Epigenetic Targets and Their Inhibitors in Thyroid Cancer Treatment
Abstract
1. Introduction
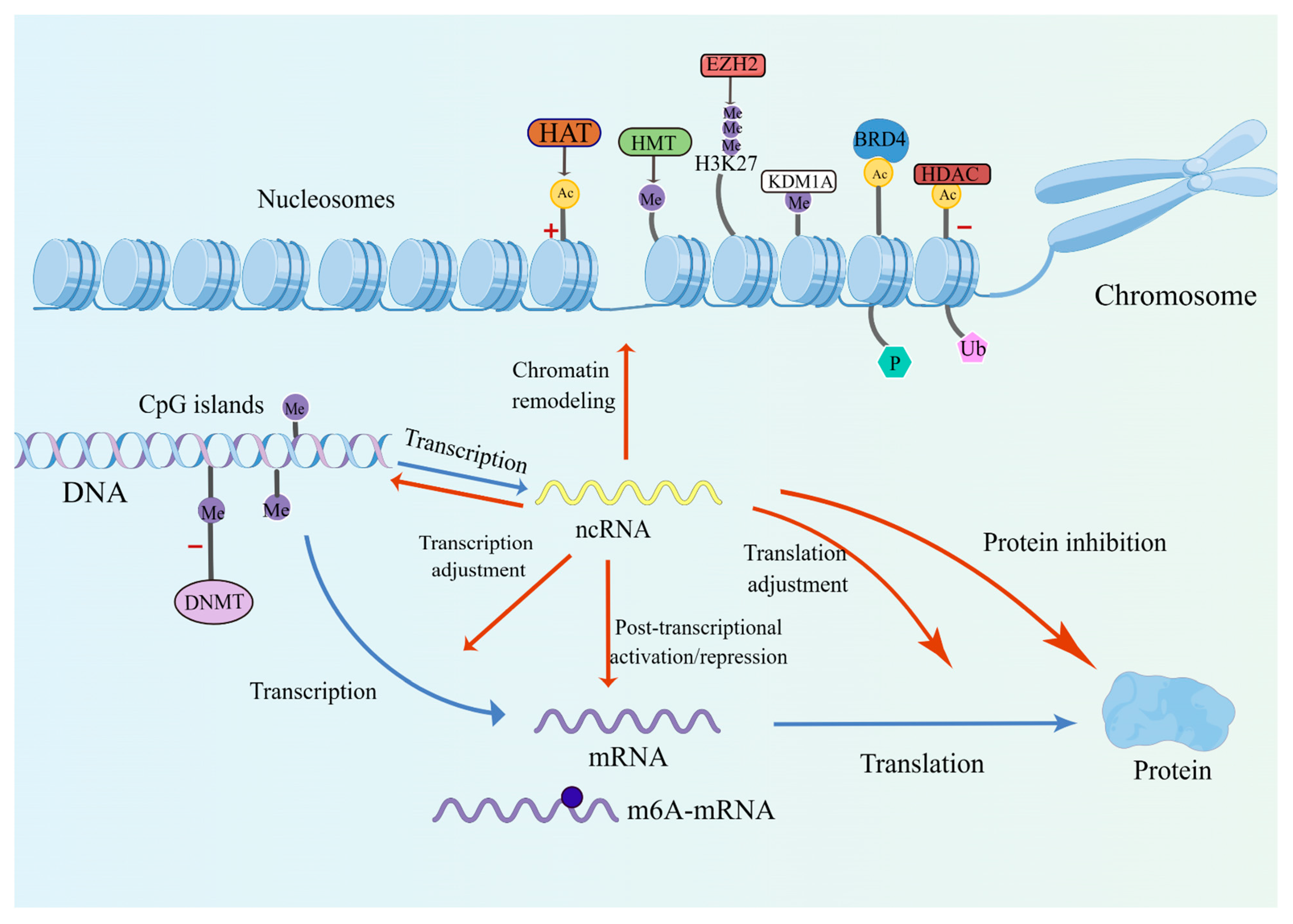
2. Epigenetic Modifications in Thyroid Cancer
2.1. DNA Methylation
2.2. Histone Modifications
2.3. Non-Coding RNAs
2.4. Chromatin Remodeling
2.5. RNA Alterations
3. Epigenetic Inhibitors in Thyroid Cancer
3.1. DNA Methyltransferase Inhibitors
3.2. Histone Deacetylase Inhibitors
3.3. Others
3.4. Combined Therapies
4. Conclusions and Future Direction
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Baloch, Z.W.; Asa, S.L.; Barletta, J.A.; Ghossein, R.A.; Juhlin, C.C.; Jung, C.K.; LiVolsi, V.A.; Papotti, M.G.; Sobrinho-Simoes, M.; Tallini, G.; et al. Overview of the 2022 WHO Classification of Thyroid Neoplasms. Endocr. Pathol. 2022, 33, 27–63. [Google Scholar] [CrossRef]
- Catalano, M.G.; Fortunati, N.; Boccuzzi, G. Epigenetics modifications and therapeutic prospects in human thyroid cancer. Front. Endocrinol. 2012, 3, 40. [Google Scholar] [CrossRef] [PubMed]
- Kurata, K.; Onoda, N.; Noda, S.; Kashiwagi, S.; Asano, Y.; Hirakawa, K.; Ohira, M. Growth arrest by activated BRAF and MEK inhibition in human anaplastic thyroid cancer cells. Int. J. Oncol. 2016, 49, 2303–2308. [Google Scholar] [CrossRef]
- Martelli, M.P.; Martino, G.; Cardinali, V.; Falini, B.; Martinelli, G.; Cerchione, C. Enasidenib and ivosidenib in AML. Minerva Med. 2020, 111, 411–426. [Google Scholar] [CrossRef]
- Julia, E.; Salles, G. EZH2 inhibition by tazemetostat: Mechanisms of action, safety and efficacy in relapsed/refractory follicular lymphoma. Future Oncol. 2021, 17, 2127–2140. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Li, C.; Liu, J.; Tang, X.; Li, Z. DNA methylation alterations as therapeutic prospects in thyroid cancer. J. Endocrinol. Investig. 2019, 42, 363–370. [Google Scholar] [CrossRef]
- Zafon, C.; Gil, J.; Perez-Gonzalez, B.; Jorda, M. DNA methylation in thyroid cancer. Endocr. Relat. Cancer 2019, 26, R415–R439. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Rodero, S.; Fernandez, A.F.; Fernandez-Morera, J.L.; Castro-Santos, P.; Bayon, G.F.; Ferrero, C.; Urdinguio, R.G.; Gonzalez-Marquez, R.; Suarez, C.; Fernandez-Vega, I.; et al. DNA methylation signatures identify biologically distinct thyroid cancer subtypes. J. Clin. Endocrinol. Metab. 2013, 98, 2811–2821. [Google Scholar] [CrossRef]
- Broekhuis, J.M.; James, B.C.; Cummings, R.D.; Hasselgren, P.O. Posttranslational Modifications in Thyroid Cancer: Implications for Pathogenesis, Diagnosis, Classification, and Treatment. Cancers 2022, 14, 1610. [Google Scholar] [CrossRef]
- Bates, S.E. Epigenetic Therapies for Cancer. N. Engl. J. Med. 2020, 383, 650–663. [Google Scholar] [CrossRef]
- Oh, J.M.; Ahn, B.C. Molecular mechanisms of radioactive iodine refractoriness in differentiated thyroid cancer: Impaired sodium iodide symporter (NIS) expression owing to altered signaling pathway activity and intracellular localization of NIS. Theranostics 2021, 11, 6251–6277. [Google Scholar] [CrossRef]
- Spartalis, E.; Athanasiadis, D.I.; Chrysikos, D.; Spartalis, M.; Boutzios, G.; Schizas, D.; Garmpis, N.; Damaskos, C.; Paschou, S.A.; Ioannidis, A.; et al. Histone Deacetylase Inhibitors and Anaplastic Thyroid Carcinoma. Anticancer Res. 2019, 39, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Puppin, C.; Passon, N.; Lavarone, E.; Di Loreto, C.; Frasca, F.; Vella, V.; Vigneri, R.; Damante, G. Levels of histone acetylation in thyroid tumors. Biochem. Biophys. Res. Commun. 2011, 411, 679–683. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Nakazawa, T.; Ma, D.; Niu, D.; Mochizuki, K.; Kawasaki, T.; Nakamura, N.; Yamane, T.; Kobayashi, M.; Katoh, R. Epigenetic silencing of TTF-1/NKX2-1 through DNA hypermethylation and histone H3 modulation in thyroid carcinomas. Lab. Investig. 2009, 89, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Wu, X.; Zhang, X.; Hua, W.; Zhang, Y.; Maimaiti, Y.; Gao, Z.; Zhang, Y. Inhibition of BRD4 suppresses tumor growth and enhances iodine uptake in thyroid cancer. Biochem. Biophys. Res. Commun. 2016, 469, 679–685. [Google Scholar] [CrossRef]
- Zhang, X.; Wen, H.; Shi, X. Lysine methylation: Beyond histones. Acta Biochim. Biophys. Sin. 2012, 44, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Sun, W.; Qin, Y.; Wu, C.; He, L.; Zhang, T.; Shao, L.; Zhang, H.; Zhang, P. Knockdown of KDM1A suppresses tumour migration and invasion by epigenetically regulating the TIMP1/MMP9 pathway in papillary thyroid cancer. J. Cell. Mol. Med. 2019, 23, 4933–4944. [Google Scholar] [CrossRef] [PubMed]
- Sasanakietkul, T.; Murtha, T.D.; Javid, M.; Korah, R.; Carling, T. Epigenetic modifications in poorly differentiated and anaplastic thyroid cancer. Mol. Cell. Endocrinol. 2018, 469, 23–37. [Google Scholar] [CrossRef]
- Liao, T.; Wang, Y.J.; Hu, J.Q.; Wang, Y.; Han, L.T.; Ma, B.; Shi, R.L.; Qu, N.; Wei, W.J.; Guan, Q.; et al. Histone methyltransferase KMT5A gene modulates oncogenesis and lipid metabolism of papillary thyroid cancer in vitro. Oncol. Rep. 2018, 39, 2185–2192. [Google Scholar] [CrossRef]
- Ahmed, A.A.; Essa, M.E.A. Potential of epigenetic events in human thyroid cancer. Cancer Genet. 2019, 239, 13–21. [Google Scholar] [CrossRef]
- Mahmoudian-Sani, M.R.; Jalali, A.; Jamshidi, M.; Moridi, H.; Alghasi, A.; Shojaeian, A.; Mobini, G.R. Long Non-Coding RNAs in Thyroid Cancer: Implications for Pathogenesis, Diagnosis, and Therapy. Oncol. Res. Treat. 2019, 42, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Pellecchia, S.; Sepe, R.; Decaussin-Petrucci, M.; Ivan, C.; Shimizu, M.; Coppola, C.; Testa, D.; Calin, G.A.; Fusco, A.; Pallante, P. The Long Non-Coding RNA Prader Willi/Angelman Region RNA5 (PAR5) Is Downregulated in Anaplastic Thyroid Carcinomas Where It Acts as a Tumor Suppressor by Reducing EZH2 Activity. Cancers 2020, 12, 235. [Google Scholar] [CrossRef] [PubMed]
- Asa, S.L.; Ezzat, S. The epigenetic landscape of differentiated thyroid cancer. Mol. Cell. Endocrinol. 2018, 469, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, Z.; Hu, J.; Wan, X.; Huang, W.; Zhang, H.; Jiang, N. MiR-1246 regulates the PI3K/AKT signaling pathway by targeting PIK3AP1 and inhibits thyroid cancer cell proliferation and tumor growth. Mol. Cell. Biochem. 2022, 477, 649–661. [Google Scholar] [CrossRef]
- Riesco-Eizaguirre, G.; Wert-Lamas, L.; Perales-Paton, J.; Sastre-Perona, A.; Fernandez, L.P.; Santisteban, P. The miR-146b-3p/PAX8/NIS Regulatory Circuit Modulates the Differentiation Phenotype and Function of Thyroid Cells during Carcinogenesis. Cancer Res. 2015, 75, 4119–4130. [Google Scholar] [CrossRef]
- Hou, S.; Xie, X.; Zhao, J.; Wu, C.; Li, N.; Meng, Z.; Cai, C.; Tan, J. Downregulation of miR-146b-3p Inhibits Proliferation and Migration and Modulates the Expression and Location of Sodium/Iodide Symporter in Dedifferentiated Thyroid Cancer by Potentially Targeting MUC20. Front. Oncol. 2020, 10, 566365. [Google Scholar] [CrossRef]
- Lakshmanan, A.; Wojcicka, A.; Kotlarek, M.; Zhang, X.; Jazdzewski, K.; Jhiang, S.M. microRNA-339-5p modulates Na+/I- symporter-mediated radioiodide uptake. Endocr. Relat. Cancer 2015, 22, 11–21. [Google Scholar] [CrossRef]
- Russo, D.; Damante, G.; Puxeddu, E.; Durante, C.; Filetti, S. Epigenetics of thyroid cancer and novel therapeutic targets. J. Mol. Endocrinol. 2011, 46, R73–R81. [Google Scholar] [CrossRef]
- Saini, S.; Tulla, K.; Maker, A.V.; Burman, K.D.; Prabhakar, B.S. Therapeutic advances in anaplastic thyroid cancer: A current perspective. Mol. Cancer 2018, 17, 154. [Google Scholar] [CrossRef]
- Saqcena, M.; Leandro-Garcia, L.J.; Maag, J.L.V.; Tchekmedyian, V.; Krishnamoorthy, G.P.; Tamarapu, P.P.; Tiedje, V.; Reuter, V.; Knauf, J.A.; de Stanchina, E.; et al. SWI/SNF Complex Mutations Promote Thyroid Tumor Progression and Insensitivity to Redifferentiation Therapies. Cancer Discov. 2021, 11, 1158–1175. [Google Scholar] [CrossRef]
- Allegri, L.; Baldan, F.; Molteni, E.; Mio, C.; Damante, G. Role of m6A RNA Methylation in Thyroid Cancer Cell Lines. Int. J. Mol. Sci. 2022, 23, 11516. [Google Scholar] [CrossRef]
- Chen, Z.; Hu, Y.; Jin, L.; Yang, F.; Ding, H.; Zhang, L.; Li, L.; Pan, T. The Emerging Role of N6-Methyladenosine RNA Methylation as Regulators in Cancer Therapy and Drug Resistance. Front. Pharmacol. 2022, 13, 873030. [Google Scholar] [CrossRef]
- Zhao, W.; Qi, X.; Liu, L.; Ma, S.; Liu, J.; Wu, J. Epigenetic Regulation of m(6)A Modifications in Human Cancer. Mol. Ther. Nucleic Acids 2020, 19, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Sa, R.; Liang, R.; Qiu, X.; He, Z.; Liu, Z.; Chen, L. IGF2BP2-dependent activation of ERBB2 signaling contributes to acquired resistance to tyrosine kinase inhibitor in differentiation therapy of radioiodine-refractory papillary thyroid cancer. Cancer Lett. 2022, 527, 10–23. [Google Scholar] [CrossRef]
- Sa, R.; Liang, R.; Qiu, X.; He, Z.; Liu, Z.; Chen, L. Targeting IGF2BP2 Promotes Differentiation of Radioiodine Refractory Papillary Thyroid Cancer via Destabilizing RUNX2 mRNA. Cancers 2022, 14, 1268. [Google Scholar] [CrossRef]
- Catalano, M.G.; Pugliese, M.; Gargantini, E.; Grange, C.; Bussolati, B.; Asioli, S.; Bosco, O.; Poli, R.; Compagnone, A.; Bandino, A.; et al. Cytotoxic activity of the histone deacetylase inhibitor panobinostat (LBH589) in anaplastic thyroid cancer in vitro and in vivo. Int. J. Cancer 2012, 130, 694–704. [Google Scholar] [CrossRef] [PubMed]
- Catalano, M.G.; Fortunati, N.; Pugliese, M.; Marano, F.; Ortoleva, L.; Poli, R.; Asioli, S.; Bandino, A.; Palestini, N.; Grange, C.; et al. Histone deacetylase inhibition modulates E-cadherin expression and suppresses migration and invasion of anaplastic thyroid cancer cells. J. Clin. Endocrinol. Metab. 2012, 97, E1150–E1159. [Google Scholar] [CrossRef]
- Chan, D.; Zheng, Y.; Tyner, J.W.; Chng, W.J.; Chien, W.W.; Gery, S.; Leong, G.; Braunstein, G.D.; Koeffler, H.P. Belinostat and panobinostat (HDACI): In vitro and in vivo studies in thyroid cancer. J. Cancer Res. Clin. Oncol. 2013, 139, 1507–1514. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Park, K.C.; Jeon, J.Y.; Kim, B.W.; Kim, H.K.; Chang, H.J.; Choi, S.H.; Park, C.S.; Chang, H.S. Potential anti-cancer effect of N-hydroxy-7-(2-naphthylthio) heptanomide (HNHA), a novel histone deacetylase inhibitor, for the treatment of thyroid cancer. BMC Cancer 2015, 15, 1003. [Google Scholar] [CrossRef]
- Zhu, X.; Enomoto, K.; Zhao, L.; Zhu, Y.J.; Willingham, M.C.; Meltzer, P.; Qi, J.; Cheng, S.Y. Bromodomain and Extraterminal Protein Inhibitor JQ1 Suppresses Thyroid Tumor Growth in a Mouse Model. Clin. Cancer Res. 2017, 23, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ruan, X.; Li, Y.; Zhi, J.; Hu, L.; Hou, X.; Shi, X.; Wang, X.; Wang, J.; Ma, W.; et al. KDM1A promotes thyroid cancer progression and maintains stemness through the Wnt/beta-catenin signaling pathway. Theranostics 2022, 12, 1500–1517. [Google Scholar] [CrossRef]
- Wang, Z.; Dai, J.; Yan, J.; Zhang, Y.; Yin, Z. Targeting EZH2 as a novel therapeutic strategy for sorafenib-resistant thyroid carcinoma. J. Cell. Mol. Med. 2019, 23, 4770–4778. [Google Scholar] [CrossRef]
- Kelly, W.K.; O’Connor, O.A.; Krug, L.M.; Chiao, J.H.; Heaney, M.; Curley, T.; MacGregore-Cortelli, B.; Tong, W.; Secrist, J.P.; Schwartz, L.; et al. Phase I study of an oral histone deacetylase inhibitor, suberoylanilide hydroxamic acid, in patients with advanced cancer. J. Clin. Oncol. 2005, 23, 3923–3931. [Google Scholar] [CrossRef]
- Woyach, J.A.; Kloos, R.T.; Ringel, M.D.; Arbogast, D.; Collamore, M.; Zwiebel, J.A.; Grever, M.; Villalona-Calero, M.; Shah, M.H. Lack of therapeutic effect of the histone deacetylase inhibitor vorinostat in patients with metastatic radioiodine-refractory thyroid carcinoma. J. Clin. Endocrinol. Metab. 2009, 94, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Amiri-Kordestani, L.; Luchenko, V.; Peer, C.J.; Ghafourian, K.; Reynolds, J.; Draper, D.; Frye, R.; Woo, S.; Venzon, D.; Wright, J.; et al. Phase I trial of a new schedule of romidepsin in patients with advanced cancers. Clin. Cancer Res. 2013, 19, 4499–4507. [Google Scholar] [CrossRef] [PubMed]
- Sherman, E.J.; Su, Y.B.; Lyall, A.; Schoder, H.; Fury, M.G.; Ghossein, R.A.; Haque, S.; Lisa, D.; Shaha, A.R.; Tuttle, R.M.; et al. Evaluation of romidepsin for clinical activity and radioactive iodine reuptake in radioactive iodine-refractory thyroid carcinoma. Thyroid 2013, 23, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Nilubol, N.; Merkel, R.; Yang, L.; Patel, D.; Reynolds, J.C.; Sadowski, S.M.; Neychev, V.; Kebebew, E. A phase II trial of valproic acid in patients with advanced, radioiodine-resistant thyroid cancers of follicular cell origin. Clin. Endocrinol. 2017, 86, 128–133. [Google Scholar] [CrossRef]
- Choi, Y.W.; Kim, H.J.; Kim, Y.H.; Park, S.H.; Chwae, Y.J.; Lee, J.; Soh, E.Y.; Kim, J.H.; Park, T.J. B-RafV600E inhibits sodium iodide symporter expression via regulation of DNA methyltransferase 1. Exp. Mol. Med. 2014, 46, e120. [Google Scholar] [CrossRef]
- Gunda, V.; Cogdill, A.P.; Bernasconi, M.J.; Wargo, J.A.; Parangi, S. Potential role of 5-aza-2′-deoxycytidine induced MAGE-A4 expression in immunotherapy for anaplastic thyroid cancer. Surgery 2013, 154, 1456–1462. [Google Scholar] [CrossRef]
- Luong, Q.T.; O’Kelly, J.; Braunstein, G.D.; Hershman, J.M.; Koeffler, H.P. Antitumor activity of suberoylanilide hydroxamic acid against thyroid cancer cell lines in vitro and in vivo. Clin. Cancer Res. 2006, 12, 5570–5577. [Google Scholar] [CrossRef] [PubMed]
- Wachter, S.; Damanakis, A.I.; Elxnat, M.; Roth, S.; Wunderlich, A.; Verburg, F.A.; Fellinger, S.A.; Bartsch, D.K.; Di Fazio, P. Epigenetic Modifications in Thyroid Cancer Cells Restore NIS and Radio-Iodine Uptake and Promote Cell Death. J. Clin. Med. 2018, 7, 61. [Google Scholar] [CrossRef] [PubMed]
- Kitazono, M.; Bates, S.; Fok, P.; Fojo, T.; Blagosklonny, M.V. The histone deacetylase inhibitor FR901228 (desipeptide) restores expression and function of pseudo-null p53. Cancer Biol. Ther. 2002, 1, 665–668. [Google Scholar] [CrossRef]
- Xu, J.; Hershman, J.M. Histone deacetylase inhibitor depsipeptide represses nicotinamide N-methyltransferase and hepatocyte nuclear factor-1beta gene expression in human papillary thyroid cancer cells. Thyroid 2006, 16, 151–160. [Google Scholar] [CrossRef]
- Xiao, X.; Ning, L.; Chen, H. Notch1 mediates growth suppression of papillary and follicular thyroid cancer cells by histone deacetylase inhibitors. Mol. Cancer Ther. 2009, 8, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.T.; Wong, T.S.; Chung, W.Y.; Wong, M.G.; Kebebew, E.; Duh, Q.Y.; Clark, O.H. Valproic acid inhibits growth, induces apoptosis, and modulates apoptosis-regulatory and differentiation gene expression in human thyroid cancer cells. Surgery 2005, 138, 979–984. [Google Scholar] [CrossRef]
- Puppin, C.; D’Aurizio, F.; D’Elia, A.V.; Cesaratto, L.; Tell, G.; Russo, D.; Filetti, S.; Ferretti, E.; Tosi, E.; Mattei, T.; et al. Effects of histone acetylation on sodium iodide symporter promoter and expression of thyroid-specific transcription factors. Endocrinology 2005, 146, 3967–3974. [Google Scholar] [CrossRef]
- Dong, X.; Korch, C.; Meinkoth, J.L. Histone deacetylase inhibitors upregulate Rap1GAP and inhibit Rap activity in thyroid tumor cells. Endocr. Relat. Cancer 2011, 18, 301–310. [Google Scholar] [CrossRef]
- Mio, C.; Lavarone, E.; Conzatti, K.; Baldan, F.; Toffoletto, B.; Puppin, C.; Filetti, S.; Durante, C.; Russo, D.; Orlacchio, A.; et al. MCM5 as a target of BET inhibitors in thyroid cancer cells. Endocr. Relat. Cancer 2016, 23, 335–347. [Google Scholar] [CrossRef]
- Hou, P.; Bojdani, E.; Xing, M. Induction of thyroid gene expression and radioiodine uptake in thyroid cancer cells by targeting major signaling pathways. J. Clin. Endocrinol. Metab. 2010, 95, 820–828. [Google Scholar] [CrossRef]
- Copland, J.A.; Marlow, L.A.; Williams, S.F.; Grebe, S.K.; Gumz, M.L.; Maples, W.J.; Silverman, V.E.; Smallridge, R.C. Molecular diagnosis of a BRAF papillary thyroid carcinoma with multiple chromosome abnormalities and rare adrenal and hypothalamic metastases. Thyroid 2006, 16, 1293–1302. [Google Scholar] [CrossRef]
- Mitmaker, E.J.; Griff, N.J.; Grogan, R.H.; Sarkar, R.; Kebebew, E.; Duh, Q.Y.; Clark, O.H.; Shen, W.T. Modulation of matrix metalloproteinase activity in human thyroid cancer cell lines using demethylating agents and histone deacetylase inhibitors. Surgery 2011, 149, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Cha, H.Y.; Lee, B.S.; Chang, J.W.; Park, J.K.; Han, J.H.; Kim, Y.S.; Shin, Y.S.; Byeon, H.K.; Kim, C.H. Downregulation of Nrf2 by the combination of TRAIL and Valproic acid induces apoptotic cell death of TRAIL-resistant papillary thyroid cancer cells via suppression of Bcl-xL. Cancer Lett. 2016, 372, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Cheng, L.; Jin, Y.; Cheng, L.; Liu, M.; Chen, L. MAPK Inhibitors Enhance HDAC Inhibitor-Induced Redifferentiation in Papillary Thyroid Cancer Cells Harboring BRAF (V600E): An In Vitro Study. Mol. Ther. Oncolytics 2019, 12, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kang, J.G.; Kim, C.S.; Ihm, S.H.; Choi, M.G.; Yoo, H.J.; Lee, S.J. Gemigliptin, a novel dipeptidyl peptidase-IV inhibitor, exerts a synergistic cytotoxicity with the histone deacetylase inhibitor PXD101 in thyroid carcinoma cells. J. Endocrinol. Investig. 2018, 41, 677–689. [Google Scholar] [CrossRef]
- Lee, Y.S.; Kim, S.M.; Kim, B.W.; Chang, H.J.; Kim, S.Y.; Park, C.S.; Park, K.C.; Chang, H.S. Anti-cancer Effects of HNHA and Lenvatinib by the Suppression of EMT-Mediated Drug Resistance in Cancer Stem Cells. Neoplasia 2018, 20, 197–206. [Google Scholar] [CrossRef]
- Fu, H.; Cheng, L.; Sa, R.; Jin, Y.; Chen, L. Combined tazemetostat and MAPKi enhances differentiation of papillary thyroid cancer cells harbouring BRAF(V600E) by synergistically decreasing global trimethylation of H3K27. J. Cell. Mol. Med. 2020, 24, 3336–3345. [Google Scholar] [CrossRef]
- Zhu, X.; Park, S.; Lee, W.K.; Cheng, S.Y. Potentiated anti-tumor effects of BETi by MEKi in anaplastic thyroid cancer. Endocr. Relat. Cancer 2019, 26, 739–750. [Google Scholar] [CrossRef]
| Drug | Target | Chemical Structure | Model | Type of Cancer | Observed Effect | Ref. |
|---|---|---|---|---|---|---|
| LBH589 | HDACs (class I, IIa, IIb, IV) | 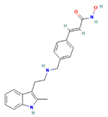 | Combined immune-deficiency xenograft model implanted with CAL-62 cells | ATC | Growth inhibition | [37] |
| Combined immune-deficiency xenograft model implanted with CAL-62 cells | ATC | Weakening of invasive capacity | [38] | |||
| Belinostat | HDACs (class I, IIa and IIb) | 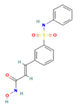 | Immunodeficient mice with BHP2-7 xenografts | PTC | Inhibition of tumor | [39] |
| HNHA | HDAC | 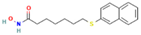 | Mice with SNU-790 xenografts | PTC | Proliferation inhibition | [40] |
| JQ1 | BRD4 | 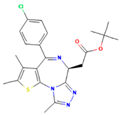 | ThrbPV/PVKrasG12D | ATC | Growth inhibition | [41] |
| Mice with PTC xenografts | PTC | Growth inhibition and restoration of radioiodine uptake | [16] | |||
| GSK-LSD1 | KDM1A | 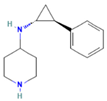 | Mice with ATC xenografts | ATC | Growth inhibition | [42] |
| EPZ-6438 | EZH2 | 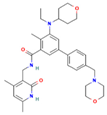 | Mice implanted with TC-07 and TC-13 cells | NA | Restoration of sorafenib resistant cells’ sensitivity | [43] |
| Inhibitor | Target | Chemical Structure | Phase and Status | Main Result | Ref. |
|---|---|---|---|---|---|
| Decitabine | DNMT | 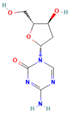 | Phase 2, completed | Increase in RAI uptake | NCT00085239 |
| Azacitidine | DNMT | 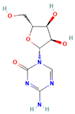 | Phase 1, completed | No results posted | NCT00004062 |
| SAHA | HDACs (class I and class II) | 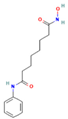 | Phase 1, completed | Increase in RAI uptake | [44] |
| Phase 2, completed | Faint increase in RAI uptake | [45] | |||
| Depsipeptide | HDAC1, HDAC2 | 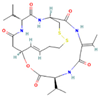 | Phase 1, completed | Faint increase in RAI uptake | [46] |
| Phase 2, completed | Increase in RAI uptake | [47] | |||
| Valproic acid | HDACs (class I) | 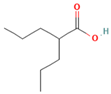 | Phase 2, completed | No increase in RAI uptake | [48] |
| LBH589 | HDACs (class I, IIa, IIb, IV) | 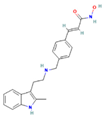 | Phase 2, completed | NA | NCT01013597 |
| Combined Therapy | Target | Cellular Model | Type of Cancer | Observed Effect | Ref. |
|---|---|---|---|---|---|
| SAHA + RDEA119 + temsirolimus + perifosine | HDAC + MEK + mTOR+ Akt | TPC-1, BCPAP, K1 | PTC | Growth inhibition and induction of radioiodine uptake | [60] |
| Depsipeptide + Paclitaxel, or lovastatin, or gefitinib | HDAC + Microtubule + HMG-CoA + EGFR | Primary culture of a papillary thyroid carcinoma harboring BRAFV600E | PTC | Growth inhibition | [61] |
| Trichostatin A + 5-azacytidine | HDAC + DNMT | TPC-1, FTC-133, FTC-236, FTC-238 | PTC and FTC | Growth inhibition | [62] |
| Valproic acid + 5-azacytidine | HDAC + DNMT | TPC-1, FTC-133, FTC-236, FTC-238 | PTC and FTC | Growth inhibition | [62] |
| Valproic acid + TRAIL | HDAC + Death receptor | TPC-1, BCPAP and BHP10-3 | PTC | Apoptosis | [63] |
| Panobinostat + dosatinib or pazopanib | HDAC + MAPK | BCPAP, K1 | PTC | Growth inhibition | [64] |
| Belinostat + Gemigliptin | HDAC + DPP4 | BCPAP | PTC | Apoptosis | [65] |
| HNHA + Levatinib | HDAC + TKI | patient-derived PTC | PTC | Apoptosis, cell cycle arrest and growth inhibition | [66] |
| Tazemetostat + dabrafenib or selumetinib | EZH2 + MAPK | BCPAP, K1, TPC-1 | PTC | Enhancement of differentiation | [67] |
| PLX51107 + PD0325901 | BRD4 + MEK | THJ-11T, THJ-16T | ATC | Apoptosis and proliferation inhibition | [68] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, K.; Wang, J.; He, Z.; Qiu, X.; Sa, R.; Chen, L. Epigenetic Targets and Their Inhibitors in Thyroid Cancer Treatment. Pharmaceuticals 2023, 16, 559. https://doi.org/10.3390/ph16040559
Zhang K, Wang J, He Z, Qiu X, Sa R, Chen L. Epigenetic Targets and Their Inhibitors in Thyroid Cancer Treatment. Pharmaceuticals. 2023; 16(4):559. https://doi.org/10.3390/ph16040559
Chicago/Turabian StyleZhang, Ke, Junyao Wang, Ziyan He, Xian Qiu, Ri Sa, and Libo Chen. 2023. "Epigenetic Targets and Their Inhibitors in Thyroid Cancer Treatment" Pharmaceuticals 16, no. 4: 559. https://doi.org/10.3390/ph16040559
APA StyleZhang, K., Wang, J., He, Z., Qiu, X., Sa, R., & Chen, L. (2023). Epigenetic Targets and Their Inhibitors in Thyroid Cancer Treatment. Pharmaceuticals, 16(4), 559. https://doi.org/10.3390/ph16040559






