossRef]
- Shi, G.F.; Yao, R.X.; Wang, G.Y.; Wang, Z.J.; Chen, F.W. Liquid Chromatography-Tandem Mass Spectrometry Screening Method for the Detection of Radical-Scavenging Natural Antioxidants from the Whole Scutellariae (Radix, Stem and Leaf). J. Chromatogr. Sci. 2015, 53, 1140–1146. [Google Scholar] [CrossRef]
- Islam, M.N.; Chung, H.J.; Kim, D.H.; Yoo, H.H. A Simple Isocratic HPLC Method for the Simultaneous Determination of Bioactive Components of Scutellariae Radix Extract. Nat. Prod. Res. 2012, 26, 1957–1962. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Liqiong, S.; Yingduo, Y.; Jin, Q.; Boyang, Y. Application of Multi-Dimensional and Multi-Informational (MD-MI) Integrated Xanthine Oxidase and Superoxide Anion Fingerprint in Quality Evaluation of Scutellariae Radix. J. Pharm. Biomed. Anal. 2020, 191, 113595. [Google Scholar] [CrossRef] [PubMed]
- Weiping, L.; Yukui, R. Short Communication Evaluation of Baicalin in Scutellaria baicalensis georgi Using HPLC Method. Bull. Chem. Soc. Ethiop. 2010, 24, 115–119. [Google Scholar] [CrossRef]
- Chen, H.; Li, Z.; Li, Y.; Wu, X.; Wang, S.; Chen, K.; Zheng, X.; Du, Q.; Tang, D. Simultaneous Determination of Baicalin, Oroxylin A-7-O-Glucuronide and Wogonoside in Rat Plasma by UPLC-DAD and Its Application in Pharmacokinetics of Pure Baicalin, Radix Scutellariae and Yinhuang Granule. Biomed. Chromatogr. 2015, 29, 1819–1825. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Pi, C.; Yang, G.; Xiong, X.; Lan, Y.; Yang, H.; Zhou, Y.; Ye, Y.; Zou, Y.; Zheng, W.; et al. LC-UV Determination of Baicalin in Rabbit Plasma and Tissues for Application in Pharmacokinetics and Tissue Distribution Studies of Baicalin after Intravenous Administration of Liposomal and Injectable Formulations. Molecules 2016, 21, 444. [Google Scholar] [CrossRef]
- Pang, H.; Shi, A.; Li, M.; Xue, W.; Li, Y.; Cao, G.; Yan, B.; Dong, F.; Xiao, W.; He, G.; et al. Simultaneous Determination of Baicalein and Baicalin in Human Plasma by High Performance Liquid Chromatograph-Tandem Spectrometry and Its Application in a Food-Effect Pharmacokinetic Study. Drug Res. 2016, 66, 394–401. [Google Scholar] [CrossRef]
- Tu, Y.; Zhou, L.; Li, L.; Wang, L.; Gao, S.; Hu, M. Development and Validation of an LC-MS/MS Method for the Quantification of Flavonoid Glucuronides (Wogonoside, Baicalin, and Apigenin-Glucuronide) in the Bile and Blood Samples: Application to a Portal Vein Infusion Study. Anal. Biochem. 2020, 601, 113723. [Google Scholar] [CrossRef]
- Navarro Escamilla, M.; Rodenas Sanz, F.; Li, H.; Schönbichler, S.A.; Yang, B.; Bonn, G.K.; Huck, C.W. Rapid Determination of Baicalin and Total Baicalein Content in Scutellariae Radix by ATR-IR and NIR Spectroscopy. Talanta 2013, 114, 304–310. [Google Scholar] [CrossRef]
- Li, C.; Xu, Y. Determination of Puerarin, Daidzein, Baicalin and Wogonin in Composite Preparations by Capillary Electrophoresis. J. Chem. Soc. Pak. 2022, 44, 350–355. [Google Scholar] [CrossRef]
- Ran, X.; Yang, L.; Zhao, G.; Ye, H.; Zhang, Y.; Fan, S.; Xie, X.; Zhao, H.; Li, C.P. Simultaneous Determination of Two Flavonoids Based on Disulfide Linked β-Cyclodextrin Dimer and Pd Cluster Functionalized Graphene-Modified Electrode. RSC Adv. 2015, 5, 60775–60785. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, A.; Guo, Y.; Dong, C. Electrochemical Sensor for Ultrasensitive Determination of Isoquercitrin and Baicalin Based on DM-β-Cyclodextrin Functionalized Graphene Nanosheets. Biosens. Bioelectron. 2014, 58, 242–248. [Google Scholar] [CrossRef]
- Sheng, K.; Wang, L.; Li, H.; Zou, L.; Ye, B. Green Synthesized Co Nanoparticles Doped Amino-Graphene Modified Electrode and Its Application towards Determination of Baicalin. Talanta 2017, 164, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Lu, W.; Yang, L.; Li, G.; Ye, B. A Voltammetry Sensor Platform for Baicalein and Baicalin Simultaneous Detection in Vivo Based on Ta2O5-Nb2O5@CTS Composite. Talanta 2017, 170, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, T.; Qiu, Y.; Fu, F.F.; Yu, Y. Electrochemical Behavior and Determination of Baicalin on a Glassy Carbon Electrode Modified with Molybdenum Disulfide Nano-Sheets. J. Electroanal. Chem. 2016, 775, 286–291. [Google Scholar] [CrossRef]
- Rao, L.; Zhou, P.; Liu, P.; Lu, X.; Duan, X.; Wen, Y.; Zhu, Y.; Xu, J. Green Preparation of Amorphous Molybdenum Sulfide Nanocomposite with Biochar Microsphere and Its Voltametric Sensing Platform for Smart Analysis of Baicalin. J. Electroanal. Chem. 2021, 898, 115591. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Wang, C.; Wang, Y.; Yang, Y.; Chen, J.; Li, C.; Xie, Y.; Zhao, P.; Fei, J. Uncovering the Optimal Pyrolysis Temperature of NH2-MIL-88B-Derived FeOX/Fe@porous Carbon Composites for the Ultrasensitive Electrochemical Detection of Baicalin in Natural Plant Samples. Carbon 2022, 202, 125–136. [Google Scholar] [CrossRef]
- Jiang, X.; Jin, H.; Sun, Y.; Sun, Z.; Gui, R. Assembly of Black Phosphorus Quantum Dots-Doped MOF and Silver Nanoclusters as a Versatile Enzyme-Catalyzed Biosensor for Solution, Flexible Substrate and Latent Fingerprint Visual Detection of Baicalin. Biosens. Bioelectron. 2020, 152, 112012. [Google Scholar] [CrossRef]
- Cheng, W.; Wu, S.; Wang, D.; Lou, Y. Detection of Baicalin Capsule and Scutellariae Radix Based on Nitrogen-Doped Carbon Dots as a Fluorescence Probe. Results Chem. 2022, 4, 100353. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, J.J.; Bai, X.L.; Liu, H.P.; Qi, X.W.; Liao, X. Fast Screening of Tyrosinase Inhibitors from Traditional Chinese Medicinal Plants by Ligand Fishing in Combination with in Situ Fluorescent Assay. Anal. Bioanal. Chem. 2022, 414, 2265–2273. [Google Scholar] [CrossRef]
- Xia, J.; Liu, C.; Niu, H.; Hou, W.; Li, S. Screening and Isolation of Potential Lipoxidase and Superoxide Dismutase Inhibitors from Scutellaria baicalensis Georgi Using High-Speed Countercurrent Chromatography Target-Guided by Ultrafiltration-Liquid Chromatography-Mass Spectrometry. J. Sep. Sci. 2021, 44, 1371–1382. [Google Scholar] [CrossRef]
- Lu, Q.Y.; Zhang, L.; Moro, A.; Chen, M.C.; Harris, D.M.; Eibl, G.; Go, V.L.W. Detection of Baicalin Metabolites Baicalein and Oroxylin-a in Mouse Pancreas and Pancreatic Xenografts. Pancreas 2012, 41, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Cai, W.; Zhou, Y.; Liu, Y.; Wu, X.; Li, Y.; Lu, J.; Qiao, Y. Profiling and Identification of the Metabolites of Baicalin and Study on Their Tissue Distribution in Rats by Ultra-High-Performance Liquid Chromatography with Linear Ion Trap-Orbitrap Mass Spectrometer. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2015, 985, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Tang, H.; Xie, L.; Zheng, Y.; Ma, Z.; Sun, Q.; Li, X. Scutellaria baicalensis Georgi. (Lamiaceae): A Review of Its Traditional Uses, Botany, Phytochemistry, Pharmacology and Toxicology. J. Pharm. Pharmacol. 2019, 71, 1353–1369. [Google Scholar] [CrossRef]
- Tan, Y.Q.; Lin, F.; Ding, Y.K.; Dai, S.; Liang, Y.X.; Zhang, Y.S.; Li, J.; Chen, H.W. Pharmacological Properties of Total Flavonoids in Scutellaria baicalensis for the Treatment of Cardiovascular Diseases. Phytomedicine 2022, 107, 154458. [Google Scholar] [CrossRef] [PubMed]
- Song, J.W.; Long, J.Y.; Xie, L.; Zhang, L.L.; Xie, Q.X.; Chen, H.J.; Deng, M.; Li, X.F. Applications, Phytochemistry, Pharmacological Effects, Pharmacokinetics, Toxicity of Scutellaria baicalensis Georgi. And Its Probably Potential Therapeutic Effects on COVID-19: A Review. Chin. Med. 2020, 15, 102. [Google Scholar] [CrossRef] [PubMed]
- Gaire, B.P.; Moon, S.K.; Kim, H. Scutellaria baicalensis in Stroke Management: Nature’s Blessing in Traditional Eastern Medicine. Chin. J. Integr. Med. 2014, 20, 712–720. [Google Scholar] [CrossRef]
- Wu, J.; Nakashima, S.; Shigyo, M.; Yamasaki, M.; Ikuno, S.; Morikawa, A.; Takegami, S.; Nakamura, S.; Konishi, A.; Kitade, T.; et al. Antihypertensive Constituents in Sanoshashinto. J. Nat. Med. 2020, 74, 421–433. [Google Scholar] [CrossRef]
- Li, C.; Lin, G.; Zuo, Z. Pharmacological Effects and Pharmacokinetics Properties of Radix Scutellariae and Its Bioactive Flavones. Biopharm. Drug Dispos. 2011, 32, 427–445. [Google Scholar] [CrossRef]
- Bao, M.; Ma, Y.; Liang, M.; Sun, X.; Ju, X.; Yong, Y.; Liu, X. Research Progress on Pharmacological Effects and New Dosage Forms of Baicalin. Vet. Med. Sci. 2022, 8, 2773–2784. [Google Scholar] [CrossRef]
- Hu, Z.; Guan, Y.; Hu, W.; Xu, Z.; Ishfaq, M. An Overview of Pharmacological Activities of Baicalin and Its Aglycone Baicalein: New Insights into Molecular Mechanisms and Signaling Pathways. Iran J. Basic Med. Sci. 2022, 25, 14–26. [Google Scholar] [CrossRef]
- de Oliveira, M.R.; Nabavi, S.F.; Habtemariam, S.; Erdogan Orhan, I.; Daglia, M.; Nabavi, S.M. The Effects of Baicalein and Baicalin on Mitochondrial Function and Dynamics: A Review. Pharmacol. Res. 2015, 100, 296–308. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.R.; Do, C.W.; To, C.H. Potential Therapeutic Effects of Baicalein, Baicalin, and Wogonin in Ocular Disorders. J. Ocul. Pharmacol. Ther. 2014, 30, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Sowndhararajan, K.; Deepa, P.; Kim, M.; Park, S.J.; Kim, S. Neuroprotective and Cognitive Enhancement Potentials of Baicalin: A Review. Brain Sci. 2018, 8, 104. [Google Scholar] [CrossRef] [PubMed]
- Dinda, B.; Dinda, S.; DasSharma, S.; Banik, R.; Chakraborty, A.; Dinda, M. Therapeutic Potentials of Baicalin and Its Aglycone, Baicalein against Inflammatory Disorders. Eur. J. Med. Chem. 2017, 131, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Liang, Y.; Cheng, A.; Wang, Q.; Li, Y.; Wei, H.; Zhou, C.; Wan, X. Antiviral Properties of Baicalin: A Concise Review. Rev. Bras. Farmacogn. 2021, 31, 408–419. [Google Scholar] [CrossRef]
- Xin, L.; Gao, J.; Lin, H.; Qu, Y.; Shang, C.; Wang, Y.; Lu, Y.; Cui, X. Regulatory Mechanisms of Baicalin in Cardiovascular Diseases: A Review. Front. Pharmacol. 2020, 11, 583200. [Google Scholar] [CrossRef] [PubMed]
- Noh, K.; Kang, Y.; Nepal, M.R.; Jeong, K.S.; Oh, D.G.; Kang, M.J.; Lee, S.; Kang, W.; Jeong, H.G.; Jeong, T.C. Role of Intestinal Microbiota in Baicalin-Induced Drug Interaction and Its Pharmacokinetics. Molecules 2016, 21, 337. [Google Scholar] [CrossRef]
- Jiang, M.; Li, Z.; Zhu, G. Immunological Regulatory Effect of Flavonoid Baicalin on Innate Immune Toll-like Receptors. Pharmacol. Res. 2020, 158, 104890. [Google Scholar] [CrossRef]
- Wang, L.; Feng, T.; Su, Z.; Pi, C.; Wei, Y.; Zhao, L. Latest Research Progress on Anticancer Effect of Baicalin and Its Aglycone Baicalein. Arch. Pharm. Res. 2022, 45, 535–557. [Google Scholar] [CrossRef]
- Chen, H.; Gao, Y.; Wu, J.; Chen, Y.; Chen, B.; Hu, J.; Zhou, J. Exploring Therapeutic Potentials of Baicalin and Its Aglycone Baicalein for Hematological Malignancies. Cancer Lett. 2014, 354, 5–11. [Google Scholar] [CrossRef]
- Gong, W.; Zhao, Z.X.; Liu, B.J.; Lu, L.W.; Dong, J.C. Exploring the Chemopreventive Properties and Perspectives of Baicalin and Its Aglycone Baicalein in Solid Tumors. Eur. J. Med. Chem. 2017, 126, 844–852. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Meena, A.; Luqman, S. Baicalin Mediated Regulation of Key Signaling Pathways in Cancer. Pharmacol. Res. 2021, 164, 105387. [Google Scholar] [CrossRef] [PubMed]
- Alsharairi, N.A. Scutellaria baicalensis and Their Natural Flavone Compounds as Potential Medicinal Drugs for the Treatment of Nicotine-Induced Non-Small-Cell Lung Cancer and Asthma. Int. J. Environ. Res. Public Health 2021, 18, 5243. [Google Scholar] [CrossRef]
- Liang, W.; Huang, X.; Chen, W. The Effects of Baicalin and Baicalein on Cerebral Ischemia: A Review. Aging Dis. 2017, 8, 850–867. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Cho, K.S.; Yi, I.; To, C.H.; Chen, D.F.; Do, C.W. Baicalein, Baicalin, and Wogonin: Protective Effects against Ischemia-Induced Neurodegeneration in the Brain and Retina. Oxid. Med. Cell Longev. 2021, 2021, 8377362. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Wang, Q.; Qi, J.; Yu, H.; Wang, C.; Wang, X.; Ren, Y.; Yang, F. Promoting Effect of Baicalin on Nitric Oxide Production in CMECs via Activating the PI3K-AKT-ENOS Pathway Attenuates Myocardial Ischemia–Reperfusion Injury. Phytomedicine 2019, 63, 153035. [Google Scholar] [CrossRef]
- Hu, S.; Jiang, L.; Yan, Q.; Zhou, C.; Guo, X.; Chen, T.; Ma, S.; Luo, Y.; Hu, C.; Yang, F.; et al. Evidence Construction of Baicalin for Treating Myocardial Ischemia Diseases: A Preclinical Meta-Analysis. Phytomedicine 2022, 107, 154476. [Google Scholar] [CrossRef]
- Cui, L.; Yuan, T.; Zeng, Z.; Liu, D.; Liu, C.; Guo, J.; Chen, Y. Mechanistic and Therapeutic Perspectives of Baicalin and Baicalein on Pulmonary Hypertension: A Comprehensive Review. Biomed. Pharmacother. 2022, 151, 113191. [Google Scholar] [CrossRef]
- Ding, L.; Jia, C.; Zhang, Y.; Wang, W.; Zhu, W.; Chen, Y.; Zhang, T. Baicalin Relaxes Vascular Smooth Muscle and Lowers Blood Pressure in Spontaneously Hypertensive Rats. Biomed. Pharmacother. 2019, 111, 325–330. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, Y.; Chu, J.; Wu, M.; Yan, M.; Wang, D.; Xie, Q.; Ali, F.; Fang, Y.; Wei, L.; et al. Baicalin Attenuates Angiotensin II-Induced Blood Pressure Elevation and Modulates MLCK/p-MLC Signaling Pathway. Biomed. Pharmacother. 2021, 143, 112124. [Google Scholar] [CrossRef]
- Hu, Q.; Zhang, W.; Wu, Z.; Tian, X.; Xiang, J.; Li, L.; Li, Z.; Peng, X.; Wei, S.; Ma, X.; et al. Baicalin and the Liver-Gut System: Pharmacological Bases Explaining Its Therapeutic Effects. Pharmacol. Res. 2021, 165, 105444. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, M.; Zhang, C.L.; Liu, D. Pharmacological Properties of Baicalin on Liver Diseases: A Narrative Review. Pharmacol. Rep. 2021, 73, 1230–1239. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, R.; Gupta, A.; Pandey, A.K. Role of Baicalin as a Potential Therapeutic Agent in Hepatobiliary and Gastrointestinal Disorders: A Review. J. Gastroenterol. 2022, 28, 3047–3062. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Du, J.; Cao, L.; Feng, W.; Xu, P.; Yin, G. Effects of Dietary Baicalin Supplementation on Growth Performance, Antioxidative Status and Protection against Oxidative Stress-Induced Liver Injury in GIFT Tilapia (Oreochromis Niloticus). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 240, 108914. [Google Scholar] [CrossRef]
- Li, P.; Chen, Y.; Ke, X.; Zhang, R.; Zuo, L.; Wang, M.; Chen, Z.; Luo, X.; Wang, J. Baicalin Ameliorates Alcohol-Induced Hepatic Steatosis by Suppressing SREBP1c Elicited PNPLA3 Competitive Binding to ATGL. Arch. Biochem. Biophys. 2022, 722, 109236. [Google Scholar] [CrossRef]
- Fang, P.; Yu, M.; Shi, M.; Bo, P.; Gu, X.; Zhang, Z. Baicalin and Its Aglycone: A Novel Approach for Treatment of Metabolic Disorders. Pharmacol. Rep. 2020, 72, 13–23. [Google Scholar] [CrossRef]
- Baradaran Rahimi, V.; Askari, V.R.; Hosseinzadeh, H. Promising Influences of Scutellaria baicalensis and Its Two Active Constituents, Baicalin, and Baicalein, against Metabolic Syndrome: A Review. Phytother. Res. 2021, 35, 3558–3574. [Google Scholar] [CrossRef]
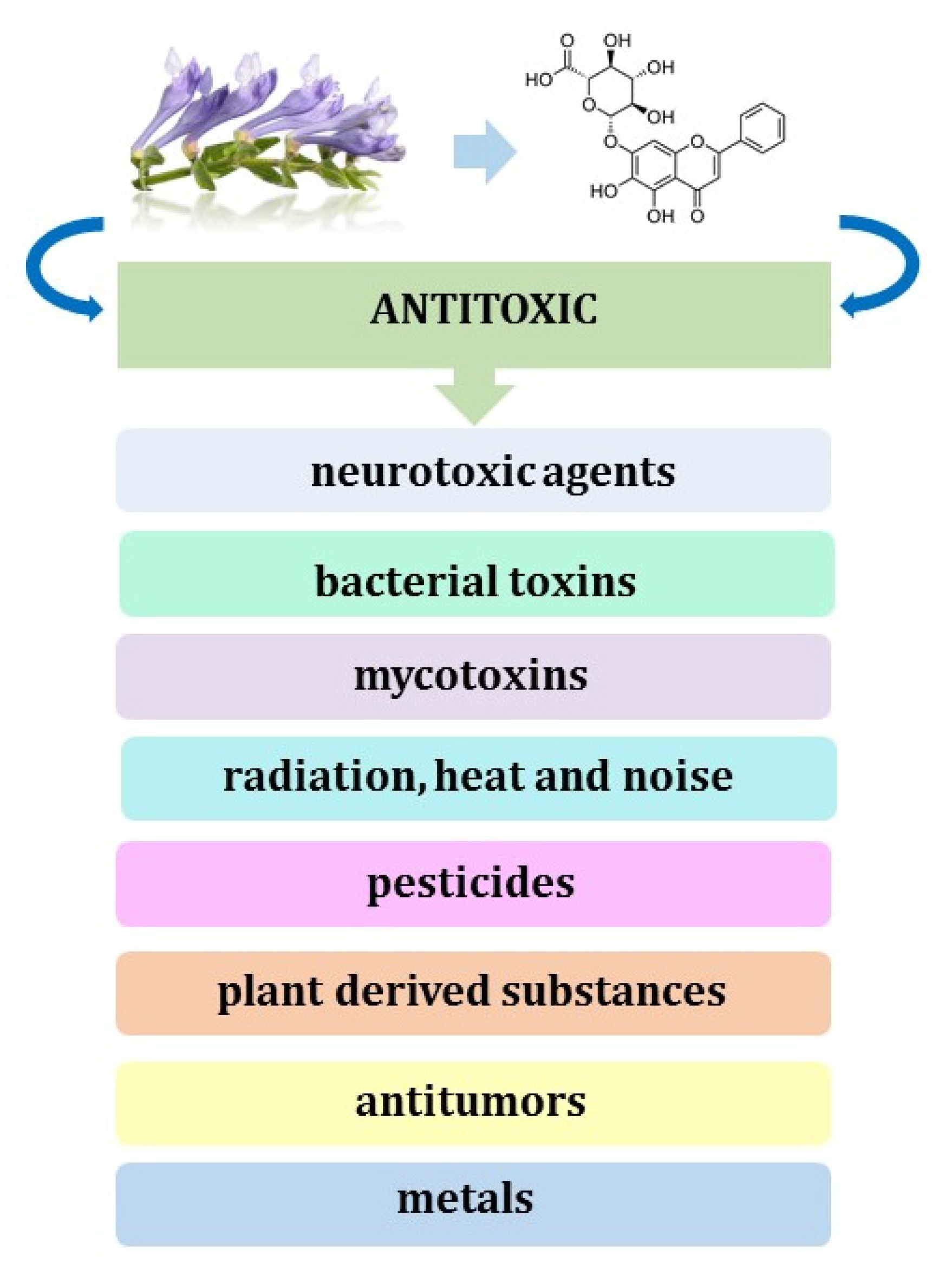
- Ahmadi, A.; Mortazavi, Z.; Mehri, S.; Hosseinzadeh, H. Protective and Therapeutic Effects of Scutellaria baicalensis and Its Main Active Ingredients Baicalin and Baicalein against Natural Toxicities and Physical Hazards: A Review of Mechanisms. DARU J. Pharm. Sci. 2022, 30, 351–366. [Google Scholar] [CrossRef]
- Ahmadi, A.; Mortazavi, Z.; Mehri, S.; Hosseinzadeh, H. Scutellaria baicalensis and Its Constituents Baicalin and Baicalein as Antidotes or Protective Agents against Chemical Toxicities: A Comprehensive Review. Naunyn Schmiedebergs Arch. Pharmacol. 2022, 395, 1297–1329. [Google Scholar] [CrossRef]
- Zha, A.; Cui, Z.; Qi, M.; Liao, S.; Yin, J.; Tan, B.; Liao, P. Baicalin-Copper Complex Modulates Gut Microbiota, Inflammatory Responses, and Hormone Secretion in Don-Challenged Piglets. Animals 2020, 10, 1535. [Google Scholar] [CrossRef]
- Zha, A.; Tu, R.; Cui, Z.; Qi, M.; Liao, S.; Wang, J.; Tan, B.; Liao, P. Baicalin–Zinc Complex Alleviates Inflammatory Responses and Hormone Profiles by Microbiome in Deoxynivalenol Induced Piglets. Front. Nutr. 2021, 8, 717. [Google Scholar] [CrossRef] [PubMed]
- Ming, J.; Zhuoneng, L.; Guangxun, Z. Protective Role of Flavonoid Baicalin from Scutellaria baicalensis in Periodontal Disease Pathogenesis: A Literature Review. Complement. Ther. Med. 2018, 38, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Kunimatsu, R.; Kimura, A.; Sakata, S.; Tsuka, Y.; Yoshimi, Y.; Abe, T.; Kado, I.; Yashima, Y.; Izumino, J.; Nakatani, A.; et al. Effects of Baicalin on the Proliferation and Expression of OPG and RANKL in Human Cementoblast-Lineage Cells. J. Dent. Sci. 2022, 17, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Kimura, A.; Kunimatsu, R.; Yoshimi, Y.; Tsuka, Y.; Awada, T.; Horie, K.; Gunji, H.; Abe, T.; Nakajima, K.; Kitagawa, M.; et al. Baicalin Promotes Osteogenic Differentiation of Human Cementoblast Lineage Cells via the Wnt/β Catenin Signaling Pathway. Curr. Pharm. Des. 2018, 24, 3980–3987. [Google Scholar] [CrossRef] [PubMed]
- Kunimatsu, R.; Kimura, A.; Tsuka, Y.; Horie, K.; Yoshimi, Y.; Awada, T.; Gunji, H.; Abe, T.; Nakajima, K.; Sakata, S.; et al. Baicalin Inhibits Root Resorption during Tooth Movement in a Rodent Model. Arch. Oral Biol. 2020, 116, 104770. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Hao, X.; Lau, B.W.-M.; Wang, S.; Li, Y. Baicalin Regulates Stem Cells as a Creative Point in the Treatment of Climacteric Syndrome. Front. Pharmacol. 2022, 13, 4638. [Google Scholar] [CrossRef]
- Zhou, R.; Han, X.; Wang, J.; Sun, J. Baicalin May Have a Therapeutic Effect in Attention Deficit Hyperactivity Disorder. Med. Hypotheses 2015, 85, 761–764. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhong, L.; Peng, J.; Yuan, Q.; Xu, A. The Therapeutic Effects of Baicalin on Vitiligo Mice. Biol. Pharm. Bull 2019, 42, 1450–1455. [Google Scholar] [CrossRef]
- Zhang, J.A.; Luan, C.; Huang, D.; Ju, M.; Chen, K.; Gu, H. Induction of Autophagy by Baicalin through the AMPK-MTOR Pathway Protects Human Skin Fibroblasts from Ultraviolet B Radiation-Induced Apoptosis. Drug Des. Dev. Ther. 2020, 14, 417–428. [Google Scholar] [CrossRef]
- Zheng, G.; Gan, L.; Jia, L.Y.; Zhou, D.C.; Bi, S.; Meng, Z.Q.; Guan, G.J.; Huang, M.M.; He, X.; Zhang, C.F.; et al. Screen of Anti-Migraine Active Compounds from Duijinsan by Spectrum-Effect Relationship Analysis and Molecular Docking. J. Ethnopharmacol. 2021, 279, 114352. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, Q.; Zhang, L. Baicalin Protects against Renal Interstitial Fibrosis in Mice by Inhibiting the TGF-β/Smad Signalling Pathway. Pharm. Biol. 2022, 60, 1407–1416. [Google Scholar] [CrossRef] [PubMed]
- Lai, N.; Fu, X.; Hei, G.; Song, W.; Wei, R.; Zhu, X.; Guo, Q.; Zhang, Z.; Chu, C.; Xu, K.; et al. The Role of Dendritic Cell Subsets in Recurrent Spontaneous Abortion and the Regulatory Effect of Baicalin on It. J. Immunol. Res. 2022, 2022, 9693064. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Fan, B.; Gu, H.; Yang, L.; Li, X. Effects of Baicalin on Alopecia and the Associated Mechanism. Biomed. Res. Int. 2022, 2022, 3139123. [Google Scholar] [CrossRef]
- Xu, G.; Dou, J.; Zhang, L.; Guo, Q.; Zhou, C. Inhibitory effects of baicalein on the influenza virus in vivo is determined by baicalein in the serum. Biol. Pharm. Bull. 2010, 33, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Nayak, M.K.; Agrawal, A.S.; Bose, S.; Naskar, S.; Bhowmick, R.; Chakrabarti, S.; Sarkar, S.; Chawla-Sarkar, M. Antiviral activity of baicalin against influenza virus H1N1-pdm09 is due to modulation of NS1-mediated cellular innate immune responses. J. Antimicrob. Chemother. 2014, 69, 1298–1310. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Dong, Y.; Shan, X.; Li, L.; Xia, B.; Wang, H. Anti-depressive effectiveness of baicalin in vitro and in vivo. Molecules 2019, 24, 326. [Google Scholar] [CrossRef]
- Lin, C.C.; Shieh, D.E. In vivo hepatoprotective effect of baicalein, baicalin and wogonin from Scutellaria rivularis. Phytother. Res. 1996, 10, 651–654. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, X.; Zhao, L.; Jiao, Y.; Liu, J.; Zhai, G. In vitro and in vivo study of baicalin-loaded mixed micelles for oral delivery. Drug Deliv. 2016, 23, 1933–1939. [Google Scholar]
- Hu, L.; Li, L.; Yan, C.; Cao, Y.; Duan, X.; Sun, J. Baicalin inhibits airway smooth muscle cells proliferation through the RAS signaling pathway in murine asthmatic airway remodeling model. Oxid. Med. Cell. Longev. 2023, 2023, 4144138. [Google Scholar] [CrossRef]
- Yanxia, G.; Jingbo, L.; Zhili, H.; Na, S.; Jianhua, G.; Xiaozhong, Z.; Panpan, S.; Wei, Y.; Kuohai, F.; Hongquan, L. Baicalin ameliorates high fat diet-induced nonalcoholic fatty liver disease in mice via adenosine monophosphate-activated protein kinase-mediated regulation of SREBP1/Nrf2/NF-κB signaling pathway. Phytother. Res. 2023, 1–14. [Google Scholar] [CrossRef]
- Kim, E.; Ham, S.; Jung, B.K.; Park, J.W.; Kim, J.; Lee, J.H. Effect of baicalin on wound healing in a mouse model of pressure ulcers. Int. J. Mol. Sci. 2023, 24, 329. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Cheng, Y.T.; Lu, Y.; Sun, G.Q.; Pei, L. Baicalin ameliorates corticosterone-induced depression by promoting neurodevelopment of hippocampal via mTOR/GSK3 β pathway. Chin. J. Integr. Med. 2023. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Li, C.; Jin, X.; Zhu, L.; Shen, J.; Bai, M.; Li, Y.; Xu, E. Baicalin improves the energy levels in the prefrontal cortex of mice exposed to chronic unpredictable mild stress. Heliyon 2023, 8, e12083. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Cong, X.; Yu, W.; Jiang, Z.; Fu, K.; Cao, R.; Tian, W.; Feng, Y. Baicalin inhibits oxidative injures of mouse uterine tissue induced by acute heat stress through activating the Keap1/Nrf2 signalling pathway. Res. Vet. Sci. 2023, 152, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Pisano, M.; Xu, L.; Sun, F.; Xu, J.; Zheng, W.; Liu, X.; Zhang, Y.; Sun, R.; Cui, X. Baicalin regulates autophagy to onterfere with small intestinal acute graft-versus-host disease. Sci. Rep. 2023, 12, 6551. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bajek-Bil, A.; Chmiel, M.; Włoch, A.; Stompor-Gorący, M. Baicalin—Current Trends in Detection Methods and Health-Promoting Properties. Pharmaceuticals 2023, 16, 570. https://doi.org/10.3390/ph16040570
Bajek-Bil A, Chmiel M, Włoch A, Stompor-Gorący M. Baicalin—Current Trends in Detection Methods and Health-Promoting Properties. Pharmaceuticals. 2023; 16(4):570. https://doi.org/10.3390/ph16040570
Chicago/Turabian StyleBajek-Bil, Agata, Marcelina Chmiel, Aleksandra Włoch, and Monika Stompor-Gorący. 2023. "Baicalin—Current Trends in Detection Methods and Health-Promoting Properties" Pharmaceuticals 16, no. 4: 570. https://doi.org/10.3390/ph16040570
APA StyleBajek-Bil, A., Chmiel, M., Włoch, A., & Stompor-Gorący, M. (2023). Baicalin—Current Trends in Detection Methods and Health-Promoting Properties. Pharmaceuticals, 16(4), 570. https://doi.org/10.3390/ph16040570







