Angiotensin II-Related Activation of Scleral Fibroblasts and Their Role on Retinal Ganglion Cell Death in Glaucoma
Abstract
1. Introduction
2. Results
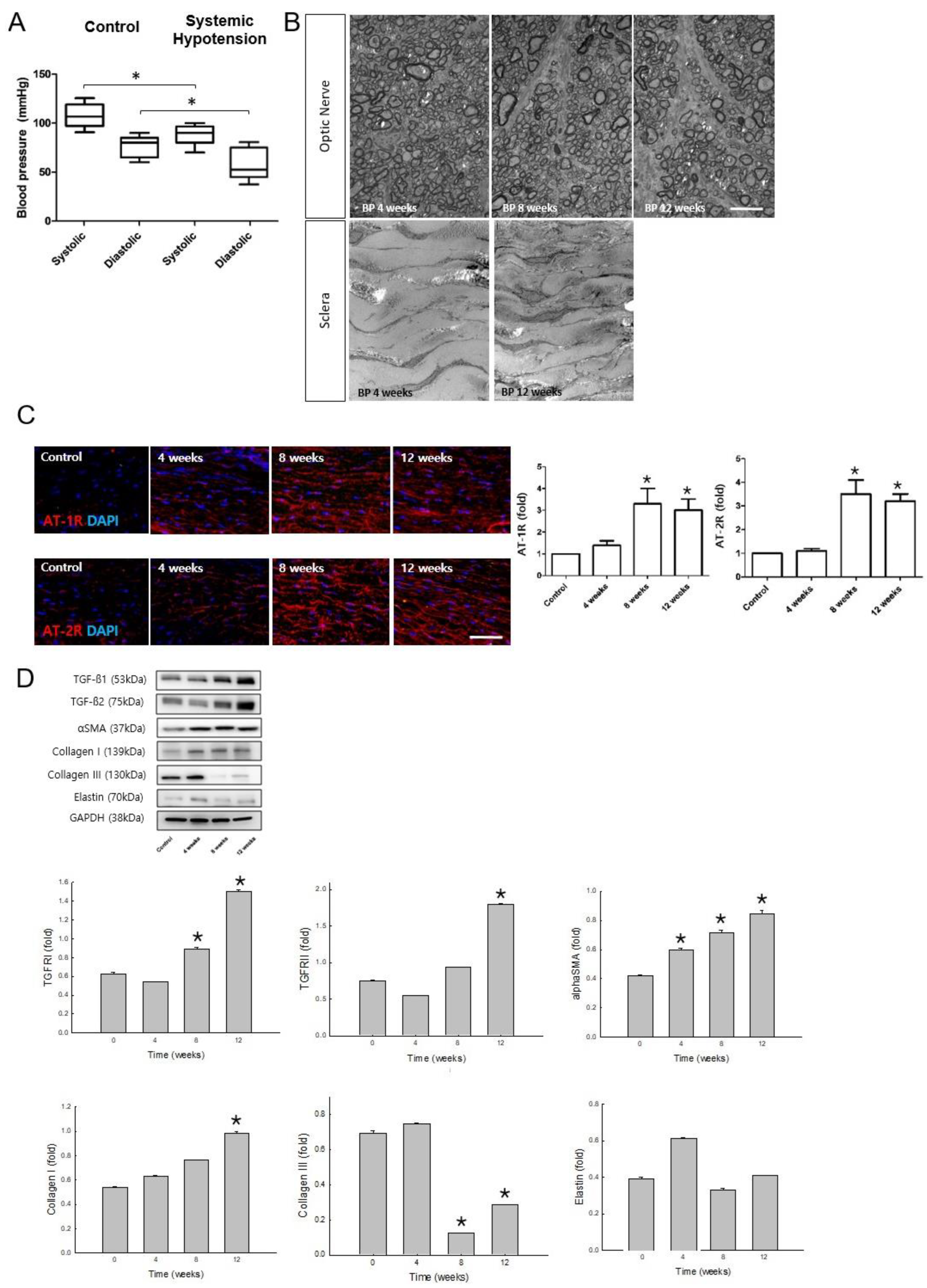
2.1. Confirmation of the Systemic Hypotensive Model and Involvement of the RAAS in the Sclera
2.2. Changes in the Biomechanical Properties of the Sclera after Systemic Hypotension
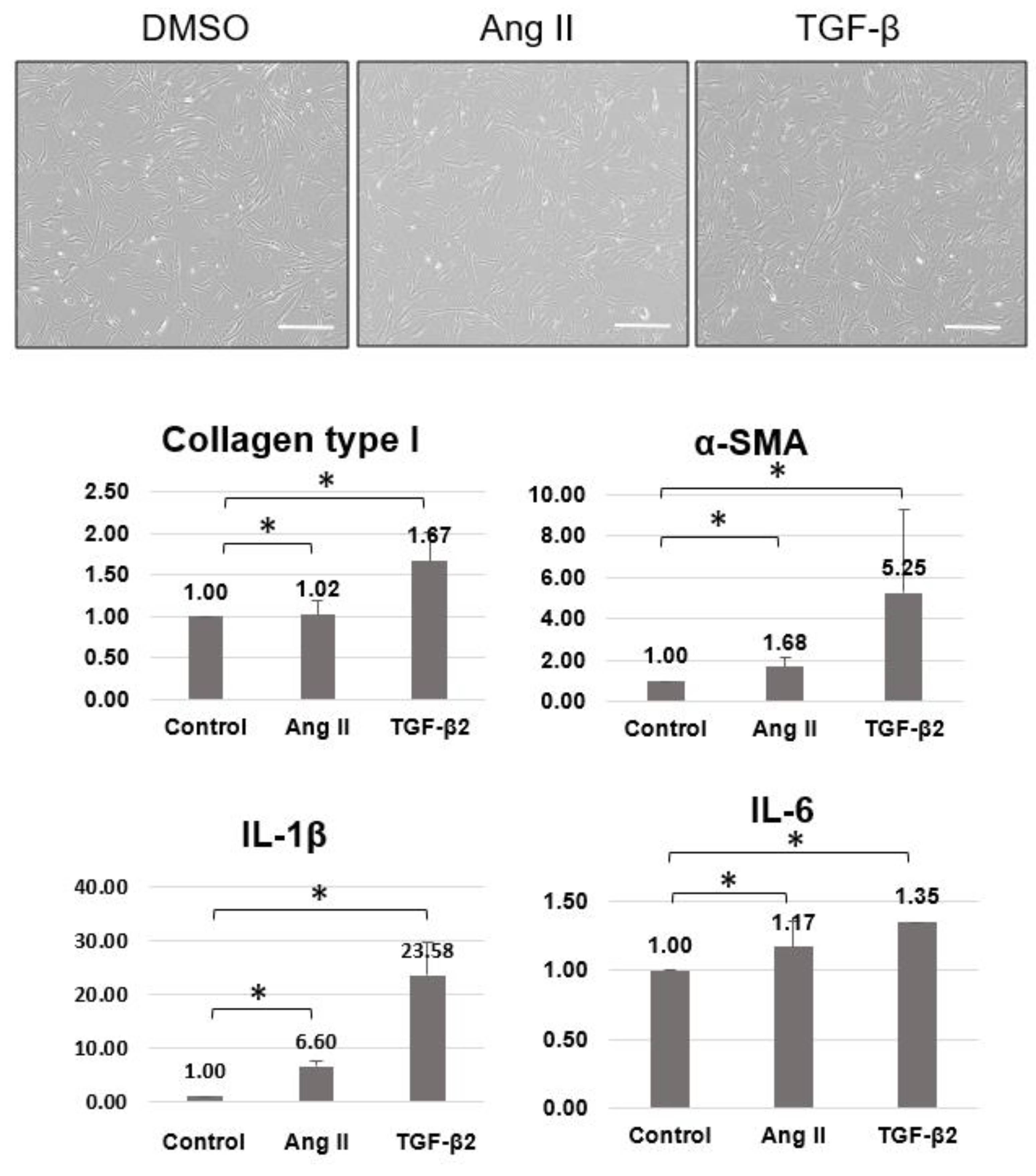
2.3. Involvement of the RAAS and ECM Changes in the Sclera
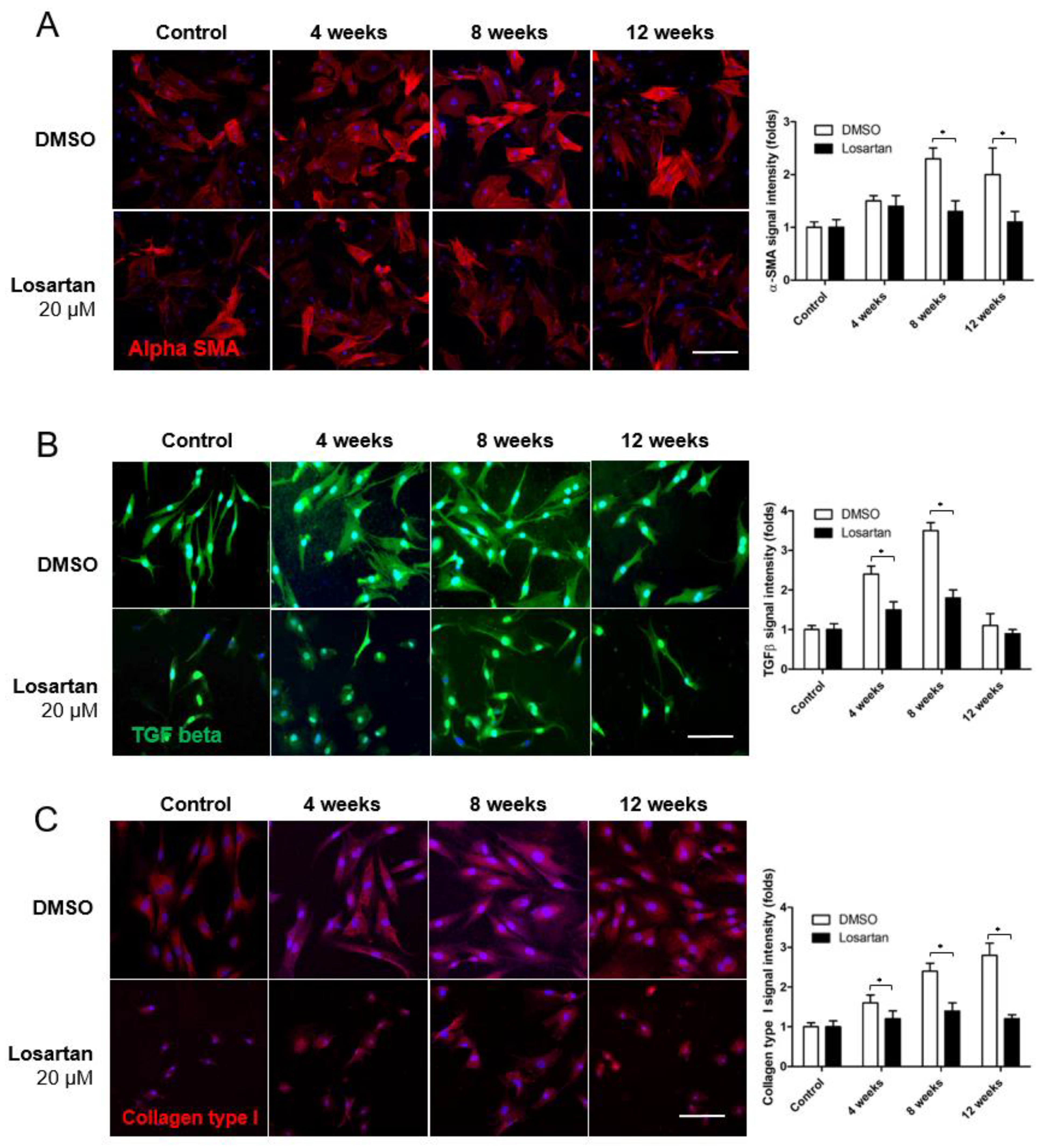
2.4. Effect of Inhibiting Angiotensin on RGCs
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Systemic Hypotensive Rats
4.3. Immunohistochemistry of the Sclera and Retina
4.4. Transmission Electron Microscopy (TEM)
4.5. Biomechanical Analysis
4.6. Fibroblast Culture
4.7. Quantitative Real-Time Polymerase Chain Reaction (RT-PCR)
4.8. Immunocytochemistry
4.9. Western Blotting Analysis
4.10. Immunohistochemistry of Flat-Mounted Retinas
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AngII | Angiotensin II |
| AT-1R | Angiotensin II receptor type I |
| AT-2R | Angiotensin II receptor type II |
| ECM | Extracellular matrix |
| HCTZ | Hydrochlorothiazide |
| IL | Interleukin |
| ONH | Optic nerve head |
| RAAS | Renin–angiotensin–aldosterone system |
| RGC | Retinal ganglion cell |
| SMA | Smooth muscle actin |
| TGF | Transforming growth factor |
References
- Weinreb, R.N.; Khaw, P.T. Primary open-angle glaucoma. Lancet 2004, 363, 1711–1720. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-Y.L.; Kim, J.H.; Jung, Y.; Park, C.K. Racial Differences in the Extracellular Matrix and Histone Acetylation of the Lamina Cribrosa and Peripapillary Sclera. Investig. Opthalmology Vis. Sci. 2017, 58, 4143–4154. [Google Scholar] [CrossRef] [PubMed]
- Galassi, F.; Giambene, B.; Varriale, R. Systemic Vascular Dysregulation and Retrobulbar Hemodynamics in Normal-Tension Glaucoma. Investig. Opthalmology Vis. Sci. 2011, 52, 4467–4471. [Google Scholar] [CrossRef] [PubMed]
- Kosior-Jarecka, E.; Wróbel-Dudzińska, D.; Łukasik, U.; Żarnowski, T. Ocular and Systemic Risk Factors of Different Morphologies of Scotoma in Patients with Normal-Tension Glaucoma. J. Ophthalmol. 2017, 2017, 1480746. [Google Scholar] [CrossRef]
- Mudumbai, R.C. Clinical Update on Normal Tension Glaucoma. Semin. Ophthalmol. 2013, 28, 173–179. [Google Scholar] [CrossRef]
- Flammer, J.; Mozaffarieh, M. Autoregulation, a balancing act between supply and demand. Can. J. Ophthalmol. 2008, 43, 317–321. [Google Scholar] [CrossRef]
- Hamed, S.A.; Hamed, E.A.; Eldin, A.M.E.; Mahmoud, N.M. Vascular Risk Factors, Endothelial Function, and Carotid Thickness in Patients with Migraine: Relationship to Atherosclerosis. J. Stroke Cerebrovasc. Dis. 2010, 19, 92–103. [Google Scholar] [CrossRef]
- Stoyneva, Z. Laser Doppler-recorded venoarteriolar reflex in Raynaud’s phenomenon. Auton. Neurosci. 2004, 116, 62–68. [Google Scholar] [CrossRef]
- Navar, L.G. Physiology: Hemodynamics, endothelial function, renin–angiotensin–aldosterone system, sympathetic nervous system. J. Am. Soc. Hypertens. 2014, 8, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Rauf, A.; Khan, H.; Abu-Izneid, T. Renin-angiotensin-aldosterone (RAAS): The ubiquitous system for homeostasis and pathologies. Biomed. Pharmacother. 2017, 94, 317–325. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, M.; Wang, Y.; Chen, R.; Xu, S.; Sun, X.; Yang, Y.; Lin, Z.; Wang, S.; Huang, H. Gentiopicroside Ameliorates Diabetic Renal Tubulointerstitial Fibrosis via Inhibiting the AT1R/CK2/NF-κB Pathway. Front. Pharmacol. 2022, 13, 848915. [Google Scholar] [CrossRef]
- Lu, W.; Zhu, H.; Wu, J.; Liao, S.; Cheng, G.; Li, X. Rhein attenuates angiotensin II-induced cardiac remodeling by modulating AMPK–FGF23 signaling. J. Transl. Med. 2022, 20, 305. [Google Scholar] [CrossRef]
- Arruda-Junior, D.F.; Salles, T.A.; Martins, F.L.; Antonio, E.L.; Tucci, P.J.; Gowdak, L.H.W.; Tavares, C.A.; Girardi, A.C. Unraveling the interplay between dipeptidyl peptidase 4 and the renin-angiotensin system in heart failure. Life Sci. 2022, 305, 120757. [Google Scholar] [CrossRef]
- Zhang, J.; Hui, Y.; Liu, F.; Yang, Q.; Lu, Y.; Chang, Y.; Liu, Q.; Ding, Y. Neohesperidin Protects Angiotensin II-Induced Hypertension and Vascular Remodeling. Front. Pharmacol. 2022, 13, 890202. [Google Scholar] [CrossRef]
- Huang, Z.; Khalifa, M.O.; Li, P.; Huang, Y.; Gu, W.; Li, T.-S. Angiotensin receptor blocker alleviates liver fibrosis by altering the mechanotransduction properties of hepatic stellate cells. Am. J. Physiol. Liver Physiol. 2022, 322, G446–G456. [Google Scholar] [CrossRef] [PubMed]
- White, A.J.; Cheruvu, S.C.; Sarris, M.; Liyanage, S.S.; Lumbers, E.; Chui, J.; Wakefield, D.; McCluskey, P. Expression of classical components of the renin-angiotensin system in the human eye. J. Renin-Angiotensin-Aldosterone Syst. 2015, 16, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Senanayake, P.D.; Drazba, J.; Shadrach, K.; Milsted, A.; Rungger-Brandle, E.; Nishiyama, K.; Miura, S.-I.; Karnik, S.; Sears, J.E.; Hollyfield, J.G. Angiotensin II and Its Receptor Subtypes in the Human Retina. Investig. Opthalmology Vis. Sci. 2007, 48, 3301–3311. [Google Scholar] [CrossRef]
- Quigley, H.A.; Pitha, I.F.; Welsbie, D.S.; Nguyen, C.; Steinhart, M.; Nguyen, T.D.; Pease, M.; Oglesby, E.N.; Berlinicke, C.A.; Mitchell, K.L.; et al. Losartan Treatment Protects Retinal Ganglion Cells and Alters Scleral Remodeling in Experimental Glaucoma. PLoS ONE 2015, 10, e0141137. [Google Scholar] [CrossRef]
- Wollensak, G.; Iomdina, E. Long-term biomechanical properties after collagen crosslinking of sclera using glyceraldehyde. Acta Ophthalmol. 2008, 86, 887–893. [Google Scholar] [CrossRef] [PubMed]
- Korneva, A.; Schaub, J.; Jefferys, J.; Kimball, E.; Pease, M.E.; Nawathe, M.; Johnson, T.V.; Pitha, I.; Quigley, H. A method to quantify regional axonal transport blockade at the optic nerve head after short term intraocular pressure elevation in mice. Exp. Eye Res. 2020, 196, 108035. [Google Scholar] [CrossRef]
- Kimball, E.C.; Nguyen, C.; Steinhart, M.R.; Nguyen, T.D.; Pease, M.E.; Oglesby, E.N.; Oveson, B.C.; Quigley, H.A. Experimental scleral cross-linking increases glaucoma damage in a mouse model. Exp. Eye Res. 2014, 128, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Binggeli, T.; Schoetzau, A.; Konieczka, K. In glaucoma patients, low blood pressure is accompanied by vascular dysregulation. EPMA J. 2018, 9, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.J.; Huh, J.; Jeong, E.; Park, C.K.; Park, H.Y.L. Angiotensin II related glial cell activation and necroptosis of retinal ganglion cells after systemic hypotension in glaucoma. Cell Death Dis. 2022, 13, 323. [Google Scholar] [CrossRef]
- Yoo, C.; Eom, Y.S.; Suh, Y.-W.; Kim, Y.Y. Central Corneal Thickness and Anterior Scleral Thickness in Korean Patients With Open-angle Glaucoma: An Anterior Segment Optical Coherence Tomography study. J. Glaucoma 2011, 20, 95–99. [Google Scholar] [CrossRef]
- Aghaian, E.; Choe, J.E.; Lin, S.; Stamper, R.L. Central corneal thickness of Caucasians, Chinese, Hispanics, Filipinos, African Americans, and Japanese in a glaucoma clinic. Ophthalmology 2004, 111, 2211–2219. [Google Scholar] [CrossRef]
- Park, H.-Y.L.; Jeon, S.H.; Park, C.K. Enhanced Depth Imaging Detects Lamina Cribrosa Thickness Differences in Normal Tension Glaucoma and Primary Open-Angle Glaucoma. Ophthalmology 2012, 119, 10–20. [Google Scholar] [CrossRef]
- Hannon, B.G.; Luna, C.; Feola, A.J.; Ritch, M.D.; Read, A.T.; Stinnett, S.S.; Vo, H.; Pardue, M.T.; Gonzalez, P.; Ethier, C.R. Assessment of Visual and Retinal Function Following In Vivo Genipin-Induced Scleral Crosslinking. Transl. Vis. Sci. Technol. 2020, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Oglesby, E.N.; Tezel, G.; Cone-Kimball, E.; Steinhart, M.R.; Jefferys, J.; Pease, M.E.; Quigley, H.A. Scleral fibroblast response to experimental glaucoma in mice. Mol. Vis. 2016, 22, 82–99. [Google Scholar]
- Iomdina, E.N.; Tikhomirova, N.K.; Bessmertny, A.M.; Serebryakova, M.V.; Baksheeva, V.E.; Zalevsky, A.O.; Kotelin, V.I.; Kiseleva, O.A.; Kosakyan, S.M.; Zamyatnin, A.A., Jr.; et al. Alterations in proteome of human sclera associated with primary open-angle glaucoma involve proteins participating in regulation of the extracellular matrix. Mol. Vis. 2020, 26, 623–640. [Google Scholar]
- Chen, H.-Y.; Chou, H.-C.; Chang, S.-J.; Liao, E.-C.; Tsai, Y.-T.; Wei, Y.-S.; Li, J.-M.; Lin, L.-H.; Lin, M.-W.; Chen, Y.-J.; et al. Proteomic Analysis of Various Rat Ocular Tissues after Ischemia–Reperfusion Injury and Possible Relevance to Acute Glaucoma. Int. J. Mol. Sci. 2017, 18, 334. [Google Scholar] [CrossRef]
- Shi, H.; Zhang, Y.; Fu, S.; Lu, Z.; Ye, W.; Xiao, Y. Angiotensin II as a Morphogenic Cytokine Stimulating Fibrogenesis of Human Tenon’s Capsule Fibroblasts. Investig. Opthalmology Vis. Sci. 2015, 56, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.; Danser, A.H.J.; Derkx, F.H.; De Jong, T.V.; Paul, M.; Mullins, J.J.; Schalekamp, A.M.A.; Ganten, D. Demonstration of renin mRNA, angiotensinogen mRNA, and angiotensin converting enzyme mRNA expression in the human eye: Evidence for an intraocular renin-angiotensin system. Br. J. Ophthalmol. 1996, 80, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Berka, J.L.; Stubbs, A.J.; Wang, D.Z.; DiNicolantonio, R.; Alcorn, D.; Campbell, D.J.; Skinner, S.L. Renin-containing Müller cells of the retina display endocrine features. Investig. Opthalmology Vis. Sci. 1995, 36, 1450–1458. [Google Scholar]
- Sarlos, S.; Rizkalla, B.; Moravski, C.J.; Cao, Z.; Cooper, M.E.; Wilkinson-Berka, J.L. Retinal Angiogenesis Is Mediated by an Interaction between the Angiotensin Type 2 Receptor, VEGF, and Angiopoietin. Am. J. Pathol. 2003, 163, 879–887. [Google Scholar] [CrossRef]
- Yang, H.; Hirooka, K.; Fukuda, K.; Shiraga, F. Neuroprotective Effects of Angiotensin II Type 1 Receptor Blocker in a Rat Model of Chronic Glaucoma. Investig. Opthalmology Vis. Sci. 2009, 50, 5800–5804. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T.; Hirooka, K.; Nakamura, T.; Itano, T.; Nishiyama, A.; Nagai, Y.; Shiraga, F. Neuroprotective Effects of Angiotensin II Type 1 Receptor (AT1-R) Blocker via Modulating AT1-R Signaling and Decreased Extracellular Glutamate Levels. Investig. Opthalmology Vis. Sci. 2012, 53, 4099–4110. [Google Scholar] [CrossRef]
- White, A.J.; Heller, J.; Leung, J.; Tassoni, A.; Martin, K.R. Retinal ganglion cell neuroprotection by an angiotensin II blocker in an ex vivo retinal explant model. J. Renin-Angiotensin-Aldosterone Syst. 2015, 16, 1193–1201. [Google Scholar] [CrossRef]
- Yadav, H.; Singh, P.K. Effect of thiazide drug compound on the testicular protein and cholesterol contents of albino rat. Indian J. Clin. Biochem. 2003, 18, 206–208. [Google Scholar] [CrossRef]
- Reungjui, S.; Hu, H.; Mu, W.; Roncal, C.; Croker, B.; Patel, J.; Nakagawa, T.; Srinivas, T.; Byer, K.; Simoni, J.; et al. Thiazide-induced subtle renal injury not observed in states of equivalent hypokalemia. Kidney Int. 2007, 72, 1483–1492. [Google Scholar] [CrossRef]
- Nakagawa, T.; Hu, H.; Zharikov, S.; Tuttle, K.; Short, R.A.; Glushakova, O.; Ouyang, X.; Feig, D.; Block, E.R.; Herrera-Acosta, J.; et al. A causal role for uric acid in fructose-induced metabolic syndrome. Am. J. Physiol. Physiol. 2006, 290, F625–F631. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, S.-E.; Kim, J.-H.; Shin, H.-J.; Kim, S.-A.; Park, C.-K.; Park, H.-Y.L. Angiotensin II-Related Activation of Scleral Fibroblasts and Their Role on Retinal Ganglion Cell Death in Glaucoma. Pharmaceuticals 2023, 16, 556. https://doi.org/10.3390/ph16040556
Oh S-E, Kim J-H, Shin H-J, Kim S-A, Park C-K, Park H-YL. Angiotensin II-Related Activation of Scleral Fibroblasts and Their Role on Retinal Ganglion Cell Death in Glaucoma. Pharmaceuticals. 2023; 16(4):556. https://doi.org/10.3390/ph16040556
Chicago/Turabian StyleOh, Si-Eun, Jie-Hyun Kim, Hee-Jong Shin, Seong-Ah Kim, Chan-Kee Park, and Hae-Young Lopilly Park. 2023. "Angiotensin II-Related Activation of Scleral Fibroblasts and Their Role on Retinal Ganglion Cell Death in Glaucoma" Pharmaceuticals 16, no. 4: 556. https://doi.org/10.3390/ph16040556
APA StyleOh, S.-E., Kim, J.-H., Shin, H.-J., Kim, S.-A., Park, C.-K., & Park, H.-Y. L. (2023). Angiotensin II-Related Activation of Scleral Fibroblasts and Their Role on Retinal Ganglion Cell Death in Glaucoma. Pharmaceuticals, 16(4), 556. https://doi.org/10.3390/ph16040556





