Head-to-Head Comparison between [68Ga]Ga-DOTA.SA.FAPi and [18F]F-FDG PET/CT Imaging in Patients with Breast Cancer
Abstract
1. Introduction
2. Results
2.1. Patients
2.2. Patient-Based Detection Rate Analysis
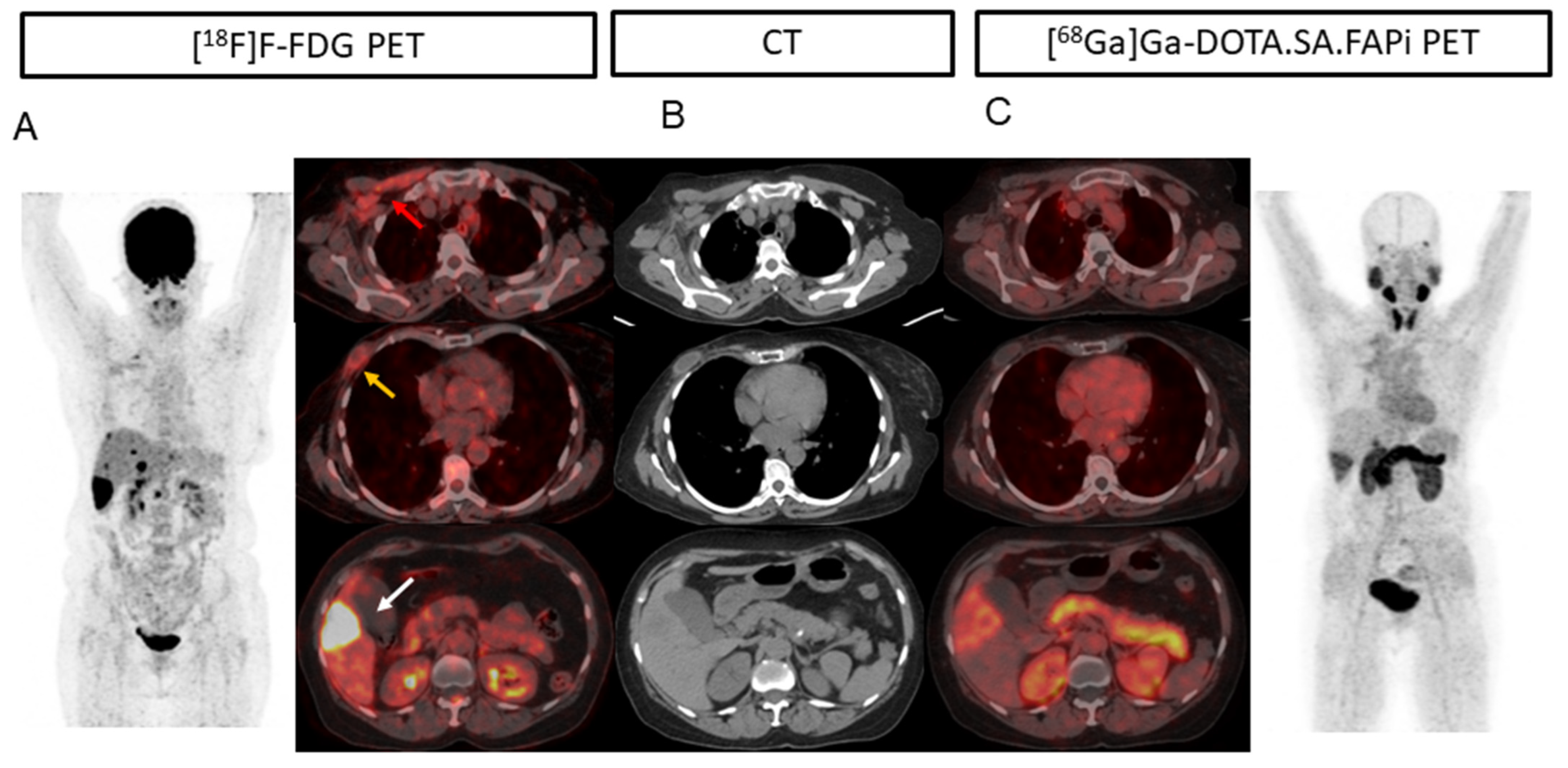
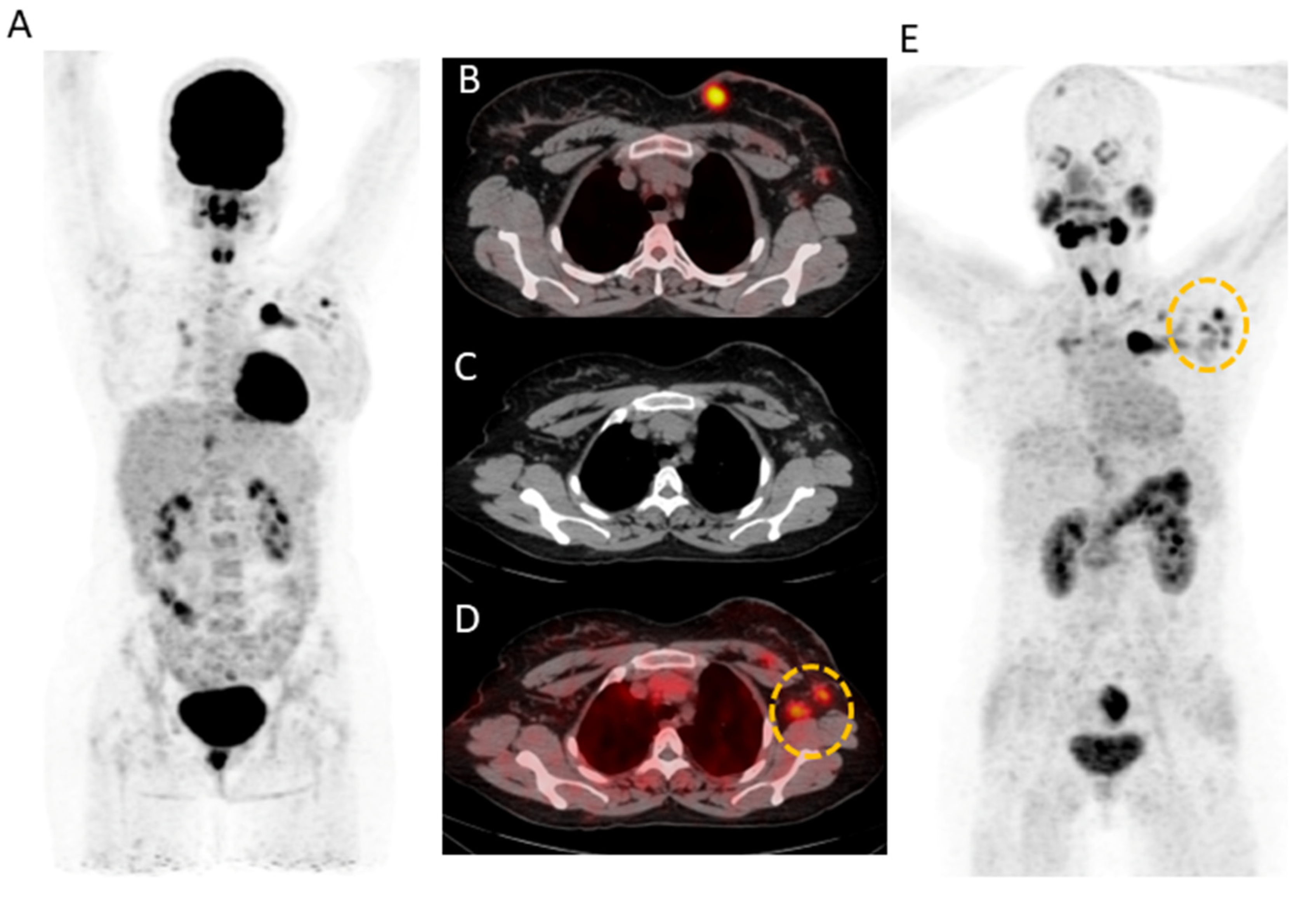
2.3. Lesion-Based Analysis
2.4. Comparison of Uptake and TBRs in Tumor Lesions
3. Discussion
3.1. Limitations
3.2. Future Prospects
4. Materials and Methods
4.1. Patient Selection
4.2. PET/CT Acquisition
4.3. Data Interpretation
4.4. Data Analysis and Processing
4.5. Definitions
- True-positive (TP) lesion: active uptake in the lesion seen on [18F]F-FDG/[68Ga]Ga-DOTA.SA.FAPi PET/CT images and found to be positive on diagnostic CT/histological examination.
- False-positive (FP) lesion: active uptake in the lesion seen on [18F]F-FDG/[68Ga]Ga-DOTA.SA.FAPi PET/CT images and found to be negative on diagnostic CT/histological examination/clinical or radiological follow-up.
- True-negative (TN) lesion: No uptake seen on [18F]F-FDG/[68Ga]Ga-DOTA.SA.FAPi PET/CT images and the results on diagnostic CT/histological examination/clinical or radiological follow-up were also negative.
- False-negative (FN) lesion: a lesion that was missed in [18F]F-FDG/[68Ga]Ga-DOTA.SA.FAPi PET/CT images were found to be positive for malignancy at diagnostic CT/histological examination/clinical or radiological follow-up.
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- The Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/cancer/breast/basic_info/index.htm (accessed on 2 January 2023).
- Lind, P.; Igerc, I.; Beyer, T.; Reinprecht, P.; Hausegger, K. Advantages and limitations of FDG PET in the follow-up of breast cancer. Eur. J. Nucl. Med. Mol. Imaging 2004, 31 (Suppl. S1), S125–S134. [Google Scholar] [PubMed]
- Huang, Y.; Simms, A.E.; Mazur, A.; Wang, S.; León, N.R.; Jones, B.; Aziz, N.; Kelly, T. Fibroblast activation protein-α promotes tumor growth and invasion of breast cancer cells through non-enzymatic functions. Clin. Exp. Metastasis 2011, 20, 567–579. [Google Scholar] [CrossRef] [PubMed]
- Kömek, H.; Can, C.; Güzel, Y.; Oruç, Z.; Gündoğan, C.; Yildirim, Ö.A.; Kaplan, I.; Erdur, E.; Yıldırım, M.S.; Çakabay, B. 68Ga-FAPI-04 PET/CT, a new step in breast cancer imaging: A comparative pilot study with the 18F-FDG PET/CT. Ann. Nucl. Med. 2021, 35, 744–752. [Google Scholar] [CrossRef]
- Elboga, U.; Sahin, E.; Kus, T.; Cayirli, Y.B.; Aktas, G.; Uzun, E.; Cinkir, H.Y.; Teker, F.; Sever, O.N.; Aytekin, A.; et al. Superiority of 68Ga-FAPI PET/CT scan in detecting additional lesions compared to 18FDG PET/CT scan in breast cancer. Ann. Nucl. Med. 2021, 35, 1321–1331. [Google Scholar] [CrossRef]
- Moon, E.S.; Ballal, S.; Yadav, M.P.; Bal, C.; Van Rymenant, Y.; Stephan, S.; Bracke, A.; Van der Veken, P.; De Meester, I.; Roesch, F. Fibroblast Activation Protein (FAP) targeting homodimeric FAP inhibitor radiotheranostics: A step to improve tumor uptake and retention time. Am. J. Nucl. Med. Mol. Imaging 2021, 11, 476–491. [Google Scholar]
- Moon, E.S.; Van Rymenant, Y.; Battan, S.; De Loose, J.; Bracke, A.; Van der Veken, P.; De Meester, I.; Rösch, F. In Vitro Evaluation of the Squaramide-Conjugated Fibroblast Activation Protein Inhibitor-Based Agents AAZTA5.SA.FAPi and DOTA.SA.FAPi. Molecules 2021, 26, 3482. [Google Scholar] [CrossRef] [PubMed]
- Moon, E.S.; Elvas, F.; Vliegen, G.; De Lombaerde, S.; Vangestel, C.; De Bruycker, S.; Bracke, A.; Eppard, E.; Greifenstein, L.; Klasen, B.; et al. Targeting fibroblast activation protein (FAP): Next generation PET radiotracers using squaramide coupled bifunctional DOTA and DATA5m chelators. EJNMMI Radiopharm. Chem. 2020, 29, 19. [Google Scholar] [CrossRef] [PubMed]
- Ballal, S.; Yadav, M.P.; Moon, E.S.; Kramer, V.S.; Roesch, F.; Kumari, S.; Tripathi, M.; ArunRaj, S.T.; Bal, C. Biodistribution, pharmacokinetics, dosimetry of [68Ga]GaDOTA.SA.FAPi, and the head-to-head comparison with [18F]F-FDG PET/CT in patients with various cancers. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1915–1931. [Google Scholar] [CrossRef] [PubMed]
- Ballal, S.; Yadav, M.P.; Kramer, V.; Moon, E.S.; Roesch, F.; Tripathi, M.; Mallick, S.; ArunRaj, S.T.; Bal, C. A theranostic approach of [68Ga]Ga-DOTA.SA.FAPi PET/CT-guided [177Lu]Lu-DOTA.SA.FAPi radionuclide therapy in an end-stage breast cancer patient: New frontier in targeted radionuclide therapy. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 942–944. [Google Scholar] [CrossRef] [PubMed]
- Ballal, S.; Yadav, M.P.; Moon, E.S.; Kramer, V.S.; Roesch, F.; Kumari, S.; Bal, C. First-In-Human Results on the Biodistribution, Pharmacokinetics, and Dosimetry of [177Lu]Lu-DOTA.SA.FAPi and [177Lu]Lu-DOTAGA.(SA.FAPi)2. Pharmaceuticals 2021, 14, 1212. [Google Scholar] [CrossRef] [PubMed]
- Ballal, S.; Yadav, M.P.; Moon, E.S.; Roesch, F.; Kumari, S.; Agarwal, S.; Tripathi, M.; Sahoo, R.K.; Mangu, B.S.; Tupalli, A.; et al. Novel Fibroblast Activation Protein Inhibitor-Based Targeted Theranostics for Radioiodine-Refractory Differentiated Thyroid Cancer Patients: A Pilot Study. Thyroid 2022, 32, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Karnoub, A.E.; Dash, A.B.; Vo, A.P.; Sullivan, A.; Brooks, M.W.; Bell, G.W.; Richardson, A.L.; Polyak, K.; Tubo, R.; Weinberg, R.A. Mesenchymalstem cells within tumour stroma promote breast cancer metastasis. Nature 2007, 449, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Shekhar, M.P.; Santner, S.; Carolin, K.A.; Tait, L. Direct involvement of breast tumor fibroblasts in the modulation of tamoxifen sensitivity. Am. J. Pathol. 2007, 170, 1546–1560. [Google Scholar] [CrossRef] [PubMed]
- Hasebe, T.; Sasaki, S.; Imoto, S.; Ochiai, A. Highly proliferative fibroblasts forming fibrotic focus govern metastasis of invasive ductal carcinoma of the breast. Mod. Pathol. 2001, 14, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Orimo, A.; Gupta, P.B.; Sgroi, D.C.; Arenzana-Seisdedos, F.; Delaunay, T.; Naeem, R.; Carey, V.J.; Richardson, A.L.; Weinberg, R.A. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell 2005, 121, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Stuelten, C.H.; Busch, J.I.; Tang, B.; Flanders, K.C.; Oshima, A.; Sutton, E.; Karpova, T.S.; Roberts, A.B.; Wakefield, L.M.; Niederhuber, J.E. Transient tumor-fibroblast interactions increase tumor cell malignancy by a TGF-Beta mediated mechanism in a mouse xenograft model of breast cancer. PLoS ONE 2010, 5, e9832. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Peluffo, G.; Chen, H.; Gelman, R.; Schnitt, S.; Polyak, K. Role of COX-2 in epithelial-stromal cell interactions and progression of ductal carcinoma in situ of the breast. Proc. Natl. Acad. Sci. USA 2009, 106, 3372–3377. [Google Scholar] [CrossRef] [PubMed]
- Finak, G.; Bertos, N.; Pepin, F.; Sadekova, S.; Souleimanova, M.; Zhao, H.; Chen, H.; Omeroglu, G.; Meterissian, S.; Omeroglu, A.; et al. Stromal gene expression predicts clinical outcome in breast cancer. Nat. Med. 2008, 14, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Tchou, J.; Paul, J.; Zhang, P.J.; Ying, T.; Bi, Y.; Satija, C.; Marjumdar, R.; Stephen, T.L.; Lo, A.; Chen, H.; et al. Fibroblast activation protein expression by stromal cells and tumor-associated macrophages in human breast cancer. Hum. Pathol. 2013, 44, 2549–2557. [Google Scholar] [CrossRef] [PubMed]
- Ariga, N.; Sato, E.; Ohuchi, N.; Nagura, H.; Ohtani, H. Stromal expression of fibroblast activation protein/seprase, a cell membrane serine proteinase and gelatinase, is associated with longer survival in patients with invasive ductal carcinoma of breast. Int. J. Cancer 2001, 95, 67–72. [Google Scholar] [CrossRef] [PubMed]



| Parameters | Imaging Method | Primary | Lymph Node Metastasis | Lung Metastasis | Pleural Metastases | Liver Metastases | Bone Metastases | Brain Metastases |
|---|---|---|---|---|---|---|---|---|
| Patient-based analysis | ||||||||
| CT | 30 | 41 | 26 | 8 | 17 | 25 | 15 | |
| [18F]F-FDG | 27 (90%) | 38 (92.6%) | 25 (96.1%) | 6 (75%) | 17 (100%) | 24 (96%) | 13 (86.6%) | |
| [68Ga]Ga-DOTA. SA.FAPi | 29 (96.6%) | 40 (97.5%) | 25 (96.1%) | 8 (100%) | 17 (100%) | 25 (100%) | 15 (100%) | |
| p-value | 0.3107 | 0.309 | 1.000 | 0.1432 | 1.000 | 0.865 | 0.748 | |
| Lesion-based analysis | ||||||||
| CT | 44 | 248 | - | 15 | 88 | - | 42 | |
| [18F]F-FDG | 36 (81.8%) | 208 (83.8%) | - | 11 (73%) | 85 (96.6%) | - | 25 (59.5%) | |
| [68Ga]Ga-DOTA. SA.FAPi | 39 (88.6%) | 221 (89.1%) | - | 14 (93.3%) | 88 (100%) | - | 42 (100%) | |
| p-value | 0.001 | 0.0001 | - | 0.096 | 0.785 | - | 0.0001 | |
| SULpeak | ||||||||
| [18F]F-FDG | 5.8 (2.5–11.7) | 3.8 (2.4–6.6) | 3.7 (2.3–9) | 5.3 (2–8) | 5.3 (3.1–11.9) | 8.1 (4.5–11.6) | 5.5 (3.5–6.6) | |
| [68Ga]Ga-DOTA. SA.FAPi | 6.6 (4.1–11.9) | 4.9 (2.9–7.8) | 4.6 (3.2–7.2) | 9.8 (3.2–9.2) | 10.3 (6–4) | 9.4 (5.4–15.4) | 8.8 (7.6–10.7) | |
| p-value | 0.3369 | 0.0453 | 0.523 | 0.0001 | 0.0419 | 0.114 | 0.1309 | |
| SULavg | ||||||||
| [18F]F-FDG | 3.8 | 2.1 (1.2–3.8) | 3.7 (2.3–9) | 2.6 (1.7–4.4) | 3.9 (1.6–7.1) | 4.7 (2.4–7.02) | 3.2 (1.7–3.9) | |
| [68Ga]Ga-DOTA. SA.FAPi | 5.4 | 2.8 (1.6–5.2) | 4.6 (3.2–7.2) | 5.8 (3–7) | 5.5 (3.3–11.7) | 5.3 (2.9–8.6) | 5.3 (4.3–6.8) | |
| p-value | 0.351 | 0.0431 | 0.523 | 0.0001 | 0.017 | 0.330 | 0.139 | |
| TBR (SUVpeak) | ||||||||
| [18F]F-FDG | 0.9 (0.1–3.4) | 1.3 (0.4–3.6) | 1.62 (1.1–4.08) | 1.6 (0.6–2.3) | 1.8 (1.2–3.2) | 2.87 (1.96–0.13) | 6.8 (1.9–9.8) | |
| [68Ga]Ga-DOTA. SA.FAPi | 1.2 (0.2–4.3) | 2.3 (0.9–4.2) | 1.8 (1.1–2.9) | 3.2 (1.1–4.2) | 3.8 (2.7–6.6) | 4.4 (3.2–9.3) | 22.3 (11.6–35.6) | |
| p-value | 0.5434 | 0.0129 | 0.3570 | 0.0023 | 0.0472 | 0.134 | <0.0001 |
| Patient S.No | CT | [68Ga]Ga-DOTA.SA.FAPi | [18F]F-FDG |
|---|---|---|---|
| 11. | 0 | 0 | 1 |
| 12. | 1 | 1 | 0 |
| 18. | 1 | 1 | 0 |
| 21. | 2 | 1 | 1 |
| 24. | 2 | 1 | 1 |
| 25. | 0 | 0 | 1 |
| 29. | 2 | 2 | 1 |
| 32. | 4 | 2 | 2 |
| 35. | 1 | 0 | 0 |
| 37. | 0 | 0 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ballal, S.; Yadav, M.P.; Roesch, F.; Wakade, N.; Raju, S.; Sheokand, P.; Mishra, P.; Moon, E.S.; Tripathi, M.; Martin, M.; et al. Head-to-Head Comparison between [68Ga]Ga-DOTA.SA.FAPi and [18F]F-FDG PET/CT Imaging in Patients with Breast Cancer. Pharmaceuticals 2023, 16, 521. https://doi.org/10.3390/ph16040521
Ballal S, Yadav MP, Roesch F, Wakade N, Raju S, Sheokand P, Mishra P, Moon ES, Tripathi M, Martin M, et al. Head-to-Head Comparison between [68Ga]Ga-DOTA.SA.FAPi and [18F]F-FDG PET/CT Imaging in Patients with Breast Cancer. Pharmaceuticals. 2023; 16(4):521. https://doi.org/10.3390/ph16040521
Chicago/Turabian StyleBallal, Sanjana, Madhav P. Yadav, Frank Roesch, Nicky Wakade, Shobhana Raju, Parvind Sheokand, Prashant Mishra, Euy Sung Moon, Madhavi Tripathi, Marcel Martin, and et al. 2023. "Head-to-Head Comparison between [68Ga]Ga-DOTA.SA.FAPi and [18F]F-FDG PET/CT Imaging in Patients with Breast Cancer" Pharmaceuticals 16, no. 4: 521. https://doi.org/10.3390/ph16040521
APA StyleBallal, S., Yadav, M. P., Roesch, F., Wakade, N., Raju, S., Sheokand, P., Mishra, P., Moon, E. S., Tripathi, M., Martin, M., & Bal, C. (2023). Head-to-Head Comparison between [68Ga]Ga-DOTA.SA.FAPi and [18F]F-FDG PET/CT Imaging in Patients with Breast Cancer. Pharmaceuticals, 16(4), 521. https://doi.org/10.3390/ph16040521







