Production, Characterization, and In Vitro and In Vivo Studies of Nanoemulsions Containing St. John’s Wort Plant Constituents and Their Potential for the Treatment of Depression
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of the System
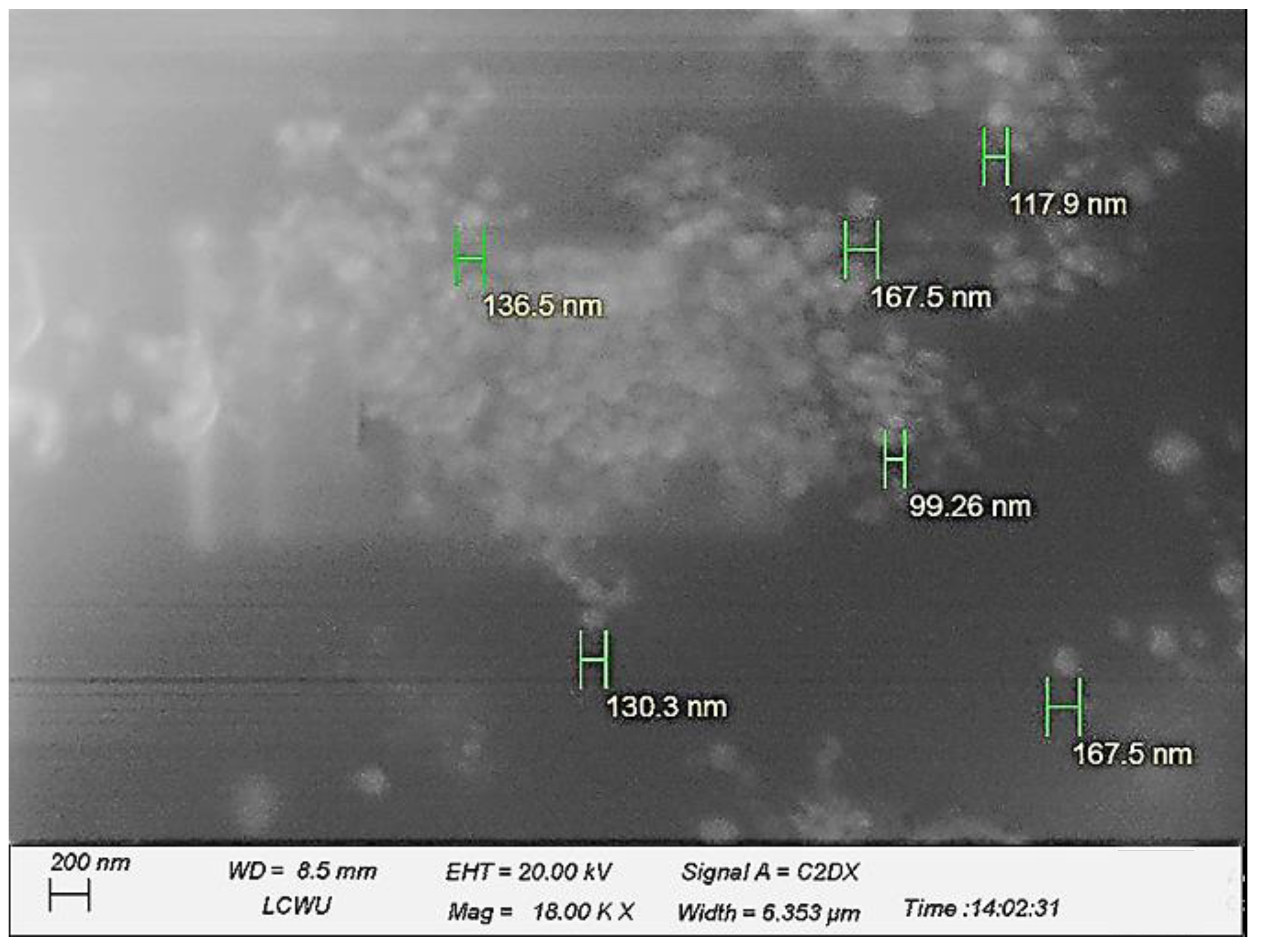
2.2. Morphology Analysis of the System
2.3. Dilutability Test
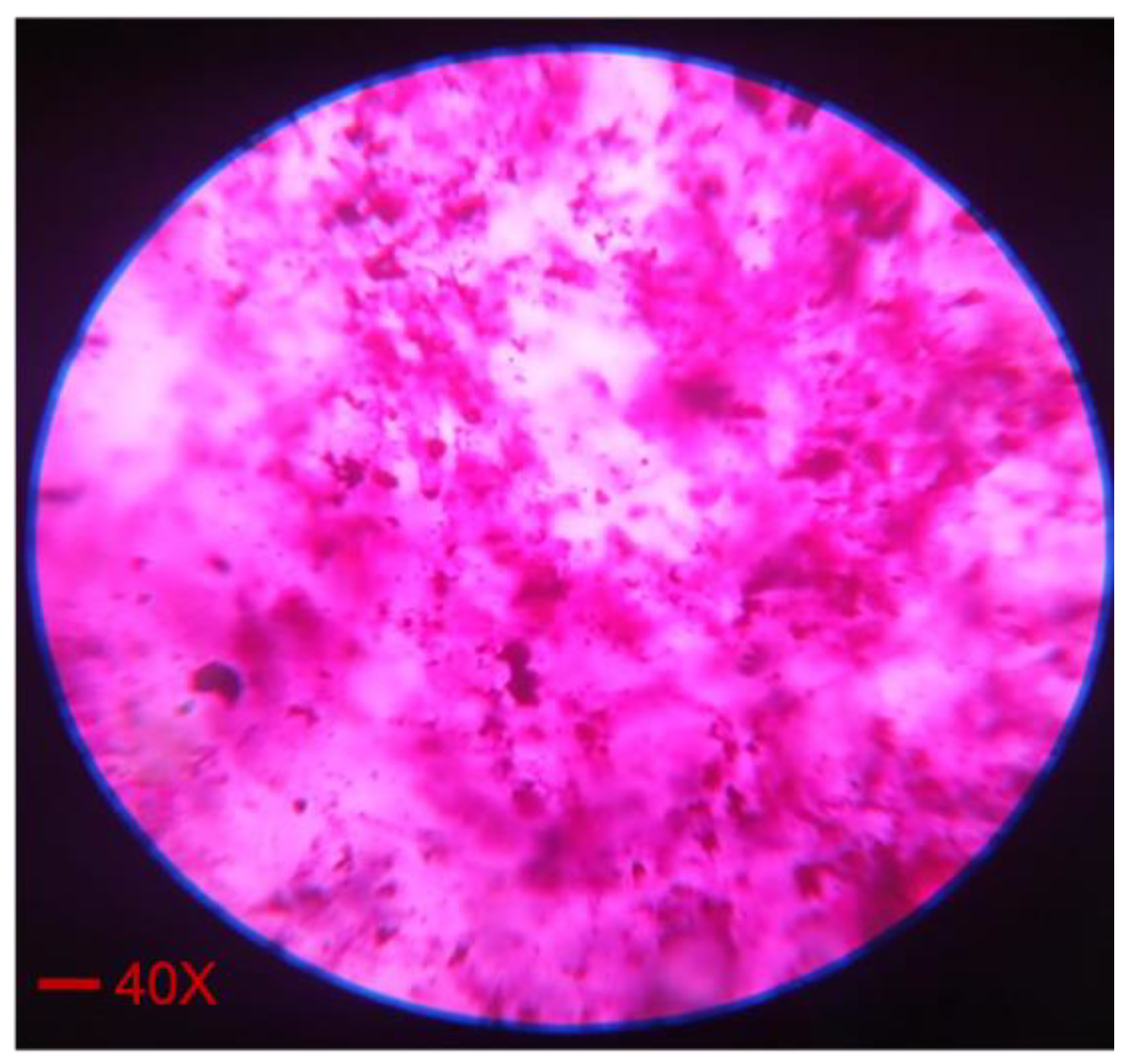
2.4. Dye Solubility Test
2.5. pH, Refractive Index, and Viscosity
2.6. In Vitro Drug Release Studies
2.7. Drug Solubility Studies
2.8. Stability Studies
2.9. In Vivo Studies
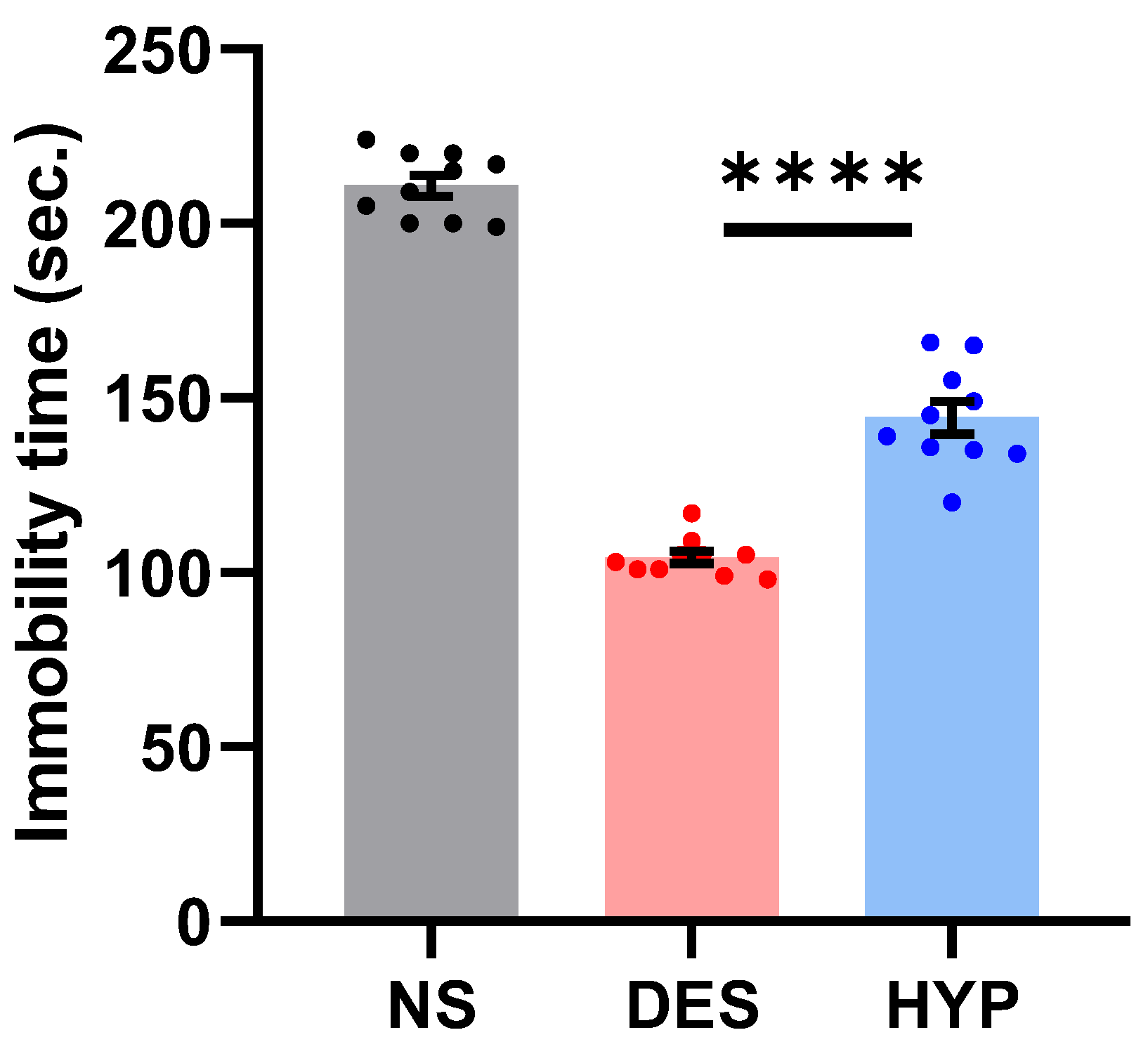
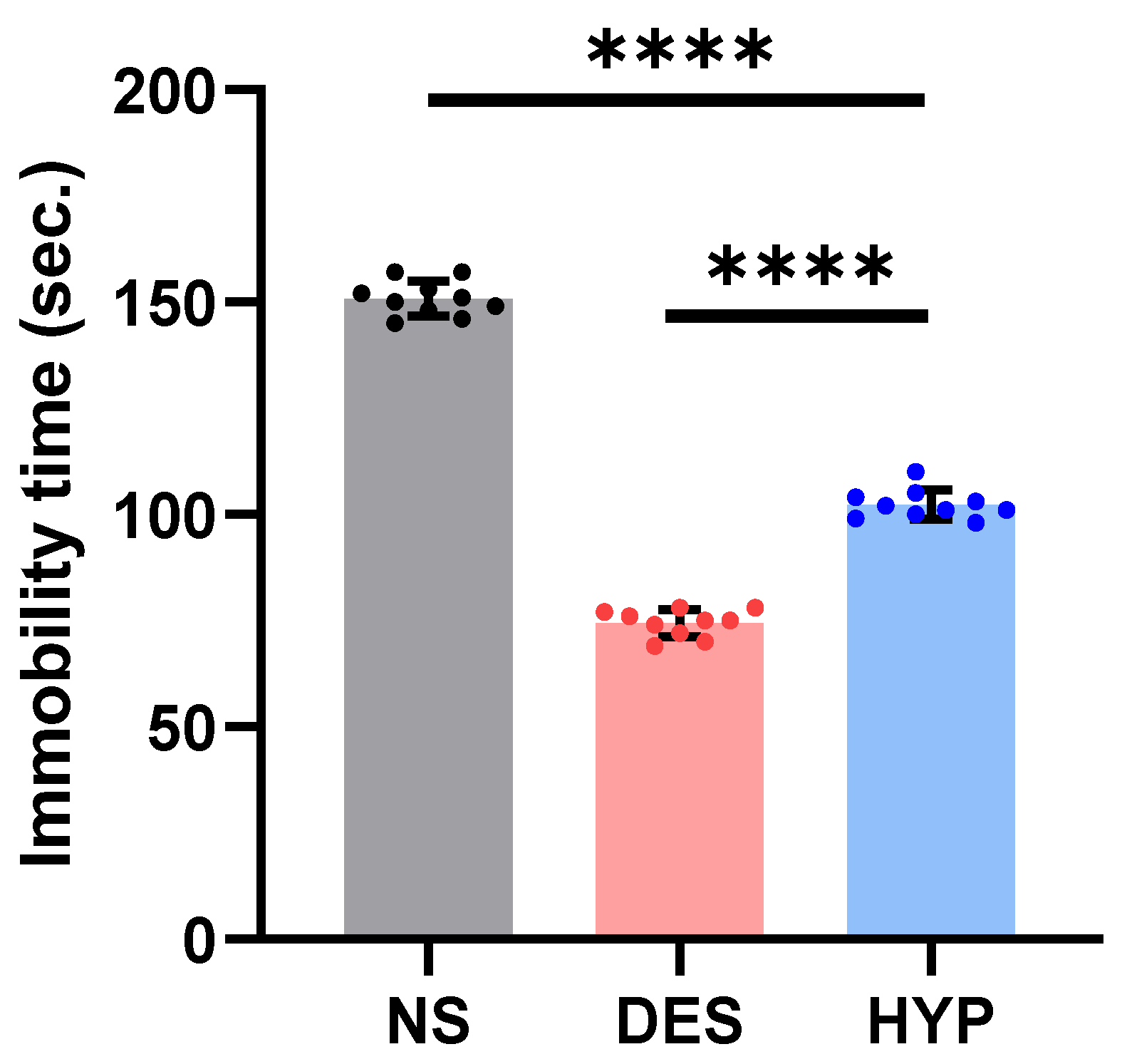
2.9.1. Forced Swim Test (FST)
2.9.2. Tail Suspension Test
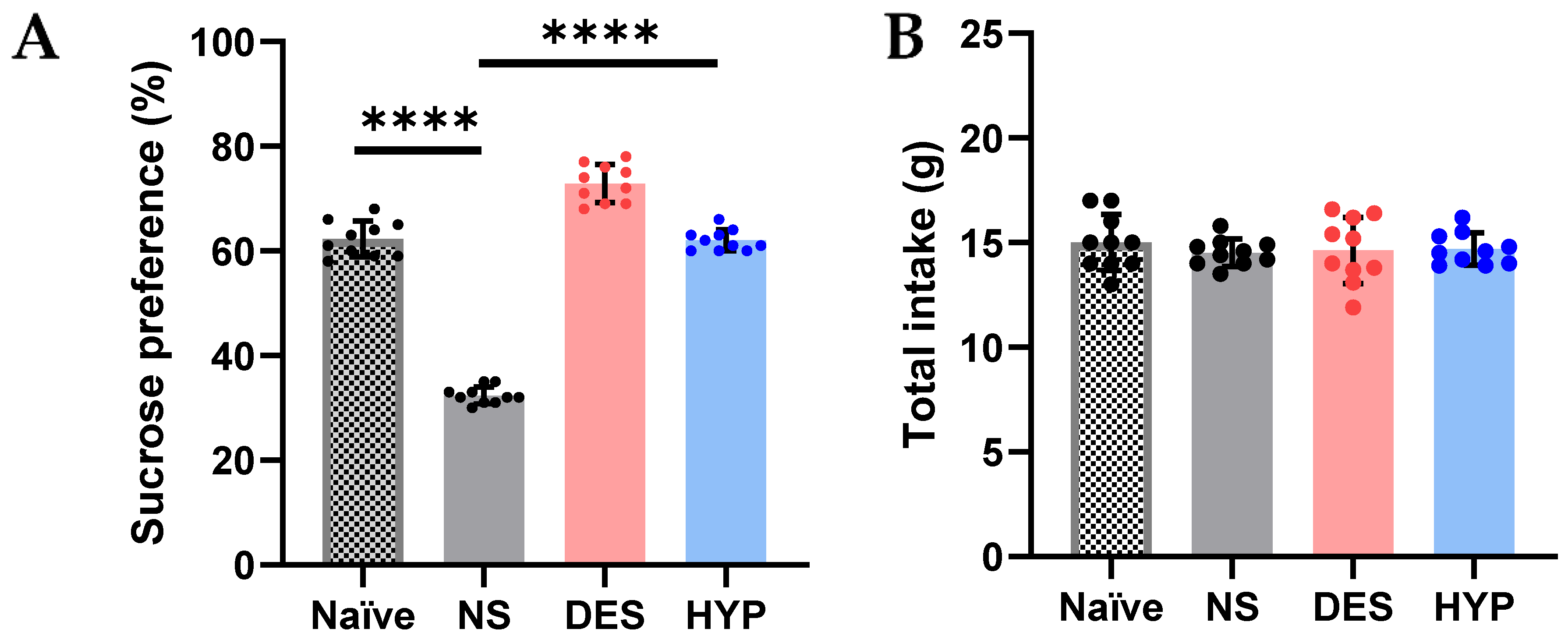
2.9.3. Sucrose Preference Test
3. Materials and Methods
3.1. Materials
3.2. Method
3.3. Characterization of Nanoemulsions
3.3.1. Zeta Potential
3.3.2. Dilutability Test
3.3.3. Dye Solubility Test
3.3.4. Scanning Electron Microscopy (SEM)
3.3.5. In Vitro Drug Release Studies
3.3.6. Drug Solubility Studies
3.3.7. Stability Studies
3.3.8. In Vivo Studies
Forced Swim Test (FST)
Tail Suspension Test
3.3.9. Chronic Mild Stress Mouse Model and Sucrose Preference Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- Singh, Y.; Meher, J.G.; Raval, K.; Khan, F.A.; Chaurasia, M.; Jain, N.K.; Chourasia, M.K. Nanoemulsion: Concepts, development and applications in drug delivery. J. Control. Release 2017, 252, 28–49. [Google Scholar] [CrossRef] [PubMed]
- McClements, D. Nanoemulsions versus microemulsions: Terminology, differences, and similarities. Soft Matter 2012, 8, 1719–1729. [Google Scholar] [CrossRef]
- Amselem, S.; Friedman, D. Submicron Emulsions as Drug Carriers for Topical Administration. In Submicron Emulsions in Drug Targeting and Delivery; CRC Press: Boca Raton, FL, USA, 2019; pp. 153–174. [Google Scholar] [CrossRef]
- Tan, S.L.; Stanslas, J.; Basri, M.; Abedi Karjiban, R.A.; Kirby, B.P.; Sani, D.; Basri, H.B. Nanoemulsion-based Parenteral Drug Delivery System of Carbamazepine: Preparation, Characterization, Stability Evaluation and Blood-Brain Pharmacokinetics. Curr. Drug Deliv. 2015, 12, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Tayeb, H.H.; Sainsbury, F. Nanoemulsions in drug delivery: Formulation to medical application. Nanomedicine 2018, 13, 2507–2525. [Google Scholar] [CrossRef]
- Wimalasiri, V.W.; Dunuweera, S.P.; Dunuweera, A.N.; Rajapakse, R.M.G. Noyes-Whitney Dissolution Model-Based pH-Sensitive Slow Release of Paclitaxel (Taxol) from Human Hair-Derived Keratin Microparticle Carriers. Biomed. Res. Int. 2021, 2021, 6657482. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.P.; He, S.N.; Li, Y.L.; Feng, D.L.; Lu, X.Y.; Du, Y.Z.; Yu, H.Y.; Hu, F.Q.; Yuan, H. Preparation and characteristics of lipid nanoemulsion formulations loaded with doxorubicin. Int. J. Nanomed. 2013, 8, 3141–3150. [Google Scholar] [CrossRef]
- Cuomo, F.; Perugini, L.; Marconi, E.; Messia, M.C.; Lopez, F. Enhanced Curcumin Bioavailability through Nonionic Surfactant/Caseinate Mixed Nanoemulsions. J. Food Sci. 2019, 84, 2584–2591. [Google Scholar] [CrossRef]
- Hilbig, J.; Ma, Q.; Davidson, P.M.; Weiss, J.; Zhong, Q. Physical and antimicrobial properties of cinnamon bark oil co-nanoemulsified by lauric arginate and Tween 80. Int. J. Food Microbiol. 2016, 233, 52–59. [Google Scholar] [CrossRef]
- Hu, D.; Ogawa, K.; Kajiyama, M.; Enomae, T. Characterization of self-assembled silver nanoparticle ink based on nanoemulsion method. R. Soc. Open Sci. 2020, 7, 200296. [Google Scholar] [CrossRef]
- Jadhav, C.; Kate, V.; Payghan, S.A. Investigation of effect of non-ionic surfactant on preparation of griseofulvin non-aqueous nanoemulsion. J. Nanostruct. Chem. 2015, 5, 107–113. [Google Scholar] [CrossRef]
- Mohamed, F.F.; Anhlan, D.; Schöfbänker, M.; Schreiber, A.; Classen, N.; Hensel, A.; Hempel, G.; Scholz, W.; Kühn, J.; Hrincius, E.R.; et al. Hypericum perforatum and Its Ingredients Hypericin and Pseudohypericin Demonstrate an Antiviral Activity against SARS-CoV-2. Pharmaceuticals 2022, 15, 530. [Google Scholar] [CrossRef] [PubMed]
- Kubin, A.; Wierrani, F.; Burner, U.; Alth, G.; Grünberger, W. Hypericin—The facts about a controversial agent. Curr. Pharm. Des. 2005, 11, 233–253. [Google Scholar] [CrossRef] [PubMed]
- Brockmann, H.; Haschad, M.N.; Maier, K.; Pohl, F. Über das Hypericin, den photodynamisch wirksamen Farbstoff aus Hypericum perforatum. Naturwissenschaften 1939, 27, 550. [Google Scholar] [CrossRef]
- Brockmann, H.; Pohl, F.; Maier, K.; Haschad, M.N. Über das Hypericin, den photodynamischen Farbstoff des Johanniskrautes (Hypericum perforatum). Liebigs Ann. Chem. 1942, 553, 1–52. [Google Scholar] [CrossRef]
- Brockmann, H.; Falkenhausen, E.-H.F.v.; Dorlars, A. Die Konstitution des Hypericins. Naturwissenschaften 1950, 37, 540. [Google Scholar] [CrossRef]
- Birt, D.F.; Widrlechner, M.P.; Hammer, K.D.; Hillwig, M.L.; Wei, J.; Kraus, G.A.; Murphy, P.A.; McCoy, J.; Wurtele, E.S.; Neighbors, J.D.; et al. Hypericum in infection: Identification of anti-viral and anti-inflammatory constituents. Pharm. Biol. 2009, 47, 774–782. [Google Scholar] [CrossRef]
- Chen, H.; Muhammad, I.; Zhang, Y.; Ren, Y.; Zhang, R.; Huang, X.; Diao, L.; Liu, H.; Li, X.; Sun, X.; et al. Antiviral Activity against Infectious Bronchitis Virus and Bioactive Components of Hypericum perforatum L. Front. Pharmacol. 2019, 10, 1272. [Google Scholar] [CrossRef]
- Van Beveren, M.L.; Mueller, S.C.; Braet, C. Emotion dysregulation, temperamental vulnerability, and parental depression in adolescents: Correspondence between physiological and informant-report measures. Dev. Psychopathol. 2019, 31, 1023–1035. [Google Scholar] [CrossRef]
- Lowe, H.; Toyang, N.; Steele, B.; Grant, J.; Ali, A.; Gordon, L.; Ngwa, W. Psychedelics: Alternative and Potential Therapeutic Options for Treating Mood and Anxiety Disorders. Molecules 2022, 27, 2520. [Google Scholar] [CrossRef]
- Och, A.; Och, M.; Nowak, R.; Podgórska, D.; Podgórski, R. Berberine, a Herbal Metabolite in the Metabolic Syndrome: The Risk Factors, Course, and Consequences of the Disease. Molecules 2022, 27, 1351. [Google Scholar] [CrossRef] [PubMed]
- Zirak, N.; Shafiee, M.; Soltani, G.; Mirzaei, M.; Sahebkar, A. Hypericum perforatum in the treatment of psychiatric and neurodegenerative disorders: Current evidence and potential mechanisms of action. J. Cell. Physiol. 2019, 234, 8496–8508. [Google Scholar] [CrossRef] [PubMed]
- Barnes, J.; Anderson, L.A.; Phillipson, J.D. St John’s wort (Hypericum perforatum L.): A review of its chemistry, pharmacology and clinical properties. J. Pharm. Pharmacol. 2001, 53, 583–600. [Google Scholar] [CrossRef] [PubMed]
- Ishkeh, S.R.; Shirzad, H.; Asghari, M.; Alirezalu, A.; Pateiro, M.; Lorenzo, J.M. Effect of Chitosan Nanoemulsion on Enhancing the Phytochemical Contents, Health-Promoting Components, and Shelf Life of Raspberry (Rubus sanctus Schreber). Appl. Sci. 2021, 11, 2224. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, S.; Jiang, W.; Zhao, H.; Fu, J. Ability of casein hydrolysate-carboxymethyl chitosan conjugates to stabilize a nanoemulsion: Improved freeze-thaw and pH stability. Food Hydrocoll. 2020, 101, 105452. [Google Scholar] [CrossRef]
- Bozkir, A.; Saka, O.M. Chitosan nanoparticles for plasmid DNA delivery: Effect of chitosan molecular structure on formulation and release characteristics. Drug Deliv. 2004, 11, 107–112. [Google Scholar] [CrossRef]
- Gurpreet, K.; Singh, S.K. Review of Nanoemulsion Formulation and Characterization Techniques. Indian J. Pharm. Sci. 2018, 80, 781–789. [Google Scholar] [CrossRef]
- Bhosale, R.; Osmani, R.; Ghodake, P.; Shaikh, S.; Chavan, S. Nanoemulsion: A Review on Novel Profusion in Advanced Drug Delivery. Indian J. Pharm. Biol. Res. 2014, 2, 122. [Google Scholar] [CrossRef]
- Bonferoni, M.C.; Sandri, G.; Rossi, S.; Usai, D.; Liakos, I.; Garzoni, A.; Fiamma, M.; Zanetti, S.; Athanassiou, A.; Caramella, C.; et al. A novel ionic amphiphilic chitosan derivative as a stabilizer of nanoemulsions: Improvement of antimicrobial activity of Cymbopogon citratus essential oil. Colloids Surf. B 2017, 152, 385–392. [Google Scholar] [CrossRef]
- Choi, A.-J.; Kim, C.-J.; Cho, Y.-J.; Hwang, J.-K.; Kim, C.-T.J.F.; Technology, B. Characterization of capsaicin-loaded nanoemulsions stabilized with alginate and chitosan by self-assembly. Food Bioprocess Technol. 2011, 4, 1119–1126. [Google Scholar] [CrossRef]
- Sun, X.; Sheng, Y.; Li, K.; Sai, S.; Feng, J.; Li, Y.; Zhang, J.; Han, J.; Tian, B. Mucoadhesive phenylboronic acid conjugated chitosan oligosaccharide-vitamin E copolymer for topical ocular delivery of voriconazole: Synthesis, in vitro/vivo evaluation, and mechanism. Acta Biomater. 2022, 138, 193–207. [Google Scholar] [CrossRef] [PubMed]
- Ranaldi, G.; Marigliano, I.; Vespignani, I.; Perozzi, G.; Sambuy, Y. The effect of chitosan and other polycations on tight junction permeability in the human intestinal Caco-2 cell line(1). J. Nutr. Biochem. 2002, 13, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Dou, H.-S.; Chen, X. Effect of particle diameter and injection position on the separation performance of cyclone separators. J. Comput. Multiph. Flows 2016, 8, 40–47. [Google Scholar] [CrossRef]
- Pan, J.; Shen, Q.; Cui, X.; Wu, J.; Ma, L.; Tian, C.; Fu, P.; Wang, H. Cyclones of different sizes and underflow leakage for aerosol particles separation enhancement. J. Clean. Prod. 2021, 280, 124379. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Ebrahimzadeh, M.A.; Abdi, M.; Arimi, Y.; Fathi, H. Antidepressant activities of Feijoa sellowiana fruit. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 2510–2513. [Google Scholar]
- Peng, W.H.; Lo, K.L.; Lee, Y.H.; Hung, T.H.; Lin, Y.C. Berberine produces antidepressant-like effects in the forced swim test and in the tail suspension test in mice. Life Sci. 2007, 81, 933–938. [Google Scholar] [CrossRef]
- Galdino, P.M.; Nascimento, M.V.; Sampaio, B.L.; Ferreira, R.N.; Paula, J.R.; Costa, E.A. Antidepressant-like effect of Lafoensia pacari A. St.-Hil. ethanolic extract and fractions in mice. J. Ethnopharmacol. 2009, 124, 581–585. [Google Scholar] [CrossRef]
- Gunaydin, L.A.; Kreitzer, A.C. Cortico-Basal Ganglia Circuit Function in Psychiatric Disease. Annu. Rev. Physiol. 2016, 78, 327–350. [Google Scholar] [CrossRef]
- Kobayashi, T.; Hayashi, E.; Shimamura, M.; Kinoshita, M.; Murphy, N.P. Neurochemical responses to antidepressants in the prefrontal cortex of mice and their efficacy in preclinical models of anxiety-like and depression-like behavior: A comparative and correlational study. Psychopharmacology 2008, 197, 567–580. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, Z.; Pan, X.; Yang, J.; Wu, C. Antidepressant-like effects of Xiaochaihutang in perimenopausal mice. J. Ethnopharmacol. 2020, 248, 112318. [Google Scholar] [CrossRef]
- Bouvier, E.; Brouillard, F.; Molet, J.; Claverie, D.; Cabungcal, J.H.; Cresto, N.; Doligez, N.; Rivat, C.; Do, K.Q.; Bernard, C.; et al. Nrf2-dependent persistent oxidative stress results in stress-induced vulnerability to depression. Mol. Psychiatry 2017, 22, 1701–1713. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xiu, Z.; Du, Y.; Li, Y.; Yang, J.; Gao, Y.; Li, F.; Yin, X.; Shi, H. Brazilin Treatment Produces Antidepressant- and Anxiolytic-Like Effects in Mice. Biol. Pharm. Bull. 2019, 42, 1268–1274. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Cao, F.; Liu, Q.; Li, X.; Xu, G.; Liu, G.; Zhang, Y.; Yang, X.; Yi, S.; Xu, F.; et al. Behavioral, inflammatory and neurochemical disturbances in LPS and UCMS-induced mouse models of depression. Behav. Brain Res. 2019, 364, 494–502. [Google Scholar] [CrossRef]
- Su, W.J.; Zhang, Y.; Chen, Y.; Gong, H.; Lian, Y.J.; Peng, W.; Liu, Y.Z.; Wang, Y.X.; You, Z.L.; Feng, S.J.; et al. NLRP3 gene knockout blocks NF-κB and MAPK signaling pathway in CUMS-induced depression mouse model. Behav. Brain Res. 2017, 322, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.; Li, M.; Wen, J.; Ren, F.; Yang, Z.; Jiang, X.; Chen, Y. The bioactivity and applications of pomegranate peel extract: A review. J. Food Biochem. 2022, 46, e14105. [Google Scholar] [CrossRef] [PubMed]
- Popescu, I.D.; Codrici, E.; Mihai, S.; Luntraru, C.M.; Neagu, M.; Tanase, C. In vitro assessment of the cytotoxicity and anti-inflammatory properties of a novel dietary supplement. Exp. Ther. Med. 2021, 22, 1170. [Google Scholar] [CrossRef]
- Jantas, D. Cell-Based Systems of Depression: An Overview. In Herbal Medicine in Depression: Traditional Medicine to Innovative Drug Delivery; Springer: Berlin/Heidelberg, Germany, 2016; pp. 75–117. [Google Scholar] [CrossRef]
- Waqas, M.K.; Sadia, H.; Khan, M.I.; Omer, M.O.; Siddique, M.I.; Qamar, S.; Zaman, M.; Butt, M.H.; Mustafa, M.W.; Rasool, N. Development and characterization of niosomal gel of fusidic acid: In-vitro and ex-vivo approaches. Des. Monomers Polym. 2022, 25, 165–174. [Google Scholar] [CrossRef]
- Salawi, A.; Khan, A.; Zaman, M.; Riaz, T.; Ihsan, H.; Butt, M.H.; Aman, W.; Khan, R.; Majeed, I.; Almoshari, Y. Development of Statistically Optimized Chemically Cross-Linked Hydrogel for the Sustained-Release Delivery of Favipiravir. Polymers 2022, 14, 2369. [Google Scholar] [CrossRef]
- Khan, R.; Zaman, M.; Salawi, A.; Khan, M.A.; Iqbal, M.O.; Riaz, R.; Ahmed, M.M.; Butt, M.H.; Alvi, M.N.; Almoshari, Y. Synthesis of Chemically Cross-Linked PH-Sensitive Hydrogels for the Sustained Delivery of Ezetimibe. Gels 2022, 8, 281. [Google Scholar] [CrossRef]
- Zaman, M.; Hanif, M. In vitro and ex vivo assessment of hydrophilic polymer- and plasticizer-based thin buccal films designed by using central composite rotatable design for the delivery of meloxicam. Adv. Polym. Technol. 2018, 37, 1823–1836. [Google Scholar] [CrossRef]
- Shafiq, S.; Shakeel, F.; Talegaonkar, S.; Ahmad, F.J.; Khar, R.K.; Ali, M. Development and bioavailability assessment of ramipril nanoemulsion formulation. Eur. J. Pharm. Biopharm. 2007, 66, 227–243. [Google Scholar] [CrossRef] [PubMed]
- Cekic, N.D.; Savic, S.M.; Savic, S.D. Dynamic-mechanical thermoanalysis test: A rapid alternative for accelerated freeze-thaw stability evaluation of W/O emulsions. Drug Dev. Ind. Pharm. 2019, 45, 1896–1906. [Google Scholar] [CrossRef] [PubMed]
- Al Haj Sleiman, S.; Izoret, L.; Alam, S.Y.; Grondin, F.; Loukili, A. Freeze–thaw field exposure and testing the reliability of performance test temperature cycle for concrete scaling in presence of de-icing salts. Mater. Struct. 2021, 55, 2. [Google Scholar] [CrossRef]
- Kwon, S.; Lee, B.; Kim, M.; Lee, H.; Park, H.J.; Hahm, D.H. Antidepressant-like effect of the methanolic extract from Bupleurum falcatum in the tail suspension test. Prog. Neuropsychopharmacol. Biol. 2010, 34, 265–270. [Google Scholar] [CrossRef]


| Formulations | Particle Size (nm) | Zeta Potential (mV) |
|---|---|---|
| F1 | 130 ± 06 | +72.7 |
| F2 | 133 ± 04 | +58.5 |
| F3 | 139 ± 07 | +56.5 |
| F4 | 141 ± 05 | +89.6 |
| Formulations | pH | Refractive Index (n) | Viscosity (cP) |
|---|---|---|---|
| F1 | 5.34 | 1.3425 | 11.61 |
| F2 | 6.11 | 1.3432 | 15.70 |
| F3 | 5.90 | 1.3422 | 28.09 |
| F4 | 5.14 | 1.3491 | 49.28 |
| Formulations | Oil Phase (mL) | Aqueous Phase (mL) | Chitosan (%) |
|---|---|---|---|
| F1 | 0.5 | 9.5 | 0.4 |
| F2 | 0.5 | 9.5 | 0.5 |
| F3 | 0.5 | 9.5 | 0.6 |
| F4 | 0.5 | 9.5 | 0.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salawi, A.; Almoshari, Y.; Sultan, M.H.; Madkhali, O.A.; Bakkari, M.A.; Alshamrani, M.; Safhi, A.Y.; Sabei, F.Y.; Al Hagbani, T.; Ali, M.S.; et al. Production, Characterization, and In Vitro and In Vivo Studies of Nanoemulsions Containing St. John’s Wort Plant Constituents and Their Potential for the Treatment of Depression. Pharmaceuticals 2023, 16, 490. https://doi.org/10.3390/ph16040490
Salawi A, Almoshari Y, Sultan MH, Madkhali OA, Bakkari MA, Alshamrani M, Safhi AY, Sabei FY, Al Hagbani T, Ali MS, et al. Production, Characterization, and In Vitro and In Vivo Studies of Nanoemulsions Containing St. John’s Wort Plant Constituents and Their Potential for the Treatment of Depression. Pharmaceuticals. 2023; 16(4):490. https://doi.org/10.3390/ph16040490
Chicago/Turabian StyleSalawi, Ahmad, Yosif Almoshari, Muhammad H. Sultan, Osama A. Madkhali, Mohammed Ali Bakkari, Meshal Alshamrani, Awaji Y. Safhi, Fahad Y. Sabei, Turki Al Hagbani, Md Sajid Ali, and et al. 2023. "Production, Characterization, and In Vitro and In Vivo Studies of Nanoemulsions Containing St. John’s Wort Plant Constituents and Their Potential for the Treatment of Depression" Pharmaceuticals 16, no. 4: 490. https://doi.org/10.3390/ph16040490
APA StyleSalawi, A., Almoshari, Y., Sultan, M. H., Madkhali, O. A., Bakkari, M. A., Alshamrani, M., Safhi, A. Y., Sabei, F. Y., Al Hagbani, T., Ali, M. S., & Alam, M. S. (2023). Production, Characterization, and In Vitro and In Vivo Studies of Nanoemulsions Containing St. John’s Wort Plant Constituents and Their Potential for the Treatment of Depression. Pharmaceuticals, 16(4), 490. https://doi.org/10.3390/ph16040490








