Conductance Changes of Na+ Channels during the Late Na+ Current Flowing under Action Potential Voltage Clamp Conditions in Canine, Rabbit, and Guinea Pig Ventricular Myocytes
Abstract
1. Introduction
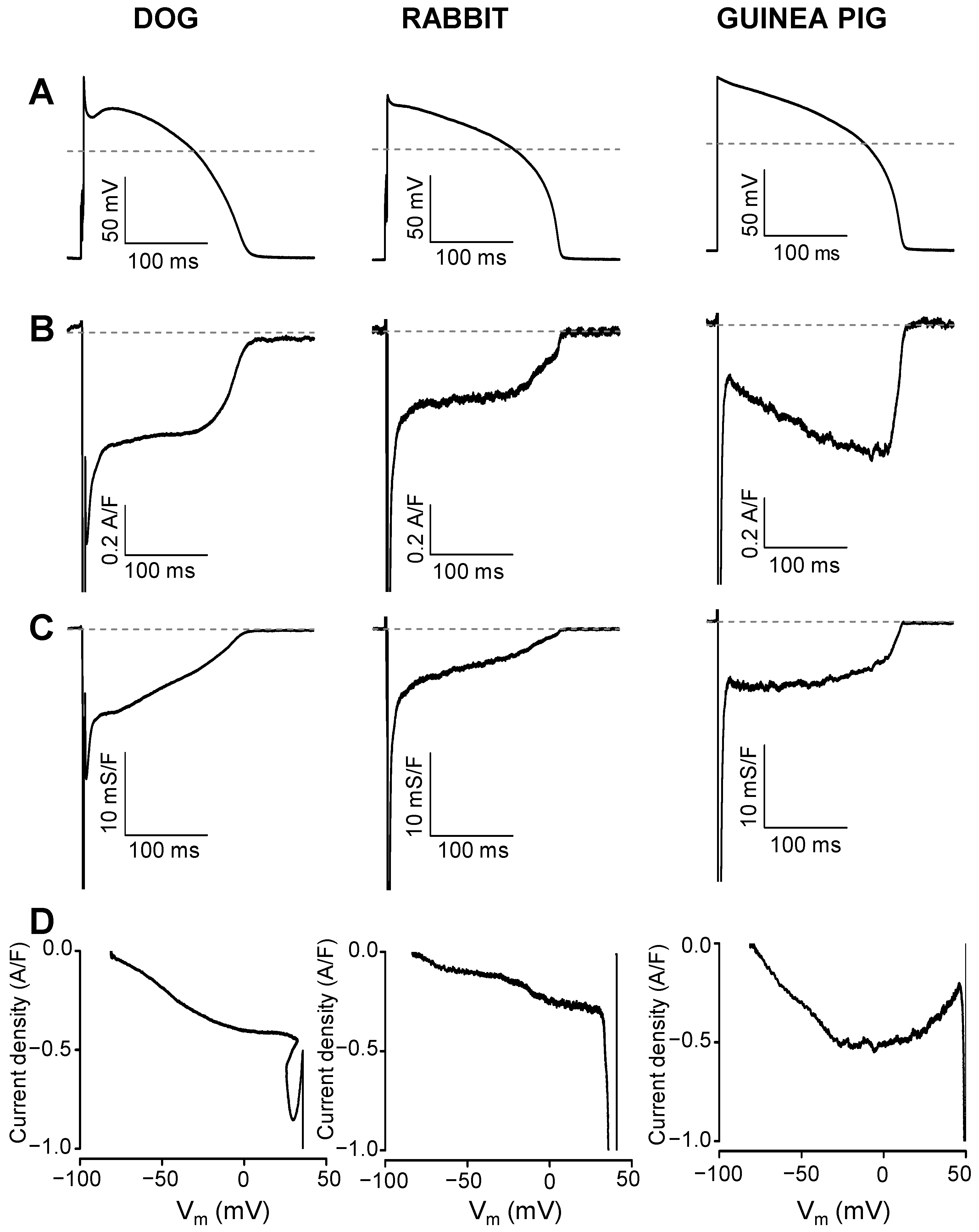
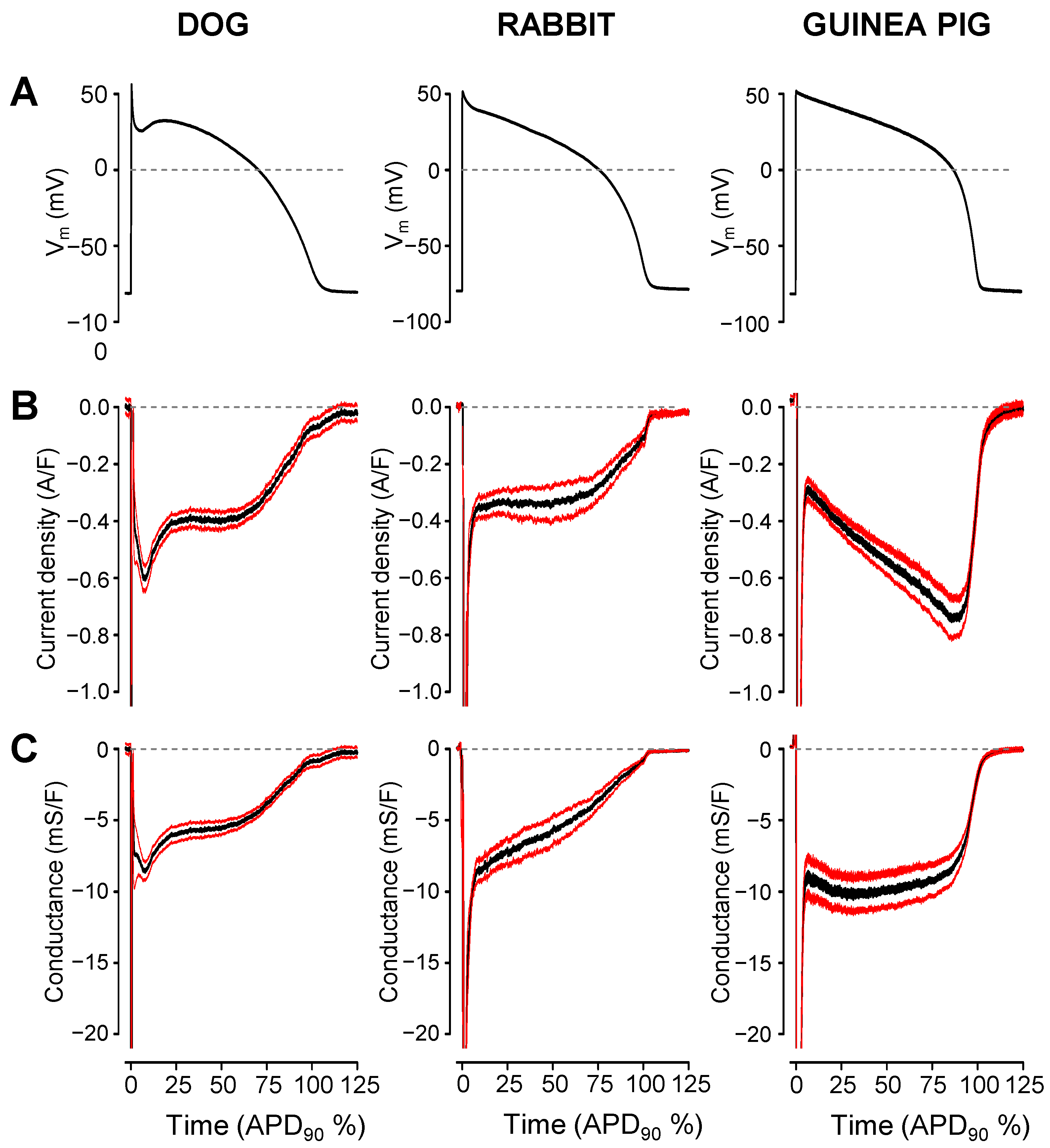
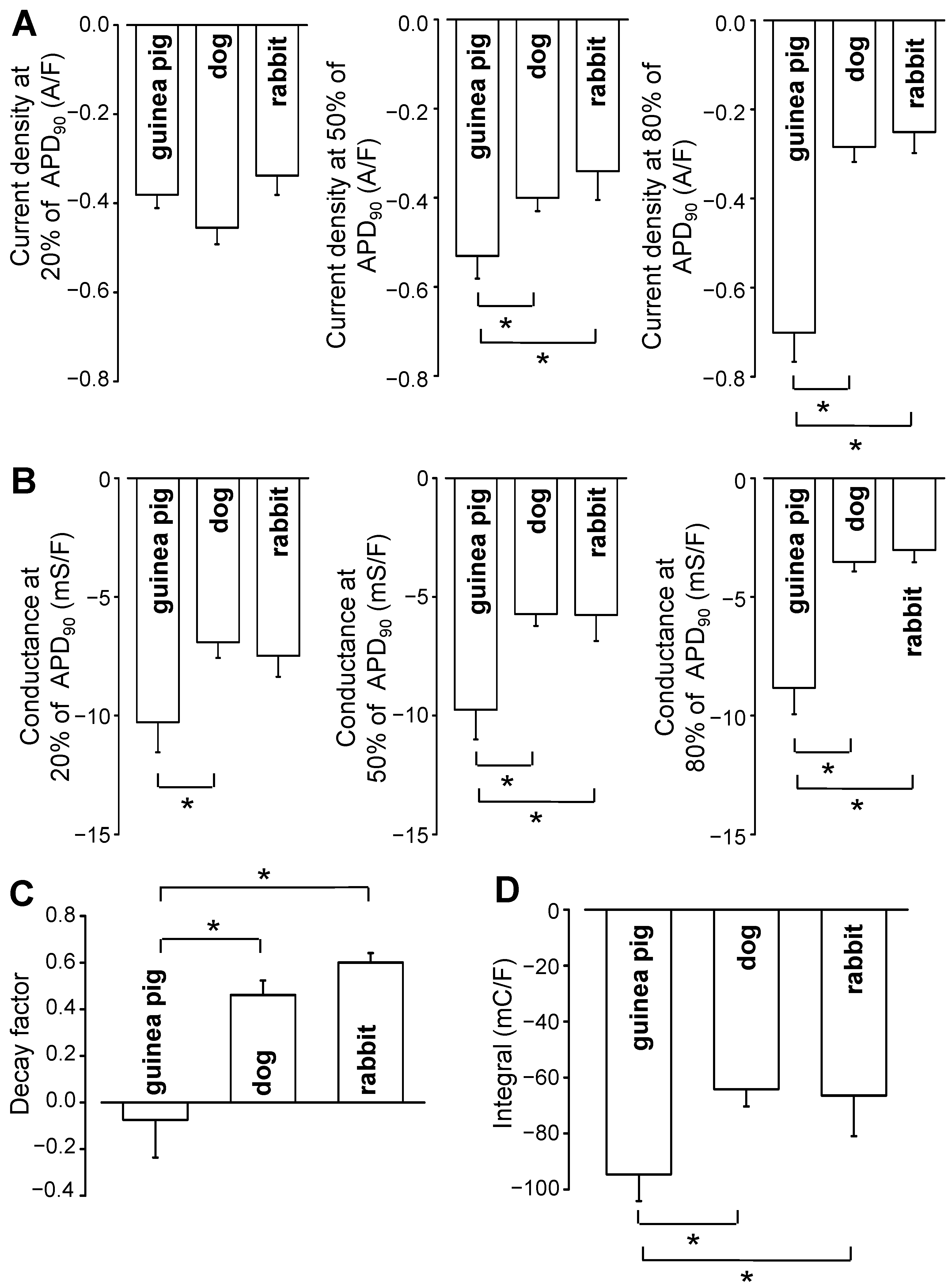
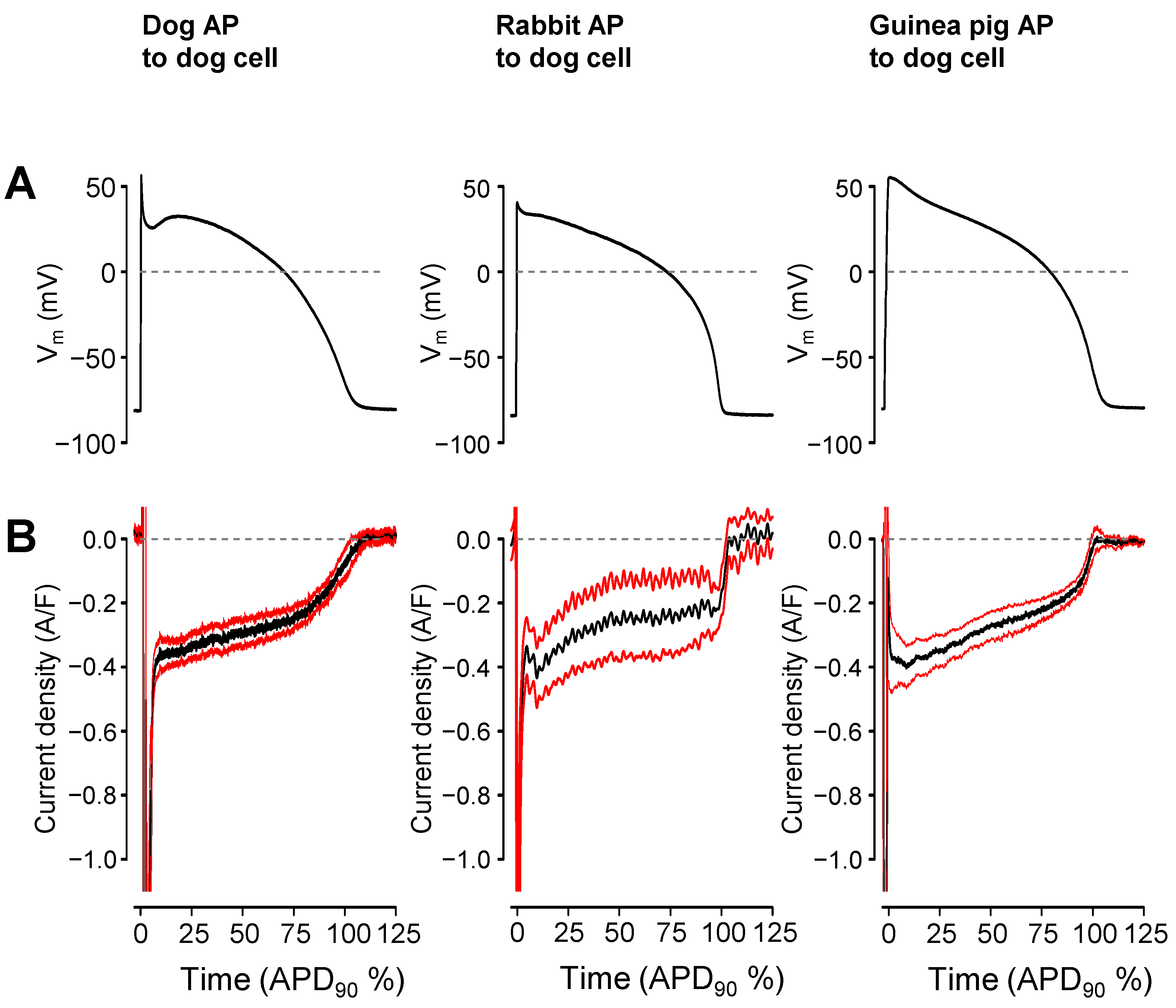
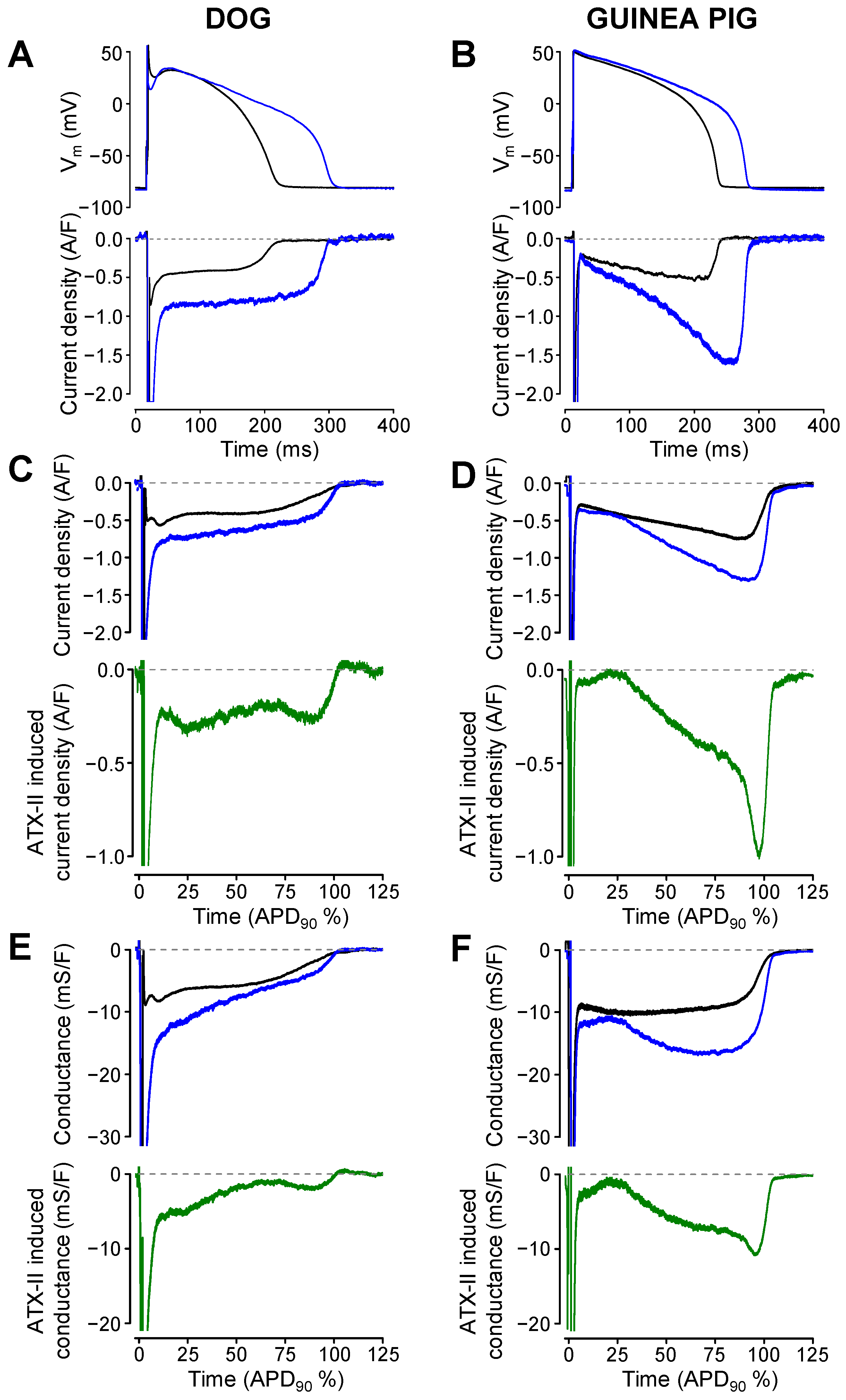
2. Results
3. Discussion
4. Methods
4.1. Animals
4.2. Isolation of Cardiomyocytes
4.3. Electrophysiology
4.4. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Coraboeuf, E.; Deroubaix, E.; Coulombe, A. Effect of tetrodotoxin on action potentials of the conducting system in the dog heart. Am. J. Physiol. Circ. Physiol. 1979, 236, H561–H567. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, E. Slow inactivation of sodium current and voltage-dependent block by tetrodotoxin in rabbit cardiac Purkinje fibers. Biomed. Biochim. Acta 1986, 45, S163–S166. [Google Scholar] [PubMed]
- Carmeliet, E. Voltage-Dependent block by tetrodotoxin of the sodium channel in rabbit cardiac Purkinje fibers. Biophys. J. 1987, 51, 109–114. [Google Scholar] [CrossRef]
- Chadda, K.R.; Jeevaratnam, K.; Lei, M.; Huang, C.L. Sodium channel biophysics, late sodium current and genetic arrhythmic syndromes. Pflug. Arch. Eur. J. Physiol. 2017, 469, 629–641. [Google Scholar] [CrossRef] [PubMed]
- Horváth, B.; Hézső, T.; Kiss, D.; Kistamas, K.; Magyar, J.; Nánási, P.P.; Bányász, T. Late Sodium Current Inhibitors as Potential Antiarrhythmic Agents. Front. Pharmacol. 2020, 11, 413. [Google Scholar] [CrossRef]
- Undrovinas, A.I.; Maltsev, V.; Kyle, J.W.; Silverman, N.; Sabbah, H.N. Gating of the Late Na+ Channel in Normal and Failing Human Myocardium. J. Mol. Cell Cardiol. 2002, 34, 1477–1489. [Google Scholar] [CrossRef]
- Valdivia, C.R.; Chu, W.W.; Pu, J.; Foell, J.D.; Haworth, R.A.; Wolff, M.R.; Kamp, T.; Makielski, J.C. Increased late sodium current in myocytes from a canine heart failure model and from failing human heart. J. Mol. Cell Cardiol. 2005, 38, 475–483. [Google Scholar] [CrossRef]
- Maltsev, V.; Silverman, N.; Sabbah, H.N.; Undrovinas, A.I. Chronic heart failure slows late sodium current in human and canine ventricular myocytes: Implications for repolarization variability. Eur. J. Hearth Fail. 2007, 9, 219–227. [Google Scholar] [CrossRef]
- Song, Y.; Belardinelli, L. Basal late sodium current is a significant contributor to the duration of action potential of guinea pig ventricular myocytes. Physiol. Rep. 2017, 5, e13295. [Google Scholar] [CrossRef]
- Maltsev, V.; Undrovinas, A.I. A multi-modal composition of the late Na+ current in human ventricular cardiomyocytes. Cardiovasc. Res. 2006, 69, 116–127. [Google Scholar] [CrossRef]
- Noble, D.; Noble, P.J. Late sodium current in the pathophysiology of cardiovascular disease: Consequences of sodium-calcium overload. Heart 2006, 92 (Suppl. 4), iv1–iv5. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Li, G.; Huang, C.L.-H.; Lei, M.; Wu, L. Late sodium current associated cardiac electrophysiological and mechanical dysfunction. Pflügers Arch. Eur. J. Physiol. 2018, 470, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Horváth, B.; Szentandrássy, N.; Almássy, J.; Dienes, C.; Kovács, Z.M.; Nánási, P.P.; Banyasz, T. Late Sodium Current of the Heart: Where Do We Stand and Where Are We Going? Pharmaceuticals 2022, 15, 231. [Google Scholar] [CrossRef]
- Ton, A.T.; Nguyen, W.; Sweat, K.; Miron, Y.; Hernandez, E.; Wong, T.; Geft, V.; Macias, A.; Espinoza, A.; Truong, K.; et al. Arrhythmogenic and antiarrhythmic actions of late sustained sodium current in the adult human heart. Sci. Rep. 2021, 11, 12014. [Google Scholar] [CrossRef]
- Qi, D.; Yang, Z.; Robinson, V.M.; Li, J.; Gao, C.; Guo, D.; Kowey, P.R.; Yan, G.-X. Heterogeneous distribution of INa-L determines interregional differences in rate adaptation of repolarization. Heart Rhythm. 2015, 12, 1295–1303. [Google Scholar] [CrossRef]
- Kaplan, A.; Amin, G.; Abidi, E.; Altara, R.; Booz, G.W.; Zouein, F.A. Role of ranolazine in heart failure: From cellular to clinic perspective. Eur. J. Pharmacol. 2022, 919, 174787. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Zhang, Z.; Fragakis, N.; Korantzopoulos, P.; Letsas, K.P.; Li, G.; Yan, G.-X.; Liu, T. Role of ranolazine in the prevention and treatment of atrial fibrillation: A meta-analysis of randomized clinical trials. Heart Rhythm. 2017, 14, 3–11. [Google Scholar] [CrossRef]
- Rasalingam, R.; Boden, W.E. Role of Ranolazine in Reducing Angina, Subsequent Revascularization, and Healthcare Expenditures in Stable Ischemic Heart Disease. Am. J. Cardiol. 2019, 123, 1729–1731. [Google Scholar] [CrossRef]
- Mehta, P.K.; Sharma, S.; Minissian, M.; Harsch, M.R.; Martinson, M.; Nyman, J.A.; Shaw, L.J.; Merz, C.N.B.; Wenger, N.K. Ranolazine Reduces Angina in Women with Ischemic Heart Disease: Results of an Open-Label, Multicenter Trial. J. Womens Health 2019, 28, 573–582. [Google Scholar] [CrossRef]
- Cattaneo, M.; Porretta, A.P.; Gallino, A. Ranolazine: Drug overview and possible role in primary microvascular angina management. Int. J. Cardiol. 2015, 181, 376–381. [Google Scholar] [CrossRef]
- Rayner-Hartley, E.; Sedlak, T. Ranolazine: A Contemporary Review. J. Am. Heart Assoc. 2016, 5, e003196. [Google Scholar] [CrossRef] [PubMed]
- Attwell, D.; Cohen, I.; Eisner, D.; Ohba, M.; Ojeda, C. The steady state TTX-sensitive (“window”) sodium current in cardiac Purkinje fibres. Pflügers Arch. 1979, 379, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Kistamás, K.; Hézső, T.; Horváth, B.; Nánási, P.P. Late sodium current and calcium homeostasis in arrhythmogenesis. Channels 2021, 15, 1–19. [Google Scholar] [CrossRef]
- Belardinelli, L.; Liu, G.; Smith-Maxwell, C.; Wang, W.-Q.; El-Bizri, N.; Hirakawa, R.; Karpinski, S.; Li, C.H.; Hu, L.; Li, X.-J.; et al. A Novel, Potent, and Selective Inhibitor of Cardiac Late Sodium Current Suppresses Experimental Arrhythmias. Experiment 2013, 344, 23–32. [Google Scholar] [CrossRef]
- Horvath, B.; Banyasz, T.; Jian, Z.; Hegyi, B.; Kistamas, K.; Nanasi, P.P.; Izu, L.T.; Chen-Izu, Y. Dynamics of the late Na+ current during cardiac action potential and its contribution to afterdepolarizations. J. Mol. Cell Cardiol. 2013, 64, 59–68. [Google Scholar] [CrossRef]
- Horvath, B.; Hezso, T.; Szentandrassy, N.; Kistamas, K.; Arpadffy-Lovas, T.; Varga, R.; Gazdag, P.; Veress, R.; Dienes, C.; Baranyai, D.; et al. Late sodium current in human, canine and guinea pig ventricular myocardium. J. Mol. Cell. Cardiol. 2020, 139, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Hegyi, B.; Bossuyt, J.; Griffiths, L.G.; Shimkunas, R.; Coulibaly, Z.; Jian, Z.; Grimsrud, K.N.; Sondergaard, C.S.; Ginsburg, K.S.; Chiamvimonvat, N.; et al. Complex electrophysiological remodeling in postinfarction ischemic heart failure. Proc. Natl. Acad. Sci. USA 2018, 115, E3036–E3044. [Google Scholar] [CrossRef] [PubMed]
- Zygmunt, A.C.; Eddlestone, G.T.; Thomas, G.P.; Nesterenko, V.V.; Antzelevitch, C. Larger late sodium conductance in M cells contributes to electrical heterogeneity in canine ventricle. Am. J. Physiol. Circ. Physiol. 2001, 281, H689–H697. [Google Scholar] [CrossRef]
- Murphy, L.; Renodin, D.; Antzelevitch, C.; Di Diego, J.M.; Cordeiro, J.M. Extracellular proton depression of peak and late Na+ current in the canine left ventricle. Am. J. Physiol. Circ. Physiol. 2011, 301, H936–H944. [Google Scholar] [CrossRef]
- Kiss, D.; Horvath, B.; Hezso, T.; Dienes, C.; Kovacs, Z.; Topal, L.; Szentandrassy, N.; Almassy, J.; Prorok, J.; Virag, L.; et al. Late Na+ Current Is [Ca2+]i-Dependent in Canine Ventricular Myocytes. Pharmaceuticals 2021, 14, 1142. [Google Scholar] [CrossRef]
- Maltsev, V.; Sabbah, H.N.; Higgins, R.S.D.; Silverman, N.; Lesch, M.; Undrovinas, A.I. Novel, Ultraslow Inactivating Sodium Current in Human Ventricular Cardiomyocytes. Circulation 1998, 98, 2545–2552. [Google Scholar] [CrossRef]
- Poulet, C.; Wettwer, E.; Grunnet, M.; Jespersen, T.; Fabritz, L.; Matschke, K.; Knaut, M.; Ravens, U. Late Sodium Current in Human Atrial Cardiomyocytes from Patients in Sinus Rhythm and Atrial Fibrillation. PLoS ONE 2015, 10, e0131432. [Google Scholar] [CrossRef]
- Hegyi, B.; Banyasz, T.; Izu, L.T.; Belardinelli, L.; Bers, D.M.; Chen-Izu, Y. beta-adrenergic regulation of late Na+ current during cardiac action potential is mediated by both PKA and CaMKII. J. Mol. Cell. Cardiol. 2018, 123, 168–179. [Google Scholar] [CrossRef]
- Bányász, T.; Fülöp, L.; Magyar, J.; Szentandrássy, N.; Varró, A.; Nánási, P.P. Endocardial versus epicardial differences in L-type calcium current in canine ventricular myocytes studied by action potential voltage clamp. Cardiovasc. Res. 2003, 58, 66–75. [Google Scholar] [CrossRef]
- Bányász, T.; Magyar, J.; Szentandrássy, N.; Horváth, B.; Birinyi, P.; Szentmiklósi, J.; Nánási, P.P. Action potential clamp fingerprints of K+ currents in canine cardiomyocytes: Their role in ventricular repolarization. Acta Physiol. 2007, 190, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Sala, L.; Hegyi, B.; Bartolucci, C.; Altomare, C.; Rocchetti, M.; Vaczi, K.; Mostacciuolo, G.; Szentandrassy, N.; Severi, S.; Nanasi, P.P.; et al. Action potential contour contributes to species differences in repolarization response to beta-adrenergic stimulation. Europace 2018, 20, 1543–1552. [Google Scholar] [CrossRef] [PubMed]
- Catterall, W.A. Neurotoxins that Act on Voltage-Sensitive Sodium Channels in Excitable Membranes. Annu. Rev. Pharmacol. Toxicol. 1980, 20, 15–43. [Google Scholar] [CrossRef] [PubMed]
- Monastyrnaya, M.M.; Kalina, R.S.; Kozlovskaya, E.P. The Sea Anemone Neurotoxins Modulating Sodium Channels: An Insight at Structure and Functional Activity after Four Decades of Investigation. Toxins 2022, 15, 8. [Google Scholar] [CrossRef]
- Bielen, F.V.; Glitsch, H.G.; Verdonck, F. Changes of the subsarcolemmal Na+ concentration in internally perfused cardiac cells. Biochim. Biophys. Acta (BBA) Biomembr. 1991, 1065, 269–271. [Google Scholar] [CrossRef]
- Fujioka, Y.; Matsuoka, S.; Ban, T.; Noma, A. Interaction of the Na+-K+ pump and Na+-Ca2+ exchange via [Na+]i in a restricted space of guinea-pig ventricular cells. J. Physiol. 1998, 509 Pt 2, 457–470. [Google Scholar] [CrossRef]
- Wendt-Gallitelli, M.F.; Voigt, T.; Isenberg, G. Microheterogeneity of subsarcolemmal sodium gradients. Electron probe microanalysis in guinea-pig ventricular myocytes. J. Physiol. 1993, 472, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Boyett, M.R.; Hart, G.; Levi, A.J.; Roberts, A. Effects of repetitive activity on developed force and intracellular sodium in isolated sheep and dog Purkinje fibres. J. Physiol. 1987, 388, 295–322. [Google Scholar] [CrossRef] [PubMed]
- Verdonck, F.; Volders, P.G.; Vos, M.A.; Sipido, K.R. Increased Na+ concentration and altered Na/K pump activity in hypertrophied canine ventricular cells. Cardiovasc. Res. 2003, 57, 1035–1043. [Google Scholar] [CrossRef] [PubMed]
- Clancy, C.E.; Tateyama, M.; Liu, H.; Wehrens, X.H.; Kass, R.S. Non-Equilibrium gating in cardiac Na+ channels: An original mechanism of arrhythmia. Circulation 2003, 107, 2233–2237. [Google Scholar] [CrossRef]
- Biet, M.; Barajas-Martínez, H.; Ton, A.-T.; Delabre, J.-F.; Morin, N.; Dumaine, R. About half of the late sodium current in cardiac myocytes from dog ventricle is due to non-cardiac-type Na+ channels. J. Mol. Cell Cardiol. 2012, 53, 593–598. [Google Scholar] [CrossRef]
- Ahmad, S.; Tirilomis, P.; Pabel, S.; Dybkova, N.; Hartmann, N.; Molina, C.E.; Tirilomis, T.; Kutschka, I.; Frey, N.; Maier, L.S.; et al. The functional consequences of sodium channel NaV 1.8 in human left ventricular hypertrophy. ESC Heart Fail. 2019, 6, 154–163. [Google Scholar] [CrossRef]
- Hegyi, B.; Horváth, B.; Váczi, K.; Gönczi, M.; Kistamás, K.; Ruzsnavszky, F.; Veress, R.; Izu, L.T.; Chen-Izu, Y.; Bányász, T.; et al. Ca2+-activated Cl− current is antiarrhythmic by reducing both spatial and temporal heterogeneity of cardiac repolarization. J. Mol. Cell Cardiol. 2017, 109, 27–37. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horváth, B.; Kovács, Z.M.; Dienes, C.; Óvári, J.; Szentandrássy, N.; Magyar, J.; Bányász, T.; Varró, A.; Nánási, P.P. Conductance Changes of Na+ Channels during the Late Na+ Current Flowing under Action Potential Voltage Clamp Conditions in Canine, Rabbit, and Guinea Pig Ventricular Myocytes. Pharmaceuticals 2023, 16, 560. https://doi.org/10.3390/ph16040560
Horváth B, Kovács ZM, Dienes C, Óvári J, Szentandrássy N, Magyar J, Bányász T, Varró A, Nánási PP. Conductance Changes of Na+ Channels during the Late Na+ Current Flowing under Action Potential Voltage Clamp Conditions in Canine, Rabbit, and Guinea Pig Ventricular Myocytes. Pharmaceuticals. 2023; 16(4):560. https://doi.org/10.3390/ph16040560
Chicago/Turabian StyleHorváth, Balázs, Zsigmond M. Kovács, Csaba Dienes, József Óvári, Norbert Szentandrássy, János Magyar, Tamás Bányász, András Varró, and Péter P. Nánási. 2023. "Conductance Changes of Na+ Channels during the Late Na+ Current Flowing under Action Potential Voltage Clamp Conditions in Canine, Rabbit, and Guinea Pig Ventricular Myocytes" Pharmaceuticals 16, no. 4: 560. https://doi.org/10.3390/ph16040560
APA StyleHorváth, B., Kovács, Z. M., Dienes, C., Óvári, J., Szentandrássy, N., Magyar, J., Bányász, T., Varró, A., & Nánási, P. P. (2023). Conductance Changes of Na+ Channels during the Late Na+ Current Flowing under Action Potential Voltage Clamp Conditions in Canine, Rabbit, and Guinea Pig Ventricular Myocytes. Pharmaceuticals, 16(4), 560. https://doi.org/10.3390/ph16040560





